Summary
This document provides a short summary of the T3MPO-3 study, which was published in Neurogastroenterology and Motility in September 2023. At the end of this summary, there are links to websites where you can find more information about this study.
Tenapanor (IBSRELA®) is a newly available medicine in the United States. It is used to treat irritable bowel syndrome with constipation (IBS-C) in people aged 18 years and older. In 2 large clinical trials, tenapanor was shown to improve the symptoms of IBS-C. People with IBS-C often need to take long-term medicines to keep their symptoms under control. As a result, this study, called T3MPO-3, was undertaken to investigate whether there are any possible side effects associated with the long-term use of tenapanor in adults with IBS-C. There were 312 people who had taken part in either of the original 2 trials and took part in this study, with 90 receiving tenapanor for at least 1 year. Loose or watery stool (diarrhea) was the most common side effect, and most cases were mild or moderate. No people died in any of these studies.
Who is this article for?
This summary may help people with irritable bowel syndrome with constipation (IBS-C) and their family members and/or caregivers better understand the long-term safety of tenapanor for treating IBS-C. Two IBS-C patient advocates coauthored this summary.
How to say….
Tenapanor: ten-AP-an-or
Placebo: plah-SEE-bow
T3MPO: tehm-po
Where is the T3MPO-3 study in the medicine development timeline?
The T3MPO-3 study was completed in 2018. In 2019, the Food and Drug Administration (FDA) approved tenapanor in the United States to treat IBS-C in adults.
• The FDA is responsible for public health in the United States and makes sure that medicines are safe and effective.
The medicine development process starts with laboratory and animal testing. Once a potential medicine has been identified and proven to be safe and effective in animals, it is tested in humans in phase I, phase II, and phase III studies. T3MPO-3 was a phase III study.

What were the overall results?
- Diarrhea was the most common side effect in the three T3MPO studies.
- A side effect is something (expected or unexpected) that you feel was caused by a medicine or treatment you take.
Side effects were similar to those reported by researchers in previous studies. No new safety issues were found with long-term use of tenapanor.
Where can I find the original article on which this summary is based?
You can read the original article published in the journal Neurogastroenterology and Motility at http://doi.org/10.1111/nmo.14658.
Who sponsored this study?
This study was sponsored by Ardelyx, Inc.
What is IBS?
• Between 4 and 10 in 100 (4–10%) people have IBS, a common condition affecting the digestive system.
• The cause of IBS is unknown. Some things that might be linked to it are a family history of IBS, stress, anxiety, oversensitive nerves in the digestive system, and food passing through the digestive system too quickly or too slowly.
• IBS is generally a lifelong condition; there is no cure, but making changes to the diet and using medicines can sometimes help to lessen symptoms.
• The symptoms of IBS can flare up for no obvious reason, or they can be triggered (e.g. by stress/anxiety, caffeine, alcohol, and certain foods).
• Symptoms of IBS include the following:

- ○ Some people with IBS have also reported less common symptoms, such as extreme tiredness, feeling sick, being unable to control bowel movements, and problems passing urine.
• These symptoms are accompanied by changes in bowel movements, which can be divided into different types:

• The symptoms of IBS-C are bothersome and can come and go over time.
• In a survey of people with IBS-C, 53 of 100 people (53%) said their symptoms were very/extremely bothersome.
• Despite receiving the standard medical treatment, many people often continue to experience symptoms.
What is tenapanor?
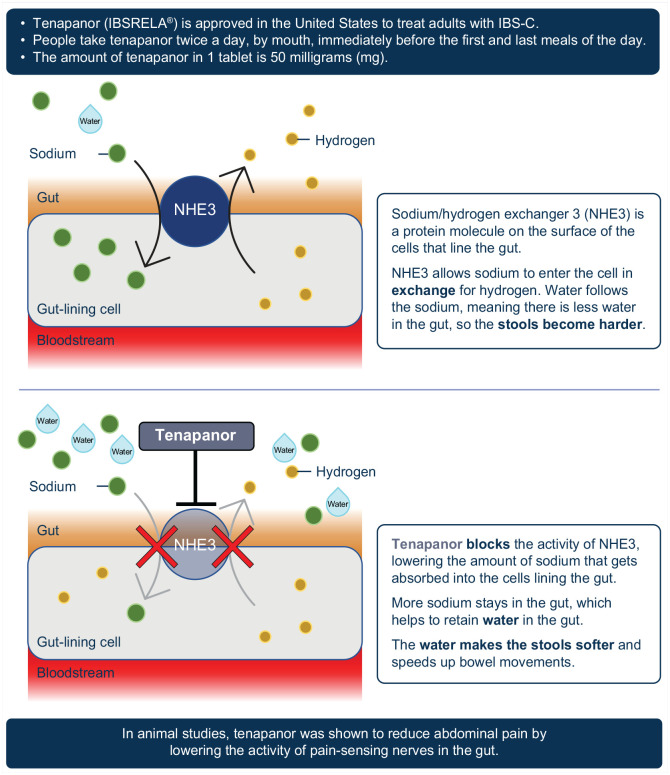
Why was the T3MPO-3 study conducted?
• Tenapanor was approved by the US FDA in 2019 to treat adults with IBS-C.
- • Tenapanor was approved after three phase III studies in humans were completed. These studies were called:
- 1. T3MPO-1 (called Study 1 from now onwards)
- 2. T3MPO-2 (called Study 2 from now onwards)
- 3. T3MPO-3
- • In Studies 1 and 2, tenapanor significantly improved people’s symptoms of abdominal pain and constipation.
- ○ Diarrhea was the most common side effect.
- ○ Most cases of diarrhea happened early in treatment and lasted for 1 week or less.
• As IBS-C is a long-term condition, it is important to be sure that tenapanor is safe for long-term use.
• People who completed either Study 1 or Study 2 were able to take part in T3MPO-3 to look at the potential side effects of taking tenapanor long term.
How was the T3MPO-3 study conducted?
- • In Studies 1 and 2, people were assigned by chance (also known as randomized) to tenapanor or placebo.
- ○ A placebo (a dummy tablet) is a treatment that does not contain active chemical ingredients (often referred to as a sugar pill).
• All people who took part in T3MPO-3 received tenapanor (there was no placebo).
Who took part in the T3MPO-3 study?
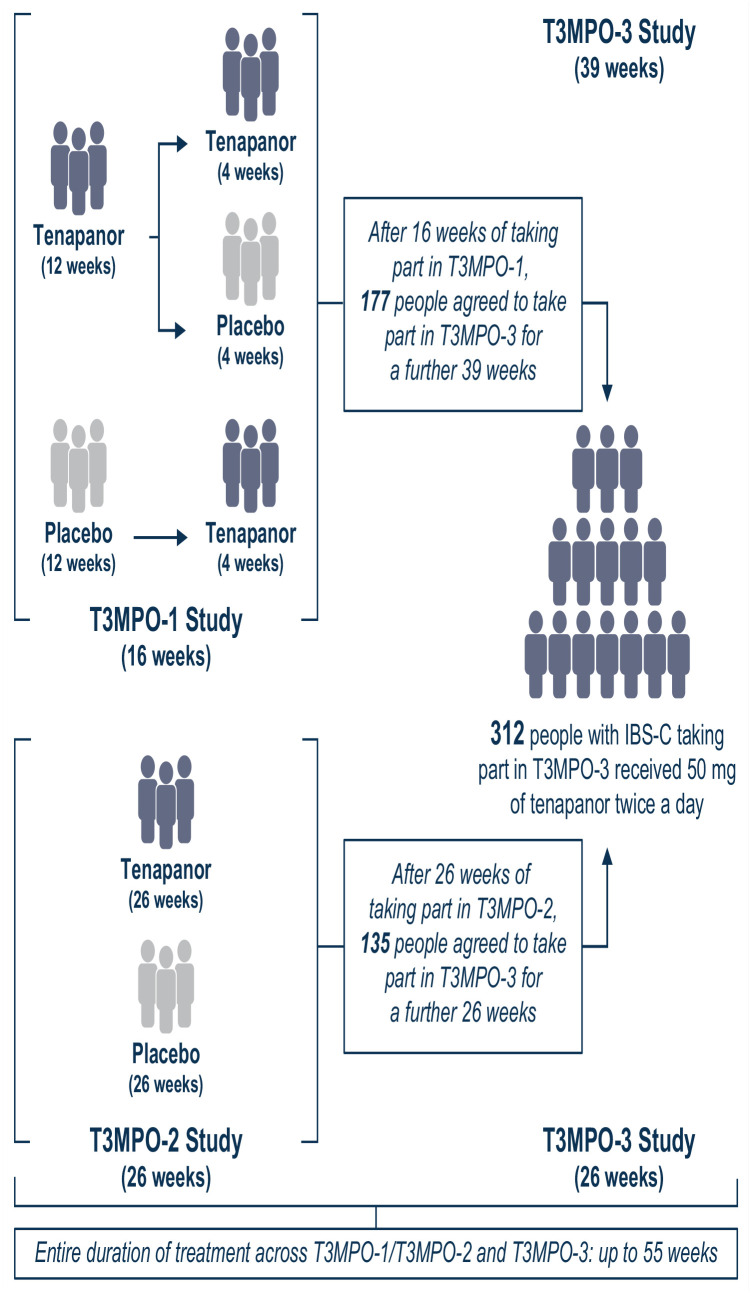
What were the results of the T3MPO-3 study?
• This diagram shows how many people took part in the T3MPO-3 study, how many completed it, and how many people received treatment with tenapanor.

- • Side effects were experienced by 38 out of 100 (38%) people who took part in the T3MPO-3 study.
- ○ A side effect is an undesirable event (expected or unexpected) that may or may not be caused by a medicine or treatment you take.
- ○ In this instance, these side effects may not be caused by tenapanor.
- • Most of the side effects reported in T3MPO-3 were considered mild by researchers.
- ○ Diarrhea was the most common side effect in people treated with tenapanor in the three T3MPO studies. In Studies 1 and 2, diarrhea occurred early and lasted for a short time.
- ○ Among people who received tenapanor in T3MPO-3 for 52 weeks (1 year) or more, 11 out of 100 people (11%) had diarrhea at some point during Study 1, Study 2, and/or T3MPO-3.
- ○ In the entire group of people who received at least one dose of study medicine in T3MPO-3, diarrhea occurred at a lower rate [11 out of 100 (11%)] compared with Study 1 [15 out of 100 (15%)] and Study 2 [16 out of 100 (16%)].
- ○ This is consistent with the observation that diarrhea is most common early in tenapanor treatment and reduces with longer-term use.
• The study showed that people with IBS-C can safely take tenapanor for at least 1 year at the recommended dosage of 50 mg twice a day.

How severe was the diarrhea in the T3MPO-3 study?
• Among all 312 people in T3MPO-3, almost all cases of diarrhea were mild or moderate. There were only two cases of severe diarrhea.

• Among the 90 people who received tenapanor for at least 1 year, all cases of diarrhea were mild or moderate except for one severe case, which was reported in Study 2.
• No people permanently stopped treatment due to diarrhea.
Did any people die because of treatment with tenapanor?
• No people died due to side effects caused by tenapanor.
What do the results of the T3MPO-3 study mean?
• This study provides long-term safety data for taking tenapanor for up to 1 year.
• Long-term side effects were similar to those reported in earlier studies of tenapanor.
• The most common side effect was diarrhea. This usually occurred early and lasted for a short time.
• These results support the use of tenapanor as a long-term treatment for people with IBS-C.
Where can readers find more information on the T3MPO-3 study?
• The original article discussed in this summary was published in Neurogastroenterology and Motility in September 2023. You can read the article here: http://doi.org/10.1111/nmo.14658.
• You can read more about the T3MPO-3 study here: https://clinicaltrials.gov/ct2/show/NCT02727751.
Original publication citation
• Lembo AJ, Friedenberg KA, Fogel RP, et al. Long-term safety of tenapanor in patients with irritable bowel syndrome with constipation in the T3MPO-3 study. Neurogastroenterology and Motility. 2023;00:e14658. doi: 10.1111/nmo.14658
Acknowledgments
We would like to thank the patients and investigators who took part in the study. Plain language summary writing and editorial support for this report were provided by Katherine Cashell, BSc, Danielle Hirsch, PhD, and Kelsey Gribbon, MS, of Ashfield MedComms (New York, NY, USA), an Inizio company, and funded by Ardelyx, Inc. (Waltham, MA, USA).
Footnotes
ORCID iDs: David P. Rosenbaum  https://orcid.org/0000-0002-9371-391X
https://orcid.org/0000-0002-9371-391X
William D. Chey  https://orcid.org/0000-0002-4584-4026
https://orcid.org/0000-0002-4584-4026
Declarations
Ethics approval and consent to participate: Data for the T3MPO-3 study were collected at 51 hospitals throughout the United States. The T3MPO-3 study protocol was approved by the Quorum Review Institutional Review Board (now Advarra Institutional Review Board), approval number 31412, and the study was conducted in accordance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice guidelines. All people who took part in the study provided written informed consent before study entry, and all participating hospitals obtained institutional review board approval.
Consent for publication: People who took part in this study provided written informed consent for publication. The authors were involved in preparing this summary and provided their final approval of all content.
Author contributions: Anthony J. Lembo: Conceptualization; Data curation; Writing – review & editing.
Susan Edelstein: Conceptualization; Writing – review & editing.
David P. Rosenbaum: Conceptualization; Writing – review & editing.
Ceciel Rooker: Writing – review & editing.
Jeffrey D. Roberts: Writing – review & editing.
William D. Chey: Conceptualization; Data curation; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Ardelyx, Inc., Waltham, MA, USA. Email: info@ardelyx.com.
AJL is a consultant for Allergan, Ardelyx, Atmo, Allakos, BioAmerica, AEON, Arena, Takeda, Evoke Pharma, Ironwood Pharmaceuticals, Gemelli, Alkermes, Pfizer, OrthoMed, Vibrant, and has stock with Johnson & Johnson, Bristol Myer Squibb, and Allurion. SE and DPR are employees of Ardelyx, Inc. CR declares no conflict of interest. JDR declares no conflict of interest. WDC is a consultant for Abbvie, Ardelyx, Arena, Baush, Biomerica, Gemelli, Ironwood, Isothrive, Nestle, Progenity, Salix, Takeda, Urovant, and Vibrant, and has stock options with GI on Demand/Gastro Girl, Isothrive, and Modify Health.
Availability of data and materials: Ardelyx will consider reasonable requests for data sharing such as the study protocol, SAP, and ICF on a case-by-case basis based on data availability, burden, and data privacy issues. This will go into effect immediately after publication for a period of up to 1 year. Data will be shared to achieve aims in an investigator-submitted proposal, which has been approved by Ardelyx. Proposals should be directed to medinfo@ardelyx.com. To gain access to data, requestors will need to sign a data access agreement.


