Abstract
Sulforaphane (SFN) is a bioactive phytonutrient found in cruciferous vegetables. There is a lack of detailed information on the lactational transfer of SFN and SFN metabolites, and potential pharmacological effects on breastfeeding infants. We carried out two maternal supplementation studies in a mouse model, wherein lactating dams received either vehicle, 300 or 600 ppm SFN from postnatal day (PND) 1 to 5, or in a second experiment, vehicle or 600 ppm SFN from PND 1 to 14. The parent compound was only detectable in milk and plasma from dams receiving 600 ppm SFN for five days. The predominant metabolite SFN-N-acetylcysteine (SFN-NAC) was readily detected in milk from dams receiving 300 and 600 ppm SFN for five days or 600 ppm for 14 days. Maternal SFN-NAC plasma levels were elevated in both 600 ppm groups. Maternal hepatic and pulmonary expression of NRF2-related genes, Nqo1, Gsta2, Gstm1, and Gstp1, were significantly increased, generally following a dose-response; however, offspring induction varied. PND5 neonates in the 600-ppm group exhibited significantly elevated expression of Nqo1, Gsta2, and Gstp1 in liver, and Gstm1 and Gstp1 in lung. Findings support maternal dietary supplementation with SFN induces NRF2-related gene expression in neonates via lactational transfer of SFN-NAC. However, NQO1 enzyme activity was not significantly elevated, highlighting the need to optimize dosing strategy. Additionally, in a pilot investigation of lactating women consuming a typical diet, without any purified SFN supplementation, 7 out of 8 breast milk samples showed SFN-NAC above the limit of quantification (LOQ). Notably, the one sample below the LOQ was collected from the only participant who reported no consumption of cruciferous vegetables in the past 24 hours. The parent compound was not detected in any of the human breast milk samples. Overall, these data indicate lactational transfer of SFN-NAC at dietary relevant levels. Future studies are needed to evaluate pharmacokinetics and pharmacodynamics of lactational transfer for potential preventive or therapeutic effects in breastfeeding children.
Keywords: Nutraceutical, cruciferous vegetables, breast milk, lactational transfer, NRF2
1. Introduction
Sulforaphane (SFN) is an isothiocyanate commonly found in cruciferous vegetables as its precursor glucoraphanin (GF). Numerous preclinical studies and clinical trials with cruciferous vegetables, GF or purified SFN describe the pharmacokinetics, pharmacodynamics, and efficacy in diverse disease mitigation, including cancer chemoprevention (Yagishita et al., 2019). SFN activates the Nrf2 antioxidant response pathway through reactivity with cysteines in the KEAP1 repressor protein, with evidence of increased downstream target gene expression, including glutathione S-transferases (Gst) and NAD(P)H dehydrogenase quinone 1 (Nqo1) (Kensler et al. 2013). The chemoprotective efficacy of SFN is diminished in Nrf2-deficient mice, shown by several models, supporting a central role of Nrf2 in the mechanism of action of SFN (Dinkova-Kostova et al., 2017). Indeed, SFN is a potent Nrf2 inducer; moreover, its high bioavailability facilitates practical doses which can yield significant clinical response (Houghton, 2019). Collectively, daily SFN dosing from ~20–40 mg produced beneficial outcomes in several prior clinical trials. These include improvements in conditions such as asthma and type 2 diabetes (Brown et al., 2015; Bahadoran et al., 2011). Clinical data also highlight the ability of SFN, administered via broccoli sprout beverage, to activate phase II metabolism to enhance detoxication of air toxics (Egner et al., 2014). Biomarker data from a randomized, placebo-controlled, multidose trial demonstrated a dose-dependent detoxication of benzene, even at a dose yielding urinary elimination of ~25 μmol SFN metabolites per day (Chen et al., 2019).
Past clinical trials also investigated the ability of SFN to mitigate infectious disease. For instance, previous studies demonstrate SFN’s bactericidal effect on Helicobacter pylori infection (Galan et al., 2004; Yanaka et al., 2009). Furthermore, broccoli sprout treatment suppressed the upregulation of inflammatory markers associated with H. pylori infection in a wild type but not in Nrf2-knockout mice (Yanaka et al., 2009). Additionally, Cho et al. (2009) demonstrated SFN pretreatment protected against sequalae from respiratory syncytial virus (RSV) infection in wild type, but not Nrf2 null mice. RSV is a common airway pathogen associated with severe lower respiratory illness characterized by bronchiolitis and respiratory failure (Piedimonte and Perez, 2014). RSV infection is the leading cause of infant hospitalization, and in the United States an estimated 58,000 children under the age of 5 require hospitalization every year (Rha et al., 2020). RSV disease severity in infants results from an exaggerated Th2 response, in addition to a substantial production of reactive oxygen species (Russell et al., 2017). Moreover, the downregulation of several antioxidant-related genes (i.e., Nqo1, Gsta2, Gpx2) is due in part to increased proteasomal degradation of NRF2 (Hosakote et al., 2012a; Hosakote et al., 2012b; Komaravelli et al., 2015). Currently, there are limited prophylactic or therapeutic options. A recently approved vaccine for pregnant women offers protection; however, lagging uptake and options for children born to unvaccinated mothers warrants additional options. Thus, a nutraceutical-based approach may offer a promising strategy for this susceptible population, as infants six months and younger are at high risk for RSV morbidity (Palliyaguru et al., 2018).
Among infants born in the United States in 2019, the majority (83.2%) received some breast milk, with over 75% receiving any breast milk at 1 month of age. At 6 months, 55.8% of infants received any breast milk, and 24.9% received breast milk exclusively (CDC, 2020). Phytochemicals in breast milk may contribute to antioxidant protection, however limited studies have investigated their role (mainly carotenoids and flavonoids) in infant oxidative status and disease protection (Tsopmo, 2018). In a mouse model of maternal immune activation, Fujita et al. (2020) demonstrated dietary intake of GF during pregnancy and lactation prevented behavioral abnormalities in offspring. Other mouse models employing SFN supplementation during pregnancy show induction of Nqo1 and Gstm1 in dams with potential implication for fetal toxicant protection (Philbrook and Winn, 2014). While placental transfer of SFN is established (Shorey et al., 2013), details on lactational transfer are not fully known. Since breastfeeding is an important window for infant protection, the objective of this study was to investigate the lactational transfer of SFN and its predominant metabolite, SFN-N-acetylcysteine (NAC), in vivo in a mouse model and in a pilot investigation of human breast milk.
2. Materials and Methods
2.1. Study Design and Animals
All experiments were approved by the Texas A&M Institutional Animal Care and Use Committee (IACUC) under AUP 2019–0025, originally approved on 07/01/2021. C57Bl/6 male and female mice (6–8 weeks old) were procured in house from the Texas A&M Institute for Genomic Medicine (College Station, Texas) and housed on a 12:12-hour light-dark cycle. Mice were checked daily and provided food and water ad libitum. Mice were mated overnight by placing one male with two females. Potentially pregnant dams were co-housed (two females per cage), and weight gain was assessed daily. When pregnancy was apparent, dams were housed individually and provided cage stimulation and a small hut for nesting to reduce stress prior to delivery, defined as postnatal day (PND) 1. We carried out two separate experiments, wherein lactating dams (3–5 per group) received either vehicle, 300 or 600 ppm SFN from PND 1 to 5, or in a second experiment, vehicle or 600 ppm SFN from PND 1 to 14.
D,L-sulforaphane (S699115) was purchased from Toronto Research Chemicals (Toronto, Canada). To avoid stress on the animals in the postnatal period, we opted to administer the SFN orally in 1 g aliquots of peanut butter, which was placed in the cage each morning. For the 600 ppm doses, 1 mL SFN (1 g) was diluted with 19 mL DMSO to make a 50 mg/mL stock solution. 300 μL of the stock solution was then diluted in 14 mL dH2O, vortexed, then mixed with 10.29 g of powdered peanut butter (PB2 Organic Powdered Peanut Butter; Tifton, Georgia). Next, 1 g aliquots were prepared and stored at −20°C until use (within one week). To make the 300 ppm doses, the stock solution added to dH2O was halved. For the vehicle control, 300 μL DMSO was diluted in 14 mL dH2O and added to 10.29 g of powdered peanut butter. Following the final dose, either on PND5 (experiment 1) or PND14 (experiment 2), pups were separated from dams the following morning (approximately 24 hours after the last dose) for maternal and neonate tissue collection, including both male and female offspring.
2.2. Tissue Collection in vivo
First, pups were euthanized via i.p. injection of EUTHASOL® Euthanasia Solution (pentobarbital sodium and phenytoin sodium). Liver and lung tissue were immediately frozen in liquid nitrogen and stored at −80°C until analysis. Dams were separated for an additional 6 hours to allow for milk accumulation. Dams were anesthetized under isoflurane and injected (i.p.) with 2 IU/kg of oxytocin. Milk was collected in a vacutainer tube using a custom-made system that allowed for gentle pressure to pump milk through sterile tubing. Samples were immediately acidified with 10% trifluoroacetic acid (by vol). Samples were then centrifuged at 16,000 g for 1 min at 4°C, and the supernatant was filtered through a 0.22 μm spin filter. Samples were then flash frozen and stored at −80°C until analysis. Next, dams were euthanized via i.p. injection of EUTHASOL®, and blood and tissues were collected. To prepare plasma, blood was collected in a BD Microtainer® MAP K2EDTA tube and centrifuged at 16,000 g for 1 min at 4°C. Plasma was acidified with 10% trifluoroacetic acid (by vol) and stored at −80°C. Liver and lung tissue were immediately frozen in liquid nitrogen and stored at −80°C until analysis.
2.3. Sample Preparation and UHPLC-MS/MS Analysis of Sulforaphane (SFN) and SFN-NAC in Mouse Plasma and Milk
Mass spectrometry analysis followed methods adapted by Wang et al. (2011) and Egner et al. (2008). For mouse plasma, samples were thawed and centrifuged at 16,000 g for 5 min at 4 °C. The supernatant was filtered using a 0.22 μm spin filter tube, and 100 μL of the supernatant was transferred to amber vials for extraction with methanol-formic acid (100:0.1, v/v). For mouse milk, samples were thawed, and 20 μL was added to an Eppendorf tube containing 200 μL methanol (w/ 0.1% formic acid). Samples were shaken at room temperature for 4 min at 1400 rpm and then centrifuged at 10,000 g for 3 min at 4°C. The supernatant was collected and placed in a glass test tube. The pelleted milk remaining in the Eppendorf tube was extracted a second time. After the second extraction, the supernatants were combined and evaporated to dryness using nitrogen gas. Samples were reconstituted in 100 μL acetonitrile/water (1:1, v/v) and filtered using a 0.22 μm spin filter tube.
Analysis of SFN and SFN-NAC was carried out on an Agilent triple quadrupole mass spectrometer coupled to an Agilent 1290 Infinity II LC System. Chromatographic separation was carried out at 35°C at a flow rate of 200 μL/min on a ZORBAX RRHD StableBond C18 column (2.1 mm x 50 mm, 1.8 μm particle size), using a ZORBAX RRHD StableBond C18 Guard Column. The mobile phase compositions were as follows: (A) water/acetonitrile/formic acid (95/5/0.1) and (B) acetonitrile/formic acid (100/0.1). The initial composition was held at 100% A for 5 min and then ramped to 95% B from 5 to 10 mins. The column was then re-equilibrated to initial conditions. Positive ESI-MS-MS was conducted with MS/MS transitions monitored for SFN at 178.0 → 114.1 and SFN-NAC at 341.0 → 178.1. A linear 9-point standard curve (r2 = 0.999) was generated and used for quantification. The limit of quantification (LOQ) for SFN and SFN-NAC was 0.49 ng/mL.
2.4. RNA Isolation and qRT-PCR Analysis of NRF2-regulated Genes in vivo
We measured expression of NRF2-regulated genes in maternal and neonate liver and lung tissues, in order to assess systemic and localized responses, respectively, for potential mitigation of infant RSV infection. Total RNA was isolated from tissues through mechanical lysis using a glass tissue grinder and pestle and Trizol solution (Invitrogen). RNA isolation included steps involving chloroform + isoamyl alcohol mixture (4.9 mL chloroform + 100 μL isoamyl alcohol), isopropanol, and 3M sodium acetate (pH 5.7). All utensils were sterilized with RNaseZAP™ (Sigma). RNA quality and purity were assessed using a Nanodrop Spectrophotometer (DeNovix DS-11 FX+ V3.35) with 260/280 absorbance values ≤ 1.8. After RNA isolation a gDNA (genomic DNA) wipeout step was used to eliminate any contamination. Total RNA was converted to genomic cDNA using a Quantitect® Reverse Transcriptase Kit (Qiagen). Quantitative real-time PCR was performed with an Applied Biosystems™ Power SYBR™ Green PCR materser mix on a Roche LightCycler® 96. The pre-incubation step had 1 cycle (50°C for 120 s and 95°C for 60 s), 3 step amplification for 45 cycles (94°C for 15 s, 60°C for 30 s, and 72°C for 30 s), and a cooling step for 1 cycle (37°C for 60 s). Primer sequences for selected genes were based on published sequences or specific design (Supplemental Table 1). Gene transcription levels were analyzed using 2–ΔΔCT method (Livak and Schmittgen, 2001).
2.5. NQO1 Activity Assay in vivo
NQO1 activity was assessed in tissues by measuring the conversion rate of NADH to NAD+ using 2,6-dichlorophenolindophenol (DCPIP) as a substrate based on established methods (Benson et al., 1980). Briefly, tissue (~ 20 mg) was homogenized in a 0.25M sucrose solution. Samples were centrifuged at 9000 g for 20 min at 4°C. Next, 0.2 vol of 0.1M CaCl2 was added; samples were incubated on ice for 30 min and centrifuged at 27,000 g for 20 min at 4°C. The reaction mixture was then prepared in a final volume of 3 mL with final concentrations of 25 mM Tris-HCl (pH 7.4), 0.7 mg BSA, 0.01% Tween 20, 5μM FAD, and 0.2mM NADH. The reaction mixture was incubated for 5 min, then 2μL (40mM) DCPIP was added and mixed. The rate of DCPIP reduction was measured over 1 min at 600 nm on a Denovix DS-11FX+ spectrophotometer. In a separate reaction mixture 10μM dicoumarol was added to inhibit the reduction of DCPIP. NQO1 activity was determined by subtracting the rate (with dicoumarol) from the rate (without dicoumarol). Samples were standardized by the amount of protein determined from a 6-point standard curve using a Coomassie blue-based assay.
2.6. Analysis of Sulforaphane (SFN) and SFN-NAC in Human Breast Milk
A pilot study, entitled “Analyzing Breast Milk for Green Nutrients” was initiated in the fall of 2021. The study protocol (IRB2021- 0638) was reviewed and approved by the Texas A&M Institutional Review Board. Eligibility criteria included: being at least 18 years of age, currently breastfeeding and willing to provide a breast milk sample, and weekly consumption of at least one cruciferous vegetable (e.g., broccoli, cauliflower, Brussels sprouts, cabbage, arugula, bok choy or collard greens). Participants were recruited from local daycares and underwent informed consent prior to enrollment. Participants were asked to fill out a short survey, including demographic and dietary information. Participants were asked about their typical weekly consumption of cruciferous vegetables and to specifically to answer if they ate any cruciferous vegetables in the past 24 hours. If participants answered yes, then they weer asked to specify the type and approximate amount, at greater than 1 cup (about two cupped hands), equal to 1 cup , ½ cup (about one cupped hand), or less than ½ cup. Participants provided a sample of expressed breastmilk (~50 mL). Samples were immediately taken to the Texas A&M School of Public Health where they were acidified with 10% formic acid and frozen at −80 °C until analysis. Samples (200 μL) were analyzed as detailed above (section 2.3). However, we added an internal standard (IS) to milk samples since they were hypothesized to be at much lower level. D8-SFN-NAC (250 ppb) IS was provided as a gift from Dr. John Groopman, which was synthesized following methods outlined by Egner et al. (2008). Samples were spiked with 10 μL IS prior to extraction and analysis with MS/MS transitions monitored for SFN at 178.0 → 114.1, D8-SFN at 184.0 → 122.1, SFN-NAC at 341.0 → 178.1, and D8-SFN-NAC at 349.0 → 186.1.
2.6. Statistical Analysis
Statistical analysis was performed with GraphPad Prism 9 (GraphPad Software, San Diego, CA). Murine data from experiment 1 (PND 5) was analyzed via one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons tests to compare dosing groups (0, 300 and 600 ppm SFN). Murine data from experiment 2 (PND 14) was analyzed via Student’s T test to compare dosing groups (0 and 600 ppm SFN). Summary statistics were compiled for human dietary surveys and metabolites in breast milk samples.
3. Results
3.1. Sulforaphane (SFN) and SFN-NAC concentrations in vivo in murine maternal plasma and milk
In the first experiment, lactating dams received vehicle, 300 or 600 ppm SFN in 1 g aliquots of peanut butter from PND 1 through 5. Dams appeared to consume the entire dose upon visual inspection, and maternal plasma and milk samples were collected ~30 hours following the final dose administration. The parent compound was only detected in two of three milk samples in the 600-ppm group in dams fed SFN for five days (mean 1.29 ng/mL) (Supplemental Figure 1). Correspondingly, plasma SFN was also detectable in this group (Supplemental Figure 1). Milk and plasma SFN was not detectable in any of the dams receiving 600 ppm SFN for 14 days.
SFN-NAC levels in milk samples were detectable in all samples from dams receiving SFN and were significantly higher in the 600-ppm group, receiving SFN for five days, on average compared to control (Figure 1A). The SFN-NAC levels from the 600-ppm group were also significantly higher than those of the 300-ppm group. Furthermore, SFN-NAC levels in milk from dams consuming 600 ppm SFN from PND 1 though 14 were significantly higher compared to control (Figure 1C). Additionally, SFN-NAC was detected in maternal plasma in the 300- and 600-ppm groups on PND 5 and PND 14 but did not vary significantly from control (Figure 1 B and D).
Figure 1.
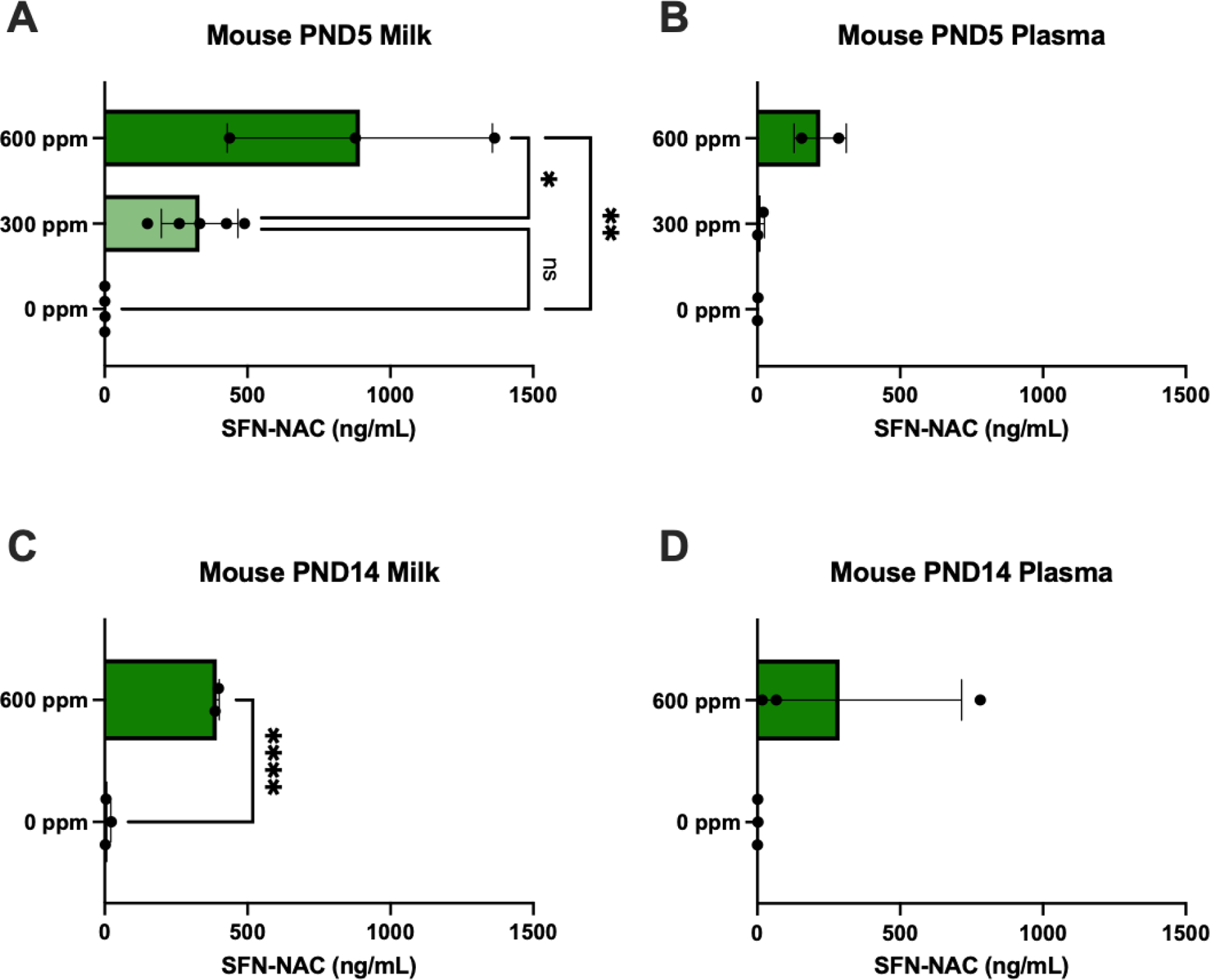
Sulforaphane-N-acetylcysteine (SFN-NAC) metabolite levels (mean ± SD) detected in murine milk and plasma following 5 days of 0, 300 or 600 ppm maternal dietary SFN supplementation post-delivery, i.e., offspring postnatal day (PND) 5 (A-B, analyzed via ANOVA) or 2 weeks of 0 or 600 ppm maternal SFN dietary supplementation, i.e., offspring PND 14 (C-D, analyzed via t test). Experimental groups represent 2–5 dams/group. *p≤0.05; **p≤0.01; ***p≤0.001; ****p≤0.0001.
3.2. NRF2-related gene expression in vivo in murine maternal and neonate tissues
We investigated Nqo1 gene expression initially in maternal and neonate liver and lung tissues. In the PND5 group, we observed a ~4-fold significant increase in hepatic and pulmonary maternal gene expression in the 600-ppm group (Figure 2A–B). Offspring hepatic Nqo1 expression was also significantly increased in the 300-ppm and 600-ppm groups by ~2- and 2.5-fold, respectively (Figure 2C). However, neonate Nqo1 expression in the lungs did not vary significantly between groups (Figure 2D). Gene expression of specific glutathione S-transferase isoforms were analyzed in the same tissues. In maternal liver samples, there was significantly increased expression of Gsta2 (~2-fold in the 300-ppm and ~4-fold in the 600-ppm groups), Gstm1 (~4-fold in the 600-ppm group), and Gstp1 (~4-fold in the 600-ppm group) (Figure 3A). Similarly in the maternal lung (Figure 3B), Gsta2 was significantly increased in the 300-ppm group (~2-fold). Gstm1 was significantly increased in the 300-ppm (~1.5-fold) and 600-ppm groups (~2-fold), and Gstp1 was significantly increased in the 300-ppm (~2-fold) and 600-ppm groups (~2.5-fold). In neonatal tissues, significant increases were observed in the high dose, 600-ppm group. This was the case for Gsta2 (~1.2-fold higher) and Gstp1 (~1.2-fold higher) in offspring liver samples (Figure 3C). In neonate lung, we observed increased expression of Gstm1 and Gstp1 by ~2.0- and 1.5-fold, respectively (Figure 3D).
Figure 2.
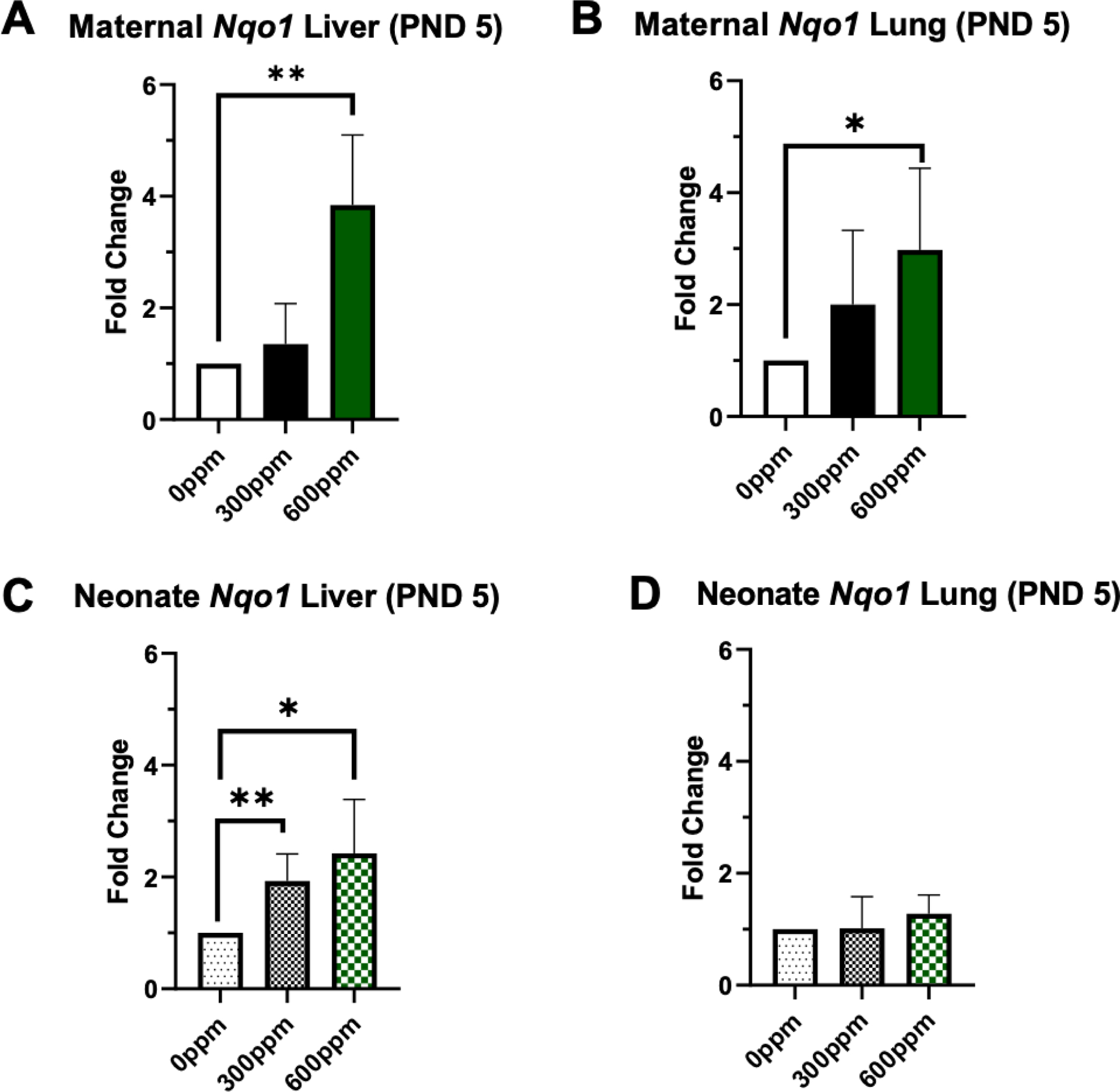
Fold change of Nqo1 in murine maternal (A-B) and neonate (C-D) liver and lung tissue collected following 5 days of 0, 300 or 600 ppm maternal dietary sulforaphane (SFN) supplementation post-delivery, i.e., offspring PND 5. Error bars represent SD. Experimental groups represent 2–5 dams/group and at least 3 neonates per group (across litters). Data analyzed via ANOVA. *p≤0.05; **p≤0.01; ***p≤0.001; ****p≤0.0001.
Figure. 3.
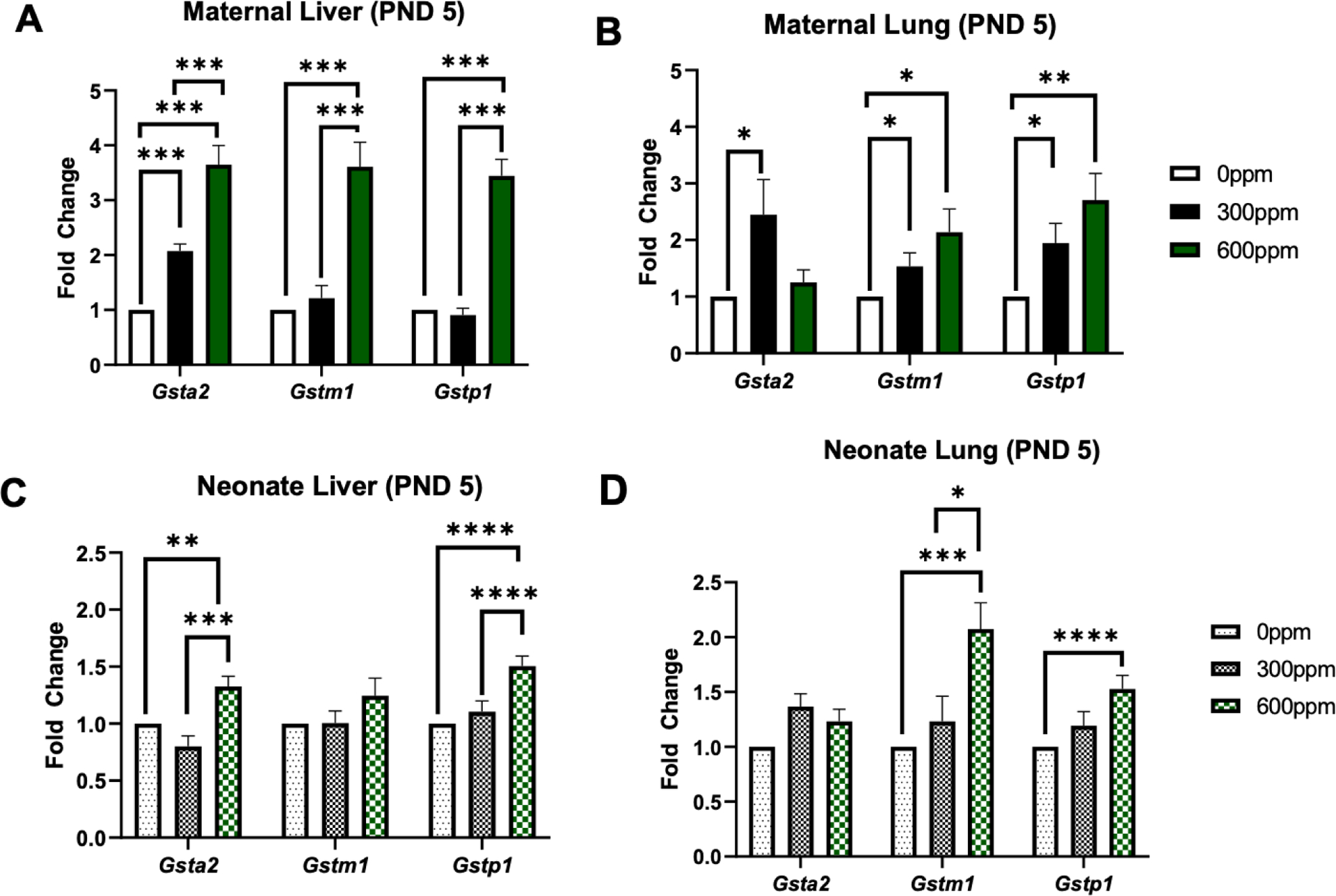
Fold change of Gsta2, Gstm1, and Gstp1 in murine maternal (A-B) and neonate (C-D) liver and lung tissue collected following 5 days of 0, 300 or 600 ppm maternal dietary sulforaphane (SFN) supplementation post-delivery, i.e., offspring PND 5. Error bars represent SD. Experimental groups represent 2–5 dams/group and at least 5 neonates per group (across litters). Data analyzed via ANOVA. *p≤0.05; **p≤0.01; ***p≤0.001; ****p≤0.0001.
In the second experiment, which entailed dams consuming 600 ppm SFN from PND 1 though 14, maternal Nqo1 hepatic expression did not vary significantly between the vehicle or 600-pppm group (Figure 4A). Maternal pulmonary Nqo1 was significantly increased (~2.5 fold) (Figure 4B). Similarly in offspring tissues, neonate Nqo1 was not elevated in liver but was significantly increased (~2.5 fold) in lung tissue (Figure 4C–D). Lastly, maternal pulmonary Gsta2 and Gstm1 were the only genes significantly increased, approximately 1.5-fold (Figure 5).
Figure 4.
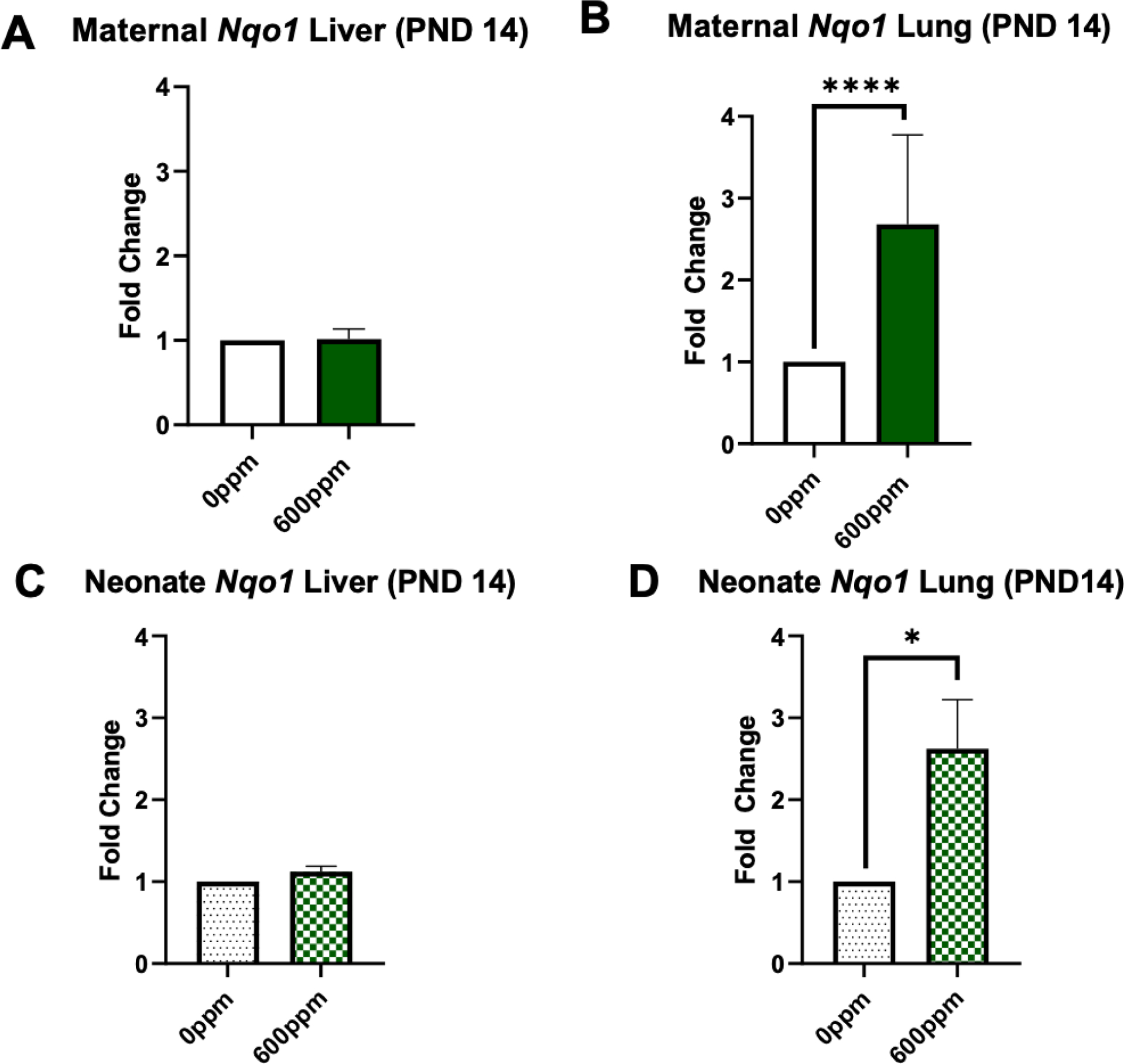
Fold change of Nqo1 in murine maternal (A-B) and neonate (C-D) liver and lung tissue collected following 2 weeks of 0 or 600 ppm maternal dietary sulforaphane (SFN) supplementation post-delivery, i.e., offspring PND 14. Error bars represent SD. Experimental groups represent 3 dams/group and at least 6 neonates per group (across litters). Data analyzed via t test. *p≤0.05; **p≤0.01; ***p≤0.001; ****p≤0.0001.
Figure 5.
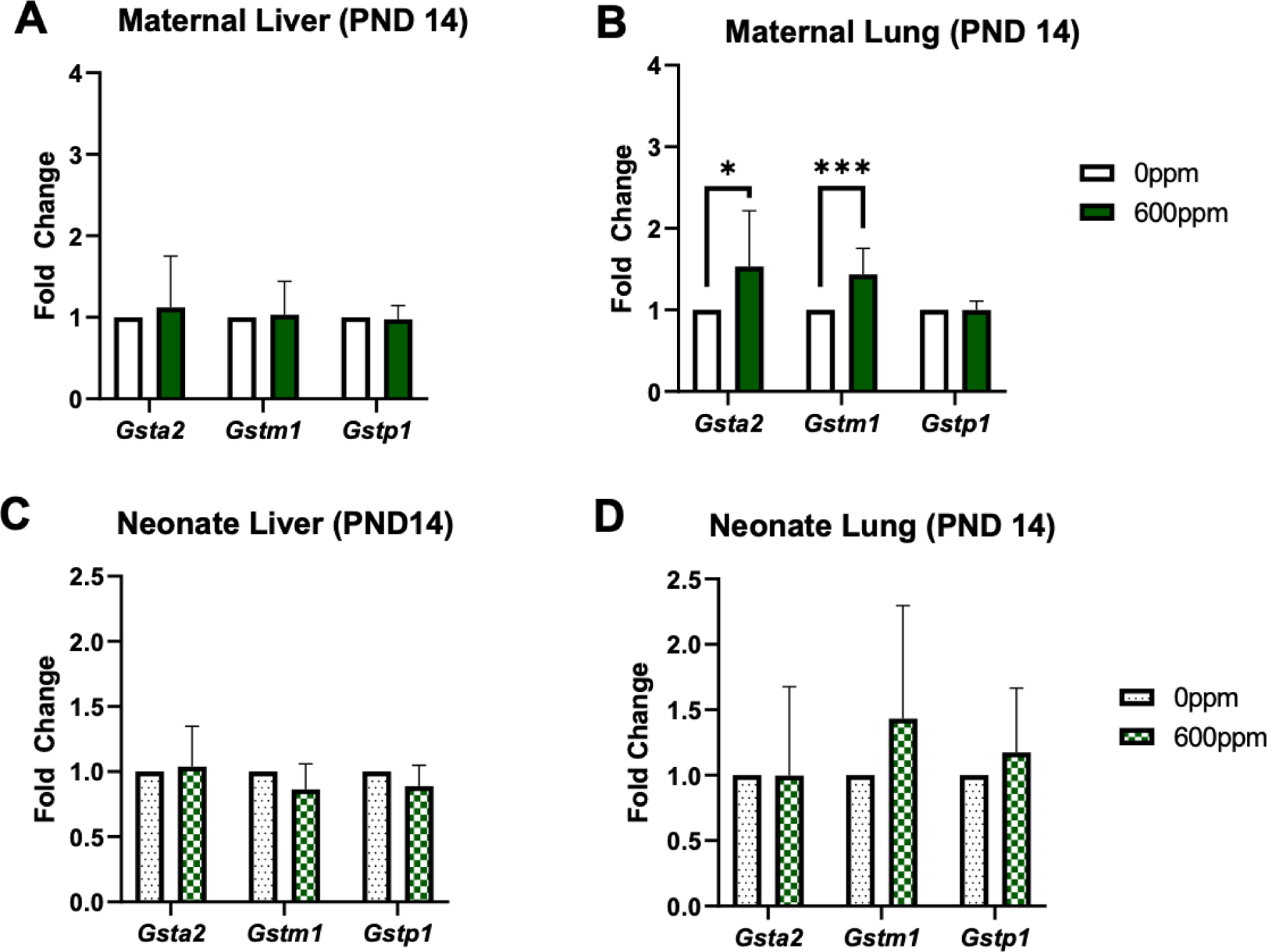
Fold change of Gsta2, Gstm1, and Gstp1 in murine maternal (A-B) and neonate (C-D) liver and lung tissue collected following 2 weeks of 0 or 600 ppm maternal dietary sulforaphane (SFN) supplementation post-delivery, i.e., offspring PND 14. Error bars represent SD. Experimental groups represent 3 dams/group and at least 6 neonates per group (across litters). Data analyzed via t test. *p≤0.05; **p≤0.01; ***p≤0.001; ****p≤0.0001.
3.3. NQO1 activity in vivo in murine maternal and neonate tissues
We also carried out an assay to measure NQO1 enzyme activity. None of the averages varied significantly across maternal or neonatal groups (Supplemental Figure 2–3).
3.4. Sulforaphane (SFN) and SFN-NAC concentrations in human breast milk samples
A total of 8 participants were recruited and enrolled in a pilot study, “Analyzing Breast Milk for Green Nutrients.” Demographic information is shown in Table 1. The average maternal age was 33.9 years, and most participants were white (87.5%). The average infant age was 8 months. Participants filled out a brief questionnaire related to consumption of cruciferous vegetables. Most participants (75%) typically consumed cruciferous vegetables less than twice per week, with a few greater than this. In total, 7 out of the 8 participants reported consuming cruciferous vegetables within 24 hours of providing the milk sample, with 5 of the 7 consuming an estimated 1 cup, and 2 of the 7 consuming an estimated ½ cup (Table 2). None of the breast milk samples had detectable levels of SFN. Overall, 87.5% (7 out of the 8 participants) had detectable levels of SFN-NAC. Levels ranged from 0.79 to 2.96 ng/mL, with an average concentration 1.38 ng/mL. Notably, dietary survey results indicated the one sample with SFN-NAC below the LOQ was the only participant who did not report consumption of cruciferous vegetables in the prior 24 hours.
Table 1.
Demographic characteristics and dietary information of study participants.
| Mother’s age in years, Mean (SD) | 33.9 (3.4) |
| Infant age in months, Mean (SD) | 8.1 (4.2) |
| Race/ethnicity, n (%) | |
| White | 7 (87.5%) |
| Asian, Asian American or Pacific Islander | 1 (12.5%) |
| Cruciferous vegetable intake, n (%) | |
| <2 times per week | 6 (75%) |
| 3–5 times per week | 1 (12.5%) |
| >6 times per week | 1 (12.5%) |
Table 2.
Sulforaphane (SFN) and SFN-N-acetylcysteine (SFN-NAC) Concentrations in Human Breast Milk Samples.
| Participant # | SFN (ng/mL) | SFN-NAC (ng/mL) | Ate cruciferous vegetables in the past 24 h? | Approximate amount of cruciferous vegetables consumed in the past 24 h | Vegetable type consumed |
|---|---|---|---|---|---|
| 1 | <LOQ | 1.20 | Yes | ½ cup | Broccoli |
| 2 | <LOQ | 1.33 | Yes | 1 cup | Kale and collard greens |
| 3 | <LOQ | 1.40 | Yes | 1 cup | Broccoli and Brussels sprouts |
| 4 | <LOQ | 2.96 | Yes | ½ cup | Broccolini |
| 5 | <LOQ | 0.80 | Yes | 1 cup | Broccoli |
| 6 | <LOQ | <LOQ | No | 0 cup | None |
| 7 | <LOQ | 1.16 | Yes | 1 cup | Broccoli and cauliflower |
| 8 | <LOQ | 0.79 | Yes | 1 cup | Broccoli and cauliflower |
Limit of quantification (LOQ) for SFN and SFN-NAC = 0.49 ng/mL.
4. Discussion
This is the first study to our knowledge to report the lactational transfer of a SFN metabolite into breast milk. Other phytochemicals/metabolites are previously reported in human breast milk, which included flavonoids, carotenoids, epicatechin metabolites, caffeine and its catabolic products (Tsopmo, 2018). Globally, traditional plant use during lactation is a common cultural practice with broad potential benefits for maternal and child health that warrant further exploration (Sibeko et al., 2021). Sulforaphane (SFN), a Brassica-derived phytochemical with high bioavailability and potent NRF2-inducing ability, has been extensively investigated in mouse models and clinical trials in the prevention and treatment of chronic disease, as well as infection (Houghton, 2019). Since pregnancy and fetal/infant development represent windows of vulnerability to environmental exposures, transplacental SFN supplementation has been investigated in previous studies, mainly as a cancer prevention strategy. Shorey and colleagues evaluated maternal dietary supplementation with SFN to protect against offspring cancer mortality in a model of transplacental and lactational dibenzo[def,p]chrysene exposure that produced an aggressive T-cell lymphoblastic lymphoma in offspring (Shorey et al., 2013). Differential dietary formulations produced varying outcomes, including an enhanced effect in one group, yet protective effects from other formulations. Notably, maternal dietary SFN intake (administered orally starting on gestational day 9) correlated with neonate plasma metabolite concentrations after birth indicating placental transfer. Moreover, SFN-NAC had the highest concentration in plasma, in comparison to SFN-glutathione (GSH) and -cysteine metabolites (Shorey et al., 2013). In our study, we measured SFN and SFN-NAC in maternal plasma following 300 or 600 ppm of dietary SFN supplementation for either 5 or 14 days. The parent compound was only detected in dams receiving 600 ppm SFN for five days. The percent of circulating plasma SFN-NAC was similar to SFN-NAC percentages previously reported in rat plasma 24 hours after acute SFN dosing (Wang et al., 2011). Additionally, we observed a significant increase in average SFN-NAC concentrations in milk samples from the 600-ppm groups after 5 or 14 days of SFN supplementation. SFN is rapidly metabolized to SFN-GSH and SFN-NAC. Wang et al. demonstrated the quick disappearance of parent SFN from plasma in rats revealing a half-life of ~3h. Additionally, in a clinical trial where investigators administered a broccoli sprout beverage, participant urinary concentrations of SFN-NAC were substantially higher than SFN in overnight voids of urine (Egner et al., 2008). We also hypothesized SFN-NAC would be similarly higher in milk as compared with SFN. Prior studies in human breast milk show similar xenobiotic conjugation patterns and metabolite in urine and breast milk (Fareed et al. 2022). However, few studies have investigated the presence of the detoxification products in breast milk.
Prior research also affirms activation of NRF2-related genes, including phase II detoxification enzymes NQO1 and GSTs, following acute SFN dosing (Fahey & Talalay, 1999) NQO1 catalyzes the two-electron reduction of quinones to hydroquinones; GSTs catalyze GSH conjugation with all the types of electrophiles. There are several GST isoforms, which are variably expressed in different tissues in mice (Knight et al., 2007). In prior acute studies in CD-1 pregnant and non-pregnant mice administered a single dose of 50 or 100 mg/kg via oral gavage yielded increased Nqo1 and Gstm1 hepatic expression ~2–3-fold (Philbrook & Winn, 2014). However, in that model, acute as well as sub-acute (repeated SFN administration) maternal supplementation did not yield a significant increase in fetal liver NRF2-related gene expression. In our model, maternal Nqo1 was significantly elevated ~4-fold in maternal liver and lung following 5 days of 600 ppm SFN supplementation. Moreover, GSTs were also significantly increased in maternal liver and lung, generally following a dose-response across isoforms with fold increases ranging from ~2–4x. After 14 days of 600 ppm SFN supplementation, only lung transcripts were significantly elevated in maternal tissues, ~2.5-fold (Nqo1) and ~1.5-fold (Gsta2 and Gstm1). Similar to past studies (Philbrook & Winn, 2014), our longer dosing (14 days) did not necessarily increase gene expression more than shorter (5 day) dosing. This may be attributable to the relatively high constitutive activity of murine NQO1 and GSTs (Knight et al., 2007; Nioi et al., 2003), as well as limitations in our dosing administration (ad libitum) versus timed oral gavage, which was selected to reduce stress on nursing animals. Thus, the time from last estimated dose administration and maternal tissue collection varied by litter. Still, maternal dosing indicated activation of NRF2-related genes in two different tissues (liver and lung) confirming SFN induced NRF2-related gene expression in our model. NQO1 activity was not significantly elevated in maternal liver or lung tissues; however, enzyme activity was measured at a later timepoint. It is possible tissue previously thawed and stored over time led to diminished enzyme activity. Future studies should present paired protein expression and enzyme activity measurements for clarity.
Additionally, we hypothesized since SFN-NAC is the predominant metabolite, it would be transferred through milk to activate neonate NRF2-related gene expression. Prior findings support the SFN-NAC metabolite also has NRF2-inducing activity, evidenced by the in vitro-based Prochaska assay showing increased NQO1 activity (Hwang & Jeffery, 2005). In our model, neonate hepatic Nqo1, Gsta2, and Gstp1 were elevated in 300- and 600-ppm groups receiving maternal SFN for 5 days. Moreover, Gstm1 and Gstp1 were also elevated in the neonate lung in the 600-ppm group after 5 days of supplementation. Conversely after 14 days of 600 ppm maternal SFN supplementation, pulmonary Nqo1 was the only transcript significantly elevated. Again, one limitation was the maternal dosing strategy. Also, importantly, neonate milk consumption may be variable, which would impact neonate dose concentration and timing, leading to variable downstream gene expression. Moreover, the baseline expression of Phase II enzymes is age- and tissue-specific, with substantial changes in baseline Gst expression in the first two weeks of life (Lu et al., 2013). Since the ontogeny of human phase II enzymes is also a function of age, future studies employing controlled dosing across important windows of development could further inform SFN-NAC’s induction potential. Additional evaluation of functional activity is also warranted since as stated above, NQO1 activity did not significantly vary across groups.
To complement our in vivo data, we carried out a pilot study in lactating mothers to evaluate the presence of SFN and SFN-NAC in human breast milk in participants consuming typical dietary intake of cruciferous vegetables. Eight participants were recruited into our pilot study. Study participants filled out a short questionnaire and provided an expressed sample of breast milk for SFN and SFN-NAC analysis. We did not detect the parent compound in any of the breat milk samples. We detected SFN-NAC in 87.5% of milk samples provided, with 7/8 above the LOQ. The presence of this metabolite correlated with self-reported dietary consumption of cruciferous vegetables in the last 24 hours. We did not collect detailed information on the exact time of consumption or cooking method, which can impact GF and SFN concentrations (Lu et al., 2020), so we could not draw any conclusions on the relationship between SFN-NAC levels in milk and type of vegetable consumed. Still, using a random sampling approach, we were able to successfully detect SFN-NAC in breast milk. The average concentration was smaller compared to urinary SFN-NAC levels reported from a past human clinical study (Egner et al., 2008). This was expected since clinical trial participants received broccoli sprouts containing a total of 400 μmol GF. In that study, the average urinary SFN-NAC level was 42 nmol/mg creatinine. Assuming an average of 30 mg creatinine/dL, this equates to ~4,296 ng/mL. Thus, if our study participants consumed mature plants containing ~1.1 μmol/g (Yagishita et al., 2019), SFN-NAC urinary levels could be expected in equal ~11.8 ng/mL. The kinetics of excretion are likely different between milk and urine, however we reasoned we may be able to detect SFN-NAC in breast milk, even at a lower anticipated GF intake from mature plants. The average concentration detected in breast milk in our study (1.38 ng/mL) was lower than expected range in urine. Future studies detailing the pharmacokinetics in human breast milk are needed.
Our study had several limitations. First, we did not measure protein expression of the NRF2-related genes. We attempted to quantify NQO1 activity; however, it is possible that the prior thawing of tissues and/or longer storage time of the samples led to inability to detect differences in enzyme activity. Second, as discussed, the variability in murine maternal SFN treatment likely led to the high variability of SFN metabolite concentrations and downstream effects in tissues. A well-controlled dosing study is warranted. Also, sex as a biological variable may impact SFN’s ability to induce NRF2-related gene expression during development and should be considered in future studies. Due to low numbers, we could not tease apart sex-specific effects. Last, in the human samples, we regret we did not run technical replicates, which would increase the accuracy of the reported SFN-NAC concentration data. We can report with certainty the detection of SFN-NAC in human milk samples; however, further studies should measure concentrations more closely related to dietary intake.
Overall, our in vivo findings demonstrate lactational transfer of SFN-NAC activates NRF2-realetd gene expression in neonates. Furthermore, our preliminary human data show the detection of SFN-NAC in breast milk at dietary relevant concentrations. Detailed pharmacodynamics in breastfeeding infants remains to be determined. Lactation represents an important window for potential preventive or therapeutic effects for children.
Supplementary Material
Acknowledgements
This research is devoted in honor of Patricia Egner, dedicated scientist, mother, grandmother, and steadfast mentor. We appreciate the laboratory of Dr. John Groopman for providing the internal standard. A special thanks to all the participants in the pilot study willing to donate their breast milk for this study. We also appreciate the excellent animal husbandry and care at the Texas A&M Institute for Genomic Medicine and contributions from Drs. Andrei Golovko and Ben Morpurgo. This research was supported in part by the National Institute of Environmental Health Sciences (R01 ES028866 and P30 ES029067). R. Shore was supported by T32 ES026568.
Footnotes
CRediT authorship contribution statement
Ross Shore: Conceptualization, Methodology, Investigation, Formal analysis, Writing – original draft. Jonathan Behlen: Methodology, Investigation, Formal analysis. Dylan McBee: Investigation. Keerthana Prayaga: Investigation. Faith Haugen: Investigation. Lenore Craig: Investigation. Michael Shields: Methodology, Validation. Toriq Mustapha: Methodology, Investigation. Navada Harvey: Methodology, Writing – Review & Editing. Natalie Johnson: Conceptualization, Methodology, Writing – review & editing, Supervision, Funding acquisition.
References
- Bahadoran Z, Mirmiran P, Hosseinpanah F, Hedayati M, Hosseinpour-Niazi S, & Azizi F (2011). Broccoli sprouts reduce oxidative stress in type 2 diabetes: a randomized double-blind clinical trial. Eur J Clin Nutr, 65(8), 972–977. 10.1038/ejcn.2011.59 [DOI] [PubMed] [Google Scholar]
- Benson AM, Hunkeler MJ, & Talalay P (1980). Increase of NAD(P)H:quinone reductase by dietary antioxidants: possible role in protection against carcinogenesis and toxicity. Proc Natl Acad Sci U S A, 77(9), 5216–5220. 10.1073/pnas.77.9.5216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RH, Reynolds C, Brooker A, Talalay P, & Fahey JW (2015). Sulforaphane improves the bronchoprotective response in asthmatics through Nrf2-mediated gene pathways. Respir Res, 16(1), 106. 10.1186/s12931-015-0253-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. (2022). Breastfeeding Report Card. https://www.cdc.gov/breastfeeding/data/reportcard.htm
- Chen JG, Johnson J, Egner P, Ng D, Zhu J, Wang JB, Xue XF, Sun Y, Zhang YH, Lu LL, Chen YS, Wu Y, Zhu YR, Carmella S, Hecht S, Jacobson L, Muñoz A, Kensler K, Rule A, … Groopman J (2019). Dose-dependent detoxication of the airborne pollutant benzene in a randomized trial of broccoli sprout beverage in Qidong, China. Am J Clin Nutr, 110(3), 675–684. 10.1093/ajcn/nqz122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HY, Imani F, Miller-DeGraff L, Walters D, Melendi GA, Yamamoto M, Polack FP, & Kleeberger SR (2009). Antiviral activity of Nrf2 in a murine model of respiratory syncytial virus disease. Am J Respir Crit Care Med, 179(2), 138–150. 10.1164/rccm.200804-535OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Fahey JW, Kostov RV, & Kensler TW (2017). KEAP1 and Done? Targeting the NRF2 Pathway with Sulforaphane. Trends Food Sci Technol, 69(Pt B), 257–269. 10.1016/j.tifs.2017.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner PA, Kensler TW, Chen JG, Gange SJ, Groopman JD, & Friesen MD (2008). Quantification of sulforaphane mercapturic acid pathway conjugates in human urine by high-performance liquid chromatography and isotope-dilution tandem mass spectrometry. Chem Res Toxicol, 21(10), 1991–1996. 10.1021/tx800210k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner PA, Chen JG, Zarth AT, Ng DK, Wang JB, Kensler KH, Jacobson LP, Muñoz A, Johnson JL, Groopman JD, Fahey JW, Talalay P, Zhu J, Chen TY, Qian GS, Carmella SG, Hecht SS, & Kensler TW (2014). Rapid and sustainable detoxication of airborne pollutants by broccoli sprout beverage: results of a randomized clinical trial in China. Cancer Prev Res (Phila), 7(8), 813–823. 10.1158/1940-6207.Capr-14-0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey JW, & Talalay P (1999). Antioxidant functions of sulforaphane: a potent inducer of Phase II detoxication enzymes. Food Chem Toxicol, 37(9–10), 973–979. 10.1016/s0278-6915(99)00082-4 [DOI] [PubMed] [Google Scholar]
- Fujita Y, Fujita A, Ishima T, Hirai A, Suzuki S, Suganuma H, & Hashimoto K (2020). Dietary intake of glucoraphanin during pregnancy and lactation prevents the behavioral abnormalities in the offspring after maternal immune activation. Neuropsychopharmacol Rep, 40(3), 268–274. 10.1002/npr2.12112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan MV, Kishan AA, & Silverman AL (2004). Oral broccoli sprouts for the treatment of Helicobacter pylori infection: a preliminary report. Dig Dis Sci, 49(7–8), 1088–1090. 10.1023/b:ddas.0000037792.04787.8a [DOI] [PubMed] [Google Scholar]
- Houghton CA (2019). Sulforaphane: Its “Coming of Age” as a Clinically Relevant Nutraceutical in the Prevention and Treatment of Chronic Disease. Oxid Med Cell Longev, 2019, 2716870. 10.1155/2019/2716870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang ES, & Jeffery EH (2005). Induction of quinone reductase by sulforaphane and sulforaphane N-acetylcysteine conjugate in murine hepatoma cells. J Med Food, 8(2), 198–203. 10.1089/jmf.2005.8.198 [DOI] [PubMed] [Google Scholar]
- Kensler TW, Egner PA, Agyeman AS, Visvanathan K, Groopman JD, Chen JG, Chen TY, Fahey JW, & Talalay P (2013). Keap1-nrf2 signaling: a target for cancer prevention by sulforaphane. Top Curr Chem, 329, 163–177. 10.1007/128_2012_339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight TR, Choudhuri S, & Klaassen CD (2007). Constitutive mRNA Expression of Various Glutathione S-Transferase Isoforms in Different Tissues of Mice. Toxicological Sciences, 100(2), 513–524. 10.1093/toxsci/kfm233 [DOI] [PubMed] [Google Scholar]
- Livak KJ, & Schmittgen TD (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods, 25(4), 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lu H, Gunewardena S, Cui JY, Yoo B, Zhong XB, & Klaassen CD (2013). RNA-sequencing quantification of hepatic ontogeny and tissue distribution of mRNAs of phase II enzymes in mice. Drug Metab Dispos, 41(4), 844–857. 10.1124/dmd.112.050211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Pang X, & Yang T (2020). Microwave cooking increases sulforaphane level in broccoli. Food Sci Nutr, 8(4), 2052–2058. 10.1002/fsn3.1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nioi P, McMahon M, Itoh K, Yamamoto M, & Hayes JD (2003). Identification of a novel Nrf2-regulated antioxidant response element (ARE) in the mouse NAD(P)H:quinone oxidoreductase 1 gene: reassessment of the ARE consensus sequence. Biochem J, 374(Pt 2), 337–348. 10.1042/bj20030754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palliyaguru DL, Yuan JM, Kensler TW, & Fahey JW (2018). Isothiocyanates: Translating the Power of Plants to People. Mol Nutr Food Res, 62(18), e1700965. 10.1002/mnfr.201700965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philbrook NA, & Winn LM (2014). Sub-chronic sulforaphane exposure in CD-1 pregnant mice enhances maternal NADPH quinone oxidoreductase 1 (NQO1) activity and mRNA expression of NQO1, glutathione S-transferase, and glutamate-cysteine ligase: potential implications for fetal protection against toxicant exposure. Reprod Toxicol, 43, 30–37. 10.1016/j.reprotox.2013.10.009 [DOI] [PubMed] [Google Scholar]
- Piedimonte G, & Perez MK (2014). Respiratory syncytial virus infection and bronchiolitis. Pediatr Rev, 35(12), 519–530. 10.1542/pir.35-12-519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rha B, Curns AT, Lively JY, Campbell AP, Englund JA, Boom JA, Azimi PH, Weinberg GA, Staat MA, Selvarangan R, Halasa NB, McNeal MM, Klein EJ, Harrison CJ, Williams JV, Szilagyi PG, Singer MN, Sahni LC, Figueroa-Downing D, … Gerber SI (2020). Respiratory Syncytial Virus-Associated Hospitalizations Among Young Children: 2015–2016. Pediatrics, 146(1). 10.1542/peds.2019-3611 [DOI] [PubMed] [Google Scholar]
- Russell CD, Unger SA, Walton M, & Schwarze J (2017). The Human Immune Response to Respiratory Syncytial Virus Infection. Clin Microbiol Rev, 30(2), 481–502. 10.1128/cmr.00090-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorey LE, Madeen EP, Atwell LL, Ho E, Löhr CV, Pereira CB, Dashwood RH, & Williams DE (2013). Differential modulation of dibenzo[def,p]chrysene transplacental carcinogenesis: maternal diets rich in indole-3-carbinol versus sulforaphane. Toxicol Appl Pharmacol, 270(1), 60–69. 10.1016/j.taap.2013.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibeko L, Johns T, & Cordeiro LS (2021). Traditional plant use during lactation and postpartum recovery: Infant development and maternal health roles. J Ethnopharmacol, 279, 114377. 10.1016/j.jep.2021.114377 [DOI] [PubMed] [Google Scholar]
- Tsopmo A (2018). Phytochemicals in Human Milk and Their Potential Antioxidative Protection. Antioxidants (Basel), 7(2). 10.3390/antiox7020032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Lin W, Shen G, Khor TO, Nomeir AA, & Kong AN (2011). Development and validation of an LC-MS-MS method for the simultaneous determination of sulforaphane and its metabolites in rat plasma and its application in pharmacokinetic studies. J Chromatogr Sci, 49(10), 801–806. 10.1093/chrsci/49.10.801 [DOI] [PubMed] [Google Scholar]
- Yagishita Y, Fahey JW, Dinkova-Kostova AT, & Kensler TW (2019). Broccoli or Sulforaphane: Is It the Source or Dose That Matters? Molecules, 24(19), 3593. https://www.mdpi.com/1420-3049/24/19/3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanaka A, Fahey JW, Fukumoto A, Nakayama M, Inoue S, Zhang S, Tauchi M, Suzuki H, Hyodo I, & Yamamoto M (2009). Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in Helicobacter pylori-infected mice and humans. Cancer Prev Res (Phila), 2(4), 353–360. 10.1158/1940-6207.Capr-08-0192 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


