Abstract
BACKGROUND:
Reducing child mortality in low-income countries is constrained by a lack of vital statistics. In the absence of such data, verbal autopsies provide an acceptable method to determining attributable causes of death. The objective was to assess potential causes of pediatric postdischarge mortality in children younger than age 5 years (under-5) originally admitted for suspected sepsis using verbal autopsies.
METHODS:
Secondary analysis of verbal autopsy data from children admitted to 6 hospitals across Uganda from July 2017 to March 2020. Structured verbal autopsy interviews were conducted for all deaths within 6 months after discharge. Two physicians independently classified a primary cause of death, up to 4 alternative causes, and up to 5 contributing conditions using the Start-Up Mortality List, with discordance resolved by consensus.
RESULTS:
Verbal autopsies were completed for 361 (98.6%) of the 366 (5.9%) children who died among 6191 discharges (median admission age: 5.4 months [interquartile range, 1.8–16.7]; median time to mortality: 28 days [interquartile range, 9–74]). Most deaths (62.3%) occurred in the community. Leading primary causes of death, assigned in 356 (98.6%) of cases, were pneumonia (26.2%), sepsis (22.1%), malaria (8.5%), and diarrhea (7.9%). Common contributors to death were malnutrition (50.5%) and anemia (25.7%). Reviewers were less confident in their causes of death for neonates than older children (P < .05).
CONCLUSIONS:
Postdischarge mortality frequently occurred in the community in children admitted for suspected sepsis in Uganda. Analyses of the probable causes for these deaths using verbal autopsies suggest potential areas for interventions, focused on early detection of infections, as well as prevention and treatment of underlying contributors such as malnutrition and anemia.
In 2021, there were more than 5 million deaths of children younger than age 5 years (under-5) globally. Most of these deaths occurred in low- and middle-income countries (LMICs), with more than half occurring in sub-Saharan Africa alone.1 It is increasingly recognized that a significant proportion of child mortality occurs outside of the hospital following an admission in LMIC settings.2,3 For children living in these settings, postdischarge mortality rates often exceed in-hospital mortality rates, with most deaths taking place in the community rather than during a readmission to the hospital.3 Thus, an improved understanding of the causes of postdischarge deaths is a crucial step if we are to address this under-5 mortality among low-income countries.
A lack of standard reporting on causes of mortality remains a serious challenge to cause-specific child mortality estimation, monitoring, and public health policy and planning. In recent years, the Institute for Health Metrics and Evaluation’s Global Burden of Disease (GBD) studies have provided several cause-specific estimates for LMICs. Yet, because of resource constraints, these are often based on infrequent demographic and health surveys and a lack of rigorous diagnostic procedures to identify the causes of illness.4 Further, most low-income settings have no reliable community-level mortality data. In Uganda, only those deaths that occur in health facilities are reported through a facility-based health management information system.4 This results in a biased estimate given that two thirds of postdischarge deaths are estimated to occur in the community outside of the health system and, therefore, go largely unreported.3
In the absence of robust vital registration systems, verbal autopsy (VA) has been previously shown to be a useful tool for characterizing causes of death.5–8 This method typically consists of a trained interviewer using a questionnaire with a close relative of the deceased to collect information on the signs, symptoms, and circumstances leading up to the death.9 The data collected are then most often interpreted by a physician to determine the cause of death, helping to close significant gaps in cause-specific mortality data. Although a growing body of literature has focused on the epidemiology of postdischarge mortality in recent years,10–12 very few studies discuss the causes of postdischarge deaths in detail. The objective of this study was to characterize the causes of postdischarge mortality among a cohort of children initially admitted for suspected sepsis in Uganda using VA.
METHODS
Study Population and Procedures
This is a secondary analysis of a multisite prospective cohort study performed at 6 hospitals across Uganda (Mbarara Regional Referral Hospital, Holy Innocents Children’s Hospital, Masaka Regional Referral Hospital, Jinja Regional Referral Hospital, Ibanda Hospital, Kitovu Hospital, and Villa Maria Hospital). The combined catchment areas for these hospitals span 30 districts and are representative of both rural and urban populations in Uganda. Full details of the study selection criteria have been previously described.13 In the parent study, children younger than 5 years of age with suspected sepsis (defined as suspected or proven infection requiring admission) were consecutively enrolled between July 2017 to March 2020. After enrollment, clinical, social, and demographic variables were captured during data collection at admission. Suitability for discharge was deemed by the treating team at each health facility. Following discharge, a telephone follow-up was completed at 2 and 4 months, and an in-person visit was conducted at 6 months postdischarge. A VA was conducted for children who died during the 6-month postdischarge period; the current study focuses on the analysis of these VA data.
Verbal Autopsy Method
Structured VA interviews were conducted by a trained field officer with the child’s caregiver during community visits shortly after notification of death. The VA questionnaire (Supplemental Table 2) was adapted from the World Health Organization and Child Acute Illness and Nutrition Network VA instruments,14,15 capturing information pertaining to the circumstances and symptoms the deceased may have experienced during the most recent illness that led to death, including medical history, general signs, risk factors, and the reported cause of death if available. All field officers received 1-on-1 training on conducting VA interviews, with a required observation period before independent assessment. VA data were collected using the Research Electronic Database Capture (REDCap)16 mobile app.
Outcomes
Following data collection, 22 pediatricians, neonatologists, and other specialists with relevant experience working within various regions of sub-Saharan Africa were recruited globally to interpret the VA data along with clinical, social, and demographic variables collected for each child as part of the larger cohort study to determine the most likely cause of death (primary outcome). The primary cause of death was defined as the disease, injury, or complication that led to death. Secondary outcomes included possible alternative causes of death (physician reviewers could list up to 4), significant contributing preexisting conditions (up to 5) either presumed or based on diagnoses or conditions listed in the VA, and level of confidence in the disease or condition assignment on a 3-point scale of (1) confident, (2) somewhat confident, or (3) not confident. Notably, a chronological ordering of any antecedent causes of death was deemed unfeasible because of a lack of available data on time course. Diseases and conditions listed were classified according to the Start-Up Mortality List (SMoL), developed by the World Health Organization as an application of the International Classification of Diseases 10th Revision (ICD-10) for low-resource settings.17 Every VA case was reviewed independently by 2 physicians and any discordant results were reconciled by consensus between the 2 reviewers.
Statistical Analysis
Children were divided into neonates (died before 28 days of age) or older children (died at 28 days or older) sub-groups for all analyses. Descriptive statistics were calculated using Microsoft Excel (Microsoft Corporation, Redmond, WA). All available data were included in the calculations. To determine significance, χ2 tests were performed and P values less than .05 were considered statistically significant for all comparisons.
Ethics
Before beginning study activities, study approval was received from the institutional review boards at the University of British Columbia (REB #H16-02679), the Mbarara University of Science and Technology (No. 15/10–16), and the Uganda National Council for Science and Technology (HS 2207). Participation in this study was voluntary and written informed consent was obtained in the local language from a parent or guardian of all children enrolled.
RESULTS
In the parent study, 6545 under-5 children hospitalized for a suspected or proven infection were enrolled between July 2017 and March 2020. There were 354 (5.4%) in-hospital deaths. Among the 6191 children who were discharged alive, 366 (5.9%) deaths occurred within 6 months after discharge (Fig 1). A VA was completed for 361 (98.6%) children. For the children who died after discharge, 163 (44.5%) were female and the median age at the initial time of hospital admission was 5.4 months (interquartile range [IQR], 1.8–16.7). Of these, 54 (14.8%) were admitted during the neonatal period and 19 (5.2%) died before reaching the age of 28 days (Table 1). Most deaths (62.3%) occurred in the community (at home or in-transit to a health facility), and the median time to death after discharge was 28 (IQR, 9–74) days after discharge.
FIGURE 1.
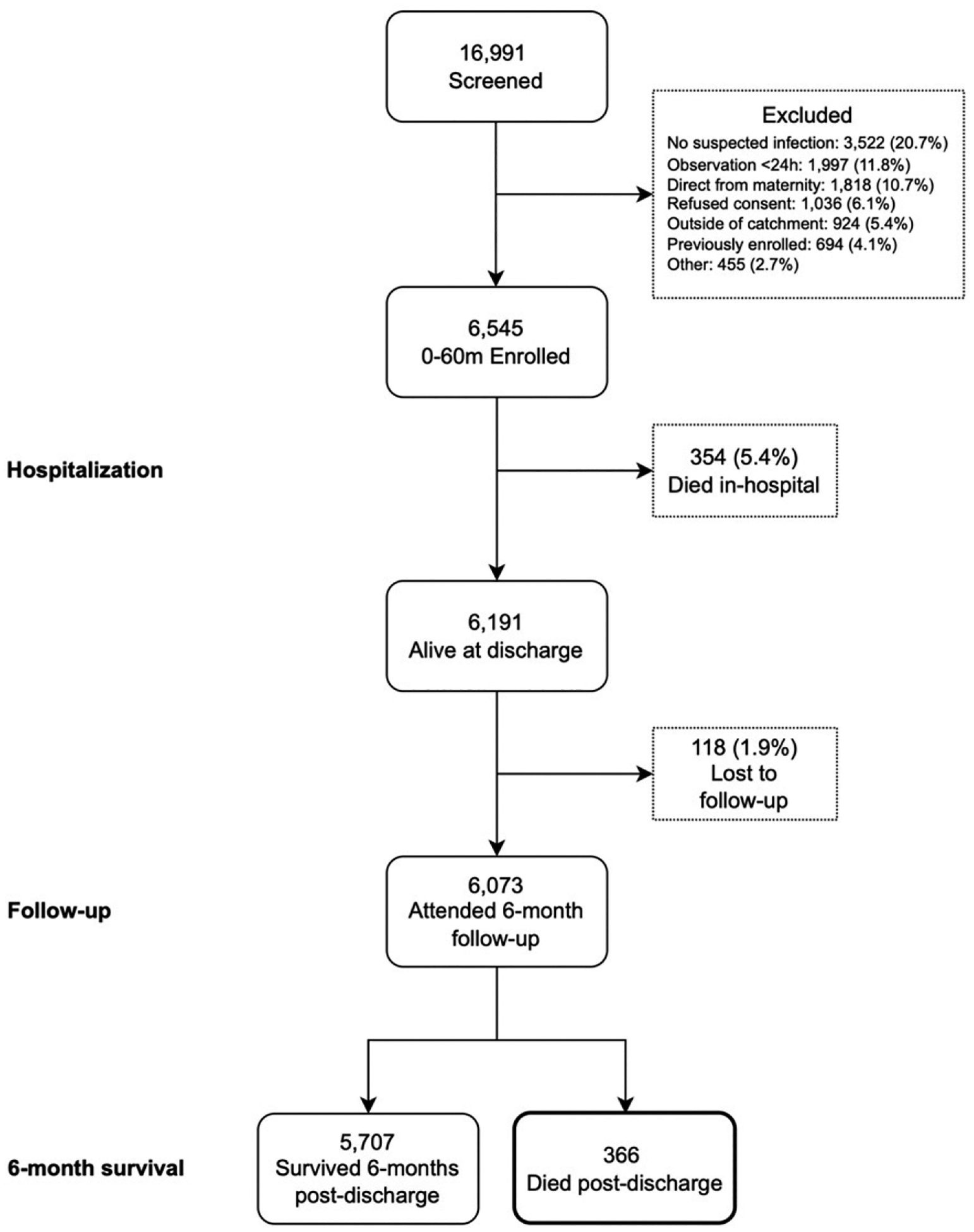
Flow diagram of children under-5 admitted with suspected sepsis enrolled in the prospective cohort study from July 2017 to March 2020 and outcomes 6 months after discharge.
TABLE 1.
Demographic and Clinical Characteristics of Children who Died After Discharge
| <28 d (N = 19) |
28 d–5 y (N = 347) |
Overall (N = 366) |
|
|---|---|---|---|
| Demographic characteristics | |||
| Age at admission, mo, median (IQR) | 0.2 (0.1–0.4) | 5.8 (2.4–17.6) | 5.4 (1.8–16.7) |
| Female sex, N (%) | 5 (26) | 158 (45.5) | 163 (44.5) |
| Maternal education | |||
| No school/≤P3, N (%) | 7 (37) | 221 (63.7) | 228 (62.3) |
| P4–P7, N (%) | 10 (53) | 96 (27.7) | 106 (29.0) |
| S1–S6, N (%) | 2 (11) | 22 (6.3) | 24 (6.6) |
| Postsecondary, N (%) | 0 (0) | 8 (2.3) | 8 (2.2) |
| Household size, median (IQR) | 4 (3–5) | 5 (4–6) | 5 (4–6) |
| Distance to facility, km, median (IQR) | 24.0 (12.8–36.4) | 26.2 (11.4–42.2) | 26.2 (11.4–41.9) |
| Time from discharge to death, in days, median (IQR)a | 1.0 (0.5–5.5) | 32.0 (11.0–79.0) | 27.5 (9.0–74.0) |
| Index admission clinical and laboratory characteristics | |||
| Prior admission, N (%) | |||
| <7 d | 1 (5) | 22 (6.3) | 23 (6.3) |
| 7 d–1 mo | 0 (0) | 48 (13.8) | 48 (13.1) |
| >1 mo | 0 (0) | 72 (20.7) | 72 (19.6) |
| Admission Sp02 | |||
| 95%–100%, N (%) | 9 (47) | 200 (57.6) | 209 (57.1) |
| 90%–94%, N (%) | 5 (26) | 60 (17.3) | 65 (17.8) |
| <90%, N (%) | 5 (26) | 87 (25.1) | 92 (25.1) |
| Admission fever (temperature >37.5°C), N (%) | 7 (37) | 122 (35.2) | 129 (35.1) |
| Abnormal Blantyre Coma Scale (<5), N (%) | 5 (26) | 51 (14.7) | 56 (15.3) |
| HIV-positive, N (%) | 1 (5) | 19 (5.5) | 20 (5.5) |
| Malaria RDT positive, N (%) | 0 (0) | 62 (17.9) | 62 (16.9) |
| Admission hemoglobin | |||
| No anemia (Hb ≥11 g/dL), N (%) | 16 (84) | 183 (52.7) | 199 (54.4) |
| Mild anemia (Hb 7–11 g/dL), N (%) | 3 (16) | 99 (28.5) | 102 (27.9) |
| Severe anemia (Hb ≤7 g/dL), N (%) | 0 (0) | 65 (18.7) | 65 (17.8) |
| Admission lactate | |||
| Normal lactate (≤2 mmol), N (%) | 4 (21) | 152 (43.8) | 156 (42.6) |
| Hyperlactemia (2–4 mmol), N (%) | 10 (53) | 126 (36.3) | 136 (37.2) |
| Severe hyperlactemia (≥4 mmol), N (%) | 5 (26) | 69 (19.9) | 74 (20.2) |
| Referred, N (%) | 18 (95) | 205 (59.1) | 223 (60.9) |
| Duration of bad health, N (%) | |||
| In good health before this illness | 12 (63) | 243 (70.0) | 255 (69.7) |
| <1 wk | 5 (26) | 9 (2.6) | 14 (3.8) |
| 1 wk–≤1 mo | 2 (11) | 41 (11.8) | 43 (11.7) |
| >1 mo | 0 (0) | 54 (15.6) | 54 (14.8) |
| WFA Z-score | |||
| No malnutrition (Z > −2), N (%) | 9 (47) | 153 (44.1) | 162 (44.3) |
| Moderate malnutrition (−2 < Z < −3), N (%) | 3 (16) | 65 (18.7) | 68 (18.6) |
| Severe malnutrition (>−3), N (%) | 7 (37) | 129 (37.2) | 136 (37.2) |
| Index admission discharge characteristics | |||
| Discharge status | |||
| Routine discharge, N (%) | 6 (32) | 202 (58.2) | 208 (56.8) |
| Referred to higher level of care, N (%) | 7 (37) | 48 (13.8) | 55 (15.0) |
| Unplanned discharge, N (%) | 6 (32) | 97 (28.0) | 103 (28.1) |
| Length of stay, days, median (IQR) | 4.0 (3.0–6.5) | 6.0 (3.0–9.0) | 5.0 (3.0–9.0) |
| Circumstances of postdischarge death | |||
| Location of postdischarge deathb | |||
| At home, N (%) | 8 (42) | 154 (45.2) | 162 (45.0) |
| In-transit to seeking care, N (%) | 5 (26) | 61 (17.9) | 66 (18.3) |
| During readmission, N (%) | 6 (32) | 126 (37.0) | 132 (36.7) |
| Caregiver sought care between discharge and death, N (%) | 6 (32) | 162 (46.7) | 168 (45.9%) |
| Initial source of care seekingc | |||
| Self-referral, N (%) | 2 (33) | 122 (75.3) | 124 (73.8) |
| Referral from other health care facility, N (%) | 3 (50) | 21 (13.0) | 24 (14.3) |
| Other/unknown, N (%) | 1 (17) | 19 (11.7) | 20 (11.9) |
| Location of care seekingc | |||
| Untrained health worker/drug shop, N (%) | 0 (0) | 8 (4.9) | 8 (4.8) |
| Traditional healer, N (%) | 0 (0) | 2 (1.2) | 2 (1.2) |
| Health center, N (%) | 1 (17) | 26 (16.0) | 27 (16.1) |
| Hospital, N (%) | 5 (83) | 118 (72.8) | 123 (73.2) |
| Unknown | 0 (0) | 8 (4.9) | 8 (4.8) |
Abbreviations: Hb, hemoglobin; P3, primary 3; P4–P7, primary 4 to primary 7; RDT, Rapid Diagnostic Test; S1–S6, secondary 1 to secondary 6; WFA, weight-for-age.
n = 364; 2 children missing exact death date.
n = 360; 6 children missing data on location of death.
n = 168; 198 children/caregivers did not seek any care between discharge and death.
Cause-Specific Mortality
Reviewing physicians felt comfortable assigning a cause of death in all but 5 cases (98.6%). However, reviewers were confident (n = 140, 39.3%), somewhat confident (n = 172, 48.3%), and not confident (n = 44, 12.4%) in their cause of death assignments after reaching a consensus. Among neonates, the leading causes of death after discharge were sepsis and other infectious conditions of the newborn (n = 15, 78.9%), followed by other and unspecified perinatal conditions (n = 3, 15.8%) and other and unspecified congenital malformations (n = 1, 5.3%) (Fig 2). Leading causes of death among older children were pneumonia (n = 96, 27.7%) and sepsis (n = 81, 23.3%), followed by malaria (n = 31, 8.9%) and other and unspecified diarrheal diseases (n = 29, 8.4%) (Fig 3). Physician confidence was generally higher among nonneonates, in which 88.7% (n = 299) of cases were categorized as at least somewhat confident, compared with neonatal cases in which only 68.4% (n = 13) of cases were categorized as at least somewhat confident (P < .05). When analyzing individual diagnosis certainty among the top 5 diagnoses, physicians most often reported a confident level of certainty when assigning malaria (n = 16, 51.6%) as the primary cause of death and least often for meningitis (n = 7, 25.9%).
FIGURE 2.
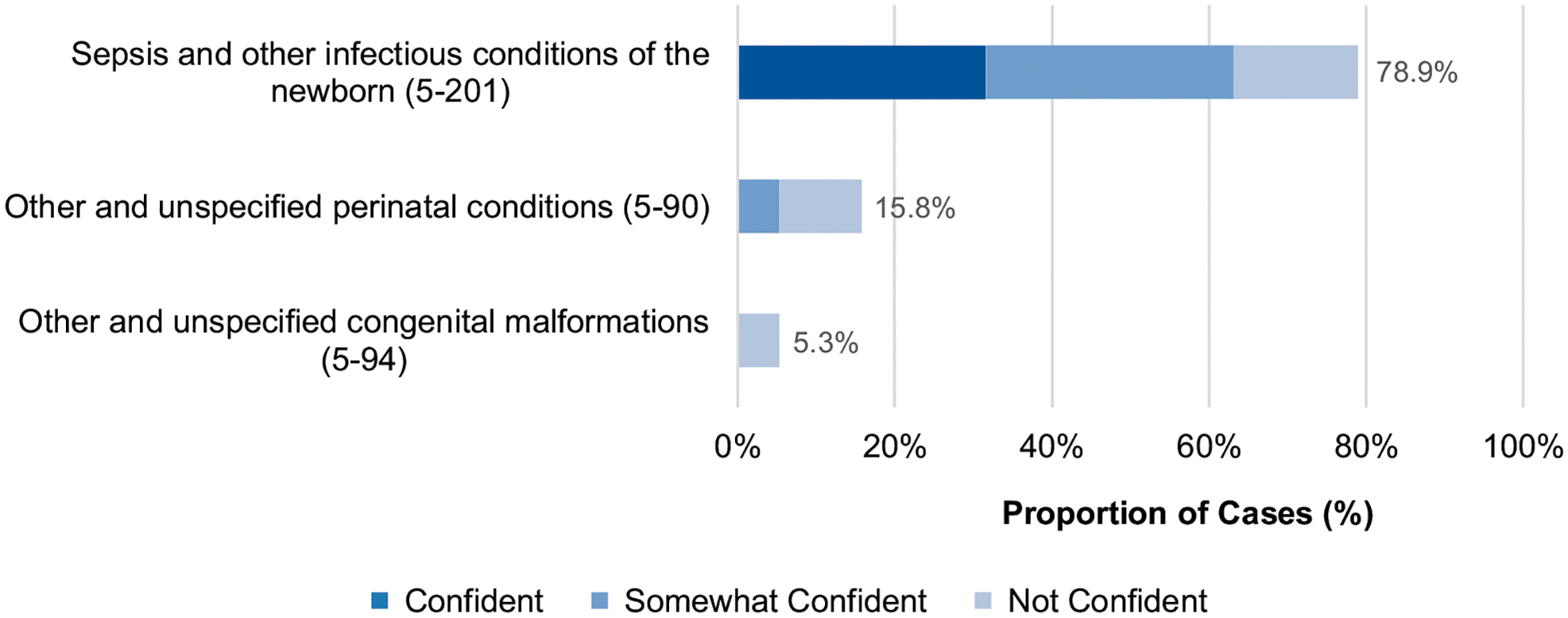
Causes of death proportions (ICD-10-SMoL Causes of Death Code) and physician certainty level for children aged <28 days at death, N = 19. Abbreviation: ICD-10-SMoL, Start-Up Mortality List. Note: Other and unspecified congenital malformations (5–94) includes other congenital malformations of the digestive system, congenital malformations of the respiratory system, cleft lip and cleft palate, etc. Other and unspecified perinatal conditions (5–90) includes hemorrhagic and hematological disorders of fetus and newborn, transitory endocrine and metabolic disorders specific to fetus and newborn, respiratory and cardiovascular disorders specific to the perinatal period, etc.
FIGURE 3.
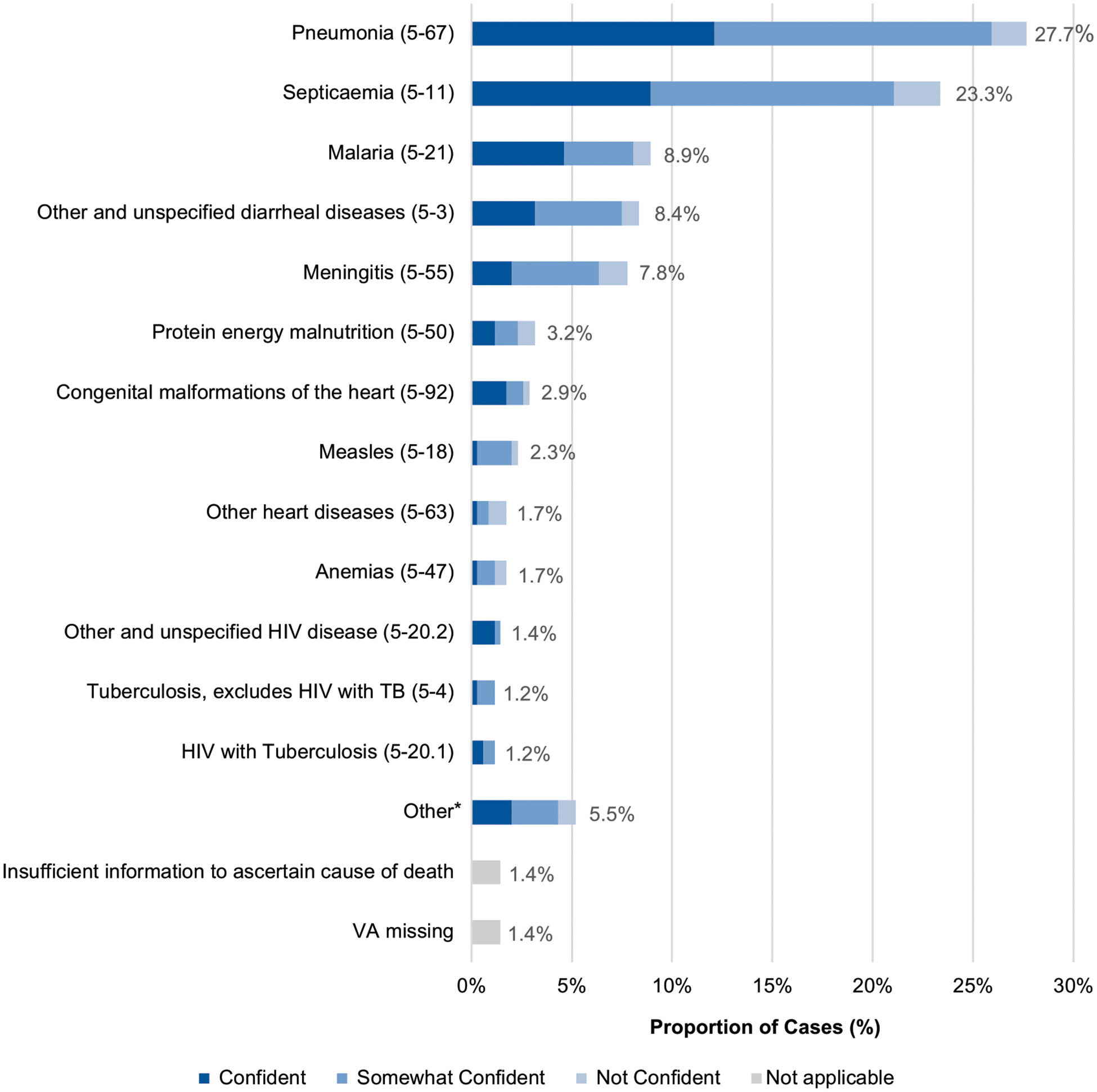
Causes of death proportions (ICD-10-SMoL Causes of Death Code) and physician certainty level for children 28 days to 5 years, N = 347. Abbreviations: ICD-10-SMoL, Start-Up Mortality List; TB, tuberculosis; VA, verbal autopsy. *Other: Other and unspecified diseases of the respiratory system (5–70), 3 (0.9%); Other and unspecified congenital malformations (5–94), 2 (0.6%); Other diseases of the digestive system (5–74), 2 (0.6%); Chronic lower respiratory diseases (5–69), 2 (0.6%); Other and unspecified malignant neoplasms (5–45), 2 (0.6%); Leukemia (5–44), 2 (0.6%); Intrauterine hypoxia and birth asphyxia (5–89), 1 (0.3%); Other and unspecified diseases of the genitourinary system (5–78), 1 (0.3%); Renal failure (5–77), 1 (0.3%); Acute rheumatic fever and chronic rheumatic heart diseases (5–60), 1 (0.3%); Other and unspecified endocrine, nutritional and metabolic diseases (5–51), 1 (0.3%).
Alternative Causes of Death
In most cases (80.4%), at least 1 alternative cause of death was listed in addition to the primary cause of death. Between 0 and 4 alternate causes of death were assigned, with a median number of 1 (IQR, 1–2). For neonates, the most common alternative causes listed were other and unspecified perinatal conditions (n = 5, 26.3%) and low birth weight (n = 3, 15.8%). In older children, the most common were sepsis (n = 96, 27.7%), pneumonia (n = 85, 24.5%), meningitis (n = 49, 14.1%), and malaria (n = 39, 11.2%).
Significant Contributing Conditions
The most commonly identified significant conditions contributing to death were malnutrition (n = 185, 50.5%), anemia (n = 94, 25.7%), and HIV-related illnesses (n = 15, 4.1%). When analyzing cause of death by significant condition, a greater proportion of children in which malnutrition was listed as a contributory cause had sepsis (n = 54, 29.2%) listed as the primary cause of death compared with those without malnutrition (n = 27, 15.8%) (P < .01). Similarly, when looking at cases with anemia listed, there was a higher proportion of sepsis (n = 32, 34.0% vs n = 49, 18.7%, P < .01), malaria (n = 17, 18.1% vs n = 14, 5.4%, P < .001), and HIV-related illnesses (n = 5, 4.3% vs n = 4, 1.5%, P < .05) reported as the primary cause of death.
Differences in Cause of Death Over Time
To determine if causes of death varied by the time elapsed since discharge from hospital, deaths were divided into those occurring early (less than 1 month from discharge) and those occurring late (1 to 6 months from discharge) and the prevalence of cause of death in each temporal group was compared (Fig 4). Compared with those who died more than 1 month after discharge, children who died early were more commonly assigned malaria (n = 18, 9.5% vs n = 12, 6.9%), diarrheal diseases (n = 17, 8.9% vs n = 12, 6.9%), or congenital malformations of the heart (n = 9, 4.7% vs n = 1, 0.6%) as the most probable cause of death. Only congenital malformations of the heart was found to be statistically significant (P < .05). Alternatively, later deaths were more commonly assigned malnutrition (n = 10, 5.7% vs n = 1, 0.5%), pneumonia (n = 50, 28.4% vs n = 45, 23.7%), and anemia (n = 5, 2.8% vs n = 1, 0.5%) as probable causes of death, with malnutrition as the only cause found to be statistically significant (P < .01). However, anemia was more likely to be listed as a significant condition contributing to death in children who died more than 1 month after discharge (n = 55, 31.6% vs n = 37, 19.5%, P < .01). To provide some insight on whether children died after discharge from the same illness for which they were admitted, we reviewed whether the assigned postdischarge cause of death was mentioned in the index admission discharge diagnosis, if provided. Concordance overall between discharge diagnosis and primary cause of death was 56.9%, increasing to 65.6% for children who died within 1 month after discharge, and decreasing to 47.0% thereafter (P < .001).
FIGURE 4.
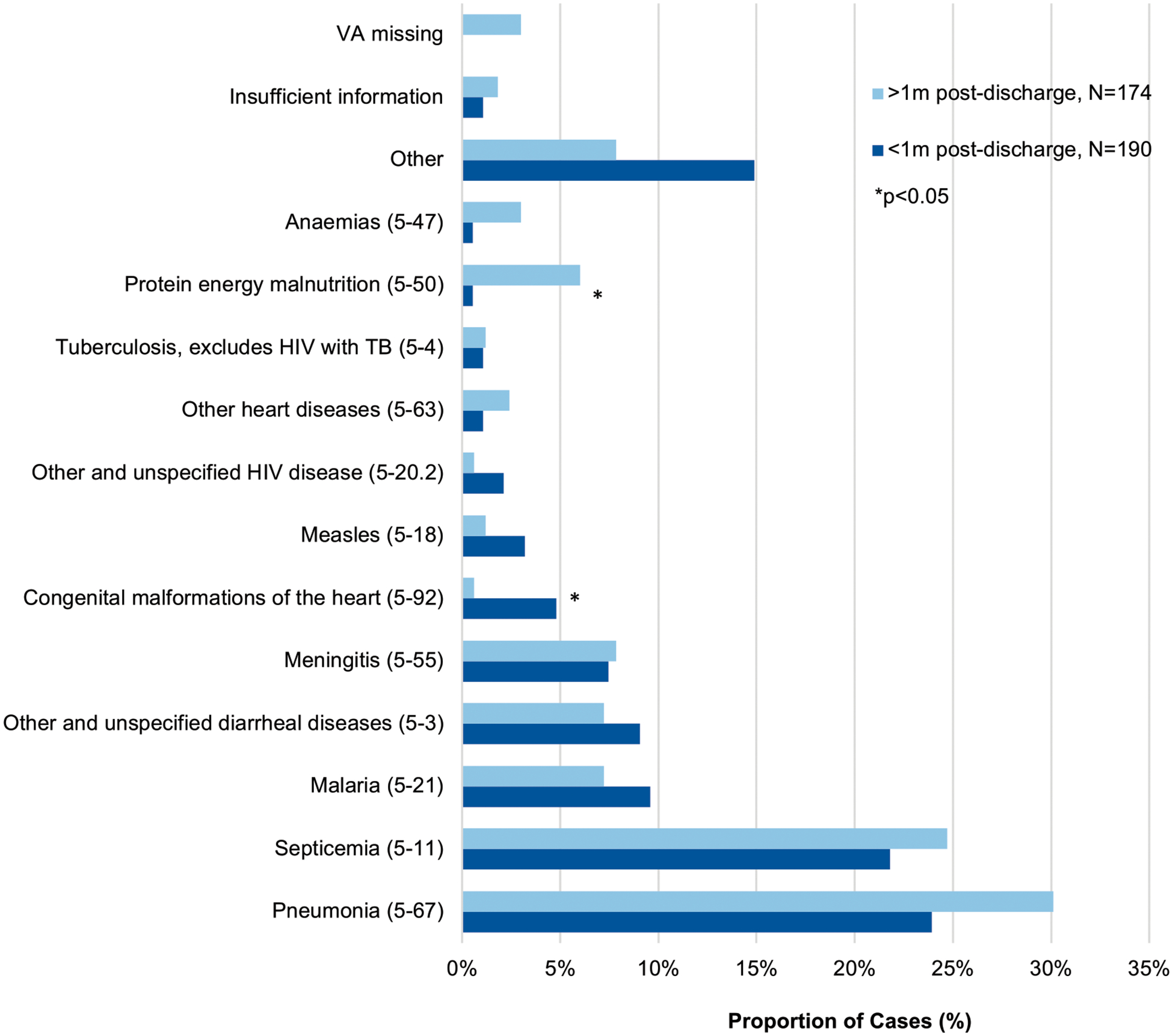
Causes of death proportions (ICD-10 SMoL Causes of Death Code) <1 month and >1 month after discharge among children aged 0 to 5 years, N = 364a. Abbreviations: ICD-10-SMoL, Start-Up Mortality List; TB, tuberculosis. aTwo children missing an exact death date and were excluded from this analysis.
DISCUSSION
In this study of a large cohort of neonates and children under-5 admitted for suspected sepsis, postdischarge deaths occurred as frequently as in-hospital deaths. The use of VA captured postdischarge deaths in the community, including deaths while children were in transit to a health facility. Further information from these VAs allowed expert reviewers to assign potential causes and significant contributors of these postdischarge deaths, demonstrating the utility of VA for characterizing the causes of out-of-facility deaths. This study provides crucial data informing the epidemiology of the incidence and causes of mortality in children under-5 with suspected sepsis to guide more effective public health policies and interventions in LMIC settings.
Physician reviewers most commonly attributed the causes of neonatal postdischarge death to sepsis and unspecified perinatal conditions. Similarly, a study of infants younger than 60 days admitted to hospitals in Kenya reported that clinician-reported causes of death within 1 year after discharge were most commonly from neonatal sepsis and preterm complications.18 However, the latter study reported the causes of death for those children who died during readmission to a health facility, representing less than one third of all study deaths. For the older children under-5, the leading causes of death assigned were pneumonia, sepsis, malaria, and diarrheal disease. These results are similar to estimates reported in another study of an acutely ill, pediatric population in sub-Saharan Africa and south Asia, where severe sepsis, pneumonia, and diarrhea were the leading causes of postdischarge death.10 Comparatively, the most recent GBD study conducted in 2019 reported neonatal disorders as the most common cause of death for children under-5 in Uganda, followed by malaria and lower respiratory infection.19 A higher proportion of deaths attributable to sepsis in this study is expected given that it was an inclusion criterion for enrolment in the prospective cohort. Sepsis may have also been overrepresented in the assigned causes because of its protean clinical presentation, lack of specific diagnostic criteria, and broad definition. Furthermore, many neonatal deaths occur during the initial birth admission. Because admissions from birth were excluded in the parent study, many neonatal conditions leading to death are likely to be underrepresented.
Anemia was a common contributing factor of postdischarge mortality in this study and has been increasingly recognized as such in the literature.3,20 A prior analysis of this cohort found that, relative to children without anemia, those with anemia have an increasing risk for mortality over the postdischarge period, rising from hazard ratio, 1.7 (95% confidence interval, 0.9–3.0) to hazard ratio, 5.2 (95% confidence interval, 3.1–8.5) between the early and late postdischarge periods.13 Treatment of anemia following discharge with a multivitamin multimineral supplement, iron and folate, or co-trimoxazole prophylaxis, however, has been shown in a randomized controlled trial in Uganda and Malawi to be ineffective at improving survival,21 suggesting an urgent need for alternative strategies to be explored. Among children with severe anemia associated with malaria, intermittent malaria chemoprophylaxis has been shown to impart a survival advantage.22 Among those with nonmalarial anemia, it is possible that the confluence of anemia with multiple comorbidities such as HIV or malnutrition imparts an increased risk of postdischarge mortality; further studies are required to better understand the complex relationships among these illnesses and their effects.20
Return visits to seek care at health facilities in Uganda occur infrequently because of socioeconomic barriers, including poor health care access, high out-of-pocket costs, lack of trust in the system, low caregiver health knowledge, and negative prior experiences.23,24 Thus, many deaths occur at home and most mortality estimates fail to capture these deaths as a consequence of underdeveloped civil registration and vital statistics systems.25 The ability to capture this often-largely overlooked population is a significant strength of our study, emphasizing the importance of capturing the causes of community-occurring deaths. A previous study in rural western Uganda demonstrated that community health workers (CHWs) were able to conduct high-quality, standardized VA interviews to provide a more complete estimate of the burden and causes of mortality in rural community settings.4 The CHW model is widespread in Africa,26 and utilization of CHWs for collection of vital statistics data using VA could be a strategy to ensure capture and inclusion of community deaths in mortality metrics systems for these settings. This CHW program can be scaled to improve the measurement of vital statistics, as well as facilitate appropriate public health interventions in rural areas of sub-Saharan Africa.
A child-centered approach to follow-up, leveraging CHWs to provide education and follow-up to children at high risk (such as those with anemia or malnutrition) during the periods of highest risk, may be an efficient strategy to address postdischarge mortality.13,27 Furthermore, concerted efforts to overcome the complex barriers to care often experienced by those most vulnerable to postdischarge outcomes must be prioritized.28 These may include interventions such as incentivization of follow-up and development of better linkages across the various tiers of the health system to improve transitions of care.
Although VA presents a useful strategy for estimating causes of death in many parts of sub-Saharan Africa, this method has known limitations.29–31 One of the major drawbacks of physician-performed VA analysis is the substantial time physicians require to review the data. Numerous computational VA algorithms have been developed to save time and circumvent physician subjectivity, including InterVA, InSillicoVA, Naïve Bayes Classifier, Tariff, and Tariff2.32 However, the accuracy of these methods in determining community-based causes of death remains relatively lower than physician-certified verbal autopsy,29 and no assessment to date has afforded preference to any specific algorithm.32 Another limitation is that clinician-determined cause of death has been previously shown to have poor sensitivity in comparison with complete diagnostic autopsy,33–35 and physician review of VA is not immune to such challenge. Without laboratory test confirmation, illnesses with overlapping symptom profiles, such as malaria and meningitis, are often detected by VA with poor sensitivity.31 To address this, we provided reviewers with all available study data from the index admission, including laboratory test results. Yet, this information may be less helpful in cases where children died much later in the postdischarge period. As an alternative strategy, some studies have demonstrated the utility of minimally invasive tissue sampling for determining causes of death.36,37 When supplemented with VA data, this has been shown to reasonably enhance the precision of community cause of death assignment, though requires additional resources.36 Future improvements are necessary to enhance the reliability of VA, and the use of minimally invasive tissue sampling should be encouraged for future research in this area. Last, further investigation of the social and clinical circumstances surrounding the deaths of children in sub-Saharan Africa may also improve understanding of postdischarge mortality.38,39 Increased knowledge and awareness of the social factors contributing to death may lead to more effective interventions than estimations and prevention measures aimed at decreasing cause-specific mortality. Further studies are needed to explore the social circumstances and potentially avoidable factors surrounding pediatric postdischarge mortality.
CONCLUSIONS
For children under-5 admitted for suspected sepsis in Uganda, postdischarge mortality within 6 months after discharge occurred as frequently as in-hospital deaths, most often in the community. Physician-performed VA analysis of the probable causes for these deaths suggests their potential preventability through increased engagement with the health system during the vulnerable post discharge period, and through the treatment and prevention of underlying contributors such as malnutrition and anemia.
Supplementary Material
WHAT’S KNOWN ON THIS SUBJECT:
Pediatric postdischarge mortality after admission for sepsis is a significant contributor to children younger than age 5 years’ mortality in low-income settings. Few studies have examined the causes and location of postdischarge deaths.
WHAT THIS STUDY ADDS:
We present verbal autopsy data for a large cohort of children with suspected sepsis who died within 6 months following discharge from 6 health facilities. Postdischarge deaths were most often attributable to infections. Malnutrition and anemia were frequent contributing factors.
ACKNOWLEDGMENTS
The authors express their gratitude to the following individuals from the Smart Discharges research program for their support in all study activities:
Stephen Businge, Abner Tagoola, Sheila Oyella Sherine, Emmanuel Byaruhanga, Edward Ssemwanga, Celestine Barigye, Jesca Nsungwa, Charles Olaro, Joel Singer, Charles P Larson, Stefanie Novakowski, Clare Komugisha, Mellon Tayebwa, Nicholas West, Nathan Kenya Mugisha, Tayebwa Mellon, Komugisha Clare, Agaba Collins, Twinomujuni Annet, Mutungi Alexander, Kembabazi Brenda, Kamazima Justine, Ankwatse Christine, Muhangi Benedicto, Atuhaire Obed, Naigaga Shamina, Nabweteme Mary Annette, Namulondo Lamulatih, Nakabiri Zaituni, Opuko Wilson, Hassan Baryahikwa, Nabawanuka Abbey Onyachi, Kugumikiriza Brenda, Juwa Ruth, Mwoya Yumani, Macklin Naturinda, Mugumya Cleophus, Tumwebaze Godfrey, Mubiru Ronald, Bulage Mary, Mwesigye Isaac, Kamba Ayub, Kamusiime Olivia, Twebaze Florence, Twesigye Leonidas, Tamusange Vincent, Kiiza Israel, Asiimwe Abibu, Ainembabazi Harriet, Nakafero Joan, Kairangwa Racheal, Nuwasasira Agaston, Kayegi Maliza, Kisaame Zorah, Tumukunde Goreth, Tukoreki Evas, Dyonisius Tuhame, Joan Namuddu, Julius Kiwanuka, Joseph Mugerwa, Albert Kamugisha, Winfred Kyobejja, Kitenda Julius, Mwigarire Provia, Asiimwe Bernadette, Tugumenawe Darius, Ounyesiga Thomas, Muhumuza Deudant, Waiswa Peter, Bamwesigye Ezrah, Okeny Louis, Kalyango Daniel, Tusingwire Fredson, Oweka Jimmy, Mwaka Savio, Kabajaasi Olive, Nsangi Damalie, Charlene Kanyali, Catherine Kiggundu, Tamara Dudley, Sahar Zandi Nia, Rishika Bose, Alishah Mawji, Brooklyn Nemetchek, Michelle Langlois, Sichen Liu, Peter Lewis, Maryum Chaudhry, Teresa Johnson, Alexia Krepiakevich, Dustin Dunsmuir, Jeffrey Bone, Vuong Nguyen, Cherri Zhang, and Jessica Trawin.
FUNDING:
This study was funded by Grand Challenges Canada (grant TTS-1809-1939), the Thrasher Research Fund (grant 13878), the BC Children’s Hospital Foundation, Mining4Life. Dr Kortz supported by the National Institute of Allergy and Infectious Diseases (award K23144029).
ABBREVIATIONS
- CHW
community health worker
- GBD
global burden of disease
- ICD-10
International Classification of Diseases 10th Revision
- IQR
interquartile range
- LMIC
low- and middle-income countries
- SMoL
Start-Up Mortality List
- VA
verbal autopsy
Footnotes
CONFLICT OF INTEREST DISCLOSURES: The authors have indicated they have no potential conflicts of interest to disclose.
REFERENCES
- 1.United Nations Inter-Agency Group for Child Mortality Estimation. Levels and trends in child mortality: report 2022. Available at: https://data.unicef.org/wp-content/uploads/2023/01/UN-IGME-Child-Mortality-Report-2022_Final-online-version_9Jan.pdf. Accessed February 12, 2023
- 2.Wiens MO, Pawluk S, Kissoon N, et al. Pediatric post-discharge mortality in resource poor countries: a systematic review. PLoS One. 2013;8(6):e66698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nemetchek B, English L, Kissoon N, et al. Paediatric postdischarge mortality in developing countries: a systematic review. BMJ Open. 2018;8(12):e023445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nabukalu D, Ntaro M, Seviiri M, et al. Community health workers trained to conduct verbal autopsies provide better mortality measures than existing surveillance: results from a cross-sectional study in rural western Uganda. PLoS One. 2019;14(2):e0211482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de André CDS, Bierrenbach AL, Barroso LP, et al. Validation of physician certified verbal autopsy using conventional autopsy: a large study of adult non-external causes of death in a metropolitan area in Brazil. BMC Public Health. 2022;22(1):748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mpimbaza A, Filler S, Katureebe A, Quick L, Chandramohan D, Staedke SG. Verbal autopsy: evaluation of methods to certify causes of death in Uganda. PLoS One. 2015;10(6):e0128801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soofi SB, Ariff S, Khan U, et al. Diagnostic accuracy of WHO verbal autopsy tool for ascertaining causes of neonatal deaths in the urban setting of Pakistan: a hospital-based prospective study. BMC Pediatr. 2015;15:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran HT, Nguyen HP, Walker SM, Hill PS, Rao C. Validation of verbal autopsy methods using hospital medical records: a case study in Vietnam. BMC Med Res Methodol. 2018;18(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Institute for Health Metrics and Evaluation. Verbal autopsy tool. Available at: https://www.healthdata.org/data-tools-practices/verbal-autopsy#:~:text=Verbal%20autopsy%20(VA)%20is%20a,a%20complete%20vital%20registration%20system. Accessed December 14, 2022
- 10.Childhood Acute Illness and Nutrition (CHAIN) Network. Childhood mortality during and after acute illness in Africa and south Asia: a prospective cohort study. Lancet Glob Health. 2022;10(5):e673–e684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madrid L, Casellas A, Sacoor C, et al. Postdischarge mortality prediction in sub-Saharan Africa. Pediatrics. 2019;143(1):e20180606 [DOI] [PubMed] [Google Scholar]
- 12.Hau DK, Chami N, Duncan A, et al. Post-hospital mortality in children aged 2–12 years in Tanzania: a prospective cohort study. PLoS One. 2018;13(8):e0202334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiens MO, Bone JN, Kumbakumba E, et al. Mortality after hospital discharge among children younger than 5 years admitted with suspected sepsis in Uganda: a prospective, multisite, observational cohort study. Lancet Child Adolesc Health. 2023;7(8):555–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Verbal autopsy standards: the 2016 WHO verbal autopsy instrument. Available at: https://www.who.int/publications/m/item/verbal-autopsy-standards-the-2016-who-verbal-autopsy-instrument. Accessed July 10, 2022
- 15.Child Acute Illness and Nutrition (CHAIN) Network. CHAIN inpatient verbal autopsy V1. Available at: https://chainnetwork.org/wp-content/uploads/2018/11/CHAIN_Inpatient-Verbal-Autopsy-CRF-v1.60.pdf. Accessed July 10, 2022
- 16.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. WHO application of ICD-10 for low-resource settings initial cause of death collection: The Startup Mortality List (ICD-10-SMoL), V2.1. Available at: https://cdn.who.int/media/docs/default-source/classification/other-classifications/mortality-list/who-application-of-icd-10-for-low-resource-settings-initatial-cause-of-death-collection.pdf. Accessed July 10, 2022
- 18.Talbert A, Ngari M, Obiero C, et al. Trends in inpatient and post-discharge mortality among young infants admitted to Kilifi County Hospital, Kenya: a retrospective cohort study. BMJ Open. 2023;13(1):e067482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwambai TK, Mori AT, Nevitt S, et al. Post-discharge morbidity and mortality in children admitted with severe anaemia and other health conditions in malaria-endemic settings in Africa: a systematic review and meta-analysis. Lancet Child Adolesc Health. 2022;6(7):474–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maitland K, Olupot-Olupot P, Kiguli S, et al. ; TRACT trial group. Co-trimoxazole or multivitamin multimineral supplement for post-discharge outcomes after severe anaemia in African children: a randomised controlled trial. Lancet Glob Health. 2019;7(10):e1435–e1447 10.1016/S2214-109X(19)30345-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwambai TK, Dhabangi A, Idro R, et al. Malaria chemoprevention in the postdischarge management of severe anemia. N Engl J Med. 2020;383(23):2242–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krepiakevich A, Khowaja AR, Kabajaasi O, et al. Out of pocket costs and time/productivity losses for pediatric sepsis in Uganda: a mixed-methods study. BMC Health Serv Res. 2021;21(1):1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.English L, Kumbakumba E, Larson CP, et al. Pediatric out-of-hospital deaths following hospital discharge: a mixed-methods study. Afr Health Sci. 2016;16(4):883–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molyneux E, Molyneux S. Learning to listen. Pediatrics. 2021; 147(4):e2020044081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ministry of Health Uganda. National village health teams (VHT) assessment in Uganda. Available at: http://library.health.go.ug/sites/default/files/resources/National%20VHT%20Assessment%20in%20Uganda%20Report%202015.pdf. Accessed February 13, 2023
- 27.Wiens MO, Kissoon N, Kabakyenga J. Smart hospital discharges to address a neglected epidemic in sepsis in low- and middle-income countries. JAMA Pediatr. 2018;172(3):213–214 [DOI] [PubMed] [Google Scholar]
- 28.Akech S, Kwambai T, Wiens MO, Chandna A, Berkley JA, Snow RW. Tackling post-discharge mortality in children living in LMICs to reduce child deaths. Lancet Child Adolesc Health. 2023;7(3): 149–151 [DOI] [PubMed] [Google Scholar]
- 29.Tunga M, Lungo J, Chambua J, Kateule R. Verbal autopsy models in determining causes of death. Trop Med Int Health. 2021; 26(12):1560–1567 [DOI] [PubMed] [Google Scholar]
- 30.Herrera S, Enuameh Y, Adjei G, et al. A systematic review and synthesis of the strengths and limitations of measuring malaria mortality through verbal autopsy. Malar J. 2017;16(1):421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garenne M, Fauveau V. Potential and limits of verbal autopsies. Bull World Health Organ. 2006;84(3):164. [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization. Verbal autopsy standards: ascertaining and attributing causes of death tool. Available at: https://www.who.int/standards/classifications/other-classifications/verbal-autopsy-standards-ascertaining-and-attributing-causes-of-death-tool. Accessed July 10, 2022
- 33.Ordi J, Castillo P, Garcia-Basteiro AL, et al. Clinico-pathological discrepancies in the diagnosis of causes of death in adults in Mozambique: a retrospective observational study. PLoS One. 2019; 14(9):e0220657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menéndez C, Quintó L, Castillo P, et al. Quality of care and maternal mortality in a tertiary-level hospital in Mozambique: a retrospective study of clinicopathological discrepancies. Lancet Glob Health. 2020;8(7):e965–e972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng J, Deng X, Wang G, Duan Y, Peng J, Yin F. A retrospective analysis of pathological and clinical diagnoses: report of 240 pediatric autopsies. Fetal Pediatr Pathol. 2012;31(2):63–73 [DOI] [PubMed] [Google Scholar]
- 36.Caballero MT, Grigaites SD, De la Iglesia Niveyro PX, et al. ; Community Mortality Network. Uncovering causes of childhood death using the minimally invasive autopsy at the community level in an urban vulnerable setting of Argentina: a population-based study. Clin Infect Dis. 2021;73(suppl 5):S435–S441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanko NM, Bakytkaly I, Issanov A, Poddighe D, Terzic M. Validating a Minimally Invasive Tissue Sampling (MITS) method in determining cause of death in stillbirths and neonates. Children (Basel). 2021;8(12):1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willcox ML, Kumbakumba E, Diallo D, et al. Circumstances of child deaths in Mali and Uganda: a community-based confidential enquiry. Lancet Glob Health. 2018;6(6):e691–e702 [DOI] [PubMed] [Google Scholar]
- 39.Lapidot R, Larson Williams A, MacLeod WB, et al. Verbal autopsies for out-of-hospital infant deaths in Zambia. Pediatrics. 2021;147(4):e20201767 10.1542/peds.2020-1767 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


