Abstract
Background:
Although blood pressure (BP) control is critical to prevent cardiovascular diseases, hypertension control rates in Canada are in decline.
Objective:
To assess this issue, we sought to evaluate the differences in antihypertensive medication prescription profiles in the province of Quebec between 2009 and 2021.
Design:
This is a retrospective cohort study.
Setting:
We used data from the CARTaGENE population–based cohort linked to administrative health databases.
Patients:
Participants with any drug claim in the 6 months prior to the end of follow-up were included.
Measurements:
Guideline-recommended antihypertensive drug prescription profiles were assessed at the time of enrollment (2009-2010) and end of follow-up (March 2021).
Methods:
Prescriptions practices from the 2 time periods were compared using Pearson’s chi-square tests. A sensitivity analysis was performed by excluding participants in which antihypertensive drugs may not have been prescribed solely to treat hypertension (presence of atrial fibrillation/flutter, ischemic heart disease, heart failure, chronic kidney disease, or migraines documented prior to or during follow-up).
Results:
Of 8447 participants included in the study, 31.4% and 51.3% filled prescriptions for antihypertensive drugs at the beginning and end of follow-up. In both study periods, guideline-recommended monotherapy was applied in most participants with hypertension (77.9% vs 79.5%, P = .3), whereas optimal 2 and 3-drug combinations were used less frequently (62.0% vs 61.4%, P = .77, 51.9% vs 46.7%, P = .066, respectively). Only the use of long-acting thiazide-like diuretics (9.5% vs 27.7%, P < .001) and spironolactone as a fourth-line agent (8.3% vs 15.9%, P = .054) increased with time but nonetheless remained infrequent. Results were similar in the sensitivity analysis.
Limitations:
Specific indication of the prescribed antihypertensive medications and follow-up BP data was not available.
Conclusions:
Application of hypertension guidelines for the choice of antihypertensive drugs remains suboptimal, highlighting the need for education initiatives. This may be an important step to raise BP control rates in Canada.
Keywords: blood pressure, therapy, antihypertensive drugs, cohort study
Abrégé
Contexte:
Bien que le contrôle de la pression artérielle (PA) soit essentiel pour prévenir les maladies cardiovasculaires, les taux de maitrise de l’hypertension artérielle sont en déclin au Canada.
Objectifs:
Pour traiter cette problématique, nous avons cherché à évaluer les différences dans les profils de prescription de médicaments antihypertenseurs dans la province de Québec entre 2009 et 2021.
Conception:
Étude de cohorte rétrospective.
Cadre:
Nous avons utilisé les données de la cohorte populationnelle CARTaGENE reliées aux bases de données administratives en santé.
Sujets:
Ont été inclus les participants qui ont présenté une demande de remboursement de médicament dans les six mois précédant la fin du suivi.
Mesures:
Les profils de prescription de médicaments antihypertenseurs recommandés dans les lignes directrices ont été évalués au moment de l’inclusion (2009-2010) et à la fin du suivi (mars 2021).
Méthodologie:
Les profils de prescription des deux périodes ont été comparés à l’aide des tests Chi-Square de Pearson. Une analyse de sensibilité a été réalisée en excluant les participants pour lesquels les antihypertenseurs n’avaient possiblement pas été prescrits uniquement pour traiter l’hypertension (présence de fibrillation auriculaire, cardiopathie ischémique, insuffisance cardiaque, insuffisance rénale chronique ou migraines documentées avant ou pendant le suivi).
Résultats:
Des 8 447 participants inclus dans l’étude, 31,4 % avait rempli des prescriptions de médicaments antihypertenseurs au début du suivi et 51,3 % à la fin du suivi. Dans les deux périodes à l’étude, la monothérapie recommandée par les directives a été appliquée chez la plupart des participants avec hypertension artérielle (77,9 % c. 79,5 %; P = 0,3), tandis que les combinaisons optimales de deux médicaments (62,0 % c. 61,4 %; P = 0,77) et de trois médicaments (51,9 % c. 46,7 % P = 0,066) ont été utilisées moins fréquemment. Seules les utilisations de diurétiques thiazidiques à action prolongée (9,5 % c. 27,7 %; P < 0,001) et de spironolactone en quatrième intention (8,3 % c. 15,9 %; P = 0,054) ont augmenté avec le temps, mais sont demeurées néanmoins peu fréquentes. Les résultats étaient similaires dans l’analyse de sensibilité.
Limites:
L’indication précise pour la prescription de médicaments antihypertenseurs et les données de suivi sur la pression artérielle n’étaient pas disponibles.
Conclusion:
L’application des lignes directrices sur l’hypertension artérielle pour le choix des médicaments antihypertenseurs reste sous-optimale, ce qui souligne un besoin pour des initiatives en matière d’éducation. Cela pourrait constituer une étape importante d’une stratégie visant l’augmentation des taux de contrôle de la PA au Canada.
Introduction
Hypertension affects nearly 1 in 4 Canadian adults and accounts for a significant proportion of health care spending.1,2 Controlling blood pressure (BP) is critical to preventing cardiovascular disease which are the leading causes of mortality and major contributors to disabilities and poor quality of life.3-5 Since the mid-2000s, Canada has emerged as an international leader in hypertension screening, diagnosis, and management.6-8 As rates of treatment and BP control significantly increased between 1992 and 2009 (34% vs 82% and 13% vs 69%, respectively), consistent decrease in national cardiovascular deaths has been reported.8-10 In the past decade, however, hypertension control rates have declined, particularly in women. According to a survey conducted in 2016 to 2017, only 58% of adults treated for hypertension were adequately controlled, despite growing evidence and availability of safe and effective antihypertensive agents. 8
A previous study has assessed hypertension management trends in Canada until 2006, but there is an absence of data since. 11 In the past decade, multiple studies were published on hypertension pharmacological treatments, prompting several updates in national hypertension guidelines that should have influenced accordingly prescription profiles. Using a large population-based cohort, we sought to evaluate trends in antihypertensive medication use in regard to national guidelines recommendations in a representative sample of the general population of the province of Quebec from 2009 to 2021.
Methods
Study Design and Population
This retrospective cohort study uses the CARTaGENE database (https://www.cartagene.qc.ca), a population-based survey of 19 996 randomly selected 40- to 69-year-old adults, recruited between 2009 and 2010 from the following 4 metropolitan regions of the province of Quebec (Canada): Montréal, Québec, Sherbrooke, and Saguenay. This biobank was designed to help investigate health determinants using a nationally representative cohort highly comparable to the overall population of Canada. 12 Details regarding its recruitment and data collection processes have been published previously.12-16 Data from the CARTaGENE biobank were cross-referenced with the governmental health administrative database from the sole provincial health insurance board, the Régie de l’Assurance Maladie du Québec (RAMQ). For the purpose of this study, we only included participants under the public RAMQ medication coverage at the end of follow-up, as medication data are not available for participants with private insurance. In the province of Quebec, all adults aged over 65 years old and those without access to a private insurance are eligible to public medication coverage by the RAMQ. To identify such individuals, we included participants with any RAMQ drug claim within 6 months prior to the administrative end of follow-up (March 31, 2021). The study adhered to the Helsinki Declaration and was approved by the local Ethics Review Board (Comité d’éthique de la recherche du CIUSSS du Nord-de-l’Île-de-Montréal, #2017-1358) and follows the STROBE statement for data reporting 17 (see Supplemental Material). Written consent was obtained from all participants at the time of recruitment.
Data Collection
At enrollment, all participants completed standardized questionnaires regarding demographics, medical history, and life habits. Participants were asked to bring all their medications, which were then reviewed by a research nurse. Physical measurements, including body mass index (BMI), BP measurements and electrocardiogram (ECG) were performed. Brachial BP was measured with the Omron 907L device (Omron, Lake Forest, Illinois) in accordance with guidelines recommendations 18 and the average of 3 measurements taken automatically every 2 minutes was recorded. Blood samples were collected to assess the estimated glomerular filtration rate (eGFR, using the 2009 Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI] formula 19 ), lipid profile, and presence of diabetes. Diabetes was defined by either a glycated hemoglobin A1c (HbA1c) ≥6.5%, a fasting glucose ≥7.0 mmol/L, a non-fasting glucose ≥11.1 mmol/L, or the use of a glucose-lowering medication. 20 Presence of cardiovascular disease (myocardial infarction, unstable angina, stroke, transient ischemic attack, heart failure) and atrial fibrillation/flutter prior to recruitment and during follow-up were determined using governmental health administrative databases from the RAMQ and the Ministère de la Santé et des Services sociaux (MSSS) and questionnaires at enrollment. Such databases compile diagnostic codes, procedure codes for ambulatory and inpatient care, data on hospital discharges, and prescribed medication. Electrocardiogram results were also reviewed to identify other participants with atrial fibrillation/flutter.
Blood Pressure Control and Profiles of Antihypertensive Drug Prescriptions
Controlled BP was defined as mean systolic blood pressure (SBP) <140 mm Hg and mean diastolic blood pressure (DBP) <90 mm Hg in individuals without diabetes, and as mean SBP <130 mm Hg and mean DBP <80 mm Hg in participants with diabetes, as per the Canadian guidelines at the time of enrollment. 21 We assessed prescription patterns of antihypertensive drugs at the time of participants’ recruitment (2009 to 2010; obtained from the medication list provided by the participant and reviewed by a research nurse) and in the 6 months prior to the last available date (March 31, 2021; from the health administrative database). As most participants were not eligible for public RAMQ medication coverage at the time of enrollment and several subsequently transited from private to public coverage during the 10-year follow-up period, this dual strategy for medication use identification allowed us to be more representative of the natural health coverage cycle in the province of Québec.
Based on the current Canadian and American Hypertension Guidelines (Supplemental Table S1), 6 optimal prescription profiles were assessed between the 2 study periods22-26:
Monotherapy: Angiotensin-converting enzyme inhibitor (ACEI), angiotensin II receptor blocker (ARB), calcium channel blocker (CCB), or thiazide/thiazide-like diuretic as initial therapy.
Two-drug combination: Combination of ACEI/ARB with CCB or thiazide/thiazide-like diuretic.
Three-drug combination: Combination of ACEI/ARB, CCB, and thiazide/thiazide-like diuretic.
Four-drug combination: 3-drug combination + spironolactone.
Use of single-pill combinations (SPCs).
Use of long-acting thiazide-like diuretic in preference to thiazide diuretic (chlorthalidone or indapamide).
Other prescription patterns and recommendations that can influence the choice of antihypertensive medication were also taken into consideration. First, ACEI/ARB is considered the most appropriate first-line agents in individuals with diabetes. Second, in contrast to the American College of Cardiology (ACC)/American Heart Association (AHA), Hypertension Canada suggests beta-blockers may be acceptable as a first-line therapy, but only in individuals aged < 60 years old.
Statistical Analysis
Analyses were conducted using IBM SPSS Statistics software, version 25. Statistical significance was set at P < .05. Continuous variables are presented as means (standard deviations) or median (interquartile range [IQR]) as appropriate and discrete numerical as frequencies (percentages). Antihypertensive drugs were classified according to their pharmacologic class. Data were compared with Pearson’s chi-square test, Student’s t-test, or Mann-Whitney U-test, as appropriate. Results were also stratified by the achievement of the BP target or not (only for baseline visit), sex, and baseline BMI category. In the subset of participants in which health administrative medication claims, data were also available at enrollment, the concordance between the medication list obtained using this method and the medication list retrieved from the CARTaGENE database (from a participant-provided list reviewed by a research nurse) was assessed using Cohen’s kappa coefficient.
As most antihypertensive agents may be prescribed for indications other than to treat high BP, such as in the context of arrhythmias, ischemic heart disease, heart failure, chronic kidney disease, and migraines, we conducted a sensitivity analysis to assess prescription patterns after excluding all participants with atrial fibrillation, flutter, acute and chronic ischemic heart disease, angina pectoris, any form of heart failure, chronic kidney disease (defined as an eGFR < 60 mL/min/1.73 m2 at baseline or with administrative data), and migraines documented prior to recruitment or at any time during the follow-up period. Furthermore, patients with these conditions may require a different sequence of antihypertensive drugs to treat their condition (eg, use of spironolactone before CCBs or thiazide diuretics).
Results
Of the 19 967 CARTaGENE participants, 17 975 had available BP measurements and prospective data from the health administrative database. Of these participants, 8447 had an RAMQ drug claim within 6 months prior to end of follow-up and were considered covered by the public RAMQ coverage and thus included in the study (Supplemental Figure 1). Median follow-up was 10.1 years (10.7-11.1). Comparison of baseline characteristics between study participants and the overall CARTaGENE cohort is presented in Table 1. Our study cohort had a higher prevalence of diabetes, cardiovascular disease as well as use of statin and aspirin than the overall cohort. In 3751 participants, the medication list was available at baseline both through the patient-provided list at study enrollment and from the medico-administrative health database. In this subset of participants, both lists were concordant in 91.4% with a kappa of 0.857 (P < .001), suggestive of almost perfect agreement. 27
Table 1.
Baseline Characteristics of the Study and the CARTaGENE Cohorts.
| Characteristics | Overall CARTaGENE cohort n = 17 967 | Study cohort n = 8447 | P |
|---|---|---|---|
| Age (years) | 53 (48-61) | 59 (53-64) | < .001 |
| Women | 9135 (50.4) | 4533 (53.7) | < .001 |
| White race | 16 062 (89.4) | 7625 (90.3) | .02 |
| Income < 50 000 CAD per year | 6052 (33.4) | 3599 (42.6) a | < .001 |
| High school education or less | 4624 (25.5) | 2560 (30.3) a | < .001 |
| BMI (kg/m2) | 27.6 ± 5.3 | 28.0 ± 5.4 a | < .001 |
| Current smoker | 3353 (18.8) | 1578 (18.7) a | .85 |
| Diabetes | 1632 (9.1) | 997 (11.8) | < .001 |
| Self-reported hypertension | 4434 (24.7) | 2701 (32.0) | < .001 |
| Cardiovascular disease | 2524 (14.0) | 1586 (18.8) | < .001 |
| eGFR (mL/min/1.73 m2) | 88 ± 15 | 85 ± 15 a | < .001 |
| Brachial SBP (mm Hg) | 124 ± 16 | 126 ± 16 | < .001 |
| Brachial DBP (mm Hg) | 74 ± 10 | 74 ± 10 | .58 |
| Heart rate | 70 ± 11 | 70 ±11 | .58 |
| Total cholesterol (mmol/L) | 5.1 ± 1.0 | 5.1 ± 1.1 a | .74 |
| HDL cholesterol (mmol/L) | 1.2 ± 0.4 | 1.2 ± 0.4 a | .68 |
| Statin use | 3352 (18.7) | 2212 (26.2) | < .001 |
| Aspirin use | 2600 (14.5) | 1733 (20.5) | < .001 |
Note. Results are presented as number (proportion), median (25th-75th percentiles), or mean ± standard deviation, as appropriate. BMI = body mass index; DBP = diastolic blood pressure; eGFR = estimated glomerular filtration rate; SBP = systolic blood pressure.
Missing data as follows [n (%)]: income, 655 (7.8%); education, 60 (0.7%); BMI 92, (1.1); smoking, 56 (0.7%); eGFR, 216 (2.6%); total cholesterol, 216 (2.6%); HDL cholesterol, 217 (2.6%).
At the time of enrollment, 2654 (31.4%) of participants received at least 1 antihypertensive drug. Of those receiving antihypertensive drugs, the BP control rate was 75.8% for a target of <140/90 mm Hg and 69.2% when the target of <130/80 mm Hg for individuals with diabetes was taken into consideration. Participants achieving their BP target had a tendency toward a higher likelihood of receiving suboptimal treatment regimens (Supplemental Table S2). At the end of follow-up, the number of participants taking at least 1 antihypertensive drug increased to 4292 (50.8% of the cohort; P < .001 compared to baseline). At the end of follow-up, 62% had an increase of their number of antihypertensive drugs (Supplemental Figure S2).
Distribution of drug classes among participants prescribed at least 1 antihypertensive medication is shown in Figure 1. During both periods, ACEI/ARB was the most used antihypertensive drug class, present in 72.9% of all patients with an antihypertensive drug prescription in 2009 to 2010 and 70.9% in 2021 (P = .07). Calcium channel blocker prescription rates increased to become the second most prescribed drug class at the end of follow-up. Likewise, between the 2 periods, beta-blocker prescription rates increased but those of thiazide/thiazide-like diuretics decreased. Use of specific antihypertensive drug molecules per pharmacologic class is presented in Supplemental Figure S3.
Figure 1.
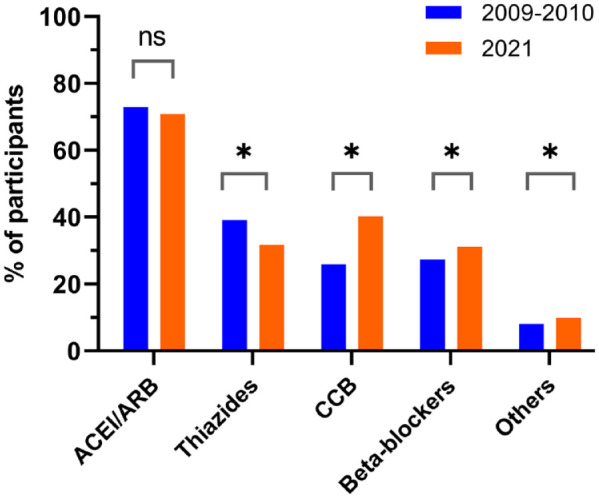
Distribution of drug classes among participants prescribed at least 1 antihypertensive medication.
Note. ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; CCB = calcium channel blocker; ns = not statistically significant.
*P < .05.
In this decade, there were no significant changes in the proportions of participants with optimal mono- or combination therapies for hypertension (Table 2), with optimal 2-, 3- and 4-drug combinations remaining considerably underused. Although use of an ACEI/ARB with a thiazide/thiazide-like diuretic decreased, it remained the most commonly prescribed 2-drug combination throughout the study (47.2% vs 35.4%), whereas the combination of an ACEI/ARB with a CCB significantly increased (14.8% vs 26.9%; Supplemental Tables S3 and S4). There was a significant decrease in the prescription of hydrochlorothiazide (90.5% vs 72.3%, P < .001) and consequently a significant increase in the prescription of long-acting thiazide-like diuretics (9.4% vs 27.7%, P < .001), both as SPCs or as standalone medications (Supplemental Table S5). Similar findings were observed in the sensitivity analysis where participants who may have had a clinical indication for antihypertensive agents outside of BP control were excluded (Supplemental Table S6). The only difference was an increase in the use of optimal monotherapy at the end of follow-up. Findings were also similar after stratification for sex and baseline BMI category (Supplemental Tables S7 and S8).
Table 2.
Optimal Profiles of Antihypertensive Drug Prescriptions From 2009 to 2021.
| Overall cohort (n = 17 967) |
Study cohort (n = 8447) |
|||
|---|---|---|---|---|
| At enrollment (2009-2010) a | At enrollment (2009-2010) a | End of follow-up (2021) | P | |
| Monotherapy
b
ACEI/ARB or CCB or thiazide |
76.6% (1557/2033) |
77.9% (964/1238) |
79.5% (1480/1861) |
.29 |
| Two-drug combination
c
Combination of ACEI/ARB with CCB or thiazide |
62.1% (893/1438) |
62.0% (596/961) |
61.4% (898/1462) |
.77 |
| Three-drug combination
d
Combination of ACEI/ARB with CCB and thiazide |
50.8% (359/706) |
51.9% (236/455) |
46.7% (471/1009) |
.066 |
| Four-drug combination
e
Spironolactone |
9.6% (17/177) |
8.3% (9/109) |
15.9% (40/252) |
.054 |
| Use of SPC f | 47.6% (1021/2144) |
47.1% (667/1416) |
35.1% (868/2471) |
< .001 |
| Use of long-acting thiazide-like diuretic g | 10.9% (172/1571) |
9.5% (99/1040) |
27.7% (381/1373) |
< .001 |
Note. ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; CCB = calcium channel blocker; SPC = single-pill combination.
P > .3 between the study cohort and the entire CARTaGENE cohort at the time of recruitment for all prescription profiles.
The denominators are participants with the following number of antihypertensive drugs.
Only 1.
Only 2.
Three or more.
Four or more.
Two or more.
Any thiazide diuretic (hydrochlorothiazide, chlorthalidone, indapamide).
In participants with diabetes, use of ACEI/ARB as initial therapy marginally decreased from 85.2% (n = 548/643) to 81.5% (n = 1065/1306; P < .001). Among participants taking beta-blockers as monotherapy, 53% (n = 121/230) were aged ≥60 years at the time of recruitment, increasing to 95% (n = 295/311) at the end of follow-up. Moreover, in the sensitivity analysis, rates of beta-blocker monotherapy among participants taking only 1 antihypertensive medication class remained stable from 11.8% (n = 79/668) to 9.1% (n = 100/1103, P = .068) despite a 10-year increase in age. When considering beta-blockers as an optimal first-line therapy only in participants <60 years (as per Hypertension Canada guidelines), use of optimal monotherapy decreased from 86.7% at enrollment to 80.4% at the end of follow-up (P < .001). In the sensitivity analysis excluding patients who may have a clinical indication for any antihypertensive agent outside of BP control, similar findings to the overall analysis are found (from 91.3% at enrollment to 86.7% at the end of follow-up; P = .0022).
Discussion
In this large population-based cohort study, the prevalence of participants with antihypertensive drug prescriptions increased by 20% between 2009 and 2021, reflecting the rise of hypertension with age.28,29 In both study periods, guideline-recommended monotherapy was applied in most but not all participants, whereas optimal 2-, 3-, and 4-drug combination therapies remained used less frequently in both study periods. Angiotensin-converting enzyme inhibitor/ARB remained the most prescribed antihypertensive drug class, whereas CCB prescriptions significantly increased to become the second most prescribed class in 2021. Although prescriptions of long-acting thiazide diuretics increased, their use remained suboptimal, with the majority of participants still receiving hydrochlorothiazide as a diuretic at the end of follow-up contrary to what is recommended by the guidelines.
Our findings are concordant with data from other countries. Several high-income countries reported prevalence of hypertension between 20% to 38% and 46% to 68% in adults aged between 50 to 59 years and 60 to 69 years, respectively.30,31 In a population-based cohort study from the United Kingdom, ACEI (40%) and CCBs (32%) were the most common first-line drugs reported between 2007 and 2018. 32 In that period, guideline-recommended first-line therapy was applied in 82% of patients. Likewise, in a US cross-sectional study analyzing national survey data from 2005 to 2016, there was no evidence of changes in the proportions of adults taking antihypertensive monotherapy (40%) and we can extrapolate from published data that optimal first-line therapy was prescribed in 84% of patients between 2013 and 2016. 33 Conversely, in the same period, 2- and 3-drug combination therapies were applied in a significantly lower proportion of patients (60% and 26%, respectively).
Suboptimal use of guideline-recommended lines of therapy may mirror therapeutic inertia in the management of hypertension. Therapeutic inertia is the failure to initiate or intensify treatment when it is clinically indicated. 34 It is due, in part, to overestimation of the care provided, use of inappropriate justifications to avoid intensification of therapy, and lack of education on the benefits of achieving therapeutic targets. 34 For instance, use of spironolactone for resistant hypertension has not yet been implemented in routine clinical practice, even though there is growing evidence of its efficacy and that it has been accepted by several organizations as the optimal fourth-line agent.24,26,35-37 In addition, throughout the study period, a significant proportion of participants on beta-blocker monotherapy aged over 60 years old, increasing to >90% at the end of follow-up. Even in our sensitivity analysis excluding all participants with a potential clinical indication for any antihypertensive agent outside of BP control, most users of beta-blockers as a monotherapy were aged over 60 years old. Several studies have suggested that beta-blockers may be less effective for prevention of stroke and all-cause mortality, primarily in adults aged over 60 years, which led the AHAs to no longer recommend beta-blockers as initial therapy. 25 ,38-40 Consequently, although Canadian guidelines still recommend their use only in patients <60 years, health practitioners should consider prioritizing other antihypertensive agents as initial therapy in patients without compelling clinical indications. 23 Our data also suggest individuals in whom the BP is below the target are less likely to receive an optimal treatment regimen. However, randomized trials, notably ASCOT-BPLA 41 and ACCOMPLISH, 42 demonstrated that specific drugs, in combination or not, result in a lower risk of cardiovascular events despite similar achieved BP targets. Therefore, even in individuals meeting their BP target, switching to more optimal antihypertensive agents may be beneficial. Convincing physicians to do so remains a challenge that may need to be further emphasized in the next iterations of the clinical practice guidelines.
Our study has shown a decrease in the use of SPC between 2009 and 2021, which correlated with concurrent decrease in hydrochlorothiazide prescriptions and the rise of dual therapy with a combination of an ACEI/ARB and a CCB. The overwhelming majority of current available SPC sold in Canada and reimbursed by the RAMQ are combinations of ACEI/ARB with a thiazide/thiazide-like diuretic, almost exclusively hydrochlorothiazide. In 2009, the ACCOMPLISH trial demonstrated that the combination of an ACEI and a CCB was superior to dual therapy with an ACEI and hydrochlorothiazide in reducing the risk of cardiovascular events even in presence of a similar achieved BP. 42 Since 2017, guidelines recommend long-acting thiazide-like diuretics in preference to thiazide diuretics.23,43 It should be noted that a recent trial of US male veterans showed no difference in occurrence of major cardiovascular outcome events in patients who were switched from hydrochlorothiazide to chlorthalidone compared with those remaining on hydrochlorothiazide. 44 Nevertheless, a major limitation of this study was that the primary comparison was between 25 mg of hydrochlorothiazide and 12.5 mg of chlorthalidone, both of which may have been suboptimally dosed to show cardiovascular benefits.45,46 Finally, intensive BP control often requires combining 2 or more antihypertensive medication classes.47,48 In this context, SPC regimens have shown to be key factors in BP management and prevention of cardiovascular disease by decreasing pill burden and improving treatment adherence.49-51
Our study has several strengths. To our knowledge, this is the first Canadian study to comprehensively assess the use of optimal antihypertensive agent profiles in clinical practice in the past decade. Access to the RAMQ drug claims, database allowed accurate and extensive analyses of antihypertensive drug prescriptions. Moreover, our study used the CARTaGENE population–based cohort, which is highly representative of the Canadian population. 12 Important limitations, however, need to be considered. First, the retrospective nature of the study elicits a potential risk of selection bias, by solely including participants who survived until the end of follow-up and who were on the public insurance regimen at the end of follow-up. This selected from the overall cohort a subgroup of participants without private medical coverage and more likely to have comorbid conditions and of lower socio-economic status, which may not be representative of the general population. Nevertheless, prescription profiles in our subgroup were similar to the ones of the overall CARTaGENE cohort at the time of enrollment, suggesting our results may have broader generalizability. Second, we used distinct methods to extract the medication list at enrollment and at the end of follow-up, although in a subset of participant where both types of data were available, the agreement in the medication list was almost perfect. Third, we could not assess if the absence of a specific indication antihypertensive drug class in a participant’s regimen was due to an intolerance or side effects before enrollment or during the follow-up. However, the proportion of participant in which a specific medication is not taken is likely similar for both study periods. Fourth, some participants may have been taking antihypertensive drugs, particularly beta-blockers, for other indications than hypertension. However, our results were highly concordant in a sensitivity analysis excluding participants with potential clinical indication antihypertensive agents outside of BP control. Fifth, as BP measurements were only taken at the time of recruitment, hypertension control could not be assessed at the end of follow-up. Furthermore, hypertension was deemed controlled or not solely based on single series of BP measurement at enrollment. Also, home BP was not measured, which may have help assess BP control rates. Finally, we studied prescriptions of medications, which do not accurately reflect their real use by participants, although all medications needed to be filled out at the pharmacy to be registered in the RAMQ registry and our aim was to assess prescription patterns of antihypertensive medications, not their efficacy.
Conclusions
The implementation of hypertension guidelines for the choice of antihypertensive drugs remains suboptimal in clinical practice, more so when multiple antihypertensive drugs are required. In a decade of declining BP control rates in Canada, our findings highlight the need for education initiatives and emphasis on implementation of evidence-based guidelines to reach BP targets and prevent cardiovascular disease.
Supplemental Material
Supplemental material, sj-docx-1-cjk-10.1177_20543581241234729 for Differences in Antihypertensive Medication Prescription Profiles Between 2009 and 2021: A Retrospective Cohort Study of CARTaGENE by Victoria Ivensky, Pitchou Zonga, Gabriel Dallaire, Louis-Charles Desbiens, Annie-Claire Nadeau-Fredette, Guy Rousseau and Rémi Goupil in Canadian Journal of Kidney Health and Disease
Acknowledgments
R.G. holds a research scholarship from the Fonds de recherche du Québec—Santé and is a recipient of the Société québécoise d’hypertension artérielle—Bourse Jacques-de-Champlain scholarship. A.-C.N.F. holds a research scholarship from the Fonds de recherche du Québec—Santé and is a recipient of the Société québécoise de néphrologie scholarship.
Footnotes
Ethics Approval and Consent to Participate: The study adhered to the Helsinki Declaration and was approved by the local Ethics Review Board (Comité d’éthique de la recherche du CIUSSS du Nord-de-l’Île-de-Montréal, #2017-1358). Written consent was obtained from all participants at the time of recruitment.
Consent for Publication: Consent for publication was obtained from all authors.
Availability of Data and Materials: Data is available upon request to CARTaGENE (subject to approval).
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Canadian Institutes of Health Research (PJT-173313) and the Heart and Stroke Foundation (G-20-0028656).
ORCID iDs: Gabriel Dallaire  https://orcid.org/0000-0001-9333-3039
https://orcid.org/0000-0001-9333-3039
Annie-Claire Nadeau-Fredette  https://orcid.org/0000-0002-7755-1404
https://orcid.org/0000-0002-7755-1404
Rémi Goupil  https://orcid.org/0000-0002-0098-3735
https://orcid.org/0000-0002-0098-3735
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Weaver CG, Clement FM, Campbell NR, et al. Healthcare costs attributable to hypertension: Canadian population-based cohort study. Hypertension. 2015;66(3):502-508. [DOI] [PubMed] [Google Scholar]
- 2. DeGuire J, Clarke J, Rouleau K, et al. Blood pressure and hypertension. Health Rep. 2019;30(2):14-21. [PubMed] [Google Scholar]
- 3. Bundy JD, Li C, Stuchlik P, et al. Systolic blood pressure reduction and risk of cardiovascular disease and mortality: a systematic review and network meta-analysis. JAMA Cardiol. 2017;2(7):775-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. GBD 2019. Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. GBD Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1659-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Joffres M, Falaschetti E, Gillespie C, et al. Hypertension prevalence, awareness, treatment and control in national surveys from England, the USA and Canada, and correlation with stroke and ischaemic heart disease mortality: a cross-sectional study. BMJ Open. 2013;3(8):e003423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leung AA, Bell A, Tsuyuki RT, Campbell NRC. Refocusing on hypertension control in Canada. CMAJ. 2021;193(23):E854-E855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leung AA, Williams JVA, McAlister FA, et al. ; Hypertension Canada’s Research and Evaluation Committee. Worsening hypertension awareness, treatment, and control rates in Canadian women between 2007 and 2017. Can J Cardiol. 2020;36(5):732-739. [DOI] [PubMed] [Google Scholar]
- 9. McAlister FA, Wilkins K, Joffres M, et al. Changes in the rates of awareness, treatment and control of hypertension in Canada over the past two decades. CMAJ. 2011;183(9):1007-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Campbell NR, Brant R, Johansen H, et al. Increases in antihypertensive prescriptions and reductions in cardiovascular events in Canada. Hypertension. 2009;53(2):128-134. [DOI] [PubMed] [Google Scholar]
- 11. Hemmelgarn BR, Chen G, Walker R, et al. Trends in antihypertensive drug prescriptions and physician visits in Canada between 1996 and 2006. Can J Cardiol. 2008;24(6):507-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Awadalla P, Boileau C, Payette Y, et al. Cohort profile of the CARTaGENE study: Quebec’s population-based biobank for public health and personalized genomics. Int J Epidemiol. 2013;42(5):1285-1299. [DOI] [PubMed] [Google Scholar]
- 13. Lamarche F, Agharazii M, Madore F, Goupil R. Prediction of cardiovascular events by type I central systolic blood pressure: a prospective study. Hypertension. 2021;77(2):319-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Desbiens LC, Fortier C, Nadeau-Fredette AC, et al. Prediction of cardiovascular events by pulse waveform parameters: analysis of CARTaGENE. J Am Heart Assoc. 2022;11(17):e026603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goupil R, Dupuis D, Agharazii M, et al. Central blood pressures in early chronic kidney disease: an analysis of CARTaGENE. Nephrol Dial Transplant. 2017;32(6):976-983. [DOI] [PubMed] [Google Scholar]
- 16. Dupuis ME, Nadeau-Fredette AC, Madore F, et al. Association of glomerular hyperfiltration and cardiovascular risk in middle-aged healthy individuals. JAMA Network Open. 2020;3(4):e202377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344-349. [DOI] [PubMed] [Google Scholar]
- 18. Quinn RR, Hemmelgarn BR, Padwal RS, et al. The 2010 Canadian Hypertension Education Program recommendations for the management of hypertension: part I—blood pressure measurement, diagnosis and assessment of risk. Can J Cardiol. 2010;26(5):241-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ivers NM, Jiang M, Alloo J, et al. Diabetes Canada 2018 clinical practice guidelines: key messages for family physicians caring for patients living with type 2 diabetes. Can Fam Physician. 2019;65(1):14-24. [PMC free article] [PubMed] [Google Scholar]
- 21. Campbell NR, Khan NA, Hill MD, et al. 2009 Canadian Hypertension Education Program recommendations: the scientific summary—an annual update. Can J Cardiol. 2009;25(5):271-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Khan NA, Hemmelgarn B, Herman RJ, et al. The 2009 Canadian Hypertension Education Program recommendations for the management of hypertension: part 2—therapy. Can J Cardiol. 2009;25(5):287-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rabi DM, McBrien KA, Sapir-Pichhadze R, et al. Hypertension Canada’s 2020 comprehensive guidelines for the prevention, diagnosis, risk assessment, and treatment of hypertension in adults and children. Can J Cardiol. 2020;36(5):596-624. [DOI] [PubMed] [Google Scholar]
- 24. Hiremath S, Sapir-Pichhadze R, Nakhla M, et al. Hypertension Canada’s 2020 evidence review and guidelines for the management of resistant hypertension. Can J Cardiol. 2020;36(5):625-634. [DOI] [PubMed] [Google Scholar]
- 25. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71(19):e127-e248. [DOI] [PubMed] [Google Scholar]
- 26. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72(5):e53-e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174. [PubMed] [Google Scholar]
- 28. Robitaille C, Dai S, Waters C, et al. Diagnosed hypertension in Canada: incidence, prevalence and associated mortality. CMAJ. 2012;184(1):E49-E56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chobufo MD, Gayam V, Soluny J, et al. Prevalence and control rates of hypertension in the USA: 2017-2018. Int J Cardiol Hypertens. 2020;6:100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. NCD Risk Factor Collaboration. Long-term and recent trends in hypertension awareness, treatment, and control in 12 high-income countries: an analysis of 123 nationally representative surveys. Lancet. 2019;394(10199):639-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Statistics Canada. Hypertension prevalence, awareness, treatment and control, combined cycles, by age group and sex, Canada (excluding territories). doi: 10.25318/1310038401-eng. [DOI] [Google Scholar]
- 32. Rouette J, McDonald EG, Schuster T, et al. Treatment and prescribing trends of antihypertensive drugs in 2.7 million UK primary care patients over 31 years: a population-based cohort study. BMJ Open. 2022;12(6):e057510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Derington CG, King JB, Herrick JS, et al. Trends in antihypertensive medication monotherapy and combination use among US adults, national health and nutrition examination survey 2005-2016. Hypertension. 2020;75(4):973-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135(9):825-834. [DOI] [PubMed] [Google Scholar]
- 35. Williams B, MacDonald TM, Morant S, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386(10008):2059-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de Souza F, Muxfeldt E, Fiszman R, Salles G. Efficacy of spironolactone therapy in patients with true resistant hypertension. Hypertension. 2010;55(1):147-152. [DOI] [PubMed] [Google Scholar]
- 37. Mancia Chairperson G, Kreutz Co-Chair R, Brunstrom M, et al. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension: endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH). J Hypertens. 2023;41(12):1874-2071. [DOI] [PubMed] [Google Scholar]
- 38. Wiysonge CS, Bradley HA, Volmink J, et al. Beta-blockers for hypertension. Cochrane Database Syst Rev. 2012;8:CD002003. [DOI] [PubMed] [Google Scholar]
- 39. Thomopoulos C, Bazoukis G, Tsioufis C, Mancia G. Beta-blockers in hypertension: overview and meta-analysis of randomized outcome trials. J Hypertens. 2020;38(9):1669-1681. [DOI] [PubMed] [Google Scholar]
- 40. Gupta A, Mackay J, Whitehouse A, et al. Long-term mortality after blood pressure-lowering and lipid-lowering treatment in patients with hypertension in the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) Legacy study: 16-year follow-up results of a randomised factorial trial. Lancet. 2018;392(10153):1127-1137. [DOI] [PubMed] [Google Scholar]
- 41. Dahlöf B, Sever PS, Poulter NR, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366(9489):895-906. [DOI] [PubMed] [Google Scholar]
- 42. Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359(23):2417-2428. [DOI] [PubMed] [Google Scholar]
- 43. Leung AA, Daskalopoulou SS, Dasgupta K, et al. Hypertension Canada’s 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol. 2017;33(5):557-576. [DOI] [PubMed] [Google Scholar]
- 44. Ishani A, Cushman WC, Leatherman SM, et al. Chlorthalidone vs. Hydrochlorothiazide for hypertension-cardiovascular events. N Engl J Med. 2022;387(26):2401-2410. [DOI] [PubMed] [Google Scholar]
- 45. Amery A, Birkenhager W, Brixko P, et al. Mortality and morbidity results from the European working party on high blood pressure in the elderly trial. Lancet. 1985;1(8442):1349-1354. [DOI] [PubMed] [Google Scholar]
- 46. The ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981-2997. [DOI] [PubMed] [Google Scholar]
- 47. Cushman WC, Ford CE, Einhorn PT, et al. Blood pressure control by drug group in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). J Clin Hypertens. 2008;10(10):751-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wright JT, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373(22):2103-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bangalore S, Kamalakkannan G, Parkar S, Messerli FH. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med. 2007;120(8):713-719. [DOI] [PubMed] [Google Scholar]
- 50. Lauffenburger JC, Landon JE, Fischer MA. Effect of combination therapy on adherence among US patients initiating therapy for hypertension: a cohort study. J Gen Intern Med. 2017;32(6):619-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Borghi C, Wang J, Rodionov AV, et al. Projecting the long-term benefits of single pill combination therapy for patients with hypertension in five countries. Int J Cardiol Cardiovasc Risk Prev. 2021;10:200102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cjk-10.1177_20543581241234729 for Differences in Antihypertensive Medication Prescription Profiles Between 2009 and 2021: A Retrospective Cohort Study of CARTaGENE by Victoria Ivensky, Pitchou Zonga, Gabriel Dallaire, Louis-Charles Desbiens, Annie-Claire Nadeau-Fredette, Guy Rousseau and Rémi Goupil in Canadian Journal of Kidney Health and Disease


