Abstract
Background
The 2023 Duke–International Society of Cardiovascular Infectious Diseases (ISCVID) criteria for infective endocarditis (IE) were introduced to improve classification of IE for research and clinical purposes. External validation studies are required.
Methods
We studied consecutive patients with suspected IE referred to the IE team of Amsterdam University Medical Center (from October 2016 to March 2021). An international expert panel independently reviewed case summaries and assigned a final diagnosis of “IE” or “not IE,” which served as the reference standard, to which the “definite” Duke-ISCVID classifications were compared. We also evaluated accuracy when excluding cardiac surgical and pathologic data (“clinical” criteria). Finally, we compared the 2023 Duke-ISCVID with the 2000 modified Duke criteria and the 2015 and 2023 European Society of Cardiology (ESC) criteria.
Results
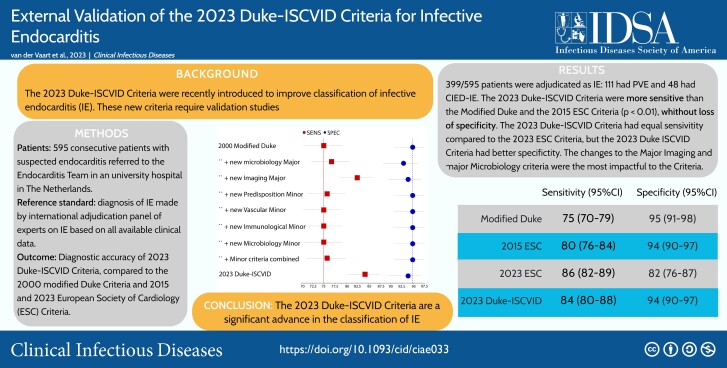
A total of 595 consecutive patients with suspected IE were included: 399 (67%) were adjudicated as having IE; 111 (19%) had prosthetic valve IE, and 48 (8%) had a cardiac implantable electronic device IE. The 2023 Duke-ISCVID criteria were more sensitive than either the modified Duke or 2015 ESC criteria (84.2% vs 74.9% and 80%, respectively; P < .001) without significant loss of specificity. The 2023 Duke-ISCVID criteria were similarly sensitive but more specific than the 2023 ESC criteria (94% vs 82%; P < .001). The same pattern was seen for the clinical criteria (excluding surgical/pathologic results). New modifications in the 2023 Duke-ISCVID criteria related to “major microbiological” and “imaging” criteria had the most impact.
Conclusions
The 2023 Duke-ISCVID criteria represent a significant advance in the diagnostic classification of patients with suspected IE.
Keywords: infective endocarditis, diagnosis, Duke criteria, validation
In a cohort of 595 patients with suspected infective endocarditis (IE), the 2023 Duke-ISCVID criteria had better sensitivity than the 2000 modified Duke and 2015 European Society of Cardiology (ESC) criteria for IE and better specificity than the 2023 ESC criteria.
Graphical Abstract
Graphical Abstract.
This graphical abstract is also available at Tidbit: https://tidbitapp.io/tidbits/external-validation-of-the-2023-duke-international-society-for-cardiovascular-infectious-diseases-diagnostic-criteria-for-infective-endocarditis/update
(See the Invited Commentary by Chambers et al. on pages 964–7.)
Infective endocarditis (IE) is often difficult to diagnose. To ensure comparability of data sets, von Reyn et al in 1981 [1] advocated the use of case definitions with strict criteria to diagnose IE. In 1994, Durack et al [2] extensively revised these criteria to improve sensitivity, while maintaining specificity. These criteria became known as the “Duke criteria” and were validated by multiple cohort studies using varying populations and reference standards [3–8].
In 2000, the criteria were updated as the “modified Duke criteria,” which have become the diagnostic standard in both clinical and research settings [9–13]. Since then, the epidemiology of and diagnostic approach to IE have changed. For example, cardiac implantable electronic devices (CIEDs) became more common, transcatheter aortic valve implantation was introduced, and newer diagnostic imaging techniques, such as fluorine 18–fludeoxyglucose (18F-FDG) positron emission tomography (PET)/computed tomography (CT) and cardiac CT, have been improved and widely implemented [14, 15]. The 2015 European Society of Cardiology (ESC) guidelines for the diagnosis and treatment of IE included 18F-FDG PET/CT and cardiac CT results as major criteria in order to improve on the modified Duke criteria [16], with the 2023 ESC criteria providing further update on these criteria [17].
The 2023 Duke–International Society of Cardiovascular Infectious Diseases (ISCVID) criteria aim to provide further improvement in the diagnosis of IE by updating and expanding multiple aspects of the prior modified Duke criteria [18]. Validation studies are needed to inform users of the real-world strengths and limitations of the 2023 Duke-ISCVID criteria. The goal of the current investigation was to evaluate the diagnostic accuracy of the 2023 Duke-ISCVID criteria and compare it with that of the 2000 modified Duke criteria and the 2015 and 2023 ESC criteria.
METHODS
Study Design
We used a consecutive series of patients referred to the Amsterdam University Medical Center (UMC) IE team between October 2016 and March 2021. To complete the data set for the current investigation, a new data extraction was performed from the electronic health records in May 2023. This project was exempted from formal ethical approval by the institutional review board of the Amsterdam UMC because all analyses involved routinely available and deidentified data. This report was prepared according to the 2015 Standards for Reporting Diagnostic Accuracy studies guideline [19].
Participants
All patients aged ≥18 years with suspected or confirmed IE were referred to the multidisciplinary IE team and subsequently recorded in its registry. Patients could be referred to the IE team with any level of suspicion for IE by attending or consulting physicians. Patients could also be referred by outside hospitals for academic or surgical consultation on cases not directly admitted to the Amsterdam UMC.
Data Sources, Variables, and Procedures
All information for evaluation by the 2023 Duke-ISCVID, 2023 ESC, 2015 ESC, and 2000 modified Duke classification (“definite,” “possible,” or “rejected” IE) was derived from patient records. Information on demographic and clinical characteristics, treatment and outcome was collected by the coordinating investigator and trained collaborators. Each patient was followed for up to 1 year after the initial evaluation by the IE team, and relapses of bloodstream infection or new IE with the same pathogen were recorded. Episodes of IE with a new pathogen, or with the same pathogen after >1 year, were recorded as a new case.
Definitions
For each criteria set (2000 modified Duke, 2015 ESC, 2023 ESC, and 2023 Duke-ISCVID), we used the definitions for definite, possible, and rejected IE, as well as their definitions of major and minor criteria, as described elsewhere [16–18, 20]. Comorbidity was classified using the Charlson comorbidity index. Alternative and concurrent diagnoses (eg, vertebral osteomyelitis or marantic endocarditis) were based on the report of the local IE team and treating physician. For each case, 2 classifications were made: one with all available data including results from surgery and, if applicable, postmortem examination, and another using only the clinical data available before surgery or death (the “clinical” criteria).
Reference Standard
The clinical diagnosis of IE is based on a collection of symptoms and test results, but it can only be diagnosed with certainty by direct examination of the infected endocardium at surgery or necropsy, preferably in the acute phase. This definitive reference standard is impossible to obtain for many patients, and using pathologic results alone would result in considerable selection bias. To overcome this lack of a usable reference standard, we implemented an adjudication committee to determine a final diagnosis of “IE” or “not IE” in each case.
Adjudication Panel
The adjudication panel consisted of an international panel of 12 experienced clinicians, all experts in the field of IE (D. T. D., L. M. B., A. S. B., E. D. M., T. L. H., A. W. K., J. M. M., P. M., M. R., C. S. S., V. G. F., and J. T. M. v. d. M.). The panel adjudicated cases as described elsewhere [21]; each case was independently adjudicated by 2 experts. When there was agreement, the diagnosis was accepted as final. In the event of disagreement, a third adjudicator ruled on the diagnosis. Each adjudicator had the option to request additional clinical information from the study coordinator (T. W. v. d. V.). Adjudication occurred independently and anonymously, and cases were randomly assigned. Adjudicators were aware of the study purpose and were specifically instructed to use their overall clinical knowledge, reasoning and “gestalt,” and to not apply any version of diagnostic criteria for IE.
Experts used an anonymized electronic case record form that contained essential variables to attain their final diagnosis. The written instructions to the adjudication committee members and a printout of the adjudication form are provided as Supplementary Materials. Adjudicators were also asked to specify whether they would treat or not treat for IE in each case, to quantify treatment variation and diagnostic uncertainty. Culture results, clinical variables, and outcomes could be missing for cases referred from other hospitals. Details on handling of missing data are provided in the Supplementary Materials.
Statistical Analysis
We calculated the sensitivity and specificity of the full 2023 Duke-ISCVID, 2023 ESC, 2015 ESC, and 2000 modified Duke criteria compared with the reference standard provided by the adjudication panel. For analysis purposes, “definite IE” was selected as a positive test result, while both “possible IE” and “rejected IE” constituted a negative test result. We also calculated the same measures for the clinical criteria, where surgical and pathologic findings were excluded. Diagnostic accuracy measures were calculated, and 95% exact binomial confidence intervals were obtained. Differences in sensitivity and specificity between criteria sets were tested using the McNemar test.
False-negative cases—for which the adjudication panel diagnosis was IE but the 2023 Duke-ISCVID criteria classification was possible or rejected IE—were explored descriptively, as were cases where the panel diagnosis was “no IE” but the Duke-ISCVID classification was definite IE (false-positives). Agreement between adjudicators was assessed using Cohen κ values. To evaluate the added value of the changes proposed by the 2023 Duke-ISCVID criteria, we compared the sensitivity and specificity of the 2000 modified Duke criteria alone and with the respective changes to these criteria.
We also assessed the diagnostic value of each 2023 Duke-ISCVID major and minor criterion by removing them from the full set of criteria and calculating the resulting diagnostic accuracy measures and true-positive rates. All statistical analyses were done using R software, version 4.2.2 [22]. The epiR and DTComPair R software packages were used for the main analyses.
Sensitivity and Subgroup Analyses
We performed 3 separate sensitivity analyses: (1) excluding patients who did not undergo transesophageal echocardiography (TEE), (2) excluding those without surgical and/or postmortem results, and (3) considering both definite and possible IE using 2023 Duke-ISCVID criteria as a positive test result. For subgroup analyses, we calculated sensitivity and specificity of the 4 criteria sets separately for patients with prosthetic valves and patients with CIED.
Power Calculation and Sample Size
Including the 595 patients in the registry, assuming a 65% prevalence of IE, a sensitivity of 90%, and a specificity of 95%, would give us 90% power to detect a difference of 5% for sensitivity and 78% power to detect a difference of 5% for specificity of the 2023 Duke-ISCVID criteria compared with other criteria sets. A 2-sided α value of 5% was set for determination of statistical significance.
RESULTS
Patient Characteristics
Between October 2016 and March 2021, 595 patients were referred to the Amsterdam UMC IE team and were included in the current analysis (Table 1). Of those patients, 285 were directly admitted to the Amsterdam UMC, 226 were transferred from an outside hospital, and 84 were referred for academic or surgical consultation but were not admitted to the Amsterdam UMC. One-year follow-up was complete for 398 patients (67%); the median follow-up duration in patients alive at last contact but without complete 1-year follow-up was 47 days (interquartile range, 7–127 days) since first discussion by the IE team. Blood cultures were collected in all patients, 558 (94%) underwent transthoracic echocardiography at least once, and 445 (75%) underwent TEE at least once; only 7 patients (1%) did not undergo echocardiography. 18F-FDG PET/CT and cardiac CT were performed in 331 (56%) and 136 (23%) of the patients, respectively.
Table 1.
Epidemiologic and Clinical Characteristics
| Characteristic | Patients by Diagnosis, No. (%)a | |
|---|---|---|
| IE (n = 399) | No IE (n = 196) | |
| Demographics and medical history | ||
| Age, median (IQR), y | 67 (56–75) | 63 (54–73) |
| Male sex | 294 (73.7) | 127 (64.8) |
| Charlson comorbidity index, median (IQR) | 2.0 (1–4) | 2.0 (1–4) |
| Congenital heart disease | 35 (8.8) | 12 (6.1) |
| Previous IE | 42 (10.5) | 13 (6.6) |
| Active injection drug use | 7 (1.8) | 1 (0.5) |
| Native valve disease | 105 (26.3) | 47 (24.0) |
| Prosthetic heart valve | 162 (40.6) | 55 (28.1) |
| Aortic | 111 (27.8) | 36 (18.4) |
| TAVR | 25 (6.3) | 4 (2.0) |
| Mitral | 36 (9.0) | 22 (11.2) |
| Pulmonic | 11 (2.8) | 3 (1.5) |
| Tricuspid | 7 (1.8) | 3 (1.5) |
| CIED | 76 (19.0) | 27 (13.8) |
| Clinical characteristics | ||
| Feverb | 291 (72.9) | 116 (59.2) |
| Janeway lesionsc | 23 (5.8) | 1 (0.5) |
| Osler nodesc | 13 (3.3) | 1 (0.5) |
| Roth spotsd | 4 (1.0) | 4 (2.0) |
| Cerebral emboli | 66 (16.5) | 12 (6.1) |
| Splenic emboli | 31 (7.8) | 3 (1.5) |
| Vertebral osteomyelitis | 27 (6.8) | 15 (7.7) |
| Causative microorganism | ||
| Streptococcus species | 121 (30.3) | 13 (6.6) |
| Staphylococcus aureus | 98 (24.6) | 73 (37.2) |
| Coagulase-negative staphylococci | 34 (8.5) | 16 (8.2) |
| Enterococcus species | 39 (9.8) | 11 (5.6) |
| HACEK group | 14 (3.5) | 2 (1.0) |
| Culture negative | 27 (6.8) | 53 (27.0) |
| Other | 66 (16.5) | 28 (14.3) |
| Diagnostic procedures and management | ||
| TTE | 374 (93.7) | 184 (93.9) |
| TEE | 337 (84.5) | 108 (55.1) |
| 18F-FDG PET/CT | 226 (56.6) | 105 (53.6) |
| Cardiac CT | 114 (28.6) | 23 (11.7) |
| Surgery | 144 (36.1) | 13 (6.6) |
| Duration of antimicrobial treatment, median (IQR), d | 42.0 (34–48) | 26.0 (12–43) |
| Relapse infection with same pathogen within 1 y | 17 (4.3) | 2 (1.0) |
| 2023 Duke-ISCVID criteria | ||
| Major criteria | ||
| Predisposing condition | 281 (70.4) | 106 (54.1) |
| Imaging | 363 (91.0) | 38 (19.4) |
| Microbiology | 313 (78.4) | 98 (50.0) |
| Minor criteria | ||
| Fever | 291 (72.9) | 116 (59.2) |
| Vascular phenomena | 131 (32.8) | 45 (23.0) |
| Immunologic phenomena | 28 (7.0) | 11 (5.6) |
| Microbiology | 43 (10.8) | 36 (18.4) |
Abbreviations: 18F-FDG, fluorine 18 fludeoxyglucose; CIED, cardiac implantable electronic device; CT, computed tomography; HACEK, Haemophilus, Aggregatibacter, Cardiobacterium, Eikenella, Kingella; IE, infective endocarditis; IQR, interquartile range; ISCVID, International Society of Cardiovascular Infectious Diseases; PET, positron emission tomography; TAVR, transcatheter aortic valve replacement; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography.
aData represent no. (%) of patients unless otherwise specified.
bData missing in 34 patients.
cData missing in 40 patients.
dData missing in 429 patients (no fundoscopy performed).
IE Diagnosis
The adjudication panel concluded that 399 (67%) patients had IE. Patient characteristics are given in Table 1. Native valve IE was present in 198 patients (33%), prosthetic valve IE in 111 (19%), and CIED IE in 48 (8%); 42 patients (7%) had another cardiac nidus or negative imaging findings. In 521 cases (88%), both adjudicators agreed on the diagnosis, while in 74 (12%) the opinion of a third adjudicator was needed. Interrater variability was good (Cohen κ = 0.72). The most common diagnoses in the 196 patients (33%) adjudicated as “no IE” were gram-positive bacteremia with an alternative focus (n = 71) and gram-positive bacteremia without an alternative focus (n = 44) (Table 2).
Table 2.
Alternative Diagnoses in 196 Patients Without Infective Endocarditis
| Diagnosis | Patients, No (%) |
|---|---|
| CIED pocket or device infection, without IE | 4 (2) |
| Fever after cardiac surgery | 7 (4) |
| Fever of unknown origin | 7 (4) |
| Gram-negative sepsis with alternative focus | 6 (3) |
| Gram-negative sepsis without alternative focus | 2 (1) |
| Gram-positive sepsis with alternative focus | 71 (36) |
| Gram-positive sepsis without alternative focus | 44 (22) |
| Marantic endocarditis | 2 (1) |
| Multiple strokes of suspected cardiac origin | 6 (3) |
| Native valve abnormalities (new regurgitation, new valvular mass, or degenerative valve disease) | 20 (10) |
| Other | 15 (11) |
| Prosthetic valve dehiscence | 12 (6) |
Abbreviations: CIED, cardiac implantable electronic device; IE, infective endocarditis.
Diagnostic Accuracy of the 2023 Duke-ISCVID Criteria
With the adjudication panel diagnosis as the reference standard, the sensitivity of the “full” 2023 Duke-ISCVID criteria (including surgical and pathologic) was 84.2% (95% confidence interval, 80.3%–87.7%), and the specificity was 93.9% (89.6%–96.8%). Compared with the 2015 ESC criteria, the 2023 Duke ISCVID criteria were more sensitive (P < .001), with equal specificity (P > .99), while compared with the 2023 ESC criteria, the 2023 Duke-ISCVID criteria had similar sensitivity (P = .23) but significantly greater specificity (93.9% vs 82.1%; P < .001). Table 3 lists diagnostic accuracy measures for the 4 different criteria sets and statistical comparisons between the criteria. Supplementary Table 1 provides raw numbers per classification for each set of criteria, compared with the reference standard. Supplementary Table 2 provides the interval likelihood ratios for each classification.
Table 3.
Diagnostic Accuracy of Criteria Sets Compared With Reference Standard: “Full” Criteriaa
| Criteria | Sensitivity (95% CI), % | Specificity (95% CI), % | NPV (95% CI), % | PPV (95% CI), % | P Valueb | |
|---|---|---|---|---|---|---|
| Sensitivity vs Duke-ISCVID Sensitivity | Specificity vs Duke-ISCVID Specificity | |||||
| Modified Duke criteria | 74.9 (70.4–79.1) | 94.9 (90.8–97.5) | 65.0 (59.2–70.6) | 96.8 (94.1–98.4) | <.001 | .16 |
| 2015 ESC criteria | 80.0 (75.7–83.8) | 93.9 (89.6–96.8) | 69.7 (63.8–75.2) | 96.4 (93.8–98.1) | <.001 | >.99 |
| 2023 ESC criteria | 85.5 (81.6–88.8) | 82.1 (76.1–87.2) | 73.5 (67.2–79.2) | 90.7 (87.3–93.4) | .22 | <.001 |
| Duke-ISCVID criteria | 84.2 (80.3–87.7) | 93.9 (89.6–96.8) | 74.5 (68.6–79.8) | 96.6 (94.1–98.2) | … | … |
Abbreviations: CI, confidence interval; ESC, European Society of Cardiology; ISCVID, International Society of Cardiovascular Infectious Diseases; NPV, negative predictive value; PPV, negative predictive value.
aDiagnostic accuracy with adjudication panel as the reference standard. “Full” criteria include histologic and microbiological results obtained from cardiac surgery. The absolute numbers for each classification-diagnosis combination are listed in Supplementary Table 1.
b P values based on McNemar test statistics [23].
Diagnostic Accuracy of Clinical Criteria
When excluding surgical and pathologic results, the sensitivity and specificity of the 2023 Duke-ISCVID criteria were 79% (95% confidence interval, 74.6%–82.3%) and 93.9% (89.6%–96.8%), respectively (Table 4). Again, the 2023 Duke-ISCVID and 2023 ESC criteria had statistically comparable sensitivity (P = .09), but specificity was better for the 2023 Duke-ISCVID criteria (P < .001).
Table 4.
Diagnostic Accuracy of Criteria Sets Compared With Reference Standard: “Clinical” Criteriaa
| Criteria | Sensitivity (95% CI), % | Specificity (95% CI), % | NPV (95% CI), % | PPV (95% CI), % | P Valueb | |
|---|---|---|---|---|---|---|
| Sensitivity vs Duke-ISCVID Sensitivity | Specificity vs Duke-ISCVID Specificity | |||||
| Modified Duke criteria | 69.9 (65.2–74.4) | 94.9 (90.8–97.5) | 60.8 (55.1–66.3) | 96.5 (93.7–98.3) | <.001 | .16 |
| 2015 ESC criteria | 74.9 (70.4–79.1) | 93.9 (89.6–96.8) | 64.8 (58.9–70.3) | 96.1 (93.4–98.0) | <.001 | 1 |
| 2023 ESC criteria | 80.7 (76.5–84.5) | 82.1 (76.1–87.2) | 67.7 (61.3–73.6) | 90.2 (86.6–93.1) | .09 | <.001 |
| Duke-ISCVID criteria | 79.0 (74.6–82.9) | 93.9 (89.6–96.8) | 68.7 (62.7–74.2) | 96.3 (93.7–98.1) | … | … |
Abbreviations: CI, confidence interval; ESC, European Society of Cardiology; ISCVID, International Society of Cardiovascular Infectious Diseases; NPV, negative predictive value; PPV, positive predictive value.
aDiagnostic accuracy with adjudication panel as the reference standard. The “clinical” criteria exclude histologic and microbiological results obtained from cardiac surgery. The absolute numbers for each classification-diagnosis combination are listed in Supplementary Table 1.
b P values based on McNemar test statistics [23].
Sensitivity Analyses
Diagnostic accuracy results for patients who underwent TEE and surgically confirmed cases were similar to the results from the main analysis, indicating that the 2023 Duke-ISCVID criteria had improved sensitivity without a statistically significant loss in specificity compared with the previous criteria (Supplementary Tables 3A and 3B and 4A and 4B). If definite plus possible IE designations were classified as a positive test result, the 2023 Duke-ISCVID criteria had 99% sensitivity, but the specificity decreased to 21%, with a corresponding negative predictive value of 98% and positive predictive value of 72%.
Performance of the 2023 Duke-ISCVID Criteria in Selected Subgroups
The 2023 Duke-ISCVID criteria were also more sensitive than the 2000 modified Duke and 2015 ESC criteria for diagnosing IE in patients with a prosthetic valve (n = 217) and in patients with a CIED (n = 103). Full data are shown in Supplementary Table 5A and 5B.
Patients Misclassified by the 2023 Duke-ISCVID Criteria
Among the 399 patients adjudicated as having IE, 336 had definite IE according to the 2023 Duke-ISCVID criteria (true-positives). Twelve patients were classified as false-positive, and 63 as false-negative. Supplementary Table 6 details clinical and epidemiologic data stratified for true- and false-positive and for true- and false-negative cases. Of the 63 false-negative cases, 52% met the major imaging criterion of the 2023 Duke-ISCVID criteria, and 24% had macroscopic evidence of IE at cardiac surgery, albeit without histopathologic confirmation. Negative blood cultures were more common in false-negative cases (22% vs 3.9% in true-positive cases). Of the 12 patients classified as false-positive, all had gram-positive bacteremia, 5 had an alternative focus for infection, while 7 had no alternative infectious focus for bacteremia. In 8 of the 12 false-positive cases, one or both adjudicators would treat the patient for IE despite adjudicating the case as “no IE,” indicating a level of diagnostic uncertainty for these cases.
Improvement in Individual Criteria of 2023 Duke-ISCVID Criteria Compared With 2000 Modified Duke Criteria
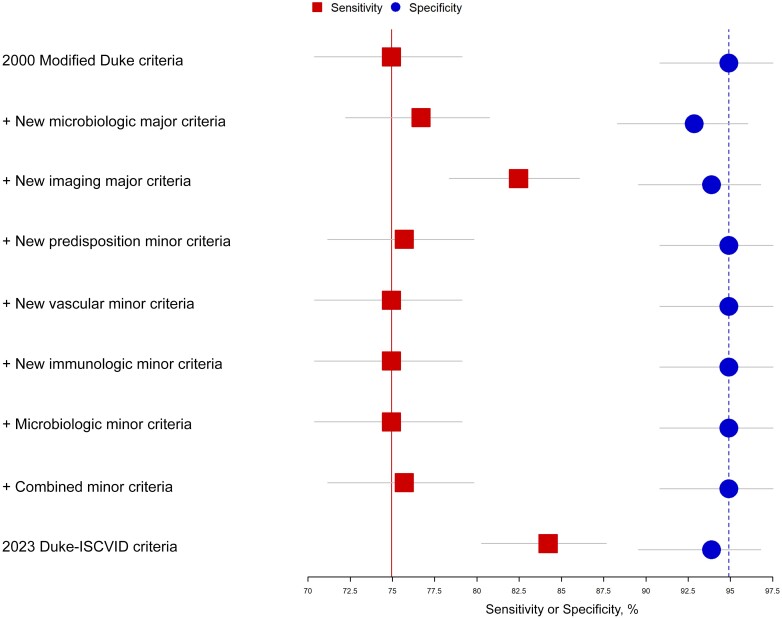
The effect of each addition of the 2023 Duke-ISCVID criteria on sensitivity and specificity, compared with the 2000 modified Duke criteria, is shown in Figure 1. The changes in “major microbiological” and “imaging” criteria resulted in the greatest increase in sensitivity, while the effects of the changes to the minor criteria were minimal.
Figure 1.
Added value of each change in the Duke–International Society of Cardiovascular Infectious Diseases (ISCVID) criteria compared with the modified Duke criteria. Each point (square for sensitivity, circle for specificity) provides the diagnostic accuracy measure for the 2000 modified Duke criteria with the addition of a specific change from the Duke-ISCVID criteria. Horizontal bars represent 95% confidence intervals for the point estimates; top points, modified Duke criteria without addition; bottom points, complete Duke-ISCVID criteria.
Individual Contribution of Each Criterion of the 2023 Duke-ISCVID Criteria to True-Positive Classifications
The major microbiological and major imaging criteria were the most important modifications for correctly classifying patients as having definite IE; removal of either criterion from the 2023 Duke-ISCVID criteria would result in a >50% reduction in patients being correctly classified as having definite IE. Modifications to the minor criteria had less impact but still resulted in establishment of a correct “definite IE” classification in 0.3%–7.4% of patients (Supplementary Table 7 and Supplementary Figure 1). Eliminating the new surgical major criterion (for direct surgical observation, without subsequent histopathologic or microbiological confirmation) did not result in loss in true-positive classifications by the 2023 Duke-ISCVID criteria.
DISCUSSION
In this cohort study, the 2023 Duke-ISCVID criteria would have resulted in improved sensitivity and similar specificity compared with both the 2000 modified Duke and the 2015 ESC criteria while also having comparable sensitivity and better specificity than the 2023 ESC criteria. Changes in the major imaging and major microbiological criteria were largely responsible for these diagnostic advancements.
Validation of diagnostic criteria for IE is challenging when a perfect reference standard, in this case histopathology for all patients, is lacking. .An expert adjudication panel was the best alternative; moreover, this approach has been used in earlier studies validating previous versions of the Duke criteria [8, 24, 25].
Our investigation provides evidence that the 2023 Duke-ISCVID criteria should replace previous versions of the Duke criteria because they have superior sensitivity and comparable specificity. While the 2023 Duke-ISCVID criteria can provide a useful framework to assist in the diagnosis of IE, clinicians should be aware that the sensitivity of the criteria is not perfect. This is apparent in the sensitivity analysis of the 2023 Duke-ISCVID clinical criteria in surgically treated patients. The sensitivity of the clinical criteria in this group was 75%, indicating that 25% of patients who will have surgically confirmed IE did not meet the clinical criteria for definite IE before surgery. Using a combination of both the definite and the possible classifications to define IE would be of limited use, as the specificity of this combination would be unacceptably low (21%). Clinicians should always consider pretest probability and the full clinical picture when deciding on treatment for individual patients, because not meeting the “definite” classification of the criteria does not mean that IE is excluded. In fact, in circumstances with a high pretest probability such as bacteremia with Streptococcus gordonii, the probability of IE in the event of a “possible IE” classification may still be >10% (Supplementary Table 8).
We identified areas for possible improvement of the criteria. The addition of the direct surgical observation as a new major criterion resulted in no additional classifications of true-positive cases. All patients fulfilling this criterion either met the major imaging criterion or had histologic or microbiological confirmation of IE with surgery, both of which excluded the use of the surgical observation major criterion. We also found that the minor criteria for immunologic phenomena rarely resulted in additional classification of true-positive cases. If these findings are confirmed in subsequent validation studies, future versions of the 2023 Duke-ISCVID criteria could potentially alter or remove this criterion. It is also noteworthy that the 2023 ESC criteria had a much lower specificity than the 2023 Duke-ISCVID criteria and older versions of the Duke criteria (82% vs 94%, respectively). This lower specificity is likely the result of the addition of findings that are not specific for IE, such as adding valve thickening as an imaging lesion characteristic of IE and adding osteoarticular infection as a vascular phenomenon [17, 26, 27].
A major strength of the current study is the relatively unselected study population, which included patients without IE, but in whom the diagnosis of IE was clinically considered. Because sensitivity and specificity can vary based on the prevalence of a disease, one should evaluate diagnostic criteria in a cohort that closely resembles the population in which they will be used [28]. We believe our cohort is an appropriate representation of this population.
This study has a number of limitations. First, only a single referral center was used. Second, the prevalence of people who inject drugs in this cohort was low (as is characteristic in the Netherlands). Third, the use of 18F-FDG PET/CT was relatively high, which will not be generalizable to many healthcare settings. Fourth, the use of an adjudication panel may have led to occasional misclassifications. This possibility however, is, inevitable when using an adjudication panel, because it is impossible to eliminate all diagnostic uncertainty. However, agreement between our expert adjudicators was high (87.6%), which bolsters our confidence in its use as a reference standard. Finally, not all patients underwent TEE, and documentation of fever and peripheral IE stigmata was missing in 7% of patients.
In conclusion, this study showed that the 2023 Duke-ISCVID criteria improve the diagnostic classification of patients with suspected IE. Pending validations of these new criteria in other cohorts, our findings support the introduction of the 2023 Duke-ISCVID criteria into current research and clinical practice.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Thomas W van der Vaart, Division of Infectious Diseases, Amsterdam University Medical Center, Universiteit van Amsterdam, Amsterdam, The Netherlands; Division of Infectious Diseases, Duke University Medical Center, Durham, North Carolina, USA; Duke Clinical Research Institute, Durham, North Carolina, USA.
Patrick M M Bossuyt, Department of Epidemiology and Data Science, Amsterdam University Medical Center, Universiteit van Amsterdam, Amsterdam, The Netherlands.
David T Durack, Division of Infectious Diseases, Duke University Medical Center, Durham, North Carolina, USA.
Larry M Baddour, Division of Public Health, Infectious Diseases and Occupational Medicine, Department of Medicine, Mayo Clinic, Rochester, Minnesota, USA; Department of Medicine and Cardiovascular Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Arnold S Bayer, Division of Infectious Diseases, The Lundquist Institute at Harbor-UCLA, Torrance, California, USA; Division of Infectious Diseases, The Geffen School of Medicine at UCLA, Los Angeles, California, USA.
Emanuele Durante-Mangoni, Department of Precision Medicine, University of Campania ‘L. Vanvitelli’, Monaldi Hospital, Naples, Italy.
Thomas L Holland, Division of Infectious Diseases, Duke University Medical Center, Durham, North Carolina, USA; Duke Clinical Research Institute, Durham, North Carolina, USA.
Adolf W Karchmer, Division of Infectious Diseases, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts, USA.
Jose M Miro, Infectious Diseases Service, Hospital Clinic-IDIBAPS, University of Barcelona, Barcelona, Spain; CIBERINFEC, Instituto de Salud Carlos III, Madrid, Spain.
Philippe Moreillon, Department of Fundamental Microbiology, UNIL—Université de Lausanne, Lausanne, Switzerland.
Magnus Rasmussen, Department of Clinical Sciences, Division of Infection Medicine, Lund University, Lund, Sweden.
Christine Selton-Suty, Centre Hospitalier Régional Universitaire (CHRU) Nancy, Cardiology Department, CIC-EC, Nancy, France; Association pour l’Étude et la Prévention de l’Endocardite Infectieuse (AEPEI), France.
Vance G Fowler, Jr, Division of Infectious Diseases, Duke University Medical Center, Durham, North Carolina, USA; Duke Clinical Research Institute, Durham, North Carolina, USA.
Jan T M van der Meer, Division of Infectious Diseases, Amsterdam University Medical Center, Universiteit van Amsterdam, Amsterdam, The Netherlands.
Notes
Acknowledgments. The authors thank the members of the Amsterdam University Medical Center IE team, without whom the database would not have been possible: Matthijs Boekholdt, Navin Bindraban, Berto Bouma, Danielle Robbers-Visser, Antoine Driessen, Robert Klautz, Wieke Freudenburg, Caroline Visser, and Bram Goorhuis.
Financial support. This work was supported by the Duke Clinical Research Institute, the Amsterdam University Medical Centers, the Universitair Medisch Centrum Utrecht, Institut d’Investigacions Biomèdiques August Pi i Sunyer, Universitat de Barcelona (IDIBAPS; personal 80:20 research grant to J. M. M., during 2017–2024), and grant 1R01-AI165671 from the National Institutes of Health (V. G. F.).
References
- 1. Von Reyn CF, Levy BS, Arbeit RD, Friedland G, Crumpacker CS. Infective endocarditis: an analysis based on strict case definitions. Ann Intern Med 1981; 94(4 pt 1):505–18. [DOI] [PubMed] [Google Scholar]
- 2. Durack DT, Lukes AS, Bright DK, Duke Endocarditis Service . New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Am J Med 1994; 96:200–9. [DOI] [PubMed] [Google Scholar]
- 3. Bayer AS, Ward JI, Ginzton LE, Shapiro SM. Evaluation of new clinical criteria for the diagnosis of infective endocarditis. Am J Med 1994; 96:211–9. [DOI] [PubMed] [Google Scholar]
- 4. Cecchi E, Parrini I, Chinaglia A, et al. New diagnostic criteria for infective endocarditis: a study of sensitivity and specificity. Eur Heart J 1997; 18:1149–56. [DOI] [PubMed] [Google Scholar]
- 5. Dodds GA 3rd, Abramson MA, Corey GR, Kisslo J, Sexton DJ. Use of the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 1995; 21:448–9. [DOI] [PubMed] [Google Scholar]
- 6. Habib G, Derumeaux G, Avierinos JF, et al. Value and limitations of the Duke criteria for the diagnosis of infective endocarditis. J Am Coll Cardiol 1999; 33:2023–9. [DOI] [PubMed] [Google Scholar]
- 7. Hoen B, Beguinot I, Rabaud C, et al. The Duke criteria for diagnosing infective endocarditis are specific: analysis of 100 patients with acute fever or fever of unknown origin. Clin Infect Dis 1996; 23:298–302. [DOI] [PubMed] [Google Scholar]
- 8. Hoen B, Selton-Suty C, Danchin N, et al. Evaluation of the Duke criteria versus the Beth Israel criteria for the diagnosis of infective endocarditis. Clin Infect Dis 1995; 21:905–9. [DOI] [PubMed] [Google Scholar]
- 9. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132:1435–86. [DOI] [PubMed] [Google Scholar]
- 10. Cecchi E, Trinchero R, Imazio M, et al. Are the Duke criteria really useful for the early bedside diagnosis of infective endocarditis? Results of a prospective multicenter trial. Ital Heart J 2005; 6:41–8. [PubMed] [Google Scholar]
- 11. Duval X, Iung B, Klein I, et al. Effect of early cerebral magnetic resonance imaging on clinical decisions in infective endocarditis: a prospective study. Ann Intern Med 2010; 152:497–504; W175. [DOI] [PubMed] [Google Scholar]
- 12. Raoult D, Casalta JP, Richet H, et al. Contribution of systematic serological testing in diagnosis of infective endocarditis. J Clin Microbiol 2005; 43:5238–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tissieres P, Gervaix A, Beghetti M, Jaeggi ET. Value and limitations of the von Reyn, Duke, and modified Duke criteria for the diagnosis of infective endocarditis in children. Pediatrics 2003; 112(6 pt 1):e467–71. [DOI] [PubMed] [Google Scholar]
- 14. Habib G, Erba PA, Iung B, et al. Clinical presentation, aetiology and outcome of infective endocarditis: results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: a prospective cohort study. Eur Heart J 2019; 40:3222–32. [DOI] [PubMed] [Google Scholar]
- 15. Murdoch DR, Corey GR, Hoen B, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 2009; 169:463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015; 36:3075–128. [DOI] [PubMed] [Google Scholar]
- 17. Delgado V, Ajmone Marsan N, de Waha S, et al. 2023 ESC guidelines for the management of endocarditis. Eur Heart J 2023; 44:3948–4042. [DOI] [PubMed] [Google Scholar]
- 18. Fowler VG, Durack DT, Selton-Suty C, et al. The 2023 Duke-ISCVID criteria for infective endocarditis: updating the modified Duke criteria. Clin Infect Dis 2023; 77:518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015; 351:h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30:633–8. [DOI] [PubMed] [Google Scholar]
- 21. van Engelen TSR, Kanglie M, van den Berk IAH, et al. Classifying the diagnosis of study participants in clinical trials: a structured and efficient approach. Eur Radiol Exp 2020; 4:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Core Team R. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2022. [Google Scholar]
- 23. Zhou X, Obuchowski N, McClish D. Comparing the accuracy of two diagnostic tests: statistical methods in diagnostic medicine. 2nd ed. Hoboken, NJ: Wiley, 2011. [Google Scholar]
- 24. Knudsen JB, Fuursted K, Petersen E, et al. Procalcitonin in 759 patients clinically suspected of infective endocarditis. Am J Med 2010; 123:1121–7. [DOI] [PubMed] [Google Scholar]
- 25. Sekeres MA, Abrutyn E, Berlin JA, et al. An assessment of the usefulness of the Duke criteria for diagnosing active infective endocarditis. Clin Infect Dis 1997; 24:1185–90. [DOI] [PubMed] [Google Scholar]
- 26. Levitt MA, Snoey ER, Tamkin GW, Gee G. Prevalence of cardiac valve abnormalities in afebrile injection drug users. Acad Emerg Med 1999; 6:911–5. [DOI] [PubMed] [Google Scholar]
- 27. Agmon Y, Khandheria BK, Meissner I, et al. Aortic valve sclerosis and aortic atherosclerosis: different manifestations of the same disease? insights from a population-based study. J Am Coll Cardiol 2001; 38:827–34. [DOI] [PubMed] [Google Scholar]
- 28. Leeflang MM, Rutjes AW, Reitsma JB, Hooft L, Bossuyt PM. Variation of a test's sensitivity and specificity with disease prevalence. CMAJ 2013; 185:E537-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.