Abstract
Background
Numerous prognostic scores have been published to support risk stratification for patients with coronavirus disease 2019 (COVID-19).
Methods
We performed a systematic review to identify the scores for confirmed or clinically assumed COVID-19 cases. An in-depth assessment and risk of bias (ROB) analysis (Prediction model Risk Of Bias ASsessment Tool [PROBAST]) was conducted for scores fulfilling predefined criteria ([I] area under the curve [AUC)] ≥ 0.75; [II] a separate validation cohort present; [III] training data from a multicenter setting [≥2 centers]; [IV] point-scale scoring system).
Results
Out of 1522 studies extracted from MEDLINE/Web of Science (20/02/2023), we identified 242 scores for COVID-19 outcome prognosis (mortality 109, severity 116, hospitalization 14, long-term sequelae 3). Most scores were developed using retrospective (75.2%) or single-center (57.1%) cohorts. Predictor analysis revealed the primary use of laboratory data and sociodemographic information in mortality and severity scores. Forty-nine scores were included in the in-depth analysis. The results indicated heterogeneous quality and predictor selection, with only five scores featuring low ROB. Among those, based on the number and heterogeneity of validation studies, only the 4C Mortality Score can be recommended for clinical application so far.
Conclusions
The application and translation of most existing COVID scores appear unreliable. Guided development and predictor selection would have improved the generalizability of the scores and may enhance pandemic preparedness in the future.
Keywords: COVID-19, scores, prediction models, predictors, pandemic preparedness
This systematic review of clinical coronavirus disease 2019 (COVID-19) scores revealed a large quantity and heterogeneity of their quality and predictor composition. We exhibit challenges for transferability across care settings. The results may support score implementation and future pandemic preparedness.
The coronavirus disease 2019 (COVID-19) pandemic has created a state of emergency in health systems across the globe [1]. Hospitals were overcrowded with patients and decisions for their management had to be made quickly. At the same time resource constraints limit the treatment of all patients with adequate therapies. Even in 2023, when the pandemic transitioned into an endemic state [2], the dynamically evolving variants of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) still cause severe disease in individuals, regardless of immunity, vaccines, and therapeutic interventions [3, 4], especially when they are of elevated age or have comorbidities [5].
Especially during the first wave of the pandemic, scientists and clinicians rushed their efforts to support decision making, often trying to define thresholds of defined symptoms or scores. Such clinical prognostic scores are derived from models that estimate an individual's probability for a particular condition by combining and weighting predictive factors, mainly in an easy-to-apply manner (eg, additive point systems). Compared to a more complex, information-intense, and accurate (statistical) outcome prediction model, a score is a clinical decision support tool that facilitates fast applicability and unambiguous communication. Clinicians use such scores as “prediction rules” daily to reduce severe outcomes by modifying therapeutic considerations according to given risks [6, 7]. Although clinical judgments remain irreplaceable [5], a score's validity, reliability, and trustworthiness depend on the quality criteria applied during development and adequate validation. Scores can be developed for different scenarios (eg, predicting in-hospital mortality after admission or hospitalization at diagnosis) [7], making their application relevant in different settings.
Although numerous predictive models for COVID-19 were published [8–10], most are of heterogeneous methodological quality or remain unvalidated. The scores were not universally implemented in everyday clinical care and treatment instructions. The current Infectious Diseases Society of America (IDSA) guideline (05/2023) [11] does not recommend a specific tool for outcome prognosis. The World Health Organization's (WHO) guideline on Therapeutics and COVID-19 (01/2023) [4] reported that reliable tools are needed, especially for using available medication. Although it mentioned the ISARIC's (International Severe Acute Respiratory and emerging Infection Consortium) 4C Mortality Score (4C) [12], the “need for better evidence on prognosis” is emphasized [4]. The WHO's Living Guidance for clinical management of COVID-19 (01/2023) also suggests “clinical judgment […] rather than currently available prediction models” [13]. In summary, evidence for prognostic scores is poor [8–10], and the translation into clinical practice remains elusive. At the same time, the need for reliable stratification tools is emphasized in COVID-19 guidelines [4, 13].
In our systematic review, we focus on the critical appraisal of predictors and the transferability of clinical scores to support implementation in routine care. We aim to identify scores for daily clinical care, provide an effective overview for decision-makers, and pave the way for future pandemic preparedness.
METHODS
Systematic Review Question, Inclusion, and Exclusion Criteria
For this systematic review, we identified the COVID-19 prognostic clinical scores developed from the onset of the pandemic. We included original scores designed or modified for the management of COVID-19 based on individual patient data from clinically assumed or confirmed COVID-19 cases. We did not preselect publications on specific patient care levels, timings of predictor measurement, predictor types, or targeted specific outcomes. We excluded regression or other prediction models unsuitable for scoring, predictors based on single observations, scores focusing on specific subpopulations (eg, comorbidities, pharmaceutical trials), and mathematical virus transmission simulations. In the first step, we extracted information from all identified studies (termed “all scores”) that fulfilled the primary inclusion criteria (see Table 1). Second, we selected scores for an in-depth analysis (Level 2 [L2]) based on predefined criteria: (I) area under the curve (AUC) ≥ 0.75, (II) a separate validation cohort, (III) training data from a multicenter setting (≥ 2 centers), and (IV) the result of the score mapped on a point scale (for details see Supplementary Text 1). Only scores fulfilling the L2 criteria were further evaluated for risk of bias (ROB). The other scores were assigned to Level 1 (L1).
Table 1.
Inclusion and Exclusion Criteria for the Selection of Literature
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
Abbreviation: COVID-19, coronavirus disease 2019.
aA radiological score without further combination with other clinical predictors was considered a single predictor.
Data Sources, Search Strategy, and Data Extraction
We searched MEDLINE and Web of Science on 14 April 2022 and 20 February 2023 using a prespecified search strategy combining domains regarding “COVID-19”, “Prediction”, “Scoring” and “Validation metrics” (Supplementary Text 2). Our processing was based on the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [14]. For the extracted information, see Supplementary Text 3. All literature processing tasks, including screening, data extraction, and ROB assessment were independently performed by two reviewers (K. A., R. G.). In case of disagreement, consensus was reached by discussion.
If not stated otherwise, the unit of analysis was one score per outcome and predictor set. We also provide an overview of external validations identified by an ad hoc search in the same literature retrieval with a reduced set of extraction items.
Extracted AUCs are presented with range or median and interquartile range (IQR); categorical information is reported in absolute numbers and percentages (n (%)). The sample size was evaluated using the (estimated) events per variable (EPV), with low EPVs indicating a higher risk for overfitting (see Supplementary Text 3) [15].
Outcomes and Categorization of Scores
Based on the identified literature, the following outcomes were present: fatal outcomes (in-hospital mortality, death within specified time intervals), disease severity (classified as composite outcomes, eg, need for mechanical ventilation, intensive care unit [ICU] admission, or death), hospitalization, and the post-COVID condition (PCC). We categorized the scores by the type of outcome and the timing of predictor measurement (Table 2).
Table 2.
Categories by Timing of Predictor Measurement and Outcome
| No. | Category |
|---|---|
| 1 | First/early contact with healthcare facility ➝ Fatal outcome |
| 2 | First/early contact with healthcare facility ➝ Deterioration (severity, ICU admission, need for mechanical ventilation, respiratory complication, specific organ failures, or fatal outcome as a composite endpoint, etc.) |
| 3 | Severe disease or ICU admission ➝ Deterioration or fatal outcome |
| 4 | First diagnosis and contact with outpatient healthcare facility ➝ Hospitalization |
| 5 | Acute infection ➝ PCC |
Contact with healthcare facility also includes hospital or emergency department (ED) admission or the first diagnosis by SARS-CoV-2 testing. Abbreviations: ICU, intensive care unit; PCC, post-COVID-19 condition; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Risk of Bias Assessment
Flaws in a study's design, conduct, or analysis methods can cause systematic errors (bias) of effect estimates. The Prediction model Risk Of Bias Assessment Tool (PROBAST) specifies the adequacy of methods when developing a clinical prediction rule by assessing the ROB within its four subdomains: “participants,” “predictors,” “outcome,” and “analysis.” The ratings “low,” “unclear,” or “high” evaluate the validity of the study and condense to an overall ROB. A “high” rating within at least 1 question or subdomain leads to an overall ROB of “high” [15].
RESULTS
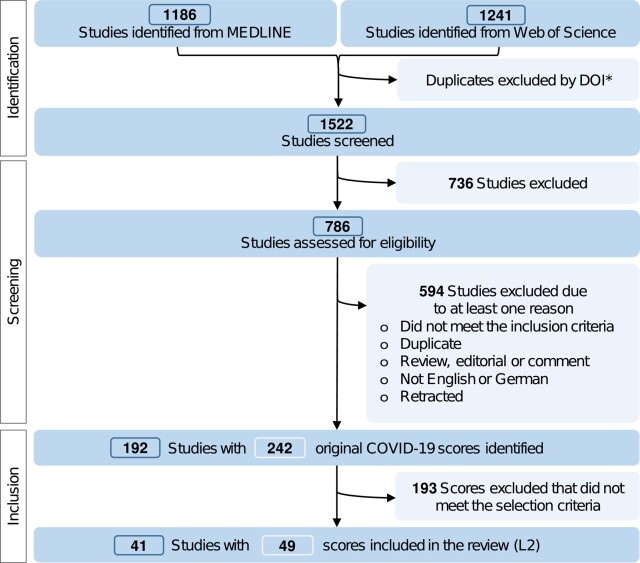
The PRISMA flow chart (Figure 1) shows the literature evaluation procedure. Of 1522 studies extracted from the database, 242 original COVID-19 scores met the primary inclusion criteria, and 49 met the L2 criteria (details for all scores in Supplementary Table 1 and L2 in Supplementary Table 2). Comparative summary statistics matching this section are presented in Table 3.
Figure 1.
PRISMA flow chart. *Not every study had a DOI or had multiple DOIs. Abbreviations: COVID-19, coronavirus disease 2019; DOI, digital object identifier; PRISMA, Preferred Reporting Items for Systematic reviews and Meta-Analyses.
Table 3.
Characteristics of the Included Scores
| Characteristics | All | Level | |
|---|---|---|---|
| N = 242 n (%)e |
Level 1d, N = 193 n (%)e |
Level 2, N = 49 n (%)e |
|
| Category | |||
| 1 First/early contact with healthcare facility ➝ Fatal outcome | 100 (41.3) | 79 (40.9) | 21 (42.9) |
| 2 First/early contact with healthcare facility ➝ Deterioration | 112 (46.3) | 94 (48.7) | 18 (36.7) |
| 3 Severe disease or ICU admission ➝ Deterioration or fatal outcome | 13 (5.4) | 12 (6.2) | 1 (2.0) |
| 4 First diagnosis and contact with outpatient healthcare facility ➝ Hospitalization | 14 (5.8) | 5 (2.6) | 9 (18.4) |
| 5 Acute infection ➝ PCC | 3 (1.2) | 3 (1.6) | 0 (0.0) |
| Study design | |||
| Prospective | 33 (13.6) | 30 (15.5) | 3 (6.1) |
| Retro- and prospective | 12 (5.0) | 3 (1.6) | 9 (18.4) |
| Retrospective | 182 (75.2) | 150 (77.7) | 32 (65.3) |
| Unknown | 15 (6.2) | 10 (5.2) | 5 (10.2) |
| Multicenter design | |||
| ≥ 2 centers | 103 (42.9) | 54 (28.3) | 49 (100.0) |
| Samples size | |||
| Cumulative number of participants ≥1000 | 87 (36.0) | 47 (24.4) | 40 (81.6) |
| Estimated events per variablea (median, IQR) | … | … | 15.6 (IQR = [6.6, 267.3]) |
| Health sector | |||
| Hospitals/emergency department | 216 (89.6) | 182 (94.8) | 34 (69.4) |
| In- or outpatient sites | 16 (6.6) | 3 (1.6) | 13 (26.5) |
| Outpatient sites | 7 (2.9) | 5 (2.6) | 2 (4.1) |
| Other | 2 (0.8) | 2 (1.0) | 0 (0.0) |
| Population | |||
| Patients in the emergency department | 25 (10.3) | 18 (9.3) | 7 (14.3) |
| Inpatients with severe disease | 37 (15.3) | 35 (18.1) | 2 (4.1) |
| Inpatients without restriction to specific conditionsb | 158 (65.3) | 132 (68.4) | 26 (53.1) |
| Inhabitants of one region | 1 (0.4) | 1 (0.5) | 0 (0.0) |
| Out- and inpatients | 11 (4.5) | 2 (1.0) | 9 (18.4) |
| Outpatients | 10 (4.1) | 5 (2.6) | 5 (10.2) |
| Study/recruitment time | |||
| 2020 | … | … | 38 (77.6) |
| 2020–2021 | … | … | 4 (8.2) |
| 2020–2022 | … | … | 7 (14.3) |
| Country | |||
| China | 45 (18.6) | 39 (20.2) | 6 (12.2) |
| Italy | 25 (10.3) | 24 (12.4) | 1 (2.0) |
| United States | 33 (13.6) | 18 (9.3) | 15 (30.6) |
| Other | 139 (57.4) | 112 (58.0) | 27 (55.1) |
| Timing of predictor measurement | |||
| Admission to hospital or emergency department | 190 (79.8) | 159 (84.1) | 31 (63.3) |
| Admission to ICU | 7 (2.9) | 6 (3.2) | 1 (2.0) |
| SARS-CoV2 testing/diagnosis | 13 (5.5) | 12 (6.3) | 1 (2.0) |
| Other | 28 (11.8) | 12 (6.3) | 16 (32.7) |
| Outcomes | |||
| Deterioration (composite, with fatal outcomes) | 109 (45.0) | 91 (47.2) | 18 (36.7) |
| Fatal outcomes (single endpoint) | 116 (47.9) | 94 (48.7) | 22 (44.9) |
| Hospitalization | 14 (5.8) | 5 (2.6) | 9 (18.4) |
| Post-acute COVID syndrome | 3 (1.2) | 3 (1.6) | 0 (0.0) |
| Handling of missing values | |||
| Any imputation method applied | … | … | 19 (38.8) |
| Multiple imputation | … | … | 11 (22.4) |
| Modeling technique | |||
| (Cox, (Bayesian) Logistic, LASSO) Regression | … | … | 41 (83.7) |
| Machine learning | … | … | 2 (4.1) |
| Mixed methods or other | … | … | 6 (12.2) |
| Validationc | |||
| Separate cohort present | 138 (57.0) | 89 (46.1) | 49 (100) |
| Geographical validation | … | … | 10 (20.4) |
| Temporal validation | … | … | 17 (34.7) |
| Temporal and geographical validation | … | … | 7 (14.3) |
| Random split | … | … | 13 (26.5) |
| Validation with different population characteristics | … | … | 1 (2.0) |
| Independent external validation | … | … | 2 (4.1) |
| Discrimination | |||
| AUC of the strongest validation ≥ 0.75 | 190 (78.5) | 141 (73.1) | 49 (100.0) |
| AUC (median, IQR) | 0.83 (IQR = [0.77, 0.90]) | 0.84 (IQR = [0.77, 0.91]) | 0.81 (IQR = [0.80, 0.85]) |
| Calibrationc | |||
| Any method applied | … | … | 30 (61.2) |
| Calibration plot or table | … | … | 23 (46.9) |
| Hosmer-Lemeshow | … | … | 12 (24.5) |
| Application | |||
| Formula | 65 (26.9) | 65 (33.7) | 0 (0.0) |
| Points-based and formula | 172 (71.1) | 123 (63.7) | 49 (100.0) |
| Formula | 3 (1.2) | 3 (1.6) | 0 (0.0) |
| Other | 2 (0.8) | 2 (1.0) | 0 (0.0) |
We present n (%) for categorical information and the median (IQR) for continuous information. The column “All” includes all scores fulfilling the a priori inclusion criteria. In contrast, Level 1 merely includes scores that did not fulfill the selection criteria and Level 2 only includes the scores fulfilling the criteria (see Methods section). As a result of two granularity levels of data extraction, some information is only available for Level 2 scores.
Abbreviations: AUC, area under the curve; COVID, coronavirus disease; ICU, intensive care unit; IQR, interquartile range; PCC, post-COVID-19 condition; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aEvents per variable (EPV) were estimated using the absolute number of candidate predictors. Some studies did not precisely name the number of candidate predictors. To generate assumptions regarding the sample size, we counted predictors indicated as candidates in tables or texts (signed by “∼” in Supplementary Table 2), even though we acknowledge that using the number of regression coefficients instead is more precise [15].
bRegarding population characteristics, “severe disease” includes ICU patients and patients with respiratory complications, pneumonia, intubation, or other severe conditions.
cMultiple options possible.
dLevel 1 (L1) includes those scores among “all” scores that did not fulfill the Level 2 selection criteria.
eOr median with IQR.
Data Basis and General Study Characteristics (All Scores)
All studies were published between 2020 and 2023. Most scores were developed based on cohorts with <1000 participants (64.0%) in a retrospective (75.2%) and/or single-center (57.1%) design. Fifty-seven percent of the models were validated in a separate cohort, including random splits, temporal, or geographical (external) validation. The median AUC was 0.83 (IQR = [0.77, 0.90]).
The study populations included hospitalized cases without restriction to specific conditions (65.3%), patients with severe disease (15.3%), or patients admitted to the emergency department (ED) (10.3%). The major timing for prediction was admission to hospital or emergency department (ED) (79.8%). Predicted outcomes (all scores) were mortality (45.0%), severity (as composite endpoints) (47.9%), hospitalization (5.8%), and PCC (1.2%).
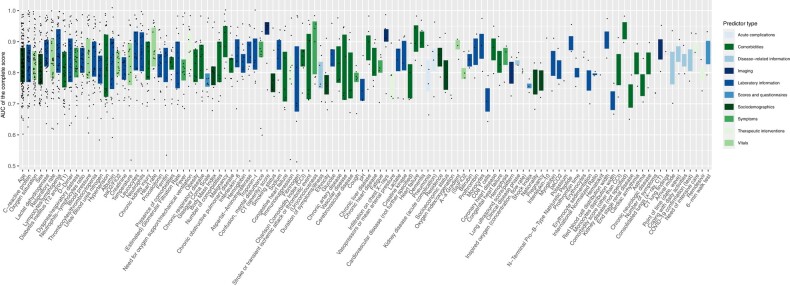
Among the 188 different predictors (extracted from all scores), age (68.2%) was the most frequently included, followed by C-reactive protein (CRP) (29.8%). This also applied to mortality or severity scores, where the importance of laboratory data, demographics, and physiological information stood out. Hospitalization scores often included age (87.8%) and dyspnea (57.1%) (for the top 20 predictors in each category, see Supplementary Table 3). The number of predictors per score ranged from two to 29. Figure 2 shows the frequency of predictor use in relation to the overall AUC of the scores. We also present the predictor domains by score, category, and inclusion level (Figure 3, Supplementary Figures 1 and 2).
Figure 2.
Relationship between predictor frequency within all scores (irrespective of category) and AUC of the overall score. The predictors were grouped by predictor type. Only predictors that were integrated at least twice in a score are presented. Abbreviations: AUC, area under the curve; COVID-19, coronavirus disease 2019.
Figure 3.
Predictor composition aggregated by predictor type for all scores assigned to category 1 stratified by the level of selection. A, Level 1, B, Level 2. The AUC is displayed on the right y-axis. The sorting of the scores is determined by (I) the absolute number of categories and (II) the relative proportion across all scores. The color gradient from green to blue indicates the availability of the category, although in case of doubt, this also depends on the level of care. Similar presentations of scores assigned to categories 2–4 are displayed in the Supplementary Material. Abbreviations: AUC, area under the curve; COVID-19, coronavirus disease 2019.
Characteristics of Scoring Systems Selected According to Predefined (Quality) Criteria (Level 2)
The most frequent outcomes among L2 scores were mortality as single endpoint (44.9%) or severity as composite outcome (36.7%). Among pure mortality and severity scores, 0.4%–51.2% and 3.7%–51.6% of the patients in the development cohorts reached the outcome, respectively. The estimated EPV ranged from 0.9 to 709.8 (eEPV < 10: 47.5%). The scores had a median AUC of 0.81 (IQR = [0.80, 0.87]).
Nine scores predicting hospitalization (18.4%) met the L2 criteria (outcomes: 4.0%–38.9%). The scores profited from larger sample sizes, with EPVs ranging from 15.6 to 120.7. The median AUC was 0.84 (IQR = [0.80, 0.85]).
The scores predicting PCC primarily used symptom information. None of them met the L2 criterium AUC ≥ 0.75 and were therefore not further analyzed.
Risk of Bias
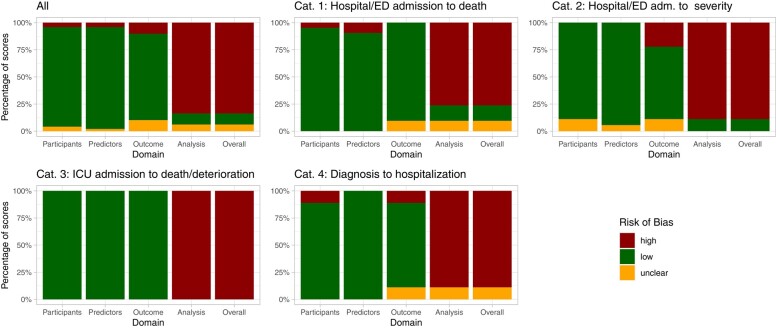
Many studies did not adhere to general guidance for developing predictive models [7, 15, 16], so that information relevant to their evaluation was unavailable. Most scores raised at least one concern within one of the PROBAST domains, leading to an overall high ROB (low 10.2%, unclear 6.1%, high 83.7%) (Figure 4, Supplementary Table 4). The primary concerns pertained especially to the “analysis” domain, namely, the absence of calibration measures (eg, adaptation of the relation of estimated and observed event probabilities) [17], failure to account for over-optimism (which could be met by, eg, bootstrapping or cross-validation) [15, 18], mishandling of missing values (eg, use of complete case analysis instead of imputation methods) [15], and insufficient validation techniques (eg, using random splits instead of geographical (external) validation) [15].
Figure 4.
Results of the ROB assessment using PROBAST. Abbreviations: ED, emergency department; ICU, intensive care unit; ROB, risk of bias.
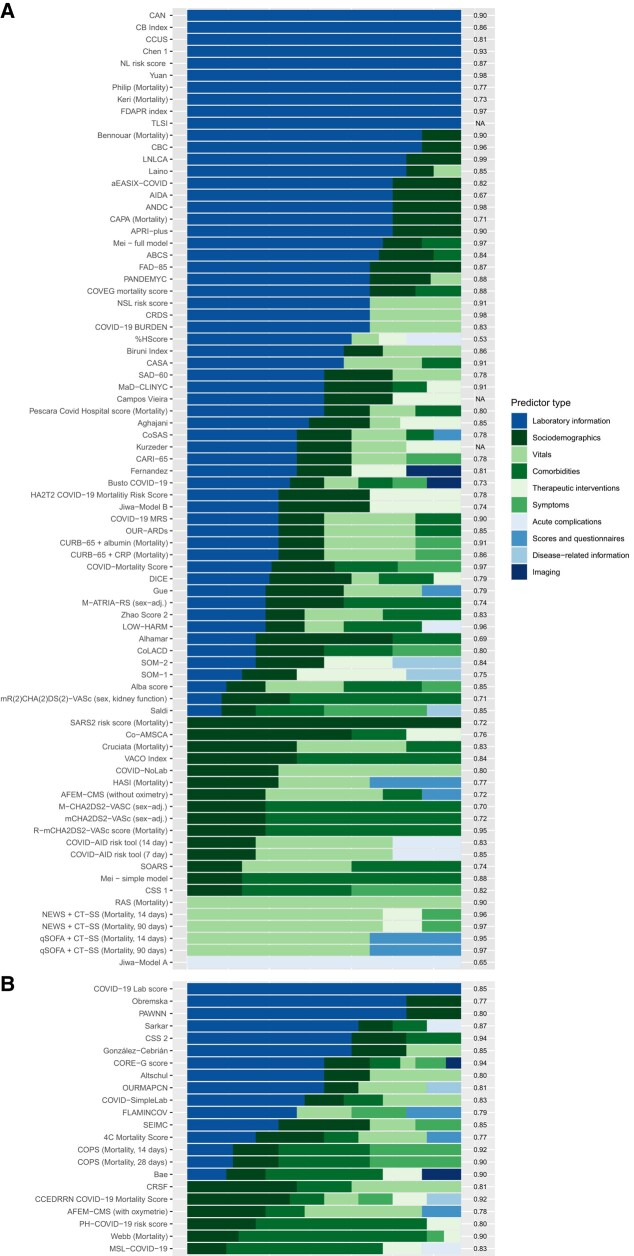
Table 4 shows scores with an overall ROB rating of “low” or “unclear.” Five scores were rated “low” ROB: (1) the ISARIC's 4C [12], (2) the CCEDRRN COVID-19 Mortality Score [20], (3) the SEIMC score [25] for the prediction of mortality, (4) the PRIEST score [23], and (5) the LMIC-PRIEST [21] for severity prognosis. Three more scores were rated with “unclear” ROB (AFEM [19], OURMAPCN [22], SARS2 [24]). These scores have common characteristics: their cumulative sample size was comparably large (except AFEM), and missing value imputation (except SEIMC) and calibration measures (except AFEM) were applied.
Table 4.
Characteristics of Scores With Low or Unclear Risk of Bias (ROB) Rating
| Score | Reference | Study Design | Population | Outcome | Year(s)a | Cumulative Sample Size; No. of Outcomesb |
Final Predictors (No.; Description) |
Strongest Type of Validation Reported | Performance of Strongest Type of Validation Reported AUC (95%-CI) |
Overall ROB |
|---|---|---|---|---|---|---|---|---|---|---|
| 4C Mortality Score | Knight et al [12] | Prospective observational cohort | Inpatients with “high likelihood” of COVID-19 | Mortality (in-hospital) | 2020 | 35 463 + 22 361; 11 426 |
n = 8: age, sex, number of comorbidities, RR, SaO2, GCS, urea level, CRP | Temporal validation with geographic subsetting | 0.77 (.76–.77) | Low |
| AFEM-CMS—with SaO2 | Pigoga et al [19] | Retrospective observational cohort | Inpatients with suspected, probable, or confirmed COVID-19 | Mortality (in-hospital) | 2020 | 374 + 93; 239* |
n = 7; sex, age, number of comorbidities, GCS, systolic BP, RR, SaO2 | Random split with cross-validation | 0.78 (.74–.81) | Unclear |
| CCEDRRN COVID-19 Mortality Score | Hohl et al [20] | Retrospective observational cohort | ED patients with confirmed or suspected COVID-19 | Mortality (in-hospital/ED) | 2020–2021 | 6758 + 2054; 471 |
n = 8, age, sex, type of residence, arrival mode, chest pain, severe liver disease, RR, level of respiratory support | Geographical validation (same country, different centers) | 0.92 (.90–.93) | Low |
| LMIC-PRIEST | Marincowitz et al [21] | Observational cohort study | ED patients with suspected or confirmed COVID | Mortality (in-hospital), intubation, NIV or ICU admission (30 d) | 2020–2022 | 305 564 + 140 520 + 20 698; 12 610 | n = 11; RR, SaO2, heart rate, systolic BP, temperature, alertness, inspired oxygen, sex, age, diabetes, heart disease | Geographical validation (other country) | 0.79 (.79–.80) | Low |
| OURMAPCN | Chen et al [22] | Retrospective observational cohort | Inpatients with confirmed COVID-19 | Mortality (in-hospital) | 2020 | 6415 + 6351 + 2169 + 553; 462 |
n = 8; CRP, SpO2, admission date, age, BUN, RR, procalcitonin, neutrophils | Geographical validation (different country) | 0.81 (.76–.86) | Unclear |
| PRIEST | Goodacre et al [23] | Retrospective observational cohort | Inpatients with suspected COVID-19 | Death or organ support (30 d) |
2020 | 11 773 + 9118; 2421 |
n = 9; age, sex, RR, systolic BP, SaO2/inspired oxygen ratio, performance status, consciousness, renal impairment, respiratory distress | Geographical validation (same country, different centers) | 0.80 (.79–.81) | Low |
| SARS2 risk score | Dashti et al [24] | Retrospective observational cohort | Out- and inpatients with confirmed COVID-19 | Hospitalization (30 d) |
2020 | 10 496 + 1851; 3197 |
n = 5; age, sex, race, socioeconomic status, smoking | Validation with different population (medical staff) | 0.77 (.73–.80) | Unclear |
| SEIMC | Berenguer et al [25] | Retrospective observational cohort | Inpatients with confirmed COVID-19 | Mortality (30 d) | 2020 | 4035 + 2126; 1047 |
n = 6; age, SaO2, NLR, GFR, dyspnea, sex | Temporal and geographical validation (same country, different centers) | 0.85 (.82–.87) | Low |
Further information on the selected set of scores and all scores assessed in Level 2 are presented in Supplementary Table 2. Abbreviations: AUC, area under the curve; BP, blood pressure; BUN, blood urea nitrogen; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; DC, development cohort; ED, emergency department; GCS, Glasgow Coma Scale; GFR, glomerular filtration rate; RR, respiratory rate; NIV, non-invasive ventilation; NLR, neutrophils-lymphocytes-ratio; SaO2, oxygen saturation; VC, validation cohort.
aRecruitment year.
bCumulative sample size consists of development cohort plus VC(s). Outcomes in the development cohort or the whole cohort (*) if not otherwise stated.
External Validations
Only a fraction of the scores (n = 33) were validated externally (see Supplementary Table 5). The 4C was replicated most frequently: 37 validations from 20 countries yielded a primarily robust median AUC of 0.80 (range: 0.55 to 0.93) for different outcomes. Based on our literature search, most COVID-19 scores remained unvalidated.
DISCUSSION
This systematic review investigated the quantity and quality of clinical scores predicting COVID-19 outcomes. Although numerous scores were developed specifically for this purpose, none were implemented in COVID-19 treatment guidelines [4, 11, 13] to become part of the clinical routine. Our analysis showed that most scores insufficiently adhered to the quality criteria required to ascertain validity, reliability, and trustworthiness.
Scores Identified With Low or Unclear ROB
Most scores (n = 41) were found to carry a significant ROB due to methodological choices. We identified only 5 scores with low and 3 scores with unclear ROB (Table 4).
The 4C score [12] can be recommended for prognostication of mortality, as it is based on a large, prospective cohort, makes use of widely available predictors (in high-resource settings at hospital admission), and was frequently validated (at least 37 validations in 20 countries) [26]. Although the CCEDRRN COVID-19 Mortality [20] and SEIMC scores [25] were also developed on large cohorts, the designs of their development studies are based on retrospective data, and both would profit from additional validations (CCEDRRN: 0 validations; SEIMC: 4 validations, 4 countries). We identified the PRIEST score [23] and the LMIC-PRIEST [21], developed on large, multicenter cohorts, to be potentially suitable for the prognosis of COVID-19 severity. The LMIC-PRIEST might especially be relevant for low- and middle-income countries (LMIC) [21]. Although disease severity seems more relevant nowadays, only a few validation studies exist (PRIEST: 3 validations in 3 countries; LMIC-PRIEST: 0 validations, but external validation within the original study). A broad clinical application should thus wait for further validation. In general, an adjustment to altered frequencies of COVID-19-related deaths and severe disease courses since score development (eg, by immunity and vaccination) should be investigated.
Predictor Selection, Applicability, and Complexities
Many scores included a wide range of predictors (Figure 2) from different domains (Figure 3). We may assume that data availability often impacts the predictor choices more than what is recommended by best practice guidelines [6, 27]. This heterogeneity is most likely a result of differences across studies, such as the scope of data sources used, entry criteria for analyzed cohorts, slight differences in endpoints and definitions, and statistical approaches employed. The heterogeneous clinical appearance of COVID-19 and changing vaccination statuses may have added to that heterogeneity. However, the review reveals a common set of predictors used in many scores (eg, age and CRP), whereas others were included in only 1 or very few (eg, nausea or hypotension).
Our results indicate that COVID-19 mortality or severity scores should include age, respiratory conditions, laboratory data, and comorbidities to predict outcomes reliably [10, 28]. Pre-hospital scores (eg, predicting hospital admission) primarily use information on comorbidities and sociodemographic information, applicable without diagnostic infrastructure. Overall, symptoms and imaging appeared to play a minor role. Among the 20 most frequently used predictors, 6 (age, sex, diabetes, hypertension (as part of metabolic syndrome), blood urea nitrogen (BUN), creatinine) represent components of baseline assessment for (organ-related) infection outcomes or differential diagnoses, 10 (CRP, lactate dehydrogenase, oxygen saturation, respiratory rate, neutrophils-lymphocyte ratio, lymphopenia, dyspnea, thrombocytopenia, blood pressure, paO2/FiO2, temperature, and leukocytes) are accepted markers of overall infection severity/sepsis [29–32]. In contrast, only 2 (D-dimer, albumin) may not be universally accepted as part of a baseline assessment for moderate to severe respiratory illnesses [33, 34]. It is not surprising that studies primarily confirm prior knowledge, since clinical practice is based on existing evidence. This in turn leads to selection of established markers for patient screening and thus limited availability of markers for score validation. Prospective determination of comprehensive metabolic panels might well lead to more effective models. This observation may partly be attributed to prior knowledge as a key criterion in defining the data sets that, in turn, were used in the analyses. It should be emphasized this means that the most used criteria are generally available during patient care in medium to high-resource settings. However, given the considerable overlap of predictors of general infection and severity/sepsis, it also suggests that the scores might not add much to existing knowledge on respiratory infection outcomes and are probably not very specific to COVID-19.
Non-routine laboratory indicators such as D-dimer and interleukine-6 restrict score applicability to high-resource settings [10]. However, because D-dimer belonged to the top 10 predictors (Figure 2), further studies are warranted to define the incremental value of such parameters for successful patient management. Overall, laboratory tests for scores (eg, indicators of kidney function or protein metabolism [urea, BUN, creatinine] or indicators for inflammation such as the CRP vs leucocytes) may restrict practicability to specific resource and management settings. Not all general practitioners and outpatient departments will perform comprehensive tests based on moderate respiratory symptoms [35]. The association between data availability, care setting, and regional standards is a likely source of bias that may limit transferability. Furthermore, non-conventional or time-dependent predictors such as arrival mode [20] or admission date [22] are less generalizable for validation in most cohorts. Only one score asked for the vaccination status [36], as most scores were developed on data from the early pandemic. Results from clinical trials indicate that vaccination status may be among the most critical outcome predictors today [37, 38].
Limitations of the Evidence Included in the Review
Differences in score development design may lead to varied performance [15]. Notably, we observed a substantial variation in sample sizes, settings, and case definitions. Population characteristics, including age [28, 39], ethnicity [40], and immunity influence COVID-19 outcomes. Additionally, we noted differences in preconditions for specific therapies or hospital admissions among countries [41]. Further complicating matters, the comparability of composite outcomes was limited due to the variation in the combination selected by different study groups.
Good performance measures, in combination with small sample sizes or inconsistent reporting of both discrimination and calibration measures, indicate a higher risk for overfitting [15, 42]. Regarding “all” scores, high AUCs (78.5% ≥ 0.75) often came together with relatively small sample sizes (64.0% ≤ 1000 patients). Scores should not be applied in clinical practice until validations show generalizability, applicability, and robust performances across various patient characteristics that match regional circumstances [8, 42, 43].
Comparison to Other Studies
With the abundance of published models and scores, identifying “all” relevant items is demanding. Therefore, complementary approaches are needed. We identified a few reviews on COVID-19 predictions or scores, all focusing on different approaches and yielding a (slightly) different set of models, both overall and in terms of low ROB [8, 9, 44, 45]. The 4C score [12], the PRIEST model [23] and the NEWS2 were repeatedly discussed as favorable prognostic tools.
Limitations of the Review Process
We restricted the detailed analysis to scores that fulfilled predefined criteria; thus, the L2 results refer to scores not representative of “all” scores. A broader approach, including additional sources, might have revealed further relevant studies. We did not contact the studies' authors for missing information and used a restricted Checklist for critical Appraisal and data extraction for systematic Reviews of prediction Modelling Studies (CHARMS) checklist [7] (see Supplementary Text 3) focused on aspects considered most relevant to our research question. Well-established early warning scores were not within our scope but are reported to have a robust performance in validation studies [46].
CONCLUSION
Our study is a comprehensive analysis of COVID-19 scores regarding predictor assessment and applicability. Most scores exhibited a marked ROB and lacked external validation. In future pandemics, data and resource sharing alongside the application of recommended model development and reporting guidelines [6, 7, 15, 16] would improve score quality and visibility, leading to better implementation for the benefit of the patients.
With currently 3 years of COVID-19 investigations at data retrieval, we also recognize the absence of reliable scoring systems for the prognosis of PCC. Because outcomes have continuously improved since the first wave of the pandemic, many experts consider PCC has surpassed severe illness and death as a health hazard. Reliable predictors of poor long-term outcomes would be an asset for decision making and the design of future clinical trials.
In conclusion, none of the numerous scores that have been developed received strong guideline recommendations on an international level. The current consensus is that the predictive tools for COVID-19 are helpful but should only support and not replace physician's judgments.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Katharina S Appel, Department II of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt am Main, Germany.
Ramsia Geisler, Department II of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt am Main, Germany.
Daniel Maier, Department II of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt am Main, Germany; German Cancer Consortium (DKTK), Partner Site Frankfurt/Mainz and German Cancer Research Center (DKFZ), Heidelberg, Germany.
Olga Miljukov, Institute of Clinical Epidemiology and Biometry, University of Würzburg, Würzburg, Germany.
Sina M Hopff, University of Cologne, Faculty of Medicine and University Hospital Cologne, Department I of Internal Medicine, Center for Integrated Oncology Aachen Bonn Cologne Duesseldorf, Cologne, Germany, University of Cologne.
J Janne Vehreschild, Department II of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt am Main, Germany; University of Cologne, Faculty of Medicine and University Hospital Cologne, Department I of Internal Medicine, Cologne, Germany; German Centre for Infection Research (DZIF), partner site Bonn-Cologne, Cologne, Germany.
Notes
Author Contributions. J. J. V. conceptualized, and J. J. V. and K. A. designed the study. K. A. and R. G. performed the systematic review including title, abstract, and full text screening and the PROBAST assessment in duplicate. K. A., D. M., R. G., O. M., and J. J. V. interpreted the findings. J. J. V. and S. M. H. supported with clinical knowledge regarding the content of the review. K. A. drafted the manuscript, and all authors critically reviewed all materials and approved the final manuscript.
Financial support. This work was supported by ORCHESTRA. The ORCHESTRA project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 101016167. The views expressed in this publication are the sole responsibility of the author, and the Commission is not responsible for any use that may be made of the information it contains. This publication was supported by the German Federal Ministry of Education and Research (BMBF) Network of University Medicine 2.0: “NUM 2.0” (grant number 01KX2121), Project: National Pandemic Cohort Network (NAPKON).
References
- 1. Filip R, Puscaselu RG, Anchidin-Norocel L, Dimian M, Savage WK. Global challenges to public health care systems during the COVID-19 pandemic: a review of pandemic measures and problems. J Pers Med 2022; 12:1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Biancolella M, Colona VL, Mehrian-Shai R, et al. COVID-19 2022 update: transition of the pandemic to the endemic phase. Hum Genomics 2022; 16:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shrestha LB, Foster C, Rawlinson W, Tedla N, Bull RA. Evolution of the SARS-CoV-2 Omicron variants BA.1 to BA.5: implications for immune escape and transmission. Rev Med Virol 2022; 32:e2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Therapeutics and COVID-19: living guideline, 13 January 2023. Geneva: World Health Organization, 2023. (WHO/2019-nCoV/therapeutics/2023.1). Licence: CC BY-NC-SA 3.0 IGO. [PubMed] [Google Scholar]
- 5. Long B, Carius BM, Chavez S, et al. Clinical update on COVID-19 for the emergency clinician: presentation and evaluation. Am J Emerg Med 2022; 54:46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cowley LE, Farewell DM, Maguire S, Kemp AM. Methodological standards for the development and evaluation of clinical prediction rules: a review of the literature. Diagn Progn Res 2019; 3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moons KGM, de Groot JAH, Bouwmeester W, et al. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLoS Med 2014; 11:e1001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wynants L, van Calster B, Collins GS, et al. Prediction models for diagnosis and prognosis of COVID-19: systematic review and critical appraisal. BMJ 2020; 369:m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miller JL, Tada M, Goto M, et al. Prediction models for severe manifestations and mortality due to COVID-19: a rapid systematic review. Acad Emerg Med 2022; 29:206–16. [DOI] [PubMed] [Google Scholar]
- 10. Chu K, Alharahsheh B, Garg N, Guha P. Evaluating risk stratification scoring systems to predict mortality in patients with COVID-19. BMJ Health Care Inform 2021; 28:e100389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Infectious Diseases Society of America . Guidelines on the treatment and management of patients with COVID-19. 2023. Available at: https://www.idsociety.org/COVID19guidelines. Accessed 21 September 2023.
- 12. Knight SR, Ho A, Pius R, et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: development and validation of the 4C Mortality Score. BMJ 2020; 370:m3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clinical management of COVID-19: living guideline, 13 January . Geneva: World Health Organization, 2023. 2023 (WHO/2019-nCoV/clinical/2023.1). Licence: CC BY-NC-SA 3.0 IGO. [PubMed] [Google Scholar]
- 14. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moons KGM, Wolff RF, Riley RD, et al. PROBAST: a tool to assess risk of bias and applicability of prediction model studies: explanation and elaboration. Ann Intern Med 2019; 170:W1–W33. [DOI] [PubMed] [Google Scholar]
- 16. Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ 2015; 350:g7594. [DOI] [PubMed] [Google Scholar]
- 17. van Calster B, McLernon DJ, van Smeden M, Wynants L, Steyerberg EW. Calibration: the Achilles heel of predictive analytics. BMC Med 2019; 17:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ramspek CL, Jager KJ, Dekker FW, Zoccali C, van Diepen M. External validation of prognostic models: what, why, how, when and where? Clin Kidney J 2021; 14:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pigoga JL, Omer YO, Wallis LA. Derivation of a contextually-appropriate COVID-19 mortality scale for low-resource settings. Ann Glob Health 2021; 87:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hohl CM, Rosychuk RJ, Archambault PM, et al. The CCEDRRN COVID-19 mortality score to predict death among nonpalliative patients with COVID-19 presenting to emergency departments: a derivation and validation study. CMAJ Open 2022; 10:E90–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marincowitz C, Hodkinson P, McAlpine D, et al. LMIC-PRIEST: derivation and validation of a clinical severity score for acutely ill adults with suspected COVID-19 in a middle-income setting. PLoS One 2023; 18:e0287091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen Z, Chen J, Zhou J, et al. A risk score based on baseline risk factors for predicting mortality in COVID-19 patients. Curr Med Res Opin 2021; 37:917–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goodacre S, Thomas B, Sutton L, et al. Derivation and validation of a clinical severity score for acutely ill adults with suspected COVID-19: the PRIEST observational cohort study. PLoS One 2021; 16:e0245840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dashti H, Roche EC, Bates DW, Mora S, Demler O. SARS2 Simplified scores to estimate risk of hospitalization and death among patients with COVID-19. Sci Rep 2021; 11:4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Berenguer J, Borobia AM, Ryan P, et al. Development and validation of a prediction model for 30-day mortality in hospitalised patients with COVID-19: the COVID-19 SEIMC score. Thorax 2021; 76:920–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. de Vito A, Colpani A, Saderi L, et al. Is the 4C score still a valid item to predict in-hospital mortality in people with SARS-CoV-2 infections in the Omicron variant era? Life 2023; 13:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schummers L, Himes KP, Bodnar LM, Hutcheon JA. Predictor characteristics necessary for building a clinically useful risk prediction model: a simulation study. BMC Med Res Methodol 2016; 16:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marin BG, Aghagoli G, Lavine K, et al. Predictors of COVID-19 severity: a literature review. Rev Med Virol 2021; 31:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016; 315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ewig S, Kolditz M, Pletz M, et al. Management of adult community-acquired pneumonia and prevention (AWMF-register-nr. 020-020). Pneumologie 2021; 75:665–72.34198346 [Google Scholar]
- 31. Cornberg M, Sandmann L, Protzer U, et al. S3-Leitlinie der Deutschen gesellschaft für gastroenterologie, verdauungs- und stoffwechselkrankheiten (DGVS) zur prophylaxe, diagnostik und therapie der hepatitis-B-virusinfektion, AWMF-Register-Nr. 021-11. Z Gastroenterol 2021; 59:691–776. [DOI] [PubMed] [Google Scholar]
- 32. Beyer G, Hoffmeister A, Michl P, et al. S3-Leitlinie pankreatitis—leitlinie der deutschen gesellschaft für gastroenterologie, verdauungs- und stoffwechselkrankheiten (DGVS), September 2021— – AWMF Registernummer 021-003. Z Gastroenterol 2022; 60:419–521. [DOI] [PubMed] [Google Scholar]
- 33. Han Y-Q, Yan L, Zhang L, et al. Performance of D-dimer for predicting sepsis mortality in the intensive care unit. Biochem Med 2021; 31:20709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yuwen P, Chen W, Lv H, et al. Albumin and surgical site infection risk in orthopaedics: a meta-analysis. BMC Surg 2017; 17:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ebell MH, Cai X, Lennon R, et al. Development and validation of the COVID-NoLab and COVID-SimpleLab risk scores for prognosis in 6 US health systems. J Am Board Fam Med 2021; 34:127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang Y, Han J, Sun F, et al. A practical scoring model to predict the occurrence of critical illness in hospitalized patients with SARS-CoV-2 omicron infection. Front Microbiol 2022; 13:1031231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. El Sahly HM, Baden LR, Essink B, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med 2021; 385:1774–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med 2020; 383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bellou V, Tzoulaki I, van Smeden M, Moons KGM, Evangelou E, Belbasis L. Prognostic factors for adverse outcomes in patients with COVID-19: a field-wide systematic review and meta-analysis. Eur Respir J 2022; 59:2002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mackey K, Ayers CK, Kondo KK, et al. Racial and ethnic disparities in COVID-19–related infections, hospitalizations, and deaths: a systematic review. Ann Intern Med 2021; 174:362–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bentivegna M, Hulme C, Ebell MH. Primary care relevant risk factors for adverse outcomes in patients with COVID-19 infection: a systematic review. JABFM 2021; 34:113–26. [DOI] [PubMed] [Google Scholar]
- 42. Alba AC, Agoritsas T, Walsh M, et al. Discrimination and calibration of clinical prediction models: users' guides to the medical literature. JAMA 2017; 318:1377–84. [DOI] [PubMed] [Google Scholar]
- 43. Shamsoddin E. Can medical practitioners rely on prediction models for COVID-19? A systematic review. Evid Based Dent 2020; 21:84–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Buttia C, Llanaj E, Raeisi-Dehkordi H, et al. Prognostic models in COVID-19 infection that predict severity: a systematic review. Eur J Epidemiol 2023; 38:355–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lombardi Y, Azoyan L, Szychowiak P, et al. External validation of prognostic scores for COVID-19: a multicenter cohort study of patients hospitalized in Greater Paris University Hospitals. Intensive Care Med 2021; 47:1426–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gupta RK, Marks M, Samuels THA, et al. Systematic evaluation and external validation of 22 prognostic models among hospitalised adults with COVID-19: an observational cohort study. Eur Respir J 2020; 56:2003498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.