Abstract
We examined changes in the proportion of people with human immunodeficiency virus (PWH) with virologic suppression (VS) in a multisite US cohort before and since the coronavirus disease 2019 (COVID-19) pandemic. Overall, prior gains in VS slowed during COVID-19, with disproportionate impacts on Black PWH and PWH who inject drugs.
Keywords: HIV, virologic suppression, post-COVID-19, Racial disparities, people who inject drugs
Clinical and psychosocial impacts of the coronavirus disease 2019 (COVID-19) pandemic could compromise past progress toward meeting the US Ending the HIV Epidemic (EHE) goals [1, 2]. Following the shelter-in-place orders in early 2020, there was a 60% drop in ambulatory care volume throughout the United States [3]. A transition to increased use of telemedicine to provide human immunodeficiency virus (HIV) care limited access to in-clinic support services, and community HIV programs transitioned to remote provision of many services, which may have limited access for some patients [4]. Although care volume has since partially or fully recovered in most clinics, many community organizations have experienced staffing and funding shortfalls, with HIV-specific resources diverted during this time to support pandemic-related activities [1]. Furthermore, the psychosocial impacts of the pandemic may have impacted the ability of people with HIV (PWH) to remain engaged in HIV care and adherent to HIV medicine, particularly given reports of worsening substance use severity, loneliness, and mental health symptoms among PWH during the pandemic [5]. Data on the pandemic’s impact on HIV virologic suppression (VS) in large multisite US cohorts, however, have been limited to date [6].
METHODS
Data were drawn from participating Center for AIDS Research Network of Integrated Clinical Systems (CNICS) sites from 1 January 2018 to 1 January 2022. CNICS is a prospective observational cohort study of PWH in routine clinical care at academic institutions throughout the United States [7]; sites in Boston, Massachusetts; Baltimore, Maryland; Birmingham, Alabama; Cleveland, Ohio; San Diego, California; San Francisco, California; Chapel Hill, North Carolina; and Seattle, Washington, contribute electronic medical record data to this study. For these analyses, the active clinic cohort was defined as participants who had at least 2 visits in the 24 months prior to 21 March 2020 and who also had at least 1 clinic visit and at least 1 HIV viral load (VL) measured in the 1 year prior to shelter-in-place orders. We assessed VS (<200 copies/mL) trajectories, comparing trends before and after 21 March 2020 across 8 HIV clinics within CNICS.
Analysis of Change in Virologic Suppression
Interrupted time series (ITS) analyses are a quasi-experimental design that is used to analyze outcomes at multiple time points before and after an intervention, with changes in slope over time providing the strongest evidence of intervention impact [8]. Mixed-effects logistic regression and ITS analyses examined changes in the trend (ie, slope) of VS over time, and maximum likelihood estimation was used to account for missing VS data among those lost to follow-up (LTFU) post-shelter in place. Analyses were adjusted for age, race/ethnicity, sex at birth, Centers for Disease Control and Prevention (CDC) transmission group (men who have sex with men [MSM], people who inject drugs [PWID], MSM/PWID, heterosexuals, other/unknown), CD4 nadir, and time on antiretroviral therapy (ART). Random effects for the CNICS site and participant were included over multiple assessments. Unstable housing was examined in separate models, adjusting for the same factors given that housing status was available only within a subset of the cohort. We tested for evidence of interaction between key factors (ie, race/ethnicity, history of injection drug use per CDC transmission group) for which the association with outcomes differed pre–/post–shelter-in-place orders (interaction P value of <.1) and present separate estimates for these subgroups.
As part of an alternate modeling strategy for VS, we first constructed a propensity score model to estimate the odds of having a VL measured pre- and post-shelter-in-place orders, adjusting for sex at birth, race/ethnicity, CDC transmission group, CD4 nadir < 200 cells/mm3, time on ART, with age and CD4+ count included via cubic splines (Supplementary Material). We then performed an inverse probability-weighted analysis to examine changes in VS trajectories weighted by the probability of having a VL checked in the post–COVID-19 period (Supplementary Material). LTFU was defined as not having any VL monitoring following shelter-in-place orders. Logistic regression was performed and adjusted for the same factors as the primary model to assess predictors of LTFU.
RESULTS
Demographics and Definition of the Active Clinic Cohort
Data from 17 999 participants were included and observed for 66 221 person-years, providing 120 918 VS assessments. The median age was 53 years (interquartile range, 42–61); 19% were female sex at birth; 44% identified as Black/African American; the mean time on ART was 9.5 years; 9% had a history of injection drug use; 18% were unsuppressed at any point; and 16% were LTFU (Supplementary Table).
HIV Viral Load Outcomes
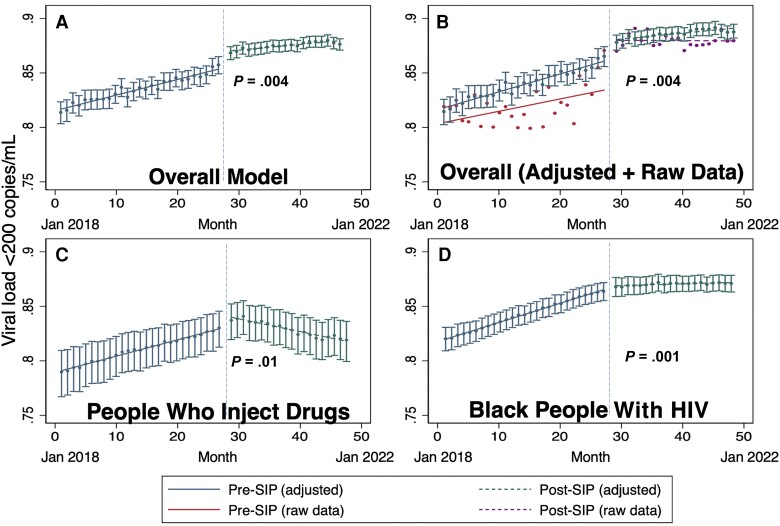
Among the overall population, prior gains in VS slowed and leveled off during the COVID-19 pandemic (adjusted odds ratio [AOR], 0.92 per quarter-year; 95% confidence interval [CI], .88–.96; P = .004; Figure 1A), with VS remaining unchanged after the pandemic (86%), after increasing from 81% to 86% in the pre-pandemic period. Mean monthly VS (ie, raw data) is compared to the adjusted analysis in Figure 1B, and findings were similar to those in the unadjusted analysis (AOR, 0.92; 95% CI, .88–.97; P = .001). Greater impacts occurred among persons with a history of injection drug use (PWID; AOR, 0.77; 95% CI, .66–.90; P = .001; test of interaction, P = .01) and among Black PWH (AOR, 0.90; 95% CI, .84–.96; P = .001; test of interaction, P = .056) in whom prior positive VS trends reversed, with VS decreasing in the post–COVID-19 period (Figure 1C, 1D). VS remained lower among those with history of unstable housing (AOR, 0.44; 95% CI, .40–.50; P < .001) but stayed unchanged from the pre-pandemic period.
Figure 1.
Virologic suppression (VS) before and after coronavirus disease 2019 (COVID-19) SIP orders. A, The difference in the slope for VS (viral load < 200 copies/mL) before and after COVID-19 SIP orders for the overall population. The horizontal solid line represents the pre–SIP period, and the horizontal dotted line represents post-SIP period. The 2 periods are separated by the dotted, vertical line. B, The overall model and the raw, mean VS each month before SIP orders (additional solid line) and after SIP orders (additional dotted line). C, The adjusted model for the subset of the population who are people who inject drugs. D, The adjusted model for the subset of the population who are Black people with HIV. Abbreviations: HIV, human immunodeficiency virus; SIP, shelter in place.
LTFU
The only significant predictor of LTFU in the post–COVID-19 period was a history of injection drug use (AOR, 1.34; 95% CI, 1.17–1.54; P < .001).
Propensity Score Sensitivity Analysis
In the alternate modeling strategy using inverse probability weighting to observations by the probability of having a VL checked in the post–COVID-19 period, the comparison of VS slopes was similar (AOR, 0.95; 95% CI, .92–.99; P = .02), with minimal impact on results when performing a trimmed analysis within the region of overlap of propensity scores (Supplementary Material, Supplementary Figure).
DISCUSSION
Previous gains in VS slowed during the COVID-19 pandemic among PWH in a multisite network of US HIV clinics. Known disparities in VS according to housing status remained unchanged during the pandemic, but VS disparities worsened for Black PWH as well as PWH with history of injection drug use, for whom prior gains in VS reversed and approached 2018 levels. Potential explanations for these findings include socioeconomic impacts of the pandemic, insurance lapses, reduction of in-person clinic services, fear of coming to clinics, and/or other factors [6]. Urgent investment in countering the HIV epidemic will be needed to achieve the US EHE goals.
Worsening disparities in HIV VS among PWH with a history of injection drug use may be related to reports of worsening substance use severity during this time, increasing mental health symptoms and isolation, and decreased access to substance use disorder treatment and syringe exchange resources, with the fentanyl and methamphetamine epidemics continuing to exacerbate during this time [9]. To address these disparities, it is crucial to improve healthcare access, expand harm reduction services, and provide targeted support to ensure equitable health outcomes. Innovative integrated care models that combine harm reduction resources, substance use treatment, and HIV treatment are needed, potentially involving use of injectable ART among viremic populations [10].
The worsening of VS disparities among Black PWH during the COVID-19 pandemic could be explained by several factors. Black individuals, who already experience higher levels of mental health challenges due to systemic racism and socioeconomic disparities, may have faced worsened psychological distress during the pandemic [11]. Black PWH experienced a disproportionate burden of COVID-19–related deaths, which may have increased fear of accessing care sites [12]. Furthermore, the pandemic’s impact on already strained healthcare systems and resources further exacerbated existing health disparities, including among populations who may have relied on social services that have not completely recovered since the onset of the pandemic. To address these disparities, HIV care systems will need to ensure that healthcare services address the needs of Black PWH, provide sufficient mental health support, and address social determinants of health that perpetuate disparities. Efforts must be made to prioritize and invest in the health needs of PWH experiencing VS disparities, including Black PWH and PWID, to mitigate the impact of both the HIV and COVID-19 epidemics and future public health crises.
There are several limitations to this analysis. Although some individuals were LTFU in the post–shelter-in-place period, we sought to account for this using maximum likelihood estimation approaches, which account for missingness in the outcome in a similar fashion to multiple imputations [13]. We sought to address potential confounding by using various methods, with qualitatively similar results obtained by maximum likelihood estimation and inverse probability weighting. However, it remains possible that residual, unmeasured confounding impacted the results. As more vulnerable persons are more likely to be LTFU, these results may underestimate negative VS trends but would not qualitatively impact results.
Renewed investment in HIV public health and clinical services will be vital to achieving the US EHE goals following the COVID-19 pandemic. Unfortunately, investment in fighting the HIV epidemic has remained flat during the COVID-19 period, with staffing and funding shortfalls in community organizations unlikely to be reversed quickly. Additional investment will be needed to provide targeted interventions for key populations with persistent disparities to recover VS and avoid worsening health disparities.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Matthew A Spinelli, Division of HIV, ID, and Global Medicine, University of California, SanFrancisco, California, USA.
Katerina A Christopoulos, Division of HIV, ID, and Global Medicine, University of California, SanFrancisco, California, USA.
Carlos V Moreira, Division of HIV, ID, and Global Medicine, University of California, SanFrancisco, California, USA.
Jennifer P Jain, Division of Prevention Science, University of California, SanFrancisco, California, USA.
Nadra Lisha, Department of Epidemiology and Biostatistics, University of California, SanFrancisco, California, USA.
David V Glidden, Department of Epidemiology and Biostatistics, University of California, SanFrancisco, California, USA.
Greer A Burkholder, Division of Infectious Diseases, University of Alabama at Birmingham, Birmingham,Alabama, USA.
Heidi M Crane, Division of Allergy and Infectious Diseases, University of Washington, Seattle, Washington, USA.
Adrienne E Shapiro, Division of Allergy and Infectious Diseases, University of Washington, Seattle, Washington, USA; Department of Global Health, University of Washington, Seattle, Washington, USA.
Jeffrey M Jacobson, Divsion of Infectious Diseases, Case Western Reserve University, Cleveland, Ohio, USA.
Edward R Cachay, Division of Infectious Diseases, University of California, San Diego, California, USA.
Kenneth H Mayer, Department of Medicine, Harvard University and the Fenway Institute/Fenway Health, Boston, Massachusetts, USA.
Sonia Napravnik, Division of Infectious Diseases, University of North Carolina, Chapel Hill, North Carolina, USA.
Richard D Moore, Division of Infectious Diseases, Johns Hopkins University, Baltimore, Maryland, USA.
Monica Gandhi, Division of HIV, ID, and Global Medicine, University of California, SanFrancisco, California, USA.
Mallory O Johnson, Division of Prevention Science, University of California, SanFrancisco, California, USA.
Notes
Financial support. This work was supported by the National Institutes of Health, National Institute of Allergy and Infectious Diseases (NIH/NIAID) R01AI158013 (M. A. S., C. V. M., N. L., D. V. G., M. G., M. O. J.), NIH/NIAID R24AI067039 (K. A. C, M. O. J.), NIH/NIAID P30AI027763 (M. G., M. O. J., M. A. S.), NIH/National Institute of Mental Health P30MH062246 (M. O. J.), NIH/National Institute on Drug Abuse (NIDA) U01DA036935 (R. D. M.), and NIH/NIDA 1K01DA056306 (J. P. J.).
References
- 1. Mitchell KM, Dimitrov D, Silhol R, et al. The potential effect of COVID-19-related disruptions on HIV incidence and HIV-related mortality among men who have sex with men in the USA: a modelling study. Lancet HIV 2021; 8:e206–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jewell BL, Mudimu E, Stover J, et al. Potential effects of disruption to HIV programmes in sub-Saharan Africa caused by COVID-19: results from multiple mathematical models. Lancet HIV 2020; 7:e629–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mehrotra A, Chernew M, Linetsky D, Hatch H, Cutler D. The impact of the COVID-19 pandemic on outpatient visits: a rebound emerges. The Commonwealth Fund. Available at: https://www.commonwealthfund.org/publications/2020/apr/impact-covid-19-outpatient-visits. Accessed 10 June 2020.
- 4. Spinelli MA, Hickey MD, Glidden DV, et al. Viral suppression rates in a safety-net HIV clinic in San Francisco destabilized during COVID-19. AIDS 2020; 34:2328–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hong C, Queiroz A, Hoskin J. The impact of the COVID-19 pandemic on mental health, associated factors and coping strategies in people living with HIV: a scoping review. J Int AIDS Soc 2023; 26:e26060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meyer D, Slone SE, Ogungbe O, Duroseau B, Farley JE. Impact of the COVID-19 pandemic on HIV healthcare service engagement, treatment adherence, and viral suppression in the United States: a systematic literature review. AIDS Behav 2023; 27:344–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kitahata MM, Rodriguez B, Haubrich R, et al. Cohort profile: the Centers for AIDS Research Network of Integrated Clinical Systems. Int J Epidemiol 2008; 37:948–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kontopantelis E, Doran T, Springate DA, Buchan I, Reeves D. Regression based quasi-experimental approach when randomisation is not an option: interrupted time series analysis. BMJ 2015; 350:h2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hochstatter KR, Akhtar WZ, Dietz S, et al. Potential influences of the COVID-19 pandemic on drug use and HIV care among people living with HIV and substance use disorders: experience from a pilot mHealth intervention. AIDS Behav 2021; 25:354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gandhi M, Hickey M, Imbert E, et al. Demonstration project of long-acting antiretroviral therapy in a diverse population of people with HIV. Ann Intern Med 2023; 176:969–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sauceda JA, Dubé K, Harris O, Campbell CK, Ndukwe S, Saberi P. Brief report: the influence of the COVID-19 pandemic on the physical, social, and mental health of Black and Latinx young people with HIV in the United States. J Acquir Immune Defic Syndr 2023; 93:187–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bogart LM, Ojikutu BO, Tyagi K, et al. COVID-19 related medical mistrust, health impacts, and potential vaccine hesitancy among Black Americans living with HIV. J Acquir Immune Defic Syndr 2021; 86:200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. von Hippel PT. New confidence intervals and bias comparisons show that maximum likelihood can beat multiple imputation in small samples. Struct Equ Model Multidiscip J 2016; 23:422–37. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.