Abstract
The recently identified human herpesvirus 8 (HHV-8, or Kaposi’s sarcoma-associated herpesvirus) has been implicated in the etiology of both Kaposi’s sarcoma (KS) and primary effusion (body cavity-based) lymphoma (PEL) (Y. Chang et al., Science 266:1865–1869, 1994; P. S. Moore et al., J. Virol. 70:549–558, 1996). An important feature of the association of HHV-8 with these malignancies is the expression of an abundant, latency-associated 0.7-kb transcript, T0.7 (W. Zhong et al., Proc. Natl. Acad. Sci. USA 93:6641–6646, 1996). T0.7 is found in all stages in nearly all KS tumors of different epidemiologic origin, including AIDS-associated, African endemic, and classical KS (K. A. Staskus et al., J. Virol. 71:715–719, 1997), as well as in a body cavity-based lymphoma-derived cell line, BCBL-1, that is latently infected with HHV-8 (R. Renne et al., Nat. Med. 2:342–346, 1996). T0.7 encodes a unique HHV-8 open reading frame, K12, also known as kaposin. In this study, we report that the kaposin gene induced tumorigenic transformation. Constructs with kaposin expressed either from its endogenous promoter or from a heterologous promoter induced focal transformation upon transfection into Rat-3 cells. All transformed Rat-3 cell lines containing kaposin sequences produced high-grade, highly vascular, undifferentiated sarcomas upon subcutaneous injection of athymic nu/nu mice. Tumor-derived cell lines expressed kaposin mRNA, suggesting a role in the maintenance of the transformed phenotype. Furthermore, kaposin protein was detected in transformed and tumor-derived cells by immunofluorescence and localized to the cytoplasm. More importantly, expression of kaposin protein was also detected in the PEL cell lines BCBL-1 and KS-1. These findings demonstrate the oncogenic potential of kaposin and suggest its possible role in the development of KS and other HHV-8-associated malignancies.
Kaposi’s sarcoma (KS) is a vascular tumor most commonly occurring in patients with AIDS (5). KS lesions are histologically complex and contain proliferating spindle-shaped cells considered to be of endothelial origin, infiltrating mononuclear cells, plasma cells, and abundant neovascular spaces (74). A new member of the herpesvirus family, human herpesvirus 8 (HHV-8), also known as KS-associated herpesvirus, has been identified in KS tumors from both human immunodeficiency virus (HIV)-positive (2, 14, 64) and HIV-negative (6, 49, 60) patients. HHV-8 sequences have also been identified in several rare lymphomas such as multicentric Castleman’s disease and primary effusion lymphoma (PEL), also known as body cavity-based large-cell lymphoma (10, 11, 13). The seroprevalence of HHV-8 in the general population exhibits variations with geographic distribution. Very low rates of prevalence have been reported for populations in Britain and North America, whereas high rates prevail in Africa and southern Europe (25, 36, 62). However, antibody kinetic studies have shown that a strong correlation exists between conversion to seropositivity and the risk for development of KS (25, 34). Thus, HHV-8 has been proposed as the etiologic agent for KS and other HHV-8-associated malignancies (25, 47, 50).
The nucleotide sequence of the HHV-8 long unique region (LUR) has been determined from viral sequences isolated from the PEL cell line BC-1 (56). Of 81 open reading frames (ORFs), 66 have homology to those in herpesvirus saimiri (HVS) and 15 (K1 to K15) are unique to HHV-8. Moreover, cellular homologs related to known oncogenes have also been identified in HHV-8; these include the genes encoding Bcl-2, cyclin D, interleukin-6 (IL-6), G-protein-coupled receptor (GPCR), and ribonucleotide reductase (1a, 12, 15, 17, 48, 52, 59). Some of these homologs have been shown to enhance cell proliferation. The HHV-8-encoded v-IL-6 supported the growth of an IL-6-dependent murine cell line, B9 (52), while the expression of v-cyclin D resulted in the induction of the S-phase in serum-starved quiescent NIH 3T3 cells (66). Expression of the v-GPCR in rat kidney fibroblasts (NRK-49F cells) enhanced cell proliferation (3). More recently, it has been reported that signaling by v-GPCR, which is associated with a switch to an angiogenic phenotype, leads to transformation and tumorigenesis in NIH 3T3 cells (4). Among the HHV-8 unique K ORFs, only K9 has been shown to induce tumorigenic transformation of NIH 3T3 cells (24). However, because K9 expression was detected in PEL cells but not KS tumors, Gao et al. concluded that K9 may play a role only in B-cell malignancies (24).
Analysis of HHV-8 gene expression in KS tumor spindle cells and the PEL cell line BCBL-1 showed a highly restricted pattern of latent HHV-8 RNA expression (74), i.e., two abundant polyadenylated transcripts of 1.1 and 0.7 kb (T1.1 and T0.7). T1.1 encoded only short ORFs and was primarily localized in the nucleus (74). Recently, Sun et al. (65) demonstrated that the T1.1 polyadenylated nuclear RNA did not associate with polyribosomes and therefore was not translationally active. On the other hand, T0.7 contained three small ORFs, one of which, ORF K12, also known as kaposin (56), encoded a highly hydrophobic 60-amino-acid (aa) peptide. The hydrophobicity of kaposin is similar to that of the 45-aa E5 oncoprotein of bovine papillomavirus type 1 (BPV-1) (7, 61). T0.7 expression was observed in KS tissue of all stages from earliest identifiable to advanced tumors (63). Moreover, in advanced KS tumors, approximately 85% of spindle cells expressed T0.7 RNA.
Due to the fact that T0.7 is an abundant, latency-associated transcript retained in KS and PEL cell line BCBL-1, its transforming ability was tested and compared to that of HHV-8 fragments containing several other unique ORFs, including K4, K5, K6 (pBS/17), and K8 (pBS/199). Each of three constructs encoding kaposin, including (i) pBS/23, a 4.4-kbp HHV-8 genomic fragment containing the T0.7 sequence cloned in the pBS vector, (ii) pBK/T0.7, the T0.7 sequence cloned in the mammalian expression vector pBK-CMV, and (iii) pBK/kap, the 225-bp kaposin gene cloned into pBK-CMV, induced morphologic transformation of Rat-3 cells. Constructs pBS/17 and pBS/199 containing the other unique HHV-8 ORFs, K4, K5, K6, and K8, were nontransforming. Focally transformed cell lines established from Rat-3 cells transfected with pBS/23, pBK/T0.7, and pBK/kap all produced high-grade, highly vascular, undifferentiated sarcomas by 1 to 2 weeks after injection into athymic nu/nu mice. Analysis of the tumor-derived cell lines demonstrated the expression of kaposin mRNA. Furthermore, kaposin protein was detected and localized to the cytoplasm of both the transformed and tumor-derived cells by an indirect immunofluorescence assay (IFA) using a polyclonal rabbit antikaposin antibody raised against a hydrophilic kaposin peptide, kap-2 (aa 42 to 55). More importantly, in this study kaposin protein was also detected in the HHV-8-containing PEL cell lines BCBL-1 (53) and KS-1 (57). The transforming ability of kaposin, its retention and expression in transformed and tumor-derived cells, and its detection in PEL cell lines demonstrate that kaposin is an HHV-8 transforming gene and suggest that kaposin could play a role in the etiology of KS and PEL as well as other HHV-8-associated malignancies.
MATERIALS AND METHODS
DNA and plasmids.
HHV-8 DNA was isolated from the PEL cell line BC-1 (47). Three clones containing unique HHV-8 ORFs were constructed by cloning 4- to 6-kbp, partially digested Sau3A fragments into the BamHI site of pBluescript (pBS; Stratagene). One of these, clone pBS/23, contained nucleotides 116121 to 120497 of the HHV-8 LUR (56) which include the sequence of the T0.7 transcript and the kaposin ORF. The sequence of kaposin in the plasmids used in these studies (56) differed from the sequence of kaposin reported by Zhong et al. (74) by a single nucleotide substitution at codon 38, resulting in a serine-to-glycine substitution.
Plasmid pBK/kap was derived by cloning a 225-bp PCR-amplified kaposin sequence into the EcoRI site of the mammalian expression vector pBK-CMV (Stratagene). The 5′ and 3′ kaposin PCR primers were TCCTCACTCCAATCCCAATGC and CTTTGGGAGGGCACGCTAGCT, respectively. The PCR-amplified fragment was first cloned into pCR2.1 (Invitrogen). The purified EcoRI kaposin fragment from pCR2.1/kap was then cloned into pBK-CMV. Plasmid pBK/T0.7 was constructed in an analogous manner by using a PCR-amplified product containing the T0.7 sequence spanning nucleotides 117431 to 118127 of HHV-8.
pET30b/kap was constructed by cloning the EcoRI fragment of pBK/kap into the EcoRI site of the bacterial expression vector pET30b (Novagen). Orientation was confirmed by sequence analysis. pET30b/kap encodes a 120-aa S-Tag/kaposin fusion protein, with the S-Tag sequence at its N terminus and the kaposin sequence at its C terminus.
Cells and transfection.
Rat-3 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM; Cell Gro/Mediatec) supplemented with 2 mM glutamine, 100 U of penicillin-streptomycin per ml, and 10% bovine calf serum. Tumor-derived cell lines were established and grown in the same medium supplemented with 100 μg of gentamicin per ml. PEL-derived BCBL-1 cells were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM glutamine, and 100 U of penicillin-streptomycin per ml. PEL-derived KS-1 cells were provided as fixed cells on slides by D. Ablashi (Advanced Biotechnologies, Inc.).
For the focal transformation assay, 5 × 104 Rat-3 cells were transfected in 35-mm-diameter petri dishes, in duplicate, with 10 μg of plasmid DNA each by the calcium phosphate method (16). The cells were subcultured into 100-mm-diameter dishes 48 h posttransfection. Morphologically transformed foci were identified and counted 3 to 4 weeks posttransfection. Independent focally transformed cell lines were established by ring isolation. Geneticin (G418)-resistant cell lines were established by selection at a concentration of 400 μg/ml. Selected cells were maintained at a concentration of 200 μg/ml.
R3/kap-1 and R3/kap-2 are Rat-3 cell lines derived from independent pBK/kap-transformed foci. R3/T0.7-1, -2, and -3 and R3/23-1 are similarly derived cell lines from pBK/T0.7- and pBS/23-transformed foci, respectively. R3/BK-G1 and R3/kap-G1 are G418 selected cell lines obtained by transfection with vectors pBK-CMV and pBK/kap, respectively. R3/kap-TL1 and R3/kap-TL2 are tumor-derived cell lines established from tumors induced by R3/kap-1 and R3/kap-2, respectively. Similarly, R3/T0.7-TL1, -TL2, and -TL3 and R3/23-TL1 are tumor-derived cell lines established from tumors induced by R3/T0.7-1, -2, and -3 and R3/23-1, respectively.
PCR analysis.
Genomic DNA was isolated from focally transformed cell lines and control Rat-3 cells by using a genomic DNA isolation kit (Promega). The presence of kaposin sequences was determined by PCR analysis of 200 ng of genomic DNA by using the 5′ and 3′ primers for kaposin described above. This resulted in amplification of a 225-bp PCR product. Negative controls included samples with either Rat-3 genomic DNA, no DNA, or mock-extracted DNA.
Tumorigenicity assay.
The tumorigenic potential of kaposin-transformed cell lines was tested in athymic Ncr nu/nu mice as previously described (18, 67). Cells (5 × 106/100 μl) were injected subcutaneously behind the neck. The mice were monitored every 3 days for the appearance of tumors. Tumor-bearing mice were sacrificed, and tumors were removed for histologic and molecular analyses as well as for establishment of tumor-derived cell lines.
Northern blot analysis.
Cells were lysed in RNAzol B solution, and total cellular RNA was isolated as specified by the manufacturer (Tel-Test, Inc.). Poly(A) RNA was isolated by using an mRNA isolation kit (Pharmacia). Total RNA (15 μg) or poly(A) RNA (5 μg) was separated by electrophoresis through a 1% formaldehyde-agarose gel and blotted overnight to a Zetabind nylon membrane (Micron Separations, Inc.). The membrane was baked at 50°C for 30 min, and the RNA was cross-linked to the membrane by using a UV Stratalinker (Stratagene). The membrane was prehybridized at 42°C for 3 h in 5× Denhardt’s solution–5× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7])–50% formamide, 150 μg of salmon sperm DNA per ml, and 0.1% sodium dodecyl sulfate (SDS). Hybridization was carried out overnight at 42°C in the prehybridization solution containing 10% dextran sulfate and 107 cpm of 32P-labeled T0.7 DNA probe, which was labeled by using a random-primer labeling kit (Amersham). Following hybridization, the membrane was washed twice for 15 min each time with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% SDS at room temperature, once for 15 min with 0.1× SSC–0.1% SDS at room temperature, and twice for 30 min each time with 0.1× SSC–0.1% SDS at 60°C. Hybridized 32P label was detected by phosphorimager analysis using ImageQuant software.
Antibodies.
Anti-kap-1 and anti-kap-2 polyclonal antibodies were generated by inoculation of rabbits with synthetic kaposin peptides, synthesized as eight-chained lysine branched molecules. The kap-1 peptide, DVLLNGWRWRLGAI (aa 15 to 29), and the kap-2 peptide, PSGQRGPVAFRTRV (aa 42 to 55), were chosen because of their hydrophilic nature. Both antibodies were generated by Chemicon International Inc.
Western blot and immunoprecipitation analyses.
Escherichia coli BL21(DE3)(pLysS) cells were transformed with pET30b or pET30b/kap. Cultures were induced at 37°C by addition of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) as specified by the manufacturer protocols (Novagen). Cell pellets were solubilized by SDS-gel loading buffer (50 mM Tris HCl [pH 6.8], 100 mM dithiothreitol, 2% SDS, 10% glycerol, 0.1% bromophenol blue). Proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE) (4 to 20% gel) and transferred to polyvinylidene difluoride (Immobilon-P; Millipore) membranes. The S-Tag/kaposin fusion protein was detected by Western blot analysis using (i) the anti-kap-2 antibody (1:500 dilution in TNET [10 mM Tris {pH 7.5}, 50 mM NaCl, 2.5 mM EDTA, 0.1% Tween 20]) as previously described (51) or (ii) the S-protein/alkaline phosphatase conjugate (1:5,000 in TBST [20 mM Tris {pH 7.6}, 137 mM NaCl, 0.1% Tween 20]) detected with Western-Light reagents as specified by the manufacturer (Tropix).
For immunoprecipitation, the kaposin protein was purified in E. coli as described elsewhere (29a), incubated with 5 μl of anti-kap-2 antibody, and processed by standard immunoprecipitation techniques (51). Immunoprecipitated kaposin protein was detected by Western blot analysis as described above.
Immunofluorescence.
Adherent cells were grown on coverslips as confluent monolayers, fixed in 4% paraformaldehyde for 10 min at room temperature, washed with phosphate-buffered saline (PBS) three times for 5 min each, and incubated with a 1:100 dilution of a rabbit polyclonal anti-kap-2 antibody or preimmune serum at 37°C for 2 h in a humidified chamber. Suspension cells (106 cells/ml) were spotted on polylysine-coated wells on a slide and placed at 4°C for 45 min. The medium was carefully removed, and the cells were air dried at room temperature for 10 min. Cells were then fixed in cold acetone at room temperature for 15 min and incubated with a 1:5 dilution of either the anti-kap-1 or anti-kap-2 antibody or with preimmune serum as described above. In both cases, following incubation with primary antibody, cells were washed with PBS as described above and incubated with fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin G at room temperature for 1 h in the dark. Following three washes with PBS, the cells were mounted with 50% glycerol in PBS containing 5% 4,6-diamidino-2-phenylindole (DAPI) to counterstain the nuclei. Immunofluorescence was detected in a Zeiss Axiophot fluorescence microscope.
RESULTS
Kaposin induced tumorigenic transformation of Rat-3 cells.
Four- to 6-kbp fragments of HHV-8 genomic DNA, partially digested with Sau3A, were cloned into the BamHI site of pBS vector. Constructs which contained unique HHV-8 ORFs, i.e., pBS/17 (ORFs K4, K5, and K6), pBS/23 (ORF K12, i.e., kaposin), and pBS/199 (ORF K8) (Fig. 1), were transfected into Rat-3 cells to test for focus-forming ability. Background levels of foci were observed for clones pBS/17 and pBS/199 as well as for mock- and pBS-transfected cells (Table 1, experiment 1). In contrast, clone pBS/23 exhibited numerous morphologically altered, highly refractile foci not seen in mock- or pBS-transfected cells, indicating the transforming activity of this clone.
FIG. 1.
Map of HHV-8 LUR showing the locations of the conserved and unique ORFs, including sequences tested for transforming ability, and a schematic diagram of the ∼140-kb LUR sequence flanked by terminal repeats (TR). HHV-8 fragments tested in the focus-forming assay (17, 23, and 199) are shown as solid bars below the map. The locations of T0.7 and kaposin (K12) sequences within fragment 23 are indicated. The solid arrows indicate HHV-8 ORFs conserved in HVS, and the open arrows indicate the unique HHV-8 ORFs. Repeat regions are shown as small filled rectangles above the ORFs (frnk, vnct, waka/jwka, zppa, moi, and mdsk). KS330 and KS631 are the fragments of HHV-8 that were identified first. CBP, complement binding protein; ss DBP, single-stranded DNA binding protein; gB, glycoprotein B; DNA pol, DNA polymerase; DHFR, dihydrofolate reductase; TS, thymidylate synthetase; MIP, macrophage inflammatory protein; nut-1, nuclear transcript 1; Teg, tegument protein; TK, thymidine kinase; gH, glycoprotein H; MCP, major capsid protein; gM, glycoprotein M; UDG, uracil DNA glucosidase; gL, glycoprotein L; R-trans, transactivator; gX, glycoprotein X; vIRF, viral interferon regulatory factor; RRS, ribonucleotide reductase, small; RRL, ribonucleotide reductase, large; CycD, cyclin D homolog; Adh, immunoglobulin family adhesion protein; GCR, G-protein-coupled receptor. The map was adapted from Fig. 1 of reference 56 with the permission of the publisher.
TABLE 1.
The HHV-8 kaposin gene induces focal transformation of Rat-3 cellsa
| Expt | Transfected DNA | Concn (μg/5 × 104 cells) | No. of foci/2 dishes |
|---|---|---|---|
| 1 | pBS/17 | 10 | 4 |
| pBS/23 | 10 | 32 | |
| pBS/199 | 10 | 0 | |
| pBS | 10 | 6 | |
| Mock | 0 | 1 | |
| 2 | pBK/mtrII | 10 | 70 |
| pBK/T0.7 | 10 | 65 | |
| pBK/kap | 10 | 45 | |
| pBK-CMV | 10 | 6 | |
| Mock | 0 | 1 |
Cloned HHV-8 DNA fragments were transfected into Rat-3 cells by the calcium phosphate method. The HCMV mtrII DNA served as a positive control. Transfected cells were subcultured at 2 days posttransfection and refed with DMEM supplemented with 7% bovine calf serum every 3 days. Foci were counted at 3 to 4 weeks posttransfection. The numbers are representative of the total foci observed in two independent experiments.
To determine if the transforming activity of pBS/23 was due to kaposin (ORF K12), both the T0.7 sequence and the kaposin gene were subcloned into the mammalian expression vector pBK-CMV and assessed for their transforming ability. Plasmid pBK/mtrII, containing the human cytomegalovirus (HCMV) transforming gene mtrII (UL111a) (68), was used as a positive control. Like clone pBS/23, both pBK/T0.7 and pBK/kap induced similar levels of morphologically altered foci (Table 1, experiment 2, and Fig. 2A). Background levels of foci were seen in mock- and vector-transfected cells. Similar levels of transforming activity observed with pBS/23, pBK/T0.7, and pBK/kap indicate that the transforming activity resides in kaposin.
FIG. 2.
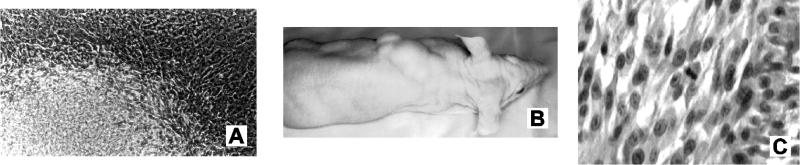
Kaposin-transformed Rat-3 cells induced high-grade undifferentiated sarcomas in athymic nu/nu mice. (A) Typical focus induced in pBK/kap-transfected Rat-3 cells. The focus of refractile randomly oriented multilayered transformed cells is shown against a background of parental Rat-3 cells (magnification, ×60). (B) Tumor development in an athymic nu/nu mouse at 2 weeks postinoculation with kaposin-transformed Rat-3 cells. (C) Representative section of a tumor induced by kaposin-transformed Rat-3 cells, fixed with formalin and stained with hematoxylin and eosin. Magnification, ×200.
Independent transformed foci were isolated and expanded into focal cell lines. Genomic DNA isolated from the cell lines was analyzed for the presence of kaposin sequences by PCR amplification. Kaposin-positive, transformed Rat-3 cell lines R3/23-1 (pBS/23 transfected), R3/T0.7-1, R3/T0.7-2, and R3/T0.7-3 (pBK/T0.7 transfected), and R3/kap-1 and R3/kap-2 (pBK/kap transfected) were tested for tumorigenicity by injection into athymic Ncr nu/nu mice (Table 2, experiment 1). All cell lines containing kaposin sequences, whether derived from transfection by pBS/23, pBK/T0.7, or pBK/kap (Fig. 2B and C), induced highly vascular, high-grade, undifferentiated sarcomas within 1 to 2 weeks of injection, whereas Rat-3 cells did not.
TABLE 2.
Kaposin-transformed Rat-3 cells are tumorigenic in athymic nu/nu micea
| Expt | Cell line | PCR positive for kaposin sequence | No. of mice with tumor(s)/no. injected | Latency (wk) |
|---|---|---|---|---|
| 1 | R3/23-1 | + | 5/5 | 1 |
| R3/T0.7-1 | + | 5/5 | 1 | |
| R3/T0.7-2 | + | 4/4 | 1 | |
| R3/T0.7-3 | + | 4/4 | 1–2 | |
| R3/kap-1 | + | 5/5 | 1 | |
| R3/kap-2 | + | 3/4 | 1–2 | |
| Rat-3 | − | 0/3 | ||
| 2 | R3/kap-G1 | NT | 4/4 | 1 |
| R3/BK-G1 | NT | 0/4 |
Each mouse was injected subcutaneously behind the neck with 5 × 106 cells and examined twice each week. Some tumors were greater than 1 cm in diameter by 2 weeks. NT, not tested.
In addition to the focal cell lines tested above, Rat-3 cells transfected with pBK-CMV or pBK/kap were selected for resistance to G418 and tested for tumorigenicity (Table 2, experiment 2). The R3/kap-G1 (pBK/kap-transfected) cell line exhibited a transformed morphology similar to focally derived R3/kap-1 and -2 cells and were tumorigenic. In contrast, the R3/BK-G1 (pBK-CMV vector-transfected) cell line maintained a morphology similar to parental Rat-3 cells and was not tumorigenic. Thus, both G418-selected as well as focally derived pBK/kap-transfected Rat-3 cells exhibited morphologic and tumorigenic transformation.
Kaposin mRNA is expressed in tumor-derived cell lines.
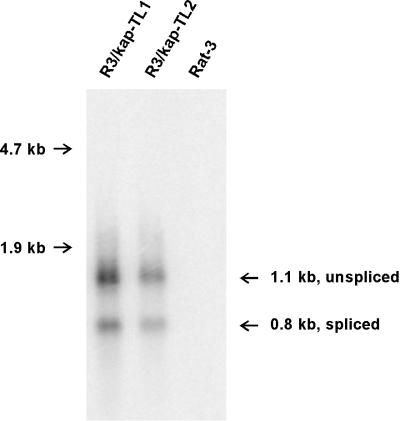
Total RNA was extracted from tumor-derived cell lines R3/kap-TL1 and R3/kap-TL2, and kaposin mRNA was detected by a Northern blot analysis using 32P-labeled kaposin DNA probe (Fig. 3). Two kaposin-specific RNA species were detected at 0.8 and 1.1 kb in the RNA from the two tumor-derived lines, whereas no kaposin signal was observed in the RNA from control Rat-3 cells. The two RNA bands observed result from splicing of the message at the simian virus 40 3′-splice acceptor site downstream of the kaposin insertion site in the pBK/kap construct. Their sizes are consistent with expected sizes of polyadenylated spliced and unspliced messages, respectively, transcribed from pBK/kap. Kaposin-specific messages were also observed in poly(A)+ mRNA isolated from the above-specified two cell lines as well as from R3/T0.7-TL1, -TL2, and -TL3 tumor-derived cell lines (data not shown). Demonstration of the retention and expression of the kaposin gene in the tumor-derived cell lines correlates with the observation of expression of the T0.7 kaposin message in all stages of KS tumors in vivo (63).
FIG. 3.
Expression of kaposin mRNA in tumor-derived cell lines. Total RNA was extracted from Rat-3, R3/kap-TL1, and R3/kap-TL2 cells. The RNA was separated on a 1% formaldehyde-agarose gel, blotted onto a Zetabind nylon membrane, and probed under stringent conditions with 32P-labeled kaposin DNA. The 1.1-kb unspliced and 0.8-kb spliced messages for kaposin are shown. Positions of 28S (4.7-kb) and 18S (1.9-kb) rRNA markers are indicated on the left.
Antibodies to hydrophilic kaposin peptides recognize bacterially expressed kaposin.
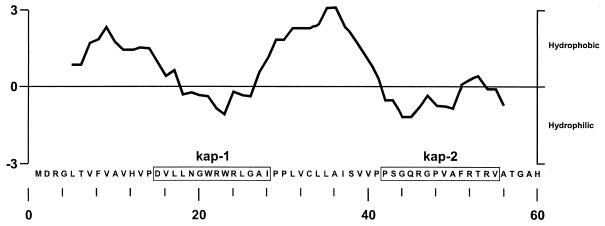
Polyclonal rabbit antisera were generated against two hydrophilic kaposin peptides, aa 15 to 29 (kap-1) and aa 42 to 55 (kap-2) (Fig. 4). The efficacy of anti-kap-2 antibody was determined by its reactivity to a 120-aa S-Tag/kaposin fusion protein produced in E. coli. A protein band with an apparent molecular mass of 17 kDa was detected in the extract of IPTG-induced cells carrying plasmid pET30b/kap by Western blot analysis using the S-protein/alkaline phosphatase conjugate, which binds to the S-Tag (Fig. 5A). No corresponding band was detected in uninduced cells or in cells carrying the vector pET30b. The membrane was then stripped and analyzed by Western blotting using the anti-kap-2 antibody (Fig. 5B). The identical 17-kDa band was observed in the extract of induced cells with pET30b/kap but not in the other samples. Moreover, the 17-kDa band was also detected with the anti-kap-1 antibody in a Western blot but not with preimmune serum (data not shown). Furthermore, kaposin purified from the S-Tag/kaposin fusion protein was immunoprecipitated by anti-kap-2 antibody but not by preimmune serum (29a). Taken together, these observations demonstrated that antikaposin antibody recognized both the linear and native conformations of kaposin protein.
FIG. 4.
Kyte-Doolittle hydrophobicity plot of kaposin showing the predominant hydrophobic domains and locations of the hydrophilic kaposin peptides used to generate the antikaposin antibodies. kap-1 and kap-2 are hydrophilic peptide sequences used to generate anti-kap-1 and anti-kap-2 antibodies, respectively.
FIG. 5.
Anti-kap-2 antibody detected bacterially expressed kaposin fusion protein. E. coli BL21(DE3)(pLysS), transformed by either pET30b-kap or pET30b, was induced for 90 min with 1 mM IPTG (I) or uninduced (U). Proteins were extracted in SDS-gel dye buffer, separated by SDS-PAGE, and electroblotted onto Immobilon-P membranes. (A) Western blot showing the 17 kDa S-Tag/kaposin fusion protein in the pET30b-kap (lane I), using the S-Tag/alkaline phosphatase conjugate (Novagen). (B) Western blot showing the S-Tag/kaposin fusion protein seen in panel A detected with anti-kap-2 antibody. Sizes on the left are indicated in kilodaltons.
Kaposin protein localized to the cytoplasm in transformed and tumor-derived cells.
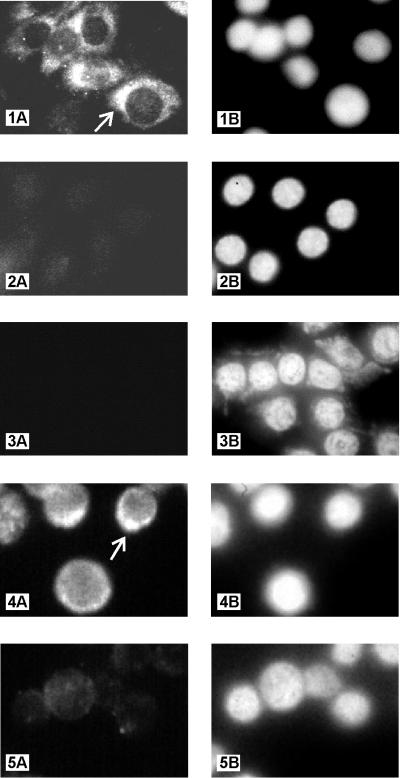
Kaposin protein was detected in transformed Rat-3 cells and tumor-derived cells by an IFA using the anti-kap-2 antibody. Positive anti-kap-2 antibody staining was observed in the tumor-derived cell line R3/kap-TL1 and predominantly localized to a restricted region of the cytoplasm (Fig. 6, panel 1A). Nuclei in the same field of cells were visualized by counterstaining with DAPI (panel 1B). No antibody staining of R3/kap-TL1 cells was observed with preimmune serum (panel 2A), while the presence of cells was demonstrated by DAPI counterstaining of nuclei (panel 2B). Moreover, no staining was observed with anti-kap-2 antibody in the stable Rat-3 cell line transfected with the vector pBK-CMV (panel 3A). Again, DAPI counterstaining revealed the presence of cells in the field (panel 3B). Positive staining was also observed with the kaposin-transformed cell line R3/kap-1, which produced tumors in athymic nu/nu mice (data not shown). These results demonstrate that kaposin protein is expressed in transformed and tumor-derived cells.
FIG. 6.
Detection and localization of kaposin protein in transformed Rat-3 cells, tumor-derived cells, and the PEL cell line BCBL-1. IFA shows cytoplasmic staining of kaposin protein in tumor-derived cell line R3/kap-TL1, using anti-kap-2 antibody (1:50) (panel 1A). Also shown are R3/kap-TL1 cells stained with preimmune serum (1:50) (panel 2A) and vector-transfected cell line R3/BK-G1 stained with anti-kap-2 antibody (1:50) (panel 3A). BCBL-1 cells stained with anti-kap-2 antibody (1:5) and preimmune serum (1:5) are shown in panels 4A and 5A, respectively. Intense staining with anti-kap-2 antibody in a restricted region of the cytoplasm of R3/kap-TL1 and BCBL-1 cells are indicated by arrows in panels 1A and 4A, respectively. DAPI staining of nuclei of the above-specified fields are shown in panels 1B, 2B, 3B, 4B, and 5B, respectively. Magnifications: panels 1 to 3 (A and B), ×1,800; panels 4 and 5 (A and B), ×3,000.
Kaposin protein is expressed in the PEL cell lines BCBL-1 and KS-1.
Kaposin protein was also detected by IFA in BCBL-1 cells. Positive anti-kap-2 antibody staining was observed with predominant staining localized to a restricted region of the cytoplasm (Fig. 6, panel 4A). Nuclei in the same field of cells was visualized by counterstaining with DAPI (panel 4B). Similar results were observed with anti-kap-1 antiserum (data not shown). Minimal background staining was observed with preimmune serum (panel 5A), and the presence of cells in the field was demonstrated by counterstaining the nuclei with DAPI (panel 5B). Kaposin protein was also detected by IFA in another HHV-8-containing PEL cell line, KS-1 (data not shown). With either anti-kap-2 or anti-kap-1 antibody, over 90% of BCBL-1 and KS-1 cells were stained. These findings demonstrate that kaposin protein is expressed in cell lines derived from HHV-8-associated lymphoid tumors like PEL and that this expression occurs in latently infected cells.
DISCUSSION
The data in this study demonstrated that the unique HHV-8 kaposin gene, ORF K12, morphologically transformed Rat-3 cells which were tumorigenic when inoculated into athymic nu/nu mice. The tumors developed with a very short latency period (1 to 2 weeks) and were highly vascular, high-grade, undifferentiated fibrosarcomas. Neoplastic transformation occurred whether kaposin was expressed from its endogenous promoter or from the heterologous CMV immediate-early promoter. Of significance, kaposin mRNA and protein were detected in both the focally transformed and tumor-derived cells (Fig. 6), suggesting that kaposin expression is required for both induction of transformation and maintenance of the tumorigenic phenotype. Retention and expression of viral oncoproteins is a hallmark of transformation by DNA tumor viruses including simian virus 40, polyomavirus, adenovirus, CMV, and HHV-6 (26, 33, 39, 51, 55, 68, 73). Although the abundant, latency-associated transcript T0.7 has been observed in all stages of KS tumors of different epidemiologic origin as well as in PEL (63, 74), expression of kaposin protein from this transcript has not been reported. This study has demonstrated, for the first time, the expression of kaposin protein in the PEL cell lines BCBL-1 and KS-1. As with the Rat-3-transformed and tumor-derived cells, kaposin protein localized predominantly to a restricted region of the cytoplasm of BCBL-1 and KS-1 cells. The ability of kaposin to induce tumorigenic transformation in rodents and its expression in PEL-derived cells suggests that kaposin may play a role in the development of KS and PEL.
The level of transforming activity of kaposin in the rodent cell focus-forming assay was 1 to 2 orders of magnitude lower than that reported for cellular or retroviral oncogenes in similar assay systems. However, the level is comparable to and representative of that reported for other herpesvirus transforming genes like HCMV mtrII (UL111a) (30, 31), HSV-2 mtrIII (UL39) (23, 32, 54), and HHV-6 ORF-1 (67). A similar low level of transforming activity has also been reported for the human papillomavirus type 16 oncogene E7 (69, 72).
Comparison of the genome of HHV-8 to those of HVS and Epstein-Barr virus (EBV) has revealed the presence of extensive conserved collinear regions as well as unique sequences (56). The transforming genes of HVS and EBV are unique to each of these herpesviruses. HVS is a T-cell-specific oncogenic virus capable of inducing lymphoproliferative disorders in natural or experimental hosts and transforming lymphoid cells in vitro (43, 44). Two HVS proteins have been associated with transforming activities (42). The saimiri transformation-associated protein is a membrane-associated phosphoprotein with a highly acidic amino terminus, central collagen-like repeats, and a hydrophobic carboxy terminus that has been shown to induce oncogenic transformation via interaction with cellular ras, resulting in activation of the Ras signaling pathway (35). Another protein, tyrosine kinase-interacting protein, binds to p56lck tyrosine kinase and significantly increases its kinase activity. It has been postulated that this activated kinase feeds into the Ras and protein kinase C signaling pathways (40). EBV, on the other hand, encodes a latent membrane protein, LMP2a, which binds stably to a Src family kinase in B cells and down-regulates Src kinase activity and thus maintains latency of the virus (9). However, none of the transforming genes of either HVS or EBV have been conserved in HHV-8. The kaposin transforming gene identified in this study is one of the unique HHV-8 ORFs.
The HHV-8 genome also contains ORFs that are functional homologs of cellular proto-oncogenes such as those encoding cyclin D (12, 15, 27, 38), GPCR (3), bcl-2 (17, 59), and IL-6 (48, 52). Of these, the v-IL-6, v-cyclin D, and v-GPCR genes have been shown to enhance cell proliferation (3, 52, 66). More recently, the v-GPCR gene has been reported to induce tumorigenic transformation in rodent cells and activate angiogenesis (4). However, v-GPCR mRNA is expressed as a lytic transcript upon tetradecanoyl phorbol acetate induction in the PEL cell line BC-1 (58). Furthermore, it has been reported that in HHV-8-containing cell lines such as BCBL-1 and BCP-1, only a minor population of cells are in the lytic state (4). In addition, using in situ hybridization with the T1.1 probe and colocalization with the major capsid protein RNA, Staskus et al. (63) have determined that only about 10% of HHV-8-infected cells in KS lesions are in the lytic state. The K9 ORF (vIRF), unique to HHV-8, has also been identified as an oncogene which inhibited the interferon signaling pathway (24). Because K9 expression was detected in an HHV-8-infected B-cell line but not in KS tissue, Gao et al. (24) proposed its putative role in B-cell malignancies. In contrast to both v-GPCR and K9, kaposin is expressed as an abundant, latency-associated transcript in both KS and PEL (63, 74).
In this study, fragments containing other ORFs unique to HHV-8 were tested for their transforming ability. Transformation was not observed with constructs of two HHV-8 fragments, pBS/17 containing unique HHV-8 ORFs K4, K5, and K6 and pBS/199 with K8. Although this observation indicated the specificity of transformation seen with pBS/23 containing kaposin, no definitive conclusions can be made about the oncogenic potential of the K4, K5, K6, and K8 ORFs because their expression in transfected cells was not analyzed. In conclusion, the kaposin transforming gene identified in this study is a unique HHV-8 ORF that is encoded by the abundant, latency-associated transcript expressed in both KS and PEL.
In vitro evidence suggests that autocrine and paracrine growth effects of cytokines, growth factors, and their receptors play an important role in the pathogenesis of KS, both AIDS associated, and non-AIDS associated. AIDS-associated KS-derived cells have been shown to respond to and express high levels of an endothelial cell growth factor, IL-1β, basic fibroblast growth factor (bFGF), and IL-6 (21, 46). In addition, the HIV type 1 Tat protein has been implicated in the pathogenesis of AIDS-associated KS, either directly by activation of HHV-8 replication (29) or indirectly by activation of cytokines (22). The Tat protein has also been shown to act in synergy with bFGF in inducing KS-like lesions in mice (20). The importance of similar autocrine and paracrine growth effects in non-AIDS-associated KS has also been documented. Various growth factors and cytokines including bFGF, IL-6, platelet-derived growth factor B (PDGF-B), vascular endothelial growth factor, and oncostatin M can serve as modulators of KS cell proliferation in vitro (21, 37, 41, 45, 70). KS-derived cells have also been shown to express a functional FGF receptor, flg (37), and PDGF-A-type and PDGF-B-type receptors (71). Constitutive expression and activation of growth factors or their specific receptors could therefore serve as a mechanism by which viral oncogenes and oncoproteins create an autocrine growth loop that leads to self-sustained aberrant growth.
Similarities in size and hydrophobicity between kaposin and the BPV-1 E5 protein suggest that they might have common mechanisms for transformation (Fig. 4) (61). BPV-1 E5 is a small 44-aa protein with a strongly hydrophobic N terminus that has been localized to Golgi apparatus and endoplasmic reticulum (ER) membranes of transformed cells (8). In the present study, kaposin, a small 60-aa protein with N- and C-terminal hydrophobic domains, was also localized to the cytoplasm of transformed and tumor-derived cells by using an antibody raised against a hydrophilic kaposin peptide. Interestingly, kaposin protein was detected predominantly in a restricted area of the cytoplasm, suggestive of the Golgi apparatus and ER membranes. In support of these findings, AU1 epitope-tagged kaposin also localized to the Golgi apparatus and ER membranes in a transient transfection assay (1). E5 has been shown to bind to the 16-kDa component of the vacuolar H+-ATPase which is important in processing growth factor receptors (28). Moreover, the interaction of E5 with the type β PDGF receptor (PDGFR) transmembrane domain which results in constitutive PDGFR activation is required for transformation (19). Therefore, it is possible that kaposin also transforms cells by activation of growth factor receptors such as PDGFR which are processed in the Golgi complex. In summary, the data presented here demonstrate that the HHV-8 kaposin gene (ORF K12) induced tumorigenic transformation and that the kaposin protein is expressed in PEL-derived cell lines. Thus, it may play a role in the development of KS, PEL, and other HHV-8-associated malignancies.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant CA 60577 from the National Institutes of Health and in part by a contract from the National Foundation for Cancer Research (Bethesda, Md.). Assistance with the tumorigenicity studies was provided by the Lombardi Cancer Research Center Animal Care Facility supported by Public Health Service grant P30 CA51008-09.
We thank Chemicon International Inc. for generating antibodies against kap-1 and kap-2 peptides and D. Ablashi of Advanced Biotechnologies Inc. for providing fixed KS-1 cells.
REFERENCES
- 1.Adduci, A., et al. Unpublished data.
- 1a.Ali M A, McWeeney D, Milosavljevic A, Jurka J, Jariwalla R J. Enhanced malignant transformation induced by expression of a distinct protein domain of ribonucleotide reductase large subunit from herpes simplex virus type 2. Proc Natl Acad Sci USA. 1991;88:8257–8261. doi: 10.1073/pnas.88.18.8257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambroziak J A, Blackbourn D J, Herndier B G, Glogau R G, Gullett J H, McDonald A R, Lennette E T, Levy J A. Herpes-like sequences in HIV-infected and uninfected Kaposi’s sarcoma patients. Science. 1995;268:582–583. doi: 10.1126/science.7725108. [DOI] [PubMed] [Google Scholar]
- 3.Arvanitakis L, Geras-Raaka E, Varma A, Gershengorn M C, Cesarman E. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature. 1997;385:347–350. doi: 10.1038/385347a0. [DOI] [PubMed] [Google Scholar]
- 4.Bais C, Santomasso B, Coso O, Arvanitakis L, Raaka E G, Gutkind J S, Asch A S, Cesarman E, Gerhengorn M C, Mesri E A. G-protein-coupled receptor of Kaposi’s sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature. 1998;391:86–89. doi: 10.1038/34193. [DOI] [PubMed] [Google Scholar]
- 5.Beral V. Epidemiology of Kaposi’s sarcoma. Cancer Surv. 1991;10:5–22. [PubMed] [Google Scholar]
- 6.Boshoff C, Whitby D, Hatziioannou T, Fisher C, van der Walt J, Hatzakis A, Weiss R, Schulz T. Kaposi’s-sarcoma-associated herpesvirus in HIV-negative Kaposi’s sarcoma. Lancet. 1995;345:1043–1044. doi: 10.1016/s0140-6736(95)90780-7. [DOI] [PubMed] [Google Scholar]
- 7.Burkhardt A, DiMaio D, Schlegel R. Genetic and biochemical definition of the bovine papillomavirus E5 transforming protein. EMBO J. 1987;6:2381–2385. doi: 10.1002/j.1460-2075.1987.tb02515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burkhardt A, Willingham M, Gay C, Jeang K-T, Schlegel R. The E5 oncoprotein of bovine papillomavirus is oriented asymmetrically in Golgi and plasma membranes. Virology. 1989;170:334–339. doi: 10.1016/0042-6822(89)90391-7. [DOI] [PubMed] [Google Scholar]
- 9.Burkhardt A L, Bolen J B, Kieff E, Longnecker R. An Epstein-Barr virus transformation-associated membrane protein interacts with src family tyrosine kinases. J Virol. 1992;66:5161–5167. doi: 10.1128/jvi.66.8.5161-5167.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cesarman E, Chang Y, Moore P S, Said J W, Knowles D M. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 11.Cesarman E, Moore P S, Rao P H, Inghirami G, Knowles D M, Chang Y. In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1 and BC-2) containing Kaposi’s sarcoma-associated herpesvirus-like (KSHV) DNA sequences. Blood. 1995;86:2708–2714. [PubMed] [Google Scholar]
- 12.Cesarman E, Nador R G, Bai F, Bohenzky R A, Russo J J, Moore P S, Chang Y, Knowles D M. Kaposi’s sarcoma-associated herpesvirus contains G protein-coupled receptor and cyclin D homologs which are expressed in Kaposi’s sarcoma and malignant lymphoma. J Virol. 1996;70:8218–8223. doi: 10.1128/jvi.70.11.8218-8223.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chadburn A, Cesarman E, Jagirdar J, Subar M, Mir R N, Knowles D M. CD30 (Ki-1) positive anaplastic large cell lymphomas in individuals infected with the human immunodeficiency virus. Cancer. 1993;72:3078–3090. doi: 10.1002/1097-0142(19931115)72:10<3078::aid-cncr2820721033>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 14.Chang Y, Cesarman E, Pessin M S, Lee F, Culpepper J, Knowles D M, Moore P S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;265:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 15.Chang Y, Moore P S, Talbot S J, Boshoff C H, Zarkowska T, Godden-Kent D, Paterson H, Weiss R A, Mittnacht S. Cyclin encoded by KS herpesvirus. Nature. 1996;382:410. doi: 10.1038/382410a0. [DOI] [PubMed] [Google Scholar]
- 16.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng E H, Nicholas J, Bellows D S, Hayward G S, Guo H G, Reitz M S, Hardwick J M. A Bcl-2 homolog encoded by Kaposi sarcoma-associated virus, human herpesvirus 8, inhibits apoptosis but does not heterodimerize with Bax or Bak. Proc Natl Acad Sci USA. 1997;94:690–694. doi: 10.1073/pnas.94.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clanton D J, Jariwalla R J, Kress C, Rosenthal L J. Neoplastic transformation by a cloned human cytomegalovirus DNA fragment uniquely homologous to one of the transforming regions of herpes simplex virus type 2. Proc Natl Acad Sci USA. 1983;80:3826–3830. doi: 10.1073/pnas.80.12.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen B D, Goldstein D J, Rutledge L, Vass W C, Lowy D R, Schlegel R, Schiller J T. Transformation-specific interaction of the bovine papillomavirus E5 oncoprotein with the platelet-derived growth factor receptor transmembrane domain and the epidermal growth factor receptor cytoplasmic domain. J Virol. 1993;67:5303–5311. doi: 10.1128/jvi.67.9.5303-5311.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ensoli B, Gendelman R, Markham P, Fiorelli V, Colombini S, Raffeld M, Cafaro A, Chang H K, Brady J N, Gallo R C. Synergy between basic fibroblast growth factor and HIV-1 Tat protein in induction of Kaposi’s sarcoma. Nature. 1994;371:674–680. doi: 10.1038/371674a0. [DOI] [PubMed] [Google Scholar]
- 21.Ensoli B, Nakamura S, Salahuddin S Z, Biberfeld P, Larsson L, Beaver B, Wong-Staal F, Gallo R C. AIDS-Kaposi’s sarcoma-derived cells express cytokines with autocrine and paracrine growth effects. Science. 1989;243:223–226. doi: 10.1126/science.2643161. [DOI] [PubMed] [Google Scholar]
- 22.Fiorelli V, Gendelman R, Samaniego F, Markham P D, Ensoli B. Cytokines from activated T cells induce normal endothelial cells to acquire the phenotypic and functional features of AIDS-Kaposi’s sarcoma spindle cells. J Clin Invest. 1995;95:1723–1734. doi: 10.1172/JCI117849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galloway D A, Nelson J A, McDougall J K. Small fragments of herpesvirus DNA with transforming activity contain insertion sequence-like structures. Proc Natl Acad Sci USA. 1984;81:4736–4740. doi: 10.1073/pnas.81.15.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao S J, Boshoff C, Jayachandra S, Weiss R A, Chang Y, Moore P. KSHV ORF K9 is an oncogene which inhibits the interferon signaling pathway. Oncogene. 1997;15:1979–1985. doi: 10.1038/sj.onc.1201571. [DOI] [PubMed] [Google Scholar]
- 25.Gao S J, Kingsley L, Li M, Zheng W, Parravicini C, Ziegler J, Newton R, Rinaldo C R, Saah A, Phair J, Detels R, Chang Y, Moore P S. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat Med. 1996;2:925–928. doi: 10.1038/nm0896-925. [DOI] [PubMed] [Google Scholar]
- 26.Garcea R L, Talmage D A, Harmatz A, Freund R, Benjamin T L. Separation of host range from transformation functions of the hr-t gene of polyomavirus. Virology. 1989;168:312–319. doi: 10.1016/0042-6822(89)90271-7. [DOI] [PubMed] [Google Scholar]
- 27.Godden-Kent D, Talbot S J, Boshoff C, Chang Y, Moore P, Weiss R A, Mittnacht S. The cyclin encoded by Kaposi’s sarcoma-associated herpesvirus stimulates cdk6 to phosphorylate the retinoblastoma protein and histone H1. J Virol. 1997;71:4193–4198. doi: 10.1128/jvi.71.6.4193-4198.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldstein D J, Finbow M E, Andresson T, McLean P, Smith K, Bubb V, Schlegel R. Bovine papillomavirus E5 oncoprotein binds to the 16k component of vacuolar H(+)-ATPases. Nature. 1991;352:347–349. doi: 10.1038/352347a0. [DOI] [PubMed] [Google Scholar]
- 29.Harrington W, Jr, Sieczkowski L, Sosa C, Chan-a-Sue S, Cai J P, Cabral L, Wood C. Activation of HHV-8 by HIV-1 tat. Lancet. 1997;349:774–775. doi: 10.1016/s0140-6736(05)60199-7. [DOI] [PubMed] [Google Scholar]
- 29a.Hassani, M., et al. Unpublished data.
- 30.Inamdar A, Thompson J, Kashanchi F, Doniger J, Brady J N, Rosenthal L J. Identification of two promoters within human cytomegalovirus morphologic transforming region II. Intervirology. 1992;34:146–153. doi: 10.1159/000150275. [DOI] [PubMed] [Google Scholar]
- 31.Jahan N, Razzaque A, Brady J, Rosenthal L J. The human cytomegalovirus mtrII colinear region in strain Tanaka is transformation defective. J Virol. 1989;63:2866–2869. doi: 10.1128/jvi.63.6.2866-2869.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jariwalla R J, Aurelian L, Ts’o P O. Tumorigenic transformation induced by a specific fragment of DNA from herpes simplex virus type 2. Proc Natl Acad Sci USA. 1980;77:2279–2283. doi: 10.1073/pnas.77.4.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kashanchi F, Araujo J C, Doniger J, Muralidhar S, Khleif S, Mendleson E, Thompson J, Azumi N, Brady J N, Luppi M, Torelli G, Rosenthal L J. Human herpesvirus type 6 (HHV-6) ORF-1 transactivating gene exhibits malignant transforming activity and its protein binds to p53. Oncogene. 1997;14:359–367. doi: 10.1038/sj.onc.1200840. [DOI] [PubMed] [Google Scholar]
- 34.Kedes D H, Operskalski E, Busch M, Kohn R, Flood J, Ganem D. The seroepidemiology of human herpesvirus 8 (Kaposi’s sarcoma-associated herpesvirus): distribution of infection in KS risk groups and evidence for sexual transmission. Nat Med. 1996;2:918–924. doi: 10.1038/nm0896-918. [DOI] [PubMed] [Google Scholar]
- 35.Lee H, Trimble J J, Yoon D W, Regier D, Desrosiers R C, Jung J U. Genetic variation of herpesvirus saimiri subgroup A transforming protein and its association with cellular Src. J Virol. 1997;71:3817–3825. doi: 10.1128/jvi.71.5.3817-3825.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lennette E T, Blackbourn D J, Levy J A. Antibodies to human herpesvirus type 8 in the general population and in Kaposi’s sarcoma patients. Lancet. 1996;348:858–861. doi: 10.1016/S0140-6736(96)03240-0. [DOI] [PubMed] [Google Scholar]
- 37.Li J J, Huang Y Q, Moscatelli D, Nicolaides A, Zhang W C, Friedman-Kien A E. Expression of fibroblast growth factors and their receptors in acquired immunodeficiency syndrome-associated Kaposi sarcoma tissue and derived cells. Cancer. 1993;72:2253–2259. doi: 10.1002/1097-0142(19931001)72:7<2253::aid-cncr2820720732>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 38.Li M, Lee H, Yoon D W, Albrecht J C, Fleckenstein B, Neipel F, Jung J U. Kaposi’s sarcoma-associated herpesvirus encodes a functional cyclin. J Virol. 1997;71:1984–1991. doi: 10.1128/jvi.71.3.1984-1991.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Linzer D I, Levine A J. Characterization of a 54k dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979;17:43–52. doi: 10.1016/0092-8674(79)90293-9. [DOI] [PubMed] [Google Scholar]
- 40.Lund T, Medveczky M M, Medveczky P G. Herpesvirus saimiri Tip-484 membrane protein markedly increases p56lck activity in T cells. J Virol. 1997;71:378–382. doi: 10.1128/jvi.71.1.378-382.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masood R, Cai J, Zheng T, Smith D L, Naidu Y, Gill P S. Vascular endothelial growth factor/vascular permeability factor is an autocrine growth factor for AIDS-Kaposi sarcoma. Proc Natl Acad Sci USA. 1997;94:979–984. doi: 10.1073/pnas.94.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Medveczky M M, Geck P, Sullivan J L, Serbousek D, Djeu J Y, Medveczky P G. IL-2 independent growth and cytotoxicity of herpesvirus saimiri-infected human CD8 cells and involvement of two open reading frame sequences of the virus. Virology. 1993;196:402–412. doi: 10.1006/viro.1993.1495. [DOI] [PubMed] [Google Scholar]
- 43.Medveczky M M, Szomolanyi E, Hesselton R, De Grand D, Geck P, Medveczky P G. Herpesvirus saimiri strains from three DNA subgroups have different oncogenic potentials in New Zealand White rabbits. J Virol. 1989;63:3601–3611. doi: 10.1128/jvi.63.9.3601-3611.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Medveczky P G. Oncogenic transformation of T cells by herpesvirus saimiri. In: Barbanti-Brodano G, Bendinelli M, Friedman H, editors. DNA tumor viruses: oncogenic mechanisms. New York, N.Y: Plenum Press; 1995. pp. 239–252. [Google Scholar]
- 45.Miles S A, Martinez-Maza O, Rezai A, Magpantay L, Kishimoto T, Nakamura S, Radka S F, Linsley P S. Oncostatin M as a potent mitogen for AIDS-Kaposi’s sarcoma-derived cells. Science. 1992;255:1432–1434. doi: 10.1126/science.1542793. [DOI] [PubMed] [Google Scholar]
- 46.Miles S A, Rezai A R, Salazar-Gonzalez J F, Vander Meyden M, Stevens R H, Logan D M, Mitsuyasu R T, Taga T, Hirano T, Kishimoto T, Martinez-Maza O. AIDS Kaposi sarcoma-derived cells produce and respond to interleukin 6. Proc Natl Acad Sci USA. 1990;87:4068–4072. doi: 10.1073/pnas.87.11.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller G, Rigsby M O, Heston L, Grogan E, Sun R, Metroka C, Levy J A, Gao S J, Chang Y, Moore P S. Antibodies to butyrate-inducible antigens of Kaposi’s sarcoma-associated herpesvirus in patients with HIV-1 infection. N Engl J Med. 1996;334:1292–1297. doi: 10.1056/NEJM199605163342003. [DOI] [PubMed] [Google Scholar]
- 48.Moore P S, Boshoff C, Weiss R A, Chang Y. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science. 1996;274:1739–1744. doi: 10.1126/science.274.5293.1739. [DOI] [PubMed] [Google Scholar]
- 49.Moore P S, Chang Y. Detection of herpesvirus-like DNA sequences in Kaposi’s sarcoma in patients with and without HIV infection. N Engl J Med. 1995;332:1181–1185. doi: 10.1056/NEJM199505043321801. [DOI] [PubMed] [Google Scholar]
- 50.Moore P S, Gao S J, Dominguez G, Cesarman E, Lungu O, Knowles D M, Garber R, Pellett P E, McGeoch D J, Chang Y. Primary characterization of a herpesvirus agent associated with Kaposi’s sarcoma. J Virol. 1996;70:549–558. doi: 10.1128/jvi.70.1.549-558.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muralidhar S, Doniger J, Mendelson E, Araujo J C, Kashanchi F, Azumi N, Brady J N, Rosenthal L J. Human cytomegalovirus mtrII oncoprotein binds to p53 and down-regulates p53-activated transcription. J Virol. 1996;70:8691–8700. doi: 10.1128/jvi.70.12.8691-8700.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nicholas J, Ruvolo V R, Burns W H, Sandford G, Wan X, Ciufo D, Hendrickson S B, Guo H G, Hayward G S, Reitz M S. Kaposi’s sarcoma-associated human herpesvirus-8 encodes homologues of macrophage inflammatory protein-1 and interleukin-6. Nat Med. 1997;3:287–292. doi: 10.1038/nm0397-287. [DOI] [PubMed] [Google Scholar]
- 53.Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D, Ganem D. Lytic growth of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat Med. 1996;2:342–346. doi: 10.1038/nm0396-342. [DOI] [PubMed] [Google Scholar]
- 54.Reyes G R, LaFemina R, Hayward S D, Hayward G S. Morphological transformation by DNA fragments of human herpesviruses: evidence for two distinct transforming regions in herpes simplex virus types 1 and 2 and lack of correlation with biochemical transfer of the thymidine kinase gene. Cold Spring Harbor Symp Quant Biol. 1980;44:629–641. doi: 10.1101/sqb.1980.044.01.066. [DOI] [PubMed] [Google Scholar]
- 55.Rosenthal L J, Choudhury S. Potential oncogenicity of human cytomegalovirus. In: Becker Y, Darai G, Huang E S, editors. Molecular aspects of human cytomegalovirus diseases. New York, N.Y: Springer-Verlag; 1993. pp. 412–436. [Google Scholar]
- 56.Russo J J, Bohenzky R A, Chien M C, Chen J, Yan M, Maddalena D, Parry J P, Peruzzi D, Edelman I S, Chang Y, Moore P S. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8) Proc Natl Acad Sci USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Said J W, Chien K, Takeuchi S, Tasaka T, Asou H, Cho S K, de Vos S, Cesarman E, Knowles D M, Koeffler H P. Kaposi’s sarcoma-associated herpesvirus (KSHV or HHV8) in primary effusion lymphoma: ultrastructural demonstration of herpesvirus in lymphoma cells. Blood. 1996;87:4937–4943. [PubMed] [Google Scholar]
- 58.Sarid R, Flore O, Bohenzky R A, Chang Y, Moore P S. Transcription mapping of the Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) genome in a body cavity-based lymphoma cell line (BC-1) J Virol. 1998;72:1005–1012. doi: 10.1128/jvi.72.2.1005-1012.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sarid R, Sato T, Bohenzky R A, Russo J J, Chang Y. Kaposi’s sarcoma-associated herpesvirus encodes a functional bcl-2 homologue. Nat Med. 1997;3:293–298. doi: 10.1038/nm0397-293. [DOI] [PubMed] [Google Scholar]
- 60.Schalling M, Ekman M, Kaaya E E, Linde A, Biberfeld P. A role for a new herpes virus (KSHV) in different forms of Kaposi’s sarcoma. Nat Med. 1995;1:707–708. doi: 10.1038/nm0795-707. [DOI] [PubMed] [Google Scholar]
- 61.Schlegel R, Wade-Glass M, Rabson M S, Yang Y-C. The E5 transforming gene of bovine papillomavirus encodes a small hydrophobic polypeptide. Science. 1986;233:464–467. doi: 10.1126/science.3014660. [DOI] [PubMed] [Google Scholar]
- 62.Simpson G R, Schulz T F, Whitby D, Cook P M, Boshoff C, Rainbow L, Howard M R, Gao S J, Bohenzky R A, Simmonds P, Lee C, de Ruiter A, Hatzakis A, Tedder R S, Weller I V, Weiss R A, Moore P S. Prevalence of Kaposi’s sarcoma associated herpesvirus infection measured by antibodies to recombinant capsid protein and latent immunofluorescence antigen. Lancet. 1996;348:1133–1138. doi: 10.1016/S0140-6736(96)07560-5. [DOI] [PubMed] [Google Scholar]
- 63.Staskus K A, Zhong W, Gebhard K, Herndier B, Wang H, Renne R, Beneke J, Pudney J, Anderson D J, Ganem D, Haase A T. Kaposi’s sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J Virol. 1997;71:715–719. doi: 10.1128/jvi.71.1.715-719.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Su I J, Hsu Y S, Chang Y C, Wang I W. Herpesvirus-like DNA sequence in Kaposi’s sarcoma from AIDS and non-AIDS patients in Taiwan. Lancet. 1995;345:722–723. [PubMed] [Google Scholar]
- 65.Sun R, Lin S F, Gradoville L, Miller G. Polyadenylylated nuclear RNA encoded by Kaposi sarcoma-associated herpesvirus. Proc Natl Acad Sci USA. 1996;93:11883–11888. doi: 10.1073/pnas.93.21.11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Swanton C, Mann D J, Fleckenstein B, Neipel F, Peters G, Jones N. Herpes viral cyclin/Cdk6 complexes evade inhibition by CDK inhibitor proteins. Nature. 1997;390:184–187. doi: 10.1038/36606. [DOI] [PubMed] [Google Scholar]
- 67.Thompson J, Choudhury S, Kashanchi F, Doniger J, Berneman Z, Frenkel N, Rosenthal L J. A transforming fragment within the direct repeat region of human herpesvirus type 6 that transactivates HIV-1. Oncogene. 1994;9:1167–1175. [PubMed] [Google Scholar]
- 68.Thompson J, Doniger J, Rosenthal L J. A 79 amino acid oncogene is responsible for human cytomegalovirus mtrII induced malignant transformation. Arch Virol. 1994;136:161–172. doi: 10.1007/BF01538825. [DOI] [PubMed] [Google Scholar]
- 69.Vousden K H, Doniger J, DiPaolo J A, Lowy D R. The E7 open reading frame of human papillomavirus type 16 encodes a transforming gene. Oncogene Res. 1988;3:167–175. [PubMed] [Google Scholar]
- 70.Weindel K, Marme D, Weich H A. AIDS-associated Kaposi’s sarcoma cells in culture express vascular endothelial growth factor. Biochem Biophys Res Commun. 1992;183:1167–1174. doi: 10.1016/s0006-291x(05)80313-4. [DOI] [PubMed] [Google Scholar]
- 71.Werner S, Hofschneider P H, Heldin C H, Ostman A, Roth W K. Cultured Kaposi’s sarcoma-derived cells express functional PDGF A-type and B-type receptors. Exp Cell Res. 1990;187:98–103. doi: 10.1016/0014-4827(90)90122-q. [DOI] [PubMed] [Google Scholar]
- 72.Yasumoto S, Burkhardt A L, Doniger J, DiPaolo J A. Human papillomavirus type 16 DNA-induced malignant transformation of NIH 3T3 cells. J Virol. 1986;57:572–577. doi: 10.1128/jvi.57.2.572-577.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yew P R, Berk A J. Inhibition of p53 transactivation required for transformation by adenovirus early 1B protein. Nature. 1992;357:82–85. doi: 10.1038/357082a0. [DOI] [PubMed] [Google Scholar]
- 74.Zhong W, Wang H, Herndier B, Ganem D. Restricted expression of Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genes in Kaposi sarcoma. Proc Natl Acad Sci USA. 1996;93:6641–6646. doi: 10.1073/pnas.93.13.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]