Abstract
The generation of fully functional oligodendrocytes, the myelinating cells of the central nervous system, is preceded by a complex maturational process. We previously showed that the timing of oligodendrocyte differentiation and rat brain myelination were altered by perinatal exposure to buprenorphine and methadone, opioid analogues used for the management of pregnant addicts. Those observations suggested the involvement of the μ-opioid receptor (MOR) and the nociceptin/orphanin FQ receptor (NOR). However, it remained to be determined if these receptors and their endogenous ligands could indeed control the timing of myelination under normal physiological conditions of brain development. We now found that the endogenous MOR ligand endomorphin-1 (EM-1) exerts a striking stimulatory action on cellular and morphological maturation of rat pre-oligodendrocytes, but unexpectedly, these effects appear to be restricted to the cells from the female pups. Critically, this stimulation is abolished by co-incubation with the endogenous NOR ligand nociceptin. Furthermore, NOR antagonist treatment of 9-day-old female pups results in accelerated brain myelination. Interestingly, the lack of sex-dependent differences in developmental brain levels of EM-1 and nociceptin, or oligodendroglial expression of MOR and NOR, suggests that the observed sex-specific responses may be highly dependent on important intrinsic differences between the male and female oligodendrocytes. The discovery of a significant effect of EM-1 and nociceptin in the developing female oligodendrocytes and brain myelination, underscores the need for further studies investigating brain sex-related differences and their implications in opioid use and abuse, pain control, and susceptibility and remyelinating capacity in demyelinating disease as multiple sclerosis.
Keywords: Oligodendrocytes, myelination, sexual dimorphism, endomorphin-1, nociceptin, μ-opioid receptor, nociceptin receptor
1. INTRODUCTION
The rapid “saltatory” transmission of nerve impulses in the brain is enabled by the presence of myelin, an insulating multilamellar structure made by the oligodendrocytes. Furthermore, these glial cells and myelin regulate axonal extension and radial growth (Yin et al., 1998), and they are both implicated in the establishment of bidirectional glial-neuronal interactions (Fields, 2008) and the formation of functional white matter networks crucial to behavior and cognition (Filley and Fields, 2016; Peer et al., 2017). Thus, oligodendrocyte generation represents one of the most critical processes that take place during brain development. Oligodendrocytes originate from bipolar progenitors that undergo a well-defined sequence of maturational changes giving rise to complex multipolar cells capable of myelin formation. Each mature oligodendrocyte produces numerous ramified processes with membrane extensions capable of wrapping multiple axons forming a large number of myelin internodes. This succession of functional and morphological changes implies the existence of control mechanisms the perturbation of which could affect the timing of myelination and its coordination with axonal outgrowth and neuronal connectivity. A main concern in this regard is the dramatic escalation in opioid use and abuse together with the resulting increase of babies exposed to opioids during pregnancy and the large percentage of these newborns that are also administered opioids for their treatment of neonatal abstinence syndrome (Stover and Davis, 2015). We have previously found that the timing of myelination in the rat brain is disturbed by in utero and postnatal exposure to buprenorphine (Sanchez et al., 2008) and methadone (Vestal-Laborde et al., 2014), the two opioid analogues currently successfully used in the management of pregnant opioid addicts (Davis et al., 2018; Kraft et al., 2017). Our studies suggested a μ-opioid receptor (MOR) dependent acceleration of myelination induced by therapeutic doses of methadone and buprenorphine. On the other hand, exposure of the pups to supra-therapeutic levels of buprenorphine resulted in a contrasting delaying effect on myelination thought to be mediated by the nociceptin/orphanin FQ receptor (NOR) (Eschenroeder et al., 2012; Sanchez et al., 2008). Early studies showed that oligodendrocytes express opioid receptors in a developmentally regulated manner (Knapp et al., 1998). Activation of MOR in oligodendrocyte progenitor cultures was shown to result in elevated DNA synthesis whereas inhibition of kappa-opioid receptor (KOR) was accompanied by increased membrane extensions (Knapp et al., 1998). However, the functions of the endogenous opioid receptor ligands in myelination are not yet clearly understood. Our previous observations questions whether methadone and buprenorphine exert purely pharmacological effects on myelination when activating MOR and NOR, or whether these receptors and their endogenous peptide ligands may indeed play a role in timing developmental brain myelination under normal physiological conditions. The two endogenous peptides that have been shown to exhibit the highest affinity and selectivity for MOR, are endomorphin-1 (EM-1) (Tyr-Pro-Trp-Phe-NH2) and endomorphin-2 (EM-2) (Tyr-Pro-Phe-Phe-NH2) (Zadina et al., 1997). EM-1 and EM-2 have been mainly respectively localized to the brain and spinal cord (Barr and Zadina, 1999; Przewlocki et al., 1999) and both endomorphins have been studied for their analgesic effects (Feehan et al., 2017). However, to our knowledge, a function in brain development has not been investigated before. The endogenous ligand of NOR is nociceptin/orphanin FQ (nociceptin), a 17-aminoacid peptide that while structurally similar to dynorphin A, does not bind to the classical MOR, KOR or delta-opioid receptors (Lapalu et al., 1997; Meunier et al., 1995; Reinscheid et al., 1995). Similarly, NOR, the most recently discovered member of the opioid receptor family, does not exhibit binding affinity for any of the classical endogenous opioids. Initial studies implicated NOR in pain modulatory functions, that depending on the site of nociceptin administration, could result in either hyperalgesia or analgesia (Heinricher et al., 1997; Pan et al., 2000; Rizzi et al., 2006). Nevertheless, findings from different laboratories indicated that the nociceptin system is also involved in a wide variety of actions, including effects on learning and memory (Noda et al., 2000), stress (Jenck et al., 2000; Koster et al., 1999), drug addiction (Chung et al., 2006; Lutfy et al., 2001; Lutfy and Zaveri, 2016; Marquez et al., 2008a; Marquez et al., 2008b; Zaveri et al., 2018), and the control of glutamate transporter levels and activity in developing rodent and human astrocytes (Meyer et al, 2017). As indicated above, our previous studies suggested that MOR and NOR mediated methadone and buprenorphine effects on myelination. The present results indicate that MOR and NOR, and their endogenous peptide ligands EM-1 and nociceptin, indeed control the expression of myelin proteins in developing oligodendrocytes and the myelinating capacity of these cells. Unexpectedly, these effects are highly sex-dependent, being specifically observed for oligodendrocytes isolated from female pups and for female rat brain myelination. These findings will be discussed in relation to the potential interference by exogenous opioid exposure during brain development, and the possible role of nociceptin in neuroinflammatory diseases with demyelination such as multiple sclerosis.
2. MATERIALS and METHODS
2.1. Materials
Papain, DNAase and Percoll for cell isolation, and all components for the preparation of chemically-defined cell culture medium were from Sigma-Aldrich (St. Louis, MO). Dulbecco’s modified Eagle’s /Ham F-12 (DMEM/F12) (1:1) medium with high glucose and L-glutamine was from GIBCO-Life Technologies (Grand Island, NY). Reduced-growth factor Matrigel extracellular matrix was from Becton Dickinson (Franklin Lakes, NJ). EM-1 (Tyr-Pro-Trp-Phe-NH2), nociceptin (Phe-Gly-Gly-Phe-Thr-Gly-Ala-Arg-Lys-Ser-Ala-Arg-Lys-Leu-Ala-Asp-Glu), BAN-ORL-24 [(2R)-1-(phenylmethyl)-N-[3-(spiro[isobenzofuran-1(3H), 4’-piperdin]-1-yl)propyl-2-pyrolidinecarboximide], and CTAP (D-Phe-Cys-Tyr-D-Trp-Arg-Thr-Pen-Thr-NH2) were purchased from Tocris Bioscience (Ellisville, MO). Rabbit anti-phospho-Akt (Ser473) (Cat. No. 4060), anti-phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (Cat. No. 9101), and anti-GAPDH (Cat. No. 5174) antibodies were from Cell Signaling Technologies (Danvers, MA). Rabbit anti-MAG (Cat. No. 34–6200) and anti-nociceptin (Cat. No. PA3–204) antibodies were from Thermo Fisher Scientific (Waltham, MA). Rabbit anti-EM-1 (Cat. No. RA21001) and anti-NOR (Cat# RA14140) antibodies were from Neuromics (Edina, MN). Mouse anti-CC1 (Cat. No. OP80) and anti-MOG (Cat. No. MAB5680), guinea pig anti-MOR (Cat. No. AB5509), and rat anti-MBP (Cat. No. MAB386) antibodies were from Millipore-Sigma (Burlington, MA). Rabbit anti-phospho-CREB (Ser133) (Cat. No. PA1–4619) and anti-phospho-p38 MAPK alpha (Thr180, Tyr182) (Cat. No. MA5–15177) were from Invitrogen/Thermo Fisher Scientific (Rockford, IL). Mouse anti-β-actin antibody (Cat. No. A5316) was from Sigma-Aldrich (St. Louis, MO). The mouse O4 antibody was kindly provided by Dr. Babette Fuss (Department of Anatomy and Neurobiology, VCU). Horseradish peroxidase (HRP)-conjugated secondary antibodies [goat anti-rat IgG(H+L) (Cat. No. 112-035-003), goat anti-rabbit IgG(H+L) (Cat. No. 111-035-003), and rabbit anti-mouse IgG(H+L) (Cat. No. 315-035-045)]; and fluorescently-labeled secondary antibodies [Alexa Fluor 488 donkey anti-mouse IgG (Cat. No. 715-546-151), Alexa Fluor 488 donkey anti-rat IgG (Cat. No. 712-546-150), Alexa Fluor 488 donkey anti-rabbit IgG (Cat. No. 711-546-152), and Alexa Fluor 594 donkey anti-mouse IgM (Cat. No. 715-586-020)] were from Jackson ImmunoResearch Laboratories (West Grove, PA). Alexa Fluor 488 goat anti-guinea pig antibody (Cat. No. A-11073) was from Thermo Fisher Scientific. 3–3’-Diaminobenzidine (DAB) was from Sigma-Aldrich. The Bradford protein determination reagent and all electrophoresis reagents and supplies were from BioRad laboratories (Hercules, CA). The protease and phosphatase inhibitor cocktail, and Supersignal West Dura and West Pico Plus chemiluminescence reagents were purchased from Thermo-Fisher Scientific. DAPI-containing Vectashield was from Vector Laboratories (Burlingame, CA).
2.2. Isolation of oligodendrocytes and preparation of cell cultures
Pre-oligodendrocytes were isolated from 9-day-old male or female pups by using a Percoll gradient and differential adhesion as previously reported (Eschenroeder et al., 2012). Sprague-Dawley female lactating rats and their pups (10 pups/litter) were obtained from Charles River (Wilmington, MA), housed under light/dark cycle and temperature-controlled conditions and allowed food and water ad libitum. Studies were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Animals in Research and under protocols approved by the Animal Care and Use Committee of Virginia Commonwealth University. After sacrifice by rapid decapitation, the cerebral hemispheres were dissected out and the meninges removed by careful rolling on sterile filter paper. The tissue was then minced into about 2-mm pieces and subjected to dissociation by incubation on a water bath with rocking platform at 280 RPM, for 25 min at 37°C in the presence of 1 unit/ml papain and 0.01 mg/ml DNAse. After washing, the tissue was then filtered through a 75μM pore size nylon mesh (Sefar, Depew, NY) and the resulting filtrate centrifuged for 15 min at 30,000 × g in an isotonic self-generated Percoll gradient using a fixed angle rotor (JLA-16–250) in a Beckman-Coulter Avanti J-E centrifuge. After collection, the fraction enriched in oligodendrocytes was subjected to differential adhesion on a tissue culture-treated Petri dish to eliminate the microglial cells and potential residual astrocytes. The floating oligodendrocytes were subsequently plated in 48-well plates pre-coated with 12.5 μl/well Matrigel (for western blot analysis) or on 13mm glass coverslips pre-coated with 25 μl Matrigel in 24 well plates (for analysis by immunocytochemistry). Cells were then maintained in chemically defined medium (CDM) [DMEM/F12 (1:1) medium with high glucose and L-glutamine, pH 7.4, supplemented with 1 mg/ml endotoxin-free fatty acid-free bovine serum albumin, 50 μg/ml transferrin, 5 μg/ml insulin, 30 nM sodium selenite, 1 mM sodium pyruvate, 10 nM biotin, 20 nM progesterone, 100 μM putrescine, and 30 nM triiodothyronine (T3)]. Cell cultures were kept in a humidified incubator at 37°C in 5% CO2. Astroglial and microglial contamination, as assessed by glial fibrillary acidic protein and ionized calcium binding adaptor molecule 1 (Iba-1) staining, was less than 5%.
2.3. Cell culture treatments for evaluation of EM-1 and nociceptin effects.
For studies of EM-1 and nociceptin effects, pre-oligodendrocytes isolated as indicated above were allowed to attach overnight and were then incubated for 72 hrs. in CDM alone (controls), or in CDM supplemented with 1 μM EM-1, 1 μM nociceptin, or a mixture of 1 μM each EM-1 and nociceptin. MOR and NOR contributions to their endogenous peptide ligand effects were evaluated in parallel cell cultures incubated in the presence of 1 μM CTAP (MOR inhibitor) or 100 nM BAN-ORL24 (NOR inhibitor). For analysis of signaling pathways, pre-oligodendrocyte cultures were treated for 0, 5, 15, and 45 minutes with CDM supplemented with either 1 μM EM-1, 1 μM nociceptin, or a mixture containing 1 μM each EM-1 and nociceptin. For western blot analysis, cells were lysed in 45–100 μl of Laemmli buffer and processed as described below.
2.4. In vivo NOR inhibitor treatment and tissue preparation
Rat pups were administered daily intraperitoneal injections of BAN-ORL24 in 50 μl phosphate-buffered saline (PBS) at a dose of 1 mg/kg/day, from postnatal days 9 to 13. All controls were similarly injected with equal volume of PBS vehicle alone. On postnatal day 14, pups were separated by sex and sacrificed following anesthesia with isofluorane. The cerebral hemispheres were rapidly removed, flash frozen, and kept at −80°C until further use. Prior to the analysis of myelin proteins, the tissue was homogenized in PBS containing a protease and phosphatase inhibitor cocktail. For processing of tissue for immunohistochemical analyses, the 14-day-old pups were anesthetized with 2.5% Avertin and then perfused transcardially with 4% paraformaldehyde (PFA) as previously described (Meyer et al., 2017). Brains were removed, post-fixed in 4% PFA, and cryopreserved overnight in 30% sucrose prior to flash freezing in OCT. Frozen sections, 20 μm-thick, were cut in the coronal plane at the level of the anterior commissure and collected on glass microscope slides that were then refrigerated until their use for immunohistochemistry as described below.
2.5. Western blot analysis
Western blot analyses were carried out as previously described with minor modifications (Sanchez et al., 2008). Cell cultures and adequate aliquots of total homogenate samples were solubilized in the appropriate volume of Laemmli buffer (60 mM Tris–HCl buffer, pH 6.8, containing 10% glycerol, 2% sodium dodecyl sulfate (SDS), and 5% 2-mercaptoethanol). For the analysis of MBPs, proteins were subjected to SDS-polyacrylamide gel electrophoresis (SDS- PAGE) in 16% polyacrylamide Tricine gels (Schägger, 2006) and then transferred to PVDF membranes for 90 min at 100V. For all other proteins, samples were subjected to SDS-PAGE in 12% polyacrylamide Tris-glycine gels and transferred to nitrocellulose (100 V, 40–25 min). For both PVDF and nitrocellulose membranes, non-specific binding was blocked with 3% nonfat dry milk and 0.05% Tween-20 in PBS (blocking solution) for 1 hr at room temperature. The membranes were then incubated overnight at 4°C with the appropriate primary antibodies in blocking solution at the following dilutions: anti-MBP (1:160), anti-MOG (1:2,000), anti-MAG (1:1,000), Anti-phospho-Akt (Ser473) (1:2,000), anti-phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (1:1,000), anti-phospho-CREB (Ser133) (1:1,000), anti-phospho-p38 MAPK alpha (Thr180, Tyr182) (1:1,000), anti-GAPDH (1:2,000), and anti-β-actin (1:5,000). After rinsing, the membranes were re-blocked for 30 min and incubated for 2–3 hrs. in blocking solution with the appropriate HRP-conjugated secondary antibodies. Following extensive rinsing, the immunoreactive bands were detected with chemiluminescent reagent and their relative expression levels were determined by scanning analysis of the X-ray films using the NIH Image J program. All relative density values were then divided by the corresponding β-actin or anti-GAPDH levels to correct for sample loading differences.
2.6. Determination of EM-1 and nociceptin concentrations by dot blot analysis
EM-1 and nociceptin concentrations along postnatal brain development in male and female pups were determined by dot blot analysis. For this, pups were sacrificed at postnatal days (2–30) and their cerebral hemispheres collected and homogenized in PBS containing a protease and phosphatase inhibitor cocktail. Different protein aliquots of the tissue homogenates were then pipetted onto a nitrocellulose membrane. Parallel sets of either EM-1 (25–200 picomoles) or nociceptin standards (12.5–1,000 femtomoles) were included in the same membrane to allow for quantitative analysis of the samples. The membranes were then dried under air for 2 hrs. followed by rehydration in PBS and incubation for 1 hr. in blocking solution (3% nonfat dry milk and 0.05% Tween-20 in PBS). After overnight incubation with anti-EM-1 antibody (1:100) or 1 hr. with anti-nociceptin antibody (1:20,000) in blocking solution, the membranes were washed, re-blocked, and incubated with HRP-conjugated secondary antibody followed by detection by chemiluminescence. After scanning of the X-ray films, relative density values of the spots were obtained by using the NIH ImageJ program. The concentrations of EM-1 and nociceptin in different dilutions of each sample were determined by interpolation to the corresponding internal standard curves.
2.7. Immunostaining analyses
For the in vitro studies, cultured oligodendrocytes growing on glass coverslips were fixed with 4% PFA for 20 min at room temperature. For immunohistochemical analysis of brain tissue, slides with frozen sections were prepared as indicated above. Coverslips and tissue sections were incubated for 1 hr. with PBS containing 5% normal goat serum and 0.3% Triton X-100 (blocking solution) to prevent non-specific antibody binding. Samples were then incubated overnight with the appropriate primary antibodies diluted at the following concentrations in blocking solution: anti-MOR (1:100), anti-NOR (1:50), O4 (1:4), anti-CC1 (1:40), and anti-MBP (1:20). Negative controls were similarly incubated in the presence of the appropriate normal pre-immune isotypic serum. After extensive rinse with PBS and re-blocking for 30 min., samples were incubated for 2 hrs. with the appropriate fluorescently-labeled secondary antibodies, rinsed with PBS, and mounted with Vectashield. Confocal images were obtained using a Zeiss LSM710 confocal microscope. To quantify MOR and NOR expression, field images were divided into 5 groups with an average of 10 cells. The NIH ImageJ program was then used to obtain the integrated fluorescence intensity of the cells along with several adjacent background readings. The Total Corrected Cellular Fluorescence (TCCF) was calculated as previously described (McCloy et al., 2014) using the following formula: TCCF = integrated density – (area of selected cell × mean fluorescence of background readings). Results are expressed as relative MOR and NOR levels.
For MBP expression in corpus callosum, tissue sections were incubated overnight as indicated above with anti-MBP antibody (1:20) followed by rinsing with PBS, re-blocking, and 2 hr. incubation with HRP-labeled goat anti-rat IgG (1:200) secondary antibody. After extensive washing, labeling with secondary antibody was detected by incubation with a solution of the HRP substrate 3–3’-diaminobenzidine (DAB) (0.25 mg DAB/ml PBS). For accurate comparison between conditions, all sections were simultaneously processed and the length of color development time in the presence of DAB kept strictly constant. Samples were then observed and photographed using a Zeiss AxioImager A1 microscope equipped with an Axiocam MRc color CCD camera. MBP immunostaining of the corpus callosum was quantified using the NIH ImageJ program according to previously reported use for the assessment of DAB staining (Fuhrich et al., 2014; Mustafa et al., 2015). The optical density (OD) was estimated as previously described using the following formula: OD = log (max intensity/mean intensity), where max intensity = 255 for 8-bit images. Results are presented as relative optical density.
2.8. Evaluation of cell morphology by Sholl analysis
The assessment of oligodendrocyte morphological complexity was carried out by using a semi-automated Sholl analysis (Bonfire) following a previously described protocol for the analysis of neurons (Kutzing et al., 2010; Langhammer et al., 2010). Briefly, the NeuronJ plugin (Meijering et al., 2004) for the NIH ImageJ program was used for the tracing of 8-bit maximum intensity projections of confocal microscopy images to obtain tracing files (*.ndf files). Using MATLAB software (The Mathworks, Inc.), the data were then transformed to SWC files (Cannon et al., 1998) and the connectivity of the tracings was examined using the NeuronStudio program (Rodriguez et al., 2006). The data were then exported to Excel using MATLAB and statistical analyses were then performed using the Prism 8 program (GraphPad).
2.9. Statistical analysis
Statistical analysis was performed by the nonparametric tests Mann–Whitney (for two group comparisons) or one-way analysis of variance on ranks (Kruskal–Wallis test) when comparing more than two experimental groups. All analyses were carried out using the GraphPad Prism 8 program (La Jolla, CA). Differences were considered statistically significant when p values were <0.05.
3. RESULTS
3.1. Brain levels of nociceptin and EM-1 are developmentally regulated
Our previous results investigating potential effects of buprenorphine and methadone suggested the involvement of the NOR and MOR in controlling the precise timing of developmental brain myelination (Eschenroeder et al., 2012; Sanchez et al., 2008; Vestal-Laborde et al., 2014). In view of these effects, our next question was if these receptors and their endogenous peptide ligands, nociceptin and EM-1, actually play a function regulating the timing of myelination under normal physiological conditions. To investigate this possibility, we first analyzed the temporal expression of nociceptin and EM-1 in the cerebral hemispheres of rat pups from postnatal days (PD) 2 to 30. This developmental period includes the beginning (PD 8–10), as well as the peak of myelin formation (PD 18–20) and the gradual decrease to basal levels by one month of age when the major wave of myelination in the rodent brain is essentially complete (Campagnoni et al., 1978; Carson et al., 1983; Wiggins, 1986). Additionally, this analysis considered potential sexual dimorphisms in peptide concentrations and compared their pattern of expression with the male and female developmental brain levels of myelin specific proteins. These included the major splicing variants of myelin basic proteins (MBPs), major components required for myelin compaction (Popko et al., 1987; Readhead et al., 1990); myelin associated glycoprotein (MAG), a protein enriched in the periaxonal layer of the myelin sheath and thought to play a role in initial glia-axonal interactions (Quarles, 2007); and myelin oligodendrocyte glycoprotein (MOG), a molecule localized in the most outer layer of the myelin sheaths (Baumann and Pham-Dinh, 2001).
Our previous analysis of nociceptin levels in the developing rat brain utilized combined pools that did not discriminate values by sex (Meyer et al., 2017). However, similar to those earlier findings, Figure 1a now shows that in both the male and female brain, nociceptin exhibits the highest expression levels between 2 and 5 days of age. However, concentrations of this peptide significantly decrease from PD9 to PD30, an age at which comparison with 2-day-old pups shows a decrease of 94.4% and 95% for the males and females, respectively. It is only at PD21 that a difference in nociceptin values between sexes could be detected, being about 40% lower in the males. Intriguingly, Figure 1 (c–e) shows that there is an inverse correlation between the developmental pattern of nociceptin expression and the progression of myelination as determined by the accumulation of MBPs, MAG and MOG. As a side observation of these studies and in contrast to previous reports of increased myelin proteins in male adult rodents (Cerghet et al., 2006) and 10-day-old mice (Abi Ghanem et al., 2017), western blot analyses in the present studies failed to detect any significant sex-dependent differences in the pattern of expression or levels of MBP, MAG or MOG protein when pups were analyzed from PD2 to PD21.
FIGURE 1. Brain levels of nociceptin and EM-1 are developmentally regulated.
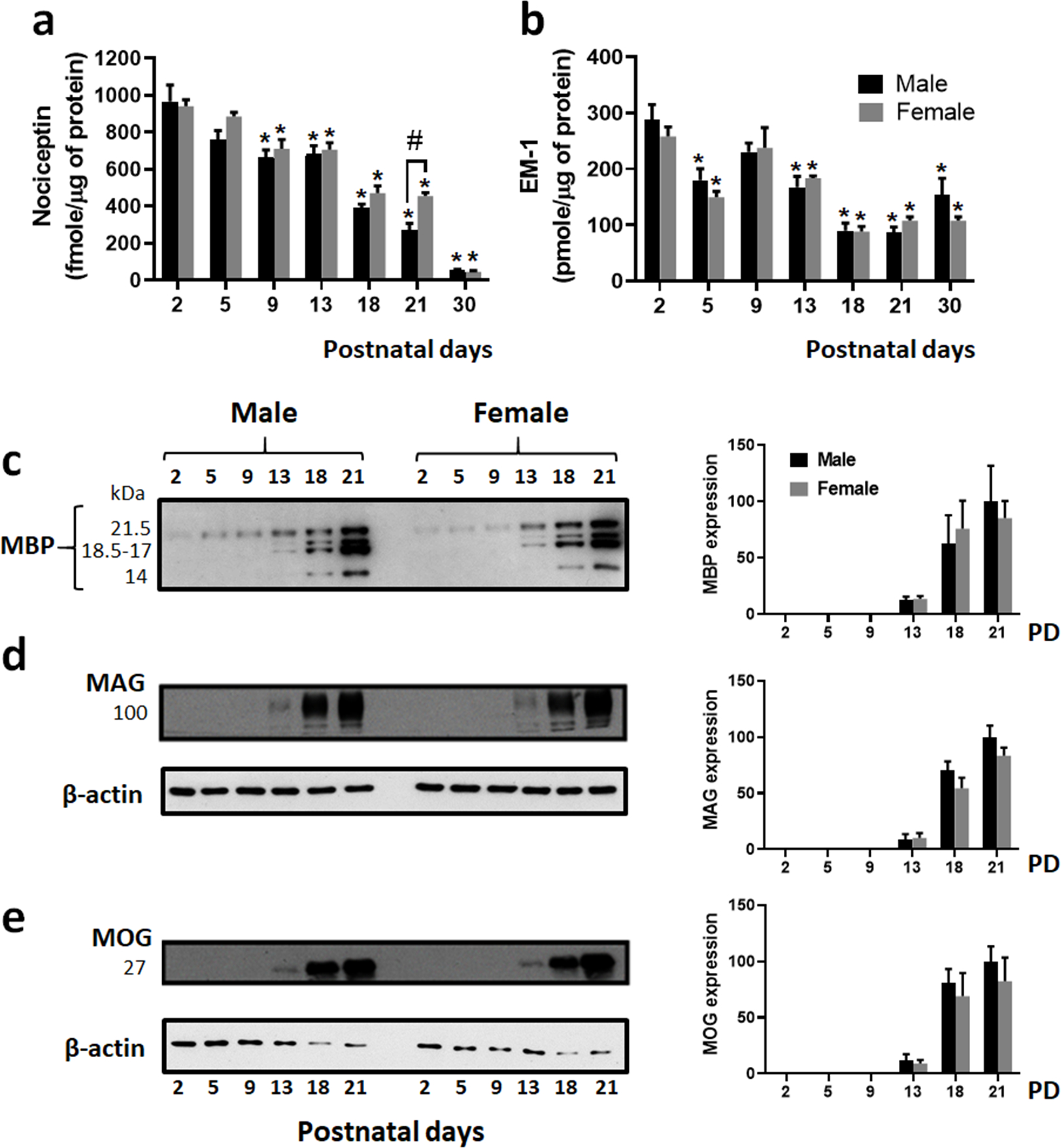
Total homogenates prepared from female and male rat cerebral hemispheres were used to analyze the developmental expression of nociceptin, EM-1 and different myelin proteins at different postnatal days (PD). Nociceptin (a) and EM-1 (b) levels were determined by dot blot analysis as described under “Methods”. For both peptides and in males and females, statistical differences for each age were determined with respect to the values for the 2-day-old pups. Each age value represents the average ± SEM from 4 animals per sex. (a) Nociceptin concentrations are expressed as fmoles/μg of protein PD2 vs. PD5, not significant; PD2 vs. PD9, PD13, PD18, PD21, and PD30, *p<0.03. PD 21 males vs. females, #p<0.03. (b) EM-1 concentrations are expressed as pmoles/μg of protein. PD2 vs. PD5, *p<0.03; PD2 vs. PD9, not significant; PD2 vs. PD13, PD18, PD21, and PD30, *p<0.03. (c-e) Western blot analysis shows the developmental expression of the myelin proteins: (c) MBP splicing isoforms, (d) MAG and (e) MOG. Bar graphs (c-e) show the expression levels calculated as relative % values with respect to the values corresponding to the PD 21 male pups. Each age value represents the average ± SEM from 4 animals per sex. No significant differences were detected between males and females.
Analysis of EM-1 levels in the postnatal brain (Fig. 1b), revealed for both sexes similar elevated levels of ~ 270 picomoles μg−1 of protein at 2 days of age. This is in contrast with a previous report in which immunohistochemical detection of EM-1 in the Long-Evans rat brain did not detect any significant staining until PD7 (Barr and Zadina, 1999). Importantly, while no significant differences in peptide concentration were detected between males and females, the developmental brain period analyzed in the present studies exhibits a biphasic pattern of EM-1 expression. In both sexes, the values observed at PD2 sharply decrease by approximately 40% at PD5. However, elevated levels of about 230 picomoles μg−1 of protein are later detected at PD9. Notably, this elevation in EM-1 peptide expression precedes the initiation of myelination and appearance of myelin specific proteins as shown in Figure 1 (c–e). Eventually, similar to nociceptin levels, there is a gradual decrease in EM-1 protein expression, reaching at 30 days of age values that are about 40–50% lower than those observed at PD2.
Altogether, these results indicate that nociceptin and EM-1 are both expressed in the maturing male and female rat brain at similar levels and their concentrations appear to be subjected to developmental regulation.
3.2. EM-1 and nociceptin exert sex-specific effects on pre-oligodendrocyte maturation
We next examined the potential effects of EM-1 and nociceptin on cultured cells directly isolated from 9-day-old male and female pups. As reported before, these cultures are composed of postmitotic pre-oligodendrocytes, the majority of which already react with the O4 antibody but are still MBP negative cells (Sato-Bigbee et al., 1999). We showed before that both MOR and NOR were expressed in pre-oligodendrocytes that were prepared from a mixed pool of male and female pup brains (Eschenroeder et al., 2012). As depicted in Figure 2, immunocytochemical staining now shows that both receptors are expressed in the vast majority of female (Fig. 2, a–d) and male (Fig. 2, e–h) pre-oligodendrocytes. In addition, comparison of relative expression levels failed to detect any sex-dependent differences for either MOR (Fig. 2, i) or NOR (Fig. 2, j).
FIGURE 2. Male and female pre-oligodendrocytes express MOR and NOR.
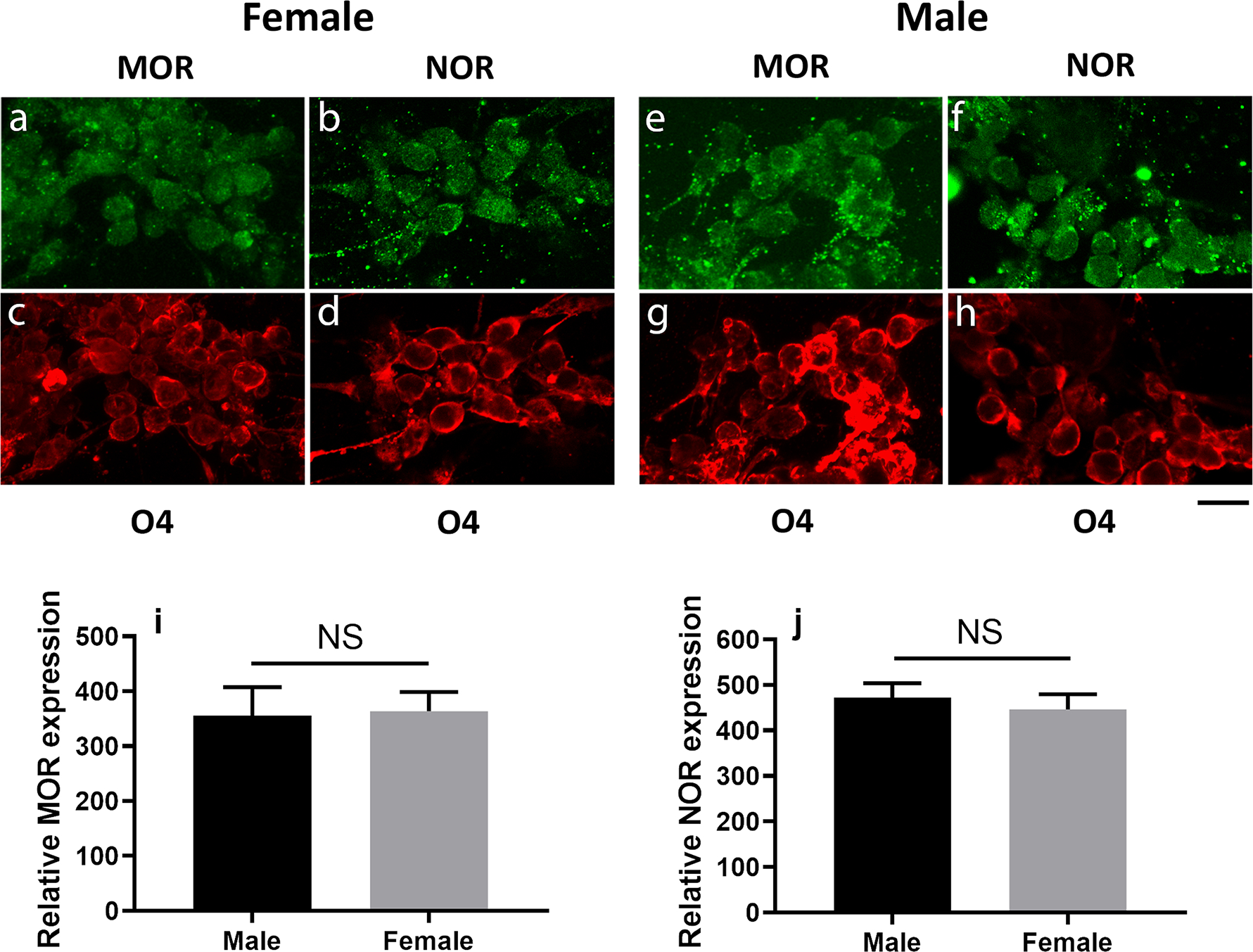
MOR and NOR expression were analyzed in pre-oligodendrocytes isolated from 9-day-old male and female pups. Following overnight attachment to the culture plates, cells were subjected to double immunostaining with O4 (red) antibody (c, d, g, and h) together with either anti-MOR (a and e) or anti- NOR (b and f) antibodies (green). Scale bar: 20 μm. Relative levels of MOR (i) and NOR (j) expression were determined as indicated under Methods. The bar graphs represent the mean ± SEM from 5 fields (average of 10 cells each) per group. Males vs. females, NS.
Levels of MBP expression in the cultures were next utilized as reporters of potential effects on the extent of pre-oligodendrocyte maturation. Interestingly, comparison with the control female cells incubated in chemically defined medium (CDM) alone, showed that supplementation with EM-1 induced a 70% increase in MBP levels (Fig. 3 a). However, this stimulation was not detected in the presence of the highly specific MOR antagonist CTAP. Since no changes in MBP levels were observed in the presence of CTAP alone, these observations indicate that MOR is the downstream effector of the stimulatory effects of EM-1.
Figure 3. Treatment of pre-oligodendrocytes with EM-1 and nociceptin induces sex-specific effects on MBP expression.
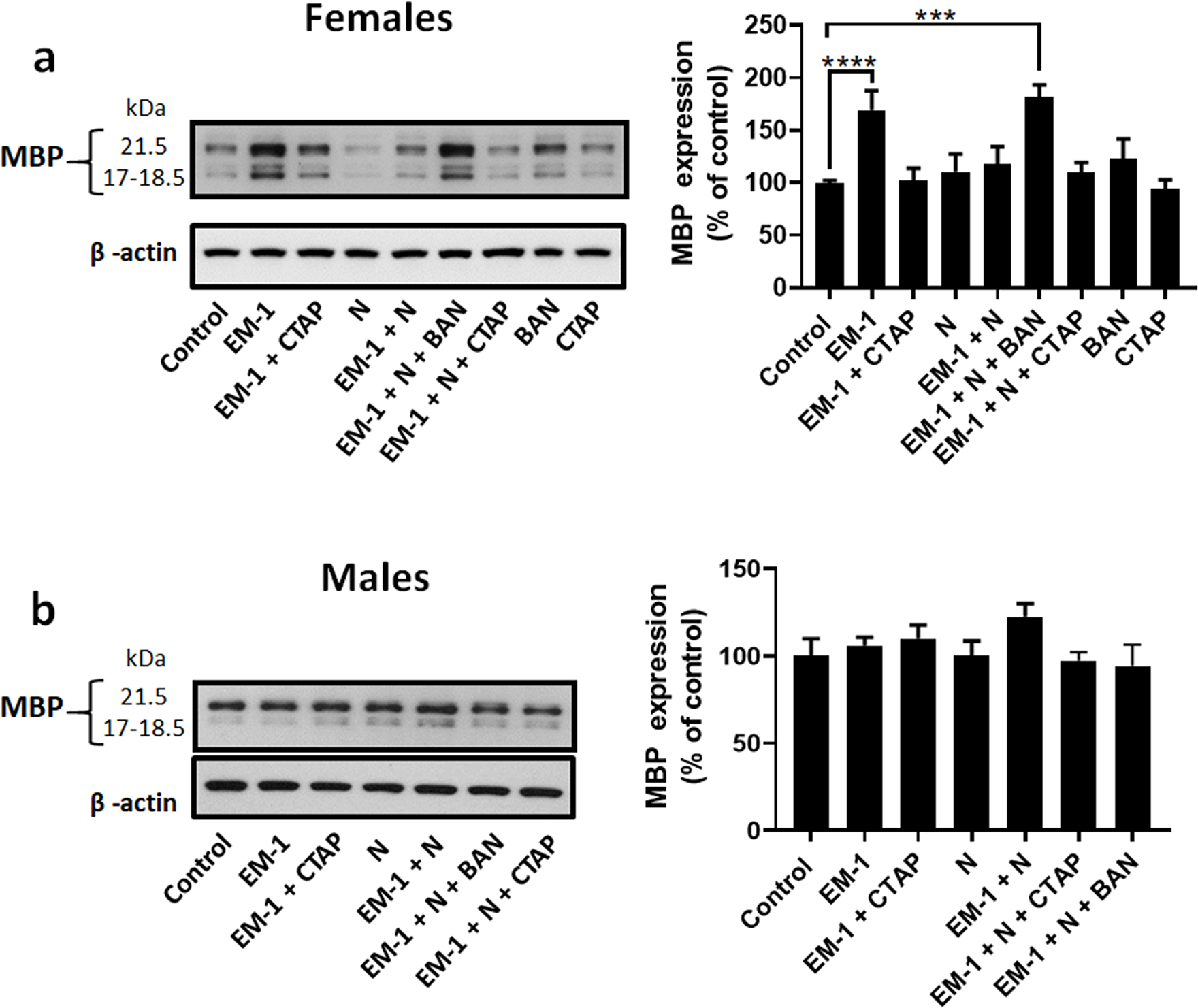
Developing oligodendrocytes from 9-day-old rat brain were cultured for 72 hours in chemically defined medium (CDM) alone (control) or in CDM supplemented with 1 μM Endomorphin-1 (EM-1), 1 μM EM-1 + 1 μM CTAP (EM-1 + CTAP), 1 μM Nociceptin (N), 1 μM EM-1 + 1 μM N (EM-1+ N), 1 μM EM-1 + 1 μM N + 100 nM BAN-ORL24 (EM-1 + N + BAN), 1 μM EM-1 + 1 μM N + 1 μM CTAP (EM-1 + N + CTAP), 100 nM BAN-ORL24or 1μM CTAP (CTAP). MBP expression levels were determined by western blot analysis using β-actin as loading control. Shown are representative western blots for cell cultures prepared from (a) female and (b) male pre-oligodendrocytes. Results in the bar graphs are expressed as % of the control values and represent the mean ± SEM from at least 3 separate experiments carried out in at least duplicates. ***p<0.0005, ****p<0.0001.
In contrast with EM-1, incubation of the female cells with nociceptin did not induce any apparent effects on MBP expression. However, nociceptin has the capacity of abolishing the stimulatory effect of EM-1, as demonstrated by the lack of changes in MBP levels when the cells were simultaneously exposed to both endogenous peptides. Significantly, this inhibitory effect is mediated by NOR as the capacity of EM-1 to elevate MBP expression in the presence of nociceptin was rescued by the NOR antagonist BAN-ORL24. Notice that BAN-ORL24 alone does not affect MBP expression.
Unexpectedly, and in sharp contrast with the observations in the female oligodendrocytes, Figure 3 b shows that the cells from male pups experience no changes in MBP expression upon exposure to either EM-1, nociceptin, a combination of both molecules, or MOR or NOR inhibitors.
These findings indicate that EM-1 has the capacity of stimulating MBP expression in the female cells and it does so by activation of MOR. This effect is counteracted by nociceptin in a mechanism that is specifically mediated by NOR. Furthermore, these actions of EM-1 and nociceptin are highly sex-specific as cells isolated from the male pups failed to demonstrate any significant response to these endogenous peptides.
3.3. EM-1 and nociceptin also induce sex-specific effects on oligodendrocyte morphological differentiation
The results described above suggested that EM-1 and nociceptin interact controlling the capacity of pre-oligodendrocytes to mature into MBP-making mature cells. However, oligodendrocyte maturation should also be accompanied by the appearance of long and highly branched processes with membrane outgrowth, a fundamental prerequisite for their myelinating activity (Osterhout et al., 1999). As shown in Figure 4, comparison with the controls (Fig. 4 a) and nociceptin alone (Fig. 4 c), indicated that cultures of female oligodendrocytes exposed to EM-1 (Fig. 4 b) appear to exhibit cells with much highly ramified processes and membrane extensions. However such EM-1 effect is abolished by co-incubation with nociceptin (Fig. 4 d). In addition, as found for the expression of MBP, no apparent differences between treatments could be detected for cells isolated from the male pups (Fig. 5, a–d).
FIGURE 4. EM-1 stimulates the morphological maturation of female oligodendrocytes and this effect is abolished by nociceptin.
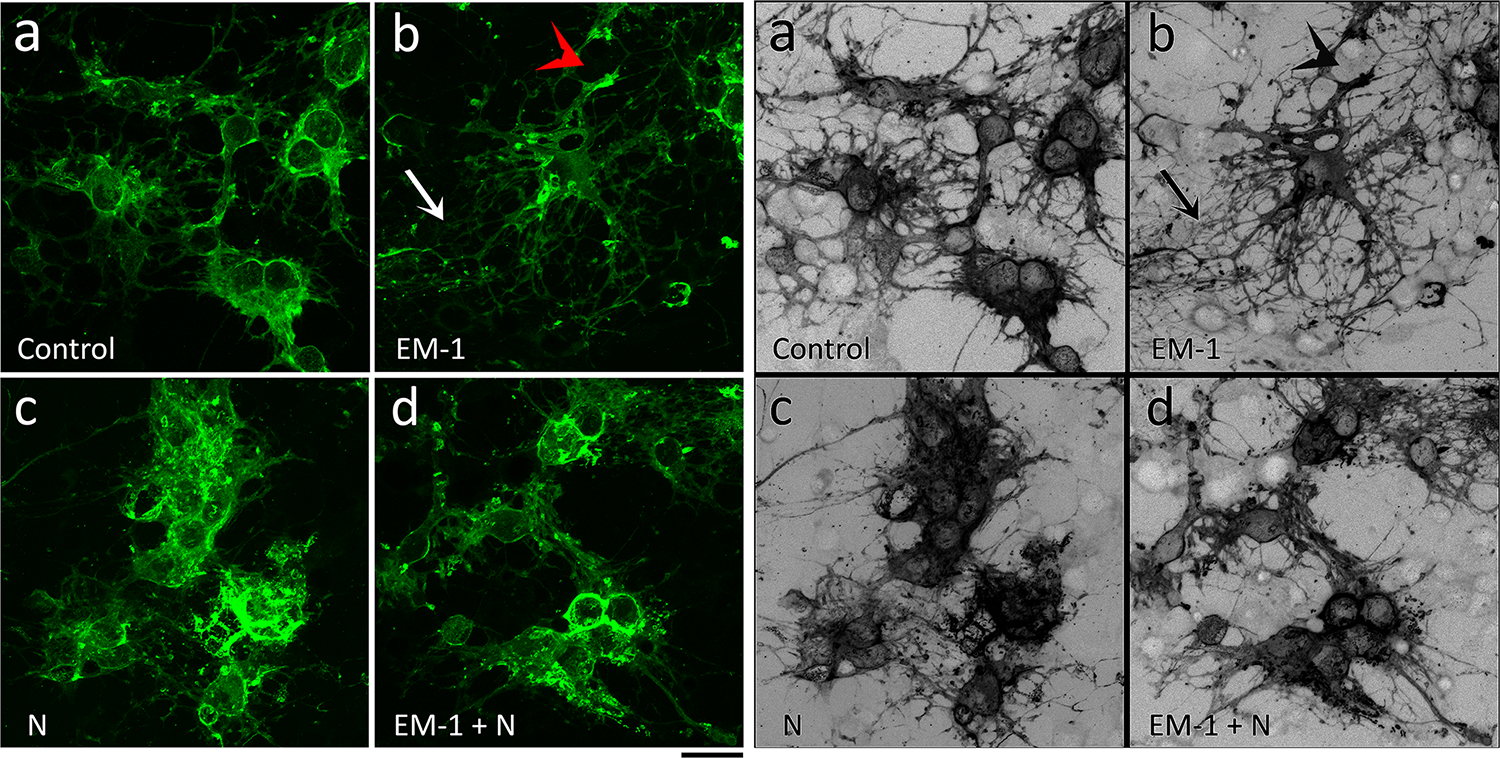
Developing OLGs from 9-day-old female rat brain were cultured for 72 hours in chemically defined medium (CDM) alone (Control) or supplemented with 1 μM EM-1, 1 μM N, or 1 μM EM-1 + 1 μM N. Cells were stained with anti-MBP antibody and visualized by confocal microscopy. Representative images demonstrate that in comparison with CDM alone (a), EM-1 (b) stimulates the formation of membrane extensions (red arrow) and the branching and length of processes (white arrow). This effect is however abolished by co-incubation with nociceptin (d). No apparent treatment effects were observed for nociceptin alone (c). Scale bar: 20 μm.
Figure 5. EM-1 does not alter the morphological maturation of male oligodendrocytes.
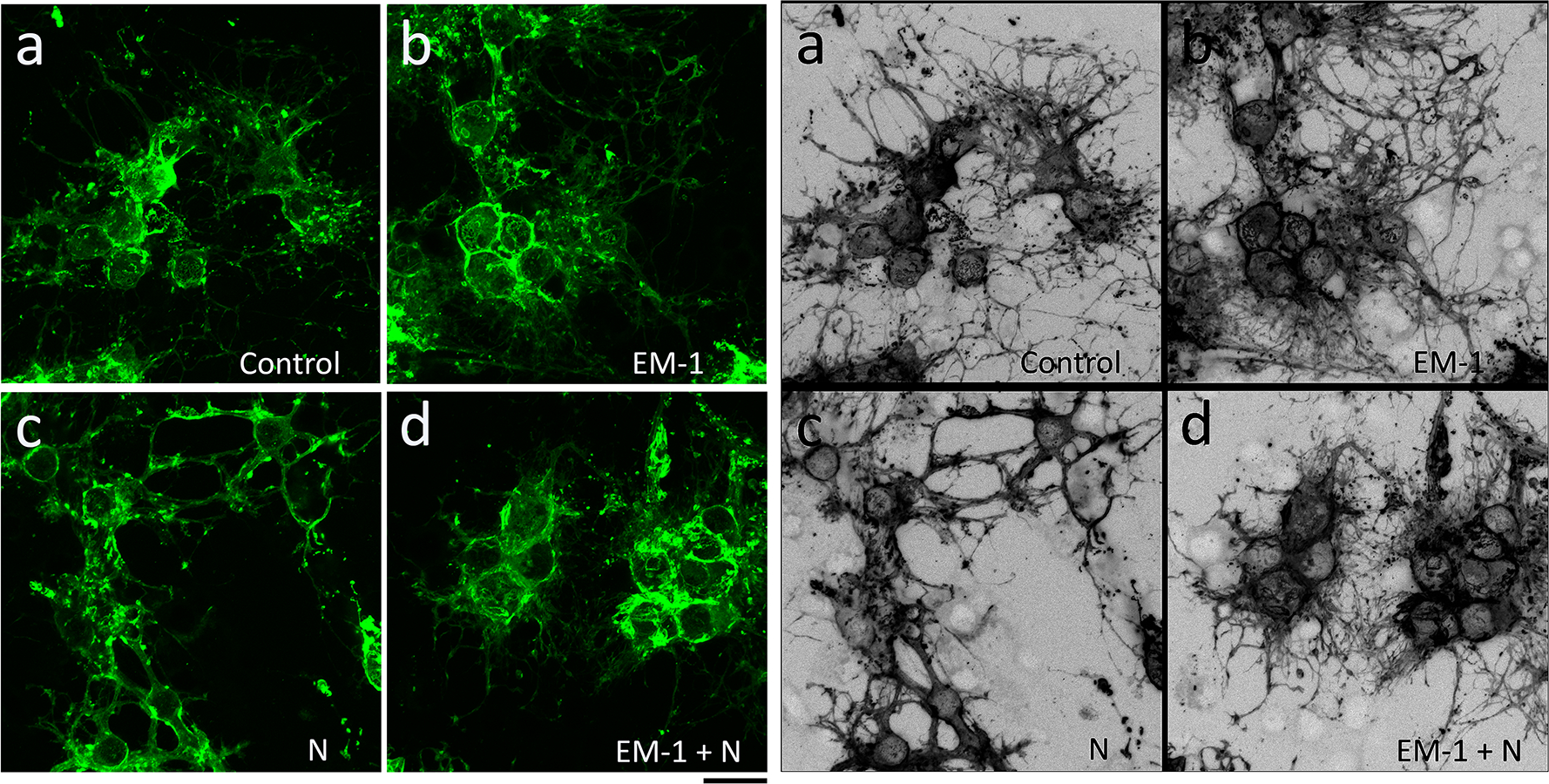
Developing OLGs from 9-day-old male rat brain were cultured for 72 hours in the conditions indicated in the legend for Figure 3. Representative images after MBP staining demonstrate that none of the experimental conditions [CDM alone (a), EM-1 (b), N (c), or EM-1 + N (d)] alter the morphological characteristics of the male cells. Scale bar: 20 μm.
Thus, we next used two unbiased approaches to quantify the effects of EM-1 and nociceptin on the morphological complexity of the cells. First, the number of branching points and different types of process segments - root, intermediate, and terminal - were determined using the Bonfire program (Kutzing et al., 2010). As depicted in Figure 6 a, “root” segments were those that directly originate from the soma, while segments that do not branch were classified as “terminals”, and any segments between the roots and the terminals were designated as “intermediates”.
FIGURE 6. Quantification of EM-1 and nociceptin effects on cell process complexity and length in female oligodendrocytes.
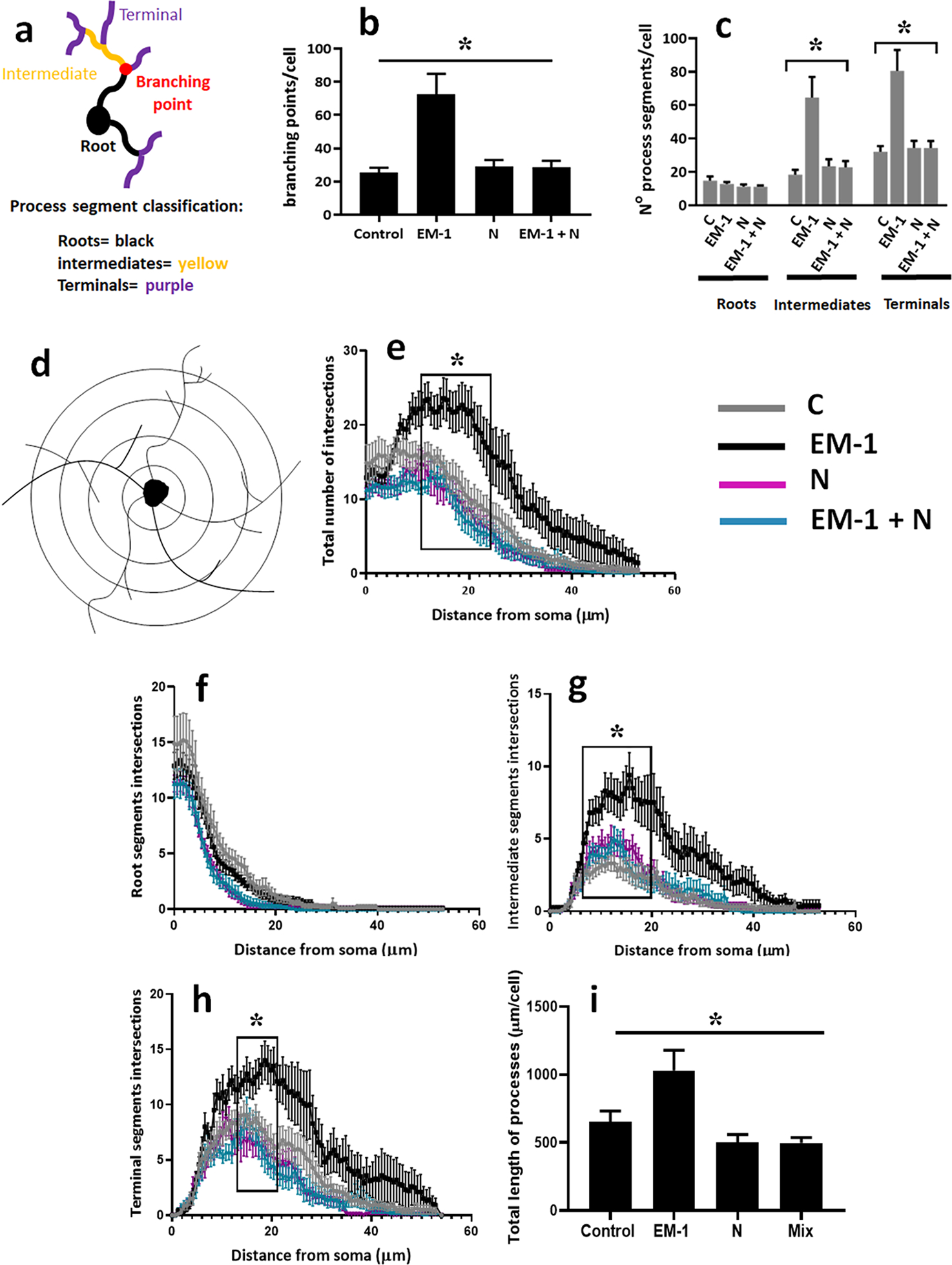
Pre-oligodendrocytes were isolated from 9-day-old female pups and cell cultures incubated for 72 hours in CDM alone (control) or CDM supplemented with 1 μM EM-1, 1 μM N, or a mixture of each 1 μM EM-1 and N. (a) Process segment classification scheme used for the Sholl analysis: roots (black) are the segments directly extending from the soma, terminals (purple) are segments with no further branching, and all other segments are considered intermediates (yellow). (b) Average number of branching points/cell. (c) Average number of process segments within each category/cell. Analysis of cell process complexity was then carried out by semi-automated Sholl analysis as indicated under “Methods”. (d) An illustration of tracing of intersections made by process segments with Sholl rings spaced at 0.6 μm increments from the cells soma. Shown are the number of intersections for the total number of segments (e), roots (f), intermediate segments (g), and terminal segments (h). (i) The bar graph represents the total length of processes; calculated as the added length of roots, intermediates and terminals under each culture condition. Results are expressed are the mean ± SEM for ten randomly selected cells analyzed per culture condition. * p <0.001.
As shown in Figure 6 b, female cells exposed to EM-1 exhibited an increased number of branching points, indicating a significant stimulation on cell process ramification. Analysis of the cells for different types of process segments (Fig. 6 c) indicated that the number of roots per cell was not affected by the treatment condition. However, cells exposed to EM-1 exhibited a significantly elevated number of both intermediate and terminal segments, an indication of increased morphological complexity. On the other hand, the number of these segments was not affected by nociceptin. Importantly, consistent with the effects on MBP expression described above, the capacity of EM-1 to stimulate complex morphology was abolished by co-incubation with nociceptin.
To further investigate the branching and complexity of the cells, we next decided to carry out a semi-automated Sholl analysis. In this analysis, the number of intersections of cell processes with equally spaced concentric rings at incremental intervals from the soma, is indicative of both the length of the cell processes and their branching (Fig. 6d). As shown in Figure 6 e, the number of total Sholl ring intersections is increased in the cells exposed to EM-1. Detailed analysis indicated that only cells exposed to EM-1 showed, with the exception of the root segments (Fig. 6 f), an overall consistent increase in the branching of cell processes, with a peak for both intermediate (Fig. 6 g) and terminal (Fig. 6 h) segments occurring at around 20 μm from the soma. Furthermore, female oligodendrocytes exposed to EM-1 exhibited an increase in the total length of processes compared to cells under each of the other conditions (Fig. 6 i).
In contrast with the observed differences for the female oligodendrocytes, a similar study failed to detect any significant differences between treatments for the cells from male pups. As shown in Figure 7, analysis of branching points (Fig. 7 a), cell processes segments (Fig. 7, b–f), and total length of processes (Fig. 7 g) did not indicate any significant differences between controls and treated male cells.
FIGURE 7. Quantification of EM-1 and nociceptin effects on cell process complexity and length in male oligodendrocytes.
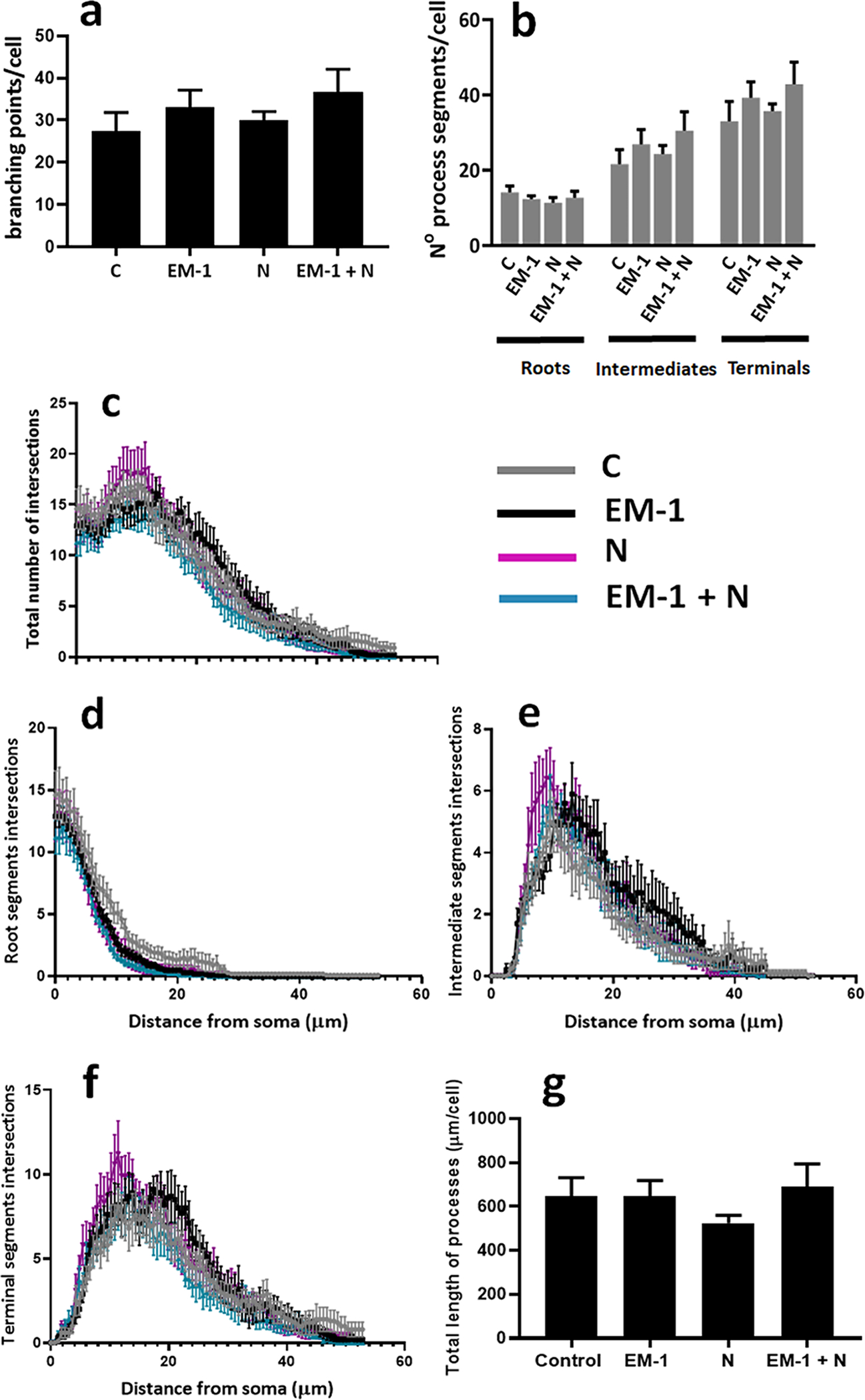
Pre-oligodendrocytes isolated from male pups were exposed to the same experimental conditions and subjected to the same analysis applied to female cells as indicated in the legend to Figure 4. (a) Average number of branching points/cell. (b) Average number of process segments within each category/cell. Shown are the results of the semi-automated Sholl analysis indicating the number of intersections for the total number of segments (c), roots (d), intermediate segments (e), and terminal segments (f). Results are expressed as the mean ± SEM for ten randomly selected cells analyzed per culture condition. (g) The bar graph represents the total length of processes; calculated as the added length of roots, intermediates and terminals under each culture condition. Ten randomly selected cells were analyzed per culture condition.
Altogether, these data suggest that EM-1 not only stimulates the expression of myelin proteins but also promotes the progression of oligodendrocyte maturation by specifically increasing the total length of cell processes and their branching distal from the soma. These differentiation effects of EM-1 are counteracted by nociceptin and appear to be solely restricted to the female cells.
3.4. Identification of p38MAPK as an early downstream target of nociceptin signaling in female oligodendrocytes
The results described above pointed to the existence of sex-specific mechanisms of EM-1 and nociceptin signaling in the oligodendrocytes. To begin to examine this possibly, on the day following isolation, cells from female and male pups were treated for different times (0, 5, 15 or 45 minutes) with either 1 μM EM-1, 1 μM nociceptin, or a mixture of 1 μM each EM-1 and nociceptin. While little is known about EM-1 effects, MOR signaling was shown to result in activation of phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) (Sánchez-Blázquez et al., 2010) and extracellular signal-regulated kinase (ERK) phosphorylation (Belcheva et al., 2002). MOR activation triggered by morphine has also been linked to both stimulatory and inhibitory effects on adenylate cyclase (Chakrabarti et al., 2016). Nevertheless, Figure 8 shows that exposure of female pre-oligodendrocytes to EM-1, nociceptin, or a mixture of both peptides did not cause any significant changes in the phosphorylation of either ERK (Fig.8 a) or AKT (Fig. 8 b). These treatments also failed to induce any variations in the phosphorylation of the transcription factor CREB (Fig. 8, c), used in the present work as a downstream reporter of potential adenylate cyclase/protein kinase A (PKA) regulation. In contrast, incubation of the cell cultures with nociceptin resulted a time-dependent decrease in the phosphorylation of the p38 mitogen-activated protein kinase alpha (p38 MAPK alpha) (Fig.8, d), a kinase that has been shown to positively regulate the expression of myelin-related genes (Haines et al., 2010) and control CNS myelination (Fragoso et al., 2007). Interestingly, the results also indicated that the inhibitory effect of nociceptin was abrogated by co-incubation with EM-1, even though EM-1 by itself did not exhibit any stimulatory effects on p38MAPK phosphorylation. This latter observation may reflect previous reports on the possibility of heterologous interaction and the existence of regulatory effects between NOR and MOR (Donica et al., 2013). In addition (see Supplemental Fig.1), just as observed for the pre-oligodendrocytes from the female pups, incubation of cells isolated from the male animals with EM-1, nociceptin, or co-treatment with EM-1 and nociceptin, failed to demonstrate any actions on ERK, AKT, or CREB phosphorylation. However, in contrast to the inhibitory effect exerted in the female cells, nociceptin exposure of the male pre-oligodendrocytes did not result in any appreciable changes in p38 MAPK phosphorylation.
FIGURE 8. Analysis of signaling pathways regulated by EM-1 and nociceptin. Identification of p38MAPK as an early downstream target of nociceptin signaling in female oligodendrocytes.
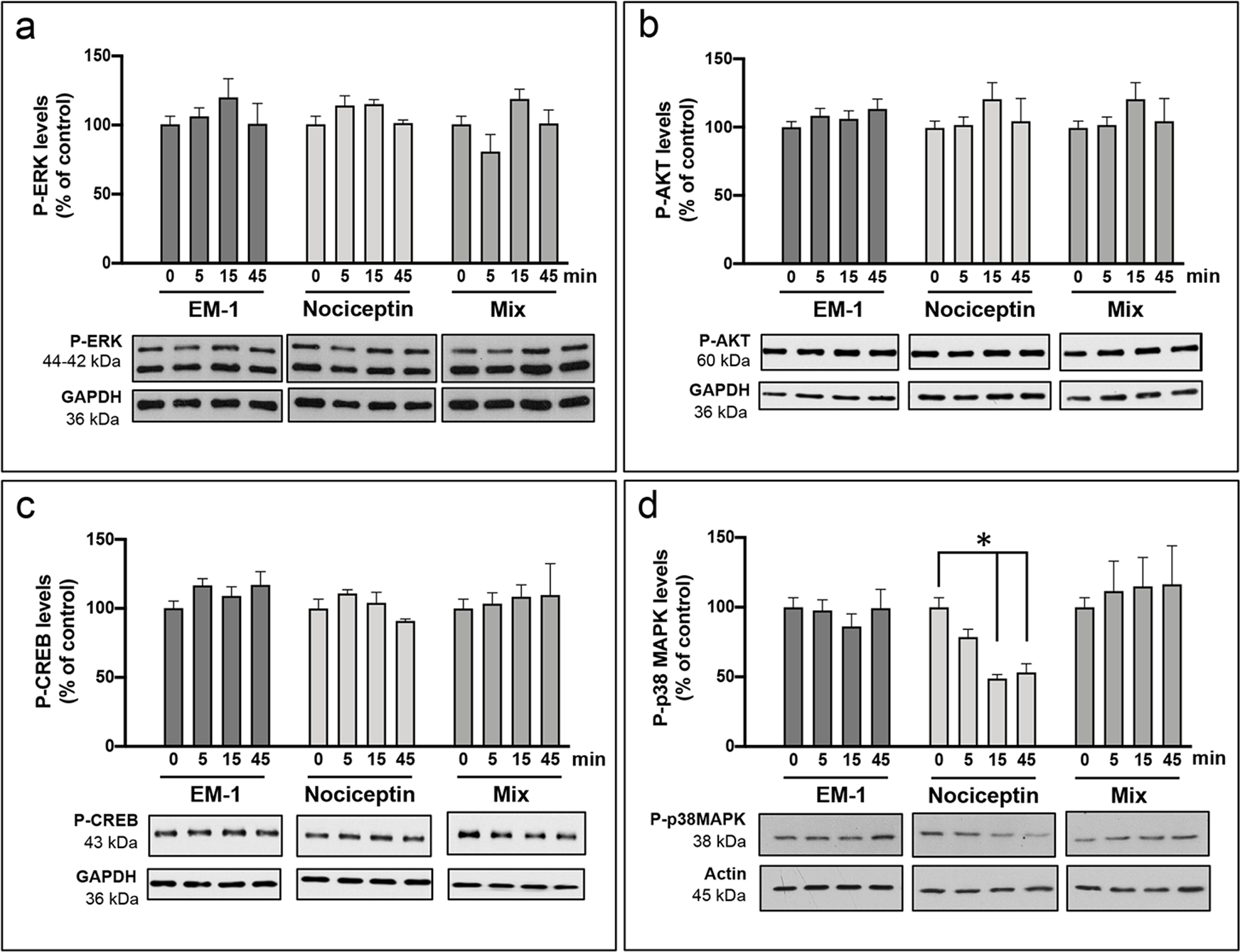
Pre-oligodendrocytes were isolated from female pups and incubated on the following day for 0, 5, 15, and 45 min. with CDM supplemented with either 1 μM EM-1, 1 μM nociceptin, or a mixture containing 1 μM each EM-1 and nociceptin. Western blot analysis of cell lysates was then used to determine the treatment effects on the levels of (a) P-ERK, (b) P-AKT, (c) P-CREB, and (d) P-p38MAPK, using β-actin or GAPDH values as loading controls. Results are expressed as % of their respective control values at 0 time. Bar graphs are the mean ± SEM from at least 3 independent experiments carried out in duplicate. *Nociceptin 0 vs. 15 and 45 min, p<0.03.
3.5. Treatment with an NOR antagonist accelerates female rat brain myelination
The cell culture studies described above showed that nociceptin did not exert any apparent effects on cell maturation. However, this peptide abrogated the increased MBP expression and morphological maturation of the female oligodendrocytes induced by EM-1. Furthermore, incubation of the female cells with nociceptin inhibited the phosphorylation of p38MAPK, a molecule shown to be important for brain myelination. All those in vitro findings raised the question of whether nociceptin could actually control myelination in the developing rat brain and whether if any, could these effects be sex-specific. To investigate this possibility, we next treated female and male rat pups with the blood–brain barrier permeable NOR antagonist BAN-ORL24. Animals were subjected to daily intraperitoneal injection (Fig. 9 a) starting at postnatal day 9, an age that not only corresponds to the time at which cells were isolated for the in vitro studies described above, but importantly, also immediately precedes the initiation of the most active period of rat brain myelination (Morell, 1999).
FIGURE 9. Administration of an NOR antagonist results in sex-specific acceleration of rat brain myelination.
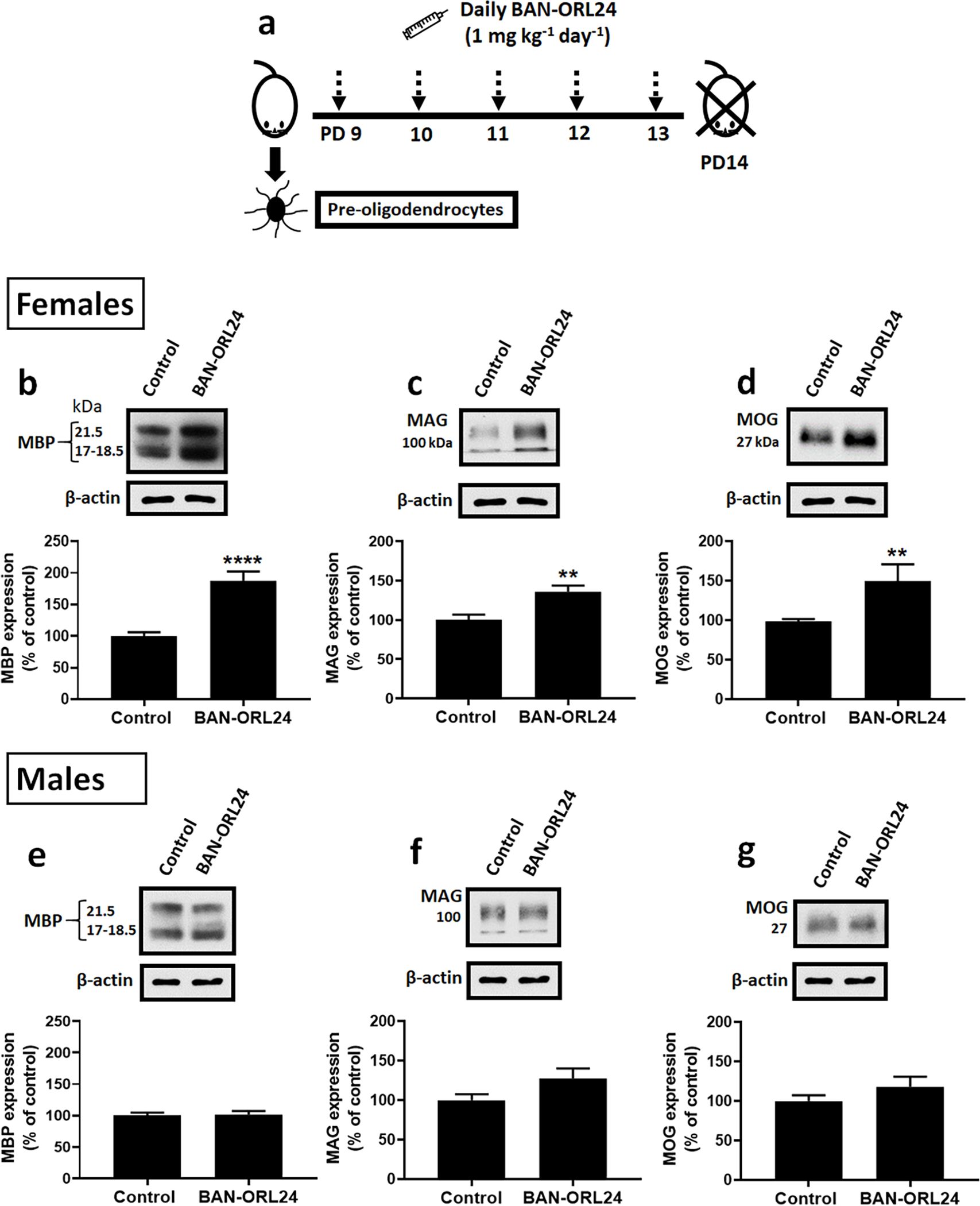
(a) Experimental schedule of BAN-ORL 24 administration: 9-day-old female and male pups were administered the blood brain barrier permeable NOR antagonist BAN-ORL24 (1mg/kg/day, IP) or vehicle (controls). Animals were sacrificed at postnatal day 14, and the cerebral hemispheres analyzed for the expression of MBP, MAG and MOG, using β-actin levels as loading controls. The figure shows representative western blots for the female (b-c) and male (e-g) pups. Bar graphs are the mean ± SEM from a total of at least 9 pups/group/sex from 3 different litters. ****p<0.001, **p<0.006.
As shown in Figure 9 (b–d), western blot analysis of the cerebral hemispheres at PD14 indicated that female pups treated with the NOR antagonist exhibited a significant increase in the expression of MBP, MAG, and MOG. This is in dramatic contrast with the apparent lack of effects in the male pups since analysis of male rat brains showed no significant differences between BAN-ORL24 injected pups and their age-matched controls (Fig. 9, e–g).
The elevated levels of myelin proteins in the female treated pups suggested that inhibition of nociceptin action resulted in sex-specific acceleration of myelination. To further investigate this problem, the brains of control and BAN-ORL24 treated female and male pups were subjected to immunohistochemical staining with anti-MBP antibody. We focused our attention on the corpus callosum, one of the largest white matter tracts that connects the cerebral hemispheres (Sargon et al., 2003). In the rat corpus callosum, myelination begins around PD 8 and gradually continuous until at least one month of age (Downes and Mullins, 2014). In agreement with the results of the western blot studies, Figure 10 (a, b and e) shows that female rat pups injected with NOR antagonist exhibited a significant increase in MBP staining of the callosal fibers as well as those extending into the cortex, an indication of accelerated myelin formation. Conversely, the intensity of MBP staining in the corpus callosum of the treated male pups was similar to that observed for their control littermates (Fig. 10; c, d, and e).
FIGURE 10. The corpus callosum of NOR antagonist-treated female pups exhibits increased myelination.
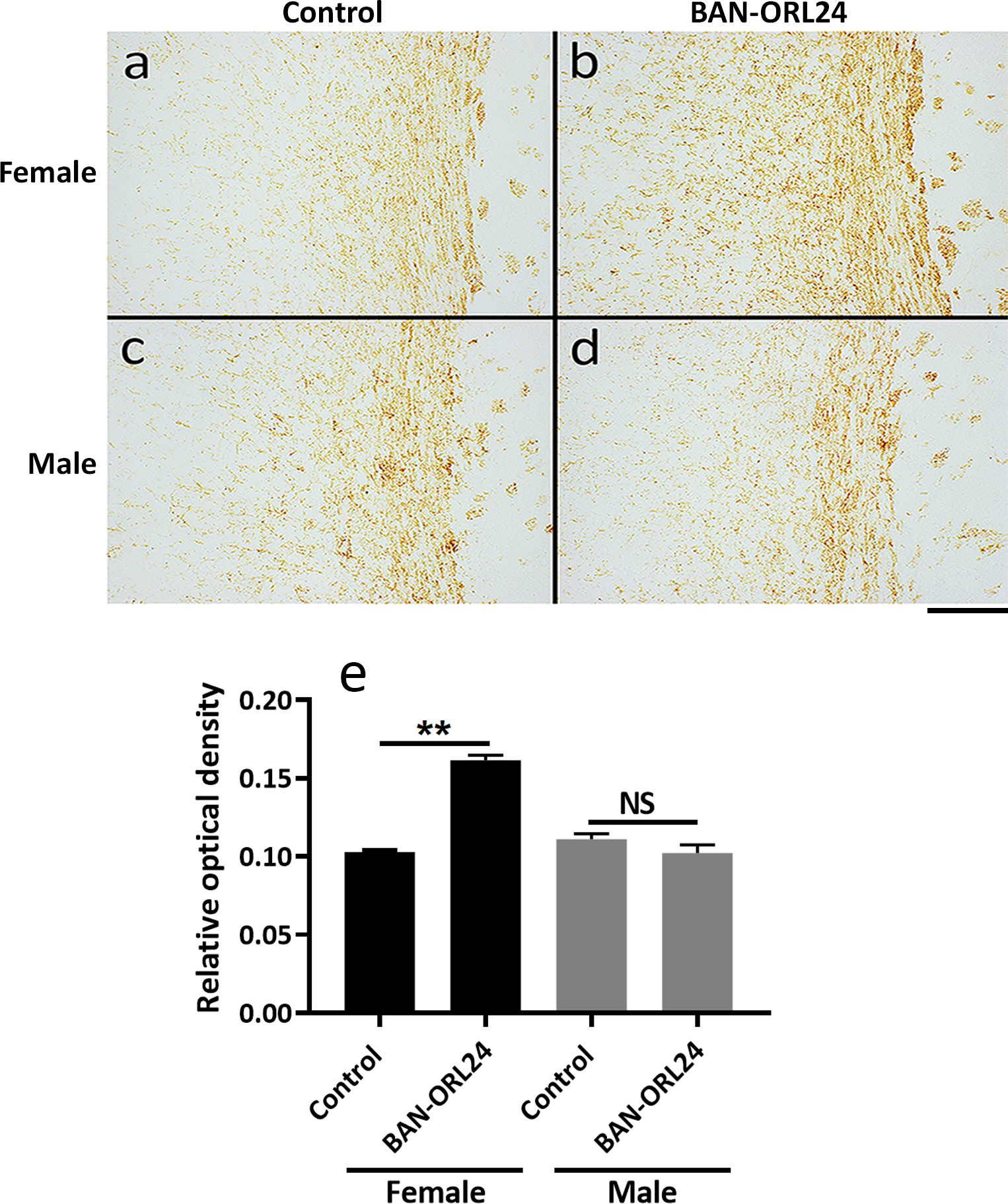
Nine-day-old female and male pups were daily administered the NOR antagonist BAN-ORL24, as indicated in the legend for Figure 9. Animals were sacrificed at postnatal day 14 and myelination of the corpus callosum was assessed after immunohistochemical staining of coronal brain sections with MBP antibody. Shown are representative images corresponding to comparable corpus callosum regions for the female (a and b) and male (c and d) pups. Scale bar: 200 μm. MBP immunostaining of the corpus callosum was quantified using the NIH ImageJ program for the assessment of DAB staining as indicated under “Methods”. ** Control vs BAN-ORL24 treated females, p<0.01.
Intriguingly, such discrepancies in the response of female versus male pups do not appear to reflect alterations in the number of mature oligodendrocytes. Oligodendrocyte maturation is accompanied by positive labeling with anti-adenomatous polyposis coli clone (CC1) antibody, which marks the cell body without staining myelin (Bhat et al., 1996). Interestingly, analysis of staining with CC1+ antibody in female (Fig. 11, a and b) and male (Fig 11, c and d) corpus callosum indicated no detectable differences in CC1+ cell numbers between controls and BAN-ORL24-treated pups of either sex (Fig. 11 e). These data suggested that the accelerated myelination observed as a result of NOR blockade in female pups is not due to an increased number of oligodendrocytes that reach maturity. Instead, it could be better explained by elevated myelinating activity of the same number of differentiated cells.
FIGURE 11. Analysis of mature oligodendrocyte numbers in the corpus callosum of BAN-ORL24 treated female and male pups.
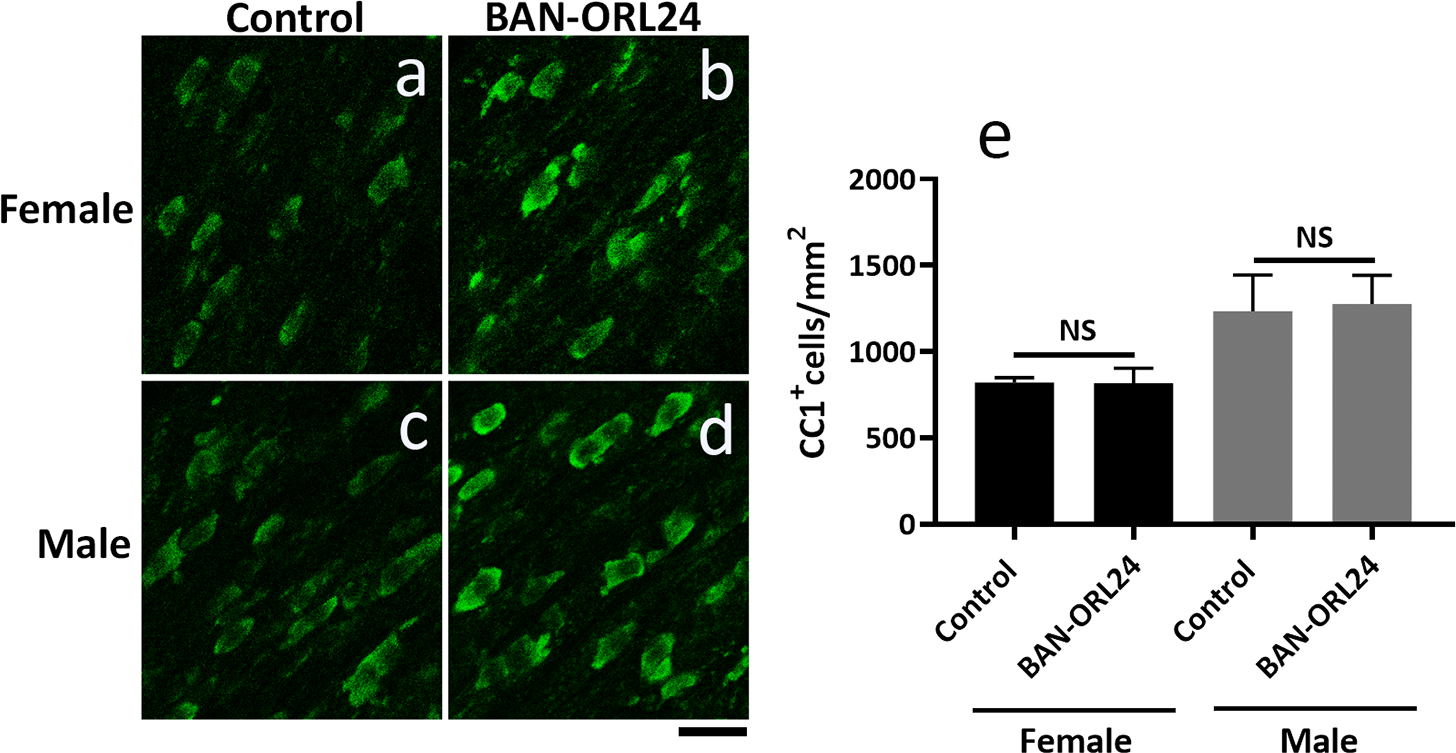
Nine-day-old female and male pups were daily administered the NOR antagonist BAN-ORL24, as indicated in the legend for Figure 9. The number of mature oligodendrocytes was analyzed in parallel comparable tissue sections stained with the CC1 antibody. (a-d) Representative images showing CC1+ cells in the corpus callosum in female and male brains. (e) Results are expressed as the number of CC1+ cells/mm2 and represent the mean ± SEM from 3 pups per group. Scale bar: 20 μm.
In summary, the present findings suggest the existence of a delicate balance between EM-1 and nociceptin that controls the proper timing of brain myelination (Figure 12). Activation of MOR by EM-1 appears to play a sex-dependent role stimulating myelin formation. On the other hand, nociceptin and NOR may have a crucial function preventing precocious myelination, a situation that could interfere with early axonal elongation and neuronal connectivity.
Figure 12. EM-1, nociceptin, and their receptors MOR and NOR, may play a crucial function controlling the proper timing of brain myelination and its coordination with axonal outgrowth.
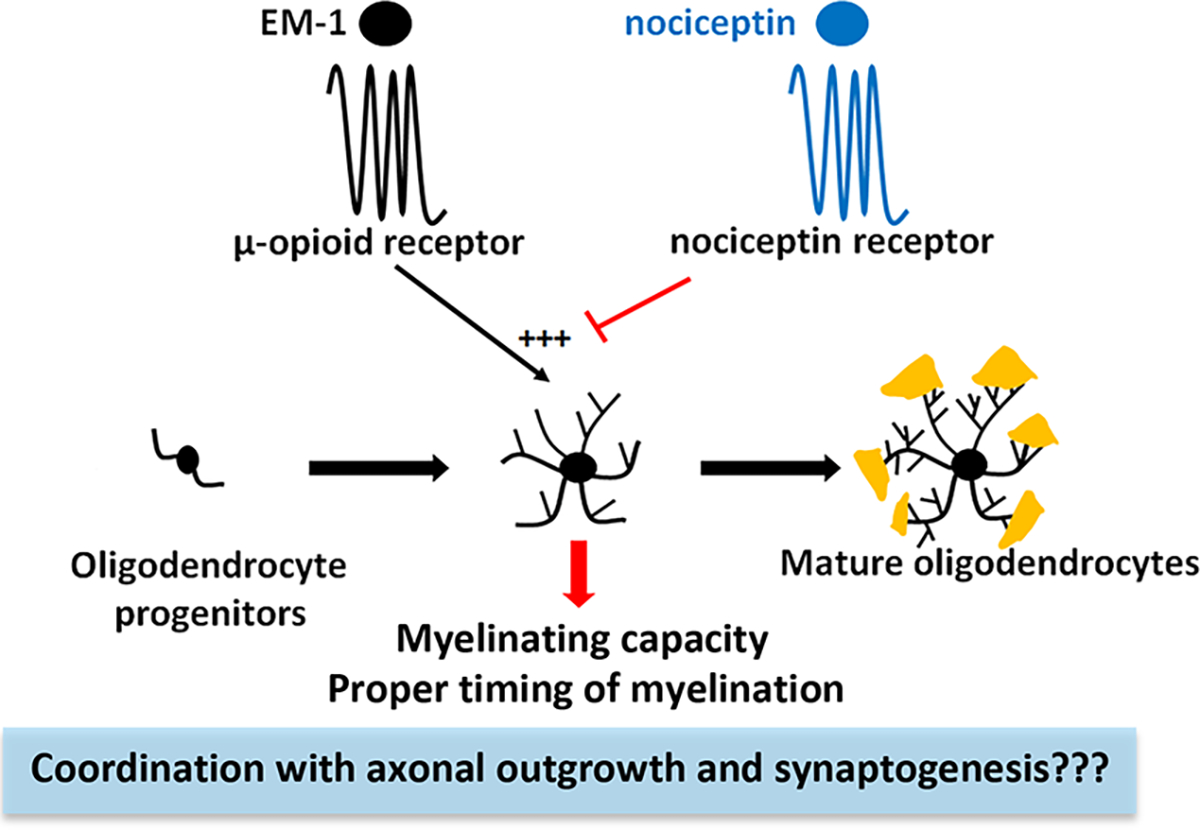
The endogenous MOR ligand EM-1 stimulates the expression of myelin proteins and morphological maturation of developing oligodendrocytes. This effect appears to be restricted to the female cells and is counteracted by nociceptin and its receptor NOR. Nociceptin may play a crucial dual function stimulating neuronal maturation while at the same time preventing premature myelination, a situation that could interfere with early axonal elongation and neuronal connectivity.
4. DISCUSSION
Neuronal generation and axonal outgrowth in the developing brain are followed by a surge in synaptogenesis that must be tightly coordinated with the differentiation of oligodendrocytes and their capacity to produce the insulating myelin sheath. Thus, multiple signals must exist to precisely synchronize oligodendrocyte maturation and myelination with neuronal activity and early plasticity of the brain. In this regard, we have previously found that the timing of myelination in the rat brain is disturbed by perinatal exposure to methadone (Vestal-Laborde et al., 2014) and buprenorphine (Sanchez et al., 2008), two opioid analogues used in the treatment of pregnant opioid addicts. Those effects implicated a MOR-dependent acceleration of myelination induced by therapeutic doses of methadone and buprenorphine, and a delaying effect induced by elevated doses of buprenorphine and NOR activation (Eschenroeder et al., 2012). Those observations raised the question of whether these synthetic opioids simply exerted pharmacological effects on myelination; or on the other hand, MOR, NOR, and their endogenous peptide ligands could play a role in timing developmental brain myelination under normal physiological conditions.
The present studies revealed that the expression of myelin proteins and morphological complexity of maturing oligodendrocytes is indeed subjected to a critical balance between a stimulatory effect induced by EM-1, the endogenous ligand of MOR; and a delaying inhibitory action mediated by nociceptin, the endogenous NOR ligand. Unexpectedly, these effects appear to be restricted to the female oligodendrocytes and the developing female rat brain. Exposure of female pre-oligodendrocytes to EM-1 results in oligodendrocytes with higher levels of MBP and morphological complexity than controls grown in differentiating medium alone. This stimulatory effect of EM-1 is counteracted by nociceptin, and furthermore, NOR antagonism results in accelerated myelination of the female brain. These sex-dependent differences are particularly intriguing as we found that the male and female rat brain not only display similar concentrations and developmental expression of EM-1 and nociceptin, but also the respective G-protein coupled receptors of these peptides are both present and expressed at similar levels in male and female developing oligodendrocytes. While specific local differences in EM-1 and/or nociceptin concentrations cannot be rejected at this time, these findings suggest that the observed sex-specific responses may be highly dependent on significant intrinsic differences between the male and female oligodendrocytes themselves.
Sex-dependent differences in oligodendrocyte biology and myelination remain poorly understood due to the fact that the majority of cell culture studies, including our own previous ones on methadone and buprenorphine effects (Eschenroeder et al., 2012; Vestal-Laborde et al., 2014), utilized oligodendrocytes isolated from a mixed pool of male and female brains. Furthermore, potential sex-dependent dissimilarities in control mechanisms of developmental myelination are difficult to assess because in vivo studies have employed diverse animal species and strains at different stages of CNS maturation. Previous studies examining the splenium of the adult Long-Evans rat corpus callosum found no overall sex-related differences in the total number of axons but a small (~8%) decrease in the number of myelinated axons in females (Kim et al., 1996). Later studies using carbonic anhydrase II protein and PLP/DM20 mRNA as in situ markers for oligodendrocytes in 13–15-month-old C57BL/6J mice and 1.5 month-old CDRS rats, indicated that the corpus callosum, fornix, and spinal cord of male rodents exhibited about 30% greater density of mature oligodendrocytes than their female counterparts (Cerghet et al., 2006). Interestingly, these authors also found that adult female rodents generate more proliferating oligodendrocytes characterized by a significantly shorter life span, concluding that proliferation and death of these cells is subjected to a sex-dependent differential regulation. In agreement with those earlier observations, our present analysis in the corpus callosum of 14-day-old rats displays a tendency towards a higher number of CC1+ oligodendrocytes in the male pups although these differences were not statistically significant and numbers of CC1+ cells may not necessarily directly correlate with the extent of myelinating activity. This suggests that the previously reported sex-dependent regulation of cell survival and proliferation in adult rodents (Cerghet et al., 2006) may already occur at the earlier stages of brain development investigated in the present studies. Importantly, our findings indicated that EM-1 not only stimulates the expression of myelin proteins but also the morphological maturation of the female cells. The results showed that these EM-1 effects are indeed mediated by MOR, but it remains to be determined what are the mechanisms downstream of MOR activation responsible for stimulating oligodendrocyte maturation. While EM-1 specifically binds to MOR, pathways classically associated to this receptor did not show major changes in these studies. Exposure of female pre-oligodendrocytes to EM-1, nociceptin, or a mixture of both peptides did not cause any significant changes in the phosphorylation of either ERK or AKT, and also failed to induced any alterations in CREB phosphorylation used in the present work as a reporter of potential adenylate cyclase/protein kinase A (PKA) activation. Importantly, EM-1 has the capacity of stimulating MBP expression and morphological complexity of the cells even in the presence of a culture medium containing triiodothyronine (T3) which is by itself a potent inducer of pre-oligodendrocyte maturation. Intriguingly, as it occurs for the expression of myelin proteins, the stimulation of branching and process outgrowth induced by EM-1 is antagonized by nociceptin, albeit the lack of any discernible actions on those parameters by nociceptin alone. However, it is important to point out that the signaling studies, carried out at the beginning of the 72 hr. treatment period, indicated that nociceptin exerted a time-dependent inhibition of p38MAPK phosphorylation. This observation is particularly important because earlier studies in cultured cells (Haines et al., 2015) showed that the p38MAPK plays a significant role in the stimulation of genes involved in cholesterol and lipid biosynthesis, cytoskeletal remodeling, vesicle trafficking and the genes encoding MOG, MBP, and MAG. It is conceivable that the inhibitory effects of nociceptin on p38MAPK are counteracted under basal conditions by the differentiating effects of the culture medium components, in particular those induced by T3. Nevertheless, the inhibition of p38MAPK phosphorylation by nociceptin could play a role in abrogating the supra-stimulatory effects induced on the oligodendrocytes by EM-1. These observations suggest the existence of a specific functional interaction between EM-1 and nociceptin signaling and not an otherwise generalized inhibitory effect of nociceptin on pre-oligodendrocyte maturation as observed by the lack of negative effects on MBP expression and morphological differentiation under basal cell culture conditions in a differentiating medium with T3. Further indication of a highly complex interaction between EM-1 and nociceptin is the observation that the inhibitory effect of nociceptin on p38MAPK phosphorylation was abrogated by co-incubation with EM-1, even though EM-1 by itself did not exhibit any stimulatory effects on this kinase. This observation may reflect previous reports on the existence of regulatory effects mediated by heterologous interactions between NOR and MOR (Donica et al., 2013). Support for this possibility stems from earlier studies in BE(2)-C human neuroblastoma cells which showed that nociceptin is capable of desensitization of both NOR and MOR by a heterologous crosstalk talk mechanism that involves PKC-alpha and the inhibition of both receptors following intracellular translocation of G protein-coupled receptor kinases (GRKs) 2 and 3 (Mandyam et al., 2002).
Regardless of the downstream mechanisms responsible for EM-1 and nociceptin interacting effects observed in these studies, particularly intriguing are the present contrasting differences between nociceptin effects in oligodendrocytes and those previously reported by others in developing neurons. Similar to our previous observation (Meyer et al., 2017) and present findings in cerebral hemispheres of Sprague Dawley rats, a recent peptidomic analysis in Wistar rats identified the presence of elevated levels of nociceptin during cerebellar development, with concentrations significantly declining beyond postnatal day 8 (Corbiere et al., 2018). Interestingly, the finding of supportive nociceptin actions on rat cerebellar granule neurons (Corbiere et al., 2018) together with earlier observations of positive effects on neurite outgrowth in mouse hippocampal cells (Ring et al., 2006) are indicative of an important stimulatory role of nociceptin on neuronal development. Thus, it is tempting to hypothesize that nociceptin may play a crucial dual function stimulating neuronal maturation while at the same time preventing premature myelination, a situation that could interfere with early axonal elongation and neuronal connectivity. Perhaps related to this function of nociceptin is the intriguing finding of our earlier studies indicating that, regardless of the dose, the brain of rat pups perinatally exposed to buprenorphine exhibited increased caliber of myelinated axons but disproportionally thinner myelin sheaths (Sanchez et al., 2008). Because no significant differences were noted for non-myelinated axons, those observations suggested that buprenorphine, a MOR and NOR partial agonist and kappa-opioid receptor antagonist, could somehow interfere with the mechanisms coordinating axonal outgrowth with myelin formation.
It is important to consider that in vivo effects on myelination triggered by endogenous EM-1 and nociceptin, or by synthetic opioid ligands of MOR and NOR, may additionally involve indirect effects mediated by cells other than oligodendrocytes. We have shown before that nociceptin also controls early expression of the glutamate transporter GLAST in both rodent and human developing astrocytes, cells which by themselves also secrete nociceptin (Meyer et al., 2017). Furthermore, astrocytes were shown to express elevated nociceptin levels when exposed to pro-inflammatory cytokines (Buzas et al., 2002), a situation which based on our present observations may exert an inhibitory effect on remyelination within the neuroinflammatory environment of demyelinating diseases like multiple sclerosis. Studies are in progress to investigate these possibilities and whether EM-1 and nociceptin signaling may play a role at earlier developmental stages when the cells are still actively proliferating oligodendrocyte progenitors. The importance of understanding the role of the endogenous opioid system in myelination and the potential interference by exogenous opioids is further emphasized by recent findings identifying changes in transcriptional responses in oligodendrocytes from mice subjected to acute morphine treatment (Avey et al., 2018). Importantly, opioid effects may not be limited to rodents as latest studies using diffusion magnetic resonance imaging showed that the brain of newborns exposed to methadone during pregnancy exhibit abnormal microstructure of major white matter tracts (Monnelly et al., 2018), and a study of teenagers and young adults indicated persistent neurocognitive differences in individuals that were prenatally exposed to opioids (Nygaard et al., 2018).
In summary, the present studies identified a novel regulatory function of the endogenous peptides EM-1 and nociceptin and their G-protein coupled receptors in the last steps of oligodendrocyte maturation and the beginning of developmental brain myelination. Importantly, the discovery of significant effects of these molecules and functions of their receptors in the developing female oligodendrocytes underscores the need for further studies investigating brain sex-related differences and their potential implications in opioid use and abuse, pain control, and neuroinflammation.
Supplementary Material
Supplemental FIGURE 1. Analysis of signaling pathways regulated by EM-1 and nociceptin in male oligodendrocytes. Pre-oligodendrocytes were isolated from male pups and incubated on the following day for 0, 5, 15, and 45 min. with CDM supplemented with either 1 μM EM-1, 1 μM nociceptin, or a mixture containing 1 μM each EM-1 and nociceptin. Western blot analysis of cell lysates was then used to determine the treatment effects on the levels of (a) P-ERK, (b) P-AKT, (c) P-CREB, and (d) P-p38MAPK, using β-actin or GAPDH values as loading controls. Results are expressed as % of their respective control values at 0 time. Bar graphs are the mean ± SEM from at least 3 independent experiments carried out in duplicate. No significant differences were detected between any of the treatment groups.
Main points:
Endomorphin-1 stimulates female oligodendrocyte myelinating activity through the μ-opioid receptor MOR
Nociceptin opposes that effect and blocking its receptor NOR hastens myelination
The MOR/NOR system may coordinate myelination with axon outgrowth
ACKNOWLEDGMENTS
This work was supported by grant RG 1501–2891 from the National Multiple Sclerosis Society, and a sub-award from NIH CTSA grant UL1TR00058 from the Virginia Commonwealth University (VCU) Center for Clinical and Translational Research. Microscopy was performed at the VCU Microscopy Facility, supported, in part, by funding from NIH-NCI Cancer Center Grant P30 CA016059.
Footnotes
The authors state no conflict of interest.
Data Availability Statement
Additional information that supports the findings of this study is available in the supplementary material of this article
REFERENCES
- Abi Ghanem C, Degerny C, Hussain R, Liere P, Pianos A, Tourpin S, Habert R, Macklin WB, Schumacher M, Ghoumari AM, 2017. Long-lasting masculinizing effects of postnatal androgens on myelin governed by the brain androgen receptor. PLoS Genet 13, e1007049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avey D, Sankararaman S, Yim AKY, Barve R, Milbrandt J, Mitra RD, 2018. Single-Cell RNA-Seq Uncovers a Robust Transcriptional Response to Morphine by Glia. Cell Rep 24, 3619–3629 e3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr GA, Zadina JE, 1999. The ontogeny of endomorphin-1- and endomorphin-2-like immunoreactivity in rat brain and spinal cord. Ann N Y Acad Sci 897, 145–153. [DOI] [PubMed] [Google Scholar]
- Baumann N, Pham-Dinh D, 2001. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev 81, 871–927. [DOI] [PubMed] [Google Scholar]
- Belcheva MM, Haas PD, Tan Y, Heaton VM, Coscia CJ, 2002. The fibroblast growth factor receptor is at the site of convergence between mu-opioid receptor and growth factor signaling pathways in rat C6 glioma cells. J Pharmacol Exp Ther 303(3), 909–918. [DOI] [PubMed] [Google Scholar]
- Bhat RV, Axt KJ, Fosnaugh JS, Smith KJ, Johnson KA, Hill DE, Kinzler KW, Baraban JM, 1996. Expression of the APC tumor suppressor protein in oligodendroglia. Glia 17, 169–174. [DOI] [PubMed] [Google Scholar]
- Buzas B, Rosenberger J, Kim KW, Cox BM, 2002. Inflammatory mediators increase the expression of nociceptin/orphanin FQ in rat astrocytes in culture. Glia 39, 237–246. [DOI] [PubMed] [Google Scholar]
- Campagnoni CW, Carey GD, and Campagnoni AT,1978. Synthesis of myelin basic proteins in the developing mouse brain. Arch Biochem Biophys 190, 118–125 [DOI] [PubMed] [Google Scholar]
- Cannon RC, Turner DA, Pyapali GK, Wheal HV, 1998. An on-line archive of reconstructed hippocampal neurons. J Neurosci Methods 84, 49–54. [DOI] [PubMed] [Google Scholar]
- Carson JH, Nielson ML, Barbarese E,1983. Developmental regulation of myelin basic protein expression in mouse brain. Developmental biology 96, 485–492 [DOI] [PubMed] [Google Scholar]
- Cerghet M, Skoff RP, Bessert D, Zhang Z, Mullins C, Ghandour MS, 2006. Proliferation and death of oligodendrocytes and myelin proteins are differentially regulated in male and female rodents. J Neurosci 26, 1439–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Pohl S, Zeng J, Civelli O, Reinscheid RK, 2006. Endogenous orphanin FQ/nociceptin is involved in the development of morphine tolerance. J Pharmacol Exp Ther 318, 262–267. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Chang A, Liu N-J, Gintzler AR, 2016. Chronic opioid treatment augments caveolin-1 scaffolding: relevance to stimulatory μ-opioid receptor adenylyl cyclase signaling. J Neurochem 139, 737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbiere A, Walet-Balieu ML, Chan P, Basille-Dugay M, Hardouin J, Vaudry D, 2018. A Peptidomic Approach to Characterize Peptides Involved in Cerebellar Cortex Development Leads to the Identification of the Neurotrophic Effects of Nociceptin. Mol Cell Proteomics 17, 1737–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Shenberger J, Terrin N, Breeze JL, Hudak M, Wachman EM, Marro P, Oliveira EL, Harvey-Wilkes K, Czynski A, Engelhardt B, D’Apolito K, Bogen D, Lester B, 2018. Comparison of Safety and Efficacy of Methadone vs Morphine for Treatment of Neonatal Abstinence Syndrome: A Randomized Clinical Trial. JAMA Pediatr 172, 741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donica CL, Awwad HO, Thakker DR and Standifer KM, 2013. Cellular Mechanisms of Nociceptin/Orphanin FQ (N/OFQ) Peptide (NOP) Receptor Regulation and Heterologous Regulation by N/OFQ. Molecular Pharmacology 83 (5) 907–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donica CL, Ramirez VI, Awwad HO, Zaveri NT, Toll L, Standifer KM, 2011. Orphanin FQ/nociceptin activates nuclear factor kappa B. J Neuroimmune Pharmacol 6, 617–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes N, Mullins P, 2014. The development of myelin in the brain of the juvenile rat. Toxicol Pathol 42, 913–922. [DOI] [PubMed] [Google Scholar]
- Eschenroeder AC, Vestal-Laborde AA, Sanchez ES, Robinson SE, Sato-Bigbee C, 2012. Oligodendrocyte responses to buprenorphine uncover novel and opposing roles of mu-opioid- and nociceptin/orphanin FQ receptors in cell development: implications for drug addiction treatment during pregnancy. Glia 60, 125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feehan AK, Morgenweck J, Zhang X, Amgott-Kwan AT, Zadina JE, 2017. Novel Endomorphin Analogs Are More Potent and Longer-Lasting Analgesics in Neuropathic, Inflammatory, Postoperative, and Visceral Pain Relative to Morphine. J Pain 18, 1526–1541. [DOI] [PubMed] [Google Scholar]
- Fields RD, 2008. Oligodendrocytes changing the rules: action potentials in glia and oligodendrocytes controlling action potentials. Neuroscientist 14, 540–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filley CM, Fields RD, 2016. White matter and cognition: making the connection. J Neurophysiol 116, 2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragoso G, Haines JD, Roberston J, Pedraza L, Mushynski WE, Almazan G, 2007. p38 Mitogen-activated protein kinase is required for central nervous system myelination. Glia 55(15), 1531–1541. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Shoda T, Morikawa H, Kato S, Mima H, Mori K, 1998. Activation of phospholipase A2 by the nociceptin receptor expressed in Chinese hamster ovary cells. J Neurochem 71, 2186–2192. [DOI] [PubMed] [Google Scholar]
- Fuhrich DG, Lessey BA, Savaris RF, 2013. Comparison of HSCORE assesment of endometrial β3 integrin subunit expression with digital HSCORE using computerized image analysis (ImageJ). Anat Quant Cytopathol Histopathol 35(4), 210–216. [PMC free article] [PubMed] [Google Scholar]
- Haines JD, Fang J, Mushynski WE, Almazan G, 2010. Mitogen-activated protein kinase activated protein kinase 2 (MK2) participates in p38 MAPK regulated control of oligodendrocyte differentiation. Glia 58(11), 1384–1393 [DOI] [PubMed] [Google Scholar]
- Haines JD, Fulton DL, Richard S, Almazan G, 2015. p38 Mitogen-Activated Protein Kinase Pathway Regulates Genes during Proliferation and Differentiation in Oligodendrocytes. PLoS ONE 10 (12): e0145843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinricher MM, McGaraughty S, Grandy DK, 1997. Circuitry underlying antiopioid actions of orphanin FQ in the rostral ventromedial medulla. J Neurophysiol 78, 3351–3358. [DOI] [PubMed] [Google Scholar]
- Jenck F, Wichmann J, Dautzenberg FM, Moreau JL, Ouagazzal AM, Martin JR, Lundstrom K, Cesura AM, Poli SM, Roever S, Kolczewski S, Adam G, Kilpatrick G, 2000. A synthetic agonist at the orphanin FQ/nociceptin receptor ORL1: anxiolytic profile in the rat. Proc Natl Acad Sci U S A 97, 4938–4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Ellman A, Juraska JM, 1996. A re-examination of sex differences in axon density and number in the splenium of the rat corpus callosum. Brain Res 740, 47–56. [DOI] [PubMed] [Google Scholar]
- Knapp PE, Maderspach K, Hauser KF, 1998. Endogenous opioid system in developing normal and jimpy oligodendrocytes: mu and kappa opioid receptors mediate differential mitogenic and growth responses. Glia 22, 189–201. [DOI] [PubMed] [Google Scholar]
- Koster A, Montkowski A, Schulz S, Stube EM, Knaudt K, Jenck F, Moreau JL, Nothacker HP, Civelli O, Reinscheid RK, 1999. Targeted disruption of the orphanin FQ/nociceptin gene increases stress susceptibility and impairs stress adaptation in mice. Proc Natl Acad Sci U S A 96, 10444–10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft WK, Adeniyi-Jones SC, Chervoneva I, Greenspan JS, Abatemarco D, Kaltenbach K, Ehrlich ME, 2017. Buprenorphine for the Treatment of the Neonatal Abstinence Syndrome. N Engl J Med 376, 2341–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutzing MK, Langhammer CG, Luo V, Lakdawala H, Firestein BL, 2010. Automated Sholl analysis of digitized neuronal morphology at multiple scales. J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhammer CG, Previtera ML, Sweet ES, Sran SS, Chen M, Firestein BL, 2010. Automated Sholl analysis of digitized neuronal morphology at multiple scales: Whole cell Sholl analysis versus Sholl analysis of arbor subregions. Cytometry A 77, 1160–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapalu S, Moisand C, Mazarguil H, Cambois G, Mollereau C, Meunier JC, 1997. Comparison of the structure-activity relationships of nociceptin and dynorphin A using chimeric peptides. FEBS Lett 417, 333–336. [DOI] [PubMed] [Google Scholar]
- Lutfy K, Do T, Maidment NT, 2001. Orphanin FQ/nociceptin attenuates motor stimulation and changes in nucleus accumbens extracellular dopamine induced by cocaine in rats. Psychopharmacology (Berl) 154, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfy K, Zaveri NT, 2016. The Nociceptin Receptor as an Emerging Molecular Target for Cocaine Addiction. Prog Mol Biol Transl Sci 137, 149–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam CD, Thakker DR, Christensen JL, Standifer KM, 2002. Orphanin FQ/nociceptin-mediated desensitization of opioid receptor-like 1 receptor and mu opioid receptors involves protein kinase C: a molecular mechanism for heterologous cross-talk. J Pharmacol Exp Ther 302, 502–509. [DOI] [PubMed] [Google Scholar]
- Marquez P, Borse J, Nguyen AT, Hamid A, Lutfy K, 2008a. The role of the opioid receptor-like (ORL1) receptor in motor stimulatory and rewarding actions of buprenorphine and morphine. Neuroscience 155, 597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez P, Nguyen AT, Hamid A, Lutfy K, 2008b. The endogenous OFQ/N/ORL-1 receptor system regulates the rewarding effects of acute cocaine. Neuropharmacology 54, 564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloy R, Rogers M, Caldon CE, Lorca T, Castro A, Burgess A, 2014. Partial inhibition of Cdk1 in G2 phase overrides the SAC and decouples mitotic events. Cell Cycle 13, 1400–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijering E, Jacob M, Sarria JC, Steiner P, Hirling H, Unser M, 2004. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry A 58, 167–176. [DOI] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, et al. , 1995. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature 377, 532–535. [DOI] [PubMed] [Google Scholar]
- Meyer LC, Paisley CE, Mohamed E, Bigbee JW, Kordula T, Richard H, Lutfy K, Sato-Bigbee C, 2017. Novel role of the nociceptin system as a regulator of glutamate transporter expression in developing astrocytes. Glia 65, 2003–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnelly VJ, Anblagan D, Quigley A, Cabez MB, Cooper ES, Mactier H, Semple SI, Bastin ME, Boardman JP, 2018. Prenatal methadone exposure is associated with altered neonatal brain development. Neuroimage Clin 18, 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell P, Quarles RH, 1999. Myelin Formation, Structure and Biochemistry. In: Siegel GJ, Agranoff BW, Alber RW, Risher SK and Uhler MD, Eds. Basic Neurochemistry, 6th Edition, Raven Press, New York, 70–93. [Google Scholar]
- Mustafa HN, El Awdan SA, Hegazy GA, Abdel Jaleel GA, 2015. Prophylactic role of coenzyme Q10 and Cynara scolymus L on doxorubicin-induced toxicity in rats: Biochemical and immunohistochemical study. Indian J Pharmacol 47(6), 649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda Y, Mamiya T, Manabe T, Nishi M, Takeshima H, Nabeshima T, 2000. Role of nociceptin systems in learning and memory. Peptides 21, 1063–1069. [DOI] [PubMed] [Google Scholar]
- Nygaard E, Slinning K, Moe V, Due-Tonnessen P, Fjell A, Walhovd KB, 2018. Neuroanatomical characteristics of youths with prenatal opioid and poly-drug exposure. Neurotoxicol Teratol 68, 13–26. [DOI] [PubMed] [Google Scholar]
- Osterhout DJ, Wolven A, Wolf RM, Resh MD, Chao MV, 1999. Morphological differentiation of oligodendrocytes requires activation of Fyn tyrosine kinase. J Cell Biol 145, 1209–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z, Hirakawa N, Fields HL, 2000. A cellular mechanism for the bidirectional pain-modulating actions of orphanin FQ/nociceptin. Neuron 26, 515–522. [DOI] [PubMed] [Google Scholar]
- Peer M, Nitzan M, Bick AS, Levin N, Arzy S, 2017. Evidence for Functional Networks within the Human Brain’s White Matter. J Neurosci 37, 6394–6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popko B, Puckett C, Lai E, Shine HD, Readhead C, Takahashi N, Hunt SW 3rd, Sidman RL, Hood L, 1987. Myelin deficient mice: expression of myelin basic protein and generation of mice with varying levels of myelin. Cell 48, 713–721. [DOI] [PubMed] [Google Scholar]
- Przewlocki R, Labuz D, Mika J, Przewlocka B, Tomboly C, Toth G, 1999. Pain inhibition by endomorphins. Ann N Y Acad Sci 897, 154–164. [DOI] [PubMed] [Google Scholar]
- Quarles RH, 2007. Myelin-associated glycoprotein (MAG): past, present and beyond. J Neurochem 100, 1431–1448. [DOI] [PubMed] [Google Scholar]
- Readhead C, Takasashi N, Shine HD, Saavedra R, Sidman R, Hood L, 1990. Role of myelin basic protein in the formation of central nervous system myelin. Ann N Y Acad Sci 605, 280–285. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ Jr., Civelli O, 1995. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science 270, 792–794. [DOI] [PubMed] [Google Scholar]
- Ring RH, Alder J, Fennell M, Kouranova E, Black IB, Thakker-Varia S, 2006. Transcriptional profiling of brain-derived-neurotrophic factor-induced neuronal plasticity: a novel role for nociceptin in hippocampal neurite outgrowth. J Neurobiol 66, 361–377. [DOI] [PubMed] [Google Scholar]
- Rizzi A, Nazzaro C, Marzola GG, Zucchini S, Trapella C, Guerrini R, Zeilhofer HU, Regoli D, Calo G, 2006. Endogenous nociceptin/orphanin FQ signalling produces opposite spinal antinociceptive and supraspinal pronociceptive effects in the mouse formalin test: pharmacological and genetic evidences. Pain 124, 100–108. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Ehlenberger DB, Hof PR, Wearne SL, 2006. Rayburst sampling, an algorithm for automated three-dimensional shape analysis from laser scanning microscopy images. Nat Protoc 1, 2152–2161. [DOI] [PubMed] [Google Scholar]
- Sanchez ES, Bigbee JW, Fobbs W, Robinson SE, Sato-Bigbee C, 2008. Opioid addiction and pregnancy: perinatal exposure to buprenorphine affects myelination in the developing brain. Glia 56, 1017–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Blázquez P, Rodríguez-Muñoz M, Garzón J 2010. Mu-opioid receptors transiently activate the Akt-nNOS pathway to produce sustained potentiation of PKC-mediated NMDAR-CaMKII signaling. PLoS One 5(6): e11278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargon MF, Mas N, Senan S, Ozdemir B, Celik HH, Cumhur M, 2003. Quantitative analysis of myelinated axons of commissural fibers in the rat brain. Anat Histol Embryol 32, 141–144. [DOI] [PubMed] [Google Scholar]
- Sato-Bigbee C, Pal S, Chu AK, 1999. Different neuroligands and signal transduction pathways stimulate CREB phosphorylation at specific developmental stages along oligodendrocyte differentiation. J Neurochem 72, 139–147. [DOI] [PubMed] [Google Scholar]
- Schägger H, 2006. Tricine-SDS-PAGE. Nat Protoc 1, 16–22. [DOI] [PubMed] [Google Scholar]
- Stover MW, Davis JM, 2015. Opioids in pregnancy and neonatal abstinence syndrome. Semin Perinatol 39, 561–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestal-Laborde AA, Eschenroeder AC, Bigbee JW, Robinson SE, Sato-Bigbee C, 2014. The opioid system and brain development: effects of methadone on the oligodendrocyte lineage and the early stages of myelination. Dev Neurosci 36, 409–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins RC, 1986. Myelination: a critical stage in development. Neurotoxicology 7, 103–120. [PubMed] [Google Scholar]
- Yin X, Crawford TO, Griffin JW, Tu P, Lee VM, Li C, Roder J, Trapp BD, 1998. Myelin-associated glycoprotein is a myelin signal that modulates the caliber of myelinated axons. J Neurosci 18, 1953–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadina JE, Hackler L, Ge LJ, Kastin AJ, 1997. A potent and selective endogenous agonist for the mu-opiate receptor. Nature 386, 499–502. [DOI] [PubMed] [Google Scholar]
- Zaveri NT, Marquez PV, Meyer ME, Hamid A, Lutfy K, 2018. The Nociceptin Receptor (NOP) Agonist AT-312 Blocks Acquisition of Morphine- and Cocaine-Induced Conditioned Place Preference in Mice. Front Psychiatry 9, 638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental FIGURE 1. Analysis of signaling pathways regulated by EM-1 and nociceptin in male oligodendrocytes. Pre-oligodendrocytes were isolated from male pups and incubated on the following day for 0, 5, 15, and 45 min. with CDM supplemented with either 1 μM EM-1, 1 μM nociceptin, or a mixture containing 1 μM each EM-1 and nociceptin. Western blot analysis of cell lysates was then used to determine the treatment effects on the levels of (a) P-ERK, (b) P-AKT, (c) P-CREB, and (d) P-p38MAPK, using β-actin or GAPDH values as loading controls. Results are expressed as % of their respective control values at 0 time. Bar graphs are the mean ± SEM from at least 3 independent experiments carried out in duplicate. No significant differences were detected between any of the treatment groups.
Data Availability Statement
Additional information that supports the findings of this study is available in the supplementary material of this article


