Abstract
The Global Program to Eliminate Lymphatic Filariasis (GPELF) relies heavily on a rapid diagnostic test (RDT) to a Wuchereria bancrofti circulating filarial antigen (Wb-CFA) to identify endemic areas and for determining when mass drug administration can stop. The antigen contains a carbohydrate epitope that is recognized by monoclonal antibody AD12. Og4C3, a monoclonal antibody that is used in a commercial ELISA for Wb-CFA recognizes the same moiety. Despite its diagnostic importance, little is known about the structure and function of this “AD12 epitope”. It is also present on other W. bancrofti glycoproteins and on glycoproteins of other filarial worms, but such antigens are not detected in the sera of individuals with most other filarial infections. We report here functional and biochemical analyses that shed light on the interaction between filarial glycoproteins and AD12 and/or Og4C3. Binding of these monoclonal antibodies to a mammalian glycan array suggests the reactive moiety has structural similarity to terminal β-D-glucuronic acid in a 1–3 linkage to other hexoses. However, sera collected from individuals with patent W. bancrofti infection had very low or undetectable serum antibodies to the GlcA-containing array glycans. Unlike other filarial glycoproteins, the Wb-CFA is relatively resistant to protease digestion by pronase and trypsin and completely resistant to the mucinase O-sialoglycoprotein endopeptidase (OSGE). The protease resistance of the Wb-CFA may contribute to its consistent detection in Wb-infected sera.
Keywords: Lymphatic filariasis, loiasis, rapid diagnostic test, Glucuronic acid, Wuchereria bancrofti, Brugia malayi
Graphical Abstract

1. INTRODUCTION
Lymphatic filariasis (LF) is a leading cause of disability worldwide. This major neglected tropical disease has been targeted for elimination [1]. The disease is caused by mosquito-borne nematode parasites. Wuchereria bancrofti is responsible for all LF in Africa and the vast majority of LF outside Africa. Brugia malayi and B. timori cause the remainder of infections. Although the Global Program to Eliminate Lymphatic Filariasis (GPELF) has reduced the at-risk population by nearly 50%, more than half a billion people remain at risk for acquisition of LF [1, 2]. Programmatic activities of GPELF rely heavily on an antigen assay that identifies active LF cases by detecting a W. bancrofti circulating filarial antigen (Wb-CFA) in the blood of infected individuals [3, 4]. GPELF currently uses the Filariasis Test Strip (FTS) that has replaced a previous related assay called the Immunochromatographic Card Test (ICT). Both of these assays use a monoclonal antibody called AD12.1 [4, 5]. A commercial ELISA test (the TropBio ELISA) detects the same antigen with monoclonal antibody Og4C3 [6] (Table 1).
Table 1:
Characteristics of commercial antibodies to circulating filarial antigens
| TropBio | ICT/FTS | |
|---|---|---|
|
| ||
| Monoclonal antibody | Og4C3 | AD12.1 |
|
| ||
| Immunogen | Onchocerca gibsoni adult male extract | Dirofilaria immitis soluble extract |
|
| ||
| Isotype | IgM | |
|
| ||
| Reactivity with human serum | Circulating antigens of W. bancrofti (Wb-CFA), intermittently present in sera from a minority of persons with heavy Loa loa infections | |
|
| ||
| Reactivity with soluble extracts | O. gibsoni | D. immitis |
| O. volvulus | B. malayi | |
| D. immitis | L. loa | |
| A. caninum | O. ochengi | |
| T. canis | A. lumbricoides | |
|
| ||
| Localization by immunohistochemistry | Junction of cuticle and hypodermis, gut, intra-uterine embryos, extra uterine microfilariae | Cuticle of adults, uterine wall, eggs, testis of adult males |
The Wb-CFA is heavily glycosylated with a carbohydrate epitope recognized by the murine IgM monoclonal antibodies AD12.1, DH6.5, and Og4C3 [5, 7]. For simplicity, we refer to the epitope recognized by these antibodies as the AD12 epitope. This epitope is not unique to W. bancrofti [5]; in fact the monoclonal antibodies recognizing it were raised against crude antigens from other related species [6, 8] (Table 1). In animal models of filarial infection, AD12 epitope-containing antigens are produced primarily by adult female worms [5]. This finding is reflected in human field studies in which Wb-CFA can be detected in infected individuals without microflaremia [9, 10].
Despite the production of AD12 epitope-containing antigens by other filarial species, LF rapid diagnostic tests (RDTs) have been considered functionally specific for W. bancrofti, since cross-reactive antigens are not commonly found in the blood of persons infected with other nematodes. In the past decade, however, it has become clear that some persons with Loa loa infections have positive LF RDT tests that limits the value of CFA tests in loiasis endemic regions [11–15]. A better understanding of the antibody to carbohydrate epitope interaction will help inform strategies for reducing L. loa related cross-reactivity in diagnostic tests for LF.
2. MATERIALS AND METHODS
2.1. Parasites
The Filariasis Research Reagent Resource Center (FR3, www.filariasiscenter.org, Athens, GA) provided adult female B. malayi worms recovered from jirds 8–10 weeks post-infection. To prepare B. malayi antigens (BmA) from whole worm lysate, thirty worms were subjected to one freeze-thaw cycle and then incubated overnight at 4°C in RIPA buffer with ProteaseArrest protease inhibitors (G-biosciences, St. Louis, MO). Samples underwent mechanical disruption by pestle and then by shearing with a 25G needle with subsequent centrifugation (13500 × g for 30 min). To collect excretory/secretory (ES) products, 60 worms were cultured for two weeks at 37°C/5% CO2 in 20 ml of RPMI 1640 media supplemented with L-glutamine, glucose and 100 U/mL penicillin/ 0.1 mg/mL streptomycin/ 0.25 μg/mL amphotericin B (Sigma). The spent media was collected on alternating days over 14 days and stored at −20°C.The ES products were concentrated to 0.1 to 0.2 μg/mL by centrifugal filtration using an Amicon Ultra 3000 MWCO filter (Millipore). L. loa adult worms were a gift from Dr. Vida Dennis, Tulane University. L. loa soluble antigen was prepared by grinding adult worms in extraction buffer (10 mM Tris pH 8.3, 2% sodium deoxycholate, 1 mM PMSF, 1 mM EDTA, 1 mM EGTA, 25 μg/mL TLCK protease inhibitor, 15 μg/mL TPCK protease inhibitor) and collecting the soluble fraction.
2.2. Chemical and enzymatic treatments of BmA
2.2.1. Periodate treatment.
BmA was treated with 10 to 50mM sodium metaperiodate overnight at 4°C in the dark. The reaction was dialyzed in slide-a-lyzer mini dialysis units (Thermo Scientific) into PBS overnight at 4°C.
2.2.2. Cleanascite delipidation.
BmA was delipidated with Cleanascite (Biotechsupportgroup.com) as per manufacturer’s instructions. Briefly, Cleanascite was added to BmA (1 mg/ml) at 1:2 dilution and mixed gently for 10 minutes. The sample was centrifuged at 16,000 × g for 1 minute, and the lipoprotein-free supernatant was stored until further use.
2.2.3. Chemical deglycosylation with TFMS.
BmA (1mg ml) was deglycosylated with GlcyoProfile IV chemical deglycosylation kit (SigmaAldrich) using Trifluoromethanesulfonic acid (TFMS), as per manufacturer’s instructions.
2.2.4. Alkaline hydrolysis.
BmA was added to 0.05 M NaOH/ 1 M NaHB4 and incubated at 50°C for 0–16 hours to remove O-linked glycans by β-elimination [16]. To stop the hydrolysis reaction, samples were neutralized with 0.5 M HCl. The mock treated sample of BmA was incubate at 50°C for 16 hours in pH 7 deionized water. AD12 antibody binding to the released glycans were analyzed by filarial antigen capture ELISA and competition ELISA as in section 2.5.
2.2.5. PNGase F.
Five micrograms of BmA or ES were digested with PNGase F (New England Biolabs) under denaturing conditions for one hour at 37°C following the manufacturer’s protocol. Two micrograms of RNase B were used as a positive control (New England Biolabs).
2.2.6. NEB O-glycosidase mix.
Five micrograms of BmA or ES were digested under reducing conditions with the protein deglosylation mix II from New England Biolabs containing PNGase F, β1–4 Galactosidase S, O-glycosidase and sialidases for 16 hours at 37°C according to the manufacturer’s protocol. Two micrograms of bovine fetuin was used as a positive control (New England Biolabs).
2.2.7. O-sialoglycoprotein endopeptidase.
Lyophilized O-sialoglycoprotein endopeptidase purified from Mannheimia haemolytica (OSGE, Ceder Lane Labs, Burlington NC) was reconstituted to 2.4 mg/mL in deionized water. One microgram of L. loa Ag, BmA or BmES were cleaved with 3 μl of OSGE at 37°C for 0–4 hours. The reaction was stopped by boiling at 100°C in 1X NuPAGE LDS sample buffer (Invitrogen). Alternatively, filarial proteins were first immunoprecipitated with 50 μl of DH6.5 conjugated affigel beads as in section 2.7 followed by OSGE cleavage.
2.2.8. Pronase and trypsin.
The amount of Pronase (Roche) and 0.5% trypsin-EDTA (Gibco) enzyme necessary to completely cleave 0.5 μg of B. malayi lysate in one hour was empirically determined for each enzyme to be 6 μg/ml and 3 ng/ml respectively. Then a cleavage reaction perfomed with either 0.5 μg of BmA or the Wb-CFA for 10 minutes to 1 hour at 37°C.
2.3. Panning of Og4C3.
To elute the reactive moiety, BmA (5 μg/ml) or BmA treated with Cleanascite or TFMS was added to well of a 96-well microtiter plate coated with Og4C3 (TropBio, Sydney, NSW). After incubation for one hour, the plate was washed, and bound antigens were eluted with 2 mM Glycine-HCl and neutralized with 1/10 volume of 1 M Tris-HCl. The eluted fraction was dialyzed and concentrated using a 3 kDa MWCO centricon filter (Millipore) and used to check the reactivity to AD12 on ICT card test.
2.4. Polyacrylamide gel electrophoresis, silver stain and western blot analysis
Equivalent amounts of proteins were boiled in 1X NuPAGE LDS reducing sample buffer (Invitrogen) then resolved by SDS-PAGE using a 4–12% bis-tris NuPAGE gel (Invitrogen). Silver staining of gels was performed with the FOCUS FAST silver staining kit (G-Biosciences, St. Louis, MO) according to the manufacturer’s instructions. For western blots, proteins transferred to nitrocellulose membranes (Amersham). Membranes were blocked with 5% milk in phosphate buffered saline with tween-20 (PBS-T) followed by incubation with peroxidase-conjugated AD12 antibody (1:3000 dilution) or Og4C3 (TropBio, Townsville, Australia, 1:50,000 dilution) for one hour at room temperature. Galectin 2 (Lec-2) was detected by probing with the 4B4 monoclonal anti-lec-2 antibody at 1:1000 dilution [17]. Paramyosin was detected using the PM-1 mouse monoclonal antibody at 1:3000 [18]. The blots were washed then incubated with the secondary goat-anti-mouse-IgG or IgM HRP antibody (1:5000, Southern Biotech). A polyclonal pan-filarial sera [19] produced by rabbits immunized with Dirofilaria immitis (dog heartworm) adult worm antigen was used at a 1:3000 dilution followed by incubation for one hour with a secondary mouse anti-rabbit-HRP used at 1:5000 (Southern Biotech). After probing, membranes were incubated with Clarity Western ECL substrate (Bio-Rad) and chemiluminescence was detected by a ChemiDoc imager and analyzed by Image Lab 5.2.1 software (Bio-Rad).
2.5. ELISAs
2.5.1. Filarial antigen capture ELISA.
Detection of circulating filarial antigen by sandwich ELISA was performed as previously described [4]. This assay uses two IgM monoclonal antibodies, AD12.1 and DH6.5, both of which recognize the AD12 carbohydrate epitope. Reagents for ELISA, immunoprecipitation and immunoblot experiments were purchased from Sigma-Aldrich (St. Louis, MO), unless otherwise noted.
2.5.2. Competition ELISA.
Wells were coated overnight at 37°C with 100 μl/well of BmES product diluted to 1.25 μg/mL in 0.6M carbonate buffer, pH 9.6. After coating, wells were washed with phosphate buffered saline (PBS) containing 0.05% Tween-20 (PBS-T) then blocked with 200 μL 5% fetal calf serum in PBS-T. Wells were then incubated with peroxidase-conjugated AD12 antibody for one hour at 37°C. Wells were washed three times with PBS-T, incubated with 100 μL O-phenylenediamine dihydrochloride (OPD) for ten minutes, stopped by addition of 30 μL of 4 M sulfuric acid, and absorbance read at 492 nm.
2.5.3. OSGE ELISA.
BmA was treated with OSGE for one hour at 37°C in tris-HCl pH 7.5 either as a pretreatment with or without dialysis during treatment (Slide-a-Lyzer MINI dialysis tubes, 10,000 MWCO, Thermo Scientific) or following antigen capture in the circulating filarial antigen sandwich ELISA.
2.6. Mammalian glycan array
Glycan microarrays were performed by the Consortium for Functional Glycomics (Atlanta, GA) following their standard protocol. The mammalian glycan array version 5.2, heparin sulfate array or heparin polymer array was probed in standard TSM binding buffer (20 mM Tris-HCl, pH 7.4 150 mM NaCl, 2 mM CaCl2, 2 mM MgCl2, 0.05% Tween-20, and 1% BSA) with AD12 at 25 μg/mL or Og4C3 (1:100 dilution). Seventy microliters were applied to the array, topped with a coverslip and incubated at room temperature in a dark humidified chamber one hour. The slide was washed four times in TSM washing buffer (20 mM Tris-HCl, pH 7.4 150 mM NaCl, 2 mM CaCl2, 2 mM MgCl2, 0.05% Tween-20) and four times in TSM washing buffer without Tween. Binding was detected with 5 μg/mL anti-mouse IgM-Alexa 488 (Invitrogen) using the same probing and washing conditions as above. For human sera, mouse anti-human IgG-Alexa 633 or mouse anti-human-IgM-Alexa-488 were used. An image of bound fluorescence was obtained using a microarray scanner (Scan Array Express, PerkinElmer Life Sciences). The integrated spot intensities were determined using ImaGene software (Biodiscovery Inc, El Segundo, CA). Once aligned, the amount of binding to each spot in quantified and the data was analyzed using Microsoft Excel, where the highest and lowest spot of the six replicates were removed, and the average of the four remaining spots was displayed graphically and, in a table, along with appropriate statistics.
2.7. Immunoprecipitation
Monoclonal antibody DH6.5 or mouse IgM isotype control (clone 11E10, Southern Biotech, Birmingham AL) were conjugated to Affigel 10 beads (Bio-Rad) according to the manufacturer’s protocol. Conjugated beads were stored as a 50% solution in PBS. 100 μL of conjugated beads were mixed with human sera (as a source of Wb-CFA), 5 μg of BmA or ES in 300 μl total volume and rocked overnight at 4°C. After washing four times with cold PBS the beads were resuspended in the appropriate buffer for enzyme assays or were analyzed by western blot as described above.
3. RESULTS
3.1. AD12 and Og4C3 antibody binds to a glycan epitope on filarial glycoproteins
To confirm the carbohydrate nature of the AD12 and Og4C3-bound epitopes on B. malayi antigens, we evaluated the periodate sensitivity of AD12-epitope containing proteins. Sodium metaperiodate treatment of B. malayi adult female soluble antigen (BmA) resulted in a loss of AD12 antibody binding at a concentration that did not affect peptide antigens such as galectin-2 and paramyosin (Figure 1A). Moreover, the epitope for AD12 was detected in the supernatant from tricholoroacetic (TCA) precipitation of BmA, and Cleanascite (CLNS)-treated BmA but not in BmA stripped of glycans by trifluoromethanesulfonic acid (TFMS). Glycoproteins with high carbohydrate content may be resistant to TCA precipitation, CLNS removes lipoproteins and TFMS completely removes N-and O-linked glycans while preserving the peptide backbone, suggesting that the major reactive molecules as either a disordered protein or proteoglycan in nature (Fig 1B and C).
Figure 1: The AD12 and Og4C3 antibodies binds to filarial glycans.

(A) AD12 reactivity is destroyed by periodate treatment. One microgram (μg) of BmA was treated overnight at 4°C with the concentration of periodate indicated, followed by SDS PAGE and western blotting to detect AD12 glycans, Lec-2 (MW 32 kDa) and paramyosin (MW 101 kDa). (B) Og4C3 reactivity of BmA before and after adsorbing out lipids/glycolipids (Cleanacite, CLNS) or chemical deglycosylation with TFMS. (C) Reactivity of AD12 (ICT card) to Og4C3 adsorbed soluble extracts of Brugia malayi (BmA) that was untreated, precipitated with TCA, treated with cleanascite (CLNS) or trifuoromethylsulfonic acid (TFMS), and pooled plasma from W. bancrofti infected patients. The bottom line represents the control line, and the upper line is the test line.
We assessed whether the AD12 antibody could bind to glycans released from the peptide core by β-elimination. Mild alkaline hydrolysis with sodium hydroxide releases O-glycans from serine and threonine sites, and when performed with excess sodium borohydride (NaBH4), the hydrolyzed glycan is protected from further degradation known as the peeling reaction [16]. Sixteen hours incubation with 50 mM NaOH/1 M NaBH4 rendered the glycan completely undetectable by sandwich ELISA (Figure 2A), which detects only antigens with multiple AD12 epitopes since the assay uses antibodies that recognize the AD12 epitope to both capture and detect bound antigen [5]. This suggests that β-elimination destroys the multivalent nature of the reactive glycoproteins, as would be expected when glycans are released from poly-glycosylated glycoproteins. To test whether the released glycans could still bind the AD12 antibody, we tested their ability to interfere with AD12 binding to filarial antigens. The hydrolyzed glycans were able to block AD12 binding to BmES (Figure 2B) at the same efficiency as the mock treated lysate, but less well than lysate mixed with hydrolysis buffer but not heat-treated at 50°C. These results suggest that released AD12 epitope glycans can be bound by the AD12 antibody, and that heating at 50°C diminishes the capacity of glycan to compete for antibody binding by an unknown mechanism. Together these data support the hypothesis that the AD12 antibody is binding O-linked glycans and that the released glycans retain affinity for the AD12 antibody, but that released glycan chains have insufficient valency to be both captured and detected by iso-specific antibodies.
Figure 2: The AD12 antibody binds to glycan irrespective of antigen protein backbone.
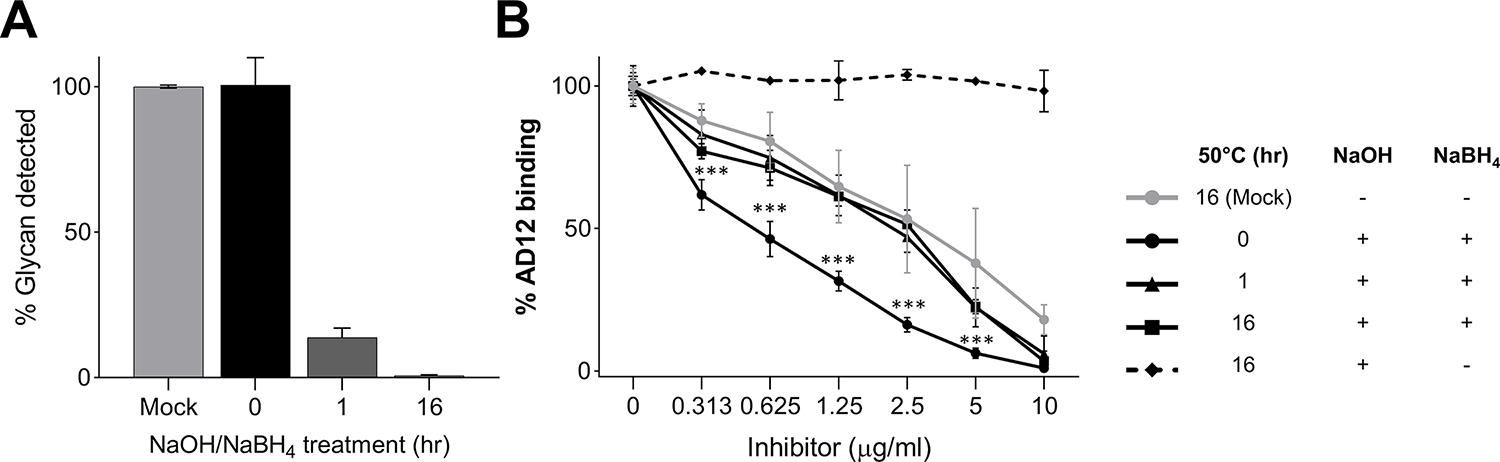
(A) Antigen capture by sandwich ELISA following β-elimination. Forty micrograms of BmA was treated at 50°C with 0.05M NaOH/ 1M NaBH4 and aliquots were removed at 0, 1, or 16 hours and analyzed by sandwich antigen ELISA using plates coated with DH6.5 and detected with HRP-conjugated AD12. A control sample (mock) was incubated for 16 hours at 50°C in water alone. The assay was repeated three times and mean absorbance values were plotted relative to the mock treated sample. (B) Competitive binding experiment. Carbohydrates hydrolyzed by β-elimination used to inhibit AD12 binding to filarial antigen in a competition ELISA. Partially, or fully hydrolyzed BmA (inhibitor) was incubated with HRP-labeled AD12 antibody in wells coated with 1.25 μg/ml B. malayi ES. A control reaction (dotted line) contained BmA that was fully hydrolyzed to monosaccharides by reacting with 0.05M NaOH in the absence of NaBH4. The assay was repeated three times and absorbance values were normalized to the inhibitor free control in each dilution series. *** denotes a p-value < 0.001 by t-test. Error bars depict standard deviation.
3.2. The Wb-CFA is protease resistant compared to non-circulating antigens
Many filarial worms express AD12 glycoproteins, yet only Wb-CFA is consistently present in individuals infected with W. bancrofti, while cross-reactive antigens are transiently detected in some patients with loiasis [14]. This suggests that Wb-CFA is uniquely stable compared to other AD12 epitope-containing filarial proteins. To test this hypothesis, we first assayed sensitivity to proteolytic cleavage. We found that 0.03 μg/ml of trypsin or 6 μg/ml of pronase efficiently degraded the AD12-reactive proteins in 0.5 μg of BmA over one hour, but that Wb-CFA was highly resistant to protease treatment (Table 2 and Figure 3).
Table 2:
Minimum protease to degrade filarial antigens
| Antigen | Trypsin (μg/ml) | Pronase (μg/ml) | OSGE (μg) |
|---|---|---|---|
|
| |||
| BmA | 0.03 | 6 | 7.2 |
| Wb-CFA | 5 | 38.5 | Resistant (>48) |
|
| |||
| fold difference | 166 | 6.4 | ND |
Figure 3: The Wb-CFA is relatively protease resistant compared to AD12 epitope-containing proteins from B. malayi.
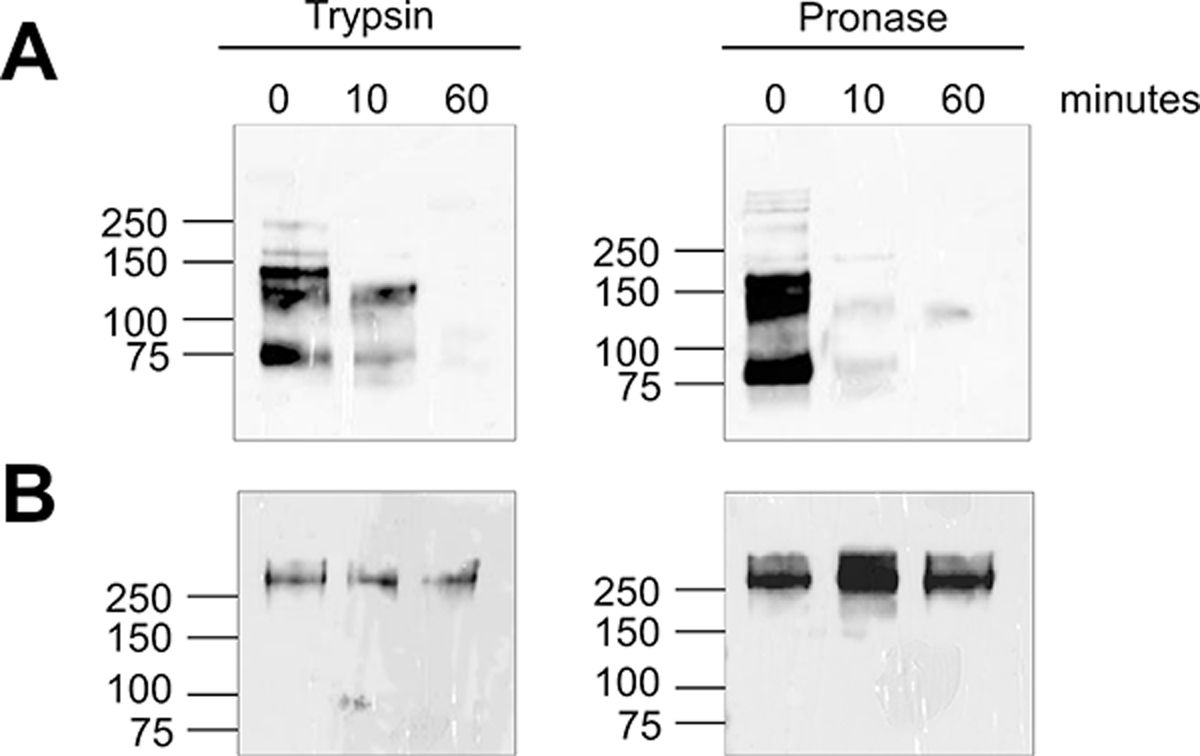
DH6.5 immunoprecipitated proteins digested with 0.03 μg/ml trypsin or 6 μg/ml pronase over one hour followed by an AD12 western blot to measure remaining intact glycoproteins. A) BmA (500 ng per condition). B) Wb-CFA from patient serum.
3.3. AD12 epitope-containing proteins other than the Wb-CFA, are sensitive to mucinase cleavage
The mucinase O-sialoglycoprotein endopeptidase (OSGE) cleaves negatively charged, mucin-like molecules [20]. Since the Wb-CFA is predicted to be a heavily O-glycosylated molecule, we tested if OSGE could cleave AD12 epitope-containing proteins. Figure 4A demonstrates that OSGE cleaves all AD12 epitope-containing proteins from B. malayi while other proteins bound by a broadly reactive polyclonal anti-filarial antisera (Rabbit C, RbC [19]) intact. In contrast, OSGE did not cleave Wb-CFA (figure 4B, Table 2).
Figure 4. OSGE selectively digests most AD12 epitope-containing Brugia malayi glycoproteins, but not Wb-CFA.

(A) Western blots following one or 4-hour OSGE digest of 0.5 μg of BmA and ES. A western blot using a polyclonal anti-filarial rabbit serum (RbC) on the OSGE-treated BmA illustrates that OSGE does not cleave all proteins. (B) Western blot of AD12 circulating antigens from 500 μl of serum collected from two W. bancrofti-infected individuals before and 24 hours-post drug treatment with ivermectin (IVM) and albendazole (ALB). Serum samples were immunoprecipitated with DH6.5 then digested with OSGE for four hours.
When people with LF are treated with anthelmintic chemotherapy there is often a spike in serum circulating filarial antigen levels [21]. We recently observed that this is not solely due to an increase of the high molecular weight Wb-CFA, but rather, to the appearance of lower molecular weight AD12-reactive proteins [22]. Such antigens are also susceptible to OSGE digest (compare lanes 3 and 4 in figure 4B). Thus, OSGE resistance is a feature unique to the >200 kDa Wb-CFA, compared to other AD12 epitope-containing proteins.
3.4. Peptidase susceptibility as a strategy to selectively deplete AD12 epitope-containing proteins from L. loa
To determine whether protease treatment could eliminate cross-reactivity in LF diagnostic tests, we used trypsin, pronase, or OSGE to digest B. malayi or L. loa antigens prior to detection. Although protease treated AD12 glycoproteins were undetectable by western blot, they were readily detectable by filarial capture ELISA (Figure 5) and by FTS (data not shown). Pretreatment with OSGE reduced but did not prevent detection of L. loa antigens by sandwich ELISA. These data indicate that glycopeptide fragments that are too small to be detected efficiently by western blot remain sufficiently polyvalent to be detected by the filarial antigen sandwich ELISA. Most immunoreactivity of the OSGE reduced material was removed by dialysis with a membrane that retained material with a molecular weight >10 kDa (Figure 5D).
Figure 5: Protease digestion diminishes, but does not eliminate, detection of AD12 glycoproteins by ELISA.
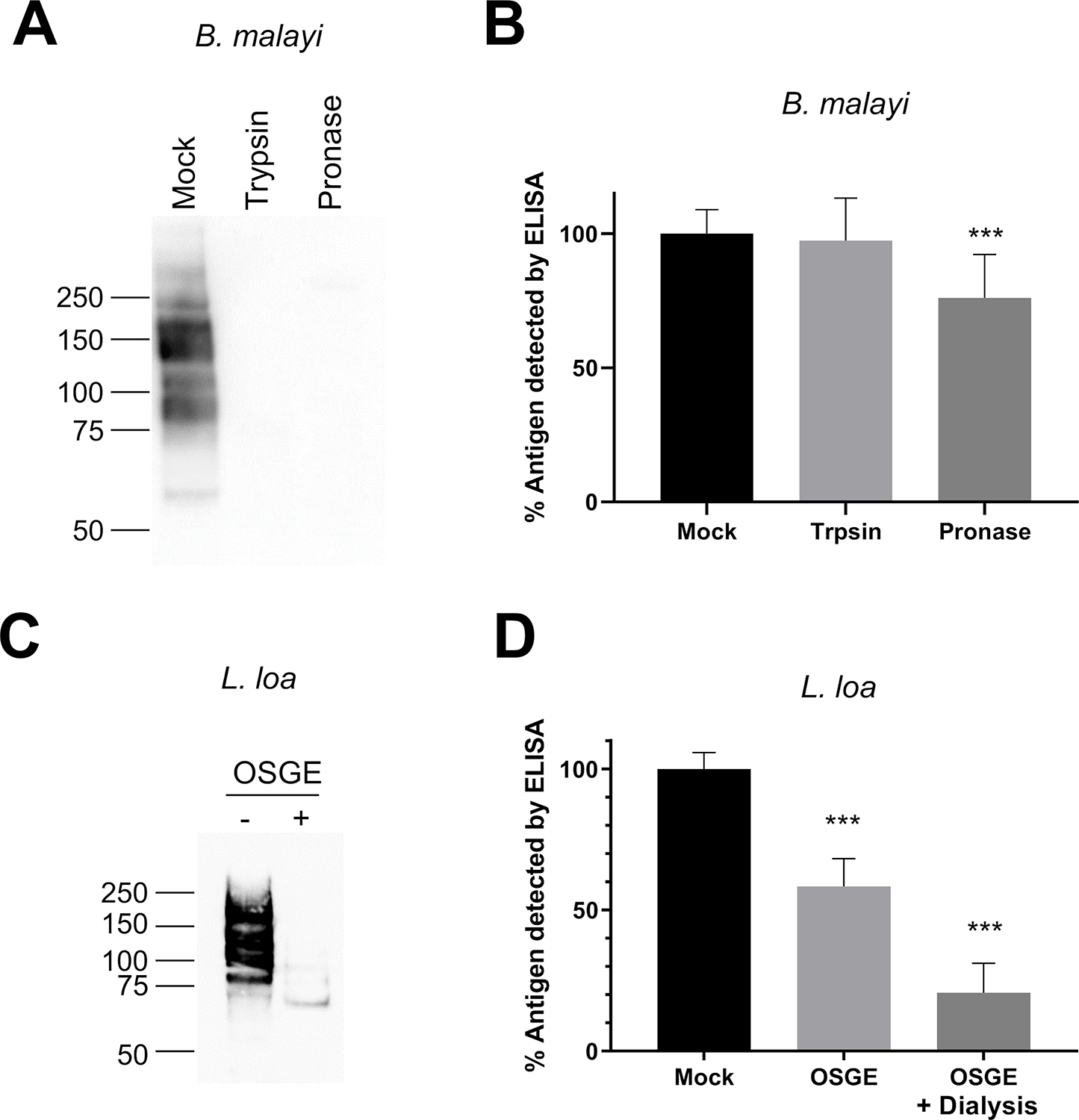
(A) Western blot of AD12 glycoproteins from 0.5 μg BmA after a 1-hour incubation at 37°C with 1.5 ng trypsin or 60 ng pronase. (B) Filarial antigen ELISA of protease digests from panel A. Assay was performed four times in triplicate and values were normalized to the mock treated sample. (C) Western blot of AD12 glycoproteins from 0.5 μg L. loa soluble antigen after a 4-hour OSGE digestion at 37°C. (D) Filarial antigen ELISA of L. loa soluble antigen featuring either mock treated, OSGE, or OSGE plus dialysis prior to antigen capture. Assay was performed twice in triplicate and values were normalized to mock treated sample. Error bars represent standard deviation. *** denotes p-value < 0.001 by unpaired t-test.
3.5. AD12 glycan is not found on typical N-glycans or core 1 or core 3 O-linked glycans
We next tested whether commercially available glycosidases could alter the molecular weight of Wb-CFA through the removal of specific glycan moieties. PNGase F, an endoglycosidase that removes most N-linked glycans, had no effect on the Wb-CFA. PNGase F did shift some AD12-reactive bands in B. malayi lysate and ES products. However, PNGase F digestion did not reduce the intensity of AD12 binding by western blot, suggesting that removal of non-AD12-binding N-glycans is responsible for the change in size of the AD12-reactive Brugia antigens (Figure 6). A glycosidase mixture of PNGase F, β1–4 galactosidase, neuraminidase and the Enterococcus faecalis O-glycosidase likewise had no effect on the Wb-CFA but did shift several bands in B. malayi lysate and ES antigens. We observed a similar shift without an overall loss of signal when L. loa soluble antigens were treated (data not shown).
Figure 6: AD12 epitope is not removed by PNGase F or O-glycosidase.

(A) The Wb-CFA was treated with PNGase F one hour at 37°C then analyzed by AD12 western blot. (B) AD12 western blot of 0.5μg B. malayi lysate or ES after a one-hour PNGase F treatment. (C) AD12 western blot of Wb-CFA following one-hour incubation with a deglycosidase mix containing O-glycosidase, PNGase F, Neuraminidase, β1–2 galactosidase, and N-acetylglucosaminidase under native or denaturing conditions. (D) AD12 western blot of B. malayi lysate and ES after a one-hour denaturing deglycosidase reaction. A corresponding silver stain of each reaction is available in supplementary figure S1 that includes the reaction positive controls RNase B for PNGase F and fetuin for deglycosidase mix.
3.6. AD12 and Og4C3 carbohydrate epitope resembles β-D-glucuronic acid in a 1–3 linkage to another hexose
To explore the glycan binding specificities of AD12 and Og4C3, we assayed the binding of each to a 609-member mammalian carbohydrate array. AD12 bound two array carbohydrates; both contained a terminal D-glucuronic acid (GlcA) in a β1–3 linkage to a second hexose (galactose [Gal]) or N-actetylhexosamine (N-acetylglucosamine [GlcNAc], Figure 7A). AD12 did not bind other array glycans containing GlcA, including GlcA alone, GlcA in a β1–6 linkage to galactose, or sulfonylated (3S)GlcA. As expected, Og4C3 bound the same glycan moieties as AD12, yet also bound several array glycans with terminal GlcA, including 3-sulfated GlcA and GlcA in β1–6 and β1–4 linkages. Further screening with sulfated glycans of heparan sulfate, confirmed reactivity with terminal glucuronic acid sulfated glycans with β1–4 linkage (Figure 7C). The reactivity of Og4C3 was not directed to the heparin itself, but to the polymerized sulfated forms (dp2 and dp4; Figure 6D), again indicating a requirement for multivalency. Table S1–S3 presents the raw data from the arrays. To confirm the array result, we assayed if GlcA or other monosaccharides could inhibit AD12 binding. GlcA blocked AD12 binding to filarial antigens while the structurally related galacturonic acid (GalA) was notably less effective. Glucose (Glu), galactose (Gal) and N-acetylglucosamine (GlcNAc) had no effect on antibody binding (Figure 7E).
Figure 7: AD12 and Og4C3 bind to glycans containing a terminal glucuronic acid (GlcA).
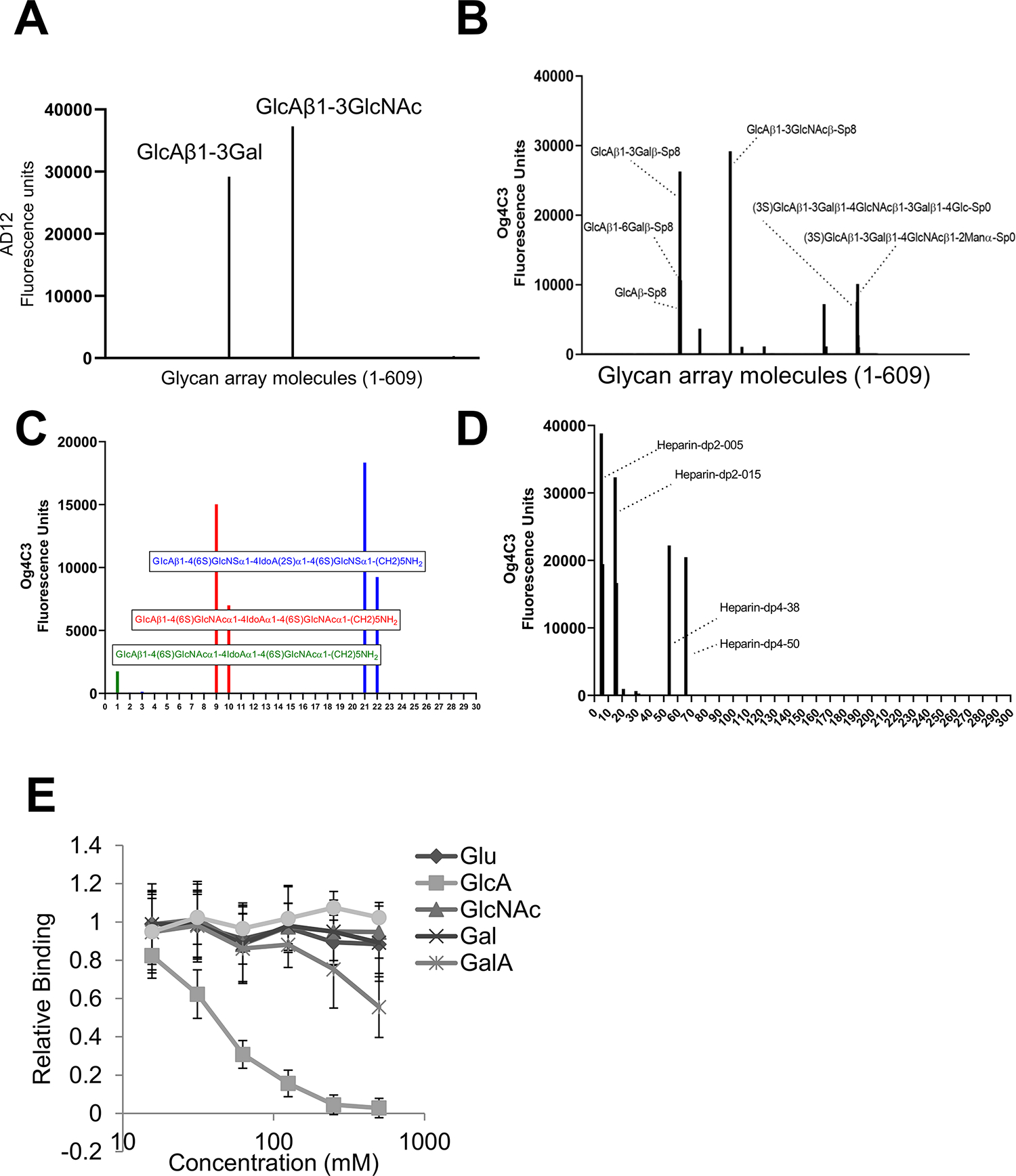
Carbohydrates on mammalian glycan array v5.2. were probed with AD12 (A) or Og4C3 (B). Og4C3 reactivity to defined heparan sulfate glycans (C) or polymerized forms of heparin (D). See tables S1–S3 for raw array data. (E) GlcA, but not other hexoses, compete with AD12 antibody binding to Brugian antigens. The hexoses shown were mixed with HRP-labeled AD12 and added to wells coated with BmES. Absorbance values are reported as a percentage of the untreated control; the data shown are the combined results of three separate experiments. Error bars represent standard deviation. Glu, glucose; GlcA, glucuronic acid; GlcNAc, N-acetylglucosamine; Gal, galactose; GalA, galacturonic acid
3.7. Antibodies to the Og4C3 targeted antigen are undetectable in patients with patent infection
To evaluate the glycan reactivity of the host humoral responses, antibody binding of sera from W. bancrofti-infected subjects was compared to that of sera from healthy uninfected blood bank donors. Differential reactivity was observed for IgG and IgM from infected individuals, with IgG having more glycan reactivity (Figure 8A). However, as shown in Figure 7B, there was minimal IgG or IgM reactivity to any of the terminal glucuronic acid containing glycans (denoted by large open circles).
Figure 8: Antibodies to the Og4C3 targeted antigen are undetectable in individuals with patent W. bancrofti infection.
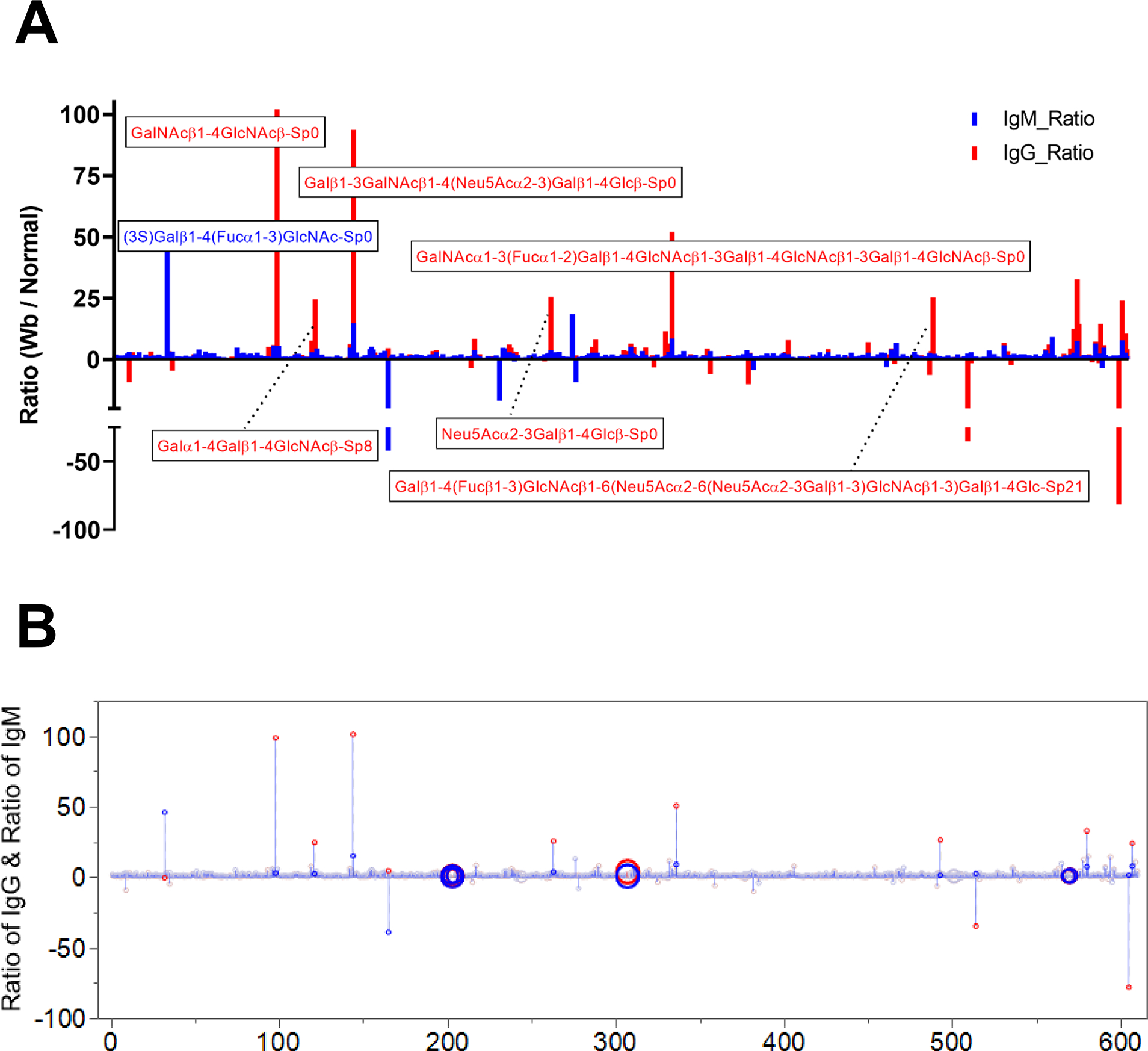
(A) Antisera from pools of 5 infected and 5 uninfected controls were used to probe the mammalian glycan array. The ratio of immunoreactive glycans in infected compared to normal sera to both human IgM (blue) and IgG responses (red) are detailed. (B) Circles indicate the position of glycans with terminal GlcA.
4. DISCUSSION
AD12 and Og4C3 recognition of carbohydrate epitope(s) serves as the foundation of two commercially available CFA detection tests. Here we confirm that the reactive moiety is a polysaccharide and show that it has structural similarity to terminal β-D-glucuronic acid, primarily in a 1–3 linkage to other hexoses. We further show that sera from patients with patent filarial infections poorly recognize such terminal GlcA-containing glycans, and that Wb-CFA is relatively resistant to protease digestion. These observations may contribute to the reliable detection of Wb-CFA in sera from infected individuals.
Several characteristics of AD12 and Og4C3-reactive antigens suggest they are mucins or proteoglycans. First, they are not precipitated by TCA. It is well known that TCA is less efficient in precipitating proteins that are unfolded, denatured or heavily glycosylated such as mucins and proteoglycans [23–25]. Second, apart from the Wb-CFA, most AD12-reactive proteins are readily digested by the mucinase OSGE. Compared to glycoproteins, proteoglycans contain long chains of repeating disaccharides, and are negatively charged due to the presence of sulfate and uronic acid groups. The TCA-resistant nature and protease-insensitivity of the Wb-CFA, in combination with the evidence that it is heavily glycosylated and may contain sulfated and terminal glucuronic acid surface groups suggests that the Wb-CFA may be a proteoglycan. The non-circulating AD12 epitope-containing proteins from B. malayi and L. loa, by contrast, are more sensitive to proteases including trypsin, pronase and OSGE than the Wb-CFA and are likely composed of glycoproteins including mucins and glycolipoproteins since the removal of lipoproteins by CLNS treatment changed the Og4C3 reactivity pattern.
The observation that Wb-CFA is protease resistant (and other AD12 glycans are not) initially suggested that integrating a protease step into diagnostic test may be a strategy to distinguish LF from cross-reactive loiasis. Unfortunately, our data indicate that proteolytic cleavage of B. malayi and L. loa antigens does not block detection by a sandwich ELISA, suggesting a protease step would still register positive by a rapid diagnostic test lateral flow assay.
The role of Wb-CFA in filarial infection is unknown and it is not understood why W. bancrofti infection maintains a stable CFA while infection with B. malayi and L. loa do not. Our data do not directly answer this question but do show that Wb-CFA is unique among Brugia, Loa, and other Wuchereria AD12 glycans in its resistance to OSGE and other protease digestion and its lack of PNGase F-labile N-glycans.
Important questions about the Wb-CFA and AD12-reactive carbohydrate epitopes remain. The complete structure of the carbohydrate remains elusive and is the subject of ongoing investigation. Although both AD12 and Og4C3 bind to carbohydrates with terminal glucuronic acid moieties, we were unable to prevent antibody binding by pre-treatment of antigen with multiple commercially available glucuronidases. It therefore remains unclear whether the AD12 epitope itself contains GlcA in a β1–3 linkage to galactose or GlcNAc, or some molecular mimic. Parasitic nematodes synthesize a rich variety of complex glycans many of which are unique compared to the host, including structures with terminal GlcA [26–29].
It is interesting that we did not detect human antibodies to the Og4C3-reactive antigens. It has previously been observed that antibodies to AD12-reactive antigens are not detected in microfilaremic or antigenemic individuals, but are detected in individuals with clinical filariasis without detectable antigenemia [4]. Whether antibodies to this antigen do not develop in the presence of persistent antigen, or whether they are bound with Wb-CFA in immune complexes and consequently depleted from serum is unclear.
Our observation that AD12 and Og4C3 have slightly different binding specificities is interesting, since the FTS/ICT RDTs utilizing AD12 and the Og4C3 ELISA are considered functionally equivalent for the purposes of identifying W. bancrofti infected individuals. The western blot analysis from figure one illustrates slight differences in the pattern of antibody binding between AD12 and Og4C3. The mammalian glycan array mapping suggest that Og4C3 is has a more permissive antigen binding cleft while AD12 is more restrictive. Og4C3 binds to GlcA in β1–3, β1–4 and β1–6 confirmations and tolerates sulfate modification on the GlcA. Og4C3 readily binds to heparan sulfate polymers while AD12 does not (data not shown). Further studies on the structure of the AD12 glycan will be required to improve our understanding of the role of molecules like Wb-CFA in filarial biology and to improve antigen detections tests for filariasis.
Supplementary Material
Figure S1: Silver stained gels of endoglycosidase treated filarial antigens
Table S3: Raw heparin polymer array data
Table S1: Raw mammalian glycan array data
Table S2: Raw heparin sulfate array data
ACKNOWLEDGEMENTS
This study utilized the NIH / NIGMS Biomedical Mass Spectrometry Resource at Washington University in St. Louis, MO, which is supported by National Institutes of Health / National Institute of General Medical Sciences Grant # 8P41GM103422. We are appreciative of the guidance and advice from the members of the Nutman and Weil labs.
FUNDING SOURCES
This work was supported by the National Institute for Allergy and Infectious Disease (NIAID) [grant number K08AI121422 (PJB)] and an NIH Loan Repayment Programs (NIAID) award (PJB). This work was also supported, in part, by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health and by the Foundation for Barnes-Jewish Hospital. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
REFERENCES
- 1.Global programme to eliminate lymphatic filariasis: progress report, 2018. Wkly Epidemiol Rec. 2019;94(41):457–72. [Google Scholar]
- 2.Hooper PJ, Chu BK, Mikhailov A, Ottesen EA, Bradley M. Assessing progress in reducing the at-risk population after 13 years of the global programme to eliminate lymphatic filariasis. PLoS Negl Trop Dis. 2014;8(11):e3333. Epub 2014/11/21. doi: 10.1371/journal.pntd.0003333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weil GJ, Ramzy RM. Diagnostic tools for filariasis elimination programs. Trends Parasitol. 2007;23(2):78–82. Epub 2006/12/19. doi: 10.1016/j.pt.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Weil GJ, Jain DC, Santhanam S, Malhotra A, Kumar H, Sethumadhavan KV, et al. A monoclonal antibody-based enzyme immunoassay for detecting parasite antigenemia in bancroftian filariasis. J Infect Dis. 1987;156(2):350–5. Epub 1987/08/01. [DOI] [PubMed] [Google Scholar]
- 5.Weil GJ, Liftis F. Identification and partial characterization of a parasite antigen in sera from humans infected with Wuchereria bancrofti. J Immunol. 1987;138(9):3035–41. [PubMed] [Google Scholar]
- 6.More SJ, Copeman DB. A highly specific and sensitive monoclonal antibody-based ELISA for the detection of circulating antigen in bancroftian filariasis. Trop Med Parasitol. 1990;41(4):403–6. Epub 1990/12/01. [PubMed] [Google Scholar]
- 7.Chanteau S, Moulia-Pelat JP, Glaziou P, Nguyen NL, Luquiaud P, Plichart C, et al. Og4C3 circulating antigen: a marker of infection and adult worm burden in Wuchereria bancrofti filariasis. J Infect Dis. 1994;170(1):247–50. Epub 1994/07/01. [DOI] [PubMed] [Google Scholar]
- 8.Weil GJ, Curtis KC, Fakoli L, Fischer K, Gankpala L, Lammie PJ, et al. Laboratory and field evaluation of a new rapid test for detecting Wuchereria bancrofti antigen in human blood. Am J Trop Med Hyg. 2013;89(1):11–5. Epub 2013/05/22. doi: 10.4269/ajtmh.13-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ismail MM, Weil GJ, Jayasinghe KS, Premaratne UN, Abeyewickreme W, Rajaratnam HN, et al. Prolonged clearance of microfilaraemia in patients with bancroftian filariasis after multiple high doses of ivermectin or diethylcarbamazine. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1996;90(6):684–8. Epub 1996/11/01. [DOI] [PubMed] [Google Scholar]
- 10.Weil GJ, Ramzy RM, Chandrashekar R, Gad AM, Lowrie RC Jr., Faris R. Parasite antigenemia without microfilaremia in bancroftian filariasis. Am J Trop Med Hyg. 1996;55(3):333–7. Epub 1996/09/01. doi: 10.4269/ajtmh.1996.55.333. [DOI] [PubMed] [Google Scholar]
- 11.Bakajika DK, Nigo MM, Lotsima JP, Masikini GA, Fischer K, Lloyd MM, et al. Filarial antigenemia and Loa loa night blood microfilaremia in an area without bancroftian filariasis in the Democratic Republic of Congo. Am J Trop Med Hyg. 2014;91(6):1142–8. doi: 10.4269/ajtmh.14-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wanji S, Amvongo-Adjia N, Koudou B, Njouendou AJ, Chounna Ndongmo PW, Kengne-Ouafo JA, et al. Cross-Reactivity of Filariais ICT Cards in Areas of Contrasting Endemicity of Loa loa and Mansonella perstans in Cameroon: Implications for Shrinking of the Lymphatic Filariasis Map in the Central African Region. PLoS Negl Trop Dis. 2015;9(11):e0004184. doi: 10.1371/journal.pntd.0004184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pion SD, Montavon C, Chesnais CB, Kamgno J, Wanji S, Klion AD, et al. Positivity of Antigen Tests Used for Diagnosis of Lymphatic Filariasis in Individuals Without Wuchereria bancrofti Infection But with High Loa loa Microfilaremia. Am J Trop Med Hyg. 2016;95(6):1417–23. doi: 10.4269/ajtmh.16-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hertz MI, Nana-Djeunga H, Kamgno J, Jelil Njouendou A, Chawa Chunda V, Wanji S, et al. Identification and characterization of Loa loa antigens responsible for cross-reactivity with rapid diagnostic tests for lymphatic filariasis. PLoS Negl Trop Dis. 2018;12(11):e0006963. Epub 2018/11/18. doi: 10.1371/journal.pntd.0006963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wanji S, Esum ME, Njouendou AJ, Mbeng AA, Chounna Ndongmo PW, Abong RA, et al. Mapping of lymphatic filariasis in loiasis areas: A new strategy shows no evidence for Wuchereria bancrofti endemicity in Cameroon. PLoS Negl Trop Dis. 2019;13(3):e0007192. Epub 2019/03/09. doi: 10.1371/journal.pntd.0007192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukuda M Beta-elimination for release of O-GalNAc-linked oligosaccharides from glycoproteins and glycopeptides. Curr Protoc Mol Biol. 2001;Chapter 17:Unit17 5B. Epub 2008/02/12. doi: 10.1002/0471142727.mb1715bs31. [DOI] [PubMed] [Google Scholar]
- 17.Hertz MI, Glaessner PM, Rush A, Budge PJ. Brugia malayi galectin 2 is a tandem-repeat type galectin capable of binding mammalian polysaccharides. Mol Biochem Parasitol. 2019:111233. Epub 2019/11/19. doi: 10.1016/j.molbiopara.2019.111233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li BW, Chandrashekar R, Alvarez RM, Liftis F, Weil GJ. Identification of paramyosin as a potential protective antigen against Brugia malayi infection in jirds. Mol Biochem Parasitol. 1991;49(2):315–23. Epub 1991/12/01. [DOI] [PubMed] [Google Scholar]
- 19.Weil GJ, Malane MS, Powers KG. Detection of circulating parasite antigens in canine dirofilariasis by counterimmunoelectrophoresis. Am J Trop Med Hyg. 1984;33(3):425–30. Epub 1984/05/01. doi: 10.4269/ajtmh.1984.33.425. [DOI] [PubMed] [Google Scholar]
- 20.Abdullah KM, Udoh EA, Shewen PE, Mellors A. A neutral glycoprotease of Pasteurella haemolytica A1 specifically cleaves O-sialoglycoproteins. Infect Immun. 1992;60(1):56–62. Epub 1992/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andersen BJ, Kumar J, Curtis K, Sanuku N, Satofan S, King CL, et al. Changes in Cytokine, Filarial Antigen, and DNA Levels Associated With Adverse Events Following Treatment of Lymphatic Filariasis. J Infect Dis. 2018;217(2):280–7. Epub 2017/11/18. doi: 10.1093/infdis/jix578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersen BJ, Rosa BA, Kupritz J, Meite A, Serge T, Hertz MI, et al. Systems analysis-based assessment of post-treatment adverse events in lymphatic filariasis. PLoS Negl Trop Dis. 2019;13(9):e0007697. Epub 2019/09/27. doi: 10.1371/journal.pntd.0007697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajalingam D, Loftis C, Xu JJ, Kumar TK. Trichloroacetic acid-induced protein precipitation involves the reversible association of a stable partially structured intermediate. Protein Sci. 2009;18(5):980–93. doi: 10.1002/pro.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dosztanyi Z, Tompa P. Prediction of protein disorder. Methods Mol Biol. 2008;426:103–15. doi: 10.1007/978-1-60327-058-8_6. [DOI] [PubMed] [Google Scholar]
- 25.Ishida T, Kinoshita K. Prediction of disordered regions in proteins based on the meta approach. Bioinformatics. 2008;24(11):1344–8. doi: 10.1093/bioinformatics/btn195. [DOI] [PubMed] [Google Scholar]
- 26.Haslam SM, Houston KM, Harnett W, Reason AJ, Morris HR, Dell A. Structural studies of N-glycans of filarial parasites. Conservation of phosphorylcholine-substituted glycans among species and discovery of novel chito-oligomers. J Biol Chem. 1999;274(30):20953–60. Epub 1999/07/20. doi: 10.1074/jbc.274.30.20953. [DOI] [PubMed] [Google Scholar]
- 27.Hokke CH, van Diepen A. Helminth glycomics - glycan repertoires and host-parasite interactions. Mol Biochem Parasitol. 2017;215:47–57. Epub 2016/12/13. doi: 10.1016/j.molbiopara.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Schiller B, Hykollari A, Yan S, Paschinger K, Wilson IB. Complicated N-linked glycans in simple organisms. Biol Chem. 2012;393(8):661–73. Epub 2012/09/05. doi: 10.1515/hsz-2012-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martini F, Eckmair B, Stefanic S, Jin C, Garg M, Yan S, et al. Highly modified and immunoactive N-glycans of the canine heartworm. Nat Commun. 2019;10(1):75. Epub 2019/01/10. doi: 10.1038/s41467-018-07948-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Silver stained gels of endoglycosidase treated filarial antigens
Table S3: Raw heparin polymer array data
Table S1: Raw mammalian glycan array data
Table S2: Raw heparin sulfate array data


