Abstract
The deposition of immune complexes, activation of complement and infiltration of the kidney by cells of the adaptive and innate immune systems have long been considered responsible for the induction of kidney damage in autoimmune, alloimmune and other inflammatory kidney diseases. However, emerging findings have highlighted the contribution of resident immune cells and of immune molecules expressed by kidney-resident parenchymal cells to disease processes. Several types of kidney parenchymal cells seem to express a variety of immune molecules with a distinct topographic distribution, which may reflect the exposure of these cells to different pathogenic threats or microenvironments. A growing body of literature suggests that these cells can stimulate the infiltration of immune cells that provide protection against infections or contribute to inflammation – a process that is also regulated by draining kidney lymph nodes. Moreover, components of the immune system, such as autoantibodies, cytokines and immune cells, can influence the metabolic profile of kidney parenchymal cells in the kidney, highlighting the importance of crosstalk in pathogenic processes. The development of targeted nanomedicine approaches that modulate the immune response or control inflammation and damage directly within the kidney has the potential to eliminate the need for systemically acting drugs.
Introduction
Kidney inflammation is associated with a variety of entities, including diseases such as lupus nephritis and vasculitis, and injury, for example, following ischaemia–reperfusion or kidney transplantation. Invariably, such inflammation exacerbates kidney damage and can lead to kidney failure. In the case of lupus nephritis and vasculitis, kidney inflammation is driven by autoimmunity. Multiple pathogenic pathways, involving genetic, epigenetic, hormonal, environmental and immunoregulatory factors, contribute to the development of autoimmunity1. However, not all patients with features of autoimmunity develop organ damage, suggesting a role for local factors in organs such as the kidney. Indeed, emerging evidence suggests that cells resident within organs can facilitate the instigation and propagation of tissue injury through a variety of processes2. The importance of these resident cells to disease processes is highlighted by the finding that the inhibition of kidney parenchymal cell (KPC) function can in some instances attenuate kidney damage, even in the presence of rampant peripheral humoral and cellular autoimmune responses3,4.
Systemic autoimmune diseases involve multiple elements and pathways of the innate and adaptive immune systems5-7. In systemic lupus erythematosus (SLE), autoantibodies form immune complexes with autoantigens, such as nucleosomes in the circulation, which can subsequently deposit in tissues, including the glomerular basement membrane8-10. The excessive production of cytokines, including type I interferon (IFN), IL-6, IL-17 and IL-23 by immune cells further induce immune cell abnormalities and can directly damage KPCs4. Moreover, autoreactive T cells, the antigen specificity of which is yet to be defined, infiltrate the kidney where they can form tertiary lymphoid organs (TLOs) that also contribute to the development of organ damage11,12. Precisely how infiltrating immune cells contribute to the demise of KPCs remains unclear, but may involve direct cell cytotoxic effects or the release of cytokines and chemokines that may alter the metabolism and/or functions of KPCs.
The prevailing belief is that the deposition of autoantibodies or immune complexes in the glomerulus or other parts of the kidney triggers and sustains an inflammatory response, resulting in kidney injury13. This assumption implies that once the peripheral autoimmune response is established, it will cause kidney damage without any contribution from the KPCs. However, this assumption is challenged by the observation that not all patients with anti-nuclear antibodies or antibodies to DNA develop clinically apparent lupus nephritis14. Furthermore, the assumption that circulating cytokines act on cells of the immune system to induce an inflammatory response has driven the development of biologics that inhibit cytokines, such as IL-6, IL-17 and IL-23, that are present in the sera of patients with autoimmune disease15,16. However, the fact that these cytokines might also directly affect KPCs to alter their metabolism and/or function has not generally been considered. Thus, despite the recognized association between kidney inflammation and morbidity and mortality13,17, it remains unclear whether peripheral autoimmunity alone – that is, the presence of autoantibodies, immune complexes and autoreactive immune cells–is sufficient to induce kidney injury.
A growing body of evidence suggests that molecular processes within the kidney parenchyma are needed for the demise of kidney tissue. This notion is in line with the idea of ‘structural immunity’, whereby non-haematopoietic, tissue-resident cells can adopt the tissue-specific and regulated expression of genes involved in immune functions18. Comprehensive characterizations of non-haematopoietic cells have demonstrated the regulation of such immune genes under physiological conditions and in response to viral infections18. This Review is aimed at providing critical discussion of the emerging information in support of the notion that KPCs provide structural immunity to the kidney by upregulating or altering the expression of immune-related genes, which in turn contributes to the infiltration of immune cells and exacerbates kidney injury.
Immune cells in the kidney
Inflammation of the kidney (nephritis) is a feature of various clinical entities, including systemic autoimmunity, vasculitis, transplantation and ischaemia–reperfusion injury19. Regardless of the aetiology, the immune response in the kidney involves the release of soluble inflammatory mediators such as cytokines and chemokines, the recruitment of immune cells and the activation of resident immune cells. These processes alter the metabolism and expression of genes that may contribute to sustenance of the inflammatory process20.
In patients with kidney disease, the accumulation of immune cells in the tubulointerstitium is strongly associated with an increased risk of progression to kidney failure21-23. However, the fact that not every patient with tubulointerstitial inflammation develops kidney failure21,23 suggests heterogeneity in the inflammatory response, which may underlie the diversity in outcomes24. Thus, better understanding of the types of infiltrating immune cells, their behaviour and their location in the kidney may provide important insights into the mechanisms that drive the progression of kidney injury in particular patients.
Cells of the innate immune response
Various components of the innate immune response have been implicated in the progression of kidney diseases. Mononuclear phagocytes, including tissue-resident macrophages, type 1 classic dendritic cells (cDC1s), type 2 classic dendritic cells (cDC2s) and plasmacytoid DCs, exist in healthy kidneys where they reside near the periphery of glomeruli or peritubular capillaries25-27. During kidney inflammation, a number of these cells expand, primarily in the tubulointerstitial area and in the periglomerular area21,28 (Fig. 1). These cells can demonstrate an activated phenotype, characterized by the production of cytokines including IFNα, or assume a “reparative” phenotype to facilitate the elimination of debris and suppress inflammation28. Mixed populations of phagocytic cells also infiltrate the kidney, further propagating the inflammatory response. It is important to note that some infiltrating phagocytes, such as cDC1s, have a protective role25,28. Similarly, infiltrating and resident macrophages in the kidney can adopt an alternatively activated reparative phenotype and can function to remove cell debris, suppress inflammation and promote the regeneration of injured tubular epithelium29,30. However, little is known about the core transcription factors that regulate renal phagocyte fate and function. In addition, other immune cell types, including natural killer (NK) cells, γδ T cells and innate lymphoid cells have been identified in kidneys from patients with lupus nephritis31,32, although the exact role of these cells in kidney injury are unclear. Importantly, the intrinsic characteristics of innate lymphoid cells are key to maintaining the homeostasis of the innate and adaptive immune response, as well as the bidirectional interactions with organ resident cells33,34.
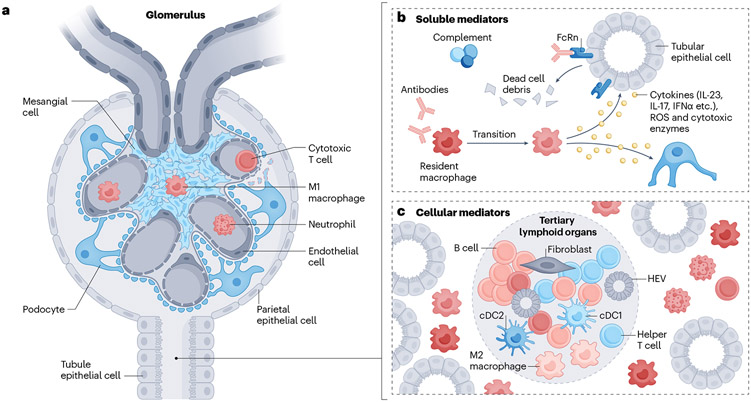
Fig. 1 ∣. Soluble and cellular mediators of inflammation in the kidney.
a, immune cells, including macrophages and dendritic cells, may enter the glomerulus to instigate damage; cytotoxic T cells might also enter and cause podocyte apoptosis when the basement membrane is injured. b, Activated components of the complement system and/or autoantibodies can attack kidney parenchymal cells (KPCs), such as tubular epithelial cells, directly, to cause tissue damage. Alternatively, they can recruit and/or activate myeloid cells to induce the release of cytokines, reactive oxygen species (ROS) and cytotoxic enzymes. c, Different immune cell clusters composed of different types of lymphocytes can be observed in the interstitial region. Some clusters display features typical of secondary lymphoid organs, including the presence of T cell and B cell zones aligned with the formation of high endothelial venules (HEVs) and new lymphatic vessels; these clusters are termed tertiary lymphoid organs.
Cells of the adaptive immune response
The adaptive immune system – comprising T lymphocytes and B lymphocytes – also has an active role in kidney inflammation and injury. Infiltrating lymphocytes accumulate mostly in the interstitial region rather than in the glomeruli, where they form clusters with each other and other immune cells35. Interestingly, some of these clusters display features of secondary lymphoid organs, including the presence of T cell and B cell zones that align with newly formed high endothelial venules (HEVs) and lymphatic vessels, and are known as TLOs35-37 (Fig. 1). TLOs are defined as lymphoid aggregates with organized stromal components consisting of follicular dendritic cells and fibroblastic reticular cells (FRCs). Remarkably, the formation and maintenance of TLOs requires molecular mechanisms, that is, lymphotoxin and lymphoid chemokines, which are very similar to those required of the formation of secondary lymphoid organs38. These structures can harbour pro-inflammatory, pathogenic lymphocytes that sustain local inflammation, but can also contain cells that provide tissue protection by restraining the magnitude of the inflammation35. Better understanding of the potential pathogenic and protective functions of TLOs is therefore crucial.
B cells and autoantibodies have long been considered to be the main drivers of autoimmune kidney diseases, such as lupus nephritis5. Remarkably, although the presence of lymphocyte clusters dominated by CD4− T cell populations comprising CD8+ T cells, γδ T cells and double-negative (CD4−CD8−; DN) T cells39,40 in the tubulointerstitium is associated with refractory disease and predicts progression to kidney failure in patients with LN, the presence of B cells in these lymphocyte clusters is associated with protection against disease progression37.
Immune cells, including macrophages and DCs, may enter the glomerulus to instigate damage. Cytotoxic T cells can also enter and cause podocyte apoptosis when the basement membrane is injured41. Meanwhile, resident myeloid and infiltrating cells can proliferate locally6,28.
In models of acute kidney injury (AKI), single-cell transcriptomic analyses of T regulatory cells identified the differential upregulation of regenerative pathways or hyperactive, pro-fibrotic pathways, dependent on environmental cues and stage of injury42. These findings suggest that immune cells are subject to plasticity and that their role in the control of damage may change in a temporal manner. Better insights into the pathogenic and regulatory properties of resident and infiltrating immune cells within the kidney parenchyma may reveal opportunities for targeted therapies.
Cytokines and chemokines
Soluble mediators, such as pro-inflammatory cytokines or antibodies, may initiate kidney inflammation by activating resident immune cells to produce various cytokines and chemokines43. These mediators are thought to promote the further recruitment of immune infiltrates, resulting in the production of additional cytokines and chemokines and amplification of the local inflammatory response44. The pathogenic role of soluble mediators in nephritis is supported by preclinical studies, which have shown that cytokine-blocking treatment suppresses kidney disease. However, the effectiveness of cytokine-blocking therapies in patients is less clear44.
Unlike cytokines, which are broadly recognized by different types of immune cells, chemokines are selectively used to promote the migration of specific cell populations to specific organs. Thus, some chemokines may exert a direct effect on the kidney through the recruitment of specific cell populations, with minimal impact on the systemic autoimmune response. Various chemokines – including CCL2, CCL20, CXCL10, CXCL12, CXCL13 and CX3CL1 – are increased in the kidneys of patients with lupus nephritis, with region-specific differences. For example, CXCL13 is highly expressed in the renal cortex, whereas levels of CXCL10 and CXCL12 were significantly increased in both the tubulointerstitial and glomerular regions45,46. These findings suggest that chemokines might orchestrate the migration of specific immune cell populations to particular regions of the kidney, and further suggest that targeting of kidney-specific cytokines and/or chemokines may represent a promising strategy for controlling nephritis. However, detailed mechanistic studies are needed to serve as the basis for future drug development.
Insights from single-cell RNA sequencing
The rapid development of single-cell RNA sequencing (scRNA-Seq) technologies has facilitated the detailed characterization of heterogeneous cell populations47. The creation of cell atlases48,49 has confirmed the presence of different immune cells in kidneys under physiological conditions50. Notably, these studies identified a unique myeloid population that is distinct from myeloid cells in the blood and a population of CD4+ T cells with an effector memory phenotype48,49. To date, scRNA-Seq has provided insights into the complexity of both parenchymal and immune cell landscapes in nephritic kidneys in the context of lupus nephritis, diabetic kidney disease, IgA nephropathy and anti-neutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis31,50-55.
Inflammatory macrophages constitute the predominant population of infiltrating immune cells in most chronic kidney diseases (CKDs), and their accumulation is closely associated with the presence of proteinuria and kidney damage28. scRNA-Seq studies have identified the presence of resident memory CD4+ T cells and B cells in kidney biopsy samples from patients with lupus nephritis, diabetic kidney disease and IgA nephropathy50, indicating the existence of shared mechanisms in the development of kidney inflammation across kidney diseases of different aetiologies. The ratio of various immune infiltrates likely varies between diseases and according to disease stage, contributing to the heterogeneous nature of the inflammatory response in individuals with kidney disease. Future scRNA-Seq studies may identify therapeutic targets that are shared between clinical entities and conversely, identify therapeutic targets that are specific to disease aetiology and stage to enable a precision medicine approach to treatment56,57.
Immune features of kidney parenchymal cells
Although KPCs are traditionally thought to participate in the core functions of the kidney, an emerging body of literature suggests that various KPCs also possess immune regulatory roles. Indeed, KPCs exhibit several characteristic features of immune cells and express components of the innate and adaptive immune cascades that are critical to the inflammatory response in the kidney58-60 (Fig. 2).
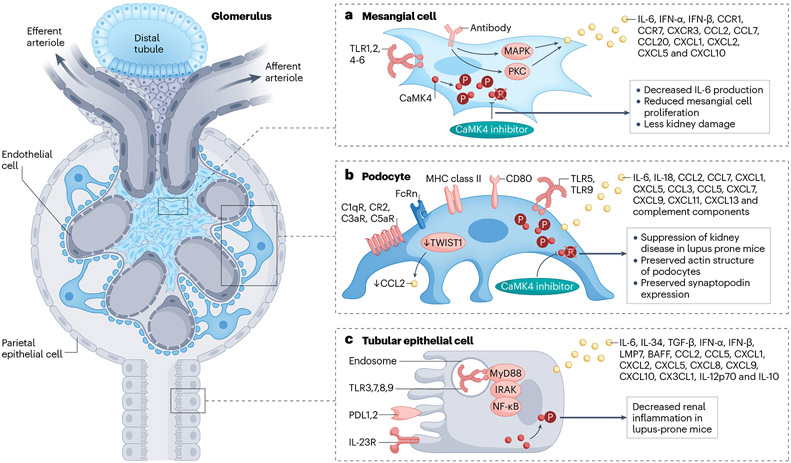
Fig. 2 ∣. Immune features of kidney parenchymal cells.
Kidney parenchymal cells, including podocytes, tubular epithelial cells and mesangial cells, express molecules that are typical of the innate and adaptive immune systems. These molecules have important roles in the recruitment of immune cells and in the development of inflammatory responses. a, Mesangial cells have long been known to express cytokines and chemokines in response to circulating inflammatory signals. For example, IL-6 can independently cause mesangial proliferation. Signalling molecules, such as CaMK4, may control the production of cytokines, and may therefore represent a treatment target. b, Podocytes produce and express molecules typically produced by cells of the immune system, including HLA, costimulatory molecules, the neonatal Fc receptor (FcRn), TLRs, and chemokines and their receptors, along with complement components and receptors. The transcription factor TWIST1 may suppress the production of CCL2 in healthy glomeruli. Podocyte injury may lead to the altered expression of molecules such as CaMK4, which can directly compromise the expression of molecules involved in their function (e.g. nephrin) or structure (e.g. synaptopodin). c, Similarly, TECs may produce cytokines and chemokines and their receptors, which may promote the recruitment of inflammatory cells. Such cells may, through the further production of cytokines, contribute to the damage of TECs. Again, alterations in the expression of molecules such as CaMK4 may contribute to the accumulation of immune cells and may therefore representa therapeutic target. FcRn, neonatal Fc receptor; TLR, Toll-like receptor; PDL1, programmed death ligand 1; IRAK, IL-1 receptor-associated kinase; TRAF6, TNF receptor-associated factor 6; BAFF, B cell–activating factor; LMP7, low–molecular mass polypeptide-7; CAMK4, calcium/calmodulin dependent protein kinase IV.
Mesangial cells
Mesangial cells are well known to share numerous molecules with immune cells, particularly with monocytes and macrophages. Moreover, they have a crucial role in preventing the accumulation of aggregated proteins and small immune complexes in the glomerular basement membrane61 and thereby interact with immune cell processes. Mesangial cell proliferation and the presence of mesangial matrix are consistently observed in kidney tissue of patients with glomerulonephritis62. Mesangial cells express Toll-like receptors (TLRs)62,63, and upon stimulation with appropriate ligands, produce type I IFN62 – a cytokine that is important in the pathogenesis of SLE62,64 (Fig. 2).
Available data suggest that the binding of antibodies, including those directed against double-stranded DNA to mesangial cells in the context of lupus nephritis activates an inflammatory signalling cascade that involves mitogen-activated protein kinase and protein kinase C, and ultimately leads to the production of pro-inflammatory cytokines64,65. In mouse models of SLE, secretion of IL-6 by mesangial cells acts as an independent driver of glomerulonephritis66. Moreover, in patients and mouse models of lupus nephritis, mesangial cells overexpress calcium/calmodulin kinase 4 (CaMK4), a serine/threonine kinase that is necessary for mesangial cell proliferation and IL-6 production (Fig. 2). Interestingly, mesangial cells from lupus-prone MRL/lpr mice that lack CaMK4 do not proliferate in response to platelet-derived growth factor and fail to produce IL-6 (ref. 67). In IgA nephropathy, under-galactosylated IgA1 containing immune complexes activate mesangial cells to produce IL-6 (ref. 68) as well as other cytokines, chemokines and complement69.
Podocytes and glomerular endothelial cells
Podocytes express MHC class II and CD86, which are required for T cell activation. Mice that lack MHC II specifically in podocytes demonstrate an attenuated response to nephrotoxic serum-induced nephritis70,71. The neonatal Fc receptor (FcRn) is expressed in glomerular endothelial cells, podocytes, and proximal tubular epithelial cells (TECs)72 and has a crucial role in salvaging protein and preventing its loss in the urine. In podocytes cultured in the presence of immune complexes, silencing of FcRn decreased immune complex trafficking to lysosomes and decreased lysosomal surface area and function, suggesting that FcRn-mediated trafficking of immune complexes modulates lysosomal function in podocytes73.
TLRs have an important role in the development and progression of kidney diseases; their dysregulation is critically involved in various conditions, including urinary tract infections, ischaemia–reperfusion injury, AKI, lupus nephritis and diabetic kidney disease12. Both podocytes and renal TECs express TLRs (Fig. 2), which enables them to recognize pathogen-associated molecular patterns and damage-associated molecular patterns. This recognition leads to the induction of chemokines and cytokines involved in the development of glomerular damage, which is achieved through the activation of downstream molecules such as NF-κB, MyD88, IRAK and TRAF6 (refs. 58,74).
In vitro studies have shown that podocytes treated with TNF produce IF-6, which promotes the migration of neutrophils to glomerular endothelial cells75. Podocytes also express TWIST1, a transcription factor that contains a basic helix–loop–helix domain and acts as a common repressor of cell-mediated immunity and cytokine production. In the context of glomerular injury, TWIST1 facilitates the production of CCF2 in glomeruli, which promotes the accumulation and activation of macrophages and exacerbates podocyte injury and proteinuria in mice injected with nephrotoxic serum or the nephrotoxin, adriamycin. However, deletion of Twist1 in podocytes promoted podocyte injury and proteinuria, suggesting that its upregulation in human and murine models of podocytopathies may represent a compensatory mechanism76.
Finally, podocytes are not only susceptible to complement-mediated injury but also actively contribute to the production of complement components77. Both primary cultured podocytes and immortalized podocytes express a wide range of complement genes under physiological conditions78. In addition, exposure of podocytes to puromycin both in vivo and in vitro increases the expression of complement component C3 (ref. 78).
Glomerular endothelial cells may also contribute to the development of nephritis in patients with autoimmune disease. In patients with lupus nephritis, these cells display an IFN signature79, and produce fractalkine (CX3CL1)80 as well as other pro-inflammatory cytokines81.
Tubular epithelial cells
Renal TECs actively contribute to the development of the kidney inflammation by producing pro-inflammatory cytokines and chemokines and by interacting with immune cells60,82-85. Renal TECs produce IFNα, which induces – probably in an autocrine manner – a type-I IFN signature that exists only in TECs86. Kidney TECs also produce B cell-activating factor (BAFF), a cytokine that is essential for B cell survival and maturation and may help B cells to survive and expand in the kidney87,88. TECs also express the receptor for BAFF and it is believed to contribute to the cell atrophy routinely seen in patients with lupus nephritis60. Proximal TECs can also produce IL-12p70 and IL-10, through which they control the function of DCs60,82-85 and B cells60,82-85, and CX3CR1, which aids the recruitment and retention of myeloid DCs60,82-85. Moreover, proximal TECs may contribute to sepsis-induced kidney inflammation and AKI through activation of TLR2–NF-κB–CCL2 signalling89. TECs also express the programmed cell death (PD) ligands, PD-L1, and PD-L2 (ref. 85). Activation of the PD-1–PD-L1 signalling in TECs is thought to exert an inhibitory effect on the development of alloreactive T-cell responses85 (Fig. 2). TECs also produce TGFβ, which induces their senescence and promotes fibroblast proliferation90.
α-Intercalated cells, traditionally known for their role in acid–base homeostasis, can sense liposaccharide through TLR4 to produce the bacteriostatic protein lipocalin 2 (ref. 91) and the anti-inflammatory cytokine IL-18 (ref. 92) to fend off infections and mitigate inflammation93. Information about the numbers and function of these cells in patients with autoimmune kidney disease is not yet available; however, given that urinary tract infections are common in patients with lupus nephritis, we suspect that their function is compromised.
Mesenchymal stromal cells
Mesenchymal stromal cells are multipotent progenitor cells that are present in all tissues; they are immunomodulatory and may exert pro-inflammatory effects in the presence of certain cytokines. Mesenchymal stem cells can be detected in the renal pelvis and the TLOs of lupus-prone mice; following stimulation, they produce a number of pro-inflammatory cytokines and facilitate the formation of TLOs94.
KPCs undergo a transition to acquire immune cell features during the postnatal period. Studies of single-cell suspensions derived from mature human kidneys and fetal kidneys have revealed that immune cells are spatially distributed throughout the kidney. This spatial distribution enables interaction between epithelial cells and immune cells and is likely important for the instigation of appropriate immune responses. For example, in the context of infection, specific interactions between epithelial cells and immune cells – most notably in the renal pelvis – may orchestrate the localization of antibacterial macrophages and neutrophils to regions of the kidney that are most susceptible. These interactions are mediated through various mechanisms. For example, the expression of TLR4 and its downstream signalling molecule MyD88 in the pelvic epithelium contributes to the localization of immune cells. In addition, epithelial cells of the renal pelvis express CXCL8, which likely promotes the chemotaxis of neutrophils to the region during infection. Notably, the innate immune capability of zonated epithelial cells and the expression of neutrophil-recruiting chemokines in the distal nephron and pelvis are acquired after birth and are not present in fetal kidneys49.
In addition to the acquisition of immune features during development, KPCs exhibit sex-based transcriptional differences49. These differences suggest higher baseline metabolic activity in males and enhanced expression of antioxidant genes in females. Specifically, KPCs from human female kidneys show increased expression of metallothionein genes and genes related to cysteine–glutathione metabolism, which are crucial for cellular antioxidant defence95. These findings provide insights into the sexual dimorphism observed for many kidney diseases96,97.
The immune function of KPCs is also affected by the natural aging process. The impact of cellular senescence and the accumulation of age-modified immune cells in aged kidneys or in kidneys with chronic injury or inflammation has been well-documented98. Renal cell senescence is associated not only with the development of AKI and CKD but also with their progression. Notable similarities between senescence and AKI in terms of pathogenic mechanisms, including the secretion of pro-inflammatory and pro-fibrotic factors, the presence of oxidative stress, mitochondrial dysfunction and loss of renoprotective factors99. Aged kidneys develop TLOs following AKI, likely because of maladaptive repair, and their presence is associated with the severity of kidney injury in both mice and humans100. Notably, in aged kidneys after ischaemia–reperfusion injury, the development of the age-dependent lymphocyte subpopulations, CD153+PD-1+CD4+ senescence-associated T (SAT) cells and CD30+T-bet+ age-associated B cells (ABCs) is spatiotemporally synchronized within TLOs100. This synchronized development is mediated through the CD153–CD30 signalling pathway as evidenced by the finding that transplantation of bone marrow from CD153-deficient mice into aged Rag2−/− mice after ischaemia–reperfusion injury attenuates TLO formation100. SAT cells in the kidney and ABCs in the spleen have shared T cell and B cell receptor sequences, respectively, suggesting the possible circulation of SAT cells and ABCs between the spleen and kidney.
Kidney draining lymph nodes
Lymph nodes are the main sites at which cells of the adaptive immune system are modified and activated to ensure specificity of the immune response. From the kidney, lymphatic vessels carry soluble antigens and cells to kidney-draining lymph nodes (KLNs) (Fig. 3). Under physiological conditions, DCs in the KLN handle kidney-filtered antigen and establish immune tolerance by upregulating PD-L1 (ref. 101). DCs also traffic from the kidney to the KLN102, where they might participate in establishing tolerance.
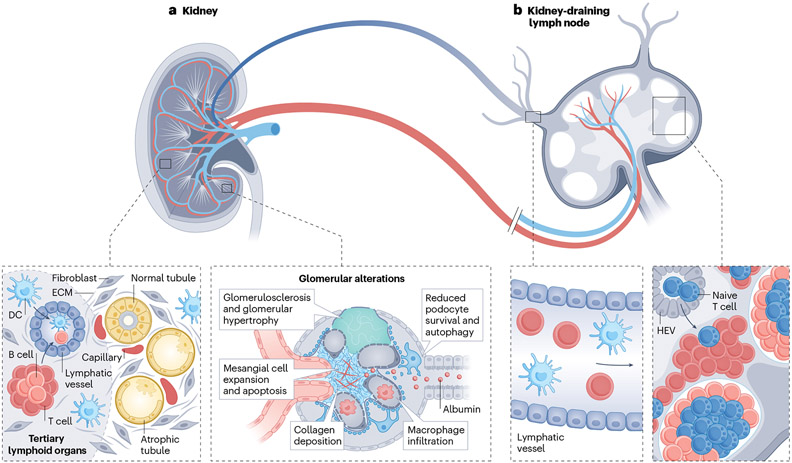
Fig. 3 ∣. Lymphoid organs in kidney disease.
a, Aged and chronically diseased kidneys can contain atrophic tubules, sclerotic glomeruli, and fibrosis, as well as tertiary lymphoid organs, which are lymphoid aggregates of T cells and B cells, high endothelial venules (HEVs) and lymphatic vessels. b, Kidney-resident dendritic cells (DCs) and T cells can circulate via lymphatic vessels from the kidney cortex to the kidney-draining lymph node (KLN), where they interact with T cells in the paracortex with the assistance of fibroblastic reticular cells. The fibroblastic reticular cells produce conduits composed of extracellular matrix (ECM), which provide structural support for the proliferation and expansion of HEVs, through which naïve T cells enter the KLN from the systemic circulation. Activation of T cells in the KLN is critical to the pathogenesis of glomerulonephritis, ischaemia–reperfusion injury and unilateral ureteral obstruction in mice.
Mesenchymal stromal cells that reside within lymph nodes are called FRCs. These cells perform a multitude of vital activities to maintain the proper functioning of the lymph node, including the deposition of extracellular matrix, support of blood vessels, facilitation of interactions between antigen-presenting cells and T cells, and promotion of lymphocyte survival103-108. A series of experiments demonstrated the importance of KLN FRCs in directing the immune response to a variety of kidney injuries. For example, in response to ischaemia-perfusion injury of the kidney, FRCs in the KLN of mice produce extracellular matrix fibres such as fibronectin. These fibres provide structural support for the proliferation and expansion of HEVs, which are lymph node-restricted segments of vasculature through which circulating naïve T cells enter from the blood108 (Fig. 3). Ablation of FRCs via administration of diphtheria toxin in transgenic Ccl19Cre × iDTR mice abrogated the expansion of HEVs and the activation of IFN-γ-producing CD4+ T cells in the KLN108. These changes were associated with a reduction in kidney damage following ischaemia–reperfusion injury108. Similar microarchitectural changes occurred in the KLNs of mice after the injection of nephrotoxic serum106. Similar to observations following ischaemia–reperfusion injury, ablation of FRCs in the KLN in Ccl19Cre × iDTR mice also ameliorated kidney injury following the injection of nephrotoxic serum106 or after unilateral ureteral obstruction107.
Of note, the phenotype of KLN FRCs changes with progression of kidney injury, such that at the end of the acute phase of the immune response to ischaemia–reperfusion injury, the FRCs support a more immunoregulatory response that dampens inflammation and promotes repair108. Use of a blocking antibody to lymphotoxin-β receptor (LTβR), a cardinal membrane protein expressed by FRCs that dictates their activity, before and after bilateral ischaemia–reperfusion injury, increased fibrogenic activity in the KLN and exacerbated kidney damage108. Together, these studies highlight the importance KLN FRCs in balancing the pro-inflammatory and regulatory immune responses in the kidney following kidney injury. The role of KLNs in shaping kidney inflammation in lupus nephritis and other autoimmune disorders remains unclear but is a topic of interest, given the role of KLNs in establishing immune tolerance to antigens and autoantigens produced in the kidney.
Cell crosstalk
Immune cells that infiltrate the kidney produce cytokines that orchestrate the phenotype and function of both resident immune cells and KPCs in nephritic kidneys43 (Fig. 4). Concomitantly, KPCs have the ability to sense pro-inflammatory signals, adopt immune functions and influence immune cells43. These interactions require engagement between cytokines and chemokines with receptors on KPCs to establish cross-communication between kidney-resident cells and immune cells109. Evidence from preclinical studies indicates that targeting of interactions between KPCs and immune cells in the kidney may be a promising strategy to attenuate renal inflammation in various forms of nephritis4,110.
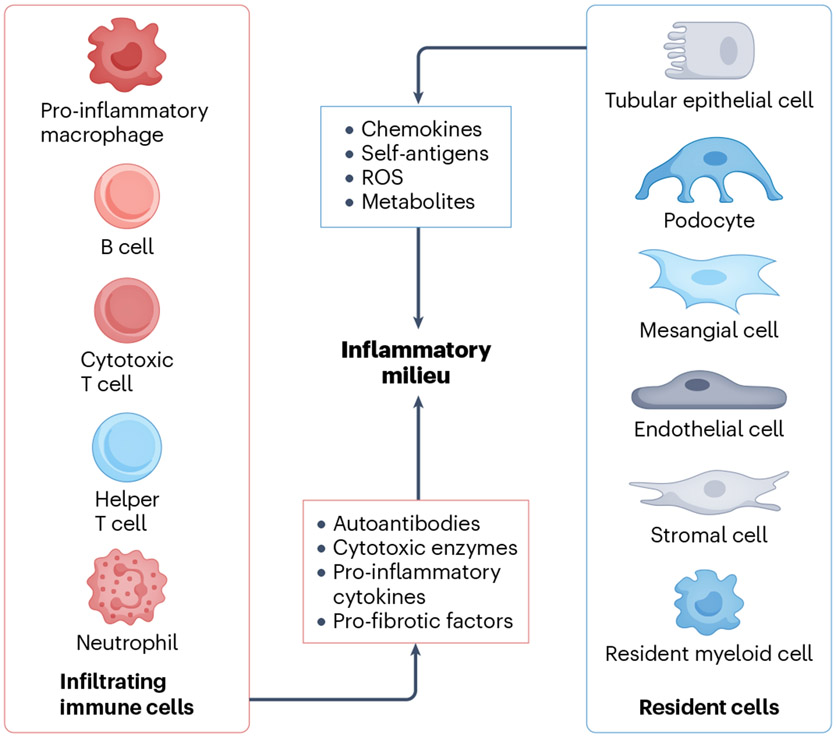
Fig. 4 ∣. Intercellular crosstalk within the kidneys between resident and infiltrating cells.
Immune cells attack tissue resident cells by producing various pathogenic factors such as antibodies, pro-inflammatory cytokines, pro-fibrotic factors and cytotoxic enzymes. Conversely, injured tissue resident cells, including resident immune cells and kidney parenchymal cells, respond by altering the local inflammatory milieu, which contributes further to the progression of renal inflammation. ROS, reactive oxygen species.
Lupus nephritis
TECs, mesangial cells and podocytes are injured in lupus nephritis, demonstrating alterations in their cellular metabolism and increased susceptibility to cell death4,39,111,112. These changes, along with aggravation of hypoxia, establish a pro-inflammatory microenvironment to which infiltrating immune cells must adapt37,113. For example, under physiological conditions, TECs maintain an immunosuppressive environment by limiting the local availability of metabolites, such as arginine4. Stimulation with IL-23 leads to the loss of this immunosuppressive ability and the increased availability of arginine for infiltrating immune cells4 resulting in the acquisition of a pro-inflammatory phenotype, characterized by the production of pro-inflammatory cytokines and chemokines114. Levels of IL-23 are, accordingly, elevated in the sera of patients with LN115 and probably also in the sera of patients with other types of glomerulonephritis.
Enhanced ferroptosis has been observed in TECs of biopsy samples from patients with lupus nephritis111. This type of cell death may be more immunogenic than other forms of necrosis by accelerating the release of self-antigens to promote and sustain local inflammation25,116. Mesangial cells and podocytes can also present antigens and produce pro-inflammatory cytokines and chemokines in the context of lupus nephritis117-119. The application of multiplexed confocal microscopy to kidney samples from patients with lupus nephritis has revealed the presence of immune cell clusters involving different types of lymphocytes in different regions of the kidney37. Notably, B cells, often along with CD4+ T cells, are organized into large cell clusters and reside in the periglomerular area, whereas CD4− T cells are always found in the interstitium within relatively small cell clusters37. This observation suggests the existence of diverse microenvironment settings in different areas of inflamed kidneys, potentially contributing to distinct types of inflammatory responses.
Acute kidney injury
AKI is a common clinical entity that is characterized by injury, predominantly of proximal TECs120. In an ideal scenario, the injured tissue undergoes self-repair mechanisms in a process that involves the activation of reparative macrophages to clear cell debris and the proliferation of surviving tubular cells, resulting in the restoration of the nephron structure121. However, failed tubule repair in the early phase of AKI can trigger a second wave of inflammation characterized by the persistent accumulation of macrophages and increased influx of neutrophils and T cells to the kidneys, which facilitate the transition from AKI to CKD122-124. This perpetuating process indicates the presence of intricate crosstalk between proximal TECs and various immune cell populations. Moreover, the heightened expression of T cell immunoglobulin and ITIM domain (TIGIT) on kidney infiltrating T cells, which has been observed in AKI125, might contribute to the adaptive response to the local microenvironment by altering intrinsic T cell metabolism. It would be of interest to investigate which kidney-resident cells engage with infiltrating T cells by expressing the TIGIT ligands CD155 (PVR) and CD112 (PVRL2).
Kidney allograft rejection
Kidney allograft rejection initiates when a donor-derived alloantigen induces an immune response against the graft; TECs are one of the main targets of inflammatory insults during this process. Kidney epithelial cells display all the factors necessary to activate CD4+ T cells, such as HLA-DR, CD80 and adhesion molecules126,127. The infiltration of different types of immune cells, especially T cells and macrophages, into the interstitium is observed in rejected grafts. DCs, NK cells, B cells and plasma cells are also present albeit in lower numbers128. Interestingly, interstitial inflammation is more frequent and severe in the cortex than in the medulla, suggesting the presence of distinct immune environments within different renal compartments129. This observation is further supported by the finding that DCs in the medulla are less well equipped than bone marrow-derived DCs to process and present antigen130. In contrast to acute rejection, wherein immune infiltrates are distributed throughout the entire kidney, chronic rejection is characterized by the formation of organized TLOs131,132. Notably, these TLOs can be identified within the grafts before the lesions develop, although studies that have examined the correlation between the formation of intrarenal TLOs and chronic rejection have reached conflicting conclusions133. Crosstalk between KPCs and immune infiltrates in different regions of the kidney, which are characterized by distinct physicochemical microenvironments, may be diverse and the processes leading to the formation of TLOs distinctly different. However, it remains unclear whether these TLOs possess pathogenic or regulatory capacity.
ANCA-associated vasculitis
Small vessels in the kidney, including capillaries, venules and arterioles are the main targets of immune cell infiltrates in ANCA-associated vasculitis. Proteinase 3 is one of the antigens recognized by ANCA and is expressed by neutrophils. Moreover, evidence from experimental models suggests that resident CD4+ T cells, especially Th17 cells, can promote the progression of ANCA-associated glomerulonephritis through the production of IL-17 (ref. 54). As described earlier, TECs can produce chemokines in response to inflammatory cytokines4,110, and may therefore participate in cellular crosstalk in inflamed kidneys to exacerbate glomerulonephritis progression. Insights into such crosstalk may facilitate the development of targeted therapies for patients with vasculitis.
Targeting KPCs to treat glomerulonephritis
Mainstay treatment strategies for patients with immune-mediated diseases include classic immunosuppressive drugs, such as prednisone and cyclophosphamide, and biologics, which interfere with the function of immune cells. Precision medicine calls for the delivery of the right drug, to the right patient, at the right time. However, the idea that a precision medicine approach could be applied to specific cells or organs to avert or mitigate injury has been insufficiently explored.
We previously established that the Ser/Thr kinase, CaMK4, which is expressed in T cells in patients with SLE, increases the production of pro-inflammatory IL-17 and decreases the production of immunoregulatory IL-2 through distinct epigenetic modulation of the two gene loci134,135. Genetic, or small molecule inhibition of CaMK4 suppressed autoimmunity and nephritis in lupus-prone mice3,4,134. More importantly, cell-targeted delivery of a CaMK4 inhibitor to CD4+ T cells, suppressed autoimmunity and nephritis in lupus-prone mice136. Based on the finding that podocytes from lupus-prone mice and patients with lupus nephritis express increased amounts of CaMK4, and that CaMK4 can suppress the expression of nephrin and enable the destruction of synaptopodin, thereby compromising podocyte structure3,137, we attempted podocyte-specific delivery of a CaMK4 inhibitor in MRL/lpr lupus-prone mice using nanoparticles loaded with a small drug inhibitor and tagged with nephrin or podocin antibodies. Indeed, such cell-specific treatment averted the development of lupus nephritis, including the deposition of immune complexes. Interestingly, humoral and cellular elements of the systemic autoimmune response remained intact3, suggesting that the glomerular deposition of immune deposits may only occur under conditions of compromised podocyte function.
We subsequently found that TECs from MRl/lpr lupus-prone mice also expressed increased amounts of CaMK4. Administration of nanoparticles loaded with a CaMK4 inhibitor targeted to TECs mitigated proteinuria and interstitial inflammation, again, without affecting systemic autoimmunity4. This observation suggests that TECs may behave as pro-inflammatory cells in lupus nephritis by attracting immune inflammatory cells and that suppression of CaMK4 mitigates this activity. As discussed above, TECs lose their immunosuppressive phenotype following stimulation with IL-23 (ref. 4). Specifically, binding of IL-23 to the IL-23 receptor expressed on the surface of TECs upregulates CaMK4 expression and alters their metabolic profile, which may enable the accumulation of immune cells4.
As described earlier, HEVs are specialized segments of the vasculature that are unique to secondary lymphoid organs, such as the lymph nodes and tonsils138. They are the gateways through which naïve T cells enter these organs, and they possess a family of membrane glycoproteins called peripheral node addressin (PNAd), which are the receptors for L-selectin, a surface protein expressed by naïve T cells138. Also, as described earlier, KLNs are the main sites for modulation of the adaptive immune response to renal damage following common types of kidney injury, such as ischaemia–reperfusion injury and glomerulonephritis106-108. Therefore, HEVs represent an attractive target for precision therapies directed at manipulating the local immune response in the context of kidney injury.
Targeted nanoparticles have already been created to target HEVs; future studies could use these carriers to deliver therapeutics to KLNs. In a mouse model of heart transplantation, delivery of a PNAd-binding IgM antibody, MECA-79, conjugated to a polymeric nanoparticle and loaded with the immunoregulatory molecule anti-CD3 – a nanoparticle that localized to activated allograft-draining lymph nodes – increased the population of T regulatory cells within draining lymph nodes and improved allograft survival139. Another antibody, MHA112, binds to PNAd with greater affinity than MECA-79; antibody–drug conjugates comprising MHA112 and the anticancer drug paclitaxel successfully targeted HEVs in tumour-draining lymph nodes, reducing tumour size and metastatic lesions140.
These preliminary successes provide proof of concept that a targeted, precision medicine approach could be used in the treatment of immune-mediated kidney diseases, either through the delivery of a targeted therapeutic to the specific cell or organ that undergoes injury or participates in perpetuation of the injurious response, or to local lymph nodes to modulate immune and autoimmune responses.
Remaining questions and future directions
There is no doubt that inflammation in the kidney occurs after immune tolerance has been lost in the periphery, or in response to an alloantigen or to kidney injury. However, it is increasingly apparent that kidney-resident cells themselves have an active role in modulating and propagating the inflammatory response. Better understanding of these novel concepts is needed as we strive to treat individuals with kidney inflammation more effectively.
As described earlier, KPCs express molecules that are shared with immune cells and the expression of these molecules is modulated during inflammation. For example, TECs or podocytes express receptors for IL-23. Activation of these receptors by circulating IL-23 can initiate a signalling process that alters their metabolism and the distribution of metabolites in the surrounding milieu. As mentioned above, IL-23 inhibits the ability of TECs to degrade arginine, which increases its availability as a fuel to infiltrating immune cells4. Technological advances should improve our understanding of the metabolic alterations that occur in the interstitium and pericellular space of the injured kidney and provide insights into how metabolites may alter the function of infiltrating immune cells141.
Podocytes, in response to aberrantly glycosylated IgG, upregulate the expression of costimulatory molecules along with major histocompatibility antigens. From an immunology point of view, podocytes exposed to autoantibodies or other inflammatory signals, may function as bona fide antigen-presenting cells and thereby inform and modulate passing lymphocytes. Whether kidney-infiltrating lymphocytes at this point have already differentiated into pro-inflammatory cells in the spleen or other secondary lymphoid organs, or if they are stimulated and become pathogenic after they enter the kidney, is unclear. We also do not know the spectrotype of the T cell receptors of infiltrating cells beyond the fact that they are limited4. Moreover, the nature of the autoantigen or antigen that they recognize is still unknown. However, we know that the kidney environment promotes a pro-inflammatory milieu for the invading T cells. Increased hypoxia in the context of LN promotes the differentiation of Th17 cells through activation of HI1α39; high levels of sodium in the interstitial space may also contribute to the generation of such cells142,143. A hypothesis to be further tested is that T cells are presented with antigen in situ and the unique kidney environment enables their differentiation into pro-inflammatory cells.
The clinical relevance of the formation of TLOs by infiltrating immune cells has been established, and the formation of such structures are predictors of poor kidney function37. However, the sequence of events leading to their formation is still unknown. HEVs are of structural and functional importance in the formation of secondary lymphoid organs, and we can predict that their appearance would facilitate the formation of TLOs in the kidney. However, we do not know what drives the appearance of HEVs in the kidney. We also do not know whether TLOs that form in the renal pelvis or cortex area differ in their cell composition and clinical importance. TECs in the renal pelvis upregulate molecules that facilitate the entry of neutrophils60, which has teleological value given the susceptibility of this region to infections. However, in-depth characterization of infiltrating lymphocytes in the context of inflammatory disease is needed. Beyond the fact that some of these cells produce IL-17 (ref. 144), our understanding is fairly limited.
The presence of B cells in the kidney tissue of patients with lupus nephritis has been proposed to protect against kidney failure37, which is counterintuitive given the importance of B cells in the pathogenesis of autoimmunity. However, these cells may be regulatory in nature or produce protective cytokines24. We do not know whether regulatory T cells are present, whether they can be expanded within the kidney tissue145 or whether they can be bestowed with tissue repair capabilities146,147. Last, we do not know how lymphocytes situated adjacent to KPCs influence their transcriptomic profile through cognate interactions. The application of new technologies, such as high-resolution spatial imaging should provide detailed insights into the interactions between resident and infiltrating cells.
An interesting concept is that in the setting of a systemic autoimmune disease, the kidney is not injured unless certain biochemical or metabolic processes are set in motion. This concept reflects clinical experience wherein not all individuals with systemic autoimmunity develop nephritis14. Our own studies have shown that podocytes, TECs and mesangial cells suffer injury only when they can upregulate CaMK4. These findings imply that if a patient with SLE cannot upregulate CaMK4 because of genetic, epigenetic, hormonal or other reasons, then they will not develop nephritis. Obviously, other molecules besides CaMK4 are likely involved in the injury of KPCs, and their regulation will likely be of comparable importance. This line of reasoning supports a therapeutic approach that enables the modulation of molecules that are specific to kidney-resident cells, including KPCs and kidney-resident immune cells. The discovery of additional pathways that are involved in the injury of KPCs in the setting of systemic autoimmunity or other inflammatory processes will enrich our understanding of mechanistic pathways and identify new approaches to treating patients with immune-mediated or inflammatory kidney diseases.
Conclusions
Inflammation in the kidney occurs after loss of immune tolerance in the periphery, or in response to an alloantigen or kidney injury, and can lead to the development of CKD and kidney failure. The inflammatory response may involve the deposition of antibody, complement and the infiltration of cells of the innate and adaptive immune systems. These infiltrating cells may expand their pro-inflammatory capacity in the hypoxic and hyperosmotic kidney interstitium, although some infiltrating cells might possess regulatory activity. KPCs can express molecules that classically belong to cells of the immune system. Such molecules most probably facilitate crosstalk between infiltrating immune and parenchymal cells. Available evidence suggests that immune cells can alter the transcriptional profile of KPCs, promoting the expression of immune molecules. Conversely, KPCs can attract and retain inflammatory cells. Upregulation of specific signalling molecules, such as CaMK4, by KPCs may be needed for cell and organ injury to occur; hence, targeted inhibition of these molecules may open up new precision medicine approaches to the treatment of kidney inflammation and disease. KLNs seem to be important for the development of kidney inflammation as they represent the site in which tolerance to kidney-derived autoantigens is developed. Targeting the immune response in the KLNs should offer additional therapeutic options.
Key points.
Non-myeloid kidney parenchymal cells (KPCs) express molecules that are shared with immune cells; the expression of these molecules may have distinct topological associations and may increase in response to inflammation.
Kidney injury can induce the infiltration of immune cells; these cells may have protective or pro-inflammatory features depending on the phase of the disease process. In addition, kidney-resident immune cells may have a surveillance or regulatory role in the kidney but can accumulate in response to injury.
Components of the immune system, such as autoantibodies, cytokines and immune cells, can influence the metabolic profile of KPCs in the kidney.
Conversely, KPCs can contribute to inflammation by providing metabolites or through the secretion of chemokines and cytokines.
The cortex and the medulla of kidney tissue can contain distinct types of tertiary lymphoid organs, which may possess pathogenic or regulatory properties.
Targeted delivery of drugs to KPCs holds promise for preventing or reversing the inflammatory response in specific forms of kidney injury and disease.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Pisetsky DS Pathogenesis of autoimmune disease. Nat. Rev. Nephrol 19, 509–524 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohan C, Zhang T & Putterman C Pathogenic cellular and molecular mediators in lupus nephritis. Nat. Rev. Nephrol 19, 491–508 (2023). [DOI] [PubMed] [Google Scholar]
- 3.Maeda K. et al. CaMK4 compromises podocyte function in autoimmune and nonautoimmune kidney disease. J. Clin. Invest 128, 3445–3459 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li H. et al. IL-23 reshapes kidney resident cell metabolism and promotes local kidney inflammation. J. Clin. Invest 131, e142428 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsokos GC Systemic lupus erythematosus. N. Engl. J. Med 365, 2110–2121 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Davidson A. What is damaging the kidney in lupus nephritis? Nat. Rev. Rheumatol 12, 143–153 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsokos GC Autoimmunity and organ damage in systemic lupus erythematosus. Nat. Immunol 21, 605–614 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Urbonaviciute V. et al. Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE. J. Exp. Med 205, 3007–3018 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suurmond J & Diamond B Autoantibodies in systemic autoimmune diseases: specificity and pathogenicity. J. Clin. Invest 125, 2194–2202 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vlahakos DV et al. Anti-DNA antibodies form immune deposits at distinct glomerular and vascular sites. Kidney Int. 41, 1690–1700 (1992). [DOI] [PubMed] [Google Scholar]
- 11.Jamaly S, Rakaee M, Abdi R, Tsokos GC & Fenton KA Interplay of immune and kidney resident cells in the formation of tertiary lymphoid structures in lupus nephritis. Autoimmun. Rev 20, 102980 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Sato Y, Silina K, van den Broek M, Hirahara K & Yanagita M The roles of tertiary lymphoid structures in chronic diseases. Nat. Rev. Nephrol 19, 525–537 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Bavel CC, Fenton KA, Rekvig OP, van der Vlag J & Berden JH Glomerular targets of nephritogenic autoantibodies in systemic lupus erythematosus. Arthritis Rheum. 58, 1892–1899 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Ge Y. et al. Cgnz1 allele confers kidney resistance to damage preventing progression of immune complex-mediated acute lupus glomerulonephritis. J. Exp. Med 210, 2387–2401 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vukelic M, Li Y & Kyttaris VC Novel treatments in lupus. Front. Immunol 9, 2658 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li H & Tsokos GC IL-23/IL-17 axis in inflammatory rheumatic diseases. Clin. Rev. Allergy Immunol 60, 31–45 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz N, Goilav B & Putterman C The pathogenesis, diagnosis and treatment of lupus nephritis. Curr. Opin. Rheumatol 26, 502–509 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krausgruber T. et al. Structural cells are key regulators of organ-specific immune responses. Nature 583, 296–302 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen CP et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet 371, 2173–2182 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Akchurin OM & Kaskel F Update on inflammation in chronic kidney disease. Blood Purif. 39, 84–92 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Hsieh C. et al. Predicting outcomes of lupus nephritis with tubulointerstitial inflammation and scarring. Arthritis Care Res. 63, 865–874 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez-Iturbe B, Johnson RJ & Herrera-Acosta J Tubulointerstitial damage and progression of renal failure. Kidney Int. Suppl 68, S82–S86 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez-Iturbe B & Garcia Garcia G The role of tubulointerstitial inflammation in the progression of chronic renal failure. Nephron Clin. Pract 116, c81–88, (2010). [DOI] [PubMed] [Google Scholar]
- 24.Li H, Tsokos MG & Tsokos GC Lymphocytes in the neighborhood: good or bad for the kidney? J. Clin. Invest 132, e160657-, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurts C, Ginhoux F & Panzer U Kidney dendritic cells: fundamental biology and functional roles in health and disease. Nat. Rev. Nephrol 16, 391–407 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Tang PM, Nikolic-Paterson DJ & Lan HY Macrophages: versatile players in renal inflammation and fibrosis. Nat. Rev. Nephrol 15, 144–158 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Salei N. et al. The kidney contains ontogenetically distinct dendritic cell and macrophage subtypes throughout development that differ in their inflammatory properties. J. Am. Soc. Nephrol 31, 257–278 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davidson A. Renal mononuclear phagocytes in lupus nephritis. ACR Open. Rheumatol 3, 442–450 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe S, Alexander M, Misharin AV & Budinger GRS The role of macrophages in the resolution of inflammation. J. Clin. Invest 129, 2619–2628 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen T, Cao Q, Wang Y & Harris DCH M2 macrophages in kidney disease: biology, therapies, and perspectives. Kidney Int. 95, 760–773 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Arazi A. et al. The immune cell landscape in kidneys of patients with lupus nephritis. Nat. Immunol 20, 902–914 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao DA, Arazi A, Wofsy D & Diamond B Design and application of single-cell RNA sequencing to study kidney immune cells in lupus nephritis. Nat. Rev. Nephrol 16, 238–250 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klose CSN & Artis D Innate lymphoid cells control signaling circuits to regulate tissue-specific immunity. Cell Res. 30, 475–491 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rabb H. The T cell as a bridge between innate and adaptive immune systems: implications for the kidney. Kidney Int. 61, 1935–1946 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Shipman WD, Dasoveanu DC & Lu TT Tertiary lymphoid organs in systemic autoimmune diseases: pathogenic or protective? F1000Res 6, 196 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang A. et al. In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J. Immunol 186, 1849–1860 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abraham R. et al. Specific in situ inflammatory states associate with progression to renal failure in lupus nephritis. J. Clin. Invest 132, e155350 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aloisi F & Pujol-Borrell R Lymphoid neogenesis in chronic inflammatory diseases. Nat. Rev. Immunol 6, 205–217 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Chen PM et al. Kidney tissue hypoxia dictates T cell-mediated injury in murine lupus nephritis. Sci. Transl. Med 12, eaay1620 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li H & Tsokos GC Double-negative T cells in autoimmune diseases. Curr. Opin. Rheumatol 33, 163–172 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen A. et al. Bowman’s capsule provides a protective niche for podocytes from cytotoxic CD8+ T cells. J. Clin. Invest 128, 3413–3424 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.do Valle Duraes F. et al. Immune cell landscaping reveals a protective role for regulatory T cells during kidney injury and fibrosis. JCI Insight 5, e130651 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhargava R, Li H & Tsokos GC Pathogenesis of lupus nephritis: the contribution of immune and kidney resident cells. Curr. Opin. Rheumatol 35, 107–116 (2023). [DOI] [PubMed] [Google Scholar]
- 44.Liao X, Pirapakaran T & Luo XM Chemokines and chemokine receptors in the development of lupus nephritis. Mediators Inflamm. 2016, 6012715 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Worthmann K. et al. Pathogenetic role of glomerular CXCL13 expression in lupus nephritis. Clin. Exp. Immunol 178, 20–27 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lema et al. Chemokine expression precedes inflammatory cell infiltration and chemokine receptor and cytokine expression during the initiation of murine lupus nephritis. J. Am. Soc. Nephrol 12, 1369–1382 (2001). [DOI] [PubMed] [Google Scholar]
- 47.Kolodziejczyk AA, Kim JK, Svensson V, Marioni JC & Teichmann SA The technology and biology of single-cell RNA sequencing. Mol. Cell 58, 610–620 (2015). [DOI] [PubMed] [Google Scholar]
- 48.Park J. et al. Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science 360, 758–763 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stewart BJ et al. Spatiotemporal immune zonation of the human kidney. Science 365, 1461–1466 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeng H, Yang X, Luo S & Zhou Y The advances of single-cell RNA-seq in kidney immunology. Front. Physiol 12, 752679 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fu J. et al. Single-cell RNA profiling of glomerular cells shows dynamic changes in experimental diabetic kidney disease. J. Am. Soc. Nephrol 30, 533–545 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson PC et al. The single-cell transcriptomic landscape of early human diabetic nephropathy. Proc. Natl Acad. Sci. USA 116, 19619–19625 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zheng Y. et al. Single-cell transcriptomics reveal immune mechanisms of the onset and progression of IgA nephropathy. Cell Rep. 33, 108525 (2020). [DOI] [PubMed] [Google Scholar]
- 54.Krebs CF et al. Pathogen-induced tissue-resident memory TH17 (TRM17) cells amplify autoimmune kidney disease. Sci. Immunol 5, eaba4163 (2020). [DOI] [PubMed] [Google Scholar]
- 55.Der E. et al. Tubular cell and keratinocyte single-cell transcriptomics applied to lupus nephritis reveal type I IFN and fibrosis relevant pathways. Nat. Immunol 20, 915–927 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sigdel TK et al. Near-single-cell proteomics profiling of the proximal tubular and glomerulus of the normal human kidney. Front. Med 7, 499 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lake BB et al. An atlas of healthy and injured cell states and niches in the human kidney. Nature 619, 585–594 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xia H, Bao W & Shi S Innate immune activity in glomerular podocytes. Front. Immunol 8, 122 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bhargava R & Tsokos GC The immune podocyte. Curr. Opin. Rheumatol 31, 167–174 (2019). [DOI] [PubMed] [Google Scholar]
- 60.Hong S, Healy H & Kassianos AJ The emerging role of renal tubular epithelial cells in the immunological pathophysiology of lupus nephritis. Front. Immunol 11, 578952 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang C. et al. Activation of Nod-like receptor protein 3 inflammasomes turns on podocyte injury and glomerular sclerosis in hyperhomocysteinemia. Hypertension 60, 154–162 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Flur K. et al. Viral RNA induces type I interferon-dependent cytokine release and cell death in mesangial cells via melanoma-differentiation-associated gene-5: implications for viral infection-associated glomerulonephritis. Am. J. Pathol 175, 2014–2022 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patole PS et al. Expression and regulation of Toll-like receptors in lupus-like immune complex glomerulonephritis of MRL-Faslpr mice. Nephrol. Dial. Transpl 21, 3062–3073 (2006). [DOI] [PubMed] [Google Scholar]
- 64.Yung S, Cheung KF, Zhang Q & Chan TM Anti-dsDNA antibodies bind to mesangial annexin II in lupus nephritis. J. Am. Soc. Nephrol 21, 1912–1927 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yung S & Chan TM Anti-dsDNA antibodies and resident renal cells — their putative roles in pathogenesis of renal lesions in lupus nephritis. Clin. Immunol 185, 40–50 (2017). [DOI] [PubMed] [Google Scholar]
- 66.Kanapathippillai P, Hedberg A, Fenton CG & Fenton KA Nucleosomes contribute to increase mesangial cell chemokine expression during the development of lupus nephritis. Cytokine 62, 244–252 (2013). [DOI] [PubMed] [Google Scholar]
- 67.Ichinose K. et al. Cutting edge: calcium/calmodulin-dependent protein kinase type IV is essential for mesangial cell proliferation and lupus nephritis. J. Immunol 187, 5500–5504 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Groza Y, Jemelkova J, Kafkova LR, Maly P & Raska M IL-6 and its role in IgA nephropathy development. Cytokine Growth Factor. Rev 66, 1–14 (2022). [DOI] [PubMed] [Google Scholar]
- 69.Lai KN et al. IgA nephropathy. Nat. Rev. Dis. Prim 2, 16001 (2016). [DOI] [PubMed] [Google Scholar]
- 70.Coers W. et al. Podocyte expression of MHC class I and II and intercellular adhesion molecule-1 (ICAM-1) in experimental pauci-immune crescentic glomerulonephritis. Clin. Exp. Immunol 98, 279–286 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goldwich A. et al. Podocytes are nonhematopoietic professional antigen-presenting cells. J. Am. Soc. Nephrol 24, 906–916 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pyzik M, Rath T, Lencer WI, Baker K & Blumberg RS FcRn: the architect behind the immune and nonimmune functions of IgG and albumin. J. Immunol 194, 4595–4603 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haddad G, Dylewski J, Evans R, Lewis L & Blaine J Knockout of the neonatal Fc receptor alters immune complex trafficking and lysosomal function in cultured podocytes. PLoS One 18, e0284636 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu M & Zen K Toll-like receptors regulate the development and progression of renal diseases. Kidney Dis. 7, 14–23 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kuravi SJ et al. Podocytes regulate neutrophil recruitment by glomerular endothelial cells via IL-6-mediated crosstalk. J. Immunol 193, 234–243 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ren J. et al. Twist1 in podocytes ameliorates podocyte injury and proteinuria by limiting CCL2-dependent macrophage infiltration. JCI Insight 6, e148109 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bruno V, Muhlig AK, Oh J & Licht C New insights into the immune functions of podocytes: the role of complement. Mol. Cell Pediatr 10, 3 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li X, Ding F, Zhang X, Li B & Ding J The expression profile of complement components in podocytes. Int. J. Mol. Sci 17, 471 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Karasawa T. et al. Glomerular endothelial expression of type I IFN-stimulated gene, DExD/H-Box helicase 60 via toll-like receptor 3 signaling: possible involvement in the pathogenesis of lupus nephritis. Ren. Fail 44, 137–145 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hirono K. et al. Endothelial expression of fractalkine (CX3CL1) is induced by Toll-like receptor 3 signaling in cultured human glomerular endothelial cells. Mod. Rheumatol 30, 1074–1081 (2020). [DOI] [PubMed] [Google Scholar]
- 81.Dimou P. et al. The human glomerular endothelial cells are potent pro-inflammatory contributors in an in vitro model of lupus nephritis. Sci. Rep 9, 8348 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kassianos AJ et al. Human proximal tubule epithelial cells modulate autologous dendritic cell function. Nephrol. Dial. Transpl 28, 303–312 (2013). [DOI] [PubMed] [Google Scholar]
- 83.Sampangi S. et al. Human proximal tubule epithelial cells modulate autologous B-cell function. Nephrol. Dial. Transpl 30, 1674–1683 (2015). [DOI] [PubMed] [Google Scholar]
- 84.Kassianos AJ et al. Fractalkine-CX3CR1-dependent recruitment and retention of human CD1c+ myeloid dendritic cells by in vitro-activated proximal tubular epithelial cells. Kidney Int. 87, 1153–1163 (2015). [DOI] [PubMed] [Google Scholar]
- 85.Starke A. et al. Renal tubular PD-L1 (CD274) suppresses alloreactive human T-cell responses. Kidney Int. 78, 38–47 (2010). [DOI] [PubMed] [Google Scholar]
- 86.Castellano G. et al. Local synthesis of interferon-alpha in lupus nephritis is associated with type I interferons signature and LMP7 induction in renal tubular epithelial cells. Arthritis Res. Ther 17, 72 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schwarting A. et al. Renal tubular epithelial cell-derived BAFF expression mediates kidney damage and correlates with activity of proliferative lupus nephritis in mouse and men. Lupus 27, 243–256 (2018). [DOI] [PubMed] [Google Scholar]
- 88.Mistry P & Kaplan MJ Cell death in the pathogenesis of systemic lupus erythematosus and lupus nephritis. Clin. Immunol 185, 59–73 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jia P. et al. Chemokine CCL2 from proximal tubular epithelial cells contributes to sepsis-induced acute kidney injury. Am. J. Physiol. Renal Physiol 323, F107–F119 (2022). [DOI] [PubMed] [Google Scholar]
- 90.Ijima S. et al. Fisetin reduces the senescent tubular epithelial cell burden and also inhibits proliferative fibroblasts in murine lupus nephritis. Front. Immunol 13, 960601 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Paragas N. et al. α-Intercalated cells defend the urinary system from bacterial infection. J. Clin. Invest 124, 2963–2976 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gauer S. et al. IL-18 is expressed in the intercalated cell of human kidney. Kidney Int. 72, 1081–1087 (2007). [DOI] [PubMed] [Google Scholar]
- 93.Breton S & Battistone MA Unexpected participation of intercalated cells in renal inflammation and acute kidney injury. Nephron 146, 268–273 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dorraji SE et al. Mesenchymal stem cells and T cells in the formation of tertiary lymphoid structures in lupus nephritis. Sci. Rep 8, 7861 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang N & Perl A Metabolism as a target for modulation in autoimmune diseases. Trends Immunol. 39, 562–576 (2018). [DOI] [PubMed] [Google Scholar]
- 96.Aufhauser DD Jr et al. Improved renal ischemia tolerance in females influences kidney transplantation outcomes. J. Clin. Invest 126, 1968–1977 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McEvoy CM et al. Single-cell profiling of healthy human kidney reveals features of sex-based transcriptional programs and tissue-specific immunity. Nat. Commun 13, 7634 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao JL, Qiao XH, Mao JH, Liu F & Fu HD The interaction between cellular senescence and chronic kidney disease as a therapeutic opportunity. Front. Pharmacol 13, 974361 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.O’Sullivan ED, Hughes J & Ferenbach DA Renal aging: causes and consequences. J. Am. Soc. Nephrol 28, 407–420 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sato Y. et al. CD153/CD30 signaling promotes age-dependent tertiary lymphoid tissue expansion and kidney injury. J. Clin. Invest 132, e146071 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gottschalk C. et al. Batf3-dependent dendritic cells in the renal lymph node induce tolerance against circulating antigens. J. Am. Soc. Nephrol 24, 543–549 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dong X. et al. Antigen presentation by dendritic cells in renal lymph nodes is linked to systemic and local injury to the kidney. Kidney Int. 68, 1096–1108 (2005). [DOI] [PubMed] [Google Scholar]
- 103.Fletcher AL, Acton SE & Knoblich K Lymph node fibroblastic reticular cells in health and disease. Nat. Rev. Immunol 15, 350–361 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lutge M, Pikor NB & Ludewig B Differentiation and activation of fibroblastic reticular cells. Immunol. Rev 302, 32–46 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Perez-Shibayama C, Gil-Cruz C & Ludewig B Fibroblastic reticular cells at the nexus of innate and adaptive immune responses. Immunol. Rev 289, 31–41 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kasinath V. et al. Activation of fibroblastic reticular cells in kidney lymph node during crescentic glomerulonephritis. Kidney Int. 95, 310–320 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li X. et al. Kidney-draining lymph node fibrosis following unilateral ureteral obstruction. Front. Immunol 12, 768412 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maarouf OH et al. Repetitive ischemic injuries to the kidneys result in lymph node fibrosis and impaired healing. JCI Insight 3, e120546 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Der E, Suryawanshi H, Buyon J, Tuschl T & Putterman C Single-cell RNA sequencing for the study of lupus nephritis. Lupus Sci. Med 6, e000329 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Disteldorf EM et al. CXCL5 drives neutrophil recruitment in TH17-mediated GN. J. Am. Soc. Nephrol 26, 55–66 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alli AA et al. Kidney tubular epithelial cell ferroptosis links glomerular injury to tubulointerstitial pathology in lupus nephritis. Clin. Immunol 248, 109213 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Giuliani KTK et al. Hypoxic human proximal tubular epithelial cells undergo ferroptosis and elicit an NLRP3 inflammasome response in CD1c+ dendritic cells. Cell Death Dis. 13, 739 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li H, Boulougoura A, Endo Y & Tsokos GC Abnormalities of T cells in systemic lupus erythematosus: new insights in pathogenesis and therapeutic strategies. J. Autoimmun 132, 102870 (2022). [DOI] [PubMed] [Google Scholar]
- 114.Krebs CF, Turner JE, Riedel JH & Panzer U Tissue-specific therapy in immune-mediated kidney diseases: new ARGuments for targeting the IL-23/IL-17 axis. J. Clin. Invest 131, e150588 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dai H, He F, Tsokos GC & Kyttaris VC IL-23 limits the production of IL-2 and promotes autoimmunity in lupus. J. Immunol 199, 903–910 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li P. et al. Glutathione peroxidase 4-regulated neutrophil ferroptosis induces systemic autoimmunity. Nat. Immunol 22, 1107–1117 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yu H. et al. Mesangial cells exhibit features of antigen-presenting cells and activate CD4+ T cell responses. J. Immunol. Res 2019, 2121849 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fu R. et al. Podocyte activation of NLRP3 inflammasomes contributes to the development of proteinuria in lupus nephritis. Arthritis Rheumatol. 69, 1636–1646 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wright RD & Beresford MW Podocytes contribute, and respond, to the inflammatory environment in lupus nephritis. Am. J. Physiol. Renal Physiol 315, F1683–F1694 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kellum JA et al. Acute kidney injury. Nat. Rev. Dis. Prim 7, 52 (2021). [DOI] [PubMed] [Google Scholar]
- 121.He L. et al. AKI on CKD: heightened injury, suppressed repair, and the underlying mechanisms. Kidney Int. 92, 1071–1083 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Venkatachalam MA, Weinberg JM, Kriz W & Bidani AK Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J. Am. Soc. Nephrol 26, 1765–1776 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Puthumana J. et al. Biomarkers of inflammation and repair in kidney disease progression. J. Clin. Invest 131, e139927 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xu L, Guo J, Moledina DG & Cantley LG Immune-mediated tubule atrophy promotes acute kidney injury to chronic kidney disease transition. Nat. Commun 13, 4892 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Noel S. et al. Immune checkpoint molecule TIGIT regulates kidney T cell functions and contributes to AKI. J. Am. Soc. Nephrol 34, 755–771 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Eleftheriadis T. et al. A role for human renal tubular epithelial cells in direct allo-recognition by CD4+ T-cells and the effect of ischemia-reperfusion. Int. J. Mol. Sci 22, 1733 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Breda PC et al. Renal proximal tubular epithelial cells exert immunomodulatory function by driving inflammatory CD4+ T cell responses. Am. J. Physiol. Renal Physiol 317, F77–F89 (2019). [DOI] [PubMed] [Google Scholar]
- 128.Metalidis C & Kuypers DR Emerging immunosuppressive drugs in kidney transplantation. Curr. Clin. Pharmacol 6, 130–136 (2011). [DOI] [PubMed] [Google Scholar]
- 129.Sis B. et al. Renal medullary changes in renal allograft recipients with raised serum creatinine. J. Clin. Pathol 59, 377–381 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chessa F. et al. The renal microenvironment modifies dendritic cell phenotype. Kidney Int. 89, 82–94 (2016). [DOI] [PubMed] [Google Scholar]
- 131.Thaunat O. et al. Lymphoid neogenesis in chronic rejection: evidence for a local humoral alloimmune response. Proc. Natl Acad. Sci. USA 102, 14723–14728 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Thaunat O. et al. Chronic rejection triggers the development of an aggressive intragraft immune response through recapitulation of lymphoid organogenesis. J. Immunol 185, 717–728 (2010). [DOI] [PubMed] [Google Scholar]
- 133.Koenig A & Thaunat O Lymphoid neogenesis and tertiary lymphoid organs in transplanted organs. Front. Immunol 7, 646 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Koga T, Ichinose K, Mizui M, Crispin JC & Tsokos GC Calcium/calmodulin-dependent protein kinase IV suppresses IL-2 production and regulatory T cell activity in lupus. J. Immunol 189, 3490–3496 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ferretti AP, Bhargava R, Dahan S, Tsokos MG & Tsokos GC Calcium/Calmodulin kinase IV controls the function of both T cells and kidney resident cells. Front. Immunol 9, 2113 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Otomo K. et al. Cutting edge: nanogel-based delivery of an inhibitor of CaMK4 to CD4+ T cells suppresses experimental autoimmune encephalomyelitis and lupus-like disease in mice. J. Immunol 195, 5533–5537 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bhargava R. et al. Aberrantly glycosylated IgG elicits pathogenic signaling in podocytes and signifies lupus nephritis. JCI Insight 6, e147789 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Blanchard L & Girard JP High endothelial venules (HEVs) in immunity, inflammation and cancer. Angiogenesis 24, 719–753 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bahmani B. et al. Targeted delivery of immune therapeutics to lymph nodes prolongs cardiac allograft survival. J. Clin. Invest 128, 4770–4786 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jiang L. et al. Simultaneous targeting of primary tumor, draining lymph node, and distant metastases through high endothelial venule-targeted delivery. Nano Today 36, 101045 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Morel L. Immunometabolism in systemic lupus erythematosus. Nat. Rev. Rheumatol 13, 280–290 (2017). [DOI] [PubMed] [Google Scholar]
- 142.Kleinewietfeld M. et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 496, 518–522 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wu C. et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 496, 513–517 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Crispin JC et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J. Immunol 181, 8761–8766 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sharabi A. et al. Regulatory T cells in the treatment of disease. Nat. Rev. Drug. Discov 17, 823–844 (2018). [DOI] [PubMed] [Google Scholar]
- 146.Munoz-Rojas AR & Mathis D Tissue regulatory T cells: regulatory chameleons. Nat. Rev. Immunol 21, 597–611 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Arpaia N. et al. A distinct function of regulatory T cells in tissue protection. Cell 162, 1078–1089 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]