Abstract
The IL-13Rα2 receptor is a high affinity receptor for IL-13 that is used only by IL-13 and is quite distinct from the well known IL-13Rα1 receptor that IL-13 shares with IL-4. It was widely considered to be a secreted receptor that is devoid of signaling activity and functional only as a decoy receptor that retarded signaling via IL-13Rα1. In recent studies, however, it was shown to be capable of robust signaling that results in production of TGF-β1 and through the latter cytokine, the induction of fibrosis occuring in various experimental inflammatory states. Thus, in initial studies, IL-13 signaling via IL-13Rα2 was shown to play an important role in the fibrosis developing in both oxazolone colitis and bleomycin-induced pulmonary fibrosis; later, it was also shown to be critical to the development of fibrosis in a model of chronic colitis induced by trinitrobenzene sulphonic acid (TNBS). These studies suggest that blockade of IL-13 or IL-13Rα2 signaling might be an excellent target for the prevention of inflammation-associated fibrosis. A second role of IL-13 signaling via IL-13Rα2 is in tumor immune surveillance. Thus, in the relevant studies it was shown that NKT cells stimulated by tumor antigens produce IL-13 that then acts on Gr-1 cells to induce TGF-β1; the latter then inhibits CD8+ T cells engaged in tumor immune surveillance; in effect, then, receptor signaling favors tumor growth. In addition to its signaling function and the induction of TGF-β1, IL-13Rα2 also influences IL-13Rα1 signaling in complex ways; thus, IL-13Rα2 emerges as a important component of IL-13 signaling, not only in its own right but also in its possible effect on its companion receptor.
INTRODUCTION
In recent years our concepts of the role of IL-13 in T cell responses has changed dramatically with the acquisition of more complete knowledge of the function of the second of the two receptors involved in its function, IL-13Rα2. To put this statement into historical prospective, one should recall that that it has been known for some time that IL-13 interacts with two receptors, a heterodimeric receptor composed of a IL-4Rα chain linked to a IL-13Rα1 chain (the IL-13Rα1 receptor) and a monomeric receptor composed of the IL-13Rα2 chain (the IL-13Rα2 receptor) [1-3]. IL-13Rα1 reacts with both IL-4 and IL-13 and signals via activation of Stat6; thus, this is the receptor (along with IL-4Rα1/γc chain receptor that binds IL-4 only) that is associated with the initiation of the Th2 response. IL-13Rα2, on the other hand, reacts only with IL-13 and does not induce Stat6 activation. The latter fact, plus the fact that IL-13Rα2 has a relatively short cytoplasmic tail (17 aa in the mouse), led to the assumption that the membrane-bound form of the receptor was a non-functional or even inhibitory form of the receptor [4]. This notion meshed with the observation that at least in mice, IL-13Rα2 also occurs as a soluble protein, most likely derived from cleavage of the membrane-bound protein, and that such soluble IL-13Rα2 acts as a “decoy” that inhibits IL-13 responses by binding to IL-13 before it can interact with IL-13Rα1 [4, 5]. Thus it appeared that the functional form of IL-13Rα2 resides in its secreted form. A somewhat different view of the function of this receptor, however, emerges from a recent molecular analysis of IL-13Rα2 [6]. This analysis showed that in mouse spleen cells IL-13Rα2 RNA is alternatively spliced to yield mRNA encoding transcripts that either do or do not contain exon 10, the exon encoding the transmembrane segment of the molecule. The mRNA retaining the transmembrane segment gives rise to a membrane-bound form of the receptor whereas the mRNA lacking the transmembrane segment gives rise to a secreted, soluble IL-13Rα2. Subsequent studies of the properties of the two forms of IL-13Rα2 showed that the soluble form had a somewhat higher affinity for IL-13 than the membrane-bound form but their tissue distribution was about the same; in addition, both forms were upregulated in lungs when the latter tissue was treated in vitro with IL-4 or IL-13 and both forms were up-regulated in the lungs of mice with experimental asthma [4, 6]. In establishing that the membrane-bound form of IL-13Rα2 results from alternative splicing and is a stable form of the receptor that is not necessarily destined to be cleaved from the membrane to become a soluble form, these studies provide molecular support for the view that IL-13Rα2 can occur as a membrane-bound signaling receptor that does in fact have signaling function. In the following review the data that directly support this thesis is discussed in some detail. The somewhat surprising fact that emerges from these data is that IL-13Rα2 function subtends immune responses very different from those involved in classical Th2 differen-tiation.
THE SIGNALING FUNCTION OF IL-13Rα2
In 2001 Lee et al. published studies showing that mice that selectively over-express IL-13 in the lung develop fibrosis in that organ and that this is associated with increased activation of TGF-β1 [7]. These seminal studies linked IL-13 for the first time with a new function, fibrosis induction, that did not have a necessary connection to the Th2 response. These studies led, several years later, to a series of studies focused on the signaling function of IL-13Rα2 and the mechanism of IL-13 induction of TGF-β1.
In the initial in vitro studies of this series, the ability of various cytokines to induce TGF-β1 was tested in THP-1 cells (a human macrophage cell line) transfected with a plasmid construct expressing a TGF-β promoter linked to a luciferase reporter [8]. It was found that exposure of these cells to a combination of IL-13 and TNF-α induced a luciferase signal whereas a combination of IL-4 and TNF-α or any of these cytokines alone did not. This result indicating that IL-13 but not IL-4 was capable of activating TGF-β1 transcription was then corroborated with studies showing that THP-1 cells, a human macrophage cell line, produce TGF-β1 protein when cultured with IL-13 and TNF-α but not when cultured with IL-4 and TNF-α. Since both IL-4 and IL-13 signal cells through IL-13Rα1 but only IL-13 induces activation of the TGF-β1 promoter, these results provide preliminary evidence that IL-13 uses another receptor, possibly IL-13Rα2, to induce TGF-β1 expression rather than the IL-13Rα1 receptor even though IL13Rα1 Is constitutively expressed on the surface of THP-1 cells whereas Rα2 expression requires some form of induction.
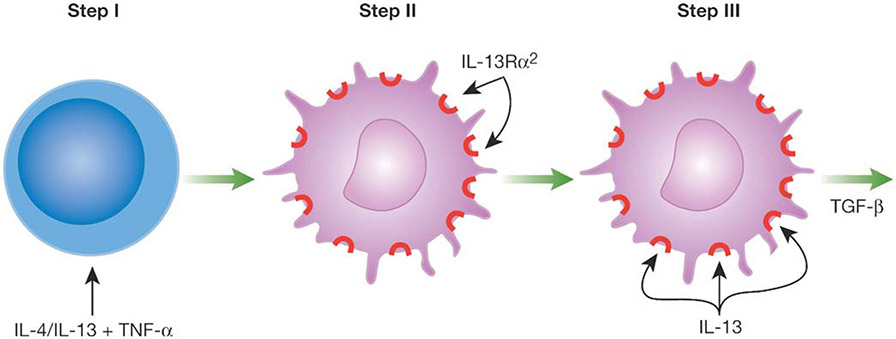
In view of the possible involvement of IL-13Rα2 in TGF-β1 production, it was logical to focus next on the factors necessary to induce IL-13Rα2 expression on THP-1 cells. Using flow cytometry to identify the induction of surface IL-13Rα2 expression, it was found that both IL-4 and IL-13 in combination with TNF-α could induce THP-1 cells to express cell surface IL-13Rα2 whereas neither of these cytokines alone could induce such expression. This result was thus consistent with a model in which the transcription of IL-13Rα2 is dependent on the generation of active NF-κB, a transcription factor induced by TNF-α and active Stat6, a transcription factor induced by either IL-4 or IL-13 acting via the IL-13Rα1 receptor (see Fig. 1) [8].
Fig. (1).
IL-13 Induction of TGF-β1 Requires Two Steps. In the first step membrane-bound IL-13Rα2 is induced by exposing cells to IL-4 or IL-13 plus TNF-α. This induces activated Stat6 and NF-κB respectively. In the second step IL-13 signals via IL-13Rα2 to induce TGF-β1. This signaling gives rise to activated AP-1.
To verify this model studies were then conducted to determine whether IL-13Rα2 expression in THP-1 cells was abolished when the cells were transfected with NF-κB- and Stat6-specific “decoy oligonucleotides” that block the activity of these factors [8]. Decoy oligonucleotides are oligonucleotides that mimic consensus binding sequences on promoters and thereby block the binding of the transcription factors to promoter sites. It was found that transfection of THP-1 cells with decoy oligonucleotides specific for either NF-κB or Stat6 did in fact abolish induction of IL-13Rα2 by IL-13 and TNF-α but had no effect on the constitutive expression of IL-13Rα1 by these cells. Furthermore, THP-1 cells transfected with either of these decoy oligonucleotides were no longer capable of supporting TGF-β1 promoter activity in the luciferase reporter system. These results led to the conclusions that IL-13 (or IL-4) signaling via IL-13Rα1 to activate Stat6 together with TNF-α signaling via a TNF receptor to activate NF-κB induce expression of IL-13Rα2 on the surface of THP-1 cells. In addition, the fact that blockade of the signaling necessary to induce IL-13Rα2 expression also abolished TGF-β1 promoter activity provided additional evidence that expression of IL-13Rα2 is necessary for the induction of TGF-β1.
Further elucidation of the function of IL-13Rα2 in TGF-β1 transcription and production were then obtained with studies of another human macrophage cell line, MonoMac6 cells (MM6 cells); this cell line, like THP-1 cells constitutively express IL-13Rα1, but unlike THP-1 cells do not express IL-13Rα2 even when activated by IL-13 and TNF-α. In an initial series of studies utilizing MM6 cells transfected with the TGF-β1-luciferase reporter plasmid, it was shown that these cells do not respond to IL-13 and TNF-α with a positive luciferase signal unless co-transfected with a plasmid expressing IL-13Rα2 and, in this case, the cells responded to IL-13 alone in the absence of TNF-α [8]. In addition, it was shown that co-transfection of MM6 cells with a plasmid expressing a truncated form of IL-13Rα2 that does not have a cytoplasmic tail could not empower these cells to produce TGF-β1. These studies thus offered incontrovertible proof that IL-13Rα2 signaling was necessary and sufficient for IL-13 induction of TGF-β1.
In yet other studies of IL-13Rα2 signaling function utilizing MM6, the role of Stat6 in IL-13 induction of TGF-β1 was further investigated [8]. It was shown that IL-4 stimulation of MM6 cells resulted in robust phosphorylation of Stat6 prior to or after transfection of a plasmid that expressed IL-13Rα2 whereas IL-13 stimulation of MM6 cells resulted in robust phosphorylation of Stat6 prior to transfection but somewhat reduced phosphorylation after transfection; such reduction after transfection probably occurred because the IL-13Rα2 does not activate Stat6 and reduces IL-13 interaction with and signaling through IL-13Rα1 because it has a much higher affinity for IL-13. In further experiments it was shown that cells that were co-transfected with a plasmid that expressed the IL-13Rα2 receptor and a TGF-β1 promoter-lucifierase reporter plasmid were able to respond to IL-13 with a positive luciferase signal, even if they were also transfected with Stat6 decoy oligonucleotides and thus lacked Stat6 function. This latter study showed that while IL-13 signaling (or IL-4 signaling) via IL-13Rα1 and Stat6 activation is necessary for the expression of IL-13Rα2 (as shown in THP-1 cells), IL-13 signaling through IL-13Rα1 to activate Stat6 is not required for IL-13 induction of TGF-β1.
In a final series of studies focusing on IL-13 signals leading to TGF-β1 induction, THP-1 cells and MM6 cells were deployed to investigate if AP-1 was one of the signals, given the fact that the TGF-β1 promoter has an AP-1 consensus binding sequence [8]. First, in studies of THP-1 cells and MM6 cells transfected with an IL-13Rα2-expressing plasmid, it was shown that transfection or co-transfection of an AP-1 decoy oligonucleotide did in fact block TGF-β1 promoter-luciferase reporter activity. Second, in electrophoretic mobility assays (EMSAs) it was shown that whereas nuclear extracts of MM6 cells not transfected with an IL-13Rα2-expressing plasmid and stimulated with IL-13 did not contain a factor that bound to a labeled AP-1 target oligonucleotide, MM6 cells that were so transfected did give a positive signal. Furthermore, in supershift studies it could be shown that the EMSA signal could be shifted with antibodies to c-Jun and Fra-2. It was thus apparent that IL-13 signaling via IL-13Rα2 to cause TGF-β1 induction acts via AP-1.
In summary, these studies establish that IL-13 induction of TGF-β1 is a two-stage process. In the first stage either IL-4 or IL-13 induce activated Stat6 by signaling through the IL-13Rα1 receptor. This, along with TNF-α induction of activated NF-κB, leads to surface expression of IL-13Rα2. In the second stage, IL-13 signals through IL-13Rα2 to induce AP-1 and posslbly other factors that cause the activation of the TGF-β1 promoter.
THE ROLE OF IL-13RA2 SIGNALING IN MODELS OF INFLAMMATION AND FIBROSIS
The mechanism of IL-13Rα2 signaling proposed above was then tested in vivo, using two models of inflammation, oxazolone-colitis and bleomycin-induced pulmonary fibrosis. Oxazolone-colitis is a murine model of human ulcerative colitis induced by the intra-rectal administration of oxazolone, a haptenating agent previously used in skin sensitization studies [9]. The colitis resulting from such treatment is mediated by the induction of NKT cells that are cytotoxic for epithelial cells. In addition, the induced NKT cells produce IL-13 that not only enhances NKT cell cytotoxicity, but also has independent deleterious effects on epithelial cell integrity and function [10]. This model is interesting in the present context because oxazolone colitis is marked by production of high levels of TGF-β1 and thus provides an opportunity to determine the role of IL-13 and IL-13Rα2 on the induction of TGF-β1 within an in vivo inflammatory model [9].
The approach taken in these studies of oxazolone colitis was to determine if inhibition of IL-13Rα2 expression (and thus of IL-13 signaling through this receptor) by blockade of TNF-α function in vivo affects IL-13 induction of TGF-β1 synthesis as previous shown in vitro [8]. Accordingly, mice with oxazolone colitis were administered etanercept (TNF-αR-Fc), a molecule that binds to TNF-α and thereby blocks it’s signaling. It was found that such treatment has no effect on the severity or the colitis or the production of IL-13; nevertheless, it inhibited IL-13Rα2 expression, and, perhaps more importantly, it inhibited TGF-β1 production by lamina propria mononuclear cells. These data therefore provided strong evidence that IL-13Rα2 expression is dependent on TNF-α stimulation and that, in turn, TGF-β1 production was dependent on IL-13Rα2 expression.
Bleomycin-induced pulmonary fibrosis was a second model utilized to verify that IL-13Rα2 signaling resulted in TGF-β1 induction. This model is a well studied fibrosis model in which severe pulmonary fibrosis is induced by bleomycin administration [11]. It is known that the fibrosis occurring in this model is dependent on the production of IL-13 and TGF-β1 so that its study in this context offers the opportunity to study the impact of IL-13 signaling via IL-13Rα2 on fibrosis as well as TGF-β1 production. In initial studies it was shown that, as in the oxazolone colitis model, while administration of etanercept had no effect on IL-13 production, it did down-regulate IL-13Rα2 expression and TGF-β1 production. In addition, it greatly decreased pulmonary fibrosis [8].
In further study of the bleomycin model, mice with bleomycin-induced fibrosis were administered IL-13Rα2-specific siRNA encapsulated in a Sendai (hemagglutinating virus of Japan (HVJ))-virus envelope which has been shown to be a highly efficient method of delivering siRNA to cells in vivo [8, 12]. Such administration did indeed lead to reduced pulmonary IL-13Rα2 expression and, more importantly, resulted in both reduced TGF-β1 production and fibrosis induction. Thus it was evident that IL-13 signaling via IL-13Rα2 is in fact necessary for TGF-β1 production and fibrosis. In a final series of studies, mice with bleomycin-induced pulmonary fibrosis were administered AP-1 decoy oligonucleotides which, as indicated above, block transcriptional function of AP-1. Such administration had no effect on IL-13Rα2 expression but greatly reduced TGF-β1 production and fibrosis. It was therefore clear that AP-1 does function as a signaling molecule of the IL-13Rα2 receptor.
Taken together, these in vivo studies fully validated the model of IL-13 signaling via IL-13Rα2 in the induction of TGF-β1 secretion and fibrosis. In addition, they showed that IL-13 induction of fibrosis in these models is an “inflammation-dependent” phenomenon in that it relies on the prior secretion of a pro-inflammatory cytokine, TNF-α, to induce expression of a key and central receptor. Thus, the IL-13/IL-13Rα2 signaling mechanism emerged as a major pathophysiologic pathway for a key feature of the inflammatory process, fibrosis.
THE ROLE OF IL-13 AND IL-13 SIGNALING VIA IL-13Rα2 IN THE FIBROSIS OCCURRING IN CHRONIC TNBS-COLITIS
In order to obtain additional insight into the role of IL-13Rα2 signaling in inflammatory disease, advantage was taken of a newly described model of chronic colitis induced by repeated intra-rectal administration of the haptenating agent trinitrobenzene sulphonic acid (TNBS) to BALB/c mice [13]. This model of colitis exhibits a prolonged inflammation that is eventually accompanied by marked fibrosis. Thus, the course of this inflammation is strikingly reminiscent of human inflammatory bowel disease, which also is associated with late-stage fibrotic changes.
Chronic TNBS-colitis is characterized by three distinct phases (see Fig. 2). In the first phase, lasting about two weeks, a severe inflammation is present, marked by an extensive transmural infiltrate similar to that found in acute TNBS-colitis originally observed in SJL/J mice. As in the latter type of colitis, chronic TNBS-colitis is associated with high level secretion IL-12p70 and IFN-β but not IL-23 and IL-17. The following two weeks usher in a more sedate phase of inflammation which dominates from day 21 to day 35 of colitis. During this phase IL-12p70 and IFN-γ levels gradually decline and by day 28 of colitis these have returned to baseline. At the same time, IL-23 and IL-17 levels gradually increase until they reach a plateau on day 49 of colitis. The inflammation during this period is less intense and the mice maintain (but do not gain) weight. On day 35 the third phase of the colitis supervenes with the abrupt onset of IL-25 (IL-17E), IL-13 secretion and TGF-β1 secretion. The inflammation continues as before but now it is accompanied by gradually increasing fibrosis. In summary, chronic TNBS-colitis is marked by a short-lived Th1 T cell-dominant phase of colitis, a prolonged Th17 T cell-dominant phase of colitis and finally, by a Th17 T cell-induced colitis accompanied by fibrosis. This model of colitis may therefore reiterate the progressive phases of inflammation occurring in localized areas of Crohn’s disease, which is also associated with fibrosis as the lesion matures.
Fig. (2).
Chronic TNBS-Colitis induced in BALB/c mice. The chronic colitis is marked by three phases dominated by a Th1 response, a Th17 response and finally, a mixed Th17/IL-13 response. Each of these phases are characterized by a unique set of cytokines. Fibrosis occurs during phase three under the influence of IL-13 and TGF-β1.
The factors leading to the onset and persistence of IL-13 in the chronic TNBS-colitis model is still somewhat unclear. However, it was found that cells extracted from the lamina propria at about the time of onset of IL-13 secretion and cultured in the presence of antibodies to IL-23 and IL-25 decreased the ability of the cells to produce IL-13, whereas antibodies to Th1 cytokines or IL-17 had no such effect. Thus, it seems likely that the cytokines accompanying the TH17 response are necessary for induction of IL-13 secretion and that the Th17 response carries within itself the means of induction of IL-13 secretion and the downstream consequences of such secretion.
Studies to address the role of IL-13Rα2 in the induction of TGF-β1 secretion and fibrosis in the chronic TNBS-colitis model were initiated with an investigation of the expression of this receptor by colonic lamina propria mononuclear cells during the course of colitis [13]. It was found that while IL-13Rα1 is a constitutively expressed receptor, IL-13Rα2 does not make its appearance until day 35 of colitis and its expression is blocked by administration of IL-13Rα2-Fc, an agent that binds to soluble IL-13 and blocks IL-13 interaction with IL-13Rα2. Thus, as in the in vitro studies discussed above, receptor expression is induced by IL-13 probably in concert with TNF-α (both cytokines produced by the inflammatory cells during this period). Finally, in vivo administration of a plasmid expressing IL-13Rα2-Fc, as well as administration of IL-13Rα2-specific siRNA led to down-regulation of c-Jun DNA binding activity, indicating that the induced receptor is actively engaged in signaling function. Interestingly, down-regulation of IL-13Rα2 in this manner also led to a decrease in IL-13 production, indicating that the receptor has an autocrine effect on the synthesis of its signaling cytokine. The mechanism of this effect is yet to be elucidated.
The above findings culminated in studies designed to address the critical question of whether the expression of IL-13Rα2 is essential to TGF-β1 production and fibrosis in mice with chronic TNBS-colitis. In these studies receptor signaling by IL-13, receptor expression and receptor induction of signaling molecule were blocked by administration of a plasmid expressing IL-13Rα2-Fc, IL-13Rα2-specific siRNA or anti-TGF-β1 respectively on day 49 of colitis following which the effect of such blockade on collagen deposition was determined [13]. It was found that, indeed, each of these treatments blocked expression of TGF-β1 as well as collagen formation. In addition, in subsequent studies of the down-stream events initiated by this signaling pathway it was shown that IL-13Rα2 induction of TGF-β1 leads to the secretion of insulinlike growth factor-1 (IGF-1) and Early Growth Response Gene-1 (Egr-1) factors that have been shown to orchestrate the fibrotic process. Taken together, these studies establish that IL-13Rα2 is a major portal to the fibrotic process occurring in chronic inflammation.
IL-13Rα2 RECEPTOR EXPRESSION AND TUMOR IMMUNE SURVEILLANCE
The important effect of IL-13 on fibrosis via IL-13α2 signaling and TGF-β1 expression in a chronic model of colitis discussed above may be one of several examples of other important effects of such signaling in other processes. Recently one such effect was uncovered in relation to immune surveillance and the control of tumor growth.
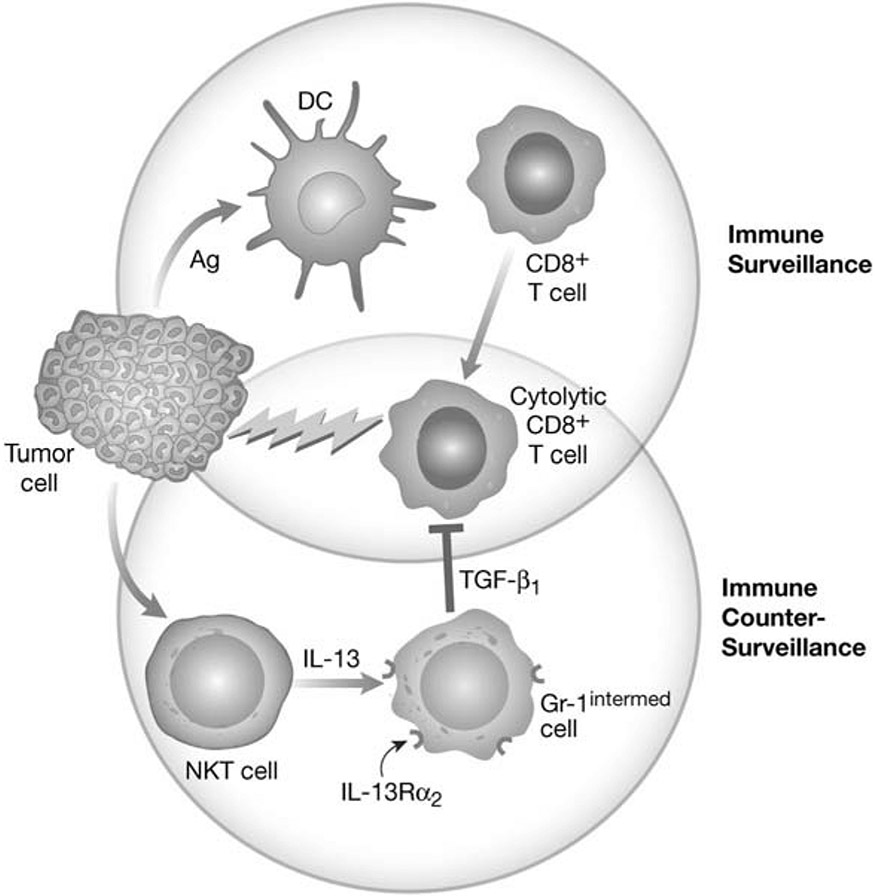
The story begins with a series of observations made over the past several years concerning a newly discovered mechanism of immune counter-surveillance involving IL-13 and TGF-β1 (see Fig. 3). In particular, it was shown that in certain experimental tumor models such as the 15-12RM fibrosarcoma and the CT-26 colon cancer model early regression of tumor gives way to re-occurrence of tumor and the death of the animal [14-17]. The regression phase was due to immune surveillance characterized by the development of tumor-specific cytolytic T cells (CD8+ T cells) that killed tumor cells. On the other hand, the recurrence phase was due to immune counter-surveillance marked by: 1)the development of NKT cells that produce IL-13: 2) IL-13-induction of TGF-β1 production by monocytelike cells bearing a Gr-1 surface marker; 3) the TGF-β-mediated inhibition of cytolytic T cell activity that allows the tumor cells to again expand [16, 17]. One unanswered question regarding this mechanism arose from the fact that the production of TGF-β1 by the Gr-1 cells was induced by IL-13 and not IL-4 and the postulated receptor for IL-13, IL-13Rα1, also responds to IL-4. Thus it was likely that a second signaling receptor for IL-13 plays a role in the process, such as the IL-13Rα2 receptor.
Fig. (3).
Immune Counter-Surveillance mediated by IL-13-induced TGF-β1. Immune surveillance and control of tumor cell growth by cytotoxic CD8+ T cells (upper circle events) can be countered by immune counter-surveillance mediated by NKT cells producing IL-13 that acts of Gr-1 cells and induces the latter to produce TGF-β1; TGF-β1 then inhibits cytotoxic CD8+ T cells to allow the tumor cells to expand (lower circle events).
Initial studies to explore this possibility centered on the CT-26 colon cancer model. Here it was shown that mice administered CT-26 cells not only begin to synthesize IL-13 and TNF-α, the cytokine shown previously to induce IL-13Rα2, but in fact such mice express the latter receptor in the malignant cell target organ, the lung, as early as seven days after tumor cell injection [18]. In prior studies it had been shown that the cells responding to IL-13 with the production of TGF-β1 were, CD11chigh, Gr-1intermediate cells [8,14]. To determine if these cells were indeed the cells responding to IL-13 in the CT-26 model, purified CD11chigh Gr-1intermediate cells and CD11chigh Gr-1high were purified from mouse spleens by flow cytometric sorting and examined for the ability to express IL-13Rα2 and to secrete TGF-β1 [18]. It was found that after CT-26 cell administration, CD11chighGr-1intermediate cells but not their Gr-1high counterparts expressed IL-13Rα2 and produced TGF-β1 in the presence of IL-13. Thus, it was shown the cells producing TGF-β1 in response to IL-13 in previous studies were also the cells bearing the IL-13Rα2 receptor.
In further studies designed to link IL-13Rα2 expression to TGF-β1 production, agents that block receptor expression or receptor signaling were administered to mice at the time of tumor cell administration to determine if such treatment also blocked TGF-β1 production [18]. To block receptor expression TNF-αR-Fc (etanercept) or plasmid encoding IL-13Rα2-specific siRNA was administered and to block receptor signaling AP-1 decoy oligonucleotide was administered, in the latter two cases using materials encased in HVJ-envelope to achieve high in vivo plasmid delivery. It was found that etanercept or IL-13Rα2-specific siRNA did indeed block receptor expression whereas decoy AP-1 oligonucleotide did not, as expected for an agent that acts “downstream” of the receptor itself. However, all three treatments resulted in greatly impaired TGF-β1 secretion by CD11chigh Gr-1intermediate spleen cells. In addition, all three treatments led to greatly increased cytolytic activity of CD8+ T cells for tumor targets in the tumor-bearing mice. Finally, it should be noted that if administration of etanercept or IL-13Rα2-specific siRNA were administered one week after tumor cell administration, one does not see either down-regulation of the Rα2 or TGF-β1 secretion, nor does one see a reversal of TGF-β1 effect on cytolytic T cells; this is presumably because, at this delayed time point, cells with the ability to produce TGF-β1 have already been generated. In contrast, delayed administration of AP-1 decoy oligonucleotide continued to down-regulate TGF-β1 and did reverse the TGF-β1 effect on cytolytic T cells presumably because this agent blocks IL-13Rα2 signaling even after the cells capable of producing TGF-β1 have been generated.
The above described effects of various agents on expression of IL-13Rα2 and TGF-β1 production by CD11chigh Gr-1intermediate cells had corresponding effects on tumor growth [18]. Thus, in the CT-26 tumor model, etanercept, IL-13Rα2-expressing plasmid or AP-1 decoy oligonucleotide administration led to markedly longer mouse survival and markedly decreased numbers of pulmonary tumor nodules three weeks following CT-26 tumor cell injection. In addition, with delayed administration of these agents, only the administration of AP-1 decoy oligonucleotides had this effect, in keeping with the effects of delayed administration mentioned above. In addition, in a similar but less complete study it was shown that administration of etanercept blocked expression of IL-13Rα2 and TGF-β1 production in the CD11chigh Gr-1intermediate cells in the 12-15RM sarcoma tumor model and in two independent studies reduced the incidence of tumor recurrence. These studies established that signaling via IL-13Rα2 is a key event in the “counter surveillance” occurring in two experimental tumor models and that by blocking the activity of this receptor, one can re-establish tumor surveillance and immunologic control of tumor development. In addition they suggest that administration of etanercept, an agent with a well-documented record of low toxicity, may prevent or delay tumor development in patients who have undergone primary treatment for tumors but are at high risk for tumor recurrence.
POSSIBLE INHIBITORY FUNCTIONS OF MEMBRANE-BOUND IL-13Rα2
So far in our discussion we have focused on studies showing that IL-13Rα2 is a membrane-bound signaling receptor with positive activity that includes, but is not necessarily limited to the induction of TGF-β1 secretion. It should be noted, however, that there is a body of evidence supporting the notion that membrane-bound IL-13Rα2 can also function as an inhibitory (negative) receptor, at least with respect to its effect on the signaling function of IL-13Rα1.
In an early study demonstrating such negative signaling function, Kawakami et al. showed that IL-13Rα2 undergoes internalization upon binding its ligand, IL-13, and thus functions as a bona fide membrane-bound IL-13 receptor [19]. As a result of such internalization, cells bearing the receptor take up a toxin (e.g., pseudomonas exotoxin) bound to IL-13 which then brings about cell death. This property of the receptor has led to ongoing studies of the treatment of glioblastoma multiforme and perhaps other neoplasms that spontaneously express IL-13Rα2 with IL-13 bound to toxin [20, 21]. Additional studies showed that total deletion of the intracellular domain led to reduced internalization, and thus provided the first evidence that the membrane-bound IL-13Rα2 is in fact a signaling receptor. However, this evidence of signaling was at least temporarily negated by the fact that cells expressing only IL-13Rα2 did not activate Stat6 and cells co-transfected with constructs expressing IL-13Rα2 and IL-13Rα1 exhibited decreased Stat6 activation compared to cells transfected with constructs expressing only IL-13Rα1 [19].
In further studies along these lines Yasunaga et al. acting on an earlier micro-array analysis disclosing that bronchial epithelial cells exposed to IL-4 or IL-13 upregulated IL-13Rα2 showed that bronchial epithelial cells from normal human donors expressed IL-13Rα2 mRNA and protein upon culture with IL-4 or IL-13, but not other cytokines [22]. Following such treatment, cytoplasmic but not membrane-bound IL-13Rα2 was detected in staining studies; however, cells transfected with an IL-13Rα2-expressing construct did bind IL-13 via this receptor, suggesting that this receptor is expressed on the cell surface. In subsequent studies of such transfected cells, it was shown that while IL-4 was able to induce Stat6, IL-13 was unable to do so despite the fact that the cells expressed IL-13Rα1 [22]. This apparent inhibition of IL-13 signaling by IL-13Rα2 was not due to receptor binding to an inhibitory moiety since chemical cross-linking data showed that IL-13 binds to single IL-13Rα2 molecules in the transfected cells. Finally, in studies of experimental asthma induced by intranasal albumin administration, it was shown that IL-13Rα2 is upregulated in tissues subject to asthmatic diathesis but not control tissue whereas constitutive expression of IL-13Rα1 remains unchanged [22]. However, expression of IL-13Rα2 had no apparent inhibitory effect on the induced asthmatic condition.
Additional studies bearing on the negative signaling function of IL-13Rα2 by Daines et al. involved the use of U937 cells (a human macrophage cell line that does not express endogenous IL-13Rα2) and normal splenocytes transfected with a plasmid expressing IL-13Rα2 expressing membrane-bound as well as intra-cytoplasmic IL-13Rα2 [4]. Here it was shown that cells expressing high levels of receptor exhibited reduced Stat6 activation of cells as compared to that induced by IL-4, but this reduction was not seen at high concentration of IL-13. While a small amount of soluble IL-13Rα2 was released from the cells, the amount released was not sufficient to account for the inhibition. Finally, in a parallel study focused on human fibroblasts, it was shown that soluble IL-13Rα2 diminishes the capacity of IL-13 but not IL-4 to induce eotaxin release, a Stat6 induced protein [23]. Furthermore, pre-stimulation of cells with IL-13 which has the effect of up-regulating membrane-bound IL-13Rα2 leads to decreased eotaxin release upon restimulation of cells with IL-13 and under these circumstances signaling does not induce activation of Stat6.
The apparent inhibition of IL-13 induction of Stat6 activation (or eotaxin production that depends on Stat6) observed in each of these studies and interpreted as a negative function of IL-13Rα2 is similar to the apparent inhibition of Stat6 activation discussed above in relation to MonoMac6 cells transfected with a plasmid expressing IL-13Rα2 [8]. However, as originally suggested by Kawakami et al., this decreased Stat6 signaling was assumed to be due to the fact that the IL-13Rα2 has a far higher affinity for IL-13 than IL-13Rα1 (100-300-fold higher) and therefore deprives the latter receptor of access to IL-13 necessary for signaling [19]. This view is strongly favored by the fact that provision of excess IL-13 in the Daines study led to restoration of Stat6 activation [4].
INHIBITION AND ENHANCEMENT OF IL-13α1 SIGNALING BY IL-13Rα2 SIGNALING
While IL-13Rα2 may not directly impair IL-13-induced IL-13Rα1 signaling studies conducted by Rahaman et al. have shown that it does impair IL-4-induced IL-13Rα1 signaling and activation of Stat6 [24]. In these studies it was shown first that 293T cells that express endogenous IL-13Rα1 exhibit reduced Stat6 activation upon exposure to IL-4 if transfected with a IL-13Rα2-expressing plasmid [24]. Moreover, it was shown that this effect was due to the intra-cellular domain of IL-13Rα1 since 293T cells that are transfected with a plasmid expressing a chimeric construct consisting of an erythropoietin (EPO) extracellular domain and a IL-13Rα1 intra-cellular domain also exhibit reduced Stat6 activation upon exposure to EPO if co-transfected with a IL-13Rα2-expressing plasmid. In addition, this effect was due to the terminal (intracellular) 6 amino-acids of IL-13Rα2 since 293T cells that are transfected with a plasmid expressing truncated IL-13Rα2 exhibit normal Stat6 activation measured by EMSA. Finally, it was shown in co-immunoprecipitation studies using 293 T cells cotransfected EPO-IL-4Ralpha2 and IL-13Ralpha2 that the molecules bind to each other.
It should be noted that the above studies were performed with cells that over-expressed IL-13Rα2 and thus raise the question as to whether the inhibitory effect occurs in normal cells. This concern is to some extent allayed by a second set of studies of pulmonary fibroblasts by Andrews et al. initially referred to above [23]. In these studies it was shown that IL-4 activation of Stat6 and induction of eotaxin as well as IL-13 stimulation of these effects is inhibited by upregulation of the IL-13Rα2 receptor by pre-stimulation of cells with IL-13. Furthermore, as in the case of the studies by Rahaman, IL-13Rα1 co-immunoprecipitates with IL-13Rα2 [24]. These studies of normal fibroblasts induced to express IL-13Rα2 under normal conditions thus suggest that IL-13Rα2 interacts with IL-13Rα1 and, doing so, inhibits Stat6 activation. Yet another study supporting the idea that IL-13Rα2 exerts inhibitory effects on IL-13Rα1 is a recent study by Zhao et al. who showed that lysophosphatidic acid (LPA) both induces IL-13Rα2 via induction of a JNK/AP-1-dependent signaling pathway and attenuates IL-13 or IL-4 activation of Stat6 [25]. In these studies the fact that LPA inhibits Stat6 activation was proven by the fact that down-regulation of IL-13Rα2 with specific siRNA prevented the inhibitory effect.
One remaining question as to whether such inhibition occurs under ordinary conditions comes from yet another finding by Andrews et al. that neither IL-4-induced eotaxin responses nor co-immunoprecipitation occurred if antibody to the IL-13Rα2 is added to the system [23]. This finding suggests that occupation of IL-13Rα2 sterically hinders its association with IL-13Rα1 and therefore the mere presence of IL-13 (which would also occupy the IL-13Rα2 binding site) would prevent the inhibition so that, in effect, IL-13 cannot inhibit Stat6 activation via IL-13Rα2. This prediction is supported by in vivo studies showing that in experimental models of asthma that depend to a great degree of Stat6-dependent Th2 responses, upregulation of IL-13Rα2 expression has no apparent effect on the severity of inflammation [22].
The above results relative to IL-13Rα2 inhibition of IL-4 induction of activated Stat6 should not be taken as evidence that IL-13Rα2 always has a down-regulatory effect on IL-4 function. This conclusion comes from a second study by Rahaman et al. in which it was shown first that glioblastoma cells exposed to IL-4 activate Stat3 and Stat3 target genes via IL-13Rα1 and second that such activation is heavily dependent on IL-13Rα2 since Stat3 activation is markedly down-regulated in cells in which IL-13Rα2 mRNA synthesis is specifically inhibited by an IL-13Rα2-specific siRNA [26]. Furthermore, such enhancement of Stat3 activation by IL-13Rα2 is again dependent on the terminal 2 amino acids of the intra-cellular tail of IL-13Rα2. These studies in providing additional evidence that IL-13Rα2 has signaling function via its cytoplasmic tail also provide some explanation of why glioblastoma cells express this receptor: they show that this receptor leads to increased IL-4 expression of an intra-cellular transcription factor (Stat3) that up-regulates factors that prevent apoptosis of the malignant cells.
THE FUNCTION OF IL-13Rα2 AS A DECOY RECEPTOR
As noted above, IL-13Rα2 can exist in mice as a secreted receptor arising from alternative splicing that results in deletion of the exon that encodes the transmembrane domain of the receptor. In addition, there is a recent report showing that the membrane-bound form of the receptor on the surface of mouse cells transfected with a receptor-expressing plasmid can be cleaved to yield a soluble form by matrix metalloproteinase 8 (MM8) and indeed, mice lacking MM8 exhibit exhibit decreased soluble IL-13Rα2 in the bronchial lavage fluid [27]. Thus, in mice, soluble IL-13Rα2 arising from either de novo synthesis or cleavage can serve as a decoy receptor because in this form it can intercept IL-13 before the latter binds to a signaling receptor. It should be noted however that in humans, there is no evidence that IL-13Rα2 exists as a secreted form and thus in humans decoy IL-13Rα2 would have to arise exclusively from proteolytic cleavage [28].
The functional significance of secreted IL-13Rα2 was first explored by Wood et al. using a mouse in which IL-13Rα2 chain expression severely impaired by deletion of the translation initiation and site a signal peptide site in exon 3 [29]. Such mice were lacking in serum IL-13Rα2 but whether or not they still expressed the membrane-bound form of this molecule was not determined. Functional analysis of these mice disclosed that mice with this deletion exhibited increased bone marrow macrophage progenitor frequency as well as decreased LPS-induced macrophage functions such as nitric oxide and IL-12 production. These changes would be predicted by the presence of increased IL-13 responses occurring as a result of decreased IL-13Rα2 decoy function and therefore provide evidence that IL-13Rα2 has “tonic” decoy function.
In further studies of the function of IL-13Rα2 as a decoy Chariamonte et al. showed that mice with experimental Shistosoma mansoni infection develop markedly increased liver fibrosis that was unassociated with changes in tissue eosinophilia or mastocytosis [5]. These liver changes were ameliorated by administration of IL-13Rα2-Fc, a substance that mimics the effect of secreted IL-13Rα2 and thus provided support for the supposition that, in the absence of secreted IL-13Rα2 there is increased IL-13 signaling. Curiously, the blocking effect of secreted IL-13Rα2 was selective in its site of action since it appeared that lack of IL-13Rα2 did not lead to increased fibrosis in tissues other than the liver despite high levels of IL-13 in the blood and the known tendency of fibrosis to occur in other organs such as the lung and the GI tract during Shistosoma infection. This suggests that fibrosis in schistosomiasis requires the presence of non-IL-13-related factors present in some tissues and not in others.
An important point relative to the above studies concerning the mechanism of inhibition of fibrosis by decoy IL-13Rα2 is that in this study and in another more complete study by the same group of authors, no evidence was found for the role of TGF-β1 in the fibrosis developing in hepatic schistosomiasis [5, 30]. The authors found that in this situation while absence of IL-13 prevented fibrosis such absence did not influence TGF-β1 production; moreover, a variety of maneuvers that blocked TGF-β1 activity directly or indirectly had no effect on the fibrotic process and IL-13 could up-regulate genes associated with fibrosis in TGF-β1 deficient mice. They thus argued that the effect of decoy IL-13Rα2 in the amelioration of fibrosis in schistosomiasis is due to its ability to block IL-13 signaling, not TGF-β1 signaling as might be argued from the data presented above showing that IL-13 induces TGF-β1 and the latter, in turn, induces fibrosis [8, 13]. This conclusion is somewhat at odds with numerous studies showing a correlation between TGF-β1 production and fibrosis in various forms of schistosomiasis and the fact that TGF-β1 signaling is clearly a key component in the induction of fibrosis in a number of inflammatory states [31-34]. It thus seems more likely that TGF-β1 has a considerable role in the fibrosis of schistosomiasis even if IL-13 can cause fibrosis via other non-TGF-β1-mediated mechanisms given the very high levels of IL-13 produced in this infection. In this view, the role of decoy IL-13Rα2 is two-fold: it blocks IL-13 signaling that results in fibrosis via both TGF-β1 and non-TGF-β1-mediated mechanisms.
A final point is that Chariamonte et al. also found that IL-13Rα2 expression and IL-13 expression were inter-dependent [5]. Thus, mice lacking IL-13 had greatly reduced IL-13Rα2 expression and administration of exogenous IL-13 induced IL-13Rα2 expression in the liver. These findings are fully consistent with those described above wherein it was shown that IL-13Rα2 expression in THP-1 cells was up regulated by IL-13 (or IL-4) plus TNF-α. More unexpectedly, they found that in the absence of IL-13Rα2 expression the mice manifested very considerable reduced IL-13 and IL-4 levels [5]. A similar observation was made by Fichtner-Feigl et al. who observed that in vivo administration of siRNA specific for IL-13Rα2 and capable of down-regulating the latter is associated with decreased IL-13 levels [8]. While the mechanism of this phenomenon is unclear, it is obvious that IL-13Rα2 must engage in signaling to bring it about and therefore this fact alone indicates that IL-13Rα2 is considerably more than a decoy.
Very recently, the role of IL-13Rα2 as a major signaling receptor for the induction of TGF-β1 has come under fire in studies of lung fibrosis conducted by Zheng et al. In these somewhat complex studies it was shown that mice that over-express IL-13 specifically in the lung due to a lung-specific IL-13 transgene develop increased lung inflammation and fibrosis in the absence of IL-13Rα2 expression (i.e., in mice with both and IL-13 transgene and IL-13Rα2 deletion) [35]. In addition, mice lacking IL-13Rα2 manifested increased ovalbumin-induced pathology suggesting that the deficiency would result in increased asthma-related inflammation. Finally, in a direct challenge to the data above relating to the role of IL-13Rα2 in intestinal and lung fibrosis, in such mice, TGF-β1 production is increased rather than decreased. Thus, as in the case of the schistosomiasis model, IL-13Rα2 appeared to be acting mainly if not exclusively as a decoy for IL-13 and therefore led to increased inflammation and fibrosis in its absence.
These results and the conclusions that flow from them are difficult to understand because in postulating that IL-13 can induce increased TGF-β1 and fibrosis in the absence of IL-13Rα2 they are, in effect, implying that IL-13 acts via IL-13Rα1, its only other known receptor, to achieve these effects. While it seems possible that IL-13 could induce fibrosis via this receptor as implied by the studies of schistosomiasis [5, 30], it is very unlikely to be inducing TGF-β1 via this receptor as shown by the fact that TGF-β1 was not upregulated in schistosomiasis and in in vitro studies of MonoMac6 cells discussed above, cells that constitutively express IL-13Rα1 but not those that could not express IL-13Rα2 (even after appropriate stimulation), could not support TGF-β1 production [8]. At the moment these studies are difficult to integrate into the overall picture of IL-13-induced TGF-β1 production and fibrosis induction and thus await further studies for clarification and verification.
SUMMARY
The weight of the studies reviewed above supports the view that IL-13Rα2 is in fact a receptor that occurs in membrane-bound form and as such is engaged in signaling activities. Perhaps somewhat surprisingly this signaling does not involve the activation of Stat6 and since such activation was assumed to be the sine qua non of IL-13 function, this led to the notion that IL-13Rα2 is a “silent” receptor that functions mainly as a secreted decoy and inhibitor of IL-13 responses. Several lines of evidence disproves this notion. First are the studies outlined above showing that IL-13 activates AP-1 via membrane-bound IL-13Rα2 and in doing so induces TGF-β1 [8]. This signaling activity has been shown to be the basis of fibrosis in several models of inflammation/fibrosis and in one model, the chronic TNBS-colitis model, shown to be an intrinsic feature of the Th17 response [13]. Second there are firm data the IL-13Rα2 can interact with IL-13Rα1 to induce activated Stat3 as well as Stat3 targets involved in cell survival [26]. This signaling function may lie at the basis of the fact that certain neoplastic cells up-regulate IL-13Rα2 expression. Finally, it has been shown that IL-13Rα2 regulates the circulating levels of both IL-13 and IL-4. Thus it is possible that signaling through this receptor has as yet undescribed autocrine or paracrine effects on ligands related to IL-13 receptors.
Evidence has been put forward showing that IL-13 can induce fibrosis via an as yet poorly understood non-TGF-β1-mediated mechanism. Since this has been derived from a model of parasitic infection wherein very high concentrations of IL-13 are present, it may be that this occurs only in the presence of high IL-13 concentrations and that at lower IL-13 concentrations fibrosis occurs via TGF-β1 and IL-13Rα2 [5, 30]. In addition, it may be that in this situation that IL-13Ra2 functions as an inhibitory decoy receptor, at least in mice.
Finally, given the fact that IL-13 signaling via IL-13Rα2 signaling does not involve Stat6, such signaling is unmoored from Th2 responses and IL-13 cannot be considered only a component of the Th2 response. In this respect, it seems likely that IL-13 secretion occur in both Th1/Th17 and Th2 responses and is responsible for the development of fibrosis under both of these conditions.
REFERENCES
- [1].Aman MJ, Tayebi N, Obiri NI, Puri RK, Modi WS, Leonard WJ (1996) J. Biol. Chem, 271, 29265–29270. [DOI] [PubMed] [Google Scholar]
- [2].Donaldson DD, Whitters MJ, Fitz LJ, Neben TY, Finnerty H, Henderson SL, O'Hara RM Jr., Beier DR, Turner KJ, Wood CR and Collins M (1998) J. Immunol, 161, 2317–2324. [PubMed] [Google Scholar]
- [3].Idzerda RL, March CJ, Mosley B, Lyman SD, Vanden Bos T, Gimpel SD, Din WS, Grabstein KH, Widmer MB, Park LS and et al. (1990) J. Exp. Med, 171, 861–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Daines MO, Tabata Y, Walker BA, Chen W, Warrier MR, Basu S and Hershey GK (2006) J. Immunol, 176, 7495–7501. [DOI] [PubMed] [Google Scholar]
- [5].Chiaramonte MG, Mentink-Kane M, Jacobson BA, Cheever AW, Whitters MJ, Goad ME, Wong A, Collins M, Donaldson DD, Grusby MJ and Wynn TA (2003) J. Exp. Med, 197, 687–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tabata Y, Chen W, Warrier MR, Gibson AM, Daines MO and Hershey GK (2006) J. Immunol, 177, 7905–7912. [DOI] [PubMed] [Google Scholar]
- [7].Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley JM, Gotwals P, Noble P, Chen Q, Senior RM and Elias JA (2001) J. Exp. Med, 194, 809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fichtner-Feigl S, Strober W, Kawakami K, Puri RK and Kitani A (2006) Nat. Med, 12, 99–106. [DOI] [PubMed] [Google Scholar]
- [9].Boirivant M, Fuss IJ, Chu A, Strober W (1998) J. Exp. Med, 188, 1929–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Heller F, Fuss IJ, Nieuwenhuis EE, Blumberg RS and Strober W (2002) Immunity, 17, 629–638. [DOI] [PubMed] [Google Scholar]
- [11].Belperio JA, Dy M, Burdick MD, Xue YY, Li K, Elias JA and Keane MP (2002) Am. J. Respir. Cell Mol. Biol, 27, 419–427. [DOI] [PubMed] [Google Scholar]
- [12].Kaneda Y, Nakajima T, Nishikawa T, Yamamoto S, Ikegami H, Suzuki N, Nakamura H, Morishita R and Kotani H (2002) Mol. Ther, 6, 219–226. [DOI] [PubMed] [Google Scholar]
- [13].Fichtner-Feigl S, Fuss IJ, Young CA, Watanabe T, Geissler EK, Schlitt HJ, Kitani A and Strober W (2007) J. Immunol, 178, 5859–5870. [DOI] [PubMed] [Google Scholar]
- [14].Park JM, Terabe M, van den Broeke LT, Donaldson DD and Berzofsky JA (2005) Int. J. Cancer, 114, 80–87. [DOI] [PubMed] [Google Scholar]
- [15].Terabe M, Khanna C, Bose S, Melchionda F, Mendoza A, Mackall CL, Helman LJ and Berzofsky JA (2006) Cancer Res., 66, 3869–3875. [DOI] [PubMed] [Google Scholar]
- [16].Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, Chen W, Wahl SM, Ledbetter S, Pratt B, Letterio JJ, Paul WE and Berzofsky JA (2003) J. Exp. Med, 198, 1741–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Terabe M, Swann J, Ambrosino E, Sinha P, Takaku S, Hayakawa Y, Godfrey DI, Ostrand-Rosenberg S, Smyth MJ and Berzofsky JA (2005) J. Exp. Med, 202, 1627–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fichtner-Feigl S, Terabe M, Kitani A, Young CA, Fuss I, Geissler EK, Schlitt HJ, Berzofsky JA and Strober W (2008) Cancer Res., 68, 3467–3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kawakami K, Taguchi J, Murata T and Puri RK. (2001) Blood, 97, 2673–2679. [DOI] [PubMed] [Google Scholar]
- [20].Kawakami K, Kioi M, Liu Q, Kawakami M and Puri RK (2005) J. Immunother, 28, 193–202. [DOI] [PubMed] [Google Scholar]
- [21].Kioi M, Kawakami K and Puri RK (2004) Clin. Cancer Res, 10, 6231–6238. [DOI] [PubMed] [Google Scholar]
- [22].Yasunaga S, Yuyama N, Arima K, Tanaka H, Toda S, Maeda M, Matsui K, Goda C, Yang Q, Sugita Y, Nagai H and Izuhara K (2003) Cytokine, 24, 293–303. [DOI] [PubMed] [Google Scholar]
- [23].Andrews AL, Nasir T, Bucchieri F, Holloway JW, Holgate STand Davies DE (2006) J. Allergy Clin. Immunol, 118, 858–865. [DOI] [PubMed] [Google Scholar]
- [24].Rahaman SO, Sharma P, Harbor PC, Aman MJ, Vogelbaum MA and Haque SJ (2002) Cancer Res., 62, 1103–1109. [PubMed] [Google Scholar]
- [25].Zhao Y, He D, Zhao J, Wang L, Leff AR, Spannhake EW, Georas S and Natarajan V (2007) J. Biol. Chem, 282, 10172–10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rahaman SO, Vogelbaum MA and Haque SJ (2005) Cancer Res., 65, 2956–2963. [DOI] [PubMed] [Google Scholar]
- [27].Chen W, Tabata Y, Gibson AM, Daines MO, Warrier MR, Wills-Karp M and Hershey GK (2008) J. Allergy Clin. Immunol, 122, 625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].O'Toole M, Legault H, Ramsey R, Wynn TA and Kasaian MT (2008) Clin. Exp. Allergy, 38, 594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wood N, Whitters MJ, Jacobson BA, Witek J, Sypek JP, Kasaian M, Eppihimer MJ, Unger M, Tanaka T, Goldman SJ, Collins M, Donaldson DD and Grusby MJ (2003) J. Exp. Med, 197, 703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kaviratne M, Hesse M, Leusink M, Cheever AW, Davies SJ, McKerrow JH, Wakefield LM, Letterio JJ and Wynn TA (2004) J. Immunol, 173, 4020–4029. [DOI] [PubMed] [Google Scholar]
- [31].Farah IO, Mola PW, Kariuki TM, Nyindo M, Blanton RE and King CL (2000) J. Immunol, 164, 5337–5343. [DOI] [PubMed] [Google Scholar]
- [32].Morais CN, Carvalho BM, Melo WG, Lopes EP, Domingues AL, Juca NT, Souza W, Abath FG and Montenegro SM (2006) Mem. Inst. Oswaldo Cruz, 101(Suppl 1), 353–354. [DOI] [PubMed] [Google Scholar]
- [33].Zhang BB, Jiao YW, Cai WM, Tao J, Zheng M, Dong FQ and Liu RH (2004) Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi, 22, 154–156. [PubMed] [Google Scholar]
- [34].Zhu H, Zeng L, Zhu D and Yuan Y (2000) J. Tongji Med. Univ, 20, 320–321, 329. [DOI] [PubMed] [Google Scholar]
- [35].Zheng T, Liu W, Oh SY, Zhu Z, Hu B, Homer RJ, Cohn L, Grusby MJ and Elias JA (2008) J. Immunol, 180, 522–529. [DOI] [PubMed] [Google Scholar]