Abstract
Forty percent of women with primary cytomegalovirus (CMV) infections during pregnancy infect their fetuses with complications for the baby varying from mild to severe. How CMV crosses the syncytiotrophoblast, the barrier between maternal blood and fetal tissue in the villous placenta, is unknown. Virus may cross by infection of maternal cells that pass through physical breaches in the syncytiotrophoblast or by direct infection of the syncytiotrophoblast, with subsequent transmission to underlying fetal placental cells. In this study, we show that pure (>99.99%), long-term and healthy (>3 weeks) cultures of syncytiotrophoblasts are permissively infected with CMV. Greater than 99% of infectious progeny virus remained cell associated throughout culture periods up to 3 weeks. Infection of term trophoblasts required a higher virus inoculum, was less efficient, and progressed more slowly than parallel infections of placental and human embryonic lung fibroblasts. Three laboratory strains (AD169, Towne, and Davis) and a clinical isolate from a congenitally infected infant all permissively infected trophoblasts, although infection efficiencies varied. The infection of first trimester syncytiotrophoblasts with strain AD169 occurred at higher frequency and progressed more rapidly than infection of term cells but less efficiently and rapidly than infection of fibroblasts. These results show that villous syncytiotrophoblasts can be permissively infected by CMV but that the infection requires high virus titers and proceeds slowly and that progeny virus remains predominantly cell associated.
Cytomegalovirus (CMV), a member of the Herpesviridae family, is endemic and results primarily in subclinical infections in normal healthy individuals (reviewed in reference 32). However, the virus causes severe disease and death in immunocompromised hosts and can be transmitted to the fetus during pregnancy. Excluding rubella epidemics, CMV is the most common congenital infection in the world, occurring in 0.5 to 2.0% of all live births (50). Ten to fifteen percent of infected infants show severe symptoms at birth (14, 41). Thirty to sixty percent of infants born with mild or clinically asymptomatic infections develop neurological deficits of various degrees later in life (41, 55). Approximately 40% of mothers with a primary infection during gestation transmit CMV to their infants, compared to <0.5% transmission during a recurring infection (65). A primary infection in the first trimester of pregnancy may result in more severe fetal consequences than one occurring in the third, but this timing is not associated with an increased risk of transmission (9, 10, 31, 42, 54).
Human CMV replicates in vivo in a variety of human cells including epithelium (56). In vitro it preferentially replicates in human fibroblasts, although low levels of replication occur in other cell types (52). Several patterns of infection occur, dependent on the cell types and virus strains involved (20, 45, 60), including (i) permissive infections during which infectious virus is produced and cytopathic effects are observed, (ii) persistent permissive infections during which virus is produced but cell loss is lower than cell replacement by proliferation, allowing the culture to survive indefinitely, or (iii) abortive infections during which immediate-early (IE) antigen, but not infectious virus, is produced.
Although the pathogenesis of CMV transmission to the fetus during pregnancy is unknown, congenital CMV infections are commonly associated with chronic villitis (38, 47) and infection of the placenta (1, 7, 18, 33, 35, 37, 38, 43, 47, 48, 51). Thus, passage likely occurs through the placenta (5, 6), which may also act as a viral reservoir (22). Since only 40% of pregnant women with primary CMV infections give birth to infected infants (54), an effective fetal barrier, either physical or immunological, must exist. Within the villous placenta lies a physical barrier of fetal cells consisting of continuous, mitotically inactive, multinucleated syncytiotrophoblasts (ST) that are in direct contact with maternal blood (6). In order to reach the fetus in the third trimester of pregnancy, molecules, cells, or organisms must cross the ST and the endothelial cells of fetal blood vessels. Underlying the ST in the first and second trimester are immature, mitotically active, mononuclear cytotrophoblasts (CT) that fuse into the ST (4). Connective tissue containing placental fibroblasts and macrophages can intervene between these CT and fetal blood vessels. Whether virus crosses the ST by direct infection or through sites of damage is not known.
The role of ST in transmission of CMV across the placental barrier is unclear. Results from in vivo studies are difficult to assess because placentas obtained from stillbirths, symptomatic congenitally infected infants at term, or those with chronic villitis at term tend to be preferentially studied (18, 33, 37, 48, 51). Such term placentas, and the trophoblast in particular, rarely display the inclusion bodies characteristic of permissive CMV infections (18, 33, 37, 38, 43, 51). Immunohistochemical analysis of sections from term placentas displaying chronic villitis revealed IE (37, 51) but not early nuclear (37) or late (p150) (51) antigens, suggesting abortive infections (51). In situ hybridization revealed CMV DNA primarily in stromal cells and rarely in the trophoblast of term placentas with chronic villitis (47). Term placentas perfused in vitro and challenged with high titers of a CMV laboratory strain for up to 9.5 h were nonpermissive within this short experimental time frame (36).
In contrast, placentas from first or second trimester abortions contain nuclear inclusions frequently in stromal cells (48) and more rarely in trophoblasts (18), with some expression of pp65 antigen in the trophoblast (61), indicating that a permissive trophoblast infection during the first half of gestation is possible. In vitro infections of first trimester placental explants show permissive infections by both morphological and immunohistochemical criteria (2, 3). In guinea pigs, detection of intranuclear inclusions and expression of CMV antigens in ST at all stages of gestation indicate permissive infections are also possible in this animal model (22). Highly purified term trophoblasts express IE antigens after CMV challenge but do not release virus into culture supernatants unless coinfected with either human immunodeficiency virus type 1 (HIV-1) (59) or human T-cell leukemia virus type 1 (HTLV-1) (58). These results are compatible with the in vivo findings of infrequent nonpermissive trophoblast infections at term. However, it remains difficult to explain the 40% transmission rate resulting from primary maternal infections or the more frequent indications of permissive infections in first trimester trophoblasts on the basis of such coinfections.
Although apparently straightforward, the development of an effective culture model of ST infection by CMV must address two interdependent problems: fibroblast contamination and long-term culture viability. Placental fibroblasts are likely preferred targets for this virus not only because laboratory strains are passaged in fibroblasts but also because placental fibroblasts, unlike primary villous trophoblasts (66), proliferate in culture. CMV replicates more rapidly in proliferating than quiescent cells (57) and would be predicted to replicate more slowly in trophoblasts than fibroblasts. Permissive infection by CMV also requires viable (healthy) cultures (32). The possibility of slow virus replication dictates that cultures must be viable longer than 2 weeks. However, primary trophoblasts have rarely been cultured for longer than 7 days because of fibroblast overgrowth or loss of viability (15, 16, 29, 65). We have developed culture models of highly purified (>99.99% [28]) term trophoblasts that maintain viability for greater than 3 weeks in culture and have modified this model to obtain highly purified first trimester trophoblasts. We demonstrate using these models that CMV laboratory strains and a clinical isolate permissively infect term and first trimester trophoblasts, but virus replication is slow and infectious virions remain cell associated.
MATERIALS AND METHODS
Cells. (i) Isolation and purification of human term villous CT.
Placentas were obtained after normal term delivery or elective cesarean section from uncomplicated pregnancies. Villous CT (>99.99% pure) were isolated by trypsin-DNase digestion of minced chorionic tissue and immunoabsorption onto immunoglobulin (Ig)-coated glass bead columns (Biotex, Edmonton, Alberta, Canada) as previously described (28, 65), using anti-CD9, anti-major histocompatibility complex (MHC) class I (W6/32; Harlan Sera-Lab, Crawley Down, Sussex, England), and anti-MHC class II (clone 7H3) antibodies for immunoelimination. The purified cells were routinely cryopreserved and after thawing were washed twice in Iscove’s modified Dulbecco’s medium (IMDM; GIBCO, Grand Island, N.Y.) supplemented with 10% fetal bovine serum (FBS; GIBCO). The cells were seeded at 105 per microwell per 100 μl of 10% FBS (GIBCO) in 96-well tissue culture dishes (Nunc no. 167008; GIBCO) and incubated for 4 h at 37°C in a 5% CO2 humidified atmosphere; the nonadherent cells and debris were removed with prewarmed IMDM, and the cells were replenished with 10% FBS–IMDM and 50 μg of gentamicin per ml. All preparations contained fewer than 10 vimentin-positive cells (fibroblasts) per microwell after the 4-h wash. Syncytialization of cultured CT was induced by treatment with 10 ng of recombinant human epidermal growth factor (EGF; Prepro-Tech, Rocky Hill, N.J.) per ml for 5 days (34) and assessed by immunostaining fixed cells with antidesmoplakin monoclonal antibody (Sigma) to visualize desmosome-containing tight junctions (15) as previously described (65). Cell numbers were estimated as described below.
(ii) Isolation, purification, and culture of human first trimester villous CT.
Placental chorionic tissue was separated microscopically from fetal material obtained from elective abortions performed at 10 to 15 weeks of gestation. Chorionic cells were isolated as described previously (65), with the following modifications: 10 ml of tissue was harvested per placenta; and trypsin-DNase digestion was performed at the same concentration for the same number of times as with term placentas, but with 1:1 volumes of tissue to trypsin-DNase at a reduced time of 5 min. Cell purification was carried out on glass bead columns as described above. CT preparations from first trimester placentas used in this study contained fewer than 0.02% vimentin-positive cells. Culture and induction of syncytialization of first trimester villous CT were performed as described for term CT.
(iii) Isolation, purification, and culture of PF.
Placental fibroblasts (PF) were isolated from first trimester chorionic cell suspensions prior to antibody treatment and column purification by plating the suspensions in 60- by 15-mm tissue culture dishes for 60 min, followed by removal of nonadherent cells and culture in 10% FBS–IMDM. Adherent cells grown to confluency were lifted by treatment with 0.05% trypsin-EDTA (GIBCO), washed in 10% FBS–IMDM, and further propagated in 100- by 20-mm tissue culture dishes. Confluent cultures were passaged at least five times to ensure >99% purity, as assessed by immunohistochemical staining for vimentin, and cryopreserved in 10% dimethyl sulfoxide in FBS. Before experimental use, PF were thawed and cultured in 10% FBS–IMDM and 50 μg of gentamicin per ml until confluent and passaged at least once.
(iv) HEL cells.
Human embryonic lung fibroblasts (HEL cells) were propagated in Eagle’s minimum essential medium (MEM) supplemented with 10% FBS and 50 μg of gentamicin per ml. For CMV infection assays, the cells were plated in 10% FBS–MEM at a concentration 4 × 104 per 100 μl in 96-well tissue culture plates. All experiments were carried out on confluent cultures with changes of media every 96 h.
(v) Determination of cell numbers in culture.
The number of trophoblasts and fibroblasts in microwells was determined from the DNA content of parallel cultures based on a DNA content for a human diploid nucleus of 6 pg. Calculations of fibroblast and CT numbers were based on one nuclei per cell, while ST numbers were based on an average of four per cell (28, 63). After virus challenge and washing (see below), the microwell cultures of trophoblasts contained from 4,000 to 16,000 cells per well, depending on the adherence properties of individual preparations, the time of culture (most preparations lose 20 to 50% of their DNA content over a 1-month culture period), and the multinucleated state of the culture.
CMV. (i) Virus stock preparations.
CMV laboratory strains AD169, Davis, and Towne and a clinical isolate from a congenitally infected infant were passaged on confluent HEL cells in 2% FBS–MEM, and infectious virus was recovered by freezing and thawing the cultures three times. The lysate was passed through 0.45-μm-pore-size filters (MILLEX-HV; Millipore Products Division, Bedford, Mass.) and stored in liquid nitrogen until use. All CMV strains were passaged <12 times, and the clinical isolate was passaged four times. Viral titers were determined by inoculating confluent HEL cultures in 96-well plates with dilutions of each virus in serum-free MEM. The plates were then centrifuged for 45 min at 2,500 rpm in a GCL-2 Sorvall centrifuge, the wells were washed five times with warm MEM, and the plates were incubated for a further 18 to 20 h in fresh 2% FBS–MEM. The cultures were fixed in ice-cold methanol and immunohistochemically stained for CMV IE antigen as described below. Each IE-positive nucleus is equated to an infection focus (IF) of infectious virus, and the titer of virus was determined within a linear dose-response concentration range as IF/milliliter.
(ii) Infection protocols.
Infection with each strain or isolate at various multiplicities of infection (MOIs) was carried out in serum-free IMDM for 2 h at 37°C in 5% CO2. MOI is the ratio of IF of inoculating virus to the total number of cells in culture to be infected. The cell number was determined at all times of culture as described above. The cultures were infected as follows: EGF-treated (+EGF) term trophoblasts at day 5 of culture, non-EGF-treated (-EGF) term trophoblasts at day 1 of culture, +EGF first trimester trophoblasts at day 3 of culture, and PF and HEL cells at confluency. The cells were then washed five times with serum-free IMDM and incubated in fresh 2% FBS–IMDM with or without EGF for various times postinfection, and the media were changed every 96 h. HEL infection was carried out as described above in serum-free MEM, followed by incubation in 2% FBS–MEM. All IE-positive nuclei strongly stained and were scored (e.g., in Fig. 1A there are eight IE-positive nuclei in the field); however, only strongly pp65-positive nuclei were scored since these were often surrounded by nuclei that stained more weakly for pp65 antigen (e.g., in Fig. 1B there are three pp65-positive nuclei in the field). All placental preparations were tested for initial or reactivated CMV infection by including uninfected control cultures stained for IE and pp65 antigens in each experiment.
FIG. 1.
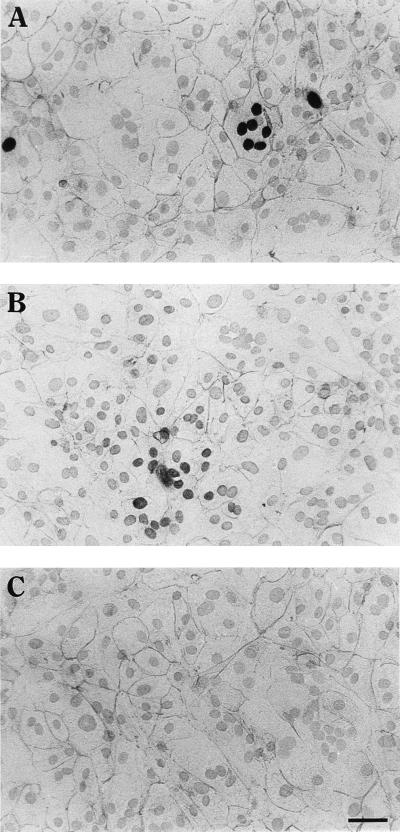
Detection of desmoplakin and nuclear expression of CMV IE or pp65 antigens in trophoblast cultures by double immunohistochemical staining. Column-purified villous CT were induced to syncytialize by the addition of 10 ng of EGF per ml and challenged on day 5 of culture with CMV strain AD169 at an MOI of 1.0. At day 12 postinfection, cultures were immunohistochemically stained for CMV antigens with Ni-DAB substrate and for desmoplakin with AEC substrate. (A) Infected culture stained for CMV IE antigen and desmoplakin; (B) infected culture stained for pp65 and desmoplakin; (C) infected culture stained for desmoplakin. Bar, 25 μm.
(iii) Determination of infectious virus titers in supernatants or cell lysates.
Supernatants were removed from cultures at various times postinfection and frozen at −80°C until assessed for virus titer on HEL cells. Adherent cells were washed three times with phosphate-buffered saline (PBS) and lysed in 100 μl of 2% FBS–IMDM by freezing and thawing three times (lysate). Viral titers in culture supernatants or cell lysates were assayed on HEL cultures as described above, and IF/milliliter of transferred supernatant or cell lysate was determined. Infectious virus found in supernatants were not from residual inoculum since in all cases, none was found 24 h after challenge (data not shown).
Immunohistochemical staining.
Infected and uninfected cultures were washed twice with PBS, fixed in ice-cold methanol for 10 min at −20°C, and washed three times with PBS. Endogenous peroxidase activity was neutralized by a 30-min incubation at room temperature with 3% H2O2, followed by a 1-h incubation at room temperature in 10% nonimmune goat serum (Zymed/Intermedico, Markham, Calif.) to block nonspecific sites. Primary antibodies detecting either CMV IE (detecting p72; Specialty Diagnostics, Dupont) or CMV pp65 (detecting pp64/pp65; Biotest, Dreieich, Germany) antigens, and their respective isotype controls, IgG2a (Zymed/Intermedico) and IgG1 (Dako Corporation, Carpinteria, Calif.), were added; the plates were sealed with Parafilm and incubated overnight at 4°C. After thorough washing with PBS, secondary antibody (biotinylated goat anti-mouse IgG) and streptavidin-peroxidase conjugate (streptavidin-biotin system, Histostain-SP kit; Zymed) were added according to the manufacturer’s instructions. Following a PBS wash, Ni-diaminobenzidine (DAB) substrate (95 mg of DAB, 1.6 g of NaCl, 0.136 g of imidazole, 2 g of NiSO4; made up to 200 ml with 0.1 M acetate buffer [pH 6.0] [21]) was added for 2 to 5 min and yields a dark brown precipitate. The plates were then washed with double-distilled H2O. The frequencies of IE- or pp65-positive nuclei and IF were determined at all time points. The number of nuclei per foci ranged from one in -EGF cultures within a week of infection to as high as 50 for +EGF cultures at 20 days of culture. In some cases, double staining by incubation with a second primary antibody, either desmoplakin (ICN ImmunoBiologicals, Costa Mesa, Calif.) or vimentin (clone V9; Dako), was carried out immediately and the secondary antibody and streptavidin-peroxidase conjugate steps were repeated as described above, using aminoethylcarbazole (AEC) as a substrate, yielding a red precipitate. The cells were counterstained with hematoxylin, and photographs were taken immediately.
Measurement of DNA.
The assay was a modification of the method described by Cesarone et al. (11). Cells cultured in 96-well plates were washed twice with PBS, 100 μl of double-distilled H2O was added to each well, and the plates were frozen and thawed three times to lyse the cells. The samples were transferred to 96-well V-bottom plates (Nunc) and mixed with equal volumes of Hoechst dye solution (1 μg of Hoechst 33258 [Sigma Chemical, St. Louis, Mo.] per ml, 10 mM Tris, 1 mM EDTA, 2.1 M NaCl [pH 7.4]), and the fluorescence was measured on an LS-5 luminescence spectrometer (Perkin-Elmer, Norwalk, Conn.), using calf thymus DNA as a standard to calculate the amount of DNA per well in nanograms/milliliter.
RESULTS
Villous trophoblasts from term placentas are infected with cell-free CMV.
Primary villous CT cultured with EGF (designated +EGF) form within 4 days a continuous cell layer that is predominantly multinuclear ST-like; Fig. 1), whereas cells cultured without EGF (designated -EGF) form a continuous layer of predominantly mononuclear cells (CT-like) (65). When +EGF cultures were challenged with AD169 and examined for CMV IE or pp65 (early-late) antigens and desmoplakin 12 days after challenge (Fig. 1A and B), both CMV antigens are expressed. Each multinucleated (syncytialized) cell, demarcated by desmoplakin staining, was generally IE positive in all nuclei or none (Fig. 1A). Characteristic cytopathic manifestations of CMV infection such as enlarged cells with nuclear inclusions (24, 27, 44) were also observed in all infected trophoblast cultures (data not shown).
Because trophoblasts do not proliferate in vitro (4, 19), cultures lose 20 to 50% of their DNA content over a 1-month period. Virus challenge of trophoblasts did not increase this loss of DNA either in the presence or in the absence of EGF over a 3-week culture period (two independent experiments with different placental preparations [data not shown]).
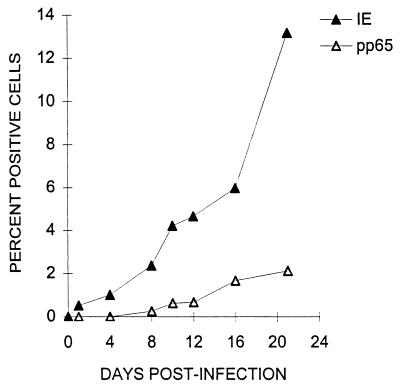
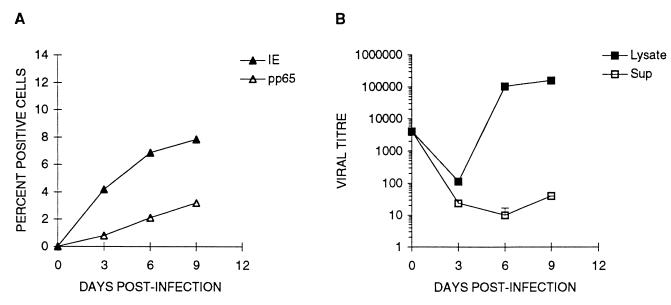
To determine the kinetics of infection, +EGF cultures were challenged with strain AD169 and the percentages of IE- and pp65-positive cells were determined at various times after challenge. The numbers of IE- and pp65-positive cells increased continuously throughout the 21-day culture period (Fig. 2). However, the increase of IE-positive cells was observed earlier, and IE-positive cells were consistently more numerous than pp65-positive cells. Figure 2 represents one of seven independent experiments carried out on five different placental trophoblast preparations. Between 18 and 21 days after challenge at an MOI of 1.0, the maximum fraction of IE-positive cells never exceeded 15%, and <3% were positive for pp65 antigen. Visual inspection of +EGF cultures at various times after virus challenge showed IE-positive nuclei to be clustered in foci which were generally equivalent to syncytialized cells until late in infection (>day 8), when some foci consisted of multiple syncytialized cells (data not shown).
FIG. 2.
Expression of CMV IE and pp65 antigen in term trophoblasts as a function of time after challenge. Villous trophoblasts from term placentas were cultured 5 days with EGF as described in Materials and Methods. The cells were challenged with CMV strain AD169 at an MOI of 1.0, cultured for the indicated periods of time (horizontal axis), and immunohistochemically stained for CMV IE and pp65 antigens (in separate wells). Percentages were calculated from the mean number of positive cells per microwell of four replicate wells from one of two independent experiments. Cell number per microwell was calculated as described in Materials and Methods.
The infected cells in culture are predominantly trophoblasts.
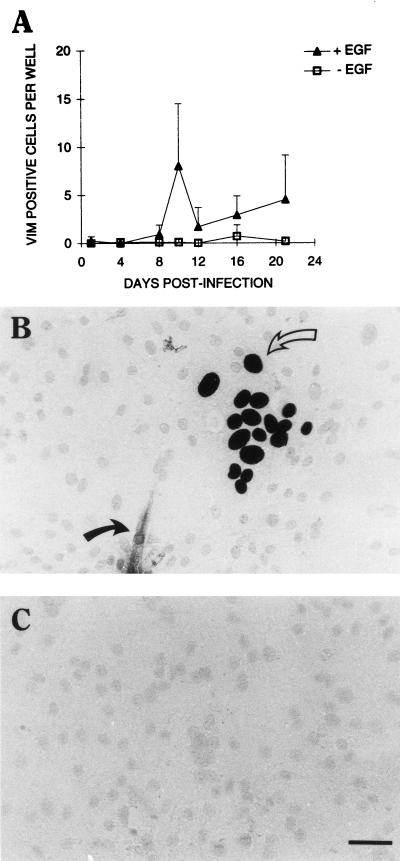
Placental fibroblasts, common contaminants of primary trophoblast cultures (29), can be infected with strain AD169 as efficiently as HEL cells (data not shown). It was therefore possible that the rather low frequency of infection observed in term trophoblast cultures could be attributed to contaminating fibroblasts. Fibroblasts, as well as other possible contaminating villous stromal cells such as macrophages and endothelial cells, can be immunohistochemically distinguished from trophoblasts by the former cells’ expression of the intermediate filament protein vimentin. Analysis of the seven preparations of placental trophoblasts used in this study for vimentin-positive cells between days 10 and 12 after infection showed 1.14 ± 1.17 (mean ± standard deviation [SD]) positive cells in +EGF microwell cultures and 1.08 ± 1.56 positive cells in -EGF cultures. Since there are between 4,000 and 16,000 cells in these cultures (see Materials and Methods), the average contamination frequency is between 0.03 and 0.007%. In an experiment using only one of these preparations (chosen for its unusually high number of vimentin-positive cells in the presence of EGF), the number of vimentin-positive cells did not exceed 10 per microwell over a 20-day infection period (Fig. 3A). Thus, it is unlikely that a significant fraction of the 15% IE-positive cells or the 2 to 3% pp65-positive nuclei observed 3 weeks after virus challenge were fibroblasts. Double staining of the cultures for IE antigen and vimentin 12 days postinfection confirmed this prediction: greater than 99% of IE-positive cells (in this experiment, 495 of 496) were vimentin negative and thus trophoblasts (Fig. 3B). Interestingly, most of the vimentin-positive cells were not IE positive (e.g., the vimentin-positive cell in Fig. 3B is IE negative).
FIG. 3.
CMV infection of placental cultures is predominantly trophoblastic. Villous trophoblasts from term placentas were cultured with or without EGF as described in Materials and Methods. (A) The cells were challenged with AD169 at an MOI of 1.0 as described in Materials and Methods. At the indicated times (horizontal axis), each well was immunohistochemically stained for CMV IE antigen by using Ni-DAB substrate and vimentin (VIM) by using AEC substrate or for vimentin alone. Total vimentin-positive cells per microwell were scored, and the mean ± SD of nine replicate cultures was plotted against the postinfection time. (B) Infected +EGF trophoblast culture at 12 days postinfection stained for CMV IE antigen (open arrow) and vimentin (closed arrow). (C) Infected +EGF trophoblast culture at 12 days postinfection stained for IgG2a and IgG1, isotype controls for CMV IE and vimentin, respectively. Bar, 25 μm.
Trophoblasts are permissively infected, but most progeny virus remains cell associated.
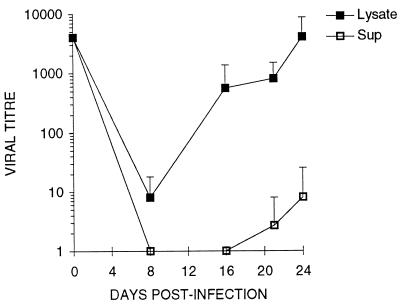
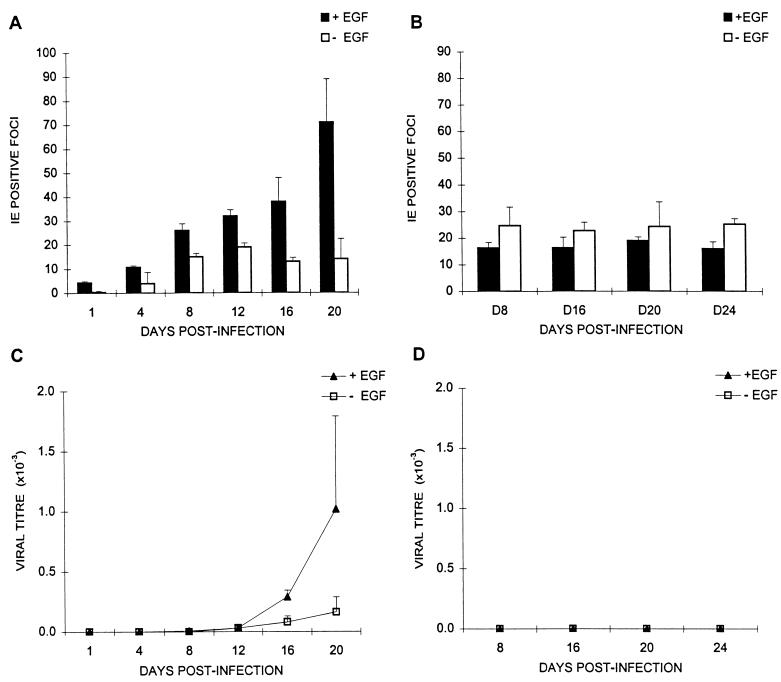
A permissive infection of trophoblasts was demonstrated by the presence of infectious progeny virus, titered on HEL cells in culture supernatants (Fig. 4 and 5C). However, exact times and extent of virus release into culture supernatants varied between trophoblast preparations, with some (Fig. 5D) releasing no detectable virus. Differences in virus release were not due to fibroblast contamination, since experiments in which there was appreciable release (mean of 531 ± 450 IF/ml) between days 8 and 20 after infection had microwells containing 1.03 ± 1.23 vimentin-positive cells, while those with very low release (mean of 1.08 ± 1.48 IF/ml) had 1.66 ± 1.65 vimentin-positive cells per microwell.
FIG. 4.
Accumulation of infectious CMV in trophoblast supernatants and cell lysates as a function of time after challenge. Villous trophoblasts from term placentas were cultured 5 days with EGF and challenged with CMV strain AD169 at an MOI of 1.0 as described in Materials and Methods. At the indicated times (horizontal axis) after challenge, 100 μl of supernatant (Sup) was removed, the adherent layer was washed with PBS, and the cells were lysed in 100 μl of medium (Lysate). Viral titer (IF/milliliter; vertical axis) was calculated from the HEL IF assay (see Materials and Methods). Each point is the mean ± SD of three replicate cultures from one of two independent experiments.
FIG. 5.
Appearance of IE-positive foci and release of infectious virus as a function of time. Panels A and C and panels B and D depict the same experiment carried out with cells isolated from two different placentas. Term trophoblasts were treated with (+EGF) or without (−EGF) epidermal growth factor and challenged with CMV strain AD169 at an MOI of 1.0 as described in Materials and Methods. (A and B) Number of IE-positive foci, determined immunohistochemically, as a function of culture time; (C and D) supernatant infectious virus titers, determined by HEL assay, as a function of time. The mean ± SD of three replicates are plotted as a function of postinfection time.
Infectious virus produced by trophoblasts is predominantly cell associated.
The variability and low titers of infectious virus released from infected trophoblasts (Fig. 5C and D) suggested intracellular accumulation of virus, a phenomenon occurring in macrophages (17). Infectious virus was observed in cell lysates, thus associated with cells, at times when none was detected in culture supernatants (Fig. 4, before day 16). In cultures where ratios of cell-associated to released virus could be calculated (those releasing detectable virus), greater than 100-fold more infectious virus was found in lysates than supernatants. This ratio for infected HEL cultures in the same experiment was approximately one (data not shown). In some preparations (Fig. 5B and D), virtually all progeny virus was cell associated over a 24-day culture period.
Susceptibility to CMV infection is independent of trophoblast differentiation state.
To determine whether the differentiation state of villous trophoblasts affected their susceptibility to CMV infection, the infection frequencies of trophoblasts from two different placentas cultured with (ST-like) and without (CT-like) EGF were compared (Fig. 5). The differences in infection frequency of +EGF and −EGF cultures during the first 2 weeks after challenge were not large and were not reproducible between trophoblast preparations (Fig. 5A and B). Any divergence between the infection frequencies of +EGF and −EGF cultures corresponded to the appearance of infectious virus in culture supernatants later in culture (day 16 for the preparation represented in Fig. 5A and C), but late release of infectious virus was not reproducible between preparations (Fig. 5D). This finding suggests that the infection progresses laterally within foci until virus is released into culture supernatants. The data also suggest that the differentiation state of the trophoblasts does not have an effect on the initial infection frequency (before progeny virus release).
Trophoblasts isolated from first trimester placentas are permissively infected with CMV.
Although in utero transmission to the fetus following primary maternal infection can occur at any point during gestation, infection during the first trimester results in the most severe consequences to the fetus (9, 10, 54). We therefore asked whether villous trophoblasts isolated from first trimester placentas could be permissively infected by CMV and, if so, whether the kinetics and extent of infection differed from term trophoblasts. The levels of expression of CMV IE and pp65 antigens were determined between days 1 to 9 after challenge with strain AD169 at an MOI of 1 (Fig. 6A). Both antigens appeared more rapidly in first trimester (Fig. 6A) than term (Fig. 2) cells, and the fraction of cells infected were higher in first trimester than term trophoblast cultures. First trimester cultures were double stained for vimentin and IE antigen (to detect infected fibroblasts). As noted above for term cells, >99% of IE-positive cells were vimentin negative and thus trophoblasts (data not shown). Infectious virus production also occurred sooner in first trimester (Fig. 6B) than term (Fig. 4 and data not shown) cells. Although the ratio of cell-associated to supernatant virus was only 6 on day 3 of culture, it increased to approximately 1,000 on days 6 and 9 (Fig. 6B). Thus, virus production in first trimester trophoblasts, as with term cells, is cell associated, but more cells are infected and the infection progresses faster.
FIG. 6.
Infection of first trimester placental trophoblasts with CMV strain AD169 as a function of time. Villous trophoblasts from first trimester placentas were cultured 3 days with EGF as described in Materials and Methods. (A) The cells were challenged with CMV strain AD169 at an MOI of 1.0, cultured for the indicated periods of time (horizontal axis), and immunohistochemically stained for CMV IE and pp65 antigens, and the percent infected cells was determined as described in the legend to Fig. 2. (B) At the indicated times (horizontal axis), released and cell-associated infectious virus titer was assessed as IF/milliliter as described in the legend to Fig. 4. The results are depicted as the mean ± SD of three replicate cultures and are representative of two independent experiments with the same results.
CMV infects a smaller fraction of trophoblasts than fibroblasts, and trophoblasts require a higher virus challenge.
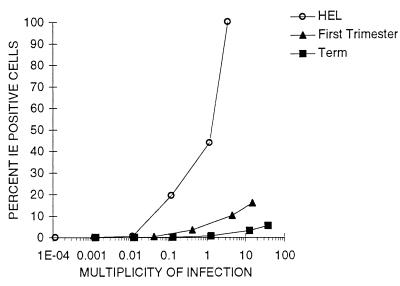
The foregoing CMV infection experiments were carried out at fixed inoculum levels of CMV for each cell type. To compare the initial interaction efficiency of virus with trophoblasts and fibroblasts, the fraction of IE-positive cells was measured 24 h after challenge with various levels of virus, expressed as MOI (allowing for multinucleated cells) for confluent HEL cell and +EGF term and first trimester trophoblast cultures (Fig. 7). The results show that EGF-treated term and first trimester trophoblasts require >100-fold-higher ratios of virus to cells for infection than fibroblasts. Increasing virus challenge increased the fraction of IE-positive fibroblasts at 24 h to 100% at an MOI of 3.5. However, fewer than 20% first trimester trophoblasts were infected at an MOI of 16, and only 6% of term cells were infected at an MOI of 38. Thus, not only do trophoblasts require higher virus concentrations for productive interaction to an IE-positive stage than fibroblasts, but the greater majority are resistant to infection.
FIG. 7.
Infection of term and first trimester placental trophoblasts and HEL cells as a function of virus concentration. HEL cells and trophoblasts from term and first trimester placentas cultured with EGF were prepared in 96-well tissue culture plates as described in Materials and Methods. Cells were challenged with CMV strain AD169 at the MOI indicated on the horizontal axis, and the number of IE-positive cells was determined after 24 h. Percentages were calculated from the mean number of positive cells per microwell of four replicate wells from one of two independent experiments. Cell number per microwell was calculated as described in Materials and Methods.
Permissive infection of trophoblasts is not unique to CMV strain AD169.
To determine whether CMV strains infected trophoblasts with differing efficiencies, cultured cells were challenged with AD169, two other laboratory strains (Davis and Towne [32]), and a low-passage clinical isolate from a congenitally infected infant. Infection was determined by using the criteria of IE and pp65 antigen expression and production of infectious virus 12 days after virus challenge. All strains permissively infected trophoblasts, albeit to different degrees, and >99% of infectious progeny virus was cell associated (Table 1). The strain variability (AD169 ∼ Towne > Davis ∼ congenital isolate) was reproducible in three independent experiments using cells from different placentas.
TABLE 1.
Permissive infection of cultured trophoblasts with different laboratory CMV strains and a congenital isolate
| CMV strain | No. of nuclei positive forc:
|
IF/ml of equal vol ofd:
|
||
|---|---|---|---|---|
| IE | pp65 | Supernatant | Lysate | |
| AD169a | 540 ± 130 | 16 ± 7.6 | 110 ± 56f | 30,000 ± 8,500f |
| Townea | 290 ± 63 | 19 ± 10 | 710 ± 530e | 110,000 ± 36,000e |
| Davisa | 25 ± 19 | 2.8 ± 0.96 | <1e | 84 ± 120e |
| Congenital isolateb | 54 ± 21 | 2.3 ± 2.1 | <1g | 200 ± 160g |
Term trophoblasts cultured with EGF as described in Materials and Methods and challenged with the laboratory CMV strains AD169, Towne, and Davis at an MOI of 1.0.
Term trophoblasts cultured without EGF challenged at an MOI of 1.0.
Mean ± SD of four microwells.
Determined as described in Materials and Methods.
Mean ± SD of six microwells.
Mean ± SD of five microwells.
Mean ± SD of three microwells.
DISCUSSION
The crucial location of placental villous trophoblasts separating maternal blood from fetal tissues suggests that it plays a role in preventing or disseminating CMV infection from mother to fetus during pregnancy. Previous studies have indicated the villous trophoblast is infected only under very specific conditions: in term placentas, trophoblasts rarely showed signs of permissive infection compared to fetal mesenchymal cells (18, 33, 37, 38, 43, 51), and permissive CMV infection in vitro occurred only after enhancement by coinfection with another virus, either HIV-1 (59) or HTLV-1 (58). Our results suggest an alternative view. We demonstrate that cultured term trophoblasts are readily infected but require a high CMV inoculum, infection progresses more slowly than in fibroblasts, and progeny virus remains predominantly cell associated. Our results argue that permissive infection of villous ST or CT in late gestation by cell-free CMV can occur but is unlikely unless the virus titer in the maternal circulation is very high. Such levels could occur during primary infections because of the transient absence of neutralizing antibody and may partially explain why vertical transmission is much more frequent in primary than recurring infections (8, 66).
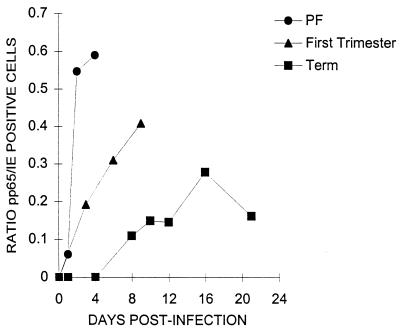
CMV crosses the placenta at all stages of gestation (54), and villous trophoblasts from first trimester placentas show frequent signs of permissive infection in vivo (18, 61). However, in vitro, Rosenthal et al. (46) found CMV-infected first trimester placental fibroblasts but not trophoblasts. We confirm that first trimester placental fibroblasts are readily infected but also find that first trimester trophoblasts are infected. The infection of first trimester trophoblasts is intermediate between placental fibroblasts and term trophoblasts in two aspects: the fraction of cells infected at near saturating virus titers and the kinetics of the infection. Twenty-four hours after challenge, all placental fibroblasts are IE antigen positive at an MOI of 3.5, 15% of first trimester trophoblasts are IE antigen positive at an MOI of 16, and only 6% of term trophoblasts are IE antigen positive at an MOI of 38 (Fig. 7). The kinetics of progression from the IE to early-late infection stage can be visualized by plotting the ratio of pp65-positive to IE-positive foci in cultures as a function of time (Fig. 8). After CMV challenge of placental fibroblasts at an MOI of 0.19, measurable pp65 antigen is observed within 24 h, and over half of infected cells have progressed to the early-late stage by 48 h. In contrast, even at a challenge MOI of 1, pp65 antigen does not appear in +EGF term trophoblasts until after day 4 and the pp65/IE ratio never exceeds 0.3 over a 21-day culture period. EGF-treated first trimester trophoblasts show intermediate progression kinetics. At an MOI challenge of 1, pp65 antigen expression appears within 24 h, but thereafter progression is slower than in fibroblasts, with a pp65/IE ratio of approximately 0.4 9 days after challenge. The larger fraction of infectable cells and more rapid progression kinetics may explain why more first trimester than term villous trophoblasts show signs of permissive infection in vivo (18, 61).
FIG. 8.
Progression of infection from IE to pp65 antigen-expressing stages in placental fibroblasts and first trimester and term trophoblasts as a function of time. Villous trophoblasts from term and first trimester placentas were cultured with EGF as described in Materials and Methods. The trophoblasts and fibroblasts were challenged with CMV strain AD169 at MOIs of 1.0 and 0.19, respectively, cultured for the indicated periods of time (horizontal axis), and immunohistochemically stained for foci of CMV IE and pp65 antigens (in separate wells). The data are expressed as the ratio of pp65- to IE-positive foci from the means of three replicate wells for each antigen and are representative of two independent experiments with very similar results.
We also find that although infectious progeny virus is rapidly released from placental fibroblasts, virus remains predominantly cell associated in both term and first trimester trophoblasts. Although basal release of infectious virus has yet to be demonstrated, such release from either first trimester or term trophoblasts would explain why vertical transmission does not appear to occur more frequently in the first than third trimester (13, 31) even though first trimester trophoblasts are more readily infected. A placental barrier that retains infectious progeny virus is in accord with studies by Griffith et al. (22) in guinea pig models showing that the placenta can accumulate CMV without transmission to the fetus.
The cell isolation procedures and culture models used in this study were essential for a complete characterization of trophoblast infection by CMV. Infectious challenge of cultures that contained very low frequencies of placental fibroblasts and direct demonstration of IE and pp65 antigen-positive cells that were vimentin negative (and thus trophoblasts) eliminate the possibility that the 5 to 15% CMV infection frequencies observed were due to preferential infection of placental fibroblasts. Crucial to the demonstrations of productive infection and the slow progression of infection in trophoblasts was the ability to maintain viable cultures for greater than 3 weeks without overgrowth by proliferating placental fibroblasts.
Interestingly, most of the very few vimentin-positive cells (fibroblasts) in these cultures were uninfected. Possible reasons include the following: (i) there is a disadvantageous target ratio (there are >4,000-fold more trophoblasts); (ii) infected vimentin-positive cells may lyse and not be detected, although lack of high titers of infectious virus in supernatants during the first week of culture argues against this; (iii) fibroblasts, which strongly adhere to tissue culture plastic, may lie beneath the trophoblasts and be protected from virus challenge; and (iv) EGF down modulates CMV production from infected placental fibroblasts as it does with other human fibroblasts (30).
Our results differ from those of Toth et al. (59), who found that CMV infection of syncytialized term trophoblasts was abortive and became fully permissive only if the cells were preinfected with HIV-1. The reasons for the different results are not clear, but it is possible that different CT subpopulations were isolated by the slightly different negative selection methods used in the two laboratories: elimination of MHC class I, MHC class II, and CD9-expressing cells in our laboratory (28) and elimination of MHC class I and II cells in their laboratory (59). Alternatively, the stocks of the laboratory strain of CMV, AD169, used in both studies may be substantially different since, according to Cha et al. (12), long-term passage can result in loss of genetic information, explaining differences in tissue tropism and virulence. To confirm that the permissive infection that we observed was not a property of our laboratory AD169 strain, we tested two other well-known laboratory-adapted strains, Towne and Davis, and a low-passage congenital isolate. Although there was considerable variation in infection efficiency (AD169 and Towne infected much more efficiently), all strains were able to permissively infect term trophoblasts.
The ST is a rather unique tissue in that it is a continuous, multinucleated cell layer that, theoretically, covers entire villous branches. The EGF-treated cultures in this study, although not continuously syncytialized, nonetheless offer a useful model of the ST. Approximately 90% of nuclei are in cells containing >2 nuclei, with approximately 20% being in cells with as many as 50 nuclei (28). It was consistently observed that either all or no nuclei in CMV-challenged syncytialized cells were positive for CMV antigens; thus, all nuclei in an infected ST participate in infection. Since ST, both in culture and in vivo, does not proliferate (4, 19), any increases in the number of infected nuclei must come from free virus infection, fusion of infected with uninfected cells, or cell-to-cell transmission (focal spread [39]). We find that the spread of virus is initially focal since the number of infected nuclei increases (data not shown) but the number of foci does not. An increase in the number of foci coincides with release of progeny virus into culture supernatants, suggesting that free virus dissemination also exists. In the absence of trophoblast proliferation in culture, cell loss due to death or shedding leads to a decrease in DNA content over time. CMV infection did not increase this loss of DNA content; thus, infected cells are not preferentially lost, a conclusion supported by the observation that the number of infected cells always increased and never peaked or decreased. These observations indicate that CMV infection, at least up to 3 weeks after virus challenge, does not damage ST, possibly because of slow virus accumulation in infected syncytia.
Our in vitro results are consistent with the more frequent and later CMV infection stages found in first trimester (18, 61) than term (18, 33, 37, 38, 40, 43, 51) ST in situ. However, detection of CMV-infected ST is less frequent than would be anticipated given the more frequent in vivo observations of infected fetal stromal cells and our in vitro observations of virus retention by cultured ST. The in vivo and in vitro observations can be reconciled by two possible explanations. (i) Virus titers in the maternal circulation are not high enough to infect the ST, and the virus enters (perhaps via CMV-infected maternal leukocytes) the stroma through breaches in the ST caused by physical trauma or sites of trophoblast damage caused by intervillous accumulations of activated monocytes (intervillositis [26]). (ii) The ST is infected as often as the stroma, but manifestations of ST infection are rapidly lost possibly because the infected ST is shed. The trophoblast, like other epithelia, would be expected to renew its outer surface. Observations of an even distribution of apoptotic nuclei (mostly in syncytial knots) in the ST of placentas from uncomplicated term deliveries suggests that turnover occurs (53). Regulated ST turnover is suggested by observations that the cytokines EGF, tumor necrosis factor alpha, and gamma interferon can up and down regulate ST apoptosis in culture (19). If CMV infection up regulates intracellular adhesion molecule 1 (which is inducible in ST [63]) as it does in fibroblasts (23, 25), T lymphocytes (62), and endothelial cells (49), infected ST may be preferentially cleared through phagocytosis by LFA-1-activated monocytes that adhere to sites of infection. Therefore, because of shedding, the steady-state level of obviously infected ST may be low even though infection occurs frequently. Thus, our data, combined with published data, tentatively describe the ST as an infectable barrier that may maintain its integrity by retaining infectious virus until shed.
ACKNOWLEDGMENTS
This work was supported by grants from the Hospital for Sick Children Foundation and the National Health Research Development of Canada to L.J.G. C.N. was supported by studentship grants from the Alberta Heritage Foundation for Medical Research, and D.G.H. was supported by a grant from University of Alberta Perinatal Research Centre.
We thank Bonnie Lowen for expert technical assistance and the University of Alberta Perinatal Research Center laboratory staff and the OB/GYN nursing staff, both at the Royal Alexandra Hospital in Edmonton, for placental cell preparations.
REFERENCES
- 1.Altshuler G, McAdams A J. Cytomegalic inclusion disease of a nineteen-week fetus. Case report including a study of the placenta. Am J Obstet Gynecol. 1971;111:295–298. doi: 10.1016/0002-9378(71)90905-7. [DOI] [PubMed] [Google Scholar]
- 2.Amirhessami-Aghili N, Lahijani R, Manalo P, St. Jeor S, Tibbitts F D, Hall M R, Afsari A. Persistence of human cytomegalovirus DNA sequences without cell-virus homology in human placental explants in culture. Int J Fertil. 1989;34:411–419. [PubMed] [Google Scholar]
- 3.Amirhessami-Aghili N, Manalo P, Hall M R, Tibbitts F D, Ort C A, Afsari A. Human cytomegalovirus infection of human placental explants in culture: histologic and immunohistochemical studies. Am J Obstet Gynecol. 1987;156:1365–1374. doi: 10.1016/0002-9378(87)90002-0. [DOI] [PubMed] [Google Scholar]
- 4.Aplin J D. Implantation, trophoblast differentiation and haemochorial placentation: mechanistic evidence in vivo and in vitro. J Cell Sci. 1991;99:681–692. doi: 10.1242/jcs.99.4.681. [DOI] [PubMed] [Google Scholar]
- 5.Becroft D M. Prenatal cytomegalovirus infection: epidemiology, pathology and pathogenesis. Perspect Pediatr Pathol. 1981;6:203–241. [PubMed] [Google Scholar]
- 6.Benirschke K, Kaufmann P. Pathology of the human placenta. 2nd ed. Berlin, Germany: Springer-Verlag; 1990. [Google Scholar]
- 7.Benirschke K, Mendoza G R, Bazeley P L. Placental and fetal manifestations of cytomegalovirus infection. Virchows Arch B. 1974;16:121–139. doi: 10.1007/BF02894070. [DOI] [PubMed] [Google Scholar]
- 8.Boppana S B, Britt W J. Antiviral antibody responses and intrauterine transmission after primary maternal cytomegalovirus infection. J Infect Dis. 1995;171:1115–1121. doi: 10.1093/infdis/171.5.1115. [DOI] [PubMed] [Google Scholar]
- 9.Boppana S B, Pass R F, Britt W J. Virus-specific antibody responses in mothers and their newborn infants with asymptomatic congenital cytomegalovirus infections. J Infect Dis. 1993;167:72–77. doi: 10.1093/infdis/167.1.72. [DOI] [PubMed] [Google Scholar]
- 10.Britt W J, Vugler L G. Antiviral antibody responses in mothers and their newborn infants with clinical and subclinical congenital cytomegalovirus infections. J Infect Dis. 1990;161:214–219. doi: 10.1093/infdis/161.2.214. [DOI] [PubMed] [Google Scholar]
- 11.Cesarone C F, Bolognesi C, Santi L. Improved microfluorometric DNA determination in biological material using 33258 Hoechst. Anal Biochem. 1979;100:188–197. doi: 10.1016/0003-2697(79)90131-3. [DOI] [PubMed] [Google Scholar]
- 12.Cha T A, Tom E, Kemble G W, Duke G M, Mocarski E S, Spaete R R. Human cytomegalovirus clinical isolates carry at least 19 genes not found in laboratory strains. J Virol. 1996;70:78–83. doi: 10.1128/jvi.70.1.78-83.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cook S M, Himebaugh K S, Frank T S. Absence of cytomegalovirus in gestational tissue in recurrent spontaneous abortion. Diagn Mol Pathol. 1993;2:116–119. [PubMed] [Google Scholar]
- 14.Doerr H W, Braun R, Munk K. Human cytomegalovirus infection: recent developments in diagnosis and epidemiology. Klin Wochenschr. 1985;63:241–251. doi: 10.1007/BF01731469. [DOI] [PubMed] [Google Scholar]
- 15.Douglas G C, King B F. Differentiation of human trophoblast cells in vitro as revealed by immunocytochemical staining of desmoplakin and nuclei. J Cell Sci. 1990;96:131–141. doi: 10.1242/jcs.96.1.131. [DOI] [PubMed] [Google Scholar]
- 16.Fazely F, Fry G N, Thirkill T L, Hakim H, King B F, Douglas G C. Kinetics of HIV infection of human placental syncytiotrophoblast cultures: an ultrastructural and immunocytochemical study. AIDS Res Hum Retroviruses. 1995;11:1023–1030. doi: 10.1089/aid.1995.11.1023. [DOI] [PubMed] [Google Scholar]
- 17.Fish K N, Depto A S, Moses A V, Britt W, Nelson J A. Growth kinetics of human cytomegalovirus are altered in monocyte-derived macrophages. J Virol. 1995;69:3737–3743. doi: 10.1128/jvi.69.6.3737-3743.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia A G, Fonseca E F, Marques R L, Lobato Y Y. Placental morphology in cytomegalovirus infection. Placenta. 1989;10:1–18. doi: 10.1016/0143-4004(89)90002-7. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Lloret M, Yui J, Winkler-Lowen B, Guilbert L J. Epidermal growth factor inhibits cytokine-induced apoptosis of primary human trophoblasts. J Cell Physiol. 1996;167:324–332. doi: 10.1002/(SICI)1097-4652(199605)167:2<324::AID-JCP17>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 20.Gonczol E, Andrews P W, Plotkin S A. Cytomegalovirus infection of human teratocarcinoma cells in culture. J Gen Virol. 1985;66:509–515. doi: 10.1099/0022-1317-66-3-509. [DOI] [PubMed] [Google Scholar]
- 21.Green M A, Sviland L, Malcolm A J, Pearson A D. Improved method for immunoperoxidase detection of membrane antigens in frozen sections. J Clin Pathol. 1989;42:875–880. doi: 10.1136/jcp.42.8.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffith B P, McCormick S R, Fong C K, Lavallee J T, Lucia H L, Goff E. The placenta as a site of cytomegalovirus infection in guinea pigs. J Virol. 1985;55:402–409. doi: 10.1128/jvi.55.2.402-409.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grundy J E, Downes K L. Up-regulation of LFA-3 and ICAM-1 on the surface of fibroblasts infected with cytomegalovirus. Immunology. 1993;78:405–412. [PMC free article] [PubMed] [Google Scholar]
- 24.Hanshaw J B. Cytomegalovirus. In: Gard S, Hallauer C, Meyer K F, editors. Virology monographs. New York, N.Y: Springer-Verlag; 1968. pp. 2–23. [Google Scholar]
- 25.Ito M, Watanabe M, Ihara T, Kamiya H, Sakurai M. Increased expression of adhesion molecules (CD54, CD29 and CD44) on fibroblasts infected with cytomegalovirus. Microbiol Immunol. 1995;39:129–133. doi: 10.1111/j.1348-0421.1995.tb02179.x. [DOI] [PubMed] [Google Scholar]
- 26.Jacques S M, Qureshi F. Chronic intervillositis of the placenta. Arch Pathol Lab Med. 1993;117:1032–1035. [PubMed] [Google Scholar]
- 27.Jesionek A, Kiolemenoglou B. Uber einen Befund von Protozoenartigen gebilden in den Organen eines heriditarluetischen Fotus. Munch Med Wochenschr. 1904;51:1905–1907. [Google Scholar]
- 28.Kilani R, Chang L-J, Hemmings D, Guilbert L J. Placental trophoblasts resist infection by multiple human immunodeficiency virus (HIV) type 1 variants even with cytomegalovirus coinfection but support HIV replication after provirus transfection. J Virol. 1997;71:6359–6372. doi: 10.1128/jvi.71.9.6359-6372.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kliman H J, Nestler J E, Sermasi E, Sanger J M, Strauss J F. Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118:1567–1582. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- 30.Knox G E, Reynolds D W, Cohen S, Alford C A. Alteration of the growth of cytomegalovirus and herpes simplex virus type 1 by epidermal growth factor, a contaminant of crude human chorionic gonadotropin preparations. J Clin Invest. 1978;61:1635–1644. doi: 10.1172/JCI109084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar M L, Prokay S L. Experimental primary cytomegalovirus infection in pregnancy: timing and fetal outcome. Am J Obstet Gynecol. 1983;145:56–60. doi: 10.1016/0002-9378(83)90339-3. [DOI] [PubMed] [Google Scholar]
- 32.Mocarski E S. Cytomegaloviruses and their replication. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2447–2492. [Google Scholar]
- 33.Monif G R, Dische R M. Viral placentitis in congenital cytomegalovirus infection. Am J Clin Pathol. 1972;58:445–449. doi: 10.1093/ajcp/58.5.445. [DOI] [PubMed] [Google Scholar]
- 34.Morrish D W, Bhardwaj D, Dabbagh L K, Marusyk H, Siy O. Epidermal growth factor induces differentiation and secretion of human chorionic gonadotropin and placental lactogen in normal human placenta. J Clin Endocrin Metab. 1987;65:1282–1290. doi: 10.1210/jcem-65-6-1282. [DOI] [PubMed] [Google Scholar]
- 35.Mostoufi-zadeh M, Driscoll S G, Biano S A, Kundsin R B. Placental evidence of cytomegalovirus infection of the fetus and neonate. Arch Pathol Lab Med. 1984;108:403–406. [PubMed] [Google Scholar]
- 36.Muhlemann K, Menegus M A, Miller R K. Cytomegalovirus in the perfused human term placenta in vitro. Placenta. 1995;16:367–373. doi: 10.1016/0143-4004(95)90094-2. [DOI] [PubMed] [Google Scholar]
- 37.Muhlemann K, Miller R K, Metlay L, Menegus M A. Cytomegalovirus infection of the human placenta: an immunocytochemical study. Hum Pathol. 1992;23:1234–1237. doi: 10.1016/0046-8177(92)90290-j. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura Y, Sakuma S, Ohta Y, Kawano K, Hashimoto T. Detection of the human cytomegalovirus gene in placental chronic villitis by polymerase chain reaction. Hum Pathol. 1994;25:815–818. doi: 10.1016/0046-8177(94)90252-6. [DOI] [PubMed] [Google Scholar]
- 39.Navarro D, Paz P, Tugizov S, Topp K, La Vail J, Pereira L. Glycoprotein B of human cytomegalovirus promotes virion penetration into cells, transmission of infection from cell to cell, and fusion of infected cells. Virology. 1993;197:143–158. doi: 10.1006/viro.1993.1575. [DOI] [PubMed] [Google Scholar]
- 40.Otto S M, Sullivan-Tailyour G, Malone C L, Stinski M F. Subcellular localization of the major immediate early protein (IE1) of human cytomegalovirus at early times after infection. Virology. 1988;162:478–482. doi: 10.1016/0042-6822(88)90490-4. [DOI] [PubMed] [Google Scholar]
- 41.Pass R F, Stagno S, Myers G J, Alford C A. Outcome of symptomatic congenital cytomegalovirus infection: results of long-term longitudinal follow-up. Pediatrics. 1980;66:758–762. [PubMed] [Google Scholar]
- 42.Preece P M, Blount J M, Glover J, Fletcher G M, Peckham C S, Griffiths P D. The consequences of primary cytomegalovirus infection in pregnancy. Arch Dis Childhood. 1983;58:970–975. doi: 10.1136/adc.58.12.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quan A, Strauss L. Congenital cytomegalic inclusion disease. Am J Obstet Gynecol. 1962;83:1240–1248. [PubMed] [Google Scholar]
- 44.Ribbert D. Uber protozoenartige Zellen in der Niere eines syphilitischen Neugeborenen und in der Parotis von Kindern. Zentralbl Allg Pathol. 1904;15:945–948. [Google Scholar]
- 45.Rice G P, Schrier R D, Oldstone M B. Cytomegalovirus infects human lymphocytes and monocytes: virus expression is restricted to immediate-early gene products. Proc Natl Acad Sci USA. 1984;81:6134–6138. doi: 10.1073/pnas.81.19.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenthal L J, Panitz P J, Crutchfield D B, Chou J Y. Cytomegalovirus replication in primary and passaged human placental cells. Intervirology. 1981;16:168–175. doi: 10.1159/000149264. [DOI] [PubMed] [Google Scholar]
- 47.Sachdev R, Nuovo G J, Kaplan C, Greco M A. In situ hybridization analysis for cytomegalovirus in chronic villitis. Pediatr Pathol. 1990;10:909–917. doi: 10.3109/15513819009064726. [DOI] [PubMed] [Google Scholar]
- 48.Schwartz D A, Khan R, Stoll B. Characterization of the fetal inflammatory response to cytomegalovirus placentitis. An immunohistochemical study. Arch Pathol Lab Med. 1992;116:21–27. [PubMed] [Google Scholar]
- 49.Sedmak D D, Knight D A, Vook N C, Waldman J W. Divergent patterns of ELAM-1, ICAM-1, and VCAM-1 expression on cytomegalovirus-infected endothelial cells. Transplantation. 1994;58:1379–1385. [PubMed] [Google Scholar]
- 50.Sessions C F, Taeusch H W. Viral infections of the newborn. In: Taeusch H W, Ballard R A, Avery M E, editors. Diseases of the newborn. W. B. Philadelphia, Pa: Saunders; 1991. pp. 331–349. [Google Scholar]
- 51.Sinzger C, Muntefering H, Loning T, Stoss H, Plachter B, Jahn G. Cell types infected in human cytomegalovirus placentitis identified by immunohistochemical double staining. Pathol Anat Histopathol. 1993;423:249–256. doi: 10.1007/BF01606887. [DOI] [PubMed] [Google Scholar]
- 52.Smith J D. Human cytomegalovirus: demonstration of permissive epithelial cells and nonpermissive fibroblastic cells in a survey of human cell lines. J Virol. 1986;60:583–588. doi: 10.1128/jvi.60.2.583-588.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith S C, Symonds E M, Baker P N. Apoptosis within the trophoblast: its distinctive electron microscopy features, and the role which it plays in the pathophysiology of intrauterine growth restriction. J Soc Gynecol Invest. 1997;4:95A. [Google Scholar]
- 54.Stagno S, Pass R F, Cloud G, Britt W J, Henderson R E, Walton P D, Veren D A, Page F, Alford C A. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA. 1986;256:1904–1908. [PubMed] [Google Scholar]
- 55.Stagno S, Reynolds D W, Amos C S, Dahle A J, McCollister F P, Mohindra I, Ermocilla R, Alford C A. Auditory and visual defects resulting from symptomatic and subclinical congenital cytomegaloviral and toxoplasma infections. Pediatrics. 1977;59:669–678. [PubMed] [Google Scholar]
- 56.Stinski M. Molecular biology of cytomegalovirus replication. In: Ho M, editor. Cytomegalovirus: biology and infection. Plenum Publishing Corp.; 1991. pp. 34–35. [Google Scholar]
- 57.Stinski M F. Synthesis of proteins and glycoproteins in cells infected with human cytomegalovirus. J Virol. 1977;23:751–767. doi: 10.1128/jvi.23.3.751-767.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Toth F D, Aboagye-Mathiesen G, Szabo J, Liu X, Mosborg-Petersen P, Kiss J, Hager H, Zdravkovic M, Andirko I, Aranyosi J, Ebbesen P. Bidirectional enhancing activities between human T cell leukemia-lymphoma virus type I and human cytomegalovirus in human term syncytiotrophoblast cells cultured in vitro. AIDS Res Hum Retroviruses. 1995;11:1495–1507. doi: 10.1089/aid.1995.11.1495. [DOI] [PubMed] [Google Scholar]
- 59.Toth F D, Mosborg-Petersen P, Kiss J, Aboagye-Mathiesen G, Hager H, Juhl C B, Gergely L, Zdravkovic M, Aranyosi J, Lampe L, Ebbesen P. Interactions between human immunodeficiency virus type 1 and human cytomegalovirus in human term syncytiotrophoblast cells coinfected with both viruses. J Virol. 1995;69:2223–2232. doi: 10.1128/jvi.69.4.2223-2232.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Turtinen L W, Saltzman R, Jordan M C, Haase A T. Interactions of human cytomegalovirus with leukocytes in vivo: analysis by in situ hybridization. Microb Pathog. 1987;3:287–297. doi: 10.1016/0882-4010(87)90062-3. [DOI] [PubMed] [Google Scholar]
- 61.van Lijnschoten G, Stals F, Evers J L, Bruggeman C A, Havenith M H, Geraedts J P. The presence of cytomegalovirus antigens in karyotyped abortions. Am J Reprod Immunol. 1994;32:211–220. doi: 10.1111/j.1600-0897.1994.tb01116.x. [DOI] [PubMed] [Google Scholar]
- 62.Waldman W J, Knight D A. Cytokine-mediated induction of endothelial adhesion molecule and histocompatibility leukocyte antigen expression by cytomegalovirus-activated T cells. Am J Pathol. 1996;148:105–119. [PMC free article] [PubMed] [Google Scholar]
- 63.Xiao J, Garcia-Lloret M I, Winkler-Lowen B, Miller R, Simpson K, Guilbert L J. ICAM-1 mediated adhesion of peripheral blood monocytes to the maternal surface of placental syncytiotrophoblasts. Am J Pathol. 1997;150:1845–1860. [PMC free article] [PubMed] [Google Scholar]
- 64.Yow M D, Williamson D W, Leeds L J, Thompson P, Woodward R M, Walmus B F, Lester J W, Six H R, Griffiths P D. Epidemiologic characteristics of cytomegalovirus infection in mothers and their infants. Am J Obstet Gynecol. 1988;158:1189–1195. doi: 10.1016/0002-9378(88)90252-9. [DOI] [PubMed] [Google Scholar]
- 65.Yui J, Garcia-Lloret M I, Brown A J, Berdan D W, Morrish D W, Wegmann T G, Guilbert L J. Functional, long-term cultures of human term trophoblasts purified by column-elimination of CD9 expressing cells. Placenta. 1994;15:231–246. doi: 10.1016/0143-4004(94)90015-9. [DOI] [PubMed] [Google Scholar]