Abstract
Background
The rVSVΔG-ZEBOV-GP vaccine (ERVEBO®) is a single-dose, live-attenuated, recombinant vesicular stomatitis virus vaccine indicated for the prevention of Ebola virus disease (EVD) caused by Zaire ebolavirus in individuals 12 months of age and older.
Methods
The Partnership for Research on Ebola VACcination (PREVAC) is a multicenter, phase 2, randomized, double-blind, placebo-controlled trial of 3 vaccine strategies in healthy children (ages 1–17) and adults, with projected 5 years of follow-up (NCT02876328). Using validated assays (GP-ELISA and PRNT), we measured antibody responses after 1-dose rVSVΔG-ZEBOV-GP, 2-dose rVSVΔG-ZEBOV-GP (given on Day 0 and Day 56), or placebo. Furthermore, we quantified vaccine virus shedding in a subset of children's saliva using RT-PCR.
Results
In total, 819 children and 783 adults were randomized to receive rVSVΔG-ZEBOV-GP (1 or 2 doses) or placebo. A single dose of rVSVΔG-ZEBOV-GP increased antibody responses by Day 28 that were sustained through Month 12. A second dose of rVSVΔG-ZEBOV-GP given on Day 56 transiently boosted antibody concentrations. In vaccinated children, GP-ELISA titers were superior to placebo and non-inferior to vaccinated adults. Vaccine virus shedding was observed in 31.7% of children, peaking by Day 7, with no shedding observed after Day 28 post-dose 1 or any time post-dose 2.
Conclusions
A single dose of rVSVΔG-ZEBOV-GP induced robust antibody responses in children that was non-inferior to the responses induced in vaccinated adults. Vaccine virus shedding in children was time-limited and only observed after the first dose. Overall, these data support the use of rVSVΔG-ZEBOV-GP for the prevention of EVD in at-risk children.
Clinical Trials Registration. The study is registered at ClinicalTrials.gov (NCT02876328), the Pan African Clinical Trials Registry (PACTR201712002760250), and the European Clinical Trials Register (EudraCT number: 2017-001798-18).
Keywords: Ebola, vaccine, pediatrics, immunogenicity, vaccine shedding
A single dose of rVSVΔG-ZEBOV-GP induced robust antibody responses in adults and children. Vaccine shedding occurred in 31.7% of children, peaking by Day 7. No shedding was observed after Day 28 post-dose 1 or after a second dose of rVSVΔG-ZEBOV-GP.
Ebola virus disease (EVD) caused by the Zaire ebolavirus (EBOV) is associated with high morbidity and mortality [1]. Past outbreaks of EVD in central and western Africa have occurred causing significant economic and social burden [2]. Vaccination to prevent EVD is a critical component of the public health response to curb EVD epidemics [3].
rVSVΔG-ZEBOV-GP (ERVEBO®) is a live-attenuated, recombinant vesicular stomatitis virus (VSV) vaccine containing the EBOV envelope glycoprotein (GP) in place of the VSV envelope gylcoprotein (G). rVSVΔG-ZEBOV-GP was found to be efficacious [4], is pre-qualified by the World Health Organization (WHO) and is approved by the Food and Drug Administration (FDA), European Medicines Agency (EMA), and numerous African countries [5]. In 2019, a single dose of rVSVΔG-ZEBOV-GP was approved for the prevention of disease caused by Zaire ebolavirus in individuals 18 years of age and older [6]. Recently, ERVEBO® was approved for pediatric populations 12 months of age and older [7, 8] who are especially vulnerable to EVD [9, 10].
The Partnership for Research on Ebola VACcination (PREVAC) is a phase 2, randomized, controlled trial of 2 leading Ebola vaccines and 3 vaccination strategies (Ad26.ZEBOV/MVABN-Filo, 1-dose rVSVΔG-ZEBOV-GP, and 2-dose rVSVΔG-ZEBOV-GP) in children ≥1 year of age and adults [11]. Primary results [12] demonstrated that a single dose of rVSVΔG-ZEBOV-GP elicited robust binding antibody responses in children and adults by Day 14 that were sustained through Month 12 and no safety concerns were identified in children receiving 1 or 2 doses of rVSVΔG-ZEBOV-GP.
At the time of initial licensure, gaps in understanding immunogenicity and vaccine virus shedding of rVSVΔG-ZEBOV-GP in children existed. Prior to PREVAC, few individuals <18 years old had been immunized in clinical trials with rVSVΔG-ZEBOV-GP. An open-label Phase 1 study in Gabon (n = 40 children ages 6–17 years) found rVSVΔG-ZEBOV-GP was immunogenic and had a similar safety profile in children compared to adults. In that study, vaccine viremia and shedding occurred more frequently in children than adults; however, the sample size was small and shedding was not assessed past Day 7 [13]. Thus, a sub-study was implemented in the PREVAC trial to expand our understanding of vaccine virus shedding in children and is reported for the first time herein.
Additionally, immunogenicity data from a single laboratory are reported for the rVSVΔG-ZEBOV-GP and matched placebo arms of PREVAC, and formal superiority and non-inferiority assessments were conducted. Antibody responses in children and adults through 12 months after 1 or 2 doses of rVSVΔG-ZEBOV-GP were determined using a validated version of the Filovirus Animal Non-Clinical Group enzyme-linked immunosorbent assay (GP-ELISA) and a validated plaque reduction neutralization test (PRNT). These immunogenicity data complement the primary paper [12] by providing confirmatory GP-ELISA results generated in a single laboratory using a validated assay and adding functional neutralizing antibody data. Furthermore, vaccine-induced GP-ELISA responses among children were formally compared to placebo and vaccinated adults based on pre-specified criteria. Finally, we evaluated the dynamics of vaccine virus shedding over a 3-month period for children receiving 1 or 2 doses of rVSVΔG-ZEBOV-GP.
METHODS
Study Design and Participants
PREVAC is a phase 2 randomized, double-blind, placebo-controlled trial conducted at 6 centers in four West-African countries [11], as previously reported [12]. Communities were engaged through social mobilization efforts as previously described [11]. Participants or their legal guardian provided written informed consent and assent (if applicable) [11]. Randomization procedures and inclusion/exclusion criteria for adults (ages ≥18) and children (ages 1–17) were previously described [11]. The study is registered at ClinicalTrials.gov (NCT02876328), was conducted in accordance with Good Clinical Practice and was approved by the ethics committees of the sponsors (INSERM-IRB00003888, LSHTM), the implementing countries (Guinea, Liberia, Mali, and Sierra Leone), and The University of Maryland, Baltimore Institutional Review Board (IRB).
The scope of the work reported herein is limited to the rVSVΔG-ZEBOV-GP and matched placebo arms only (Protocol V920-016, Version 4.0). Primary objectives reported here were to demonstrate that in children (ages 1–17), rVSVΔG-ZEBOV-GP is superior to placebo for the GP-ELISA antibody response on: (1) Day 28 post-dose 1 and (2) Month 12 after 1 or 2 doses of rVSVΔG-ZEBOV-GP. An additional primary objective was to demonstrate that rVSVΔG-ZEBOV-GP is non-inferior in children (ages 1–17) compared with adults for GP-ELISA antibody response on Day 28 post-dose 1. Secondary objectives were to demonstrate that rVSVΔG-ZEBOV-GP GP-ELISA antibody response post-dose 1 is non-inferior in children ages 3–17 and 1–17 compared to adults on Day 28 using a dependent secondary non-inferiority test (margin = 0.67) as described below.
A sub-study was conducted at the Redemption Hospital site in Liberia to estimate the proportion of children with detectable rVSVΔG-ZEBOV-GP vaccine virus in saliva by quantitative reverse transcription polymerase chain reaction (qRT-PCR) after 1 or 2 doses of rVSVΔG-ZEBOV-GP compared to placebo.
Study Vaccine
The vaccine was supplied as a sterile, aqueous, buffered solution composed of rVSVΔG-ZEBOV-GP drug product filled into single-use vials. 1-mL of rVSVΔG-ZEBOV-GP vaccine containing a minimum of 7.2 × 107 plaque-forming units (licensed dose) was administered intramuscularly in the deltoid muscle or thigh (optional for children). Placebo was matched volume of sterile 0.9% sodium chloride injection, United States Pharmacopeia. The 1-dose group received rVSVΔG-ZEBOV-GP on Day 0 and placebo on Day 56, the 2-dose group received rVSVΔG-ZEBOV-GP on Days 0 and 56, and the placebo group received placebo on Days 0 and 56.
Sample Collection
A subset of timepoints from the PREVAC trial were selected for immunogenicity assessments. Specifically, serum samples collected at: Day 0 (pre-vaccination), and post-vaccination at Day 28, Month 3, and Month 12 (Supplementary Figure 1).
From children in the vaccine shedding sub-study, approximately 0.5–1.0 mL of saliva was collected at Day 0 (pre-vaccination), Days 7, 14, 28, 56 (before dose 2), Day 63 (7 days post-dose 2), and at Month 3.
Immunogenicity
Validated assays to quantify binding antibody responses (GP-ELISA) and functional antibody responses (PRNT) were performed on gamma-irradiated serum in a central laboratory (Q2 Solutions) [14, 15] (Supplementary Methods). Formal validation was performed to demonstrate the suitability of each assay in terms of precision (intra-assay repeatability and inter-assay variance), relative accuracy/linearity, lower and upper limits of quantification, and specificity.
Vaccine Shedding
A qualified quantitative RT-PCR to measure vaccine virus shedding in saliva was performed in a centralized laboratory (Q2 Solutions). RNA was extracted with the Roche MagNa Pure 96 total nucleic acid system and a 1-step reverse transcription polymerase chain reaction (RT-PCR) was performed on an ABI QuantStudio™ 6 using a vaccine-specific primer/probe set targeting RNA sequences at the junction of the VSV matrix gene and the inserted ZEBOV GP gene. An external standard curve was used to extrapolate rVSVΔG-ZEBOV-GP RNA copies/mL in the starting sample and an internal control (MS2 phage RNA) was spiked into each sample prior to RNA extraction to verify extraction and amplification.
Statistical Analyses
The Per-Protocol (PP) population served as the primary population for the immunogenicity analyses and consisted of all randomized and vaccinated participants who did not violate inclusion/exclusion criteria or have major protocol deviations. PRNT was performed on approximately half of the participants including all available samples from children (ages 1–17) and a random sample of adults. Participants in the 1- and 2-dose rVSVΔG-ZEBOV-GP arms of PREVAC were pooled for analyses conducted at timepoints before dose 2 (given on Day 56).
Immune responses were reported as geometric mean titers (GMT), geometric mean fold increases from baseline (GMFI) and 95% confidence intervals (CI). Seroresponse rates were reported as the percentage of participants with an antibody concentration of at least 200 GP-ELISA units (EU) per milliliter (mL) and an increase from baseline by at least a factor of two [16] (as proposed to be a possible indicator of protection against EVD [17]) or a ≥ 4-fold increase from baseline (GP-ELISA and PRNT).
Analysis of GP-ELISA antibody titers for the prespecified primary and secondary immunogenicity hypotheses (superiority and non-inferiority) were conducted by log-transforming the data, performing analysis of variance (ANOVA) on the log-transformed data, and exponentiating the statistics. Participants contributing to the analyses consisted of those in the PP population who had a serum sample collected within a pre-specified day range (Supplementary Table 1). The primary non-inferiority test used a non-inferiority margin = 0.5, this 2-fold criteria was complemented by a dependent secondary non-inferiority test using a non-inferiority margin = 0.67 to bolster the stringency of the comparison. The ANOVA model included treatment group as a covariate. A fixed sequence test was used to test the four primary immunogenicity hypotheses to control the overall type 1 error at α = 0.025 (1-sided). The multiplicity adjusted power for the 4 primary hypotheses was ≥96% (0.99*0.99*0.99*0.99). Vaccine shedding was analyzed using proportions of children who had detectable Vaccine Virus RNA (defined as >0 copies/mL). Statistical analyses were performed using SAS.
RESULTS
Participant Disposition
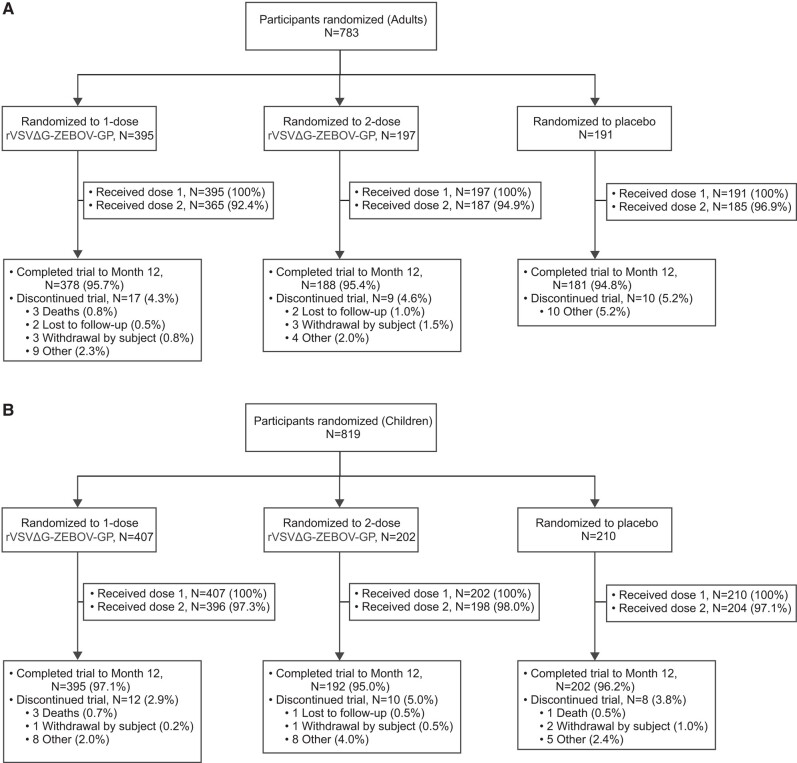
Between April and December 2018, 783 adults and 819 children were randomized to 1 or 2 doses of rVSVΔG-ZEBOV-GP or placebo (Figure 1). Of these, 1201 participants (adults and children) received rVSVΔG-ZEBOV-GP (1 or 2 doses) and 1153 (96.0%) completed the study through Month 12. The study is ongoing with a projected 5 years of follow-up. No differences in participant disposition by age (children vs adults) were observed.
Figure 1.
Participant disposition. CONSORT diagram of adults (panel A) and children (panel B) enrolled and randomized to the 1- and 2-dose rVSVΔG-ZEBOV-GP arms and the 1.0-mL placebo arm in the V4.0 PREVAC trial (NCT02876328). Shown here are only the arms analyzed in this report, see primary PREVAC study for the entire CONSORT diagram [12].
Baseline Demographics
Table 1 contains baseline demographics of participants in this study. The median age was 9 years old (range: 1–17) for children and 26 years old (range: 18–76) for adults. Approximately 50% of children were between the ages of 3 and 11 years old. At baseline, 321 (20.7%) of the 1551 PP population (adults and children) were seropositive (GP-ELISA ≥200 EU/mL) and 5 (0.6%) of 821 participants sampled had a detectable PRNT. No substantial differences in baseline serostatus were observed among children and adults.
Table 1.
Baseline Characteristics
| Adults | Children | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| rVSVΔG-ZEBOV-GP | Placebo | Total | rVSVΔG-ZEBOV-GP | Placebo | Total | |||||
| 1-dose | 2-dose | Pooleda | 1-dose | 2-dose | Pooleda | |||||
| Participants in population | 395 | 197 | 592 | 191 | 783 | 407 | 202 | 609 | 210 | 819 |
| Sex | ||||||||||
| Male | 213 (53.9%) | 110 (55.8%) | 323 (54.6%) | 110 (57.6%) | 433 (55.3%) | 222 (54.5%) | 117 (57.9%) | 339 (55.7%) | 115 (54.8%) | 454 (55.4%) |
| Female | 182 (46.1%) | 87 (44.2%) | 269 (45.4%) | 81 (42.4%) | 350 (44.7%) | 185 (45.5%) | 85 (42.1%) | 270 (44.3%) | 95 (45.2%) | 365 (44.6%) |
| Age, years | ||||||||||
| Mean | 31.1 | 29.7 | 30.6 | 30.7 | 30.6 | 8.6 | 8.2 | 8.4 | 8.3 | 8.4 |
| SD | 13 | 11.8 | 12.6 | 13 | 12.7 | 4.9 | 5.1 | 5 | 5 | 5 |
| Median | 27 | 26 | 26.5 | 26 | 26 | 9 | 8 | 9 | 8 | 9 |
| Range | 18–74 | 18–72 | 18–74 | 18–76 | 18–76 | 1–17 | 1–17 | 1–17 | 1–17 | 1–17 |
| HIV statusb | ||||||||||
| Negative | 382 (96.7%) | 195 (99.0%) | 577 (97.5%) | 188 (98.4%) | 765 (97.7%) | 407 (100.0%) | 202 (100.0%) | 609 (100.0%) | 210 (100.0%) | 819 (100.0%) |
| Positive | 13 (3.3%) | 2 (1.0%) | 15 (2.5%) | 3 (1.6%) | 18 (2.3%) | 0 | 0 | 0 | 0 | 0 |
Abbreviations: HIV, human immunodeficiency virus; SD, standard deviation.
aPooled = rVSVΔG-ZEBOV-GP 1-dose or 2-dose group.
bHIV-positive status was an exclusion criterion for participants <18 y of age.
Immunogenicity
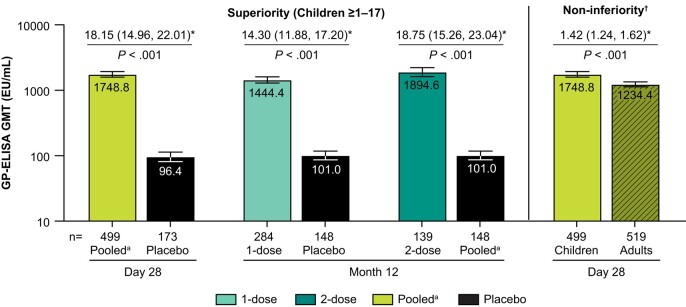
All prespecified primary immunogenicity objectives were met (Figure 2). In children (ages 1–17), the GP-ELISA GMT was superior to placebo at Day 28 post-dose 1 (P < .001) and the 1- and 2-dose GP-ELISA GMTs were superior to placebo at Month 12 (P < .001). Furthermore, at Day 28 after the first vaccination, the GP-ELISA GMT in children (ages 1–17) was non-inferior to the GP-ELISA GMT in adults (margin = 0.5, P < .001). The GMT ratio for children/adults was 1.42 (95% CI: 1.24–1.62). The secondary immunogenicity objectives were also met (Supplementary Table 2). The GP-ELISA GMT in children 3–17, and 1–17 years old were non-inferior to adults at Day 28 post-dose 1 (margin = 0.67, P < .001). The lower bound of the 2-sided 95% CI of the estimated GP-ELISA GMT ratio (children/adult) was consistently above 1.
Figure 2.
Pre-specified primary immunogenicity objectives. The bars represent GP-ELISA GMT (EU/mL) with 95% CI for the pooled arm at Day 28 (post-dose 1), and at Month 12 for the 1- and 2-dose arms, compared to placebo. GMTs from children are shown in the solid bars and GMTs from adults are shown in the hatched bars. *Estimated fold difference with 95% CI and results of hypothesis testing (superiority and non-inferiority) are shown above each bar. n = number of participants contributing to the analysis. †Non-inferiority margin =0.5. aPooled = 1- and 2-dose rVSVΔG-ZEBOV-GP arms, post-dose 1. Abbreviations: CI, confidence interval; ELISA, enzyme-linked immunosorbent assay; GMT, geometric mean titer.
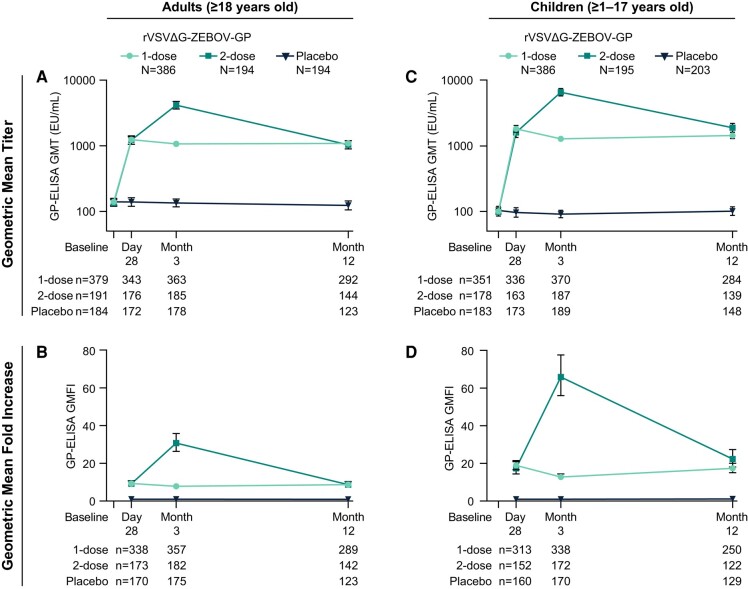
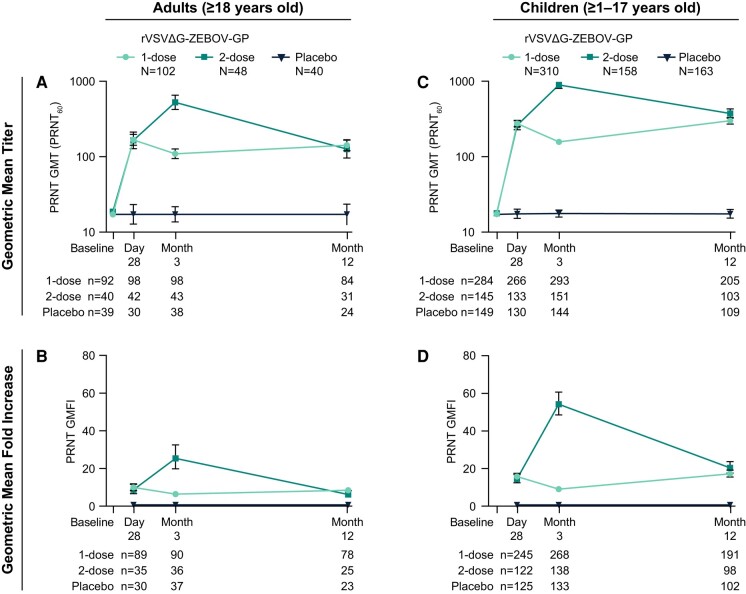
In children and adults, GP-ELISA and PRNT GMTs increased after first vaccination with rVSVΔG-ZEBOV-GP (Figures 3 and 4). By Day 28 post-vaccination, vaccine-elicited antibody responses were higher than baseline and were sustained throughout the 12-month follow-up. A second dose of rVSVΔG-ZEBOV-GP further increased antibody responses by Month 3 which subsequently declined to comparable levels elicited after a single dose. GP-ELISA titers in children at Month 12 were slightly higher among the 2-dose compared to the 1-dose group with barely non-overlapping confidence intervals.
Figure 3.
GP-ELISA antibody responses in adults and children. Binding antibody responses as assessed by GP-ELISA in adults (panels A and B) and children (panels C and D) receiving 1 dose or 2 doses of rVSVΔG-ZEBOV-GP compared to placebo. GMT (EU/mL) and GMFI are displayed with 95% CI. N = GP-ELISA Per-Protocol Population; n = number of participants contributing to the analysis. Abbreviations: CI, confidence interval; ELISA, enzyme-linked immunosorbent assay; GMFI, geometric mean fold increases; GMT, geometric mean titer.
Figure 4.
PRNT antibody responses in adults and children. Neutralizing antibody responses as assessed by PRNT in adults (panels A and B) and children (panels C and D) receiving 1 or 2 doses of rVSVΔG-ZEBOV-GP compared to placebo. GMT (PRNT60) and GMFI are displayed with 95% CI. N = PRNT Per-Protocol Population; n = number of participants contributing to the analysis. Abbreviations: CI, confidence interval; PRNT, plaque reduction neutralization test; GMFI, geometric mean fold increases; GMT, geometric mean titer.
Most vaccinated participants (91.0% of adults and 93.5% of children) had a GP-ELISA seroresponse of ≥2-fold increase from baseline and ≥200 EU/mL by Day 28 post-dose 1 (pooled 1- and 2-dose rVSVΔG-ZEBOV-GP groups); the majority (75.7% of adults and 88.4% of children) had a ≥4-fold GP-ELISA seroresponse (Supplementary Figure 2). There was a trend toward more children experiencing a ≥4-fold GP-ELISA seroresponse compared to adults after a single rVSVΔG-ZEBOV-GP dose. A similar trend was observed for a ≥4-fold PRNT seroresponse at Month 3 and Month 12 (Supplementary Figure 3).
Binding and functional antibody GMTs were higher for children (ages 1–17) compared with adults at the postvaccination timepoints (Figures 3 and 4). By Day 28, GP-ELISA GMT was 1748.8 EU/mL (95% CI: 1585.6–1928.7) for children and 1234.4 EU/mL (95% CI: 1132.5–1345.4) for adults and PRNT GMT was 277.1 (95% CI: 255.8–300.2) for children and 169.2 (95% CI: 147.4–194.3) for adults (pooled 1- and 2-dose rVSVΔG-ZEBOV-GP groups). Although not formally hypothesis tested, it was observed that children had higher, non-overlapping GMT 95% CIs (GP-ELISA and PRNT) compared to adults at Day 28 after the first vaccination that were maintained through Month 12 (Supplementary Figure 4). Likewise, GP-ELISA and PRNT GMFIs were higher for children compared with adults (Figures 3 and 4).
For each age subgroup of vaccinated children (ages <3, 3–11, and 12–17), GP-ELISA GMTs were comparable at the postvaccination timepoints (Supplementary Table 3). Baseline PRNT GMTs were comparable across age subgroups; except PRNT GMTs and GMFIs were generally higher for children <3 years old (Supplementary Table 4).
Vaccine Shedding in Children
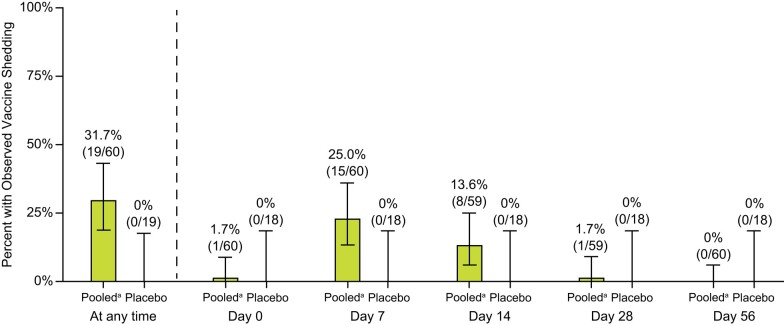
Vaccine-derived RNA in saliva was measured in a subset of children receiving 1 or 2 doses of rVSVΔG-ZEBOV-GP or matched placebo (Figure 5). Post-dose 1, 31.7% of children (ages 1–17, n = 60) had observed vaccine virus shedding (>0 copies/mL) after rVSVΔG-ZEBOV-GP vaccination while none (0%) of the 19 children receiving placebo had observed shedding. None (0%) of the 21 children receiving a 2nd dose at Day 56 had observed shedding post-dose 2 through Month 3. The greatest percentage of children with observed shedding occurred on Day 7 post-dose 1 (25.0%) and no shedding was observed after Day 28. The percentage of children (ages 3–11, n = 34) and adolescents (ages 12–17, n = 21) with observed shedding at any time post vaccination were comparable (32.4%; 95% CI: 17.4%–50.5%, and 38.1%; 95% CI:18.1%–61.6%, respectively) (Supplementary Table 5). No shedding was detected in children <3 years old after rVSVΔG-ZEBOV-GP vaccination, although the sample size was low (n = 5).
Figure 5.
Vaccine virus shedding in a subset of children after the first dose of rVSVΔG-ZEBOV-GP. The percent of children with observed vaccine shedding post the first dose of rVSVΔG-ZEBOV-GP (pooled 1- and 2-dose rVSVΔG-ZEBOV-GP, post-dose 1) compared to placebo with 95% CI. The percentage and the number of children with shedding over the total number of participants contributing to the analysis is displayed above each bar. No shedding was observed post-dose 2 (data not shown). aPooled = 1- and 2-dose rVSVΔG-ZEBOV-GP arms, post-dose 1. Abbreviation: CI, confidence interval.
DISCUSSION
The safety profile, efficacy, and immunogenicity of rVSVΔG-ZEBOV-GP in adults is established [4]. This report evaluated immunogenicity using validated GP-ELISA and PRNT assays. A single dose of rVSVΔG-ZEBOV-GP induced robust antibody responses in both children and adults. Primary and secondary objectives based upon pre-specified criteria were met, supporting the conclusion that the rVSVΔG-ZEBOV-GP vaccine is robustly immunogenic in children as young as 1 year old. Additionally, vaccine shedding through Month 3 was evaluated. Approximately 32% of children had observed vaccine shedding which peaked at Day 7 after the first dose.
Both 1 and 2 doses of rVSVΔG-ZEBOV-GP elicited robust responses through 12 months postvaccination. These findings are consistent with previous studies in adults [15, 18–21] and children [12, 13], including the primary PREVAC report. The data reported here, generated in a centralized laboratory using a GP-ELISA validated for regulatory purpose, are in concordance with the primary PREVAC data which utilized the same GP-ELISA assay method run across two independent laboratories [12]. A single dose of rVSVΔG-ZEBOV-GP was sufficient to elicit immune responses in children (ages 1–17) that were superior to placebo at both Day 28 and Month 12 postvaccination and non-inferior to adults at Day 28 post-dose 1. Furthermore, although not formally tested, children appeared to mount higher antibody responses compared to adults, in line with the published binding antibody findings [12] and expanded here to include functional antibody responses. Other live-attenuated vaccines also generally elicit stronger immune responses in children compared to adults [22].
The benefit, if any, of a booster dose of rVSVΔG-ZEBOV-GP is not known. The 2-dose rVSVΔG-ZEBOV-GP arm was included in the original trial design to match the two-dose schedule of the Ad26.ZEBOV/MVA-BN-Filo arm [11]. Most live-attenuated vaccines induce robust immune responses after the first dose [23]. Here, we observed a slight decline in titers by Month 3 after a single rVSVΔG-ZEBOV-GP dose that subsequently rose by Month 12 as seen in previous studies [12, 18, 24] potentially reflective of antibody maturation. While a second dose of rVSVΔG-ZEBOV-GP given at Day 56 boosted antibody concentrations in children and adults by Month 3, this boost was not sustained through Month 12. The primary PREVAC report included additional timepoints post-dose 2 and found that GP-ELISA titers peaked by Day 63 (7 days post-dose 2) and had begun to decay by Month 3 [12]. The single dose schedule of ERVEBO® may aid outbreak response efforts by conferring rapid and robust immune responses and has been implemented during outbreaks among children ≥6 months of age [25]. A study assessing a rVSVΔG-ZEBOV-GP booster given 18 months after the initial vaccination is ongoing (NCT02788227).
In adults, rVSVΔG-ZEBOV-GP vaccination leads to transient vaccine viremia in blood that is typically resolved within a few days, although vaccine virus shedding in saliva or urine is rare [24, 26–28]. In contrast, children (ages 6–12) and adolescents (ages 13–17) in a Phase 1 trial had a higher incidence of vaccine virus shedding in saliva compared to adults [13]. While the current study supports the finding that vaccine virus shedding in saliva occurs more frequently among children compared to adults, the incidence at Day 7 (25%) was generally lower than previously reported (35%–78%) despite using a lower threshold to define detectable vaccine virus shedding (>0 copies/mL vs >30 copies/mL) [13]. Additionally, in this study children (ages 3–11) and adolescents (ages 12–16) had a similar incidence of vaccine virus shedding, unlike in the previous report. These were distinct trials using independent assays thus precluding the ability to make direct comparisons; differences in incidence might be due to populations enrolled or the assays used.
Vaccine shedding in saliva of children had been previously measured only until Day 7 [13]. Here, we assessed vaccine shedding in saliva through 3 months post-vaccination. The peak incidence of vaccine shedding in saliva occurred by Day 7 and declined thereafter, with no shedding after Day 28. Of note, Day 28 coincides with peak antibody responses which may impact shedding. No shedding was detected at any time post-dose 2, as reported with some other live-attenuated vaccines [29–31], likely due to the sustained immune responses present.
Although rVSVΔG-ZEBOV-GP shedding was observed in less than a third of children, the implications of vaccine shedding require consideration, as for other live-attenuated vaccines [23, 32–35]. Since RT-PCR was used to measure vaccine virus shedding in saliva, infectiousness cannot be inferred. A study assessing potential transmissibility is underway (NCT05130398) to provide insights on secondary exposure risk and guide recommendations around exposure to saliva from vaccinated children.
There are some limitations of this study. This study was limited to immunogenicity (binding and neutralizing antibody responses) and, therefore, was unable to evaluate clinical efficacy. However, a GP-ELISA seroresponse of ≥200 EU/mL and 2-fold increase from baseline has been proposed as a potential indicator of protection from EVD in adults [17]. This report includes 1-year follow-up, limiting the ability to draw conclusions related to durability or immunogenicity and protection beyond that period. Immunogenicity assessments through 5 years are ongoing [11].
In conclusion, rVSVΔG-ZEBOV-GP elicits robust binding and neutralizing antibody responses through 12 months post-vaccination in children and adults. Moreover, vaccine shedding in children was of short duration and only observed after the first dose. Overall, these data support the recent approvals of rVSVΔG-ZEBOV-GP for the prevention of EVD in children.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Andrew W Lee, Merck & Co., Inc., Rahway, New Jersey, USA.
Ken Liu, Merck & Co., Inc., Rahway, New Jersey, USA.
Edouard Lhomme, Inserm, CHU Bordeaux, CIC 1401, EUCLID/F-CRIN Clinical Trials Platform, University of Bordeaux, Bordeaux, France.
Julie Blie, Partnership for Research on Ebola Vaccines in Liberia (PREVAIL), Monrovia, Liberia.
John McCullough, Advanced BioMedical Laboratories (ABML), Cinnaminson, New Jersey, USA.
Matthew T Onorato, Merck & Co., Inc., Rahway, New Jersey, USA.
Laurie Connor, Merck & Co., Inc., Rahway, New Jersey, USA.
Jakub K Simon, Merck & Co., Inc., Rahway, New Jersey, USA.
Sheri Dubey, Merck & Co., Inc., Rahway, New Jersey, USA.
Susan VanRheenen, Merck & Co., Inc., Rahway, New Jersey, USA.
Jonathan Deutsch, Merck & Co., Inc., Rahway, New Jersey, USA.
Abigail Owens, Merck & Co., Inc., Rahway, New Jersey, USA.
Amy Morgan, Merck & Co., Inc., Rahway, New Jersey, USA.
Carolee Welebob, Merck & Co., Inc., Rahway, New Jersey, USA.
Donna Hyatt, Merck & Co., Inc., Rahway, New Jersey, USA.
Sunita Nair, Merck & Co., Inc., Rahway, New Jersey, USA.
Benjamin Hamzé, Pôle Recherche Clinique, Institut National de la Santé et de la Recherche Médicale (Inserm), Paris, France.
Oumar Guindo, University Clinical Research Center (UCRC), Bamako, Mali.
Samba O Sow, CVD-Mali, Bamako, Mali.
Abdoul H Beavogui, Centre National de Formation et de Recherche en Santé Rurale (CNFRSR), Maferinyah, Guinea.
Bailah Leigh, College of Medicine & Allied Health Sciences (COMAHS), University of Sierra Leone, Freetown, Sierra Leone.
Mohamed Samai, College of Medicine & Allied Health Sciences (COMAHS), University of Sierra Leone, Freetown, Sierra Leone.
Pauline Akoo, Department of Clinical Research, Faculty of Infectious and Tropical Diseases, London School of Hygiene & Tropical Medicine (LSHTM), London, United Kingdom.
Alimamy Serry-Bangura, College of Medicine & Allied Health Sciences (COMAHS), University of Sierra Leone, Freetown, Sierra Leone.
Suzanne Fleck, Department of Clinical Research, Faculty of Infectious and Tropical Diseases, London School of Hygiene & Tropical Medicine (LSHTM), London, United Kingdom.
Fatou Secka, Department of Clinical Research, Faculty of Infectious and Tropical Diseases, London School of Hygiene & Tropical Medicine (LSHTM), London, United Kingdom.
Brett Lowe, Department of Clinical Research, Faculty of Infectious and Tropical Diseases, London School of Hygiene & Tropical Medicine (LSHTM), London, United Kingdom.
Deborah Watson-Jones, Department of Clinical Research, Faculty of Infectious and Tropical Diseases, London School of Hygiene & Tropical Medicine (LSHTM), London, United Kingdom; Mwanza Intervention Trials Unit, National Institute for Medical Research, Mwanza, Tanzania.
Céline Roy, Inserm, CHU Bordeaux, CIC 1401, EUCLID/F-CRIN Clinical Trials Platform, University of Bordeaux, Bordeaux, France; University of Bordeaux, INSERM, MART, UMS 54, F-33000 Bordeaux, France.
Lisa E Hensley, National Bio and Agro-Defense Facility (NBAF), United States Department of Agriculture (USDA), Manhattan, Kansas, USA.
Mark Kieh, Partnership for Research on Ebola Vaccines in Liberia (PREVAIL), Monrovia, Liberia.
Beth-Ann G Coller, Merck & Co., Inc., Rahway, New Jersey, USA.
the PREVAC Study Team:
Jamila Aboulhab, Michelle Aguirre-MacKenzie, Pauline Akoo, Esther Akpa, Robert Akpata, Sara Albert, Boni Maxime Ale, Serry Alimamy-Bangura, Pierre Andong, Benetta C Andrews, Stephane Anoma, Negin Atri, Augustin Augier, Ken Awuondo, Moses Badio, Aminata Bagayoko, Abby Balde, Joséphine Balssa, Lamin Molecule Bangura, Kesha Barrington, Eric Barte de Saint Fare, Beth Baseler, Ali Bauder, Claire Bauduin, Luke Bawo, Abdoul Habib Beavogui, Michael Belson, Marion Bererd, Teedoh Beyslow, Jeanne Billioux, Shere Billouin-Frazier, Blandine Binachon, Julie Blie, Viki Bockstal, Patricia Boison, Fatorma Bolay, Aliou Boly, Anne-Gaëlle Borg, Samuel Bosompem, Courtney Bozman, Tyler Brady, Sarah Browne, Barbara Cagniard, Kelly Cahill, Yingyun Cai, Aissata Abdoulaye Camara, Aboubacar Keira Camara, Alseny Modet Camara, Antoine Campagne, Cécilia Campion, Jennifer Cash, Siew Pin Chai, Francois Chambelin, Michael Chea, Geneviève Chêne, Michelle Chouinard, Florence Chung, Lucy Chung, Séverine Ciancia, Papa Ndiaga Cisse, Elfrida Cline-Cole, Céline Colin, Beth-Ann Coller, Djélikan Siaka Conde, Katherine Cone, Laurie Connor, Nicholas Connor, Joseph Boye Cooper, Sandrine Couffin-Cardiergues, Fatoumata Coulibaly, Mariam Coulibaly, Page Crew, Sandrine Dabakuyo-Yonli, Djeneba Dabitao, Thierry Damerval, Bionca Davis, Gibrilla Fadlu Deen, Eline Dekeyster, Jean-François Delfraissy, Christelle Delmas, Mahamadou Diakite, Alpha Diallo, Mamadou Saliou Diallo, Ayouba Diarra, Samba Diarra, Oualy Diawara, Bonnie Dighero-Kemp, Samba Diop, Waly Diouf, Saurabh Dixit, Barry Djenabou, Laurie Doepel, Eric D'Ortenzio, Seydou Doumbia, Moussa Moise Doumbia, Macaya Douoguih, Nelson Dozier, Natasha Dubois Cauwelaert, Alain DuChêne, Michael Duvenhage, Risa Eckes, Elizabeth Elliott, Luisa Enria, Hélène Espérou, Cécile Etienne, Allison Eyler, Lawrence Fakoli, Mosoka Fallah, Sylvain Faye, John Fayiah, Suzanne Fleck, Vemy Fofana, Karine Fouth Tchos, Kokulo Franklin, Daniela Fusco, Auguste Gaddah, Marylène Gaignet, Katherine Gallagher, Harrison Gichini, Julia Garcia Gozalbes, Greg Grandits, Maima Gray, Brian Greenwood, Nico Grobler, Robin Gross, Louis Grue, Birgit Grund, Oumar Guindo, Swati Gupta, Fadima Haidara, Benjamin Hamzé, Emma Hancox, Jean-Christophe Hébert, Jenny Hendriks, Patricia Hensley, Lisa Hensley, Elisabeth Higgs, Trudi Hilton, Horace Preston Holley, Marie Hoover, Melissa Hughes, Dicko Ilo, Skip Irvine, David Ishola, Yvonne Jato, Madison Joe, Melvin Johnson, Aboubacar Sidiki Kaba, Jonathan Kagan, Michael Kamara, Myriam Kante, Judith Katoudi, Cheick Mohamed Keita, Sakoba Keita, Seykou Keita, Stephen B Kennedy, Babajide Keshinro, Hassan Kiawu, Mark Kieh, Matthew Kirchoff, Gregory Kocher, Mamoudou Kodio, Brian Kohn, Lamine Koivogui, Richard Kojan, Cece Francis Kolié, Jacques Seraphin Kolié, David Kollie, Stacy Kopka, Bockarie Koroma, Dickens Kowuor, Catherine Kpayieli-Freeman, Liane Kwast, Christine Lacabaratz, Boris Lacarra, Laurie Lambert, Courtney Lambeth, Solange Lancrey-javal, H Clifford Lane, Shadrach Langba, Bolarinde Lawal, Andrew Wen-Tseng Lee, Shona Lee, Shelley Lees, Annabelle Lefevre, Bailah Leigh, Frederic Lemarcis, Yves Lévy, Claire Levy-Marchal, Maarten Leyssen, Edouard Lhomme, Janie Liang, Mameni Linga, Ken Liu, Brett Lowe, Julia Lysander, Ibrah Mahamadou, Marvington Mambiah, Daniela Manno, Jonathan Marchand, Lindsay Marron, Moses B F Massaquoi, Laure Masson, Charly Matard, Steven Mazur, John McCullough, Chelsea McLean, Noémie Mercier, Pauline Michavila, Tracey Miller, Niouma Pascal Millimouno, Alejandra Miranda, Soumaya Mohamed, Tom Mooney, Dally Muamba, James Mulbah, Rita Lukoo Ndamenyaa, James Neaton, Désiré Neboua, Micki Nelson, Kevin Newell, Vinh-kim Nguyen, Yusupha Njie, Wissedi Njoh, Matthew Onorato, Uma Onwuchekwa, Susan Orsega, Inmaculada Ortega-Perez, Cynthia Osborne, Tuda Otieno, Davy Oulaï, Sushma Patel, Danielle Peart, James Pettitt, Nathan Peiffer-Smadja, Robert Phillips, Jerome Pierson, Peter Piot, Micheal Piziali, Stéphany Pong, Elena Postnikova, Calvin Proffitt, Alexandre Quach, Sinead Quigley, Nadeeka Randunu, Laura Richert, Priscille Rivière, Cynthia Robinson, Céline Roy, Amy Falk Russell, Philip Sahr, Mohamed Samai, Sibiry Samake, Jen Sandrus, Ibrahim Sanogo, Yeya Sadio Sarro, Serge Sawadogo, Sani Sayadi, Maxime Schvartz, Christine Schwimmer, Fatou Secka, Heema Sharma, Denise Shelley, Bode Shobayo, Sophia Siddiqui, Jakub Simon, Shelly Simpson, Billy Muyisa Sivahera, Karen Slater, Mary Smolskis, Elizabeth Smout, Emily Snowden, Anne-Aygline Soutthiphong, Amadou Sow, Samba O Sow, Ydrissa Sow, Michael Stirratt, Jeroen Stoop, Guna Subramaniam, Léa Surugue, Nathalie Swales, Sienneh Tamba, Chan Tang, Cheick Tangara, Milagritos D Tapia, Julius Teahton, Jemee Tegli, Monique Termote, Rodolphe Thiebaut, Greg Thompson, John Tierney, Daniel Tindanbil, Abdoulaye Touré, Elvis Towalid, Stacey Traina, Awa Traore, Tijili Tyee, David Vallée, Renaud Vatrinet, Corine Vincent, Susan Vogel, Cedrick Wallet, Travis Warren, Deborah Watson-Jones, Wade Weaver, Deborah Wentworth, Cecelia Wesseh, Hilary Whitworth, Aurelie Wiedemann, Wouter Willems, Barthalomew Wilson, Jayanthi Wolf, Alie Wurie, Delphine Yamadjako, Marcel Yaradouno, Quiawiah Yarmie, Yazdan Yazdanpanah, Shuiqing Yu, Zara Zeggani, and Huanying Zhou
Notes
Author Contributions. Conceptualization: A. W. L., M. T. O., J. K. S., S. V., C. W., M. S., B. Lowe., D. W-J., C. R., L. E.H., M. K., and B-A. C. G.; Methodology: E. L., J. M., S. D., A. H. B., M. S., B. Lowe, and L. E. H.; Software: K. L., J. M., D. H., and S. N.; Validation: K. L., J. M., S. D., D. H., S. N., M. S., B. Lowe, and M. K.; Formal Analysis: A. W. L., K. L., L. C., J. K. S., S. D., J. D., A. O., A. M., D. H., and S. N.; Investigation: F. S., J. B., L. C., J. K. S., B. H., O. G., S. O. S., B. Leigh., M. S., P. A., A. S.-B., S. F., B. Lowe., L. E. H., and M. K.; Resources: B. Lowe., and J. M.; Data curation: A. W. L., D. H., J. M., S. N., and B. Lowe; Supervision: E. L., M. T. O., O. G., M. S., P. A., F. S., B. Lowe., L. E. H., M. K., and B.-A. C. G.; Project Administration: E. L., J. B., J. M., M. T. O., S. V., C. W., B. H., O. G., M. S., P. A., S. F., F. S., B. Lowe., and B.-A.C. G.; Funding Acquisition: M. S., D. W.-J., C. R., L. E. H., and B.-A. C. G.; Writing—Original Draft: A. W. L., K. L., E. L., J. B., J. M., J. K. S., C. R., L. E. H., M. K., and B.-A. G. C. supported development of the original draft; Writing—Review and Editing: All authors reviewed the manuscript, had access to the study data, and approved the manuscript for submission.
Acknowledgments. The authors would like to thank the participants who consented to the trial, the Ministries of Health of Guinea, Liberia, Sierra Leone, and Mali who permitted the conduct of the trial, and ALIMA, the PREVAC Study Team, and all site collaborators for their contribution in the implementation of the trial. Additionally, the authors would like to thank the staff at Q2 Solutions for contract research assistance for this study. Statistical support was provided by Z. Chen, PhD, medical writing assistance was provided by E. Anderson, PhD and K. Cox, MS, and editorial assistance was provided by K. Davis, BA, all of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. A full list of the PREVAC Study Team can be found in the Supplementary Materials.
Disclaimer. The dissemination represents only the authors’ views and Innovative Medicines Initiative 2 Joint Undertaking (IMI2JU) is not responsible for any use of the information contained in the dissemination. The content of this publication does not necessarily reflect the views or policies of the US Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Data sharing. The data sharing policy, including restrictions, of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD) is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the Engage Zone site or via email to the Data Access mailbox.
Financial support. The Partnership for Research on Ebola VACcination (PREVAC) trial was sponsored by the National Institutes of Health (NIH), Institut national de la santé et de la recherche médicale (Inserm), and the London School of Hygiene and Tropical Medicine (LSHTM). Statistical analyses and assays were supported by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. The clinical trial was conducted with the support of Janssen, Bavarian Nordic, and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA who provided the vaccines according to EBOVAC 1 grant agreement. This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (grant number 115854, EBOVAC1). This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA. This project has been funded, in part, with federal funds from the US Department of Health and Human Services, Administration for Strategic Preparedness & Response (ASPR), Biomedical Advanced Research and Development Authority (BARDA) (contract number HHSO100201700012C). The project has been funded by a dedicated Inserm allocation on behalf of the French Research Ministry.
References
- 1. World Health Organization . Ebola virus disease. Available at: https://www.who.int/news-room/fact-sheets/detail/ebola-virus-disease. Accessed 26 August 2022.
- 2. Huber C, Finelli L, Stevens W. The economic and social burden of the 2014 Ebola outbreak in West Africa. J Infect Dis 2018; 218(suppl_5):S698–704. [DOI] [PubMed] [Google Scholar]
- 3. Ebola in DRC: recommendations for accelerating outbreak control. WHO Scientific and Technical Advisory Group for Infectious Hazards (STAG-IH). 2019.
- 4. Henao-Restrepo AM, Camacho A, Longini IM, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!). Lancet 2017; 389:505–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wolf J, Jannat R, Dubey S, et al. Development of pandemic vaccines: ERVEBO case study. Vaccines (Basel) 2021; 9:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ERVEBO® (Ebola Zaire Vaccine, Live) suspension for intramuscular injection prescribing information. Merck Sharp & Dohme LLC., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. Available at: https://www.fda.gov/media/133748/download. Accessed 13 October 2023.
- 7.U.S. FDA approves Merck's ERVEBO® (Ebola Zaire Vaccine, Live) for use in children 12 months of age and older. Merck Sharp & Dohme LLC., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. Available at: https://www.merck.com/news/u-s-fda-approves-mercks-ervebo-ebola-zaire-vaccine-live-for-use-in-children-12-months-of-age-and-older/. Accessed 12 October 2023.
- 8. European Medicines Agency . Ervebo: EPAR—product information. Available at: https://www.ema.europa.eu/en/documents/product-information/ervebo-epar-product-information_en.pdf. Accessed 13 October 2023.
- 9. Genisca AE, Butler K, Gainey M, et al. Constructing, validating, and updating machine learning models to predict survival in children with Ebola virus disease. PLoS Negl Trop Dis 2022; 16:e0010789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. The Lancet Child Adolescent H . Children's needs in an Ebola virus disease outbreak. Lancet Child Adolesc Health 2019; 3:55. [DOI] [PubMed] [Google Scholar]
- 11. Badio M, Lhomme E, Kieh M, et al. Partnership for Research on Ebola VACcination (PREVAC): protocol of a randomized, double-blind, placebo-controlled phase 2 clinical trial evaluating three vaccine strategies against Ebola in healthy volunteers in four West African countries. Trials 2021; 22:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Team PS, Kieh M, Richert L, et al. Randomized trial of vaccines for Zaire Ebola virus disease. N Engl J Med 2022; 387:2411–24. [DOI] [PubMed] [Google Scholar]
- 13. Agnandji ST, Fernandes JF, Bache EB, et al. Safety and immunogenicity of rVSVΔG-ZEBOV-GP Ebola vaccine in adults and children in Lambarene, Gabon: a phase I randomised trial. PLoS Med 2017; 14:e1002402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rudge TL J, Sankovich KA, Niemuth NA, et al. Development, qualification, and validation of the Filovirus Animal Nonclinical Group anti-Ebola virus glycoprotein immunoglobulin G enzyme-linked immunosorbent assay for human serum samples. PLoS One 2019; 14:e0215457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mahon BE, Simon J, Widdowson MA, et al. Baseline asymptomatic malaria infection and immunogenicity of recombinant vesicular stomatitis virus-Zaire Ebola virus envelope glycoprotein. J Infect Dis 2021; 224:1907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Antonello J, Grant-Klein RJ, Nichols R, Kennedy SB, Dubey S, Simon JK. Serostatus cutoff levels and fold increase to define seroresponse to recombinant vesicular stomatitis virus—Zaire Ebola virus envelope glycoprotein vaccine: an evidence-based analysis. Vaccine 2020; 38:4885–91. [DOI] [PubMed] [Google Scholar]
- 17. Grais RF, Kennedy SB, Mahon BE, et al. Estimation of the correlates of protection of the rVSV∆G-ZEBOV-GP Zaire ebolavirus vaccine: a post-hoc analysis of data from phase 2/3 clinical trials. Lancet Microbe 2021; 2:e70–e8. [DOI] [PubMed] [Google Scholar]
- 18. Simon JK, Kennedy SB, Mahon BE, et al. Immunogenicity of rVSVΔG-ZEBOV-GP Ebola vaccine (ERVEBO®) in African clinical trial participants by age, sex, and baseline GP-ELISA titer: a post hoc analysis of three phase 2/3 trials. Vaccine 2022; 40:6599–606. [DOI] [PubMed] [Google Scholar]
- 19. Halperin SA, Das R, Onorato MT, et al. Immunogenicity, lot consistency, and extended safety of rVSV∆G-ZEBOV-GP vaccine: a phase 3 randomized, double-blind, placebo-controlled study in healthy adults. J Infect Dis 2019; 220:1127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kennedy SB, Bolay F, Kieh M, et al. Phase 2 placebo-controlled trial of two vaccines to prevent Ebola in Liberia. N Engl J Med 2017; 377:1438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boum Y, Juan-Giner A, Hitchings M, et al. Humoral and cellular immune response induced by rVSVΔG-ZEBOV-GP vaccine among frontline workers during the 2013–2016 West Africa Ebola outbreak in Guinea. Vaccine 2020; 38:4877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Block SL, Yogev R, Hayden FG, Ambrose CS, Zeng W, Walker RE. Shedding and immunogenicity of live attenuated influenza vaccine virus in subjects 5–49 years of age. Vaccine 2008; 26:4940–6. [DOI] [PubMed] [Google Scholar]
- 23. Kroger A, Bahta L, Hunter P. General Best Practice Guidelines for Immunization. Best Practices Guidance of the Advisory Committee on Immunization Practices (ACIP). Available at: www.cdc.gov/vaccines/hcp/acip-recs/general- recs/downloads/general recs.pdf. Accessed 21 August 2023.
- 24. Heppner DG J, Kemp TL, Martin BK, et al. Safety and immunogenicity of the rVSV∆G-ZEBOV-GP Ebola virus vaccine candidate in healthy adults: a phase 1b randomised, multicentre, double-blind, placebo-controlled, dose-response study. Lancet Infect Dis 2017; 17:854–66. [DOI] [PubMed] [Google Scholar]
- 25. World Health Organization . Meeting of the Strategic Advisory Group of experts on immunization, 2019—conclusions and recommendations. Wkly Epidemiol Rec 2019; 94:261–80. [Google Scholar]
- 26. Agnandji ST, Huttner A, Zinser ME, et al. Phase 1 trials of rVSV Ebola vaccine in Africa and Europe. N Engl J Med 2016; 374:1647–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. ElSherif MS, Brown C, MacKinnon-Cameron D, et al. Assessing the safety and immunogenicity of recombinant vesicular stomatitis virus Ebola vaccine in healthy adults: a randomized clinical trial. CMAJ 2017; 189:E819–E27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Regules JA, Beigel JH, Paolino KM, et al. A recombinant vesicular stomatitis virus Ebola vaccine. N Engl J Med 2017; 376:330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reinhardt B, Jaspert R, Niedrig M, Kostner C, L’Age-Stehr J. Development of viremia and humoral and cellular parameters of immune activation after vaccination with yellow fever virus strain 17D: a model of human flavivirus infection. J Med Virol 1998; 56:159–67. [DOI] [PubMed] [Google Scholar]
- 30. Russell KL, Rupp RE, Morales-Ramirez JO, et al. A phase I randomized, double-blind, placebo-controlled study to evaluate the safety, tolerability, and immunogenicity of a live-attenuated quadrivalent dengue vaccine in flavivirus-naive and flavivirus-experienced healthy adults. Hum Vaccin Immunother 2022; 18:2046960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006; 354:23–33. [DOI] [PubMed] [Google Scholar]
- 32. Anderson EJ. Rotavirus vaccines: viral shedding and risk of transmission. Lancet Infect Dis 2008; 8:642–9. [DOI] [PubMed] [Google Scholar]
- 33. Cortese MM, Parashar UD, Centers for Disease C, Prevention . Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2009; 58(RR-2):1–25. [PubMed] [Google Scholar]
- 34. Petersen BW, Harms TJ, Reynolds MG, Harrison LH. Use of vaccinia virus smallpox vaccine in laboratory and health care personnel at risk for occupational exposure to orthopoxviruses—recommendations of the Advisory Committee on Immunization Practices (ACIP), 2015. MMWR Morb Mortal Wkly Rep 2016; 65:257–62. [DOI] [PubMed] [Google Scholar]
- 35. Grossberg R, Harpaz R, Rubtcova E, Loparev V, Seward JF, Schmid DS. Secondary transmission of varicella vaccine virus in a chronic care facility for children. J Pediatr 2006; 148:842–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.