Abstract
Background
Revised diagnostic criteria for infective endocarditis (IE), the 2023 Duke-ISCVID criteria, were recently presented and need validation. Here, we compare the 2000 modified Duke criteria for IE with Duke-ISCVID among patients with bacteremia and relate the diagnostic classification to IE treatment.
Methods
We reanalyzed patient cohorts with Staphylococcus aureus, Staphylococcus lugdunensis, non–β-hemolytic streptococci, Streptococcus-like bacteria, Streptococcus dysgalactiae, Enterococcus faecalis, and HACEK (Haemophilus, Aggregatibacter, Cardiobacterium, Eikenella, Kingella) bacteremia. Episodes were classified as definite, possible, or rejected IE with the modified Duke and Duke-ISCVID criteria. Reclassification included the microbiology criteria, positron emission tomography–computed tomography, and cardiac implanted electronic devices. To calculate sensitivity, patients treated for IE were considered as having IE.
Results
In 4050 episodes of bacteremia, the modified Duke criteria assigned 307 episodes (7.6%) as definite IE, 1190 (29%) as possible IE, and 2553 (63%) as rejected IE. Using the Duke-ISCVID criteria, 13 episodes (0.3%) were reclassified from possible to definite IE, and 475 episodes (12%) were reclassified from rejected to possible IE. With the modified Duke criteria, 79 episodes that were treated as IE were classified as possible IE, and 11 of these episodes were reclassified to definite IE with Duke-ISCVID. Applying the decision to treat for IE as a reference standard, the sensitivity of the Duke-ISCVID criteria was 80%. None of the 475 episodes reclassified to possible IE were treated as IE.
Conclusions
The Duke-ISCVID criteria reclassified a small proportion of episodes to definite IE at the expense of more episodes of possible IE. Future criteria should minimize the possible IE group while keeping or improving sensitivity.
Keywords: infective endocarditis, diagnostic criteria, validation, bacteremia
The new Duke-ISCVID criteria for infective endocarditis reclassified 12% of episodes in a cohort of patients with bacteremia (n = 4050) from rejected to possible endocarditis. Only 11 of 79 episodes of possible endocarditis, treated as endocarditis, were reclassified to definite endocarditis.
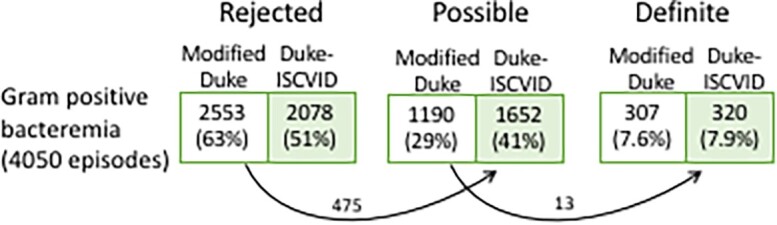
Graphical Abstract
Graphical Abstract.
(See the Invited Commentary by Chambers et al. on pages 964–7.)
Infective endocarditis (IE) is a severe disease affecting the heart valves where the typical lesion, the vegetation is a hallmark for the condition. Whereas surgery or autopsy offers the possibility for an undisputable diagnosis of IE, the clinical diagnostic criteria for IE have varied over time. A landmark in the history of criteria development was when Durack and coworkers introduced echocardiography as an important major criterion in the Duke criteria in 1994 [1]. The Duke criteria were then modified in the year 2000, after validation in a cohort of 800 patients with IE, with an aim to lower the numbers of “possible IE,” and thus increase the specificity [2]. A combination of 1 major with 1 minor criterion or ≥3 minor criteria alone were introduced as the “floor” for possible IE. In the 2015 European Society of Cardiology criteria [3], further imaging modalities, including fluorine 18 fluorodeoxyglucose positron emission tomography (PET)–computed tomography (CT) and cardiac CT were added to define major imaging criteria.
The recently presented 2023 Duke-ISCVID (hereafter referred to as Duke-ISCVID) criteria have kept the original structure of the 1994 Duke criteria, with major and minor criteria [4]. The revisions were based on opinions from a group of experts and not on systematic literature review or analysis of new cohorts. Thus, validation studies and continuous updates are called for. The Duke-ISCVID criteria have added several bacteria to the list of typical IE pathogens [4]. In addition, the presence of a cardiovascular implanted electronic device (CIED) was added as a new predisposition minor criterion. Importantly, PET-CT and cardiac CT were introduced as imaging techniques for major imaging criteria, in concordance with the ESC criteria from 2015 [3], and several features of the criteria were explained more precisely. The main aim of the Duke-ISCVID criteria for IE was to provide a relevant update on imaging criteria and bacteriology [4].
One particular clinical situation in which IE needs to be considered is the finding of gram-positive or HACEK (Haemophilus, Aggregatibacter, Cardiobacterium, Eikenella, Kingella) bacteria in blood cultures. For patients with such bacteremia, the risk for IE needs to be evaluated so that echocardiographic investigations are performed in patients at risk for IE. The finding of IE has direct implications for prolonged treatment time and also for the decision about heart valve surgery. Therefore, several studies have been performed to determine risk factors for IE and risk stratification systems for IE in patients with bacteremia caused by gram-positive bacteria, such as Staphylococcus aureus [5–7], viridans streptococci (also known as non–β-hemolytic streptococci, NBHS) [8–10], and Enterococcus faecalis [11–13].
The objective of the current study was to compare the performance of the Duke-ISCVID criteria for IE [4] with that of the 2000 modified Duke criteria [2] in patients with bacteremia from 14 different cohorts. The study also evaluated the agreements of the classifications by the sets of criteria compared with the clinical decision to treat as IE.
METHODS
Fourteen retrospective, population-based cohorts of patients with bacteremia have been published by our research group (Table 1). The cohorts were gathered from 3 geographically defined regions in Sweden and were population based. All studies included consecutive patients with bacteremia with a given bacterium or group of bacteria. The observation time for each episode was ≥1 year. In Table 1, the underlying population is given for the relevant time periods. Classification of IE status had been performed using the 2000 modified Duke criteria [2] or the 2015 European Society of Cardiology criteria [3] with some modifications with regard to the classification of Streptococcus-like bacteria [18]. For all cohorts, the absence of information about a particular feature was regarded as if that factor was missing. No imputations were made with regard to features included in the different IE criteria. A reevaluation of data from these cohort was performed, and each patient was classified as having rejected, possible, or definite IE according to the 2000 modified Duke criteria [2] and the Duke-ISCVID criteria, and the number of reclassifications was determined.
Table 1.
Details of the Bacteremia Patient Cohorts
| Bacteria | Period | Region in Sweden | Population (in Millions) | Reference | Bacteremia Episodes, No. | Episodes With IE, No. (%) |
|---|---|---|---|---|---|---|
| Staphylococcus aureus | 2016 | Skåne | 1.32 | [6] | 542 | 40 (7.4) |
| 2017 | Skåne | 1.34 | [6] | 556 | 29 (5.2) | |
| 2017–2019 | Halland | 0.32–0.33 | [14] | 267 | 24 (9.0) | |
| Staphylococcus lugdunensis | 2015–2019 | Skåne | 1.30–1.38 | [15] | 65 | 5 (7.7) |
| NBHSa | 2012–2013b | Skåne | 1.26–1.27 | [10] | 312 | 20 (6.4) |
| 2015–2016c | Skåne | 1.30 | [16] | 244 | 27 (11.1) | |
| NBHSd | 2017–2019 | Halland | 0.32–0.33 | [14] | 154 | 10 (6.5) |
| Streptococcus bovis e | 2003–2018 | Skåne | 1.15–1.36 | [17] | 210 | 28 (13.3) |
| Streptococcus-like bacteriaf | 2012–2017 | Skåne and Stockholm | 3.2 | [18] | 568 | 32 (5.6)g |
| Streptococcus dysgalactiae | 2015–2018 | Skåne | 1.30–1.36 | [19] | 287 | 4 (1.4) |
| Enterococcus faecalis | 2017–2019 | Halland | 0.32–0.33 | [14] | 62 | 3 (4.8) |
| 2012–2016 | Skåne | 1.26–1.32 | [11] | 397 | 44 (11.1) | |
| 2012–2016 | Stockholm | Karolinskah | [11] | 268 | 24 (9.0) | |
| HACEK | 2012–2017 | Skåne and Stockholm | 3.2 | [20] | 118 | 27 (22.9) |
Abbreviations: HACEK, Haemophilus, Aggregatibacter, Cardiobacterium, Eikenella, Kingella; IE, infective endocarditis; NBHS, non–β-hemolytic streptococci.
aNBHS comprising the mitis, sanguinis, mutans, salivarius, and anginosus groups; for species see reference 10. Isolates of the bovis group were excluded.
bOnly half of 2013.
cThe end of the study period was 31 March 2016.
dAll NBHS, including the bovis group.
e Streptococcus bovis–Streptococcus equinus complex: Streptococcus gallolyticus subsp pasteurianus, S. gallolyticus subsp gallolyticus, Streptococcus lutetiensis, and Streptococcus infantarius.
fIncluding Abiotrophia (n = 19), Aerococcus (n = 338), Gemella (n = 87), and Granulicatella (n = 124).
gIn this study all pathogens were regarded as “typical IE pathogens” [18].
hA tertiary center, not population based.
Changes introduced in the Duke-ISCVID criteria that were available for reanalysis in our cohorts were (1) the upgrading of Streptococcus dysgalactiae, Staphylococcus lugdunensis, and Gemella to “typical IE pathogens”; (2) the withdrawal of the demand for nonnosocomial acquisition and lack of a focal infection for E. faecalis to be regarded as a typical IE pathogen; (3) the addition of a positive PET-CT as a major imaging criterion; and (4) the addition of a CIED as a new predisposition minor criterion. The Duke-ISCVID criteria specify Abiotrophia and Granulicatella as typical IE pathogens, but our interpretation is that these organisms were implicitly included in the viridans streptococci in the previous criteria [2, 3], whereas Gemella was added to the list in the Duke-ISCVID criteria. There were no missing data for the variables regarded for reanalysis.
The clinical decision to treat a patient for IE was compared with classifications by the 2 criteria. The information on treatment as IE was lacking for one of the cohorts and this cohort was excluded from this analysis. The concordance between the clinical decision to treat as IE and the classification of patients as definite or not definite IE according to the 2000 modified Duke criteria and the Duke-ISCVID criteria was calculated and presented.
Values are given as numbers and shares of the total. Significance was defined as a P value <.05. GraphPad Prism software (version 9; GraphPad Software) was used for statistical calculations. All of the studies had been approved by relevant regional or national ethics boards, and no additional personal information was collected for the present study.
RESULTS
New Major and Minor Criteria
A total of 4050 episodes of bacteremia were reanalyzed (Table 2). Episodes caused by S. lugdunensis, S. dysgalactiae, Gemella, and E. faecalis (nosocomial acquired or with known focus) were eligible for reclassification from the minor to the major microbiology criterion since these species were changed to “typical IE pathogens” in the Duke-ISCVID criteria. Thus, those with 2 positive blood culture sets were transferred to the major microbiology criterion. Such reclassification occurred in 518 episodes (13% of all episodes). The majority of these reclassifications occurred in the S. dysgalactiae and E. faecalis cohorts (Table 2).
Table 2.
Episodes of Bacteremia and Reclassification of Minor and Major Criteria
| Microbiological Agent | Episodes of Bacteremia, No. | Change of Microbiology Minor to Major Criteria | PET-CT Providing New Major Criterion | CIED as New Minor Criterion |
|---|---|---|---|---|
| Staphylococcus aureus | 1365 | 0 | 0 | 98 |
| Staphylococcus lugdunensis | 65 | 27 | 0 | 2 |
| NBHSa | 920 | 0 | 1 | 23 |
| Streptococcus-like bacteria | 568 | 22b | 0 | 25 |
| Streptococcus dysgalactiae | 287 | 192 | 0 | 11 |
| Enterococcus faecalis | 727 | 277 | 0 | 49 |
| HACEK | 118 | 0 | 0 | 6 |
Abbreviations: CIED, cardiovascular implanted electronic device; CT, computed tomography; HACEK, Haemophilus, Aggregatibacter, Cardiobacterium, Eikenella, Kingella; NBHS, non–β-hemolytic streptococci; PET, positron emission tomography.
aNBHS comprising the mitis, sanguinis, mutans, salivarius, anginosus, and bovis groups; for species see references 10 and 17.
b Gemella was changed to a typical pathogen in the 2023 Duke-International Society of Cardiovascular Infectious Diseases (ISCVID) criteria, Abiotrophia and Granilucatella were counted as typical pathogens in both 2000 modified Duke and 2023 Duke-International Society of Cardiovascular Infectious Diseases (ISCVID) criteria, and Aerococcus was regarded as nontypical in both criteria.
The performance of PET-CT without findings of IE was not systemically recorded in all cohorts but PET-CT or CT of the heart provided a new major criterion in in only 1 episode. The patient had a CIED in 322 episodes (8.0% of all episodes). In some of these patients another predisposing factor was already present, and the CIED therefore provided a new minor criterion, according to the Duke-ISCVID criteria, in 214 episodes (5.3%) (Table 2).
Reclassification
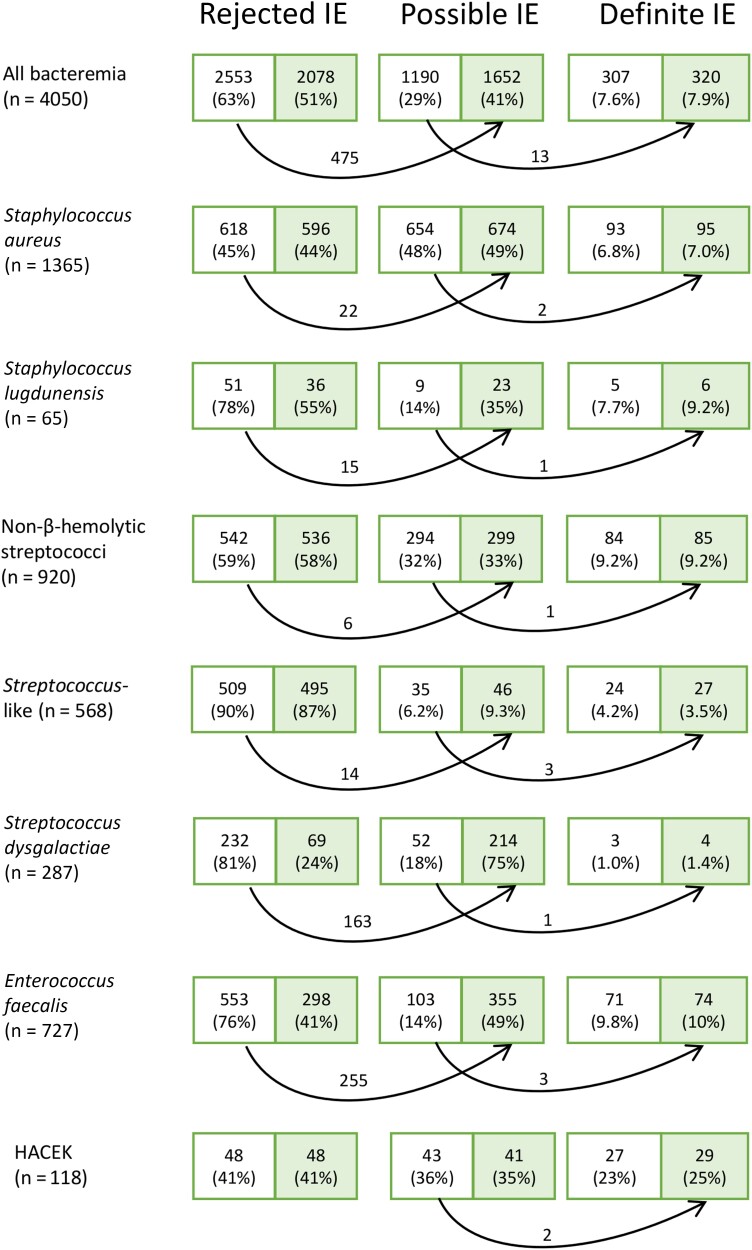
Applying the 2000 modified Duke criteria to all the cohorts resulted in 307 episodes (7.6%) of definite IE, 1188 (29%) of possible IE, and 2553 (63%) of rejected IE. With the Duke-ISCVID criteria, 13 episodes were reclassified from the possible category to definite IE, and 475 episodes from the rejected category to possible IE (Figure 1). The definite IE group thereby increased by 4.2%, and the possible IE group by 39%. Figure 1 shows the reclassification of episodes in the cohorts of patients with bacteremia caused by different bacteria. Table 3 shows a cross-tabulation of episodes with bacteremia classified as definite, possible, and rejected IE by the 2000 modified Duke criteria and the Duke-ISCVID criteria.
Figure 1.
Displayed in the boxes are the numbers of episodes (and the share [percentage] of total episodes) assigned to rejected, possible, and definite infective endocarditis (IE). The left (unshaded) part of each box shows the result using the 2000 modified Duke criteria; the right (shaded) part, the result using 2023 Duke-International Society of Cardiovascular Infectious Diseases (ISCVID) criteria. Arrows indicate reclassification of episodes, with the number of reclassified episodes displayed above the arrow. Abbreviation: HACEK, Haemophilus, Aggregatibacter, Cardiobacterium, Eikenella, Kingella.
Table 3.
Comparison of Infective Endocarditis Status by 2000 Modified Duke and 2023 Duke-International Society of Cardiovascular Infectious Diseases Criteria
| Classification by Modified Duke Criteria | Bacteremia Episodes by Duke-ISCVID Criteria Classification, No. | ||
|---|---|---|---|
| Definite IE | Possible IE | Rejected IE | |
| Definite IE | 307 | 0 | 0 |
| Possible IE | 13 | 1190 | 0 |
| Rejected IE | 0 | 475 | 2078 |
Abbreviations: IE, infective endocarditis; ISCVID, International Society of Cardiovascular Infectious Diseases.
Features of the individual patients reclassified from possible to definite IE are given in Table 4. The proportion of cases reclassified from possible to definite IE was largest among patients with Streptococcus-like bacteremia, among whom 3 episodes (8.6% of possible IE episodes) were reclassified.
Table 4.
Bacteremia Episodes Reclassified From Possible to Definite Infective Endocarditis in the 2023 Duke-International Society of Cardiovascular Infectious Diseases Criteria
| Patient Age, y (Sex) | Bacteria | Treated as IE | Positive Cultures, No./Total No. |
TEE or TTE Findings | PET-CT | Minor Criteriaa | Acquisition | Surgery | Comments |
|---|---|---|---|---|---|---|---|---|---|
| 74 (Male) | Staphylococcus aureus | Yes | 4/4 | TEE—Neg | ND | Fever, embolic event, ICD | Community | ICD extraction | No growth on extracted ICD |
| 54 (Male) | S. aureus | No | 4/4 | TEE—Neg | ND | Fever, embolic event, ICD | Community | No | Antibiotic treatment for 12 d; no recurrence |
| 85 (Female) | Staphylococcus lugdunensis | Yes | 4/4 | TEE—Veg | ND | Fever | Community | No | … |
| 76 (Male) | Streptococcus infantarius sp (bovis group) | Yes | 4/4 | TEE—Neg | PVE | Fever, prosthetic valve | Community | No | Aortic root abscess; in-hospital death |
| 88 (Male) | Granulicatella adiacens | Yes | 1/4 | TEE—Veg | ND | Microbiology, fever, PM | Nosocomial | No | No PM extraction |
| 70 (Male) | Gemella sp | Yes | 4/4 | TEE—Veg | ND | Fever | Community | Yes | 16S PCR on valve Gemella sp |
| 77 (Female) | Aerococcus urinae | Yes | 4/4 | TEE—Veg | ND | Microbiology, fever, PM | Healthcare | No | No PM extraction |
| 93 (Male) | Streptococcus dysgalactiae | Yes | 4/4 | TTE—Neg | ND | Fever, native valve disease, vascular phenomenon | Community | No | … |
| 82 (Male) | Enterococcus faecalis | No | 4/4 | TEE—Neg | ND | Prosthetic valve, previous IE, fever, stroke | Nosocomial | No | … |
| 83 (Female) | E. faecalis | Yes | 4/6 | TEE—Veg | ND | Predisposition, fever | Nosocomial | No | … |
| 80 (Male) | E. faecalis | Yes | 4/4 | TTE—Neg | ND | Predisposition, fever, embolization | Nosocomial | No | … |
| 73 (Male) | Aggregatibacter sp | Yes | 1/4 | TEE—Veg | ND | Fever, PM | Community | No | … |
| 70 (Male) | Aggregatibacter sp | Yes | 1/4 | TEE—Veg | ND | Fever, PM | Community | PM extracted | 16S PCR on PM Aggregatibacter sp |
Abbreviations: CT, computed tomography; ICD, implantable cardioverter defibrillator; IE, infective endocarditis; ND, not done; Neg, negative; PCR, polymerase chain reaction; PET, positron emission tomography; PM, pacemaker; PVE, prosthetic valve endocarditis; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography; Veg, vegetation.
aICD and PM were new additions to the 2023 Duke-International Society of Cardiovascular Infectious Diseases (ISCVID) criteria.
Agreement Between Diagnostic Criteria and Clinical Management
For all cohorts, except for the 2016 part of the S. aureus Skåne cohort [6], there was information about which episodes were diagnosed and treated as IE even though the 2000 modified Duke criteria for definite IE were not fulfilled. For this part of the study population (3508 episodes), 79 patients (2.2%) with episodes not fulfilling definite IE criteria were regarded as having IE based on being treated for IE (see above). Table 5 shows the consequences of the reclassification made by the Duke-ISCVID criteria in these patients. In Supplementary Table 1, the consequences of reclassifications are given for each bacterial species or group. If the clinical decision to treat the patient as having IE was used as the reference standard, the sensitivity of the 2000 modified Duke criteria was 77% compared with 80% for the Duke-ISCVID criteria, and this difference was not significant (P = .4 [Fisher exact test]).
Table 5.
Concordance Between Diagnostic Criteria and Treatment for Infective Endocarditis
| IE Classification by Criteria | Episodes, No. (%) | |
|---|---|---|
| Treated as IE | Not Treated as IE | |
| 2000 Modified Duke criteria | Yes | No |
| Definite IE | 267 | 0 |
| Possible IE | 79 | 841 |
| Rejected IE | 0 | 2321 |
| 2023 Duke-ISCVID criteria | Yes | No |
| Definite IE | 278 | 1 |
| Possible IE | 68 | 1307 |
| Rejected IE | 0 | 1854 |
Abbreviations: IE, infective endocarditis; ISCVID, International Society of Cardiovascular Infectious Diseases.
The largest proportions of the episodes treated as IE despite not fulfilling Duke-ISCVID criteria for definite IE were found in Streptococcus-like and HACEK bacteremia, for which 41% and 29%, respectively, of patients treated as having IE did not receive a definite IE diagnosis according to criteria. Notably, 7 patients with Aerococcus urinae bacteremia had vegetations demonstrated by echocardiography and were treated as having IE but did not fulfil definite IE criteria in either 2000 modified Duke or Duke-ISCVID criteria. Of the 475 patients reclassified from rejected IE to possible IE, none received treatment for IE or were perceived as having IE.
The specificity of the Duke-ISCVID criteria for definite IE in relation to treatment for IE was marginally lower than that of the modified Duke criteria. Only 2 episodes were reclassified to definite IE despite not being treated for IE. One of these patients likely did not have IE since his infection was cured after only 12 days of antibiotic treatment and did not relapse (see Table 4). The specificity of the criteria went from 73% with the modified Duke criteria to 59% with Duke-ISCVID (P < .001 [χ2-test]) assuming that the possible and definite IE episodes were regarded as IE and the clinical decision to treat as IE was used as the reference standard.
DISCUSSION
Applying the Duke-ISCVID 2023 criteria to our cohorts of patients who had bacteremia with IE-causing bacteria increased the proportion of patients assigned to the possible IE group by 39%, from 29% to 41%, of the entire cohort. This increase was mainly due to the recognition of E. faecalis and S. dysgalactiae as typical IE pathogens. When the Duke criteria were modified in 2000, a specific aim was to decrease the proportion of patients falling into the possible IE group [2]. With the inclusion of more bacteria as typical IE pathogens, without changing other criteria, the Duke-ISCVID criteria will classify many more patients as having possible IE. The typical patient with S. dysgalactiae bacteremia classified as possible IE in our study had cellulitis and fever. Such a patient has very low risk of having IE and would likely not benefit from workup (eg, with echocardiography) for possible IE. Thus, if more bacteria are included as typical IE pathogens, other changes are needed for the criteria not to include patients without IE in the possible IE group. For example, the usefulness of fever as a minor criterion in patients with bacteremia can be questioned, since fever and bacteremia are likely to display collinearity. This should be addressed in future studies, which need to include patients with suspected IE both with and without bacteremia. It is also problematic that IE can never be rejected in patients with bacteremia with a typical IE pathogen such as S. dysgalactiae, since the criteria for rejection demand that the bacteremia has to be caused by a “nontypical” IE pathogen [4]. Therefore, the Duke-ISCVID criteria will classify many patients with S. dysgalactiae and E. faecalis bacteremia as having possible IE, despite the extremely low probability for IE in a given patient.
Several studies have shown that the risk of IE is low in E. faecalis bacteremia of nosocomial origin and with known focus [11–13, 21, 22] as well as in S. dysgalactiae bacteremia [19, 23–25]. The study referred to in the Duke-ISCVID criteria to justify the inclusion of S. dysgalactiae as a typical IE pathogen is the only one reporting a much higher risk of IE in S. dysgalactiae bacteremia [4, 9]. It should be noted that this study was based on International Classification of Diseases codes and not on studies of medical records, thereby risking an increased proportion of misclassified episodes [9].
The Duke-ISCVID criteria assigned an additional 13 episodes (0.3% of all episodes) in our cohorts to the category of definite IE and increased the number of patients in this category from 307 to 320, an increase of 4.2%. Through the changes introduced in Duke-ISCVID criteria, 11 of 78 (14%) of patients who were treated for IE but did not fulfill 2000 modified Duke criteria for definite IE were reclassified to definite IE. Of course, it is not certain that the patients perceived as having IE really had the condition. Of those who were perceived as having IE, were treated for IE but not classified as definite IE by the Duke-ISCVID criteria, the majority had bacteremia with S. aureus (n = 18 [2.2% of all episodes]), Streptococcus bovis (n = 9 [4.3%]), HACEK (n = 11 [9.3%]), or Streptococcus-like bacteria (n = 19 [3.3%]). It was obvious that the performance of the Duke-ISCVID criteria could have been improved by the addition of A. urinae as a “typical pathogen.” By making this change to the criteria, 7 episodes with A. urinae bacteremia would have been reclassified from possible to definite IE. All of these 7 patients had imaging demonstrating IE and were treated as having IE.
The main strengths of the current study are that the cohorts are large and population based, and patient information was gathered through careful studies of medical records. In addition, the patients with bacteremia caused by these pathogens constitute a relevant study cohort for the ISCVID-Duke criteria since the suspicion of IE is often raised by the finding of a gram-positive or HACEK bacterium in a blood culture.
Our study also had many weaknesses; the most obvious one is that relevant IE investigations were not performed in all patients, thus risking the possibility that the IE diagnosis was missed in some episodes. The proportion of patients investigated with PET-CT was particularly low, reflecting the fact that this modality was not frequently used during the study periods. The recognition of PET-CT as a major criterion for IE was implemented in Sweden in 2021, after the study periods of the presented cohorts. Moreover, several new features of the Duke-ISCVID criteria could not be evaluated in the current study. As the most obvious, several important IE pathogens that changed status in the Duke-ISCVID criteria—such as coagulase-negative staphylococci and Streptococcus agalactiae [4]—were not included. Other changes in the Duke-ISCVID criteria (eg, definitions of glomerulonephritis) could not be evaluated owing to lack of data in the respective cohorts. Another weakness is that even if the cohorts were population based, more uncommon pathogens, such as HACEK and Streptococcus-like bacteria, were overrepresented relative to S. aureus. Therefore, the exact figures from this study cannot be extrapolated to the entire population, neither in Sweden nor in the rest of the world.
In conclusion, the changes introduced in the Duke-ISCVID criteria regarding microbiology and predisposition led to an increased proportion of patients with possible IE among those with gram-positive bacteremia. None of the episodes reclassified from rejected to possible IE was perceived as IE by the treating physician. The sensitivity was not significantly increased, and most patients perceived as having IE remained in the possible IE group. If other components of the criteria remain unchanged, we suggest that S. dysgalactiae should be removed from the list of typical pathogens, whereas A. urinae should be included [26]. The Duke-ISCVID criteria could likely be improved by repeated revisions based on validation studies. We believe, however, that larger changes to the diagnostic criteria for IE are needed to improve their performance. An increased emphasis could be put on the imaging criteria and the presence of an imaging major criterion together with bacteremia, irrespective of whether the bacteria are typical or nontypical IE pathogens, could constitute the basis for a definite IE diagnosis. Such a different set of criteria needs to be generated from large-volume, high-quality data, preferably from several countries and from many relevant healthcare settings.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Helena Lindberg, Department of Infectious Diseases, Hospital of Halland, Halmstad, Sweden; Division of Infection Medicine, Department of Clinical Sciences Lund, Lund University, Lund, Sweden.
Andreas Berge, Unit of Infectious Diseases, Department of Medicine, Karolinska Institutet, Stockholm, Sweden; Department of Infectious Diseases, Karolinska University Hospital Stockholm, Sweden.
Martin Jovanovic-Stjernqvist, Division of Infection Medicine, Department of Clinical Sciences Lund, Lund University, Lund, Sweden.
Malin Hagstrand Aldman, Division of Infection Medicine, Department of Clinical Sciences Lund, Lund University, Lund, Sweden; Department of Infectious Diseases, Skåne University Hospital Lund, Sweden.
David Krus, Division of Infection Medicine, Department of Clinical Sciences Lund, Lund University, Lund, Sweden; Department of Infectious Diseases, Skåne University Hospital Lund, Sweden.
Jonas Öberg, Division of Infection Medicine, Department of Clinical Sciences Lund, Lund University, Lund, Sweden; Department of Infectious Diseases, Helsingborg Hospital, Helsingborg, Sweden.
Fredrik Kahn, Division of Infection Medicine, Department of Clinical Sciences Lund, Lund University, Lund, Sweden; Department of Infectious Diseases, Skåne University Hospital Lund, Sweden.
Anna Bläckberg, Division of Infection Medicine, Department of Clinical Sciences Lund, Lund University, Lund, Sweden; Department of Infectious Diseases, Skåne University Hospital Lund, Sweden.
Torgny Sunnerhagen, Division of Infection Medicine, Department of Clinical Sciences Lund, Lund University, Lund, Sweden; Clinical Microbiology and Infection Control, Region Skåne Office for Medical Services, Lund, Sweden.
Magnus Rasmussen, Division of Infection Medicine, Department of Clinical Sciences Lund, Lund University, Lund, Sweden; Department of Infectious Diseases, Skåne University Hospital Lund, Sweden.
Notes
Acknowledgment. The authors acknowledge all coauthors of the studies that this reanalysis was based on.
Financial support. This work was supported by the Region Halland Research Council (research funding to H. L.), the Foundation of Sparbanken Varberg (research funding to H. L.), the Swedish Government Funds for Clinical Research (ALF) (to M. R.), and Skåne University Hospital (research funds to M. R.).
References
- 1. Durack DT, Lukes AS, Bright DK; Duke Endocarditis Service . New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Am J Med 1994; 96:200–9. [DOI] [PubMed] [Google Scholar]
- 2. Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30:633–8. [DOI] [PubMed] [Google Scholar]
- 3. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36:3075–128. [DOI] [PubMed] [Google Scholar]
- 4. Fowler VG, Durack DT, Selton-Suty C, et al. The 2023 Duke-ISCVID criteria for infective endocarditis: updating the modified Duke criteria. Clin Infect Dis 2023; 77:518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Palraj BR, Baddour LM, Hess EP, et al. Predicting Risk of Endocarditis Using a Clinical Tool (PREDICT): scoring system to guide use of echocardiography in the management of Staphylococcus aureus bacteremia. Clin Infect Dis 2015; 61:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kahn F, Resman F, Bergmark S, et al. Time to blood culture positivity in Staphylococcus aureus bacteraemia to determine risk of infective endocarditis. Clin Microbiol Infect 2021; 27:1345.e7–e12. [DOI] [PubMed] [Google Scholar]
- 7. Tubiana S, Duval X, Alla F, et al. The VIRSTA score, a prediction score to estimate risk of infective endocarditis and determine priority for echocardiography in patients with Staphylococcus aureus bacteremia. J Infect 2016; 72:544–53. [DOI] [PubMed] [Google Scholar]
- 8. Chamat-Hedemand S, Bruun NE, Ostergaard L, et al. Proposal for the use of echocardiography in bloodstream infections due to different streptococcal species. BMC Infect Dis 2021; 21:689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chamat-Hedemand S, Dahl A, Ostergaard L, et al. Prevalence of infective endocarditis in streptococcal bloodstream infections is dependent on streptococcal species. Circulation 2020; 142:720–30. [DOI] [PubMed] [Google Scholar]
- 10. Sunnerhagen T, Törnell A, Vikbrant M, Nilson B, Rasmussen M. HANDOC: a handy score to determine the need for echocardiography in non-beta-hemolytic streptococcal bacteremia. Clin Infect Dis 2018; 66:693–8. [DOI] [PubMed] [Google Scholar]
- 11. Berge A, Krantz A, Östlund H, Nauclér P, Rasmussen M. The DENOVA score efficiently identifies patients with monomicrobial Enterococcus faecalis bacteremia where echocardiography is not necessary. Infection 2019; 47:45–50. [DOI] [PubMed] [Google Scholar]
- 12. Bouza E, Kestler M, Beca T, et al. The NOVA score: a proposal to reduce the need for transesophageal echocardiography in patients with enterococcal bacteremia. Clin Infect Dis 2015; 60:528–35. [DOI] [PubMed] [Google Scholar]
- 13. Dahl A, Lauridsen TK, Arpi M, et al. Risk factors of endocarditis in patients with Enterococcus faecalis bacteremia: external validation of the NOVA score. Clin Infect Dis 2016; 63:771–5. [DOI] [PubMed] [Google Scholar]
- 14. Lindberg H, Löfstrom E, Rasmussen M. Risk stratification score screening for infective endocarditis in patients with gram-positive bacteraemia. Infect Dis (Lond) 2022; 54:488–96. [DOI] [PubMed] [Google Scholar]
- 15. Hagstrand Aldman M, Pahlman LI. Evaluation of penicillin G susceptibility testing methods for Staphylococcus lugdunensis. J Antimicrob Chemother 2020; 75:1206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krus D, Kahn F, Nilson B, Sunnerhagen T, Rasmussen M. Blood culture time to positivity in non-beta-hemolytic streptococcal bacteremia as a predictor of infective endocarditis—a retrospective cohort study. Eur J Clin Microbiol Infect Dis 2022; 41:325–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Öberg J, Nilson B, Gilje P, Rasmussen M, Inghammar M. Bacteraemia and infective endocarditis with Streptococcus bovis-Streptococcus equinus-complex: a retrospective cohort study. Infect Dis (Lond) 2022; 54:760–5. [DOI] [PubMed] [Google Scholar]
- 18. Berge A, Kronberg K, Sunnerhagen T, Nilson BHK, Giske CG, Rasmussen M. Risk for endocarditis in bacteremia with Streptococcus-like bacteria: a retrospective population-based cohort study. Open Forum Infect Dis 2019; 6:ofz437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bläckberg A, Lundberg K, Svedevall S, Nilson B, Rasmussen M. Time to positivity of blood cultures in bloodstream infections with Streptococcus dysgalactiae and association with outcome. Infect Dis (Lond) 2023; 55:333–9. [DOI] [PubMed] [Google Scholar]
- 20. Berge A, Morenius C, Petropoulos A, Nilson B, Rasmussen M. Epidemiology, bacteriology, and clinical characteristics of HACEK bacteremia and endocarditis: a population-based retrospective study. Eur J Clin Microbiol Infect Dis 2020; 40:525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rasmussen M, Berge A. Revision of the diagnostic criteria for infective endocarditis is needed—please do it thoroughly. Clin Infect Dis 2023; 76:2041–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dahl A, Iversen K, Tonder N, et al. Prevalence of infective endocarditis in Enterococcus faecalis bacteremia. J Am Coll Cardiol 2019; 74:193–201. [DOI] [PubMed] [Google Scholar]
- 23. Park JH, Jung J, Kim MJ, et al. Incidence, clinical characteristics, and outcomes of Streptococcus dysgalactiae subspecies equisimilis bacteremia in a tertiary hospital: comparison with S. agalactiae bacteremia. Eur J Clin Microbiol Infect Dis 2019; 38:2253–8. [DOI] [PubMed] [Google Scholar]
- 24. Trell K, Sendi P, Rasmussen M. Recurrent bacteremia with Streptococcus dysgalactiae: a case-control study. Diagn Microbiol Infect Dis 2016; 85:121–4. [DOI] [PubMed] [Google Scholar]
- 25. Nevanlinna V, Huttunen R, Aittoniemi J, Luukkaala T, Rantala S. Incidence, seasonal pattern, and clinical manifestations of Streptococcus dysgalactiae subspecies equisimilis bacteremia; a population-based study. Eur J Clin Microbiol Infect Dis 2023; 42:819–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sunnerhagen T, Senneby E, Rasmussen M. Microorganisms that commonly cause infective endocarditis—what about Aerococcus in the Duke-ISCVID criteria? Clin Infect Dis 2023; 77:1217–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.