Abstract
Background
Human immunodeficiency virus (HIV) infection leads to chronic immune activation/inflammation that can persist in virally suppressed persons on fully active antiretroviral therapy (ART) and increase risk of malignancies. The prognostic role of low CD4:CD8 ratio and elevated CD8 cell counts on the risk of cancer remains unclear.
Methods
We investigated the association of CD4:CD8 ratio on the hazard of non-AIDS defining malignancy (NADM), AIDS-defining malignancy (ADM) and most frequent group of cancers in ART-treated people with HIV (PWH) with a CD4 and CD8 cell counts and viral load measurements at baseline. We developed Cox proportional hazard models with adjustment for known confounders of cancer risk and time-dependent cumulative and lagged exposures of CD4:CD8 ratio to account for time-evolving risk factors and avoid reverse causality.
Results
CD4:CD8 ratios below 0.5, compared to above 1.0, were independently associated with a 12-month time-lagged higher risk of ADM and infection-related malignancies (adjusted hazard ratio 2.61 [95% confidence interval {CI }1.10–6.19] and 2.03 [95% CI 1.24–3.33], respectively). CD4 cell counts below 350 cells/μL were associated with an increased risk of NADMs and ADMs, as did infection, smoking, and body mass index-related malignancies.
Conclusions
In ART-treated PWH low CD4:CD8 ratios were associated with ADM and infection-related cancers independently from CD4 and CD8 cell counts and may alert clinicians for cancer screening and prevention of NADM.
Keywords: CD4:CD8 ratio, HIV infection, malignancy, observational study, antiretroviral therapy
Low CD4:CD8 ratios are independently of CD4 cell counts associated with increased risk of AIDS-defining and infection-related malignancies in antiretroviral-treated people with human immunodeficiency virus (HIV). Combined with cancer screening strategies, markers' monitoring could benefit individuals wiht inadequate immunological reconstitution.
Modern antiretroviral therapy (ART) has led to a major reduction in AIDS events and a drastic increase in life expectancy of people with human immunodeficiency virus (HIV, PWH) [1]. At the same time, HIV care providers are facing a growing number of PWH with serious comorbidities. Among these, non-AIDS defining malignancies (NADM) represent the most frequent condition and are now a major underlying cause of death in PWH [2, 3]. The risk of NADM in PWH is multifactorial and is particularly impacted by highly prevalent oncogenic viral co-infections and smoking [4–6].
HIV-induced immune activation and inflammation has been suggested as another important driver for the growing incidence of NADM [7, 8]. Pathophysiological mechanisms involve the immune response to the virus itself, the production of pro-inflammatory cytokines, the expression of HIV gene products by activated lymphocytes and macrophages, the immunodeficiency-induced reactivation and replication of other viruses or the translocation of intestinal bacterial flora into the systemic circulation [9, 10]. Chronic immune activation can persist even among virally suppressed PWH, leading to an exhaustion of the immune resources, mimicking the process of aging-associated immune-senescence and installing an immune dysfunction.
The CD4:CD8 ratio represents a surrogate marker of defective T lymphocytes in HIV infection, which in recent years has regained attention [11, 12]. Expansion of CD8 cell counts with the consequence of a low ratio, in particular in well-treated and virologically suppressed individuals, may characterize a subpopulation with distinct immunological abnormalities and chronic inflammation [13]. Some authors have concluded that ART treated patients with high CD4 cell counts but low CD4:CD8 ratio may characterize a population at risk of immuno-senescence [14, 15].
Whether CD4:CD8 ratio or CD8 cell counts may represent markers for innate and adaptive immune activation that are predictive for specific type of cancers requires additional evidence. Addressing these issues in a large observational study is important since the identification of PWH at high risk of malignancies should lead to better patient management by reinforcing prevention programs and screening strategies. For example, low-dose computed tomography screening showed a reduction in lung-cancer mortality in high-risk population [16], and active monitoring together with lesions treatment reduces the risk of anal cancer among PWH with high grade squamous intraepithelial lesions [17]. Here we assess whether CD4:CD8 ratio and CD8 cell counts may be useful biomarkers for the risk of NADM, AIDS-defining malignancies (ADM) and specific cancers, after accounting for known risk factors of cancers and sociodemographic participant characteristics.
METHODS
This prospective observational cohort study is based on data from the international cohort consortium of infectious diseases (RESPOND) [18], a collaboration of 17 observational studies across Europe and Australia including more than 30 000 PWH. CD4 and CD8 cell counts, human immunodeficiency (HI) viral load, information on ART, tobacco smoking, sociodemographic and behavioural parameters and AIDS defining illnesses are recorded at enrollment and during routine biannual or annual follow-up visits. Clinical events such as ADM and NADM, as well as information on ART, smoking and other AIDS defining illnesses, are reported annually to a central coordinating centre using standard case report forms following HIV Cohorts Data Exchange Protocol for data collection (https://hicdep.org/). Prospective follow-up started on 1 October 2017, with retrospective data collected back to 1 January 2012. Cancer events occurring during the RESPOND validation period are centrally adjudicated by clinicians at the RESPOND coordinating centre using pre-specified algorithms as detailed elsewhere [19]. Participants consented to share data with RESPOND according to local or national requirements and all cohorts had to approve the sharing of data with RESPOND. Ethical approval was obtained, if required, from the relevant bodies for collection and sharing of data. Data are stored on secure servers at the RESPOND coordinating centre in Copenhagen, Denmark, in accordance with current legislation and under approval by the Danish Data Protection Agency (approval number 2012-58-0004, RH-2018-15, 26/1/2018), under the EU General Data Protection Regulation (2016/579).
Study Population
Our analysis includes ART treated PWH above 18 years followed between 1 January 2012 (date from which cancers were retrospectively collected) and 31 December 2020 (date of the database closure). We excluded PWH with no CD4 and CD8 cell counts or viral load measurements at baseline, a record of any malignancy prior to baseline or in the first 12 months of follow-up, and individuals with a follow-up time below 12 months. Baseline is defined as the later of cohort entry and 1 January 2012.
Clinical Outcome
Our primary outcome is the occurrence of a first incident NADM. Secondary outcomes include ADM (non-Hodgkin lymphoma, Kaposi's sarcoma and invasive cervical cancer), and infection-related cancers (non-Hodgkin lymphoma, Kaposi sarcoma, Hodgkin lymphoma, and liver, invasive anal, cervical, penile, oesophageal, and stomach cancers), smoking-related malignancy (lung, head and neck, bladder, pancreas, colorectal, liver, kidney, rectum, cervical, oesophageal, lip, and stomach cancers) and body mass index (BMI)-related malignancy (pancreas, colorectal, liver, breast, kidney, rectum, gall bladder, oesophageal and thyroid cancers). Grouping of the different cancers was discussed with a panel of external oncologists at the initiation of the RESPOND consortium and defined by the RESPOND cancer working group [19, 20] for consistent use in all cohort projects explicitly allowing for not mutually exclusive classification.
Baseline and Time-Updated Exposure Variables
We considered immunological (CD4 cells and CD8 cell counts, CD4:CD8 ratio) and HI viral load factors as time-updated exposure variables. In particular, time-updated exposures are considered as point exposures observed 12 months prior to a given follow-up (12 months lagged exposure) to avoid risks of detecting associations that reflect reverse causality. Baseline sociodemographic individual characteristics include sex (male/female), age, risk group (men having sex with men [MSM], people who inject drugs [PWID], and heterosexual/other/unknown), and race (White and other/unknown). Smoking (yes/no) is regarded as a time-varying lagged exposure at 12 months. Obesity (BMI >30 kg/m2), hepatitis B (positive surface antigen or detectable DNA) and C status (positive antibody test or detectable RNA) are also considered at baseline.
Statistical Analysis
We aimed to assess the association of immunological and viral factors on the hazard of malignancies using Cox models with time-dependent covariates after adjusting for the baseline socio-demographic patient characteristics, sex, age, risk, and race. Infection-related malignancy analysis was further adjusted for hepatitis C and B status, whereas smoking-related and BMI related malignancy analyses were additionally adjusted for 12 months lagged smoking status and baseline obesity, respectively. We modeled time from 12 months after baseline to the first diagnosis of the considered malignancies in the analysis, death, or cohort administrative censoring, whichever came first. The first 12 months of follow-up were thus removed from the risk set to remove immortal bias induced by our study design. Time-updated variables were updated at the end of each follow-up month and missing information was extrapolated using the last observation carried forward (LOCF). Thus, at each follow-up month, participants who have experienced an event are compared with those currently at risk.
We assessed the robustness of our results in sensitivity analyses by considering time-updated exposure at different lags. In particular, we additionally considered point exposures lagged at 24 and 36 months, as well as cumulative exposures using simple moving averages (SMA) over the past 12 months and 24 months (12 months SMA are additionally lagged at 12 and 24 months, whereas 24 months SMA were lagged at 12 months) and baseline exposures. Additional sensitivity analyses included LOCF restricted to a maximum of 12 months. Hence, when gaps between laboratory measurements were longer than 12 months, the laboratory measurement for this specific month was considered as missing, and events observed during this specific follow-up month were excluded from the analysis. This implies that cancer diagnosis occurring during these months were censored. For smoking-related malignancy, we also performed a sensitivity analysis by excluding cohort for which smoking information was missing for more than 30% of the overall participants. All analyses were done in R Project for Statistical Computing (version 4.1.0) software [21]. Data management was performed in Stata SE 17-VA [22].
RESULTS
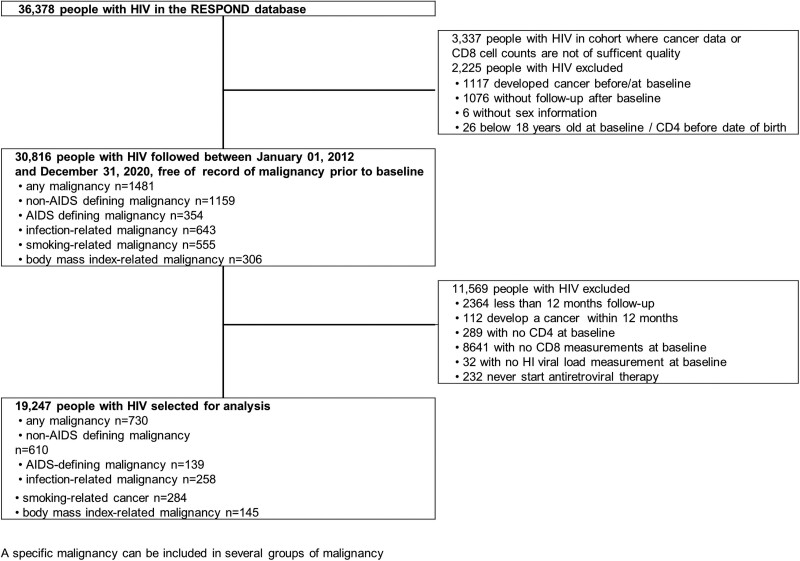
A total of 30 816 PWH above 18 years of age were followed after 1 January 2012 onward without history of a malignancy at baseline. Malignancy, irrespective of the type, was diagnosed in 1481 individuals over 183 406 person-years (PY) (incidence rate [IR] 8.08 per 1000 PY (95% confidence interval [CI] 7.67–8.50). NADM and ADM had an IR of 6.32 per 1000 PY (95% CI 5.96–6.69) and 1.93 per 1000 PY (95% CI 1.74–2.14), respectively. In total, 11 569 individuals had no CD4 and CD8 cell counts or viral load measurements recorded after January 2012, never started ART, or had < 12 months follow-up and were excluded, leaving 19 247 individuals and 730 malignancies for analysis (Figure 1). Seventy-five percent of the dropped individuals were excluded due to missing CD8 laboratory measurements; the majority (80%) being from one large cohort that only measured CD8 cell counts at enrollment and after that only for HIV/hepatitis C coinfected individuals. Further details on the classification and frequency of single malignancies are provided in Table 1.
Figure 1.
Flow chart of people with HIV selection and overview of number of specific type of malignancy. Abbreviation: HIV, human immunodeficiency virus.
Table 1.
Types and Frequency of First Cancers Diagnosed for Each Malignancy Group Among People With HIV With CD4 Cells, CD8 Cells and HI Viral Load at Baseline
| Non-AIDS Defining | AIDS Defining | Smoking-Related | ||||||
|---|---|---|---|---|---|---|---|---|
| Malignancy | n | % | Malignancy | n | % | Malignancy | n | % |
| Lung | 73 | 11.97 | Non-Hodgkin lymphoma | 73 | 52.52 | Lung | 78 | 27.46 |
| Anal | 60 | 9.84 | Kapsoi sarcoma | 56 | 40.29 | Head and neck cancera | 37 | 13.03 |
| Prostate | 54 | 8.85 | Cervical | 10 | 7.19 | Bladder | 34 | 11.97 |
| Malignant melanoma | 41 | 6.72 | Total | 139 | 100.00 | Pancreas | 32 | 11.27 |
| Head and neck cancer | 40 | 6.56 | Infection-related | … | … | Colorectal | 27 | 9.51 |
| Bladder | 32 | 5.25 | malignancy | n | % | Liver | 24 | 8.45 |
| Pancreas | 31 | 5.08 | Non-Hodgkin lymphoma | 71 | 19.19 | Kidney | 16 | 5.63 |
| Colorectal | 26 | 4.26 | Anal | 60 | 16.22 | Rectum | 12 | 4.23 |
| Hodgkin lymphoma | 24 | 3.93 | Kaposi sarcoma | 55 | 14.86 | Cervical | 10 | 3.52 |
| Liver | 24 | 3.93 | Liver | 24 | 6.49 | Oesophageal | 6 | 2.11 |
| Breast | 16 | 2.62 | Hodgkin lymphoma | 22 | 5.95 | Lip | 6 | 2.11 |
| Kidney | 16 | 2.62 | Cervical | 10 | 2.70 | Stomach | 2 | 0.70 |
| Gynecological | 15 | 2.46 | Penile | 8 | 2.16 | Total | 284 | 100.00 |
| Rectum | 12 | 1.97 | Oesophagal | 6 | 1.62 | Body mass index- | … | … |
| Connective tissue | 8 | 1.31 | Stomach | 2 | 0.54 | related | ||
| Penile | 8 | 1.31 | Total | 258 | 100.00 | malignancy | n | % |
| Testicular | 8 | 1.31 | … | … | … | Pancreas | 32 | 22.07 |
| Gall bladder | 7 | 1.15 | … | … | … | Colorectal | 28 | 19.31 |
| Oesophageal | 6 | 0.98 | … | … | … | Liver | 24 | 16.55 |
| Leukemia | 6 | 0.98 | … | … | … | Breast | 17 | 11.72 |
| Lip | 5 | 0.82 | … | … | … | Kidney | 16 | 11.03 |
| Multiple myeloma | 4 | 0.66 | … | … | … | Rectum | 12 | 8.28 |
| Brain | 2 | 0.33 | … | … | … | Gall bladder | 7 | 4.83 |
| Stomach | 2 | 0.33 | … | … | … | Oesophageal | 6 | 4.14 |
| Bone | 1 | 0.16 | … | … | … | Thyroid | 3 | 2.07 |
| Other/unclassified | 89 | 14.59 | … | … | … | Total | 145 | 100.00 |
| Total | 610 | 100.00 | … | … | … | … | … | … |
Abbreviation: HIV, human immunodeficiency virus.
aSmoking-related head and neck malignancies include oral cavity, hypo- and oro-pharyngeal, laryngeal, saliva gland, sino/nasal cavity, and unspecified sub-types. Each cancer represents the first cancer diagnosed within the group.
Baseline sociodemographic and clinical characteristics were compared between the overall population in the RESPOND database and those included in the final analysis (Supplementary Table 1). They were found to be similar. Baseline characteristics of individuals included in the analysis and of individuals with specific incident cancers are provided in Table 2. PWH who developed cancers were generally older compared to PWH included in the analysis. Individuals of White race current smokers, with a positive baseline hepatitis C status, and PWID were more prevalent among individuals who developed a smoking-related malignancy. BMI-related malignancies were observed more frequently in heterosexuals, females, and obese individuals, whereas males and individuals with positive hepatitis B status were more prevalent among individuals that developed infection-related malignancy. The median time on ART at cancer diagnosis varies from 9.1 years (95% CI 4.3–16.4) for ADM to 15.0 years (95% CI 8.8–19.0) for smoking-related malignancies, suggesting that individuals included in our analysis that developed malignancies were mainly long-term treated PWH.
Table 2.
Baseline Characteristics of Patients With CD4 Cells. CD8 Cells and HI Viral Load at Baseline, Overall and for Patients With Incident Cancers
| PWH Included in the Analysis |
PWH With Non-AIDS Defining Malignancy |
PWH With AIDS Defining Malignancy |
PWH With Infection-Related Malignancy |
PWH With Smoking-Related Malignancy |
PWH With Body Mass Index-Related Malignancy |
|
|---|---|---|---|---|---|---|
| (n = 19 247) | (n = 610) | (n = 139) | (n = 258) | (n = 284) | (n = 145) | |
| Age [y] | ||||||
| <50 | 13 698 (71.2%) | 229 (37.5%) | 84 (60.4%%) | 139 (53.9%%) | 98 (34.5%%) | 61 (42.1%%) |
| 50–65 | 4746 (24.7%) | 302 (49.5%%) | 46 (33.1%%) | 100 (38.8%%) | 151 (53.2%) | 70 (48.3%) |
| ≥65 | 803 (4.2%) | 79 (13.0%) | 9 (6.5%) | 19 (7.4%) | 35 (12.3%) | 14 (9.7%) |
| Median age (interquartile range) [y] | 44 (36–51) | 52 (47–59) | 46 (38–52) | 49 (42–55) | 52 (47–59) | 51 (46–57) |
| Sex | ||||||
| Male | 14 715 (76.5%) | 484 (79.3%) | 108 (77.7%) | 213 (82.6%) | 223 (78.5%) | 110 (75.9%) |
| Female | 4532 (23.5%) | 126 (20.7%) | 31 (22.3%) | 45 (17.4%) | 61 (21.5%) | 35 (24.1%) |
| Risk group | ||||||
| Men having sex with men | 9596 (49.9%) | 267 (43.8%) | 75 (54.0%) | 134 (51.9%) | 100 (35.2%) | 51 (35.2%) |
| People who inject drugs | 1921 (10.0%) | 111 (18.2%) | 14 (10.1%) | 31 (12.0%) | 76 (26.8%) | 33 (22.8%) |
| Heterosexual | 6544 (34.0%) | 195 (32.0%) | 39 (28.1%) | 74 (28.7%) | 91 (32.0%) | 52 (35.9%) |
| Other/unknown | 1186 (6.2%) | 37 (6.1%) | 11 (7.9%) | 19 (7.4%) | 17 (6.0%) | 9 (6.2%) |
| Race | ||||||
| White | 12 934 (67.2%) | 477 (78.2%) | 92 (66.2%) | 181 (70.2%) | 233 (82.0%) | 107 (73.8%) |
| Other/unknown | 6313 (32.8%) | 133 (21.8%) | 47 (33.8%) | 77 (29.8%) | 51 (18.0%) | 38 (26.2%) |
| CD4 cell count [cells/μL] | ||||||
| <350 | 4731 (24.6%) | 165 (27.0%) | 56 (40.3%) | 95 (36.8%) | 78 (27.5%) | 36 (24.8%) |
| 350–500 | 4233 (22.0%) | 127 (20.8%) | 34 (24.5%) | 59 (22.9%) | 58 (20.4%) | 33 (22.8%) |
| ≥500 | 10 283 (53.4%) | 318 (52.1%) | 49 (35.3%) | 104 (40.3%) | 148 (52.1%) | 76 (52.4%) |
| CD8 cell count [cells/μL] | ||||||
| <1000 | 12 533 (65.1%) | 386 (63.3%) | 77 (55.4%) | 148 (57.4%) | 181 (63.7%) | 91 (62.8%) |
| ≥1000 | 6714 (34.9%) | 224 (36.7%) | 62 (44.6%) | 110 (42.6%) | 103 (36.3%) | 54 (37.2%) |
| CD4:CD8 ratio | ||||||
| <0.5 | 7271 (37.8%) | 238 (39.0%) | 80 (57.6%) | 140 (54.3%) | 114 (40.1%) | 57 (39.3%) |
| 0.5–1.0 | 8119 (42.2%) | 247 (40.5%) | 42 (30.2%) | 83 (32.2%) | 115 (40.5%) | 57 (39.3%) |
| ≥1 | 3857 (20.0%) | 125 (20.5%) | 17 (12.2%) | 35 (13.6%) | 55 (19.4%) | 31 (21.4%) |
| HI viral load [copies/mL] | ||||||
| <200* | 12 590 (65.4%) | 485 (79.5%) | 75 (54.0%) | 167 (64.7%) | 233 (82.0%) | 119 (82.1%) |
| ≥200 | 6657 (34.6%) | 125 (20.5%) | 64 (46.0%) | 91 (35.3%) | 51 (18.0%) | 26 (17.9%) |
| Body mass index [kg/m2] | ||||||
| <30 | 12 785 (66.4%) | 435 (71.3%) | 81 (58.3%) | 168 (65.1%) | 214 (75.4%) | 110 (75.9%) |
| ≥30 | 1128 (5.9%) | 40 (6.6%) | 6 (4.3%) | 17 (6.6%) | 19 (6.7%) | 14 (9.7%) |
| Missing | 5334 (27.7%) | 135 (22.1%) | 52 (37.4%) | 73 (28.3%) | 51 (18.0%) | 21 (14.5%) |
| Hepatitis C status | ||||||
| Negative | 14 029 (72.9%) | 406 (66.6%) | 94 (67.6%) | 169 (65.5%) | 170 (59.9%) | 93 (64.1%) |
| Positive | 2981 (15.5%) | 152 (24.9%) | 19 (13.7%) | 50 (19.4%) | 95 (33.5%) | 45 (31.0%) |
| Unknown | 2237 (11.6%) | 52 (8.5%) | 26 (18.7%) | 39 (15.1%) | 19 (6.7%) | 7 (4.8%) |
| Hepatitis B status | ||||||
| Negative | 16 029 (83.3%) | 532 (87.2%) | 106 (76.3%) | 203 (78.7%) | 255 (89.8%) | 131 (90.3%) |
| Positive | 735 (3.8%) | 29 (4.8%) | 7 (5.0%) | 18 (7.0%) | 15 (5.3%) | 8 (5.5%) |
| Unknown | 2483 (12.9%) | 49 (8.0%) | 26 (18.7%) | 37 (14.3%) | 14 (4.9%) | 6 (4.1%) |
| Smoking | ||||||
| No | 7043 (36.6%) | 236 (38.7%) | 50 (36.0%) | 95 (36.8%) | 89 (31.3%) | 67 (46.2%) |
| Yes | 6018 (31.3%) | 225 (36.9%) | 32 (23.0%) | 73 (28.3%) | 128 (45.1%) | 48 (33.1%) |
| Unknown | 6186 (32.1%) | 149 (24.4%) | 57 (41.0%) | 90 (34.9%) | 67 (23.6%) | 30 (20.7%) |
| Median time on ART at diagnosis | ||||||
| (interquartile range) [y] | … | 13.7 (7.8–19.3) | 9.1 (4.3–16.4) | 12.3 (5.8–18.4) | 15.0 (8.8–19.0) | 14.9 (9.1–19.0) |
Positive hepatitis C is defined as detectable RNA or a positive immunoglobulin G (IgG) antibody test; positive hepatitis B is defined as surface antigen positivity.
Abbreviations: ART, antiretroviral therapy; PWH, people with human immunodeficiency virus (HIV).
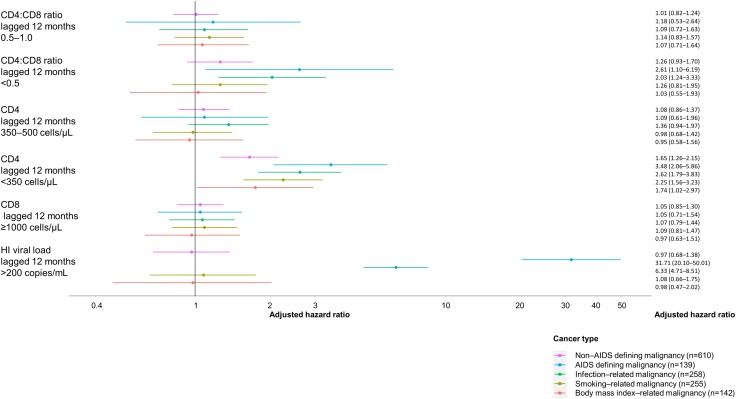
Figure 2 summarizes the adjusted hazard ratio (aHR) for cancer with 95% CI of immune function parameters that are lagged at 12 months (parameters' estimates of the full model are given in the Supplementary Table 2). The estimated mean aHR ranged from 1.03 (BMI-related malignancies) to 2.61 (AIDS-defining malignancies) when comparing CD4:CD8 ratios <0.5 to CD4:CD8 ratios >1.0. However, CD4:CD8 ratios <0.5 were significantly associated with infection-related malignancies (aHR 2.03; 95% CI 1.24–3.33) and AIDS-defining malignancies (aHR 2.61; 95% CI 1.10–6.19) when compared to CD4:CD8 ratios >1.0. CD4 cell counts <350 cells/μl increased the risk of NADMs (aHR: 1.65; 95% CI: 1.26–2.15), ADMs (aHR 3.48; 95% CI 2.06–5.86), infection-related malignancies (aHR 2.62; 95% CI 1.79–3.83), smoking-related malignancies (aHR 2.25; 95% CI 1.56–3.23) and BMI-related malignancies (aHR 1.74; 95% CI 1.02–2.97). In the adjusted analyses, none of the associations between CD8 cell counts and any of the malignancies analysed were statistically significant. HI viral loads ≥200 copies/mL were important drivers of ADM (aHR 31.71; 95% CI 20.10–50.01), and infection-related malignancy (aHR 6.33; 95% CI 4.71–8.51).
Figure 2.
Adjusted hazard ratio of immunological and virological factors for non-AIDS defining, AIDS defining, infection, smoking and body mass related malignancies. All models are adjusted for age, sex, risk, and race. The model for infection-related malignancy is further adjusted for baseline chronic hepatitis C and B status. The model for smoking-related malignancy is further adjusted for 12 m lagged smoking status. The body mass index-related malignancy model is adjusted for baseline body mass index. Reference categories are CD4:CD8 ratio lagged 12 m ≥1.0, CD4 lagged 12 m ≥50 cells/μL, HI viral lead lagged 12 m ≤200 copies/mL. Abbreviation: HI, human immunodeficiency.
Sensitivity analyses results were robust for point lagged exposures at 24 and 36 months, as well as for several cumulative lagged exposures and baseline exposure (Supplementary Figure 1). When restricting the extrapolation of immunological and virological factors to 12 months, estimates from models were similar to models fitted on data with immunological and virological exposures that were extrapolated without the restriction of time. We therefore do not expect bias due to the extrapolation procedure (Supplementary Figure 2 vs Supplementary Figure 1). In addition, results for smoking-related malignancy were robust when excluding individuals from cohorts reporting suboptimal smoking information (data completeness lower than 70%) (Supplementary Figure 3), as well as to the exclusion of individuals from cohorts that did not routinely collect CD8 cell counts (Supplementary Figure 4), limiting the risk of selection bias. Finally, we considered smoking as a binary time-varying exposure rather than categorizing participants into current and past smokers. This did not affect our results, but we estimated in an additional sensitivity analysis not presented here a stronger association with current smoking status than previous smoking status for smoking-related malignancies, in line with past RESPOND analyses [19].
All final models satisfied the proportional assumption of the Cox regression model according to the test of temporal independence of Schoenfeld residuals. Multi-collinearity was not detected according to the variance inflation factors that were all below the threshold value of 5.
DISCUSSION
In this large prospective multinational cohort study of ART treated individuals who were followed between 2012 and 2020, a low CD4:CD8 ratio was associated with significantly higher risk of ADM and infection-related cancers independent from CD4 cell counts. Elevated CD8 cell counts were not an important marker of any malignancy, whereas CD4 cell counts below 350 cells/μL were associated with a higher risk of all malignancies studied in this analysis. Unsuppressed HI viral load was associated with a substantial increase in risk of ADM and infection-related cancer, which also included ADM. Hence, altered immune function, as proxied by low CD4:CD8 ratio, might additionally affect the carcinogenesis of ADM and infection-related cancers and be an important additive marker to a low CD4 cell count for these malignancies among PWH.
Several cohort studies have investigated the association between CD4:CD8 ratio and the risk of non-AIDS defining malignancies and other clinical events [23–30]. Results are conflicting due to the lack of standardized methodology, different modelling approaches, the use of a mixture of combined endpoints that encompassed different disease entities, limitation of sample sizes, and missing smoking status data. For example, in the large ART-CC cohort, no association between CD4:CD8 ratio and non-AIDS related mortality was found, but this cohort lacked data on smoking status [23]. In the Italian ICONA cohort [24] and a Thai cohort [25] low CD4:CD8 ratio was associated with an increased risk of non-AIDS events, which included a composite endpoint of malignancies, cardiovascular, renal [25], and hepatic events [24]. However, the limited sample size did not allow differentiation among the risks for malignancy and for the remaining non-cancer events. The French APROCO cohort study was the first study to report a significant association between low CD4:CD8 ratio and increased risk of NADM [26]. A low CD4:CD8 cell ratio was associated with an increased risk of lung cancer [28] in the US Veteran cohort study, and a higher risk of Kaposi sarcoma and non-Hodgkin lymphoma in the COHERE cohort study [29]. In the Swiss HIV Cohort Study a low CD4:CD8 ratio was not associated with increased risk of anal, lung, prostate and liver cancers [27], whereas the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) showed an association of low CD4 and CD8 ratio and the risk of lung, anal, and colorectal cancers in addition to the higher risk for the ADM non-Hodgkin lymphoma and Kaposi sarcoma [30]. Our study is important because it provides further evidence about an association between surrogate parameters of chronic immune activation in the presence of ART and the risk of NADMs, and in particular for infection-related cancers.
Strengths of this analysis are the large sample size with a large number of events grouping different centrally adjudicated malignancies and our ability to control for important known risk factors for cancers. In addition we considered virological and immunological factors under several functional forms to ensure the robustness of our results and present them in form that is directly useful for targeted patient management. Latency period in cancer studies can be subject to discussion. Here we primarily considered 12 month lagged exposure to exclude reverse causality while keeping sufficient statistical power for our analyses. The robustness of our results with regards to increased latency period suggest that immunological and virological factors appear to affect cancer risk early on and reflects long duration of cancers pathogenesis. In our modeling approach we used time-updated covariates, which enabled us to account for immunological and viral parameters that change over time. Therefore, missing laboratory measurements between periods had to be imputed, which could have made our approach prone to misclassification bias; however, a sensitivity analysis that restricted the extrapolation suggests it had little impact on our results.
Limitations of this study are that we did not additionally consider cubic splines that can capture more complex non-linear relationships [23] or weighted cumulative exposures that allow a flexible modelling time-dependent exposures [31]. Because our analysis looked at four different time-varying immunological and virological factors, a more systematic look at the complex relationship between all 4 parameters was not feasible also due to the limited number of events. In addition, we lacked information on bacterial pneumonia, cytomegalovirus (CMV), and human papillomavirus (HPV) infections. Also, alcohol consumption, which was not systematically collected by the majority of the cohorts involved in RESPOND could not be considered. Accounting for those factors would have reduced the risk of confounding bias in models that include associated cancers. Despite our efforts to adjust our models to the available risk factors, we cannot rule out bias due to unmeasured confounding. We note, however, that the effect of CD4:CD8 ratio on the risk of lung cancers remained after adjusting for bacterial pneumonia episodes in the US Veteran study [28]. Finally, CD4 and CD8 cell counts were the two available markers that routinely monitor the immune system. Data on additional markers of inflammation like plasma interleukin (IL)-6 or D-Dimer levels were not available and might add value in future studies.
CONCLUSION
In this large cohort study with pooled data on PWH across Europe and Australia, a CD4 cell count of <350 cells/µL was associated with an increased risk of any studied malignancy. The study provides additional evidence that a low CD4:CD8 ratio carries an additional risk for ADM and infection-related malignancies. Therefore, regular monitoring of CD4 cells and CD4:CD8 ratios may provide benefit if it leads to enhanced cancer screening strategies for individuals who initiate ART late and do not achieve immune restoration above 350 cells/µL and >1.0, respectively. Our findings illustrate the importance of early HIV diagnosis and ART initiation with lifelong HIV suppression to reduce, in addition to other relevant clinical events, the risk of ADM and NADM. Whether PWH with insufficiently restored immune function profit on top of smoking cessation counselling from enhanced cancer screening programs should be further investigated.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments.
RESPOND Study Group
AIDS Therapy Evaluation in the Netherlands Cohort (ATHENA): F. Wit, M. van der Valk, M. Hillebregt, Stichting HIV Monitoring (SHM), Amsterdam, Netherlands
The Australian HIV Observational Database (AHOD): K. Petoumenos, M, Law, UNSW, Sydney, Australia
Austrian HIV Cohort Study (AHIVCOS): R. Zangerle, H. Appoyer, Medizinische Universität Innsbruck, Innsbruck, Austria
CHU Saint-Pierre: S, De Wit, M Delforge, Centre de Recherche en Maladies Infectieuses a.s.b.l., Brussels, Belgium
EuroSIDA Cohort: G. Wandeler, CHIP, Rigshospitalet, RegionH, Copenhagen, Denmark
Frankfurt HIV Cohort Study: C. Stephan, M. Bucht, Johann Wolfgang Goethe-University Hospital, Frankfurt, Germany
Infectious Diseases, AIDS and Clinical Immunology Research Center (IDACIRC): N. Chkhartishvili, O. Chokoshvili, Infectious Diseases, AIDS and Clinical Immunology Research Center, Tbilisi, Georgia
Italian Cohort Naive Antiretrovirals (ICONA): A. d’Arminio Monforte, A. Rodano, A. Tavelli, ASST Santi Paolo e Carlo, Milan, Italy; I. Fanti, Icona Foundation, Milan, Italy
Modena HIV Cohort: C. Mussini, V. Borghi, Università degli Studi di Modena, Modena, Italy
Nice HIV Cohort: C. Pradier, E. Fontas, K. Dollet, C. Caissotti, Université Côte d’Azur et Centre Hospitalier Universitaire, Nice, France
PISCIS Cohort Study: J. Casabona, J. M. Miro, J. M. Llibre, A. Riera, J. Reyes- Urueña, Centre Estudis Epidemiologics de ITS i VIH de Catalunya (CEEISCAT), Badalona, Spain
Royal Free Hospital Cohort: C. Smith, F. Lampe, Royal Free Hospital, University College London, London, United Kingdom
San Raffaele Scientific Institute: A. Castagna, A. Lazzarin, A Poli, Università Vita-Salute San Raffaele, Milano, Italy
Swedish InfCare HIV Cohort: A. Sönnerborg, K. Falconer, V. Svedhem, Karolinska University Hospital, Stockholm, Sweden
Swiss HIV Cohort Study (SHCS): H. Günthard, B. Ledergerber, H. Bucher, K. Kusejko, University of Zurich, Zurich, Switzerland
University Hospital Bonn: J. C. Wasmuth, J. Rockstroh, Bonn, Germany
University Hospital Cologne: J. J. Vehreschild, G. Fätkenheuer, Cologne, Germany
RESPOND Executive Committee
L. Ryom∗, M. Law∗, R. Campo, S. De Wit, H. Garges, H. Günthard, J. Lundgren, I. McNicholl, J. Rooney, C. Smith, V. Vannappagari, G. Wandeler, L. Young, R. Zangerle (*Chairs)
RESPOND Scientific Steering Committee
J. Lundgren∗, H. Günthard∗, J. Begovac, A. Bruguera, H. Bucher, A. Castagna, R. Campo, N. Chkhartishvili, A. D’Arminio Monforte, N. Dedes, H. Garges, J. Kowalska, M. Law, I. McNicholl, C. Mussini, C. Necsoi, L. Peters, K. Petoumenos, C. Pradier, D. Raben, J. Rockstroh, J. Rooney, L. Ryom, C. Smith, A. Sönnerborg, C. Stephan, V. Vannappagari, J. J. Vehreschild, A. Volny Anne, G. Wandeler, J. C. Wasmuth, E. D. Williams, F. Wit, L. Young, R. Zangerle (*Chairs)
RESPOND Outcomes Scientific Interest Group
L. Ryom, A. Mocroft, B. Neesgaard, L. Greenberg, N. Jaschinski, A. Timiryasova, L. Bansi-Matharu, D. Raben, L. Peters, E. Tusch, W. Bannister, A. Roen, D. Byonanebye, O. Fursa, A. Pelchen-Matthews, J. Reekie, V. Svedhem-Johansson, M. Van der Valk, F. Wit, K. Grabmeier-Pfistershammer, R. Zangerle, J. Hoy, M. Bloch, D. Braun, A. Calmy, G. Schüttfort, M. Youle, S. De Wit, C. Mussini, S. Zona, A. Castagna, A. Antinori, N. Chkhartishvili, N. Bolokadze, E. Fontas, K. Dollet, C. Pradier, J. M. Miro, J. M. Llibre, J. J. Vehreschild, C. Schwarze-Zander, J. C. Wasmuth, J. Rockstroh, K. Petoumenos, J. Hutchinson, M. Law, J. Begovac, C. Duvivier, G. Dragovic, R. Radoi, C. Oprea, M. Vasylyev, J. Kowalska, R. Matulionyte, V. Mulabdic, G. Marchetti, E. Kuzovatova, N. Coppola, I. Aho, S. Martini, H. Bucher, A. Harxhi, T. Wæhre, A. Pharris, A. Vassilenko, G. Fätkenheuer, J. Bogner, A. Maagaard, E. Jablonowska, D. Elbirt, G. Marrone, C, Leen, C. Wyen, L. Dahlerup Rasmussen, C. Hatleberg, M. Kundro, N. Dedes, E, Dixon Williams, J. Gallant, C. Cohen, M. Dunbar, A. Marongiu, V. Vannappagari, H. Garges, R. Campo, L. Young.
Community Representatives
A. Volny Anne, N. Dedes, L. Mendao (European AIDS Treatment Group), E. Dixon Williams (UK)
RESPOND Staff
Coordinating Centre and Data Management: N. Jaschinski, B. Neesgaard, A. Timiryasova, O. Fursa, O. Valdenmaier, J. F. Larsen, M. Gardizi, D. Raben, L. Peters, L. Ryom, T. W. Elsing, L. Ramesh Kumar, S. Shahi, K. Andersen
Statistical Staff: J. Reekie, L. Greenberg, L. Bansi-Matharu, K. Petoumenos, D. Byonanebye, E. Tusch, A. Roen, W. Bannister, A. Mocroft
Data sharing. The RESPOND Scientific Steering Committee (SSC) encourages the submission of concepts for research projects. Online research concepts should be submitted to the RESPOND secretariat (respond.rigshospitalet@regionh.dk); for guidelines of how to submit research concepts see the RESPOND governance and procedures. The secretariat will direct the proposal to the relevant Scientific Interest Group, where the proposal will initially be discussed for scientific relevance before being submitted to the SSC for review. Once submitted to the SSC, the research concept's scientific relevance, relevance to RESPOND's ongoing scientific agenda, design, statistical power, feasibility, and overlap with already approved projects will be assessed. Upon completion of the review, feedback will be provided to the proposer or proposers. In some circumstances, a revision of the concept might be requested. If the concept is approved for implementation, a writing group will be established consisting of the proposers (up to 3 people who were centrally involved in developing the concept), representatives from RESPOND cohorts, and representatives from the Statistical Department and Coordinating Center. All individuals involved in the process of reviewing these research concepts are bound by confidentiality. All data within RESPOND from individual cohorts are deidentified. The present RESPOND data structure and a list of all collected variables and their definitions can be found online. For any inquiries regarding data sharing, please contact the RESPOND secretariat and Dorthe Raben, Director of Research Coordination (Dorthe.raben@regionh.dk).
Financial support. Swiss cancer league grant KFS-4981-02-2020. The International Cohort Consortium of Infectious Disease (RESPOND) is supported by ViiV Healhtcare, Gilead Sciences, and Merck Sharp & Dohme. Additional support has been provided by participating cohorts contributing in-kind data and/or statistical support: the Austrian HIV Cohort Study, the Australian HIV Observational Database, CHU Saint-Pierre, Universtiy Hospital Cologne, EuroSIDA, the Frankfurt HIV Cohort Study, the Georgian National AIDS Health Information System, the Modena HIV Cohort, the San Raffaele Scientific Institute, the Swiss HIV Cohort Study, and the Royal Free HIV Cohort Study. The Australian HIV Observational Database is further supported by the US National Institutes of Health (grant U01-AI069907) and the National Healht and Medical Research Council, Australia (grant GNT105874), and the Swiss HIV Cohort Study is supported by the Swiss National Science Foundation.
Contributor Information
Frédérique Chammartin, Division of Clinical Epidemiology, Department of Clinical Research, University Hospital Basel and University of Basel, Basel, Switzerland.
Amanda Mocroft, CHIP, Department of Infectious Diseases, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark; Centre for Clinical Research, Epidemiology, Modelling and Evaluation (CREME), Institute for Global Health, University College London, London, United Kingdom.
Alexander Egle, Austrian HIV Cohort Study (AHIVCOS), Paracelsus Medical University Hospital, Salzburg, Austria.
Robert Zangerle, Austrian HIV Cohort Study (AHIVCOS), Medizinische Universität Innsbruck, Innsbruck, Austria.
Colette Smith, The Royal Free HIV Cohort Study, Royal Free Hospital, University College London, London, United Kingdom.
Cristina Mussini, Modena HIV Cohort, Università degli Studi di Modena, Modena, Italy.
Ferdinand Wit, AIDS Therapy Evaluation in the Netherlands (ATHENA) Cohort, HIV Monitoring Foundation, Amsterdam, The Netherlands.
Jörg Janne Vehreschild, Department I of internal Medicine, University Hospital Cologne, Cologne, Germany.
Antonella d’Arminio Monforte, Italian Cohort Naive Antiretrovirals (ICONA), ASST Santi Paolo e Carlo, Milano, Italy.
Antonella Castagna, San Raffaele Scientific Institute, Università Vita-Salute San Raffaele, Milano, Italy.
Laurent Bailly, Nice HIV Cohort, Department of Public Health, Université Côte d’Azur—Centre Hospitalier Universitaire de Nice, UR2CA, Nice, France.
Johannes Bogner, Division of Infectious Diseases, Medizinische Klinik und Poliklinik IV, LMU University Hospital, LMU Munich, Munich, Germany.
Stéphane de Wit, CHU Saint-Pierre, Centre de Recherche en Maladies Infectieuses a.s.b.l., Brussels, Belgium.
Raimonda Matulionyte, Vilnius University, Faculty of Medicine, Department of Infectious Diseases and Dermatovenerology; Vilnius University Hospital Santaros Klinikos, Vilnius, Lithuania.
Matthew Law, The Australian HIV Observational Database (AHOD), Kirby Institute, University of New South Wales, New South Wales, Australia.
Veronica Svedhem, Division of Infectious Diseases, Department of Medicine, Karolinska Institute and Karolinska University Hospital, Stockholm, Sweden.
Joan Tallada, European AIDS Treatment Group (EATG), Brussels, Belgium.
Harmony P Garges, ViiV Healthcare, Durham, North Carolina, USA.
Andrea Marongiu, Gilead Sciences, Foster City, California, USA.
Álvaro H Borges, CHIP, Department of Infectious Diseases, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark; Department of Infectious Diseases Immunology, Statens Serum Institut, Copenhagen, Denmark.
Nadine Jaschinski, CHIP, Department of Infectious Diseases, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark.
Bastian Neesgaard, CHIP, Department of Infectious Diseases, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark.
Lene Ryom, CHIP, Department of Infectious Diseases, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark; Department of Infectious Diseases 144, Hvidovre University Hospital, Copenhagen, Denmark.
Heiner C Bucher, Division of Clinical Epidemiology, Department of Clinical Research, University Hospital Basel and University of Basel, Basel, Switzerland.
for the RESPOND Study Group:
F Wit, M van der Valk, M Hillebregt, K Petoumenos, M Law, R Zangerle, H Appoyer, C Stephan, M Bucht, N Chkhartishvili, O Chokoshvili, A d’Arminio Monforte, A Rodano, A Tavelli, I Fanti, J Casabona, J M Miro, J M Llibre, A Riera, J Reyes-Urueña, C Smith, F Lampe, A Sönnerborg, K Falconer, V Svedhem, H Günthard, B Ledergerber, H Bucher, K Kusejko, J C Wasmuth, J Rockstroh, J J Vehreschild, G Fätkenheuer, L Ryom, M Law, R Campo, S De Wit, H Garges, H Günthard, J Lundgren, I McNicholl, J Rooney, C Smith, V Vannappagari, G Wandeler, L Young, R Zangerle, J Lundgren, H Günthard, J Begovac, A Bruguera, H Bucher, A Castagna, R Campo, N Chkhartishvili, A D’Arminio Monforte, N Dedes, H Garges, J Kowalska, M Law, I McNicholl, C Mussini, C Necsoi, L Peters, K Petoumenos, C Pradier, D Raben, J Rockstroh, J Rooney, L Ryom, C Smith, A Sönnerborg, C Stephan, V Vannappagari, J J Vehreschild, A Volny Anne, G Wandeler, J C Wasmuth, E D Williams, F Wit, L Young, R Zangerle, L Ryom, A Mocroft, B Neesgaard, L Greenberg, N Jaschinski, A Timiryasova, L Bansi-Matharu, D Raben, L Peters, E Tusch, W Bannister, A Roen, D Byonanebye, O Fursa, A Pelchen-Matthews, J Reekie, V Svedhem-Johansson, M Van der Valk, F Wit, K Grabmeier-Pfistershammer, R Zangerle, J Hoy, M Bloch, D Braun, A Calmy, G Schüttfort, M Youle, S De Wit, C Mussini, S Zona, A Castagna, A Antinori, N Chkhartishvili, N Bolokadze, E Fontas, K Dollet, C Pradier, J M Miro, J M Llibre, J J Vehreschild, C Schwarze-Zander, J C Wasmuth, J Rockstroh, K Petoumenos, J Hutchinson, M Law, J Begovac, C Duvivier, G Dragovic, R Radoi, C Oprea, M Vasylyev, J Kowalska, R Matulionyte, V Mulabdic, G Marchetti, E Kuzovatova, N Coppola, I Aho, S Martini, H Bucher, A Harxhi, T Wæhre, A Pharris, A Vassilenko, G Fätkenheuer, J Bogner, A Maagaard, E Jablonowska, D Elbirt, G Marrone, C Leen, C Wyen, L Dahlerup Rasmussen, C Hatleberg, M Kundro, N Dedes, E Dixon Williams, J Gallant, C Cohen, M Dunbar, A Marongiu, V Vannappagari, H Garges, R Campo, L Young, A Volny Anne, N Dedes, L Mendao, E Dixon Williams, N Jaschinski, B Neesgaard, A Timiryasova, O Fursa, O Valdenmaier, J F Larsen, M Gardizi, D Raben, L Peters, L Ryom, T W Elsing, L Ramesh Kumar, S Shahi, K Andersen, J Reekie, L Greenberg, L Bansi-Matharu, K Petoumenos, D Byonanebye, E Tusch, A Roen, W Bannister, and A Mocroft
Notes
Author Contributions. F. C. and H. C. B. conceived and designed the study. F. C. did the statistical analysis. F. C. and H. C. B. verified the underlying data. H. C. B, and F. C. acquired funding for the statistical analysis. F. C. and H. C. B. drafted the manuscript, and all authors critically revised the manuscript and consented to final publication. All authors have full access to the data included in the study and accept responsibility for the decision to submit for publication.
References
- 1. Antiretroviral Therapy Cohort Collaboration . Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet 2008; 372:293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morlat P, Roussillon C, Henard S, et al. Causes of death among HIV-infected patients in France in 2010 (national survey): trends since 2000. AIDS 2014; 28:1181–91. [DOI] [PubMed] [Google Scholar]
- 3. Engels EA, Yanik EL, Wheeler W, et al. Cancer-attributable mortality among people with treated human immunodeficiency virus infection in North America. Clin Infect Dis 2017; 65:636–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Borges ÁH, Dubrow R, Silverberg MJ. Factors contributing to risk for cancer among HIV-infected individuals, and evidence that earlier combination antiretroviral therapy will alter this risk. Curr Opin HIV AIDS 2014; 9:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clifford GM, Polesel J, Rickenbach M, et al. Cancer risk in the Swiss HIV cohort study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst 2005; 97:425–32. [DOI] [PubMed] [Google Scholar]
- 6. Marques M, Silva DA, Brites C. Cancer during HIV infection. J Pathol Microbiol Immunol 2020; 128:121–8. [DOI] [PubMed] [Google Scholar]
- 7. Borges ÁH, Silverberg MJ, Wentworth D, et al. Predicting risk of cancer during HIV infection: the role of inflammatory and coagulation biomarkers. AIDS 2013; 27:1433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu Rev Med 2011; 62:141–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Younas M, Psomas C, Reynes J, Corbeau P. Immune activation in the course of HIV-1 infection: causes, phenotypes and persistence under therapy. HIV Med 2016; 17:89–105. [DOI] [PubMed] [Google Scholar]
- 10. Zicari S, Sessa L, Cotugno N, et al. Immune activation, inflammation, and non-AIDS co-morbidities in HIV-infected patients under long-term ART. Viruses 2019; 11:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bruno G, Saracino A, Monno L, Angarano G. The revival of an “old” marker: CD4/CD8 ratio. AIDS Rev 2017; 19:81–8. [PubMed] [Google Scholar]
- 12. Ron R, Moreno E, Martínez-Sanz J, et al. CD4/CD8 ratio during human immunodeficiency virus treatment: time for routine monitoring? Clin Infect Dis 2023; 76:1688–96. [DOI] [PubMed] [Google Scholar]
- 13. Serrano-Villar S, Sainz T, Lee SA, et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog 2014; 10:e1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deeks SG, Verdin E, McCune JM. Immunosenescence and HIV. Curr Opin Immunol 2012; 24:501–6. [DOI] [PubMed] [Google Scholar]
- 15. Serrano-Villar S, Pérez-Elías MJ, Dronda F, et al. Increased risk of serious non-AIDS-related events in HIV-infected subjects on antiretroviral therapy associated with a low CD4/CD8 ratio. PLoS One 2014; 9:e85798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bonney A, Malouf R, Marchal C, et al. Impact of low-dose computed tomography (LDCT) screening on lung cancer-related mortality. Cochrane Database Syst Rev 2022; 8:CD013829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Palefsky JM, Lee JY, Jay N, et al. Treatment of anal high-grade squamous intraepithelial lesions to prevent anal cancer. N Engl J Med 2022; 386:2273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. RESPOND Study Group . How to RESPOND to modern challenges for people living with HIV: a profile for a new cohort consortium. Microorganisms 2020; 8(8):1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mocroft A, Petoumenos K, Wit F, et al. The interrelationship of smoking, CD4 + cell count, viral load and cancer in persons living with HIV. AIDS 2021; 35:747–57. [DOI] [PubMed] [Google Scholar]
- 20. CHIP - Centre of Excellence for Health, Immunity and Infections . RESPOND Manual of operations (MOOP) for clinical events v 1.8. 2023.
- 21. R Core Team . R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statitical Computing, 2022.
- 22. StataCorp . Stata statistical software: Release 16. College Station, TX: StataCorp LLC, 2023.
- 23. Trickey A, May MT, Schommers P, et al. CD4:CD8 ratio and CD8 count as prognostic markers for mortality in human immunodeficiency virus-infected patients on antiretroviral therapy: the antiretroviral therapy cohort collaboration (ART-CC). Clin Infect Dis 2017; 65:959–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mussini C, Lorenzini P, Cozzi-Lepri A, et al. CD4/CD8 ratio normalisation and non-AIDS-related events in individuals with HIV who achieve viral load suppression with antiretroviral therapy: an observational cohort study. Lancet HIV 2015; 2:e98–e106. [DOI] [PubMed] [Google Scholar]
- 25. Han WM, Apornpong T, Kerr SJ, et al. CD4/CD8 ratio normalization rates and low ratio as prognostic marker for non-AIDS defining events among long-term virologically suppressed people living with HIV. AIDS Res Ther 2018; 15:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hema MN, Ferry T, Dupon M, et al. Low CD4/CD8 ratio is associated with non AIDS-defining cancers in patients on antiretroviral therapy: ANRS CO8 (APROCO/COPILOTE) prospective cohort study. PLoS One 2016; 11:e0161594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ruffieux Y, Chammartin F, Feller A, et al. Non-AIDS defining malignancies in the combination ART era: immunological and socio-behavioral risk factors. F1000Research 2019; 8:1400. [Google Scholar]
- 28. Sigel K, Wisnivesky J, Crothers K, et al. Immunological and infectious risk factors for lung cancer in US veterans with HIV: a longitudinal cohort study. Lancet HIV 2017; 4:e67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Caby F, Guiguet M, Weiss L, et al. CD4/CD8 ratio and risks of Kaposi sarcoma and non-Hodgkin lymphoma in the context of effectively treated HIV infection: a collaborative analysis of 20 European cohort studies. Clin Infect Dis 2021; 73:50–9. [DOI] [PubMed] [Google Scholar]
- 30. Castilho JL, Bian A, Jenkins CA, et al. CD4/CD8 ratio and cancer risk among adults with HIV. J Natl Cancer Inst 2022; 114:854–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sylvestre M-P, Abrahamowicz M. Flexible modeling of the cumulative effects of time-dependent exposures on the hazard. Stat Med 2009; 28:3437–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.