Abstract
Background:
The impact of ATM, CHEK2, and PALB2, the 3 most prevalent moderate-risk breast cancer genes, on surgical decision making is not well known.
Methods:
Our retrospective study included patients with resectable non-metastatic breast cancer who underwent multigene panel testing between 07/2014-01/2020 with at least 1 genetic alteration (pathogenic or variant of uncertain significance [VUS] in ATM [n=49], CHEK [n=57], or PALB2 [n=27]). Our objectives were to determine the rate of contralateral prophylactic mastectomy (CPM) and the rate of bilateral breast cancer. Univariable (UVA) and multivariable analyses (MVA) were performed to identify factors associated with CPM and bilateral breast cancer.
Results:
The rate of CPM was 39% (n=49/127), with 54% (n=25/46) of patients with a pathogenic mutation and 30% (n=24/81) of patients with a VUS choosing CPM. On MVA, premenopausal status (OR 3.46) and a pathogenic alteration (OR 3.01) were associated with increased use of CPM. Bilateral disease was noted in 16% (n=22/138). Patients with pathogenic mutations had a 22% (n=11/51) incidence of bilateral breast cancer, while patients with VUS had a 13% (n=11/87) incidence, although this was not statistically significant on UVA or MVA. On MVA, premenopausal status was associated with a decreased risk of bilateral disease (OR 0.33, p=0.022). During follow-up, a breast cancer event occurred in 16% (n=22/138).
Conclusions:
Our study identified a high rate of CPM amongst those with ATM, CHEK2, and PALB2 alterations, including VUS. Further studies are needed to clarify reasons for CPM amongst patients with moderate-risk alterations.
Keywords: ATM, PALB2, CHEK2, contralateral prophylactic mastectomy, breast cancer, bilateral breast cancer
INTRODUCTION
Breast cancer is the most commonly diagnosed cancer in women in the United States, with an estimated more than 300,000 new cases to be diagnosed in 2023.1 It is also the second most common cause of cancer death in the United States female population and the leading cause of death in women 40-49 years of age. Among patients with a new breast cancer diagnosis, it is estimated that 5-10% can be attributed to a germline pathogenic variant, including high-penetrance genes such as BRCA1 and BRCA2, and moderate-risk genes such as PALB2, ATM, CHEK2, and others.2,3
In daily practice, multigene panel testing is being used for breast cancer risk assessment. Expert panels such as the American Society of Breast Surgeons have called for genetic testing to be available to all patients with a history of or a new diagnosis of breast cancer. Broad accessibility and declining out-of-pocket costs of genetic testing have resulted in increased performance of panel testing. In the setting of a new diagnosis, the primary purpose of germline testing is to assess contralateral breast cancer risk to guide decision making regarding prophylactic surgery and to guide perioperative systemic treatment.4 The presence of a BRCA1/2 mutation indicates a high risk of metachronous contralateral breast cancer, and 42-88% of women in this setting choose to undergo contralateral prophylactic mastectomy (CPM).5–7 There are few studies related to the surgical decisions of women with mutations in other, more moderate-risk susceptibility genes, such as ATM, CHEK2, and PALB2.8–10 In addition, the contralateral breast cancer risk in women with these mutations is poorly understood.
We therefore conducted a retrospective, single-institution study of patients diagnosed with resectable non-metastatic breast cancer with at least 1 germline alteration (pathogenic and/or variant of unknown significance [VUS]) in 1 of the 3 most prevalent moderate-penetrance breast cancer genes—PALB2, CHEK2, and/or ATM—to understand the rate of CPM in breast cancer patients with PALB2, CHEK2, and/or ATM mutations, the frequency of bilateral breast cancer (synchronous or metachronous), and patterns of recurrence.
METHODS
Our primary objective was to describe the prevalence of CPM in women with a germline alteration in ATM, CHEK2, or PALB2 in the context of a new breast cancer diagnosis. For our primary analysis, we excluded patients with synchronous bilateral breast disease as well as patients with prior unilateral mastectomy. Our secondary objectives were to describe the prevalence of synchronous or metachronous bilateral breast cancer and disease recurrence in patients with a germline alteration in these genes. For our secondary outcomes we included patients with synchronous bilateral breast cancer and patients with prior unilateral mastectomy.
Study Design
Upon institutional review board approval, the authors performed a single-center retrospective analysis of patients treated at Memorial Sloan Kettering Cancer Center (MSK)(New York, NY, USA) for resectable non-metastatic breast cancer with at least 1 germline alteration identified in PALB2, CHEK2, and/or ATM. Eligible patients were identified through MSK’s cancer genetics and breast surgery databases, and were 18 years of age or older, had a confirmed history of resectable non-metastatic breast cancer who underwent multigene panel testing between July 2014 and January 2020 at MSK. Patients had at least 1 intact breast at the time of multigene testing as well as 1 genetic alteration in ATM, CHEK2, and/or PALB2 (pathogenic and/or VUS). Referral to the MSK Clinical Genetics Service and the decision to offer germline testing was based on contemporaneous National Comprehensive Cancer Network guidelines after a shared decision-making discussion between the patient and provider.
Detailed review of electronic medical records was performed to collect patient demographics, clinical and pathologic tumor status, and treatment information. The following variables were systematically reviewed and collected: age at diagnosis; stage at diagnosis; expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) by immunohistochemistry (IHC) and FISH, where applicable, on primary disease; cancer treatment; date, type, and result of genetic testing; date of first genetic counseling appointment; family history of breast cancer, and clinical outcomes. Patients with both a pathogenic and a VUS alteration were categorized under the pathogenic category. Baseline characteristics were obtained at the time of the new breast cancer diagnosis. We defined synchronous breast cancer as occurring within 3 months of new breast cancer diagnosis, and metachronous as occurring 6 months or more from new breast cancer diagnosis. For patients with metachronous breast cancer, the clinicopathologic factors used for our analysis were collected for the cancer diagnosis that was contemporaneous with the genetic test that identified the variant that led to inclusion in the study. Follow-up time was calculated from the date of surgery for the first breast cancer diagnosis.
Statistical Analysis
Descriptive statistics were provided using counts and percentages for categorical variables, and medians and ranges for continuous variables. Comparisons between patients who underwent CPM and those who did not undergo CPM, and between patients who had bilateral breast cancer and those who did not have bilateral breast cancer, were performed using Fisher’s exact test or the Chi-square test of independence. Covariates that were significant in this univariable analysis (UVA) were then selected for multivariable logistic regression analysis. Multiple comparison correction was made using the Bonferroni adjustment, resulting in a Type I error rate of 0.025 (α) for multivariable analysis (MVA). The final MVA model was selected using backward selection procedure. All statistical analyses were conducted using R 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
A total of 138 patients with resectable non-metastatic breast cancer and a germline alteration in PALB2, ATM, and/or CHEK2 were identified. Nine patients had synchronous bilateral resectable non-metastatic breast cancer and 2 patients had a prior mastectomy to manage a previous ductal carcinoma in situ (DCIS), leaving 127 patients with intact bilateral breasts and unilateral disease evaluable for surgical decision analysis. The study populations used for primary and secondary analyses are described in Figure 1. Median follow-up from time of surgery for the first breast cancer diagnosis for the entire group was 28 months (interquartile range [IQR] 11-44 months).
Fig. 1.
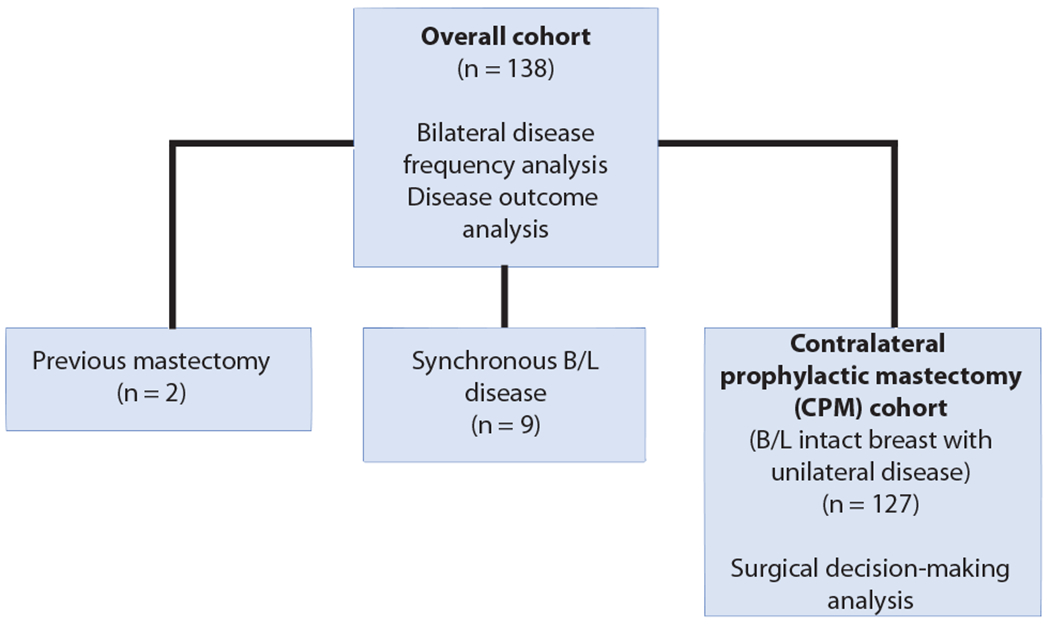
Study population used for primary and secondary analyses.
B/L bilateral
Characteristics of the overall cohort are shown in Table 1. The median age of the overall cohort was 47 years, 75% (n = 103) were premenopausal or perimenopausal, and 67% (n = 93) were White. Twenty-six percent of patients (n = 36) had a first-degree relative with breast cancer. In the overall cohort, 36% (n = 49, 15 pathogenic, 34 VUS) had an ATM alteration, 41% (n = 57, 28 pathogenic, 29 VUS) had a CHEK2 alteration, and 20% (n = 27, 8 pathogenic, 19 VUS) had a PALB2 alteration. Thirty-seven percent (n = 51) were pathogenic, while 63% (n = 87) were VUS. Four patients had both CHEK2 VUS and PALB2 VUS, and 1 patient had both a CHEK2 VUS and an ATM VUS. Fourteen percent of patients underwent a bilateral salpingo-oophorectomy. Patient characteristics of the CPM cohort were similar and are also shown in Table 1.
TABLE 1.
Patient characteristics of overall cohort and the contralateral prophylactic mastectomy cohort
| Characteristic | Overall (n = 138) |
CPM cohort (n = 127) |
|---|---|---|
| Age, years, median (range) | 47 (21-76) | 47 (21-76) |
| Menopausal status | ||
| Pre- or peri-menopausal | 103 (75%) | 97 (76%) |
| Postmenopausal | 35 (25%) | 30 (24%) |
| Race | ||
| Asian | 14 (10%) | 12 (9.4%) |
| Black | 15 (11%) | 15 (12%) |
| Other | 7 (5.1%) | 5 (3.9%) |
| White | 93 (67%) | 86 (68%) |
| Unknown | 9 (6.5%) | 9 (7.1%) |
| Gene | ||
| ATM | 49 (36%) | 45 (35%) |
| CHEK2 | 57 (41%) | 52 (41%) |
| PALB2* | 27 (20%) | 26 (20%) |
| Multiple mutations** | 5 (3.6%) | 4 (3.1%) |
| Alteration | ||
| Pathogenic | 51 (37%) | 46 (36%) |
| VUS | 87 (63%) | 81 (64%) |
| First degree relative with breast cancer | 36 (26%) | 33 (26%) |
| Neoadjuvant chemotherapy | 53 (38%) | 50 (39%) |
| Adjuvant chemotherapy | 52 (38%) | 50 (39%) |
| Histology | ||
| IDC | 99 (72%) | 94 (74%) |
| ILC | 17 (12%) | 15 (12%) |
| Invasive other | 5 (3.6%) | 4 (3.1%) |
| DCIS | 17 (12%) | 14 (11%) |
| Receptor status | ||
| HR+/HER2− | 65 (47%) | 60 (47%) |
| HER2+ | 30 (22%) | 27 (21%) |
| Triple negative | 26 (19%) | 26 (20%) |
| Unknown or not applicable | 17 (12%) | 14 (11%) |
| Clinical T stage | ||
| T0/Tis | 33 (24%) | 26 (20%) |
| T1 | 58 (42%) | 55 (43%) |
| T2 | 27 (20%) | 27 (21%) |
| T3 | 14 (10%) | 13 (10%) |
| T4 | 5 (3.6%) | 5 (3.9%) |
| Unknown | 1 (0.7%) | 1 (0.8%) |
| Clinical N stage | ||
| N0 | 105 (76%) | 95 (75%) |
| N1 | 28 (20%) | 27 (21%) |
| N2 | 2 (1.4%) | 2 (1.6%) |
| N3 | 2 (1.4%) | 2 (1.6%) |
| Unknown | 1 (0.7%) | 1 (0.8%) |
| Type of surgery | ||
| Breast-conserving surgery | 51 (37%) | |
| Mastectomy | 87 (63%) | |
| Pathologic T stage | ||
| T0/Tis | 33 (24%) | 28 (22%) |
| T1 | 80 (58%) | 75 (59%) |
| T2 | 18 (13%) | 17 (13%) |
| T3 | 6 (4.3%) | 6 (4.7%) |
| Unknown | 1 (0.7%) | 1 (0.8%) |
| Pathologic N stage | ||
| N0 | 99 (72%) | 90 (71%) |
| N1 | 27 (20%) | 25 (20%) |
| N2 | 6 (4.3%) | 6 (4.7%) |
| N3 | 3 (2.2%) | 3 (2.4%) |
| Unknown | 3 (2.2%) | 3 (2.4%) |
| Bilateral salpingo-oophorectomy | ||
| Yes | 20 (14%) | 18 (14%) |
| Unknown | 10 (7.2%) | 9 (7.1%) |
Data expressed as n (%) unless otherwise indicated
Includes 1 patient with a PALB2 pathogenic mutation and a PTEN pathogenic mutation
4 patients had CHEK2 and PALB2 alterations, while 1 patient had ATM and CHEK2 alterations
CPM contralateral prophylactic mastectomy, VUS variant of uncertain significance, IDC invasive ductal carcinoma, ILC invasive lobular carcinoma, DCIS ductal carcinoma in situ, HR hormone receptor, HER2 human epidermal growth factor receptor 2
CPM
Overall prevalence of CPM was 39% (n = 49/127), with 54% (n = 25/46) in the pathogenic group and 30% (n = 24/81) in the VUS group opting for prophylactic surgery. On UVA (Table 2), factors associated with choosing CPM included being premenopausal or perimenopausal (p = 0.029) and having a pathogenic alteration (p = 0.010). On MVA (Table 3), premenopausal or perimenopausal status was associated with increased use of CPM (odds ratio [OR] 3.46, 95% confidence interval [CI] 1.33-10.3, p = 0.016), as was having a pathogenic alteration (OR 3.01, 95% CI 1.40-6.67, p = 0.005).
TABLE 2.
UVA of factors associated with contralateral prophylactic mastectomy
| Characteristic | CPM (n = 49) | No CPM (n = 78) | p-value |
|---|---|---|---|
| Race | 0.3 | ||
| Asian | 2 (4.4%) | 10 (14%) | |
| Black | 7 (16%) | 8 (11%) | |
| Other | 1 (2.2%) | 4 (5.5%) | |
| White | 35 (78%) | 51 (70%) | |
| Unknown | 4 | 5 | |
| Menopausal status | 0.029 | ||
| Pre- or peri-menopausal | 43 (88%) | 54 (69%) | |
| Postmenopausal | 6 (12%) | 24 (31%) | |
| Gene | 0.9 | ||
| ATM | 17 (35%) | 28 (36%) | |
| CHEK2 | 22 (45%) | 30 (38%) | |
| PALB2 | 9 (18%) | 17 (22%) | |
| Multiple mutations | 1 (2.0%) | 3 (3.8%) | |
| Alteration | 0.010 | ||
| Pathogenic | 25 (51%) | 21 (27%) | |
| VUS | 24 (49%) | 57 (73%) | |
| First-degree relative with breast cancer | 8 (16%) | 25 (32%) | 0.079 |
| History of breast cancer | 2 (4.1%) | 4 (5.1%) | > 0.9 |
| Neoadjuvant chemotherapy | 18 (38%) | 32 (41%) | 0.8 |
| Unknown | 1 | 0 | |
| Adjuvant chemotherapy | 18 (37%) | 32 (41%) | 0.8 |
| Histology | 0.9 | ||
| IDC | 35 (71%) | 59 (76%) | |
| ILC | 6 (12%) | 9 (12%) | |
| Invasive/other | 2 (4.1%) | 2 (2.6%) | |
| DCIS | 6 (12%) | 8 (10%) | |
| Receptor status | 0.4 | ||
| HR+/HER2− | 25 (58%) | 35 (50%) | |
| HER2+ | 11 (26%) | 16 (23%) | |
| Triple negative | 7 (16%) | 19 (27%) | |
| Unknown | 6 | 8 | |
| Clinical T stage | 0.3 | ||
| T0/Tis | 12 (24%) | 14 (18%) | |
| T1 | 21 (43%) | 34 (44%) | |
| T2 | 9 (18%) | 18 (23%) | |
| T3 | 7 (14%) | 6 (7.8%) | |
| T4 | 0 (0%) | 5 (6.5%) | |
| Unknown | 0 | 1 | |
| Clinical N stage | 0.7 | ||
| N0 | 39 (80%) | 56 (73%) | |
| N1 | 9 (18%) | 18 (23%) | |
| N2 | 1 (2.0%) | 1 (1.3%) | |
| N3 | 0 (0%) | 2 (2.6%) | |
| Unknown | 0 | 1 | |
| Pathologic T stage | 0.5 | ||
| T0/Tis | 12 (24%) | 16 (21%) | |
| T1 | 30 (61%) | 45 (58%) | |
| T2 | 4 (8.2%) | 13 (17%) | |
| T3 | 3 (6.1%) | 3 (3.9%) | |
| Unknown | 0 | 1 | |
| Pathologic N stage | 0.2 | ||
| N0 | 36 (73%) | 54 (72%) | |
| N1 | 8 (16%) | 17 (23%) | |
| N2 | 2 (4.1%) | 4 (5.3%) | |
| N3 | 3 (6.1%) | 0 (0%) | |
| Unknown | 0 | 3 | |
| Bilateral salpingo-oophorectomy | 10 (22%) | 8 (11%) | 0.2 |
| Unknown | 4 | 5 |
Data expressed as n (%) unless otherwise indicated.
Bolded values indicate statistical significance.
UVA univariable, CPM contralateral prophylactic mastectomy, VUS variant of uncertain significance, IDC invasive ductal carcinoma, ILC invasive lobular carcinoma, DCIS ductal carcinoma in situ, HR hormone receptor, HER2 human epidermal growth factor receptor 2
TABLE 3.
Multivariable analysis of factors associated with contralateral prophylactic mastectomy
| Characteristic | OR | 95% CI | p-value |
|---|---|---|---|
| Menopausal status | |||
| Postmenopausal | — | — | |
| Premenopausal | 3.46 | 1.33, 10.3 | 0.016 |
| Alteration | |||
| VUS | — | — | |
| Pathogenic | 3.01 | 1.40, 6.67 | 0.005 |
Bolded values indicate statistical significance.
OR odds ratio, CI confidence interval, VUS variant of uncertain significance
Bilateral Breast Cancer
Twenty-two (16%) out of the 138 patients evaluable for bilateral disease had bilateral breast cancer, including 9 patients with synchronous disease and 13 patients with metachronous disease. Descriptive characteristics of patients who developed bilateral breast cancer were compared to those who had unilateral breast cancer. Patients with pathogenic mutations had a diagnosis of bilateral breast cancer in 22% (n = 11/51) of the cases, while patients with VUS had a bilateral breast cancer diagnosis in 13% (n = 11/87) of the cases.
On UVA (Table 4), premenopausal or perimenopausal status (p = 0.036) and invasive ductal histology (p = 0.046) were associated with a decreased risk of bilateral disease. Bilateral disease developed in 29% (n = 10/35) of postmenopausal patients and 12% (n = 12/103) of the premenopausal or perimenopausal patients. The differences between premenopausal or perimenopausal patients and postmenopausal patients remained statistically significant on MVA (Table 5), with an OR of 0.33 (95% CI 0.13-0.86) in favor of the premenopausal patients (p = 0.022). The difference in frequency of bilateral disease between patients with pathogenic alterations versus VUS did not achieve statistical significance on UVA or MVA.
TABLE 4.
UVA of factors associated with bilateral disease
| Characteristic | Unilateral disease (n = 116) |
Bilateral disease (n = 22) |
p-value |
|---|---|---|---|
| Race | 0.6 | ||
| Asian | 12 (11%) | 2 (9.1%) | |
| Black | 14 (13%) | 1 (4.5%) | |
| Other | 5 (4.7%) | 2 (9.1%) | |
| White | 76 (71%) | 17 (77%) | |
| Unknown | 9 | 0 | |
| Menopausal status | 0.036 | ||
| Pre- and peri-menopausal | 91 (78%) | 12 (55%) | |
| Postmenopausal | 25 (22%) | 10 (45%) | |
| Gene | 0.3 | ||
| ATM | 38 (33%) | 11 (50%) | |
| CHEK2 | 49 (42%) | 8 (36%) | |
| PALB2 | 25 (22%) | 2 (9.1%) | |
| Multiple mutations | 4 (3.4%) | 1 (4.5%) | |
| Alteration | 0.3 | ||
| Pathogenic | 40 (34%) | 11 (50%) | |
| VUS | 76 (66%) | 11 (50%) | |
| First-degree relative with breast cancer | 28 (24%) | 8 (36%) | 0.4 |
| Neoadjuvant chemotherapy | 48 (42%) | 5 (23%) | 0.2 |
| Unknown | 1 | 0 | |
| Adjuvant chemotherapy | 46 (40%) | 6 (27%) | 0.4 |
| Histology | 0.046 | ||
| IDC | 88 (76%) | 11 (50%) | |
| ILC | 11 (9.5%) | 6 (27%) | |
| Invasive/other | 4 (3.4%) | 1 (4.5%) | |
| DCIS | 13 (11%) | 4 (18%) | |
| Receptor status | 0.2 | ||
| HR+/HER2− | 52 (50%) | 13 (72%) | |
| HER2+ | 26 (25%) | 4 (22%) | |
| Triple negative | 25 (24%) | 1 (5.6%) | |
| Unknown | 13 | 4 | |
| Clinical T stage | 0.5 | ||
| T0/Tis | 25 (22%) | 8 (38%) | |
| T1 | 49 (42%) | 9 (43%) | |
| T2 | 25 (22%) | 2 (9.5%) | |
| T3 | 12 (10%) | 2 (9.5%) | |
| T4 | 5 (4.3%) | 0 (0%) | |
| Unknown | 0 | 1 | |
| Clinical N stage | 0.9 | ||
| N0 | 89 (77%) | 16 (76%) | |
| N1 | 23 (20%) | 5 (24%) | |
| N2 | 2 (1.7%) | 0 (0%) | |
| N3 | 2 (1.7%) | 0 (0%) | |
| Unknown | 0 | 1 | |
| Type of surgery | 0.5 | ||
| Breast-conserving surgery | 41 (35%) | 10 (45%) | |
| Mastectomy | 75 (65%) | 12 (55%) | |
| Pathologic T stage | 0.4 | ||
| T0/Tis | 27 (23%) | 6 (27%) | |
| T1 | 69 (60%) | 11 (50%) | |
| T2 | 13 (11%) | 5 (23%) | |
| T3 | 6 (5.2%) | 0 (0%) | |
| Unknown | 1 | 0 | |
| Pathologic N stage | 0.4 | ||
| N0 | 85 (75%) | 14 (67%) | |
| N1 | 21 (18%) | 6 (29%) | |
| N2 | 6 (5.3%) | 0 (0%) | |
| N3 | 2 (1.8%) | 1 (4.8%) | |
| Unknown | 2 | 1 | |
| Bilateral salpingo-oophorectomy | 18 (17%) | 2 (11%) | 0.7 |
| Unknown | 7 | 3 |
Data expressed as n (%) unless otherwise indicated.
Bolded values indicate statistical significance.
UVA univariable, VUS variant of uncertain significance, IDC invasive ductal carcinoma, ILC invasive lobular carcinoma, DCIS ductal carcinoma in situ, HR hormone receptor, HER2 human epidermal growth factor receptor 2
TABLE 5.
Multivariable analysis of factors associated with bilateral disease
| Characteristic* | OR | 95% CI | p-value |
|---|---|---|---|
| Menopausal status | |||
| Postmenopausal | — | — | |
| Premenopausal | 0.33 | 0.13, 0.86 | 0.022 |
Histology was eliminated from the multivariable analysis by backward selection.
Bolded values indicate statistical significance.
OR odds ratio, CI confidence interval
Patient Outcomes
Twenty-two patients (16%) had a breast cancer event during follow-up. Six patients developed metastatic disease (4 patients with VUS and 2 patients with pathogenic mutations). Six patients had locoregional recurrence, including ipsilateral breast, chest wall, or ipsilateral axilla (4 patients with VUS and 2 patients with a pathogenic mutation). Thirteen patients had bilateral breast cancer. Three patients had a history of DCIS or invasive disease which occurred prior to inclusion in this study. Ten patients developed a contralateral breast cancer (6 patients with VUS and 4 patients with a pathogenic mutation) during the follow-up period. This equated to 1 contralateral breast cancer per 37.7 person-years of follow-up for the VUS group and 1 contralateral breast cancer per 38.2 person-years of follow-up for the pathogenic group.
DISCUSSION
Our study examined the effect of the moderate-risk breast cancer genes ATM, CHEK2, and PALB2 on use of CPM in patients presenting with resectable non-metastatic breast cancer. To our knowledge, our study is amongst one of the first studies investigating CPM rates in patients with these moderate-risk gene alterations, and one of the larger series. We found a CPM rate of 39% amongst patients with pathogenic or VUS alterations in ATM, CHEK2, and/or PALB2. In those with a pathogenic alteration, 54% chose CPM. Similarly, a study by Cragun and colleagues with 28 PALB2 carriers and 21 ATM or CHEK2 carriers found a high rate of CPM of approximately 60% in patients with unilateral breast cancer in PALB2 and ATM or CHEK2 mutation carriers,9 while a population-based study conducted by Kurian et al. reported a 43% incidence of bilateral mastectomy in a group of non-BRCA pathogenic carriers that included ATM, CHEK2, PALB2, and 6 other pathogenic variants.10 Our rate of CPM falls within the lower range of the 42-88% of BRCA patients who undergo CPM5–7, but is still relatively high given ATM, CHEK2, and PALB2 confer lower risk of breast cancer compared to BRCA mutation carriers. Cragun and colleagues found that PALB2 and ATM or CHEK2 mutation carriers were less likely to undergo bilateral mastectomy compared to BRCA carriers (OR 0.34 and 0.19, respectively).9
We found premenopausal status and having a pathogenic alteration to be associated with undergoing CPM. Previous studies similarly found having a moderate-risk gene mutation and being younger were associated with increased likelihood of bilateral mastectomy.8–10 This is also similar to previous findings that younger age and being a pathogenic BRCA carrier were associated with an increased rate of CPM.5,11
In those patients in our study with a VUS alteration in ATM, CHEK2, and/or PALB2, 30% chose to undergo CPM. Although there is lack of improvement in survival with CPM in this setting, our finding is comparable to a previously published CPM rate of 27% in patients with negative genetic testing.12 Rates of CPM in patients with a VUS alteration in BRCA range from 11-22%5,13, with 1 study demonstrating similar rate of CPM between BRCA VUS patients and those without a known genetic alteration.13 Our study was not able to investigate rationale for choosing CPM in the VUS population. Interestingly, patients who test negative for a BRCA pathogenic alteration have been shown to be more likely to pursue CPM if they discussed CPM with their surgeon at the time of diagnosis, were not BCS candidates, or had higher levels of cancer-related anxiety.14 Whether this correlation existed for our patients who had VUS is difficult to determine in this retrospective study. However, given that the majority of VUS alterations are ultimately classified as benign or likely benign variants15, these alterations should not be routinely used for surgical decision making.
There are emerging data on the risk of contralateral breast cancer in patients with ATM, CHEK2, and PALB2 mutations.16 CHEK2 mutations are estimated to increase the risk of contralateral breast cancer, with a relative risk of 1.8-4.016–18, while ATM pathogenic mutations do not demonstrate a significantly increased risk of contralateral breast cancer.16,19 PALB2 pathogenic mutation carriers have recently been found to have elevated risks of ER negative breast cancer with hazard ratio of 2.9.16 A study of 41 patients with CHEK2 pathogenic mutations found a bilateral breast cancer risk of 20%20, with a mean of 7 years to development of contralateral breast cancer. A larger prospective study reported a 13% 10-year incidence of contralateral breast cancer for premenopausal women with a CHEK2 mutation and a 4% 10-year incidence in postmenopausal patients16. Our data suggest an overall higher rate of bilateral breast cancer at 22% for ATM, CHEK2, and PALB2 pathogenic mutation carriers compared to 13% for patients with ATM, CHEK2, and PALB2 VUS, although this did not achieve statistical significance.
Limitations of our study include its single-institution setting and its pooling of results for 3 moderate-risk genes, which may detract from the generalizability of our results. We did not have a large enough sample size to differentiate outcomes between ATM, CHEK2, and PALB2 mutations. Similarly, our study lacked the power to determine differences in recurrence between pathogenic and VUS alterations. The sample size and short follow-up also affected our ability to robustly determine true contralateral breast cancer risk for specific pathogenic gene alterations. Additionally, we were not able to determine patient-level discussions that lead to surgical decision making in this retrospective study. However, despite these limitations, our findings regarding the rate of CPM in patients with moderate-risk breast cancer genes can inform shared surgical decision making and counseling.
Conclusions
Our study results suggest that factors leading patients to choose CPM, such as premenopausal status and having a pathogenic mutation, are similar to the factors leading BRCA carriers to choose CPM. We also found a relatively high rate of CPM of 30% in patients with only a VUS alteration in ATM, CHEK2, and/or PALB2. Further studies are needed to clarify reasons for CPM amongst patients with moderate-risk alterations.
Synopsis.
Here we examine the impact of the 3 most-prevalent moderate-risk breast cancer genes on surgical decision making. We find that the decision for CPM is associated with premenopausal status and having a pathogenic alteration.
ACKNOWLEDGEMENTS
The preparation of this study was supported in part by NIH/NCI Cancer Center Support Grant P30 CA008748 to Memorial Sloan Kettering Cancer Center, and this study was presented in poster format at the Society of Surgical Oncology 2022 International Conference on Surgical Cancer Care, March 9-12, 2022, Dallas, TX. Dr. Mark E. Robson reports uncompensated provision of services to Artios Pharma Limited, AstraZeneca, Tempus Labs, Inc., Pfizer, Inc., and Zenith Pharma, Inc., and honoraria from Clinical Education Alliance, LLC, Genome Quebec, MJH Associates, Physicians’ Education Resources, and Change Healthcare Inc. Dr. Carlos Henrique Dos Anjos reports the following speaking engagements: AstraZeneca, Daiichi-Sankyo, Pfizer, MDHealth, Springer Healthcare, MSD, Roche, Lilly, Gilead, and Fleury. All other authors have no conflicts of interest to disclose.
REFERENCES
- 1.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. [DOI] [PubMed] [Google Scholar]
- 2.Tung N, Lin NU, Kidd J, et al. Frequency of Germline Mutations in 25 Cancer Susceptibility Genes in a Sequential Series of Patients With Breast Cancer. J Clin Oncol. 2016;34(13):1460–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turnbull C, Rahman N. Genetic predisposition to breast cancer: past, present, and future. Annu Rev Genomics Hum Genet. 2008;9:321–345. [DOI] [PubMed] [Google Scholar]
- 4.Geyer CE Jr., Garber JE, Gelber RD, et al. Overall survival in the OlympiA phase III trial of adjuvant olaparib in patients with germline pathogenic variants in BRCA1/2 and high-risk, early breast cancer. Ann Oncol. 2022;33(12):1250–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elsayegh N, Webster RD, Gutierrez Barrera AM, et al. Contralateral prophylactic mastectomy rate and predictive factors among patients with breast cancer who underwent multigene panel testing for hereditary cancer. Cancer Med. 2018;7(6):2718–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lokich E, Stuckey A, Raker C, Wilbur JS, Laprise J, Gass J. Preoperative genetic testing affects surgical decision making in breast cancer patients. Gynecol Oncol. 2014;134(2):326–330. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz MD, Peshkin BN, Isaacs C, et al. Randomized trial of proactive rapid genetic counseling versus usual care for newly diagnosed breast cancer patients. Breast Cancer Res Treat. 2018;170(3):517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergstrom C, Pence C, Berg J, et al. Clinicopathological Features and Outcomes in Individuals with Breast Cancer and ATM, CHEK2, or PALB2 Mutations. Ann Surg Oncol. 2021;28(6):3383–3393. [DOI] [PubMed] [Google Scholar]
- 9.Cragun D, Weidner A, Tezak A, Clouse K, Pal T. Cancer risk management among female BRCA1/2, PALB2, CHEK2, and ATM carriers. Breast Cancer Res Treat. 2020;182(2):421–428. [DOI] [PubMed] [Google Scholar]
- 10.Kurian AW, Ward KC, Abrahamse P, et al. Association of Germline Genetic Testing Results With Locoregional and Systemic Therapy in Patients With Breast Cancer. JAMA Oncol. 2020;6(4):e196400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elsayegh N, Kuerer HM, Lin H, et al. Predictors that influence contralateral prophylactic mastectomy election among women with ductal carcinoma in situ who were evaluated for BRCA genetic testing. Ann Surg Oncol. 2014;21(11):3466–3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King TA, Sakr R, Patil S, et al. Clinical management factors contribute to the decision for contralateral prophylactic mastectomy. J Clin Oncol. 2011;29(16):2158–2164. [DOI] [PubMed] [Google Scholar]
- 13.Welsh JL, Hoskin TL, Day CN, et al. Clinical Decision-Making in Patients with Variant of Uncertain Significance in BRCA1 or BRCA2 Genes. Ann Surg Oncol. 2017;24(10):3067–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tynan M, Peshkin BN, Isaacs C, et al. Predictors of contralateral prophylactic mastectomy in genetically high risk newly diagnosed breast cancer patients. Breast Cancer Res Treat. 2020;180(1):177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makhnoon S, Levin B, Ensinger M, et al. A multicenter study of clinical impact of variant of uncertain significance reclassification in breast, ovarian and colorectal cancer susceptibility genes. Cancer Med. 2023;12(3):2875–2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yadav S, Boddicker NJ, Na J, et al. Contralateral Breast Cancer Risk Among Carriers of Germline Pathogenic Variants in ATM, BRCA1, BRCA2, CHEK2, and PALB2. J Clin Oncol. 2023:Jco2201239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kriege M, Hollestelle A, Jager A, et al. Survival and contralateral breast cancer in CHEK2 1100delC breast cancer patients: impact of adjuvant chemotherapy. Br J Cancer. 2014;111(5):1004–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mellemkjaer L, Dahl C, Olsen JH, et al. Risk for contralateral breast cancer among carriers of the CHEK2*1100delC mutation in the WECARE Study. Br J Cancer. 2008;98(4):728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teoh V, Tasoulis MK, Gui G. Contralateral Prophylactic Mastectomy in Women with Unilateral Breast Cancer Who Are Genetic Carriers, Have a Strong Family History or Are just Young at Presentation. Cancers (Basel). 2020;12(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nizic-Kos T, Krajc M, Blatnik A, et al. Bilateral Disease Common Among Slovenian CHEK2-Positive Breast Cancer Patients. Ann Surg Oncol. 2021;28(5):2561–2570. [DOI] [PubMed] [Google Scholar]


