Abstract
Background
Organ‐at‐risk (OAR) sparing is often assessed using an overlap volume‐based parameter, defined as the ratio of the volume of OAR that overlaps the planning target volume (PTV) to the whole OAR volume. However, this conventional overlap‐based predictive parameter (COPP) does not consider the volume relationship between the PTV and OAR.
Purpose
We propose a new overlap‐based predictive parameter that consider the PTV volume. The effectiveness of proposed overlap‐based predictive parameter (POPP) is evaluated compared with COPP.
Methods
We defined as POPP = (overlap volume between OAR and PTV/OAR volume) × (PTV volume/OAR volume). We generated intensity modulated radiation therapy (IMRT) based on step and shoot technique, and volumetric modulated arc therapy (VMAT) plans with the Auto‐Planning module of Pinnacle3 treatment planning system (v14.0, Philips Medical Systems, Fitchburg, WI) using the American Association of Physicists in Medicine Task Group (TG119) prostate phantom. The relationship between the position and size of the prostate phantom was systematically modified to simulate various geometric arrangements. The correlation between overlap‐based predictive parameters (COPP and POPP) and dose‐volume metrics (mean dose, V70Gy, V60Gy, and V37.5 Gy for rectum and bladder) was investigated using linear regression analysis.
Results
Our results indicated POPP was better than COPP in predicting intermediate‐dose metrics. The bladder results showed a trend similar to that of the rectum. The correlation coefficient of POPP was significantly greater than that of COPP in < 62 Gy (82% of the prescribed dose) region for IMRT and in < 55 Gy (73% of the prescribed dose) region for VMAT regarding the rectum (p < 0.05).
Conclusions
POPP is superior to COPP for creating predictive models at an intermediate‐dose level. Because rectal bleeding and bladder toxicity can be associated with intermediate‐doses as well as high‐doses, it is important to predict dose‐volume metrics for various dose levels. POPP is a useful parameter for predicting dose‐volume metrics and assisting the generation of treatment plans.
Keywords: intensity‐modulated radiation therapy, overlap volume, pinnacle3 Auto‐Planning, plan quality, prostate cancer, step and shoot, volumetric modulated arc therapy
1. INTRODUCTION
Recently, intensity‐modulated radiation therapy (IMRT) and volumetric modulated arc therapy (VMAT) have become increasingly prevalent in radiation therapy for prostate cancer. These techniques deliver improved dose conformity to the target while minimizing the dose to surrounding organs at risk (OARs). For radiation‐induced toxicities in prostate cancer, it is well‐known that rectal bleeding is associated with high dose (70−80 Gy). 1 , 2 , 3 On the other hand, studies have reported that rectal bleeding is related to the volume receiving an intermediate dose (V30Gy–V60 Gy). 4 , 5 , 6 , 7 , 8 In addition, researchers have reported that bladder toxicity is associated with high dose (70−78 Gy) 9 , 10 , 11 , 12 and intermediate dose (V14Gy–V40 Gy). 13 , 14 To reduce the risk of developing radiation‐induced toxicities in prostate cancer, the dose to the rectum and bladder should be lowered as much as possible.
In contrast to conventional radiotherapy, the planner's skill greatly influences the treatment plan quality of an IMRT plan. Inverse optimization approaches used in IMRT planning require a large amount of manual effort. 15 , 16 To lower the OAR dose as much as possible, recent studies have discussed the effectiveness of the overlap ratio, which is the ratio of overlap between an OAR and the planning target volume (PTV). 17 , 18 , 19 Moore et al. reported that the achievable OAR sparing is related to the overlap ratio. 20 They proposed a predictive model for the OAR mean dose using the overlap ratio. Their results indicated that by using the predictive model, the OAR mean dose was lowered, and the interplanner treatment plan variability was reduced by incorporating the predictive model into the planning workflow. Chao et al. investigated the correlation between the overlap ratio and dose‐volume metrics such as V75Gy, V70Gy, V60Gy, and V40Gy for the rectum. 21 Their results also indicated that the overlap ratio was beneficial for predicting OAR sparing. Nevertheless, the weakness of the overlap ratio (which we call the conventional overlap‐based predictive parameter [COPP] in this paper) is that the PTV volume is not considered. Thus, the COPP cannot distinguish different PTV sizes. Two cases with the same overlap ratio and different PTV volumes have the same COPP values, despite the different OAR doses. This means that the COPP cannot predict the OAR dose with distinction of PTV volume. In practice, OAR doses are dependent on the PTV size and intuitively increase with the PTV size.
In this study, we hypothesize that the prediction of dose‐volume metrics using the COPP will be improved by considering the relationship between the OAR volume and the PTV volume. Based on this hypothesis, we proposed overlap‐based predicted parameter in this study. The effectiveness of proposed overlap‐based predictive parameter (POPP) is demonstrated with simulations for IMRT and VMAT in several geometry cases, in which the PTV and/or OAR positions and sizes were systematically varied using the American Association of Physicists in Medicine Task Group‐119 (TG119) prostate phantom.
2. MATERIALS AND METHODS
2.1. Definition of the predictive parameters
First, we define the COPP, which has been used for predicting OAR dose in recent studies 17 , 18 , 19 :
| (1) |
where VOV is the overlap volume of OAR and PTV and VOAR is the volume of OAR.
To consider the volume relationship between PTV and OAR, we propose the following predicted parameter, POPP:
| (2) |
where VPTV is the volume of the PTV. The POPP is defined as the product of the COPP and the ratio of PTV volume to OAR volume. We propose this parameter based on the assumption that the OAR dose should increase as the PTV volume becomes larger and the OAR volume becomes smaller.
2.2. Dosimetric simulations
We investigated the effectiveness of the POPP in two simulations. We used the TG119 prostate phantom in these simulations. The structure sets were expanded, referring to clinical studies, 22 , 23 since the structures were smaller than in the actual patients.
2.2.1. Simulation 1: Cases with two PTV sizes with the same overlap ratio
In the first simulation, we studied two PTV cases that had the same overlap ratio. This was done in order to evaluate the effectiveness of the POPP in a situation in which the COPP cannot distinguish PTV size. Figure 1 show a schematic diagram of the phantom setup in this simulation for a small PTV case and a large PTV case, respectively. PTV volumes were set at 162.7 and 188.0 cc, respectively. We set the rectum volume (RV) and bladder volume (BV) in this simulation at 49.9 and 165.0 cc, respectively. In this simulation, we studied three values of the rectum overlap volumes (ROV), which is the overlap volume of the rectum and PTV. To set the same COPP value with different PTV sizes, we set the ROV at 3.3, 5.3, and 7.7 cc for both PTV cases. We set the bladder overlap volume (BOV), which is the overlap volume of the bladder and PTV, at 13.2 cc in this simulation. Dosimetric simulation was conducted in the six cases that consisted of the combinations of two PTV sizes and three ROV values.
FIGURE 1.
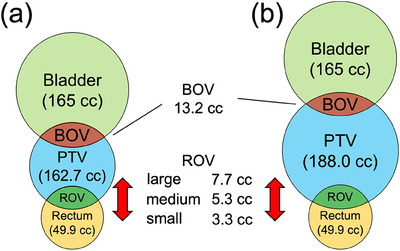
Schematic diagram of phantom geometries for (a) small PTV volume and (b) large PTV volume. The three types of ROV are small, medium, and large. RV and BV were kept constant in all cases. BOVs were kept constant in all cases by shifting the bladder structure. BOV, bladder overlap volume; BV, bladder volume; PTV, planning target volume; ROV, rectum overlap volume; RV, rectum volume.
2.2.2. Simulation 2: Various rectum and bladder geometries
For this simulation, we investigated the effectiveness of POPP compared with COPP in several phantom setups by systematically varying the volumes and positions of the rectum and bladder while considering various patient anatomies. Figure 2 shows a schematic diagram of the phantom setup in simulation 2. Figure 2a shows the volume change in rectum and bladder. The rectum was expanded from the original phantom size by 4, 5, and 6 mm in the AP and LR directions. The corresponding RV values were 42.5, 50.0, and 58.2 cc. The bladder was expanded from the original phantom size by 7, 10, and 12 mm in all directions. The corresponding BV values were 124.2, 165.0, and 197.0 cc. The PTV was expanded from the original phantom size by 16 mm in the AP and LR directions, resulting in 162.7 cc. The PTV volume and PTV position were fixed in this simulation because we were interested in the relative geometrical relationship between the PTV and OARs. Figure 2b shows the position shift of the rectum and bladder in the AP direction. The rectum position was shifted from the phantom original position by 10, 12, and 14 mm in the posterior direction. The bladder position was shifted from the original phantom position by 5, 8, and 11 mm in the anterior direction. We studied a total of 81 cases with combinations of nine rectum configurations (three volumes and three positions cases) and nine bladder configurations (three volumes and three positions cases).
FIGURE 2.
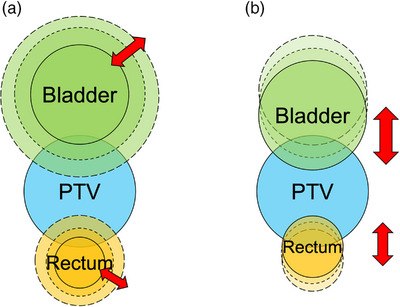
Schematic diagram of phantom geometries in simulation 2. (a) Volume change in the rectum and bladder. (b) The shift of the rectum and bladder in the AP direction. The PTV volume and position were fixed. AP, anterior–posterior; PTV, planning target volume.
2.3. Treatment planning
IMRT plans with seven fields (gantry angles: 0°, 51°, 102°, 153°, 207°, 258°, 309°) and VMAT plans with two coplanar full arcs were generated for each case described above using the Pinnacle3 treatment‐planning system (v14.0, Philips Medical Systems, Fitchburg, WI). We used the Auto‐Planning module to exclude the dependence on the planner's skill. Auto‐Planning is the function implemented in the Pinnacle3, which automatically optimize the treatment plan according to the user‐specified dose goals. In the Auto‐Planning optimization process, the following consecutive loop is repeated; (1) the ROIs to improve the target coverage and OARs sparing are automatically created, 24 (2) the objectives for these ROIs are automatically established based on the specified dose goals, (3) the weights for their objectives are automatically determined, and (4) optimization is performed. Nawa et al. reported that the treatment plans generated by Auto‐Planning could be comparable or better with less interplanner variation as compared with plans created by human planners. 24 All plans were created for Varian Clinac iX equipped with Millennium 120‐leaf multileaf collimators using 6‐MV photons. The prescription dose for the PTV was set to 75.6 Gy, and all plans required at least 95% of the volume of the PTV receiving the prescription dose. All plans followed the TG119 dose goals: V75Gy < 10% and V70Gy < 30% for the rectum and bladder. Table 1 lists the optimization goals for the Auto‐Planning used for all simulations.
TABLE 1.
Dose optimization goals.
| Target optimization goals | ||
|---|---|---|
| ROI | Dose | |
| PTV | 7800 cGy | |
| Optimization goals for target and organs at risk | ||
|---|---|---|
| ROI | Goal | Priority/Compromise |
| PTV | Dmax < 7636 cGy | High/yes |
| Rectum | V75Gy < 10% | High/no |
| Rectum | V70Gy < 30% | High/no |
| Bladder | V75Gy < 10% | Medium/yes |
| Bladder | V70Gy < 30% | Medium/yes |
| ring | Dmax < 4536 cGy | Medium/yes |
2.4. Plan evaluation and regression analysis
We evaluated the generated plans in terms of conformity number (CN) 25 and homogeneity index (HI). 26 CN is defined as CN = (TVPIV /TV)×(TVPIV /PIV), where represents the volume of PTV receiving more than the prescription dose, represents the target volume, and represents the volume receiving more than the prescription dose. HI is defined as HI = (D2 − D98)/D50, where D2, D50, and D98 are the minimum dose covering 2%, 50%, and 98% of the target volume, respectively.
We investigated the correlations between the predictive parameters (COPP or POPP) and the dose‐volume metrics using linear regression analysis. In this study, the dose‐volume metrics include mean dose (Dmean), V70Gy, V60Gy, and V37.5 Gy for the rectum and bladder.
Tests of statistical significance between the correlation coefficient of COPP and that of POPP were performed by paired‐samples t‐test at a 5% significance level.
3. RESULTS
3.1. Linear regression analysis in simulation 1
Figure 3 shows linear regression analysis of the overlap‐based predictive parameter for rectum V70Gy and rectum V37.5 Gy with different PTV volumes for IMRT and VMAT in simulation 1. Figure 3a,c show the dose‐volume metrics (V70Gy and V37.5 Gy) for the rectum plotted against the COPP. These results suggest that the rectum doses in large PTV cases were higher than those in the small PTV cases when the COPP value was the same. Figure 3b,d show the dose‐volume metrics (V70Gy and V37.5 Gy) for the rectum plotted against the POPP. These results demonstrate that the POPP appears to correlate with V70Gy and V37.5 Gy more linearly than the COPP does. In terms of the correlation coefficients, the POPP correlated with V37.5 Gy better than the COPP did (R = 0.897 and 0.860, respectively) for IMRT, and (R = 0.973 and 0.932, respectively) for VMAT. The POPP correlated with V70Gy as well as the COPP (R = 0.965 and 0.967, respectively) for IMRT, and (R = 0.988 and 0.990, respectively) for VMAT. This result suggests that the predictivity for V70Gy is not improved by considering the PTV volume, and the OAR volume receiving the prescription dose is mainly dependent on the overlap volume. The results of this simulation indicate that the POPP can be effective for predicting rectum dose, especially intermediate dose.
FIGURE 3.
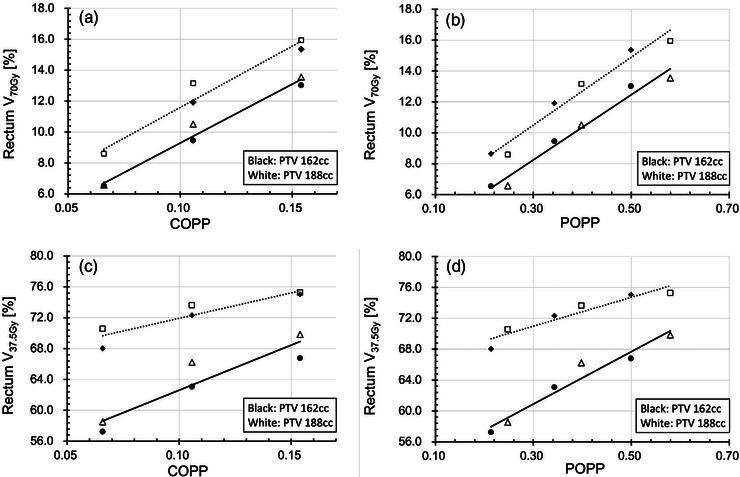
Linear regression analysis of the overlap‐based predictive parameter for rectum V70Gy and rectum V37.5 Gy with different PTV volumes for IMRT (dashed) and VMAT (solid): (a) rectum V70Gy against COPP, (b) rectum V70Gy against POPP, (c) rectum V37.5 Gy against COPP, and (d) rectum V37.5 Gy against POPP. Three different ROV patterns were used for two types of PTV volume, whereas the RV, BV, and BOV were kept constant. Data are plotted for two types of PTV volume: small for IMRT (black diamond), large for IMRT (white square), small for VMAT (black circle), and large for VMAT (white triangle). BOV, bladder overlap volume; BV, bladder volume; COPP, conventional overlap‐based predictive parameter; IMRT, intensity modulated radiation therapy; POPP, proposed overlap‐based predictive parameter; PTV, planning target volume; ROV, rectum overlap volume; RV, rectum volume; VMAT, volumetric modulated arc therapy.
3.2. Plan quality and linear regression analysis in simulation 2
The RV and BV were 50.4 ± 6.6 cc and 162.1 ± 29.8 cc, respectively. The overlap ratios (i.e., COPP) for the rectum and bladder were 0.11 ± 0.04 and 0.08 ± 0.02, respectively. The calculated CN in the present study were 0.91 for IMRT, and 0.92 for VMAT. The HI for IMRT and VMAT were 0.065 and 0.088, respectively. These outcomes were better than those reported in other prostate cancer studies. 23 , 27 Among the 81 treatment plans generated by Auto‐Planning for IMRT and VMAT, 58 IMRT plans and 63 VMAT plans met the TG119 dose goals.
Figures 4 and 5 show the dose‐volume metrics (V70Gy and V37.5 Gy) plotted against the predictive parameters (COPP and POPP) for IMRT and VMAT, respectively, in simulation 2. The results of dose‐volume metrics and the correlation coefficients between dose‐volume metrics and the predictive parameters are presented in Table 2 for IMRT and in Table 3 for VMAT. In terms of the correlation coefficients, the COPP correlated with high‐dose metrics better than the POPP did, whereas the results also show that the POPP correlated well with intermediate‐dose metrics. These trends were similar to those in simulation 1.
FIGURE 4.
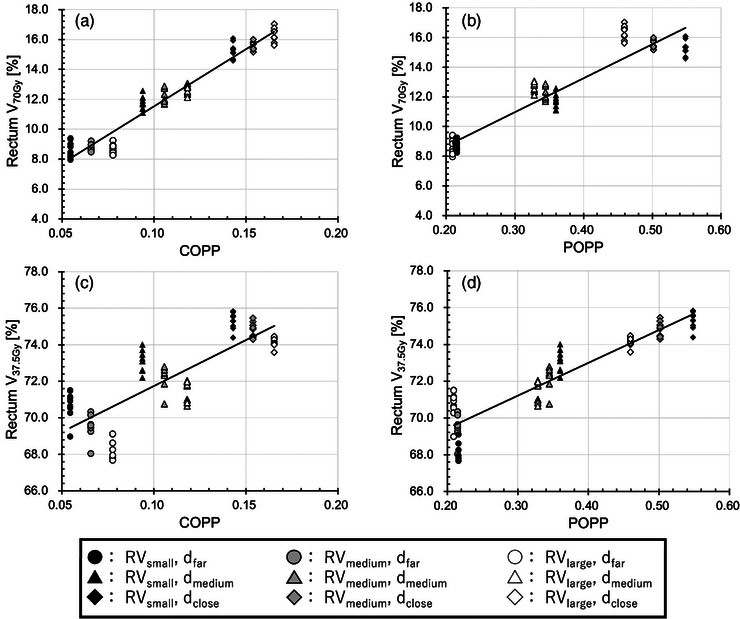
Linear regression analysis of the overlap‐based predictive parameter for rectum V70Gy and rectum V37.5 Gy with various patient cases for IMRT: (a) rectum V70Gy against COPP, (b) rectum V70Gy against POPP, (c) rectum V37.5 Gy against COPP, and (d) rectum V37.5 Gy against POPP. Data are plotted for three types of distances between PTV and rectum: far (circle), medium (triangle), and close (diamond), and for three types of volume: small (black), medium (gray), and large (white). The plot variations in markers of the same shape and same color indicate the result of varying bladder geometry. COPP, conventional overlap‐based predictive parameter; d, distance between rectum and PTV; IMRT, intensity modulated radiation therapy; POPP, proposed overlap‐based predictive parameter; PTV, planning target volume; RV, rectum volume.
FIGURE 5.
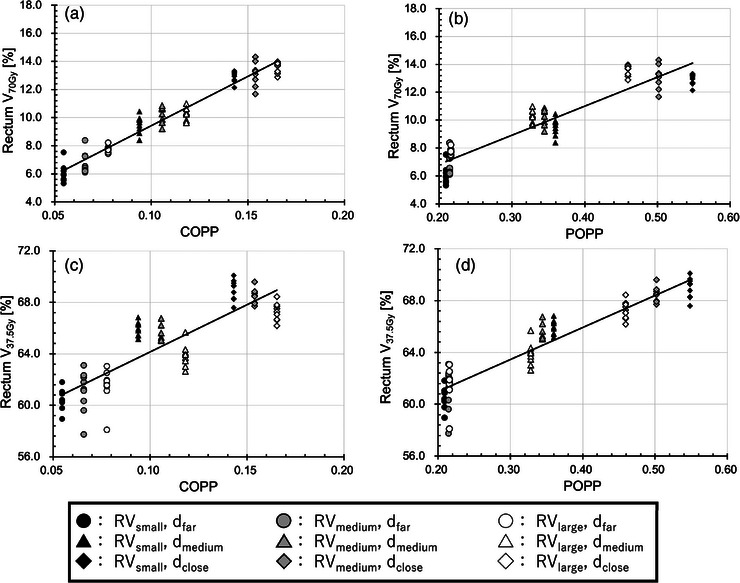
Linear regression analysis of the overlap‐based predictive parameter for rectum V70Gy and rectum V37.5 Gy with various patient cases for VMAT: (a) rectum V70Gy against COPP, (b) rectum V70Gy against POPP, (c) rectum V37.5 Gy against COPP, and (d) rectum V37.5 Gy against POPP. Data are plotted for three types of distances between PTV and rectum: far (circle), medium (triangle), and close (diamond), and for three types of volume: small (black), medium (gray), and large (white). The plot variations in markers of the same shape and same color indicate the result of varying bladder geometry. COPP, conventional overlap‐based predictive parameter; d, distance between rectum and PTV; POPP, proposed overlap‐based predictive parameter; PTV, planning target volume; RV, rectum volume; VMAT, volumetric modulated arc therapy.
TABLE 2.
Dose‐volume metrics and correlation coefficients for COPP and POPP in IMRT.
| Correlation coefficient | ||||
|---|---|---|---|---|
| OAR | Dose volume metrics | COPP | POPP | |
| Rectum | Dmean (Gy) | 44.7 ± 1.6 | 0.865 | 0.955 |
| V70Gy (%) | 12.2 ± 2.9 | 0.975 | 0.945 | |
| V60Gy (%) | 24.6 ± 3.6 | 0.921 | 0.962 | |
| V37.5 Gy (%) | 72.2 ± 2.3 | 0.806 | 0.927 | |
| Bladder | Dmean (Gy) | 30.7 ± 1.7 | 0.870 | 0.953 |
| V70Gy (%) | 11.1 ± 2.3 | 0.990 | 0.802 | |
| V60Gy (%) | 15.6 ± 2.7 | 0.977 | 0.862 | |
| V37.5 Gy (%) | 36.2 ± 3.3 | 0.881 | 0.951 | |
Abbreviations: COPP, conventional overlap predictive parameter; IMRT, intensity modulated radiation therapy; POPP, proposed overlap predictive parameter.
TABLE 3.
Dose‐volume metrics and correlation coefficients for COPP and POPP in VMAT.
| Correlation coefficient | ||||
|---|---|---|---|---|
| OAR | Dose volume metrics | COPP | POPP | |
| Rectum | Dmean (Gy) | 41.4 ± 1.8 | 0.933 | 0.964 |
| V70Gy (%) | 10.0 ± 2.7 | 0.971 | 0.933 | |
| V60Gy (%) | 19.4 ± 3.8 | 0.946 | 0.958 | |
| V37.5 Gy (%) | 64.8 ± 3.2 | 0.861 | 0.940 | |
| Bladder | Dmean (Gy) | 28.5 ± 1.7 | 0.934 | 0.924 |
| V70Gy (%) | 9.9 ± 2.3 | 0.997 | 0.774 | |
| V60Gy (%) | 13.5 ± 2.6 | 0.993 | 0.824 | |
| V37.5 Gy (%) | 31.2 ± 3.4 | 0.929 | 0.934 | |
Abbreviations: COPP, conventional overlap predictive parameter; POPP, proposed overlap predictive parameter; VMAT, volumetric modulated arc therapy.
Simulation 2 indicates that the COPP is effective for predicting high‐dose metrics and that the POPP is effective for predicting intermediate‐dose metrics. We confirmed the dose region, where the COPP or POPP is effective, by comparing the correlation coefficients of the COPP and that of the POPP for dose‐volume metrics in each 1‐Gy increment ranging from V20Gy to V75Gy. Our analysis showed a statistically significant superiority of POPP over COPP in predicting rectum doses within the range of V20Gy–V62Gy for IMRT and V20Gy–V55Gy for VMAT, and bladder doses within the range of V20Gy–V41Gy for IMRT and V20Gy–V23Gy for VMAT (p < 0.05). On the other hand, the COPP had a statistically better correlation than the POPP in rectum doses within the range of V70Gy–V75Gy for IMRT and V68Gy–V75Gy for VMAT, and bladder doses within the range of V51Gy–V75Gy for IMRT and V42Gy–V75Gy for VMAT (p < 0.05). In this simulation, the threshold dose Dth, at which correlation coefficients of the COPP and the POPP for V Dth were equal, was shown to be 67 Gy for IMRT and 63 Gy for VMAT in the rectum, and 47 Gy for IMRT and 38 Gy for VMAT in the bladder.
4. DISCUSSION
In this study, we proposed a POPP and investigated the predictivity of OAR dose using the POPP by comparing the correlation coefficients of POPP and COPP with respect to OAR dose‐volume metrics. The POPP is defined as the COPP multiplied by VPTV/VOAR based on the assumption that the predicted OAR dose should become higher as the PTV volume becomes larger or the OAR volume becomes smaller. The results of the linear regression analysis showed an improvement in the correlation with intermediate‐dose metrics when considering the volume relationship between the PTV and OAR.
As shown in Figures 4a and 5a, the V70Gy for the rectum was found to be about 10% when the COPP was about 0.1. This indicates that the RV receiving more than 70 Gy was nearly equal to the RV overlapping the PTV. In well‐conformed plans, the high‐dose region of the rectum roughly matches the overlap volume. Therefore, the COPP correlated well with the V70Gy for the rectum.
Figures 4b and 5b shows the relationship between the POPP and V70Gy for the rectum. As mentioned above, because V70Gy strongly correlates with the overlap volume, the benefit of modifying the COPP is likely to be limited. The VPTV/VOAR term in Equation (2) makes the POPP smaller as the RV becomes larger. This led to a lower correlation between the POPP and V70Gy for the rectum.
In the low‐dose index case, V37.5 Gy was about 68%, as shown in Figures 4c and 5c, which means that large regions of RV received more than 37.5 Gy. This region largely differed from the overlap volume, as the overlap volume was about 10% in this simulation. Thus, the overlap volume may be insufficient for predicting V37.5 Gy. Our simulations confirmed that the correlation between the COPP with V37.5 Gy was lower than that with V70 Gy. In terms of the relative volume relationship between PTV and the rectum, large rectum cases can be regarded as relatively small PTV cases. Thus, V37.5 Gy is smaller as the RV becomes larger. This led to a low correlation between COPP and V37.5 Gy. On the other hand, as shown in Figures 4d and 5d, POPP correlated well with V37.5 Gy, because the VPTV/VOAR term in POPP works to correct the inverted relationship.
It is reported that the high and intermediate doses to rectum and bladder associate with their toxicities. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 Thus, it is important to control both high and intermediate doses of rectum and bladder. Using COPP and POPP, it is possible to provide the treatment planners with the prediction of high and intermediate dose to OAR before starting dose optimization, which (1) improves treatment efficiency by reducing time to find achievable OAR doses, (2) helps reduce the difference of treatment planning quality among planners, and (3) allows early discussion about planning strategies with physicians in challenging cases.
As shown in Figures 4c and 5c, our results show that the COPP increases but that the V37.5 Gy decreases when the OAR volume becomes larger, resulting in a poor correlation between the COPP and V37.5 Gy. With regard to the lower dose‐volume metrics, prior studies reported that the overlap ratio (i.e., COPP) had poor correlation, but intermediate‐dose results were not mentioned in those articles. 21 , 22 , 28 From the clinical data, it is difficult to detect the cause of the poor predictivity for low dose, because OAR sparing is related to various factors such as OAR shapes, the planner's skill, and planning time. In this study, we excluded the dependence on these factors by using Auto‐Planning and systematically varying the phantom volume and position. Our study clarified that the volume relationship between PTV and OAR should be considered for predicting intermediate dose‐volume metrics.
In the recent studies, it is reported that the predictive models for OAR dose have been created using machine learning and the model performance is superior. 29 , 30 , 31 In practical implementation, the utilization of machine learning to predict OAR doses may not be feasible for every institution due to requiring a large number of training data, a development environment for machine learning, and an expertise of machine learning. On the other hand, the process of constructing predictive models for OAR doses using COPP/POPP is straightforward, because this approach solely requires geometric data; the PTV volume, OAR volume, and the overlap volume. Consequently, our proposed methodology has the potential for implementation across a wide array of institutions.
We note the following two limitations of this study:
The scope of present study was to propose a new predictive parameter and demonstrate the effectiveness of this parameter. For this purpose, we used a simple phantom in order to simulate systematic anatomical changes including the volume and position between PTV and OAR. However, the structure of human patients are complex than TG119 prostate phantom used in this study. The effectiveness of the POPP for the clinical data will be evaluated using clinical data in future studies.
All plans in this study were created using Auto‐Planning. Had the planner manually created the plans, a different result might have been obtained, because the plan includes the planner's variability and intention.
5. CONCLUSION
We proposed a POPP that considers the volume relationship between PTV and OAR. Our study showed that the POPP strongly correlated with dose‐volume metrics for low‐intermediate doses, and the COPP had a strong correlation for high doses. Because it has been reported that rectal bleeding and bladder toxicity are associated with low‐intermediate doses as well as high doses, it is important to estimate the dose‐volume metrics across a broad dose range (low‐high doses). The POPP and COPP are suitable for this purpose. This work will be beneficial to help planners to efficiently create the high‐quality treatment plans.
AUTHOR CONTRIBUTIONS
Yuki Saito, Ryusuke Suzuki, Naoki Miyamoto, and Masaya Tamura collected the data and prepared the materials. Yuki Saito, Ryusuke Suzuki, Naoki Miyamoto, Takahiro Kanehira, Masaya Tamura, Tadashi Mori, Kentaro Nishioka, Takayuki Hashimoto, and Hidefumi Aoyama contributed to the study conception and design. Yuki Saito and Ryusuke Suzuki participated in data analysis. Yuki Saito, Ryusuke Suzuki, Naoki Miyamoto, Kenneth Lee Sutherland, Takahiro Kanehira, and Masaya Tamura wrote the first draft of the manuscript. All authors commented on the manuscript and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
Yuki Saito and Ryusuke Suzuki would like to thank Atsushi Izuka for collaboration on the early stages of this work.
Saito Y, Suzuki R, Miyamoto N, et al. A new predictive parameter for dose‐volume metrics in intensity‐modulated radiation therapy planning for prostate cancer: Initial phantom study. J Appl Clin Med Phys. 2024;25:e14250. 10.1002/acm2.14250
REFERENCES
- 1. Fellin G, Fiorino C, Rancati T, et al. Clinical and dosimetric predictors of late rectal toxicity after conformal radiation for localized prostate cancer: results of a large multicenter observational study. Radiother Oncol. 2009;93(2):197‐202. doi: 10.1016/j.radonc.2009.09.004 [DOI] [PubMed] [Google Scholar]
- 2. Storey MR, Pollack A, Zagars G, Smith L, Antolak J, Rosen I. Complications from radiotherapy dose escalation in prostate cancer: preliminary results of a randomized trial. Int J Radiat Oncol Biol Phys. 2000;48(3):635‐642. doi: 10.1016/s0360-3016(00)00700-8 [DOI] [PubMed] [Google Scholar]
- 3. Fiorino C, Sanguineti G, Cozzarini C, et al. Rectal dose‐volume constraints in high‐dose radiotherapy of localized prostate cancer. Int J Radiat Oncol Biol Phys. 2003;57(4):953‐962. doi: 10.1016/s0360-3016(03)00665-5 [DOI] [PubMed] [Google Scholar]
- 4. Ebert MA, Foo K, Haworth A, et al. Gastrointestinal dose‐histogram effects in the context of dose‐volume‐constrained prostate radiation therapy: analysis of data from the RADAR prostate radiation therapy trial. Int J Radiat Oncol Biol Phys. 2015;91(3):595‐603. doi: 10.1016/j.ijrobp.2014.11.015 [DOI] [PubMed] [Google Scholar]
- 5. Karlsdóttir A, Muren LP, Wentzel‐Larsen T, Dahl O. Late gastrointestinal morbidity after three‐dimensional conformal radiation therapy for prostate cancer fades with time in contrast to genitourinary morbidity. Int J Radiat Oncol Biol Phys. 2008;70(5):1478‐1486. doi: 10.1016/j.ijrobp.2007.08.076 [DOI] [PubMed] [Google Scholar]
- 6. Fiorino C, Cozzarini C, Vavassori V, et al. Relationships between DVHs and late rectal bleeding after radiotherapy for prostate cancer: analysis of a large group of patients pooled from three institutions. Radiother Oncol. 2002;64(1):1‐12. doi: 10.1016/s0167-8140(02)00147-0 [DOI] [PubMed] [Google Scholar]
- 7. Jackson A, Skwarchuk MW, Zelefsky MJ, et al. Late rectal bleeding after conformal radiotherapy of prostate cancer. II. Volume effects and dose‐volume histograms. Int J Radiat Oncol Biol Phys. 2001;49(3):685‐698. doi: 10.1016/s0360-3016(00)01414-0 [DOI] [PubMed] [Google Scholar]
- 8. Michalski JM, Gay H, Jackson A, Tucker SL, Deasy JO. Radiation dose‐volume effects in radiation‐induced rectal injury [published correction appears in Int J Radiat Oncol Biol Phys. 2019 Aug 1;104(5):1185]. Int J Radiat Oncol Biol Phys. 2010;76(Suppl 3):S123‐S129. doi: 10.1016/j.ijrobp.2009.03.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bagalà P, Ingrosso G, Falco MD, et al. Predicting genitourinary toxicity in three‐dimensional conformal radiotherapy for localized prostate cancer: a dose‐volume parameters analysis of the bladder. J Cancer Res Ther. 2016;12(2):1018‐1024. doi: 10.4103/0973-1482.165871 [DOI] [PubMed] [Google Scholar]
- 10. Son CH, Melotek JM, Liao C, et al. Bladder dose‐volume parameters are associated with urinary incontinence after postoperative intensity modulated radiation therapy for prostate cancer. Pract Radiat Oncol. 2016;6(5):e179‐e185. doi: 10.1016/j.prro.2015.12.004 [DOI] [PubMed] [Google Scholar]
- 11. Cheung MR, Tucker SL, Dong L, et al. Investigation of bladder dose and volume factors influencing late urinary toxicity after external beam radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2007;67(4):1059‐1065. doi: 10.1016/j.ijrobp.2006.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Viswanathan AN, Yorke ED, Marks LB, Eifel PJ, Shipley WU. Radiation dose‐volume effects of the urinary bladder. Int J Radiat Oncol Biol Phys. 2010;76(Suppl 3):S116‐S122. doi: 10.1016/j.ijrobp.2009.02.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karlsdóttir A, Johannessen DC, Muren LP, Wentzel‐Larsen T, Dahl O. Acute morbidity related to treatment volume during 3D‐conformal radiation therapy for prostate cancer. Radiother Oncol. 2004;71(1):43‐53. doi: 10.1016/j.radonc.2004.01.014 [DOI] [PubMed] [Google Scholar]
- 14. Deville C, Both S, Bui V, et al. Acute gastrointestinal and genitourinary toxicity of image‐guided intensity modulated radiation therapy for prostate cancer using a daily water‐filled endorectal balloon. Radiat Oncol. 2012;7:76. doi: 10.1186/1748-717X-7-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Berry SL, Boczkowski A, Ma R, Mechalakos J, Hunt M. Interobserver variability in radiation therapy plan output: results of a single‐institution study. Pract Radiat Oncol. 2016;6(6):442‐449. doi: 10.1016/j.prro.2016.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Batumalai V, Jameson MG, Forstner DF, Vial P, Holloway LC. How important is dosimetrist experience for intensity modulated radiation therapy? A comparative analysis of a head and neck case. Pract Radiat Oncol. 2013;3(3):e99‐e106. doi: 10.1016/j.prro.2012.06.009 [DOI] [PubMed] [Google Scholar]
- 17. Powis R, Bird A, Brennan M, et al. Clinical implementation of a knowledge based planning tool for prostate VMAT. Radiat Oncol. 2017;12(1):81. doi: 10.1186/s13014-017-0814-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bandlamudi BP, Sharan K, Yathiraj PH, et al. A study on the impact of patient‐related parameters in the ability to spare parotid glands by intensity‐modulated radiotherapy for head and neck squamous cell carcinomas. J Cancer Res Ther. 2018;14(6):1220‐1224. doi: 10.4103/jcrt.JCRT_362_16 [DOI] [PubMed] [Google Scholar]
- 19. Zhang H, Cao Y, Antone J, et al. A model‐based method for assessment of salivary gland and planning target volume dosimetry in volumetric‐modulated arc therapy planning on head‐and‐neck cancer. J Med Phys. 2019;44(3):201‐206. doi: 10.4103/jmp.JMP_19_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moore KL, Brame RS, Low DA, Mutic S. Experience‐based quality control of clinical intensity‐modulated radiotherapy planning. Int J Radiat Oncol Biol Phys. 2011;81(2):545‐551. doi: 10.1016/j.ijrobp.2010.11.030 [DOI] [PubMed] [Google Scholar]
- 21. Chao M, Coburn N, Cosgriff E, Brown C, Van Tilburg K, Hayden A. A predictive model for determining rectum and bladder dose constraints in prostate volumetric modulated arc therapy. Med Dosim. 2021;46(3):269‐273. doi: 10.1016/j.meddos.2021.02.005 [DOI] [PubMed] [Google Scholar]
- 22. Ueda Y, Monzen H, Fukunaga JI, et al. Characterization of knowledge‐based volumetric modulated arc therapy plans created by three different institutions' models for prostate cancer. Rep Pract Oncol Radiother. 2020;25(6):1023‐1028. doi: 10.1016/j.rpor.2020.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mellon EA, Javedan K, Strom TJ, et al. A dosimetric comparison of volumetric modulated arc therapy with step‐and‐shoot intensity modulated radiation therapy for prostate cancer. Pract Radiat Oncol. 2015;5(1):11‐15. doi: 10.1016/j.prro.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 24. Nawa K, Haga A, Nomoto A, et al. Evaluation of a commercial automatic treatment planning system for prostate cancers. Med Dosim. 2017;42(3):203‐209. doi: 10.1016/j.meddos.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 25. Paddick I. A simple scoring ratio to index the conformity of radiosurgical treatment plans. Technical note. J Neurosurg. 2000;93(Suppl 3):219‐222. doi: 10.3171/jns.2000.93.supplement [DOI] [PubMed] [Google Scholar]
- 26. Hodapp N. The ICRU Report 83: prescribing, recording and reporting photon‐beam intensity‐modulated radiation therapy (IMRT). Strahlenther Onkol. 2012;188(1):97‐99. doi: 10.1007/s00066-011-0015-x [DOI] [PubMed] [Google Scholar]
- 27. Nguyen TTT, Arimura H, Asamura R, Hirose TA, Ohga S, Fukunaga JI. Comparison of volumetric‐modulated arc therapy and intensity‐modulated radiation therapy prostate cancer plans accounting for cold spots. Radiol Phys Technol. 2019;12(2):137‐148. doi: 10.1007/s12194-019-00502-0 [DOI] [PubMed] [Google Scholar]
- 28. Mattes MD, Lee JC, Elnaiem S, Guirguis A, Ikoro NC, Ashamalla H. A predictive model to guide management of the overlap region between target volume and organs at risk in prostate cancer volumetric modulated arc therapy. Radiat Oncol J. 2014;32(1):23‐30. doi: 10.3857/roj.2014.32.1.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kajikawa T, Kadoya N, Ito K, et al. A convolutional neural network approach for IMRT dose distribution prediction in prostate cancer patients. J Radiat Res. 2019;60(5):685‐693. doi: 10.1093/jrr/rrz051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kandalan RN, Nguyen D, Rezaeian NH, et al. Dose prediction with deep learning for prostate cancer radiation therapy: model adaptation to different treatment planning practices. Radiother Oncol. 2020;153:228‐235. doi: 10.1016/j.radonc.2020.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gronberg MP, Beadle BM, Garden AS, et al. Deep learning‐based dose prediction for automated, individualized quality assurance of head and neck radiation therapy plans. Pract Radiat Oncol. 2023;13(3):e282‐e291. doi: 10.1016/j.prro.2022.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]


