Abstract
Background
The world has witnessed one of the largest pandemics, dubbed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). As of December 2020, the USA alone reported 98,948 cases of coronavirus disease 2019 (COVID-19) infection during pregnancy, with 109 related maternal deaths. Current evidence suggests that unvaccinated pregnant women infected with SARS-CoV-2 are at a higher risk of experiencing complications related to COVID-19 compared to nonpregnant women. This review aimed to provide healthcare workers and non-healthcare workers with a comprehensive overview of the available information regarding the efficacy of vaccines in pregnant women.
Summary
We performed a systematic review and meta-analysis following PRISMA guidelines. The search through the database for articles published between December 2019 and October 2021 was performed. A comprehensive search was performed in PubMed, Scopus, and EMBASE databases for research publications published between December 2019 and October 2021. We focused on original research, case reports, case series, and vaccination side effect by authoritative health institutions. Phrases used for the Medical Subject Heading [MeSH] search included (“COVID-19” [MeSH]) or (“Vaccine” [MeSH]) and (“mRNA” [MeSH]) and (“Pregnant” [MeSH]). Eleven studies were selected and included, with a total of 46,264 pregnancies that were vaccinated with mRNA-containing lipid nanoparticle vaccine from Pfizer/BioNTech and Moderna during pregnancy. There were no randomized trials, and all studies were observational (prospective, retrospective, and cross-sectional). The mean maternal age was 32.2 years, and 98.7% of pregnant women received the Pfizer COVID-19 vaccination. The local and systemic adverse effects of the vaccination in pregnant women were analyzed and reported. The local adverse effects of the vaccination (at least 1 dose) such as local pain, swelling, and redness were reported in 32%, 5%, and 1%, respectively. The systemic adverse effects such as fatigue, headaches, new onset or worsening of muscle pain, chills, fever, and joint pains were also reported in 25%, 19%, 18%, 12%, 11%, and 8%, respectively. The average birthweight was 3,452 g. Among these pregnancies, 0.03% were stillbirth and 3.68% preterm (<37 weeks) births.
Key Messages
The systemic side effect profile after administering the COVID-19 mRNA vaccine to pregnant women was similar to that in nonpregnant women. Maternal and fetal morbidity and mortality were lowered with the administration of either one or both the doses of the mRNA COVID-19 vaccination.
Keywords: COVID-19, Vaccine, mRNA, Pregnant, Complications, Outcomes
Introduction
The world has witnessed one of the largest pandemics to date and dubbed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. This pandemic has resulted in a worldwide infection of over 280,680,256 people and over 4,400,850 deaths [2]. As of December 2020, 98,948 cases of coronavirus disease 2019 (COVID-19) infection in pregnancy were reported and 109 related maternal deaths only in the USA [3]. Current evidence suggests that unvaccinated pregnant women infected with SARS-CoV-2 are more likely to experience COVID-19-related complications compared with nonpregnant women [4]. Adverse outcomes seen during SARS-CoV-2 infection in pregnancy include severe infections, increased rates of preeclampsia/eclampsia, preterm births, maternal morbidity and mortality, neonatal morbidity, and severe perinatal morbidity and mortality [5].
On the other hand, vaccine hesitancy among pregnant individuals has been a significant concern, with studies consistently showing higher rates of hesitancy in this population compared to the general adult population. Research from Ireland and the UK found vaccine hesitancy/resistance in 35% and 31% of the general adult populations, respectively [6]. Similarly, a study in Japan reported a vaccine hesitancy rate of 50.9% among pregnant women [7]. Furthermore, studies have demonstrated a higher frequency of vaccine hesitancy and a lower frequency of vaccination among pregnant individuals compared with their nonpregnant counterparts of reproductive age [8]. This is consistent with the findings of a study that highlighted a high rate of COVID-19 vaccine hesitancy among pregnant individuals, which is concerning as vaccine hesitancy may disproportionately affect pregnant individuals [9]. Additionally, prior to the H1N1 pandemic, seasonal influenza vaccine uptake in pregnancy was low, at around 27% [10]. These findings collectively indicate a pattern of higher vaccine hesitancy in pregnant individuals compared to the general adult population.
As recommended by the American College of Obstetricians and Gynecologists (ACOG) and the Centers for Disease Control and Prevention (CDC) in August 2021, two mRNA vaccines (Pfizer-BioNTech BNT162b2, Moderna mRNA-1273) and one based on a replication-incompetent adenovirus recombinant vector (Janssen Ad26.COV2.S or JNJ-78436735) are available for use by pregnant women in the USA [11]. On August 23, 2021, the Food and Drug Administration (FDA) granted full approval for the Pfizer-BioNTech mRNA vaccine, now to be called Comirnaty, for individuals over the age of 16. These vaccines do not contain the virus that replicates; thus, they do not cause disease, but nonspecific side effects from activation of the immune system may occur [3, 12]. Both mRNA vaccines were studied during phase 3 randomized controlled trials and proved to be highly effective at preventing COVID-19 infection in nonpregnant participants; however, pregnant patients were excluded from all clinical trials [13, 14]. The use of the vaccination in pregnant women remains controversial as none of these trials initially included pregnant or lactating women prior to gaining FDA approval [15]. Pfizer and BioNTech, Janssen, and Moderna are currently undergoing interventional (clinical trial) and observational (patient registry) studies [16–18]. This review was conducted to offer healthcare workers and non-healthcare workers a comprehensive and up-to-date overview of the currently available information about the efficacy of available vaccines in pregnant women.
Methods
This systematic review and meta-analysis were done following the PRISMA guidelines [19]. A comprehensive search was performed in PubMed, Scopus, and EMBASE databases for research publications on pregnancy and COVID-19 vaccine published between December 2019 and October 2021 using the terms COVID-19, women, Pregnant, SARS-CoV-2, mRNA, and Vaccine. Phrases used for the Medical Subject Heading [MeSH] search included (“COVID-19” [MeSH]) or (“Vaccine” [MeSH]) and (“mRNA” [MeSH]) and (“Pregnant” [MeSH]). We focused on original research, case reports, case series, and vaccination side effect by authoritative health institutions. All records were retrieved, including original articles, letters to the editor, editorials, and case reports. Given the current scarcity of evidence, preprints, in-press papers, and accepted-for-publication research were also considered.
Eligibility Criteria, Search Strategy, and Study Selection
Studies were included for the systematic review if they met the following criteria: (a) pregnant women who had been vaccinated by COVID-19 mRNA vaccine; (b) at least one side effect after receiving mRNA vaccine, (c) pregnancy outcomes, (d) neonatal outcomes, and (e) accepted articles or published English articles. The exclusion criteria included (a) sampling of non-pregnancy outcomes, (b) duplicate reports, (c) studies where data could not be extracted, (d) non-accepted articles or non-published English articles.
Through abstract screening, the previously indicated inclusion criteria were applied to choose possibly relevant articles. Three authors (A.J.S., A.R.A.-Q., and M.B.A.D.) assessed full texts of relevant articles and screened them according to the inclusion criteria. Where there was difference of opinions, conversation with the senior authors (O.M.A. and R.M.A.-Z.) was held until a consensus was established.
Data Extraction
We extracted as many relevant variables as possible from the information provided (age, date, etc.) and according to the main stratification variable, author, country, data source, age range, study timeframe, baseline population group, outcome (symptoms after vaccination, severity symptoms), total sample, others.
Meta-Analysis
The prevalence of delivery complications was represented using forest plots with 95% confidence intervals. The random-effect model was used to pool the estimated prevalence, and the Cochrane Q statistics and Higgen’s I2 value were used to check heterogeneity in our study. Meta-analysis was performed for the outcome during pregnancy and delivery period, using SATA software V.17. Furthermore, detailed synthesis was used for reporting the results of clinical symptoms with pregnancy after mRNA COVID-19 vaccine and newborn complications. The Q value was applied to discover between-study heterogeneity, and I2 values were calculated to assess statistical heterogeneity. Random-effect model was used based on the level of heterogeneity. Based on the Cochrane criteria, we used the random-effect model when the heterogeneity was over 50% (21). The event rate with 95% CI was calculated for each variable. Egger’s test and visual inspection of the funnel plot were used for assessing publication bias.
Assessment of Risk of Bias
Included studies were assessed by the authors for internal and external validity using the criteria for bias assessment in prevalence and incidence studies [20]. The assessment of bias was conducted for all the eleven papers included in the quantitative analysis. The result is reported in Table 1. All the eleven studies were found of low risk.
Table 1.
Assessment of bias per Hoy criteria described
| Author (year) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Score | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rottenstreich et al. [21] (2022) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | 2 | Low |
| Bookstein Peretz et al. [22] (2021) | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | 1 | Low |
| Goldshtein et al. [23] (2021) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | 2 | Low |
| Blakeway et al. [24] (2022) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | 2 | Low |
| Wainstock et al. [25] (2021) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | 2 | Low |
| Trostle et al. [26] (2021) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | 2 | Low |
| Theiler et al. [27] (2021) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | 2 | Low |
| Jamieson et al. [28] (2006) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 | Low |
| Shimabukuro et al. [29] (2021) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | 2 | Low |
| Gray et al. [30] (2021) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 0 | Low |
| Kadali et al. [31] (2021) | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | 1 | Low |
| Risk of bias item | Risk of bias levels | Risk of bias result |
|---|---|---|
| 1. Was the study’s target population a close representation on national population in relation to relevant variables, e.g., age, sex, occupation? | ||
| 2. Was the sampling frame a true or close representation of the target population? | Yes = low risk = 0 | |
| 3. Was some form of random selection used to select the sample, OR, was a census undertaken? | Low risk = 0–3 | |
| 4. Was the likelihood of nonresponse bias minimal? | No = high risk = 1 | |
| 5. Were data collected directly from the subjects (as opposed to a proxy)? | Moderate risk = 4–6 | |
| 6. Was an acceptable case definition used in the study? | ||
| 7. Was the study instrument that measured the parameter of interest shown to have reliability and validity? | ||
| 8. Was the same mode of data collection used for all subjects? | High risk = 7–9 | |
| 9. Were the numerator(s) and denominator(s) for the parameter of interest appropriate? | ||
| 10. Summary on the overall risk of study bias | ||
Ethics
The protocol for this systematic review was registered in International Prospective Register of Systematic Reviews (PROSPERO) with unique number of CRD42022309054.
Data Analysis
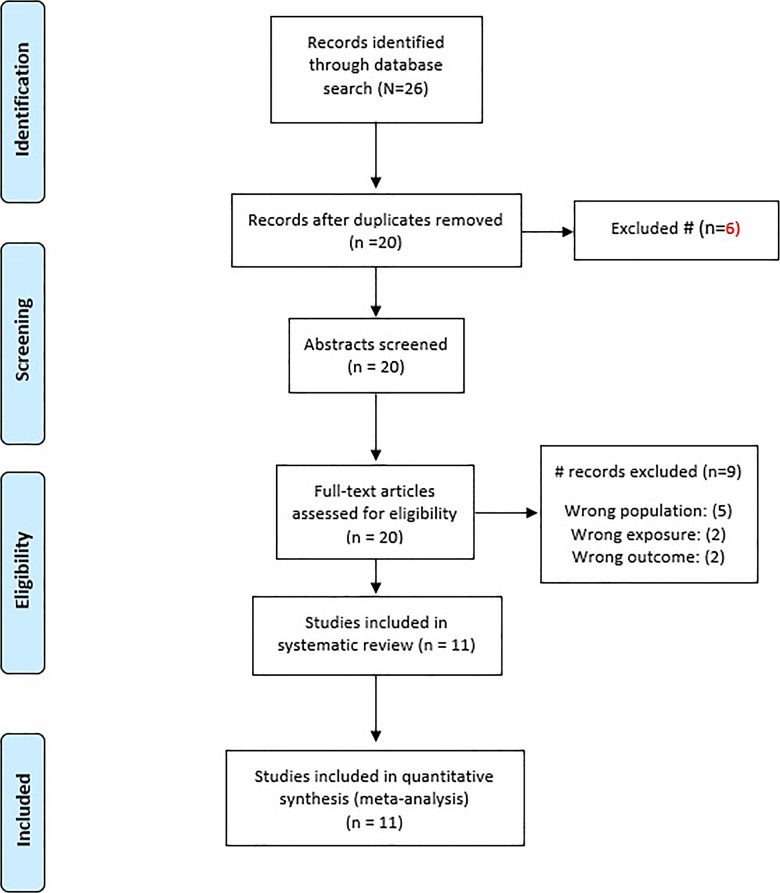
There were 26 studies found in the literature review. Six were disqualified based on their titles. Nine were excluded due to inclusion criteria not being met (wrong population, wrong exposure, and wrong outcome). No non-English articles were reported. The remaining eleven were incorporated into our research study (shown in Fig. 1).
Fig. 1.
PRISMA diagram of literature search.
Results
Study Selection
Eleven studies were included in this review with a total of 46,264 pregnant women, who were vaccinated with a mRNA-containing lipid nanoparticle vaccine Pfizer/BioNTech and Moderna during pregnancy. There were no randomized trials, and all studies were observational (prospective, retrospective, and cross-sectional). The mean maternal age was 32.2 years as shown in Table 2.
Table 2.
Summary of relevant research studies and characteristics of pregnancy after mRNA COVID-19 vaccine
| Author (year) | Country | Study design | Patients, n | Maternal age (mean), years | Enrollment period |
|---|---|---|---|---|---|
| Rottenstreich et al. [21] (2022) | Israel | Retrospective | 712 | 30.6 | (Jan–Apr 2021) |
| Bookstein Peretz et al. [22] (2021) | Israel | Case-control | 390 | 32.5 | (Jan–Feb 2021) |
| Goldshtein et al. [23] (2021) | Israel | Retrospective | 7,530 | 31.1 | (Dec 2020–Feb 2021) |
| Blakeway et al. [24] (2022) | UK | Retrospective | 140 | 35 | (Mar 2020–Jul 2021) |
| Wainstock et al. [25] (2021) | Israel | Retrospective | 913 | 30.6 | (Jan–Jun 2021) |
| Trostle et al. [26] (2021) | USA | Descriptive | 424 | 35 | (Dec 2020–Apr 2021) |
| Theiler et al. [27] (2021) | USA | Retrospective | 140 | 31.8 | (Dec 2020–Apr 2021) |
| Jamieson et al. [28] (2006) | Israel | Prospective | 202 | 31.7 | (Jan–Jan 2021) |
| Shimabukuro et al. [29] (2021) | USA | Retrospective | 35,691 | Ns | (Dec 2020–Feb 2021) |
| Gray et al. [30] (2021) | USA | Prospective | 84 | Ns | (Dec 2020–Mar 2021) |
| Kadali et al. [31] (2021) | USA | Cross-sectional | 38 | Ns | (Jan 2021–Feb 2021) |
| Total | 46,264 | 32.3 |
Medical history of the pregnant received at least one dose of mRNA. The symptoms of vaccinated adults were listed and summarized. In total, 98.7% of pregnant women received Pfizer COVID-19 vaccination as shown in Table 3.
Table 3.
Medical history of the pregnant who have received at least one dose of mRNA
| Author (year) | Medical history of women patients before pregnancy | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| patients, n | BMI, kg/m2 | medical history | vaccine type | |||||||
| ≥30 | <30 | primipara | fertility treatment | DM | HTV | others | mRNA-1273 (Moderna) | BNT162b2 (Pfizer) | ||
| Rottenstreich et al. [21] (2022) | 712 | 101 | 611 | 122 | 33 | Ns | Ns | Ns | 0 | 712 |
| Bookstein Peretz et al. [22] (2021) | 390 | Ns | 390 | Ns | Ns | 13 | 2 | 24 | 0 | 390 |
| Goldshtein et al. [23] (2021) | 7,530 | 825 | 6,705 | 3,447 | 556 | 63 | 51 | 1,561 | 0 | 7,530 |
| Blakeway et al. [24] (2022) | 140 | 15 | 125 | 78 | Ns | 6 | 13 | Ns | 18 | 109 |
| Wainstock et al. [25] (2021) | 913 | 152 | 761 | Ns | 63 | Ns | Ns | Ns | 0 | 913 |
| Trostle et al. [26] (2021) | 424 | 42 | 382 | 267 | Ns | 5 | 28 | 77 | 92 | 332 |
| Theiler et al. [27] (2021) | 140 | 33 | 91 | 0 | 6 | 2 | 6 | 15 | 12 | 127 |
| Jamieson et al. [28] (2006) | 202 | Ns | Ns | 18 | Ns | Ns | Ns | Ns | 0 | 202 |
| Total | 10,451 | 1,168 | 9,065 | 3,932 | 658 | 89 | 100 | 1,677 | 122 | 10,315 |
| Percentage | 100 | 11.2 | 86.7 | 37.6 | 6.3 | 0.9 | 1 | 16 | 1.2 | 98 |
The local and systemic adverse effects of the vaccination in pregnant women were provided in five papers [22, 29–31] as shown in Table 4. Data from 36,337 vaccinated women were analyzed. From our interpretation of the data available, we were able to deduce that the highest local adverse effect of the vaccination (at least 1 dose) was 32% pain at the injection site. Other local injection site adverse effects were swelling seen in 5% and redness in 1%. There were several systemic adverse effects. Overall, 25% complained of fatigue, 19% headaches, 18% new onset or worsening of muscle pain, 12% chills, 11% fever, and 8% joint pains. The least symptoms in pregnant vaccinated women were diarrhea and vomiting seen in 1%.
Table 4.
Clinical signs and symptoms on pregnant after mRNA COVID-19 vaccine
| Author (year) | Clinical symptoms with pregnancy after mRNA COVID-19 vaccine | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| patients, n | injection site | fever | fatigue | headache | chills | vomiting | diarrhea | pain | ||||
| pain | redness | swelling | myalgia | joint | ||||||||
| Shimabukuro et al. [29] (2021) | 35,691 | 11,274 | 660 | 1,462 | 4,242 | 8,772 | 6,800 | 4,502 | 558 | 609 | 6,638 | 3,138 |
| Bookstein Peretz et al. [22] (2021) | 390 | 360 | Ns | 360 | 35 | 220 | 40 | NA | NA | NA | 94 | 16 |
| Gray et al. [30] (2021) | 84 | 44 | 1 | Ns | 25 | 41 | 25 | 25 | N/A | N/A | 37 | NA |
| Kadali et al. [31] (2021) | 38 | 37 | Ns | 3 | 6 | 22 | 19 | 18 | 1 | 0 | 13 | 3 |
| Jamieson et al. [28] (2006) | 134 | Ns | Ns | Ns | 3 | 34 | 22 | NA | NA | NA | NA | NA |
| Total | 36,337 | 11,715 | 661 | 1,825 | 4,311 | 9,089 | 6,906 | 4,545 | 559 | 609 | 6,782 | 3,157 |
| Percentage | 100 | 32.24 | 1.82 | 5.02 | 11.86 | 25.01 | 19.01 | 12.51 | 1.54 | 1.68 | 18.66 | 8.69 |
From the review of eight studies [21, 22, 24–27, 29, 30] with data on neonatal outcomes in 2,880 completed pregnancies, we observed that the average birthweight is 3,452 g (shown in Table 5). Among these pregnancies, 0.03% were stillbirth and 3.68% preterm (<37 weeks) births. Newborn intensive care unit (ICU) admissions accounted for 2.36%, and 6.01% neonates were small for gestational age (SGA). Congenital abnormalities of 2% were observed in only three studies [24, 26, 29]. Three studies reported 21 cases of congenital anomalies in babies born to vaccinated mothers with 2% of the total completed pregnancies in them [24, 26, 29]. Spontaneous abortion was also reported in three studies to have a rate of 2.7% of the total pregnant population [23, 26, 29].
Table 5.
Newborn complications
| Author (year) | Patients, n | Newborn complications | ||||||
|---|---|---|---|---|---|---|---|---|
| newborn ICU admission | birthweight, g | SGA, weeks | stillbirth | preterm <37 weeks | congenital abnormalities | |||
| Rottenstreich et al. [21] (2022) | 712 | 29 | 3,318 | 81 | Ns | 27 | Ns | |
| Bookstein Peretz et al. [22] (2021) | 57 | 2 | 3,269 | 3 | 0 | 0 | Ns | |
| Blakeway et al. [24] (2022) | 133 | 7 | Ns | 16 | 0 | Ns | 3 | |
| Wainstock et al. [25] (2021) | 913 | 14 | 3,224 | 26 | Ns | Ns | Ns | |
| Trostle et al. [26] (2021) | 85 | 13 | Ns | 10 | 0 | 5 | 2 | |
| Theiler et al. [27] (2021) | 140 | 1 | Ns | 14 | 0 | 13 | Ns | |
| Shimabukuro et al. [29] (2021) | 827 | Ns | Ns | 23 | 1 | 60 | 16 | |
| Gray et al. [30] (2021) | 13 | 2 | 3,452 | Ns | 0 | 1 | Ns | |
| Total | 2,880 | 66 | 3,452 | 173 | One death | 106 | 21 | |
Meta-Analysis of the Prevalence of Delivery Methods and Complications among Vaccinated Pregnant Women
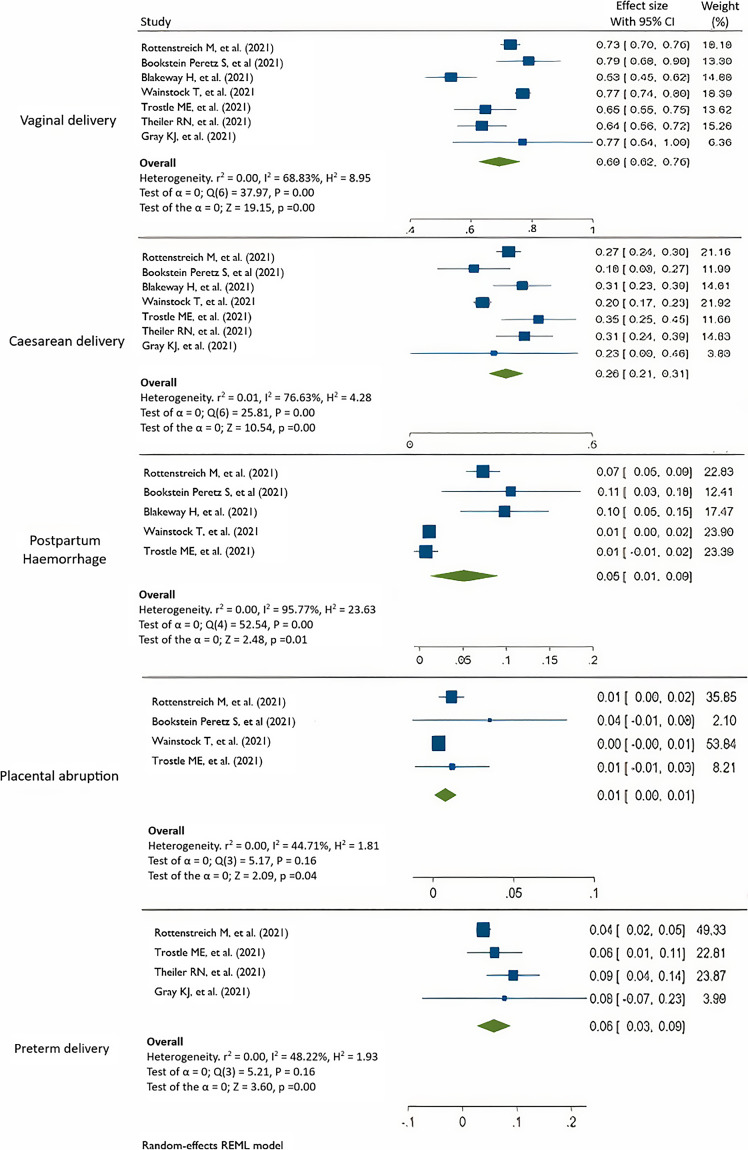
According to the Cochrane criteria, a p value of 0.05 was considered significant for the Cochrane Q, and an I2 > 50% was indicative of considerable heterogeneity. Figure 2 summarizes the meta-analysis results. As COVID-19 is a relatively new disease, which emerged in 2019, only eleven studies met our inclusion criteria. Among these, seven studies reported the rates of vaginal deliveries (VDs) and cesarean deliveries [21, 22, 24–27, 30], five studies reported rates of postpartum hemorrhage [21, 22, 24, 26, 27], and 4 studies reported rates of placental abruption [21, 22, 25, 26] and preterm deliveries [21, 26, 27, 30]. A considerable amount of heterogeneity existed when pooling prevalence I2 = 98.65. In order to detect the sources of heterogeneity, subgroup analysis and meta-regression were conducted as shown in Figure 3.
Fig. 2.
Forest plots of combined and rate of delivery complications.
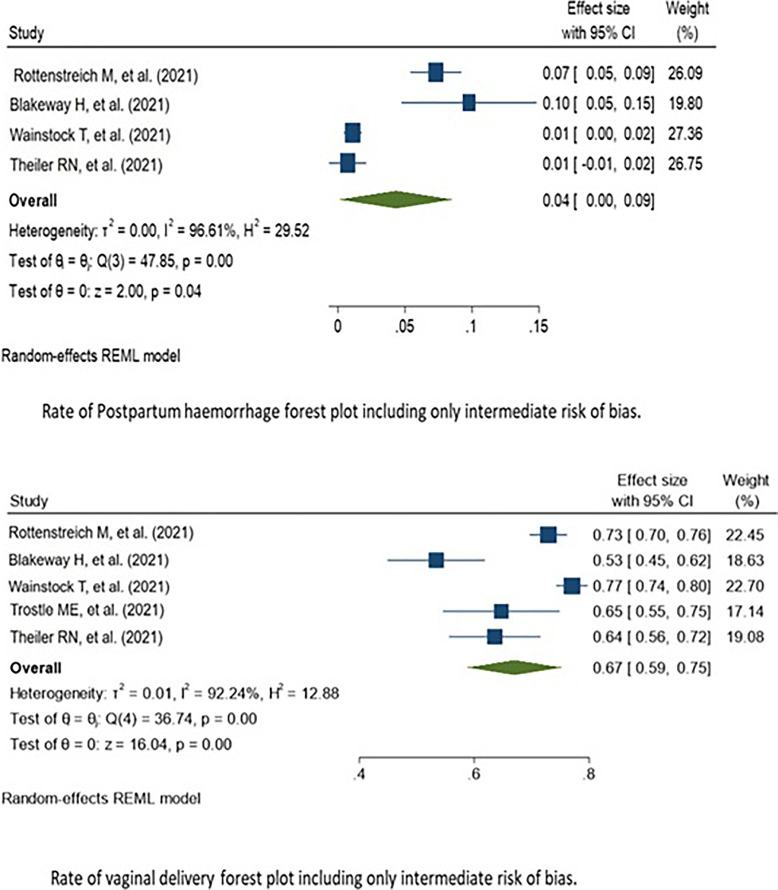
Fig. 3.
Forest plots of subgroup analysis.
Subgroup Analysis
To elucidate the origin of substantial heterogeneity in rates of VD and postpartum complication (I2 = 88% and 95% retrospectively), we conducted a retrospective analysis. After keeping the moderate risk of bias studies, the pooled prevalence of delivery complications was almost similar to the VD rate including all rating of bias (shown in Fig. 3). The prevalence in five moderate risk of bias studies was 67% (95% CI: 65–72%). The same applies to postpartum rate when only intermediate studies were included; the prevalence in 4 moderate risk of bias studies was 5% (95% CI: 0–9%), almost same to rate of postpartum hemorrhage including all different levels of bias.
Meta-Regression
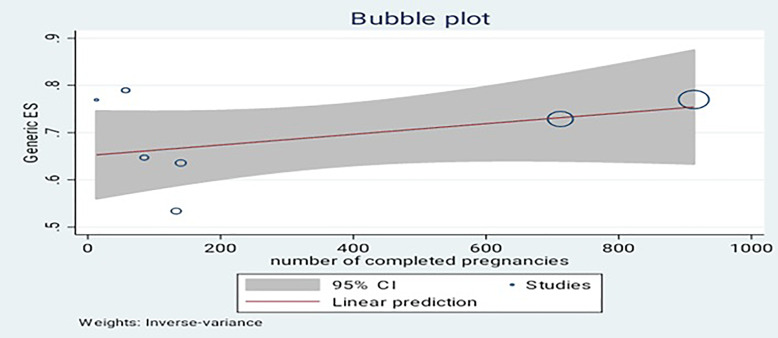
Meta-regression was used to assess if there is a significant correlation between pooled estimate and number of subjects included in each study, whether small studies tend to overestimate prevalence compared to large studies. Although the linear prediction tells us that there is a positive correlation, p value of meta-regression shows 0.234, which indicates no significant association between number of participants included in the study and summarized average prevalence (shown in Fig. 4).
Fig. 4.
Meta-regression of number of the studies.
Risk of Bias of Included Studies
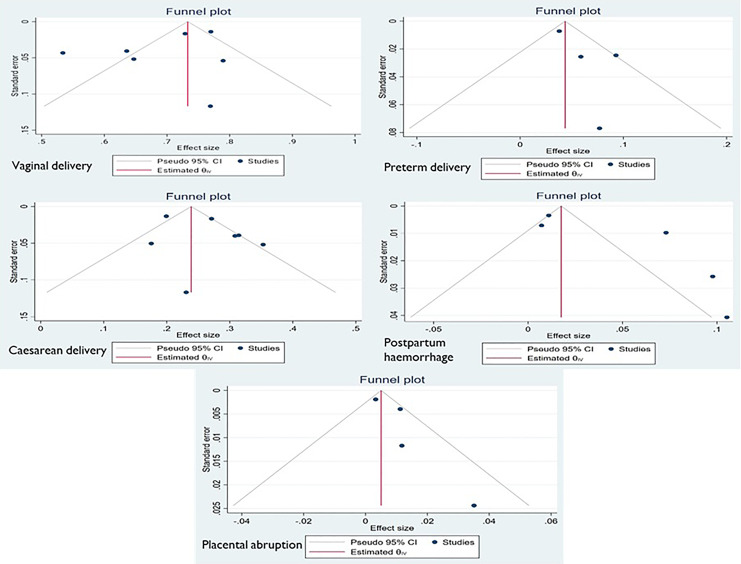
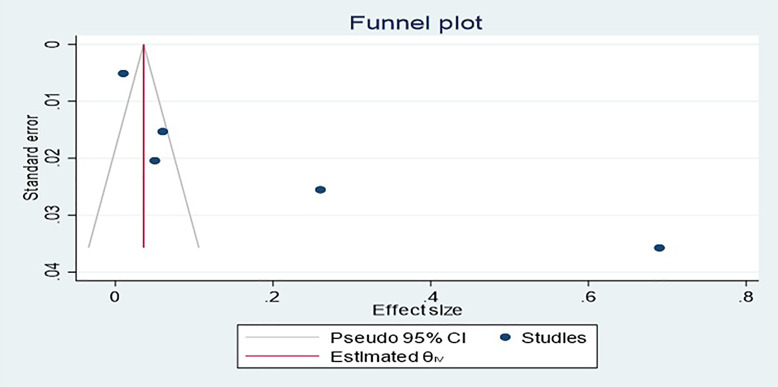
Funnel plot and Egger’s test were used to look for publication bias, and funnel plot revealed no evidence of publication bias. The plot shows approximate symmetry of the studies included. This result was confirmed by p value for Egger’s regression intercept test, which was 0.9317, showing that there was no indication of publication bias in each complication (shown in Fig. 5) as well as in all complications (shown in Fig. 6).
Fig. 5.
Publication bias of each complication of pregnancy.
Fig. 6.
Publication bias for all complications.
Discussion
Main Findings
Immunologic changes in pregnancy alter the susceptibility and severity of contracting certain infectious diseases [28]. This systematic review comprises a cohort of reproductive age pregnant women who received the mRNA-containing lipid nanoparticle vaccine Pfizer/BioNTech and Moderna during pregnancy. From recent studies and reviews, it appears that pregnant women are not at increased risk of developing COVID-19 [32]. Infected pregnant women are known to have severe complications of COVID-19 when compared with the nonpregnant, which include increased risk of hospitalization, ICU admission, invasive ventilation, and death [5, 33, 34]. Increased maternal age, high body mass index, pre-existing hypertension, ethnicity (black, Asian, Latino), and pre-existing diabetes were associated with severe COVID-19 [35]. A study including 1,219 patients conducted by the Maternal-Fetal Medicine Unit Network reported that mothers with severe COVID-19 infection and their neonates are at increased risk for perinatal complications compared to asymptomatic mothers. Increased rates of cesarean births, hypertensive disorders of pregnancy, venous thromboembolism, neonatal ICU admission, and low birthweight were reported in symptomatic pregnant women [36–38].
Injection site pain and fatigue were reported more frequently among vaccinated pregnant women. Other findings of redness at the injection site, headache, myalgia, chills, and fever were reported less frequently. Shimabukuro et al. [29] from a US surveillance published their preliminary results of mRNA COVID-19 vaccine safety in pregnant women, and our review of four other studies that discussed the elicited local and systemic reactions is akin to their findings.
Chronic hypertension, gestational hypertension, preeclampsia, and chronic hypertension with superimposed preeclampsia define hypertensive disorders in pregnancy which complicate 5–10% of pregnancies and are increasing in prevalence with increasing rates of cardiometabolic diseases in younger women [39]. The prospect of preeclampsia and eclampsia increased by 1.76 times in pregnancies diagnosed with COVID-19 [40]. Acute respiratory distress syndrome results from damage to the capillary endothelium and alveolar epithelium resulting in pulmonary edema [41], which is the main cause of mortality in COVID-19 [42, 43]. Endothelial cell dysfunction is also observed in women with preeclampsia [44]. Hypertensive disorders of pregnancy were provided in only six papers [21, 22, 24–27, 30]. Our findings are in line with expected percentage of 5–10% of noninfected pregnancies complicated by hypertensive disorders of pregnancy [45].
Body mass index higher than 30 kg/m2, diabetes, cardiovascular disorders, and advanced maternal age appear to have an independent risk for adverse maternal outcomes. The prevalence of diabetes during pregnancy (inclusive of pre- and gestational diabetes) from 1980 to 2008 increased from around 5% to ≈ 9%. In comparison, the reviewed data of pregnant vaccinated women indicate that 4.8% of vaccinated pregnant women had either pre- or gestational diabetes, which is in line with the current trend.
The average gestational age at delivery in our review of vaccinated pregnancies was approximately 39 weeks which is within the expected 38–42 weeks gestational age for a normal pregnancy. Vaginal and cesarean delivery percentage is well within the 68% vaginal and 32% cesarean delivery range reported by the CDC in 2017. The percentage of postpartum hemorrhage and placental abruption remained in the expected range for unvaccinated pregnancies [46, 47].
Been et al. [48] demonstrated in their report that the national introduction of COVID-19 mitigation measures in the Netherlands was associated with a considerable reduction in preterm births. Furthermore, they highlighted the safety of COVID-19 vaccination during pregnancy, with no major safety signals observed [24]. Additionally, they further demonstrated the safety of COVID-19 vaccination among pregnant women related to preterm birth outcomes [49]. Moreover, they indicated that COVID-19 mRNA vaccination in pregnancy appears to be safe and is associated with a reduction in stillbirth, which indirectly suggests a potential impact on preterm birth rates [50]. Furthermore, they reported that large epidemiological studies of COVID-19 vaccination during pregnancy have not identified significantly increased risks of preterm birth or SGA birth [51]. Additionally, they conducted a retrospective cohort study that included 85,162 live births and stillbirths to assess the risk of preterm birth after COVID-19 vaccination with mRNA during pregnancy, further supporting the safety of vaccination in relation to preterm birth [52]. Moreover, they demonstrated that COVID‐19 vaccination during the third trimester of pregnancy was not associated with maternal adverse outcomes and was associated with a significant reduction in the risk for neonatal adverse outcomes [21].
As defined by the WHO, preterm births are live births that occur before 37 completed weeks of pregnancy. Globally, 15 million babies are born before 37 weeks of gestation [21, 22, 26, 27, 29, 30]. The rate of preterm births in the USA in 2016 was between 9% and 14% with a variation according to race as opposed to 5–10% in Europe [53, 54]. The prevalence pooled of preterm delivery showed 6% (95% CI: 3–9%) in vaccinated pregnant women and significantly lower than 19% preterm birth rate reported by Blitz et al. [55] and Di Mascio et al. [56] in pregnant women with COVID-19. SGA prevalence did not differ by the vaccination status as stated by Lipkind et al. [49]. The overall combined pooled prevalence of delivery complications was 21% (95% CI: 3–46%), and the prevalence of VD among vaccinated women produced a pooled VD prevalence of 69% (95% CI: 62–76%), prevalence of cesarean delivery 26% (95% CI: 21–31%), prevalence of postpartum hemorrhage %5 (95% CI: 1–9%), prevalence of placental abruption 1% (95% CI: 0–1%), and prevalence of preterm delivery 6% (95% CI: 3–9%). Neonatal ICU admission in vaccinated pregnant women was lower than that reported by Braun et al. [57]. Stillbirths in COVID-19-positive pregnancies are expected to be between 0.9% and 2% as opposed to 0.64% in uninfected pregnancies [58]. Six papers included in our study reported only 1 case of stillbirth, which is substantially lower than the previously mentioned rates.
Strengths and Limitations
To our knowledge, this is the first systematic study with a full meta-analysis that quantifies the prevalence of VD among vaccinated pregnant women. We employed a rigorous review procedure and followed PRISMA principles. We employed a rigorous approach to identify papers, extract data, and appraise data after searching different electronic databases between December 2019 and October 2021. We also established operational definitions for the results, separating them using simple and repeatable formulas. We also avoided using duplicate publications, which could have skewed the interpretation of the prevalence incidence values, to reduce the effect of multiple publication bias in the study. This review has some restrictions as well. To begin, we eliminated non-English papers due to a lack of resources. There are no studies conducted in low- or middle-income countries, which limits the generalizability of the study results concerning population health. Moreover, substantial heterogeneity makes a debate whether pooling prevalence is a worthy procedure to do.
Comparison with Existing Literature
Recently, there have been some systematic reviews on COVID-19 vaccination in pregnancy [24, 49]. The articles have attempted to explore the systemic, pregnancy, delivery, and neonatal outcome of receiving the vaccination. Our review differs from these as we focus on certain key outcomes and include a meta-analysis of our findings.
From our search between December 2019 and October 2021, we found 6 retrospective studies, 2 prospective studies, 1 cross-sectional study, 1 descriptive study, and 1 case-control study that matched out review criteria. These studies were done on pregnant women who received the mRNA COVID-19 vaccine only and who were followed to study systemic effects of the vaccine, pregnancy outcomes, delivery outcomes, and neonatal outcomes.
The ACOG and CDC both recommended in August 2021 the use of the mRNA vaccines. On August 23, 2021, the FDA granted full approval for the Pfizer-BioNTech mRNA vaccine for individuals over the age of 16 [11]. These vaccines are undergoing studies in pregnant women who were initially excluded from clinical trials.
Conclusions and Implications
The systemic side effect profile after administering the COVID-19 mRNA vaccine to pregnant women was similar to that in nonpregnant women. The maternal and fetal morbidity and mortality were lowered with the administration of either one or both doses of the mRNA COVID-19 vaccines. It is quite intriguing to learn that preterm births were lowered after the administration of the vaccination. The mRNA vaccination does have a significant humoral immune response and safety profile in pregnant women. It is paramount to have more focused studies on the outcome of the vaccination in each trimester. From our review, it is evident that administering the mRNA vaccination to pregnant women significantly lowered the risks of SARS-CoV-2 infection and decreased pregnancy-related complications to both the mother and neonate. Research focusing on studying the effects of the mRNA on the pregnant women should take into consideration many of the factors highlighted in the review.
Statement of Ethics
It is not applicable because this study is based exclusively on published literature, and consent was not required.
Conflict of Interest Statement
The authors report no conflict of interest.
Funding Sources
The publication of this article was funded by the Qatar National Library.
Author Contributions
A.J.S., A.R.A.-Q., M.A.R., M.B.A.D., N.M.R., L.K.O., and M.A.E. performed the literature search, and collected and interpreted the data. A.R.A.-Q., A.J.S., and R.M.A.-Z. drafted the work and contributed to the writing of this manuscript. A.R.A.-Q. and Y.H. performed the meta-analysis. R.M.A.-Z., O.M.A., A.E.O., M.A.A.-A., and H.B. edited and drafted the final version of this manuscript. A.Y., A.Z., and A.A.A.-A. reviewed the final version to be published.
Funding Statement
The publication of this article was funded by the Qatar National Library.
Data Availability Statement
All data analyzed during this study are included in this article, and further inquiries can be directed to the corresponding author.
References
- 1. Alwani M, Yassin A, Al-Zoubi RM, Aboumarzouk OM, Nettleship J, Kelly D, et al. Sex-based differences in severity and mortality in COVID-19. Rev Med Virol. 2021;31(6):e2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO Coronavirus (COVID-19) . Dashboard | WHO coronavirus (COVID-19) dashboard with vaccination data.
- 3. Pham A, Aronoff DM, Thompson JL. Maternal COVID-19, vaccination safety in pregnancy, and evidence of protective immunity. J Allergy Clin Immunol. 2021;148(3):728–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. CDC- pregnancy and recently pregnant people- Update December 17, 2021. 2021. [Google Scholar]
- 5. Villar J, Ariff S, Gunier RB, Thiruvengadam R, Rauch S, Kholin A, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175(8):817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murphy J, Vallieres F, Bentall RP, Shevlin M, McBride O, Hartman TK, et al. Psychological characteristics associated with COVID-19 vaccine hesitancy and resistance in Ireland and the United Kingdom. Nat Commun. 2021;12(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hosokawa Y, Okawa S, Hori A, Morisaki N, Takahashi Y, Fujiwara T, et al. The prevalence of COVID-19 vaccination and vaccine hesitancy in pregnant women: an internet-based cross-sectional study in Japan. J Epidemiol. 2022;32(4):188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Germann K, Kiefer MK, Rood KM, Mehl R, Wu J, Pandit R, et al. Association of initial COVID-19 vaccine hesitancy with subsequent vaccination among pregnant and postpartum individuals. BJOG. 2022;129(8):1352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kiefer MK, Mehl R, Costantine MM, Johnson A, Cohen J, Summerfield TL, et al. Characteristics and perceptions associated with COVID-19 vaccination hesitancy among pregnant and postpartum individuals: a cross-sectional study. BJOG. 2022;129(8):1342–51. [DOI] [PubMed] [Google Scholar]
- 10. Shook LL, Kishkovich TP, Edlow AG. Countering COVID-19 vaccine hesitancy in pregnancy: the “4 Cs”. Am J Perinatol. 2022;39(10):1048–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. ACOG ACOG, SMFM Recommend COVID-19 vaccination for pregnant individuals. Am Coll Obstet Gynecol. 2021. [Google Scholar]
- 12. Ciapponi A, Bardach A, Mazzoni A, Alconada T, Anderson SA, Argento FJ, et al. Safety of components and platforms of COVID-19 vaccines considered for use in pregnancy: a rapid review. Vaccine. 2021;39(40):5891–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383(27):2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Adhikari EH, Spong CY. COVID-19 vaccination in pregnant and lactating women. JAMA. 2021;325(11):1039–40. [DOI] [PubMed] [Google Scholar]
- 16. Clinical trials.gov . Study to evaluate the safety, tolerability, and immunogenicity of SARS CoV-2 RNA vaccine candidate (BNT162b2) against COVID-19 in healthy pregnant women 18 years of age and older.
- 17. Clinical trials.gov . A study of Ad26.COV2.S in healthy pregnant participants (COVID-19) (HORIZON 1).
- 18. Clincial Trial.gov . Moderna COVID-19 vaccine mRNA-1273 observational pregnancy outcome study.
- 19. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41. [DOI] [PubMed] [Google Scholar]
- 20. Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934–9. [DOI] [PubMed] [Google Scholar]
- 21. Rottenstreich M, Sela HY, Rotem R, Kadish E, Wiener-Well Y, Grisaru-Granovsky S. Covid-19 vaccination during the third trimester of pregnancy: rate of vaccination and maternal and neonatal outcomes, a multicentre retrospective cohort study. BJOG. 2022;129(2):248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bookstein Peretz S, Regev N, Novick L, Nachshol M, Goffer E, Ben-David A, et al. Short-term outcome of pregnant women vaccinated with BNT162b2 mRNA COVID-19 vaccine. Ultrasound Obstet Gynecol. 2021;58(3):450–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goldshtein I, Nevo D, Steinberg DM, Rotem RS, Gorfine M, Chodick G, et al. Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA. 2021;326(8):728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blakeway H, Prasad S, Kalafat E, Heath PT, Ladhani SN, Le Doare K, et al. COVID-19 vaccination during pregnancy: coverage and safety. Am J Obstet Gynecol. 2022;226(2):236.e1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wainstock T, Yoles I, Sergienko R, Sheiner E. Prenatal maternal COVID-19 vaccination and pregnancy outcomes. Vaccine. 2021;39(41):6037–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Trostle ME, Limaye MA, Avtushka V, Lighter JL, Penfield CA, Roman AS. COVID-19 vaccination in pregnancy: early experience from a single institution. Am J Obstet Gynecol MFM. 2021;3(6):100464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Theiler RN, Wick M, Mehta R, Weaver AL, Virk A, Swift M. Pregnancy and birth outcomes after SARS-CoV-2 vaccination in pregnancy. Am J Obstet Gynecol MFM. 2021;3(6):100467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jamieson DJ, Theiler RN, Rasmussen SA. Emerging infections and pregnancy. Emerg Infect Dis. 2006;12(11):1638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shimabukuro TT, Kim SY, Myers TR, Moro PL, Oduyebo T, Panagiotakopoulos L, et al. Preliminary findings of mRNA covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384(24):2273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gray KJ, Bordt EA, Atyeo C, Deriso E, Akinwunmi B, Young N, et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;225(3):303.e1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kadali RAK, Janagama R, Peruru SR, Racherla S, Tirumala R, Madathala RR, et al. Adverse effects of COVID-19 messenger RNA vaccines among pregnant women: a cross-sectional study on healthcare workers with detailed self-reported symptoms. Am J Obstet Gynecol. 2021;225(4):458–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wastnedge EAN, Reynolds RM, van Boeckel SR, Stock SJ, Denison FC, Maybin JA, et al. Pregnancy and COVID-19. Physiol Rev. 2021;101(1):303–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Adhikari EH, Moreno W, Zofkie AC, MacDonald L, McIntire DD, Collins RRJ, et al. Pregnancy outcomes among women with and without severe acute respiratory syndrome coronavirus 2 infection. JAMA Netw Open. 2020;3(11):e2029256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lokken EM, Huebner EM, Taylor GG, Hendrickson S, Vanderhoeven J, Kachikis A, et al. Disease severity, pregnancy outcomes, and maternal deaths among pregnant patients with severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am J Obstet Gynecol. 2021;225(1):77.e1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Metz TD, Clifton RG, Hughes BL, Sandoval G, Saade GR, Grobman WA, et al. Disease severity and perinatal outcomes of pregnant patients with coronavirus disease 2019 (COVID-19). Obstet Gynecol. 2021;137(4):571–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Di Renzo GC, Giardina I. Coronavirus disease 2019 in pregnancy: consider thromboembolic disorders and thromboprophylaxis. Am J Obstet Gynecol. 2020;223(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ji HL, Zhao R, Matalon S, Matthay MA. Elevated plasmin(ogen) as a common risk factor for COVID-19 susceptibility. Physiol Rev. 2020;100(3):1065–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mustafa R, Ahmed S, Gupta A, Venuto RC. A comprehensive review of hypertension in pregnancy. J Pregnancy. 2012;2012:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Timircan M, Bratosin F, Vidican I, Suciu O, Tirnea L, Avram V, et al. Exploring pregnancy outcomes associated with SARS-CoV-2 infection. Medicina. 2021;57(8):796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bowman ZS, Eller AG, Bardsley TR, Greene T, Varner MW, Silver RM. Risk factors for placenta accreta: a large prospective cohort. Am J Perinatol. 2014;31(9):799–804. [DOI] [PubMed] [Google Scholar]
- 42. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–42. [DOI] [PubMed] [Google Scholar]
- 43. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: pathophysiology and clinical implications. BMJ. 2019;366:l2381. [DOI] [PubMed] [Google Scholar]
- 45. Ying W, Catov JM, Ouyang P. Hypertensive disorders of pregnancy and future maternal cardiovascular risk. J Am Heart Assoc. 2018;7(17):e009382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Evensen A, Anderson JM, Fontaine P. Postpartum hemorrhage: prevention and treatment. Am Fam Physician. 2017;95(7):442–9. [PubMed] [Google Scholar]
- 47. Ananth CV, Keyes KM, Hamilton A, Gissler M, Wu C, Liu S, et al. An international contrast of rates of placental abruption: an age-period-cohort analysis. PLoS One. 2015;10(5):e0125246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Been JV, Burgos Ochoa L, Bertens LCM, Schoenmakers S, Steegers EAP, Reiss IKM. Impact of COVID-19 mitigation measures on the incidence of preterm birth: a national quasi-experimental study. Lancet Public Health. 2020;5(11):e604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lipkind HS, Vazquez-Benitez G, DeSilva M, Vesco KK, Ackerman-Banks C, Zhu J, et al. Receipt of COVID-19 vaccine during pregnancy and preterm or small-for-gestational-age at birth — eight integrated health care organizations, United States, December 15, 2020–July 22, 2021. MMWR Morb Mortal Wkly Rep. 2022;71(1):26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Prasad S, Kalafat E, Blakeway H, Townsend R, O’Brien P, Morris E, et al. Systematic review and meta-analysis of the effectiveness and perinatal outcomes of COVID-19 vaccination in pregnancy. Nat Commun. 2022;13(1):2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fell DB, Dhinsa T, Alton GD, Török E, Dimanlig-Cruz S, Regan AK, et al. Association of COVID-19 vaccination in pregnancy with adverse peripartum outcomes. JAMA. 2022;327(15):1478–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dahlquist K, Stuart A, Källén K. Planned cesarean section vs planned vaginal delivery among women without formal medical indication for planned cesarean section: a retrospective cohort study of maternal short-term complications. Acta Obstet Gynecol Scand. 2022;101(9):1026–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. Births: final data for 2016. Natl Vital Stat Rep. 2018;67(1):1–55. [PubMed] [Google Scholar]
- 54. Delnord M, Blondel B, Zeitlin J. What contributes to disparities in the preterm birth rate in European countries? Curr Opin Obstet Gynecol. 2015;27(2):133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Blitz MJ, Gerber RP, Gulersen M, Shan W, Rausch AC, Prasannan L, et al. Preterm birth among women with and without severe acute respiratory syndrome coronavirus 2 infection. Acta Obstet Gynecol Scand. 2021;100(12):2253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Di Mascio D, Khalil A, Saccone G, Rizzo G, Buca D, Liberati M, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020;2(2):100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Braun D, Braun E, Chiu V, Burgos AE, Gupta M, Volodarskiy M, et al. Trends in neonatal intensive care unit utilization in a large integrated health care system. JAMA Netw Open. 2020;3(6):e205239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. DeSisto CL, Wallace B, Simeone RM, Polen K, Ko JY, Meaney-Delman D, et al. Risk for stillbirth among women with and without COVID-19 at delivery hospitalization: United States, March 2020–September 2021. MMWR Morb Mortal Wkly Rep. 2021;70(47):1640–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data analyzed during this study are included in this article, and further inquiries can be directed to the corresponding author.