Abstract
Cytomegalovirus (CMV) disease after bone marrow (BM) transplantation is often associated with BM graft failure. There are two possible reasons for such a correlation. First, a poor hematopoietic reconstitution of unrelated etiology could promote the progression of CMV infection by the lack of immune control. Alternatively, CMV infection could interfere with the engraftment of donor BM cells in recipient BM stroma. Evidence for a causative role of CMV in BM aplasia came from studies in long-term BM cultures and from the murine in vivo model of CMV-induced aplastic anemia. A deficiency in the expression of essential stromal hemopoietins, such as stem cell factor (SCF), has indicated a functional insufficiency of the stromal microenvironment. It remained open to question whether CMV mediates a negative regulation of hemopoietin gene expression (the downregulation model) or whether it causes the default of a positive regulator (the lack-of-induction model). Further, even though implicitly assumed, it has never been formally documented that CMV directly interferes with the engraftment of a BM cell transplant. We addressed these problems in a murine model of CMV infection after experimental male-into-female BM transplantation. The data indicate that the downregulation model applies. Quantitation of the male-sex-determining gene tdy demonstrated an impaired engraftment of donor BM cells in the BM stroma of the female recipients. This graft failure was reflected by a diminished population of SCF-receptor-expressing hematopoietic progenitor cells and correlated with a reduced level of stromal SCF gene expression. Interestingly, high doses of BM cells protected against stromal insufficiency by a mechanism unrelated to control of infection.
The reconstitution of the bone marrow (BM) after hematoablative treatment is the therapeutic aim of BM transplantation (BMT). Primary or recurrent cytomegalovirus (CMV) infection is a problem in BMT patients for two reasons. Organ manifestations of human CMV disease, interstitial CMV pneumonia in particular, are a feared complication. In addition, human CMV infection is associated with an impaired graft acceptance and, in the most extreme case, with a complete graft failure. Insufficient reconstitution of the BM prolongs the period of immunodeficiency and thus promotes the progression of CMV organ disease in a vicious cycle. It is now widely assumed that CMV does not just profit from the hematoablation but actively contributes to its maintenance (reviewed in reference 4). Most details of the mechanism of CMV pathogenesis in the BM still need to be investigated. Until recently it was even unknown whether the hematopoietic cord is an in situ target site of CMV replication and which cells in the BM serve as targets for infection (29).
In principle, CMV could infect cells of the two functional compartments of the BM, namely, the hematopoietic and stromal compartments. The hematopoietic compartment comprises stem and progenitor cells as well as their progeny in all hematopoietic differentiation lineages. These cells are mostly radiation sensitive and are therefore donor derived in post-BMT chimeras. It is established from the work of many that hematopoietic cells of the myelomonocytic lineage in particular can carry latent viral DNA without apparent cytopathic effects (9, 20, 21, 28, 31, 42), but CMV infection may induce apoptosis of hematopoietic progenitor cells by upregulating the expression of Fas (32). The stromal compartment comprises reticular stromal cells (RSC) and adipocytes that form a network that provides the matrix for the engraftment of the hematopoietic cells and that delivers cytokines, so-called hemopoietins, which are essential for hematopoiesis to occur in that they support the proliferation and differentiation of hematopoietic cells. The stromal cells are largely radiation resistant and are therefore recipient derived in post-BMT chimeras (reviewed in reference 3). Stromal cell types are the principal targets of productive CMV infection in long-term BM cultures with consequent cessation of in vitro hematopoiesis (1, 2, 24, 25, 41, 44). The in vivo model of CMV aplastic anemia in BALB/c mice after hematoablative treatment and infection with murine CMV has revealed a BM aplasia represented by a failure in the regeneration of early hematopoietic progenitor cells (33, 35). We have shown recently that the cellular target of CMV in the BM is the RSC that forms the stromal network (29). Low virus productivity in the BM, a low number of RSC reaching the late phase of the viral replicative cycle, unchanged expression of housekeeping genes in stromal cells, and an unchanged amount of tdy gene in the stroma of infected female-into-male chimeras prompted us to conclude that the mechanism of CMV pathogenesis in the BM is neither a cytolytic disruption of the stromal network nor a direct effect of CMV replication on hemopoietin gene expression in infected RSC. Instead, an overall low level of stromal hemopoietin gene expression indicated a functional deficiency of BM stroma, uninfected bystander cells included, with consequent lack of support for hematopoietic stem and progenitor cells (29).
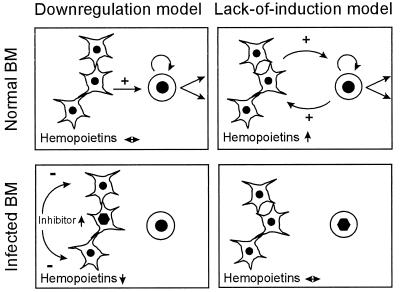
Since the basal level of stromal hemopoietin gene expression was unknown, we could not decide whether the expression was downregulated by a CMV-induced negative regulator (i.e., the downregulation model) or whether CMV prevented an upregulation by a positive regulator (i.e., the lack-of-induction model). Such a positive regulator could be a signal delivered by incoming hematopoietic cells inducing the stroma to produce hemopoietins in a positive feedback mode. In this case, the primary defect caused by CMV would lie within hematopoietic cells (see Fig. 1 for an illustration of the two hypotheses). Even though the consequences are similar, distinguishing between the two models is crucial for the understanding of CMV pathogenesis in the BM and for the direction of future investigations. We have addressed this question by an in vivo titration of the putative hematopoietic inducer cells, that is, by performing experimental BMT with graded BM cell numbers. The results demonstrate that CMV infection inhibits the engraftment of donor BM cells in the recipient stroma and provide evidence favoring stromal hemopoietin downregulation as the explanation for the deficient hematopoietic reconstitution.
FIG. 1.
Models of CMV pathogenesis in the BM. (Left panels) Downregulation model. (Left panel, top) Network-forming RSC constitutively express a basal level of hemopoietins that give positive signalling to support proliferation and differentiation of hematopoietic cells. (Left panel, bottom) Infection of RSC induces an inhibitor that downregulates hemopoietin gene expression in uninfected bystander RSC. A failure in positive signalling results in cessation of hematopoiesis. (Right panels) Lack-of-induction model. (Right panel, top) RSC constitutively express an insufficient basal level of hemopoietins. Hematopoietic cells, e.g., donor BMC immigrating into recipient stroma after BMT, induce hemopoietin gene expression in RSC to provide the positive signalling for hematopoiesis in a feedback mode. (Right panel, bottom) Infected hematopoietic cells fail to induce stromal hemopoietins, with consequent cessation of hematopoiesis. Symbols: ↔, constitutive hemopoietin gene expression; hexagon (nucleocapsid), infection of cells; arrows up and down, upregulation and downregulation of cytokines, respectively. Positive and negative signalling is indicated by arrows marked with plus and minus, respectively. Arrows at the hematopoietic cells symbolize self-renewal of stem cells and differentiation into lineages.
MATERIALS AND METHODS
Male-into-female BMT and infection.
For major histocompatibility complex (MHC)-compatible BMT, male and female mice of the CMV-susceptible inbred strain BALB/c (MHC haplotype H-2d) were used at the age of 8 weeks as BM cell (BMC) donors and recipients, respectively. For hematoablative conditioning, recipients were total body gamma irradiated with a single dose of 7 Gy from a 137Cs source. Donor femoral and tibial BMC were depleted of contaminating CD8 T cells by three cycles of treatment with rat anti-murine CD8 monoclonal antibody, clone YTS 169.4 (5), and magnetic beads coated with sheep anti-rat immunoglobulin (Dynabeads M-450; Dynal, Oslo, Norway) at a bead-to-cell ratio of 2:1. The indicated numbers of BMC were infused intravenously into the tail veins of recipients (33) at ca. 6 h after the irradiation. Infection with 105 PFU of purified murine CMV (23), strain Smith ATCC VR-194, was performed subcutaneously at the left hind footpad at ca. 2 h after BMT.
Quantitation of infectious virus in BMC suspensions.
The recently described reverse transcriptase PCR (RT-PCR)-based focus expansion assay was employed for quantitating low doses of infectious murine CMV (23). In brief, BMC were flushed quantitatively out of femurs, and homogenates thereof were used in the indicated dilutions to infect permissive mouse embryofetal fibroblasts by the technique of centrifugal infection (16). After 72 h of focus formation in culture, poly(A)+ RNA was isolated, and a fragment of 188 bp specific for exons 3 and 4 of the murine CMV immediate-early (IE) gene ie1 was amplified from 100 ng of the poly(A)+ RNA by RT-PCR and analyzed as described previously (23).
Histological analysis of BM reconstitution.
On day 14 after BMT, femurs were fixed in 4% (vol/vol) formalin buffered at pH 7.4, and the bone substance was decalcified with 20% (wt/vol) EDTA for enzyme histochemistry or with 5% (wt/vol) trichloroacetic acid for hematoxylin-eosin (HE) staining and for immunohistochemistry (IHC) analysis. Paraffin sections of 2 μm were dewaxed in xylene and processed for HE staining (hematoxylin for 8 min, eosin for 10 s) according to established procedures. The enzymatic activity of the specific esterase was used for histochemical staining of myelomonocytic cells, with naphthole AS-D chloroacetate (N0758; Sigma) as the substrate and with freshly diazotized pararosaniline (P3750; Sigma) for the red label. The counterstaining was done with hematoxylin for 1 min. IHC staining specific for the intranuclear viral IE1 protein pp89 (18) was performed as described previously (29) by using an indirect avidin-biotin-peroxidase complex method with diaminobenzidine for brown staining. The sections were counterstained with HE (hematoxylin for 4 min, eosin for 5 s). Negative controls included sections from uninfected tissues as well as replacement of the IE1 protein specific antibody by unrelated antibody of the same isotype in the staining of infected tissues.
Quantitation of male hematopoietic cells by Y-chromosome-specific PCR.
Femoral and tibial BMC were washed in PBS-A (phosphate-buffered saline [PBS] devoid of Ca2+ and Mg2+). Contaminating erythrocytes were lysed by incubation in Gey’s buffer. After extensive washing in PBS-A, cells were sedimented, and DNA was isolated by standard procedures of proteinase K digestion, phenol-chloroform-isoamyl alcohol extraction, and precipitation with ethanol. A 402-bp DNA fragment of the male-sex-determining gene tdy located at the Y chromosome (13) was amplified by PCR in 30 cycles essentially as described previously (29), except that the PCR were performed in conically welled polycarbonate 96-well (0.17 ml) microplates (Omniplate 96; Hybaid Ltd., Teddington, Middlesex, England). Amplificates (20 μl thereof) were vacuum dot blotted onto nylon membrane by using the Minifold dot blot manifold device (Schleicher & Schuell, Keene, N.H.) and hybridized with a γ-32P-end-labeled internal oligonucleotide probe. Controls in which the amplificates were analyzed by agarose gel electrophoresis and Southern blotting followed by autoradiography had verified that the probe hybridizes to a single band of correct size (not shown). Radioactivity per dot was measured with a digital phosphorimaging system (Fujifilm bioimaging system BAS 2500; Fuji, Tokyo, Japan) and is expressed in phosphostimulated luminescence units. Data analysis was performed by using Tina 2.10 software (Raytest, Straubenhardt, Germany).
Analysis of gene expression in hematopoietic cells.
The expression of the stem cell factor receptor (SCF-R [also known as CD117 and c-Kit]) gene in hematopoietic cells was measured by RT-PCR on day 14 after BMT. Femoral and tibial BMC were washed twice in PBS-A and then dissolved in the extraction buffer of a QuickPrep-Micro mRNA purification kit (Pharmacia Biotech). Poly(A)+ RNA was purified with the kit by using oligo(dT)-cellulose affinity. Reverse transcription and subsequent amplification of the resulting cDNA were performed in essence as described previously (29), but with some modifications that were found to significantly improve the sensitivity of detection. Specifically, primer annealing was performed at 58°C and oligonucleotides 5′-4361–4381 and 5′-4968–4947 were used as forward and reverse primers, respectively, resulting in an amplificate of 607 bp. Oligonucleotide 5′-4480–4500 was end labeled with γ-32P and served as the internal probe (34; GenBank accession no. Y00864).
Analysis of gene expression in stromal cells.
In order to restrict the analysis to stromal cells, gene expression in radiation-sensitive hematopoietic cells was abolished by the strategy of second irradiation, the efficacy of which was demonstrated previously by the absence of SCF-R gene expression (29). In brief, on day 13 after BMT, mice were again gamma irradiated with a dose of 7 Gy. After 24 h, that is, on day 14 after BMT, poly(A)+ RNA was isolated from the remaining BMC as outlined above. Gene expression in radiation-resistant stromal cells was analyzed by RT-PCR analyses specific for the genes encoding HPRT (hypoxanthine phosphoribosyltransferase), SCF, and CMV-IE1 (29). None of these PCRs gave amplificates if reverse transcriptase was omitted. Amplification products of 163, 543, and 280 bp, respectively, were visualized by autoradiography after separation on agarose gels, Southern blotting, and hybridization with the respective γ-32P-end-labeled internal oligonucleotide probe.
Cytofluorometric analysis of SCF-R cell membrane expression by hematopoietic cells.
BMC were labeled with an affinity-purified phycoerythrin-conjugated rat anti-mouse CD117 monoclonal antibody (clone ACK45, no. 09995B; Pharmingen, San Diego, Calif.) as recommended by the supplier. Measurements were performed with a FACSort (Becton Dickinson, San Jose, Calif.) with CellQuest software (Becton Dickinson) for data processing.
RESULTS
Lethality of CMV infection after MHC-compatible BMT.
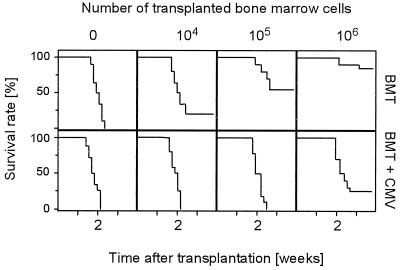
It has previously been documented that murine CMV infection causes lethal CMV disease with multiple organ involvement provided that it is preceded by a hematoimmunoablative treatment abrogating the host’s ability to develop a protective primary immune response (37; reviewed in reference 22). Accordingly, hematopoietic and lymphopoietic reconstitution by BMT should restore the capacity for controlling infection with an efficacy that is related to the efficacy of the reconstitution. To test this assumption in a murine model, we have used MHC compatible male-into-female BMT in a mouse strain that is genetically susceptible to murine CMV (26), namely, BALB/c (MHC haplotype H-2d). Increasing efficacy of reconstitution was experimentally forced by transplanting graded numbers of BMC. Accordingly, the survival rates after lethal hematoablative gamma irradiation with a dose of 7 Gy improved in uninfected recipients with increasing doses of transplanted donor BMC, and 106 donor BMC sufficed for prevention of mortality in a high percentage of the recipients (Fig. 2, upper row). A concurrent infection with murine CMV had a negative impact on the overall survival rates after BMT. Specifically, survivors were not seen below a minimum of 106 donor BMC transplanted (Fig. 2, lower row). It should be noted that survival rates in either type of sex-matched syngeneic BMT did not differ from the survival rates in sex-disparate male-into-female BMT, regardless of whether or not the recipients were infected (not shown). This indicates that the H-Y minor histocompatibility difference was not of pathogenetic significance in this particular model of transplantation and infection.
FIG. 2.
Pathogenic potential of murine CMV after MHC-compatible male-into-female BMT. (Upper panel) BMT. Kaplan-Meier survival plots for groups of 20 female BALB/c recipients, documenting the influence of the number of transplanted male BALB/c BMC on the survival rates (ordinate) as a function of time (abscissa) after hematoablative treatment with 7 Gy of gamma radiation. (Lower panel) BMT and CMV. Influence of a concurrent murine CMV infection under otherwise identical conditions.
In conclusion, murine CMV infection has a significant pathogenic potential in an experimental setting of MHC-compatible BMT.
CMV inhibits the engraftment of donor BM cells in recipient stroma.
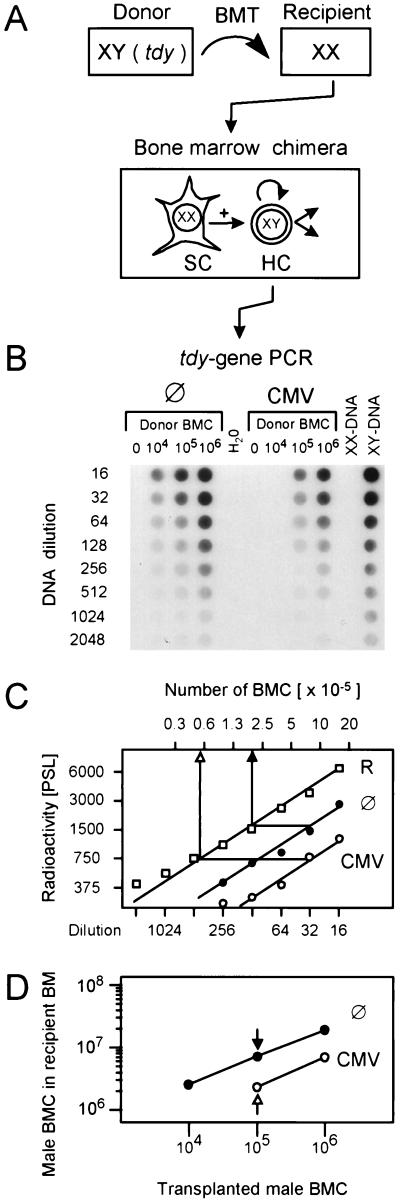
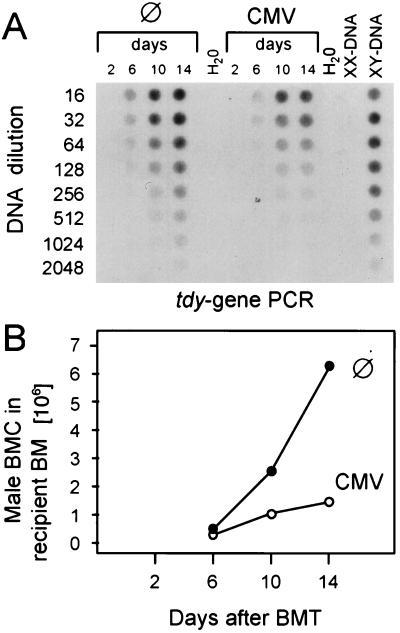
Since CMV is a cytolytic virus that can cause multiple organ failure by direct tissue destruction, organ manifestations of CMV disease, such as interstitial pneumonia (37, 39), cortical and medullary adrenalitis (36, 40), and hepatitis (38), could collectively account for the lethality of CMV infection after BMT and could thus explain the data shown in Fig. 2. However, CMV infection of long-term BM cultures (reviewed in reference 4) and the in vivo model of lethal CMV-induced BM aplasia after sublethal hematoablative treatment in the absence of BMT (29, 33) have predicted an inhibition of hematopoiesis as an additional pathomechanism of CMV. Does this proposed pathomechanism apply also in the setting of MHC-compatible BMT? If so, CMV would directly interfere with the therapeutic aim of BMT, namely, the hematopoietic and lymphopoietic reconstitution of the recipient. This important question can be directly addressed in the model system of male-into-female BMT (Fig. 3A). Hematoablative treatment of female recipients by gamma irradiation with a dose of 7 Gy affects the radiation-sensitive female (XX) hematopoietic cells but spares the radiation-resistant female BM stromal cells. By subsequent BMT with male donors, the female hematopoietic cells are replaced by male (XY) hematopoietic cells, whereas the male stromal cells are not transplantable (3, 29). As a consequence, the amount of Y chromosome in the resulting XY-XX chimeras is a direct measure of the engraftment efficacy of the donor hematopoietic cells in recipient stroma. The Y chromosome was quantitated in DNA derived from the yield of femoral and tibial BMC on day 14 after BMT by a PCR specific for the male sex (testes)-determining gene tdy (13, 29). The autoradiograph of the dot blot documents that increasing doses of donor BMC lead to increasing repopulation of recipient BM and that the amount of the tdy sequence is reduced in the BM of the infected group throughout the donor BMC titration (Fig. 3B). For an accurate quantitation, the radioactivity per dot was counted by phosphorimaging, and the number of male cells was calculated from the linear portions of the graphs, e.g., as shown for the groups with the intermediate cell number (Fig. 3C). The results from all experimental groups are compiled in a plot of calculated numbers of cells engrafted on day 14 versus the numbers of transplanted cells (Fig. 3D). The increment of repopulation was thus found to be a ca. three- to fourfold increase in the number of cells engrafted on day 14 per 10-fold increase in the number of transplanted cells. Notably, this proved to be a linear function over the range of transplanted cell numbers tested, and this was true for uninfected as well as infected recipients. However, the repopulation efficacy was generally lower in infected recipients. Under the conditions of the documented example, the repopulation efficacy was reduced to about one-third of normal, with the notable exception of a complete graft failure after transplantation of <105 BMC. In conclusion, these data demonstrate an impaired day-14 engraftment of a BM transplant in recipients suffering from a concurrent CMV infection.
FIG. 3.
Engraftment of male hematopoietic cells in the BM of female recipients. (A) Experimental design for the quantitation of donor cell engraftment. Male donor BMC carry the tdy gene located at the Y chromosome. Upon transplantation into gamma-irradiated (7 Gy) female recipients, male donor hematopoietic cells (HC) replace the radiation-sensitive female hematopoietic cells, whereas the radiation-resistant female stromal cells (SC) are not replaced. The amount of tdy gene in the resulting BM chimeras therefore provides a measure for the engraftment of donor hematopoietic cells. (B) Quantitation of the tdy gene in recipients with no infection (⊘) and recipients with murine CMV infection. BMT was performed with the indicated doses of male donor BMC. Femoral and tibial BMC of five chimeras per group were harvested on day 14 after BMT, and total cellular DNA was isolated, purified, and titrated for a tdy gene-specific PCR. The titrations started with a 1:16 aliquot of the DNA yield of one femur plus one tibia. As a negative control, BMC-derived DNA from female BALB/c mice (lane XX-DNA) was titrated and subjected to tdy gene-specific PCR accordingly. PCR with no template DNA served as a second negative control. BMC-derived DNA from male BALB/c mice (lane XY-DNA) was titrated as a positive reference for the quantitation. The autoradiograph of the dot blot obtained after hybridization of the amplificates with a γ-32P-end-labeled internal oligonucleotide probe is shown. (C) Comparison of BM repopulation efficacy in the absence and presence of infection. For quantitation of radioactivity, the dot blot was subjected to phosphorimaging. The log-log plot of radioactivity per dot (ordinate) versus the DNA dilutions (lower abscissa) is shown, as documented for the groups with 105 transplanted donor (male) BMC. Symbols: ⊘, recipients with no infection; CMV, recipients with murine CMV infection; R, XY-DNA serving as a reference. The radioactivity is given in phosphostimulated luminescence (PSL) units. Comparisons are made from the linear portions of the titrations. The upper abscissa relates the DNA dilutions to the numbers of BMC determined for the male reference. (D) Compilation of the results from all groups. Log-log plot of the numbers at day 14 of male BMC in recipient BM (ordinate) versus the numbers of male donor BMC transplanted on day 0 (abscissa).
Direct visualization of engraftment and of graft failure.
The approach of tdy gene quantitation was chosen for an objective determination of donor cell engraftment, thereby avoiding the technical problems imposed by residual female hematopoietic cells and by a microscopic discrimination between hematopoietic cells and stromal cells. It is nonetheless informative to note that the cell numbers determined by tdy gene-specific PCR were in fair accordance with the yields of BMC calculated by routine cell counting (not shown).
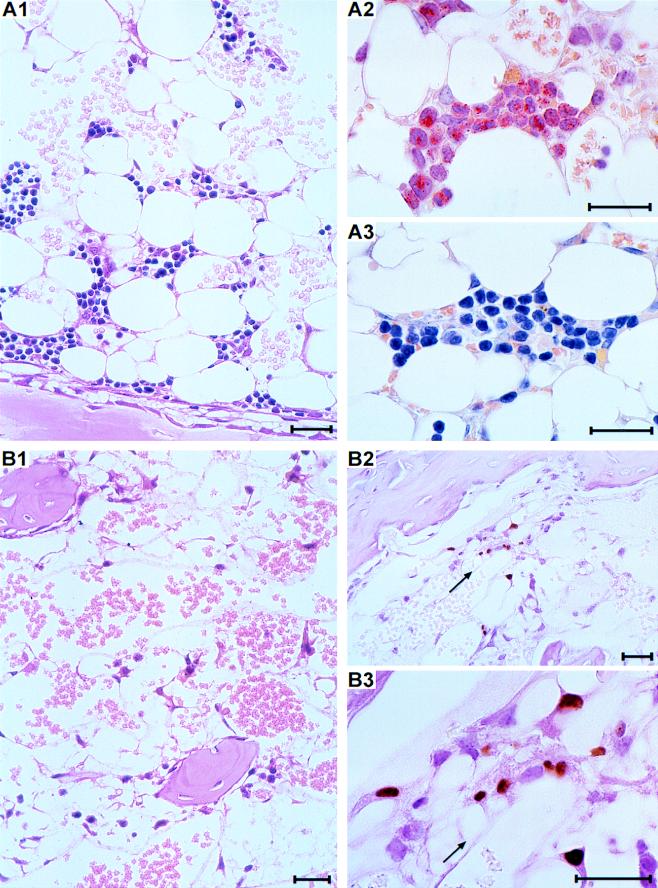
Histologic analysis of the femurs gives a direct visual impression of the success of engraftment. According to the tdy gene-specific PCR, 104 transplanted cells contained hematopoietic progenitor cells that were able to engraft and to generate a detectable male progeny in uninfected female recipients but failed in infected recipients (compare with results in Fig. 3). These on-and-off conditions are precisely reflected by the histology (Fig. 4). In uninfected recipients, foci of primordial reconstitution, so-called hematopoietic islands, are frequent in the stromal network of the hematopoietic cord on day 14 after BMT in a section of a femur (Fig. 4A1). Morphological criteria combined with in situ cytochemical staining detecting the marker enzyme naphthol AS-D chloroacetate esterase, also known as specific esterase, identified red-stained myelomonocytic colonies (Fig. 4A2) and unstained erythroblastic colonies (Fig. 4A3). By contrast, hematopoietic colonies did not develop in the BM stroma of infected recipients under otherwise identical experimental conditions. Accordingly, the stromal network remained empty, which defines a complete BM aplasia (Fig. 4B1). Foci of infection were detectable in the aplastic BM by IHC staining of the intranuclear viral IE1 protein (Fig. 4B2). When resolved to greater detail, the infected cells can be identified as RSC building up the stromal network (Fig. 4B3). The fact that hematopoietic colonies were absent suggested to us that CMV infection indeed prevented a successful engraftment of transplanted hematopoietic cells.
FIG. 4.
Histological documentation of BM repopulation and of CMV-induced graft failure. (A) Repopulation of recipient BM shown for day 14 after hematoablative treatment (7 Gy) and transplantation of 104 donor BMC with no infection. (A1) Section of a femoral diaphysis stained with HE. Overview shows hematopoietic colonies developing in the stromal network. (A2) Myelomonocytic colony characterized by the enzymatic activity of specific esterase yielding a red cytoplasmic label with the AS-D technique. Counterstaining with hematoxylin. (A3) Erythroblastic colony negative for specific esterase, counterstained with hematoxylin. (B) Aplasia of recipient BM on day 14 after the BMT specified in panel A but with murine CMV infection. (B1) Section of a femoral epiphysis stained with HE. Overview shows empty stromal network spanning bone trabeculae. (B2) IHC staining of intranuclear viral IE1 protein, counterstained with HE. The arrow points to a focus of infected cells. (B3) Detail of B2, identifying the infected cells as reticular stromal cells that form the stromal network. The bars represent 25 μm throughout.
Hematopoietic myeloid sublineages are differentially affected by CMV infection.
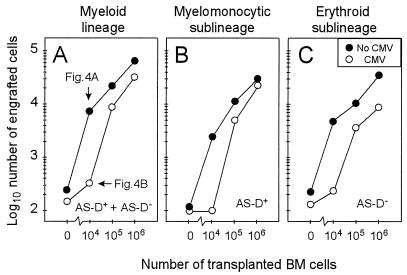
Quantitative in situ cytochemistry (see Fig. 4) was employed to discriminate and enumerate cells of the myelomonocytic and the erythroid sublineages of the myeloid lineage by positive (red) and negative AS-D staining for specific esterase, respectively (Fig. 5). For clarity, it should be recalled that cells of the lymphoid lineage are also negative for specific esterase but that erythroid cells outnumber lymphoid cells within the BM. Regarding the increment of day-14 BM repopulation after transplantation of increasing numbers of BMC, the total cell count in representative sections of femoral, tibial, and sternal BM was in good accordance with the tdy gene PCR assay (compare Fig. 5A and Fig. 3D), even though the data were derived from independent experiments. In principle, both myeloid sublineages were affected by CMV (Fig. 5B and C). Notably, however, the erythroid reconstitution was more severely inhibited. This becomes apparent in particular at higher numbers of transplanted BMC.
FIG. 5.
Histological quantitation of BM repopulation. (A) Cells of the myeloid lineage comprising cells positive for AS-D staining and cells negative for AS-D staining (AS-D+ plus AS-D−). Note that cells of the lymphoid lineage are included in the counting but are negligible in number. (B) Cells of the myelomonocytic sublineage positive for specific esterase (AS-D+). (C) Cells of the erythroid sublineage plus cells of the lymphoid lineage negative for specific esterase (AS-D−). Throughout, cell numbers represent engrafted cells per representative 10-mm2 area of femoral, tibial, and sternal BM sections compiled from five recipients per experimental group. Note that there were no differences between ipsilateral and contralateral femurs. Symbols: •, uninfected recipients; ○, recipients infected with murine CMV on day 0.
Concurrent CMV infection does not prevent the early homing of transplanted cells.
The term “engraftment” implies that donor-derived hematopoietic cells successfully repopulate the empty BM of the recipient. An absolute or relative graft failure can occur at three decisive steps: (i) the ingress of transplanted cells from the vascular-sinusoidal compartment into the BM, (ii) their homing in the BM stroma, and (iii) their clonal expansion and differentiation resulting in colony formation. An early functional damage of transplanted cells by a virus encounter within the vascular compartment was unlikely, since the intraplantar route of infection separated early local virus replication and migration of intravenously transplanted cells spatially and temporally. The approach of male-into-female BMT followed by the tdy gene PCR assay was used to determine the kinetics of the repopulation after transplantation of 105 BMC (Fig. 6). After 2 days, transplanted male cells were not present in the recipient BM in numbers detectable by this assay, although ingress and homing of hematopoietic stem and progenitor cells should be completed by that time. Male hematopoiesis became visible on day 6 in both groups of recipients, but the numbers of repopulating cells diverged with time. It is worth recalling that infectious virus in organs, the BM included, does not reach detectable levels before day 6 after intraplantar infection (29, 33, 37). Thus, as observed in previous work in a model of endogenous BM reconstitution (33), the inhibition of BM repopulation coincides with the onset of virus replication in host tissues. In conclusion, CMV infection interferes with successful engraftment of transplanted cells mainly by an inhibition of colony growth. This interpretation would be compatible with a deficiency in the support provided by stroma-derived growth-promoting hemopoietins.
FIG. 6.
Kinetics of BM reconstitution. The tdy gene PCR assay described in Fig. 3 was used for quantitating the engraftment of male donor BMC in the femoral and tibial BM of female recipients as a function of time after experimental BMT, which was performed with 105 male BMC. DNA was pooled from three chimeras per time point, and DNA dilutions started with a 1:16 aliquot of the DNA yield from BMC of one femur plus one tibia. (A) Autoradiograph of the dot blot. (B) Lin-lin plot of calculated numbers of engrafted male cells (ordinate) as a function of time (abscissa). Symbols: ⊘, recipients with no infection; CMV, recipients with concurrent murine CMV infection.
The expression of SCF-R in reconstituting BM reflects absolute differences in the numbers of engrafted cells.
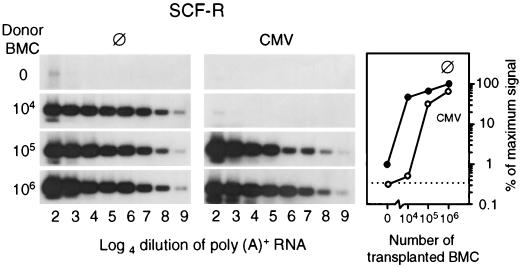
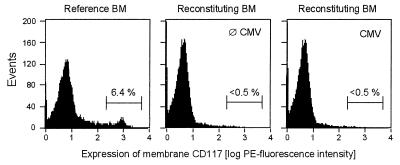
The tdy gene assay has quantitated the entire male progeny of engrafted hematopoietic cells irrespective of the differentiation stage of the cells. Hematopoietic stem and progenitor cells express SCF-R, also known as CD117 or c-Kit (reviewed in reference 45). Quantitation of c-kit gene expression in the BM by RT-PCR was therefore employed to test whether the pool of stem and progenitor cells was diminished during CMV infection (Fig. 7). Differences in the number of c-kit transcripts may comprise differences in the number of SCF-R-expressing cells, in the transcription rate of c-kit, and in transcript stability. The low level of c-kit transcription in the group with no infection and no BMT (Fig. 7; left panel, upper lane) identified residual female hematopoiesis that was by approach invisible in the tdy gene assay. In agreement with our previous work (29), this endogenous reconstitution was prevented by the infection (Fig. 7; center panel, upper lane). Notably, after BMT with graded cell numbers, the amounts on day 14 of c-kit transcripts, as determined from the linear portions of the titrations, largely paralleled the absolute differences in progeny cell numbers determined by the quantitative histological analysis of BM repopulation (compare graphs in Fig. 5 and 7). In an attempt to measure relative differences in the numbers of SCF-R-positive cells and/or in the expression of membrane SCF-R per cell, cytofluorometric analysis of CD117 in reconstituting BM was performed on day 14 after transplantation of 105 BMC (Fig. 8). Normal BM of adult BALB/c mice served as a reference. In this positive control BM, SCF-Rhigh-expressing cells are contained within a cell population characterized by small size and low granularity, and they form a distinct peak (Fig. 8, left panel). The frequency of the SCF-R-positive cells in this steady-state BM was in good accordance with data reported for BALB/c BM (17). Such a population of SCF-Rhigh-expressing cells was undetectable in reconstituting BM irrespective of whether or not the recipients were infected (Fig. 8, center and right panels). We therefore conclude that the frequency of SCF-R-positive cells is very low at this early stage of BM reconstitution and that the absolute differences observed for the c-kit gene expression reflect absolute differences in stem and progenitor cell numbers rather than differences in their frequencies or in their levels of SCF-R membrane expression.
FIG. 7.
SCF-R gene expression by repopulating hematopoietic cells. BMT was performed with graded numbers of male donor BMC. Femoral and tibial BMC were harvested on day 14 after BMT from five chimeras per experimental group. Poly(A)+ RNA was isolated, titrated, and subjected to an RT-PCR specific for SCF-R transcripts. Log4 dilutions started with a 1:16 aliquot of the yield from one femur plus one tibia. Autoradiographs were obtained after hybridization of the 607-bp amplificates with a γ-32P-end-labeled internal oligonucleotide probe. (Left and center panels) ⊘, no infection; CMV, concurrent murine CMV infection. (Right panel) Log-log plot compiling the phosphorimaging results obtained from the linear portions of the titrations for all experimental groups. The dotted line marks the background radioactivity.
FIG. 8.
Cytofluorometric analysis of SCF-R cell surface expression. BMC were harvested on day 14 after transplantation of 105 donor BMC from the reconstituting BM of five chimeras per experimental group. BMC derived from 8-week-old, untreated BALB/c mice served as a positive reference. A gate was set in the scatter plot on living cells of small size and low granularity. The gate was selected so as to include most of the SCF-R-positive cells. The histograms represent the analysis of CD117 (SCF-R) surface expression for a total of 20,000 cells gated. The percentages of SCF-Rhigh expressing cells are indicated.
Effect of BM reconstitution on the infection of BM stroma.
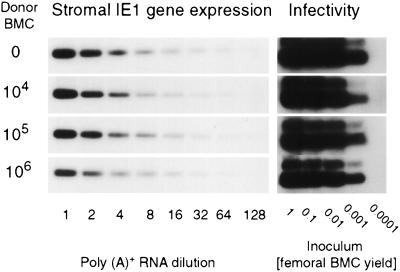
Whereas the efficacy of BM cell engraftment was generally reduced in the infected recipients, a dose-dependent engraftment did occur at higher cell numbers (recall Fig. 3 and 5). This finding is not easily explained by the theory of a functional deficiency of the stroma. Why should the stroma be unable to support the engraftment of low numbers of hematopoietic cells while being able to nourish higher numbers? It appeared to us as if the functional capacity of the stroma was influenced by the transplanted cells. The most obvious influence one can think of is the prevention of stromal deficiency by controlling the infection. We have tested this idea by two independent approaches. First, viral gene expression in stromal cells was measured on day 14 postinfection by titration of stromal poly(A)+ RNA followed by RT-PCR specific for the ie1 transcript (Fig. 9, left panel). Second, infectious virus present in femoral BM, which includes stromal and hematopoietic cells, was quantitated on day 14 by the infection of permissive indicator cells (Fig. 9, right panel). Since virus productivity in the BM is known to be low (29), the conventional plaque assay was replaced by the more sensitive RT-PCR-based focus expansion assay, an assay that detects infectivity by detecting viral transcription after several rounds of viral replication in indicator cultures inoculated with as few as five virions (23). At a glance, the viral gene expression in the BM stroma and the amount of infectious virus in the BM were independent of the number of transplanted BMC over a wide range (Fig. 9). While the data may indicate an onset of antiviral control for 106 transplanted BMC, the marked difference in the BM repopulation efficacy observed after transplantation of 104 and 105 BMC (recall Fig. 3, 5, and 7) is definitely not explained by a difference in BM infection. It is worth mentioning that this conclusion is also supported by immunohistological quantitation of infected cells in tissues (not shown), as well as by virus titers measured in various organs (33). However, it is important to emphasize that all this applies only to the early period after BMT, whereas, beginning with the third week, reconstituting CD8 T cells control the infection and are essential for the survival observed after transplantation of 106 BM cells (recall Fig. 2) (43). In conclusion, the successful early engraftment seen on day 14 for higher numbers of transplanted cells is not explained by a control of the infection.
FIG. 9.
Viral gene expression in stroma and infectivity in the BM. (Left panel) Quantitation of murine CMV IE1 transcripts in stromal cells. Transcription in hematopoietic cells was abolished by a second gamma irradiation of the recipients with a dose of 7 Gy performed on day 13 after BMT. The next day, poly(A)+ RNA derived from radiation-resistant stromal cells of five recipients per experimental group was purified, titrated, and subjected to RT-PCR. Titrations started with the yield of poly(A)+ RNA from one femur plus one tibia. The expression of the housekeeping gene HPRT was identical in all groups (not shown). Shown are the autoradiographs obtained after hybridization of IE1-specific 280-bp amplificates with a γ-32P-end-labeled internal oligonucleotide probe. (Right panel) Quantitation of infectivity in the total BM of infected recipients. Infectious virus present in the BM, including the vascular compartment, was quantitated by inoculating permissive indicator cell cultures with BM cell homogenate. BMC were harvested on day 14 postinfection from five recipients per group, and the titration was started with an aliquot of the homogenate representing the yield from one whole femur. After 72 h of virus replication in the indicator cultures, poly(A)+ RNA was isolated, and an aliquot was subjected to an IE1-specific RT-PCR. Shown are the autoradiographs obtained after hybridization of the 188-bp amplificates with a γ-32P-end-labeled oligonucleotide probe directed against the ie1 gene exon 3/4 splicing junction.
The efficacy of hematopoietic reconstitution correlates with stromal function.
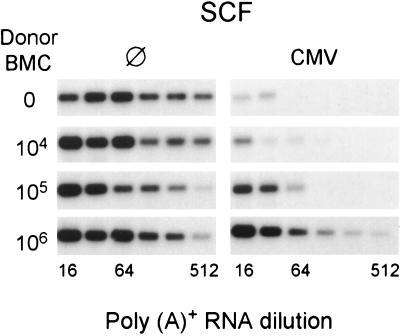
According to our previous work (29), CMV-induced BM aplasia is associated with a stromal deficiency in the expression of various essential hemopoietins, including the counter-receptor of SCF-R, the SCF, also known as Kit ligand or Steel factor (45). The downregulation of stromal SCF gene expression by CMV is again documented (Fig. 10). The finding that infected BM is repopulated after transplantation of higher doses of BMC demanded an increased expression of SCF in the respective recipients. This was indeed what we found (Fig. 10, right panel), even though the infection was not controlled (Fig. 9) and even though transplanted hematopoietic cells did not induce SCF transcription in uninfected stroma (Fig. 10, left panel). We therefore propose that the BMC dose-dependent recovery of SCF transcription observed in infected stroma does not reflect induction of SCF transcription by hematopoietic cells but rather a prevention of its downregulation.
FIG. 10.
SCF gene expression in BM stroma. BMT was performed as in all other experiments with the indicated numbers of male donor BMC. Stromal SCF gene expression was analyzed by RT-PCR on day 14 after BMT for groups of five recipients. Symbols: ⊘, no infection; CMV, concurrent murine CMV infection. The indicated dilutions refer to the poly(A)+ RNA yield from one femur plus one tibia. The expression of the housekeeping gene HPRT was identical in all groups (not shown). Autoradiographs obtained after hybridization of the SCF gene-specific 543-bp amplificates with a γ-32P-end-labeled internal oligonucleotide probe are shown.
In conclusion, transplanted BMC appear to protect the recipient stroma by a mechanism that is unrelated to the control of infection.
DISCUSSION
A link between CMV infection and insufficient hematopoietic reconstitution after BMT is established clinical experience for human CMV (8, 10). However, from the clinical data it could not be decided whether in the respective patients a graft failure of unrelated etiology had promoted CMV disease or whether CMV infection had caused the graft failure. The observation of myelosuppressive effects of human and murine CMV infection in vitro, namely, in long-term BM cultures supporting primarily the myelomonocytic differentiation lineage that yields mature monocytes and neutrophilic granulocytes, has prompted the hypothesis that CMV interferes with hematopoiesis (reviewed in reference 4). Further support was provided by murine models of CMV infection demonstrating myelosuppression in vivo (11, 32, 33, 35). Our recent work on lethal BM aplasia after murine CMV infection has given evidence for the prevention of endogenous hematopoietic reconstitution by a functional deficiency of the BM stroma regarding the expression of essential hemopoietins, such as SCF, interleukin-6, and granulocyte colony-stimulating factor (29). Collectively, these in vitro and in vivo data have strongly suggested a pathomechanism for CMV affecting specifically the supportive stromal microenvironment in the BM. However, to date, proof was missing that this all applies to the specific conditions in the setting of BMT, where exogenous hematopoietic cells have to repopulate recipient BM. One has to consider the possibility that the transplant itself may modulate the infection or protect the recipient stroma by other means. The results presented here have helped us to answer some of the open questions.
The data have definitively shown that CMV infection inhibits the engraftment of the transplanted hematopoietic cells and can, in the extreme case, cause a complete graft failure. CMV infection thus interferes directly with the therapeutic aim of BMT.
Successful engraftment implies that intravenously transplanted hematopoietic cells migrate from the vascular compartment into the hematopoietic cord, settle there in the stromal network, and grow out to form hematopoietic colonies repopulating the BM. The tdy gene assay did not detect male cells in the BM of the recipients on day 2 after BMT (Fig. 6); that is, the actually transplanted cells are not visible in this assay. This is explained by the known fact that only a minority of the transplanted cells represent SCF-R expressing stem and progenitor cells (see also Fig. 8) capable of invading and repopulating the BM. The assay thus measures the progeny of the transplanted hematopoietic cells, which became detectable by day 6 (Fig. 6). Ingress and egress across the marrow-blood barrier are highly selective events that are thought to require specific interaction between hematopoietic cells and capillary or sinusoidal endothelium at the sites of entry or exit (12, 27). CMV infection could intervene at this crucial step. However, as we have documented previously (29, 33) and have reproduced here (Fig. 7), CMV infection also inhibits the endogenous hematopoietic reconstitution originating from residual stem cells after incomplete hematoablative treatment. Per se, this finding did not exclude an influence of the infection on the ingress of transplanted cells, but it did indicate that there must exist a pathomechanism operating beyond that stage. In the present model, a beginning repopulation of recipient BM by a male donor progenitor cell progeny became visible on day 6 also in the infected recipients (Fig. 6), which implies a successful ingress, homing, and several rounds of proliferation. However, while in the uninfected recipients the reconstitution proceeded with time (Fig. 6) and eventually resulted in histologically visible myeloid lineage colonies (Fig. 4A), infection led to a stagnation in the growth of the progeny (Fig. 6), resulting in BM aplasia (Fig. 4B) or hypoplasia (Fig. 5), depending on the experimental conditions. We therefore conclude that the infection interferes with hematopoiesis at the stage of clonal expansion, a conclusion that is compatible with the previous finding of a deficiency in stroma-derived growth- and differentiation-promoting hemopoietins, such as SCF, interleukin-6, and granulocyte colony-stimulating factor (29).
An inhibition of colony growth could operate at the level of stem cell self-renewal, as well as at any later stage in the hematopoietic differentiation hierarchy. Cells expressing SCF-R are not identical to pluripotent stem cells, but they do include stem and early progenitor cells. If the renewal of the SCF-R-positive cells is blocked, this necessarily also affects their progeny, and we would therefore expect an unchanged relative number of SCF-R-positive cells. By contrast, if the inhibition operates at a later stage, SCF-R-positive cells should accumulate relative to the progeny. We have found that the frequency of SCF-Rhigh expressors is generally low in reconstituting BM and, specifically, we did not get any evidence for an accumulation of these cells in the infected BM (Fig. 8). Instead, the amount of SCF-R transcripts in reconstituting BM paralleled the progeny size (Fig. 7). These data are compatible with the interpretation that the inhibition by CMV affects the renewal of SCF-R-positive stem and progenitor cells.
The histological analysis of the early repopulation of the uninfected stroma has revealed spatially separated myelomonocytic and erythroblastic colonies. This indicates that those colonies arose from the respective myeloid sublineage-committed progenitors rather than from the common myeloid stem cells or from the pluripotent hematopoietic stem cells (PHSC). By definition, only the PHSC are capable of long-term self-renewal and can thus stably repopulate the BM and give rise to all hematopoietic differentiation lineages. Estimates of the frequency of the PHSC in the BM of untreated mice range between 1 in 3 × 104 BMC (19) and 1 in 1 × 105 BMC, with some variance according to donor age (14). It is thus obvious that the many colonies found in uninfected stroma on day 14 after transplantation of only 104 BMC (Fig. 4A1) represented the progeny of committed, relatively short-lived progenitor cells. Accordingly, all observations described here refer to progenitor cell engraftment rather than to stem cell engraftment. Even though the initial repopulation provided by committed progenitor cells does not lead to long-term reconstitution of the recipient, the immediate progenitor cell engraftment is crucial for survival after hematoablative treatment. As discussed previously by Keller (19), the few PHSC do not generate significant numbers of differentiated progeny immediately following transplantation. It takes at least 1 month before progeny of the PHSC emerge in the BM. As a consequence, PHSC are unable to provide the rapid reconstitution required for protection against radiation disease (19). With the same reasoning, we can now add that PHSC are also unable to protect against early CMV disease after BMT because in the model presented here it was shown that the fate of the infected recipients is decided by the third week after BMT (Fig. 2). Since the progenitor cells by far outnumber the PHSC, our data on the expression of SCF-R apparently refer to the size of the progenitor cell pool. Altogether, progenitor cell engraftment is the relevant parameter.
So far, we have discussed the nature of the hematopoietic deficiency but not the mechanism by which CMV causes it. Two previous findings have indicated that CMV-mediated BM aplasia is caused by an insufficiency of the supportive stromal microenvironment. First, myelomonocytic progenitor cells derived from an infected BM cell culture were shown to continue normal proliferation and differentiation upon transfer onto an intact stroma cell monolayer (2). Second, stromal cells were identified as targets of CMV infection in vitro (reviewed in reference 4) as well as in vivo (29). The straightforward explanation of a stromal failure being caused by an extensive cytocidal infection of RSC, as suggested by the in vitro studies, did not apply to the in situ infection since the stromal network was not disrupted, the number of infected RSC was low, and the virus productivity was minute (29). In contrast, there was a clear functional insufficiency of the stroma with regard to the expression of hemopoietin genes, including the gene encoding SCF, the ligand of the SCF-R expressed by the progenitor cells (29). This finding offers a plausible and attractive explanation for the failure in progenitor cell engraftment.
A lower number of hemopoietin transcripts in stromal cells of infected recipients could be explained by a negative regulation of hemopoietin gene expression and/or transcript stability or by a failure in positive regulation. In either case, the low number of infected cells in BM stroma demands a regulation that is induced by the infection but is operative also in uninfected bystander cells. We initiated the present study in order to decide between the two models illustrated in Fig. 1, namely the downregulation model and the lack-of-induction model. If negative regulation applies, constitutive SCF gene expression should be high in uninfected stroma and reduced in the infected stroma. If a failure in positive regulation applies, a low constitutive SCF gene expression should be enhanced by transplanted hematopoietic cells only in the stroma of uninfected recipients. The results indicate a higher degree of complexity, but they do answer the main question. Clearly, the constitutive SCF gene expression in uninfected stroma is high and is not further elevated by transplanted hematopoietic cells (Fig. 10). Thus, a central postulate of the lack-of-induction model is not fulfilled. On the other hand, in the absence of transplanted hematopoietic cells the high constitutive SCF gene expression is reduced by the infection. Thus, a central postulate of the downregulation model is fulfilled. As a consequence, future research will have to identify the postulated negative regulator. This could be a secreted viral product or a cytokine induced by the infection. Regarding cytokines, transforming growth factor β1 (TGF-β1) is induced by CMV infection (24, 30) and is known to inhibit SCF transcription as well as SCF-R transcript stability (7, 15). However, recent work has provided evidence against a key role for TGF-β1 in CMV-induced BM graft failure (6). Specifically, preemptive CD8 T-cell therapy of murine CMV infection was found to restore endogenous hematopoietic reconstitution, even though the induction of TGF-β1 was not prevented.
While the downregulation model proved to be essentially valid, some modification is now required. We have wondered why the stroma of infected mice was unable to support the engraftment of low numbers of hematopoietic progenitor cells, even though engraftment occurred at higher numbers. The explanation is provided by the recovery of stromal SCF gene expression in response to increasing numbers of transplanted cells (Fig. 10). Apparently, the hematopoietic cells take an active part in their own survival in that they help the stroma to maintain supportive competence. Since transplanted cells comprise hematopoietic cells of different lineages and in all stages of differentiation, the identification of the stroma-protecting cell as well as the mechanism of stroma protection will be exciting but difficult issues of future investigations. Our studies have already excluded stroma protection by an antiviral effect of the hematopoietic cells. At present we can only speculate that hematopoietic cells may abrogate CMV-induced negative regulation of SCF gene expression by positive counter-regulation or by inactivating the negative regulator.
In conclusion, engraftment of a BM transplant in the presence of CMV infection depends on a sophisticated interplay between the virus, the stromal microenvironment, and the immigrating hematopoietic cells.
ACKNOWLEDGMENTS
We thank Liane Dreher, Ulm, Germany; Anna Mayer, Munich, Germany; and F. W. Busch, Stuttgart, Germany, for their contributions earlier in this project.
This work was supported by a grant to M. J. Reddehase by the Deutsche Forschungsgemeinschaft, Sonderforschungsbereich 311.
REFERENCES
- 1.Apperley J F, Dowding C, Hibbin J, Buiter J, Matutes E, Sissons P J, Gordon M, Goldman J M. The effect of cytomegalovirus on hematopoiesis: in vitro evidence for selective infection of marrow stromal cells. Exp Hematol. 1989;17:38–45. [PubMed] [Google Scholar]
- 2.Busch F W, Mutter W, Koszinowski U H, Reddehase M J. Rescue of myeloid lineage-committed preprogenitor cells from cytomegalovirus-infected bone marrow stroma. J Virol. 1991;65:981–984. doi: 10.1128/jvi.65.2.981-984.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chabannon C, Torok-Storb B. Stem cell-stromal cell interactions. Curr Top Microbiol Immunol. 1992;177:123–136. doi: 10.1007/978-3-642-76912-2_10. [DOI] [PubMed] [Google Scholar]
- 4.Childs B, Emanuel D. Cytomegalovirus infection and compromise. Exp Hematol. 1993;21:198–200. . (Editorial.) [PubMed] [Google Scholar]
- 5.Cobbold S P, Jayasuriya A, Nash A, Prospero T D, Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature (London) 1984;312:548–550. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- 6.Dobonici M, Podlech J, Steffens H-P, Maiberger S, Reddehase M J. Evidence against a key role for transforming growth factor-β1 in cytomegalovirus-induced bone marrow aplasia. J Gen Virol. 1998;79:867–876. doi: 10.1099/0022-1317-79-4-867. [DOI] [PubMed] [Google Scholar]
- 7.Dubois C M, Ruscetti F W, Stankova J, Keller J R. Transforming growth factor-β regulates c-kit message stability and cell-surface expression in hematopoietic progenitors. Blood. 1994;83:3138–3145. [PubMed] [Google Scholar]
- 8.Einsele H, Ehninger G, Steidle M, Fischer I, Bihler S, Gerneth F, Vallbracht A, Schmidt H, Waller H D, Müller C A. Lymphocytopenia as an unfavorable prognostic factor in patients with cytomegalovirus infection after bone marrow transplantation. Blood. 1993;82:1672–1678. [PubMed] [Google Scholar]
- 9.Fish K N, Britt W, Nelson J A. A novel mechanism for persistence of human cytomegalovirus in macrophages. J Virol. 1996;70:1855–1862. doi: 10.1128/jvi.70.3.1855-1862.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fries B C, Khaira D, Pepe M S, Torok-Storb B. Declining lymphocyte counts following cytomegalovirus (CMV) infection are associated with fatal CMV disease in bone marrow transplant patients. Exp Hematol. 1993;21:1387–1392. [PubMed] [Google Scholar]
- 11.Gibbons A E, Price P, Shellam G R. Analysis of hematopoietic stem and progenitor cell populations in cytomegalovirus-infected mice. Blood. 1995;86:473–481. [PubMed] [Google Scholar]
- 12.Gordon M Y, Greaves M F. Physiological mechanisms of stem cell regulation in bone marrow transplantation and haemopoiesis. Bone Marrow Transplant. 1989;4:335–338. [PubMed] [Google Scholar]
- 13.Gubbay J, Collignon J, Koopman P, Capel B, Economou A, Münsterberg A, Vivian N, Goodfellow P, Lovell-Badge R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature (London) 1990;346:245–250. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- 14.Harrison D E. Evaluating functional abilities of primitive hematopoietic stem cell populations. Curr Top Microbiol Immunol. 1992;177:13–30. doi: 10.1007/978-3-642-76912-2_2. [DOI] [PubMed] [Google Scholar]
- 15.Heinrich M C, Dooley D C, Keeble W W. Transforming growth factor β1 inhibits expression of the gene products for steel factor and its receptor (c-kit) Blood. 1995;85:1769–1780. [PubMed] [Google Scholar]
- 16.Hudson J B, Misra V, Mosmann T R. Cytomegalovirus infectivity: analysis of the phenomenon of centrifugal enhancement of infectivity. Virology. 1976;72:235–243. doi: 10.1016/0042-6822(76)90326-3. [DOI] [PubMed] [Google Scholar]
- 17.Ikuta K, Weissman I L. Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proc Natl Acad Sci USA. 1992;89:1502–1506. doi: 10.1073/pnas.89.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keil G M, Fibi M R, Koszinowski U H. Characterization of the major immediate-early polypeptides encoded by murine cytomegalovirus. J Virol. 1985;54:422–428. doi: 10.1128/jvi.54.2.422-428.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keller G. Clonal analysis of hematopoietic stem cell development in vivo. Curr Top Microbiol Immunol. 1992;177:41–57. doi: 10.1007/978-3-642-76912-2_4. [DOI] [PubMed] [Google Scholar]
- 20.Kondo K, Kaneshima H, Mocarski E S. Human cytomegalovirus latent infection of granulocyte-macrophage progenitors. Proc Natl Acad Sci USA. 1994;91:11879–11883. doi: 10.1073/pnas.91.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondo K, Xu J, Mocarski E S. Human cytomegalovirus latent gene expression in granulocyte-macrophage progenitors in culture and in seropositive individuals. Proc Natl Acad Sci USA. 1996;93:11137–11142. doi: 10.1073/pnas.93.20.11137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koszinowski U H, Del Val M, Reddehase M J. Cellular and molecular basis of the protective immune response to cytomegalovirus infection. Curr Top Microbiol Immunol. 1990;154:189–220. doi: 10.1007/978-3-642-74980-3_8. [DOI] [PubMed] [Google Scholar]
- 23.Kurz S, Steffens H-P, Mayer A, Harris J R, Reddehase M J. Latency versus persistence or intermittent recurrences: evidence for a latent state of murine cytomegalovirus in the lungs. J Virol. 1997;71:2980–2987. doi: 10.1128/jvi.71.4.2980-2987.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lagneaux L, Delforge A, Snoeck R, Bosmans E, Schols D, DeClercq E, Stryckmans P, Bron D. Imbalance in production of cytokines by bone marrow stromal cells following cytomegalovirus infection. J Infect Dis. 1996;174:913–919. doi: 10.1093/infdis/174.5.913. [DOI] [PubMed] [Google Scholar]
- 25.Lagneaux L, Delforge A, Snoeck R, Stryckmans P, Bron D. Decreased production of cytokines after cytomegalovirus infection of marrow-derived stromal cells. Exp Hematol. 1994;22:26–30. [PubMed] [Google Scholar]
- 26.Lathbury L J, Allan J E, Shellam G R, Scalzo A A. Effect of host genotype in determining the relative roles of natural killer cells and T cells in mediating protection against murine cytomegalovirus infection. J Gen Virol. 1995;77:2605–2613. doi: 10.1099/0022-1317-77-10-2605. [DOI] [PubMed] [Google Scholar]
- 27.Lichtman M A, Packman C H, Constine L S. Molecular and cellular traffic across the marrow sinuses. In: Tavassoli M, editor. Handbook of the hematopoietic microenvironment. Clifton, N.J: Humana Press; 1989. pp. 87–140. [Google Scholar]
- 28.Maciejewski J P, Bruening E E, Donahue R E, Mocarski E S, Young N S, St. Jeor S C. Infection of hematopoietic progenitor cells by human cytomegalovirus. Blood. 1992;80:170–178. [PubMed] [Google Scholar]
- 29.Mayer A, Podlech J, Kurz S, Steffens H-P, Maiberger S, Thalmeier K, Angele P, Dreher L, Reddehase M J. Bone marrow failure by cytomegalovirus is associated with an in vivo deficiency in the expression of essential stromal hemopoietin genes. J Virol. 1997;71:4589–4598. doi: 10.1128/jvi.71.6.4589-4598.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michelson S, Alcami J, Kim S-J, Danielpour D, Bachelerie F, Picard L, Bessia C, Paya C, Virelizier J L. Human cytomegalovirus infection induces transcription and secretion of transforming growth factor β1. J Virol. 1994;68:5730–5737. doi: 10.1128/jvi.68.9.5730-5737.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minton E J, Tysoe C, Sinclair J H, Sissons J G. Human cytomegalovirus infection of the monocyte/macrophage lineage in bone marrow. J Virol. 1994;68:4017–4021. doi: 10.1128/jvi.68.6.4017-4021.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mori T, Ando K, Tanaka K, Ikeda Y, Koga Y. Fas-mediated apoptosis of the hematopoietic progenitor cells in mice infected with murine cytomegalovirus. Blood. 1997;89:3565–3573. [PubMed] [Google Scholar]
- 33.Mutter W, Reddehase M J, Busch F W, Bühring H J, Koszinowski U H. Failure in generating hematopoietic stem cells is the primary cause of death from cytomegalovirus disease in the immunocompromised host. J Exp Med. 1988;167:1645–1658. doi: 10.1084/jem.167.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiu F, Ray P, Brown K, Barker P E, Jhanwar S, Ruddle F H, Besmer P. Primary structure of c-kit: relationship with the CSF-1/PDGF receptor kinase family. EMBO J. 1988;7:1003–1011. doi: 10.1002/j.1460-2075.1988.tb02907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reddehase, M. J., L. Dreher-Stumpp, P. Angele, M. Balthesen, and M. Susa. 1992. Hematopoietic stem cell deficiency resulting from cytomegalovirus infection of bone marrow stroma. Ann. Hematol. 64(Suppl.):A125–A127. [DOI] [PubMed]
- 36.Reddehase M J, Jonjic S, Weiland F, Mutter W, Koszinowski U H. Adoptive immunotherapy of murine cytomegalovirus adrenalitis in the immunocompromised host: CD4-helper-independent antiviral function of CD8-positive memory T lymphocytes derived from latently infected donors. J Virol. 1988;62:1061–1065. doi: 10.1128/jvi.62.3.1061-1065.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reddehase M J, Weiland F, Münch K, Jonjic S, Lüske A, Koszinowski U H. Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J Virol. 1985;55:264–273. doi: 10.1128/jvi.55.2.264-273.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shanley J D, Biczak L, Forman S J. Acute murine cytomegalovirus infection induces lethal hepatitis. J Infect Dis. 1993;167:264–269. doi: 10.1093/infdis/167.2.264. [DOI] [PubMed] [Google Scholar]
- 39.Shanley J D, Pesanti E L. The relation of viral replication to interstitial pneumonitis in murine cytomegalovirus lung infection. J Infect Dis. 1985;151:454–458. doi: 10.1093/infdis/151.3.454. [DOI] [PubMed] [Google Scholar]
- 40.Shanley J D, Pesanti E L. Murine cytomegalovirus adrenalitis in athymic nude mice. Arch Virol. 1986;88:27–35. doi: 10.1007/BF01310887. [DOI] [PubMed] [Google Scholar]
- 41.Simmons P, Kaushansky K, Torok-Storb B. Mechanisms of cytomegalovirus-mediated myelosuppression: perturbation of stromal cell function versus direct infection of myeloid cells. Proc Natl Acad Sci USA. 1990;87:1386–1390. doi: 10.1073/pnas.87.4.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soderberg-Naucler C, Fish K N, Nelson J A. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell. 1997;91:119–126. doi: 10.1016/s0092-8674(01)80014-3. [DOI] [PubMed] [Google Scholar]
- 43.Steffens H-P, Kurz S, Holtappels R, Reddehase M J. Preemptive CD8 T-cell immunotherapy of acute cytomegalovirus infection prevents lethal disease, limits the burden of latent viral genomes, and reduces the risk of virus recurrence. J Virol. 1998;72:1797–1804. doi: 10.1128/jvi.72.3.1797-1804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taichman R S, Nassiri M R, Reilly M J, Ptak R G, Emerson S G, Drach J C. Infection and replication of human cytomegalovirus in bone marrow stromal cells: effects on the production of IL-6, MIP-1α, and TGF-β1. Bone Marrow Transplant. 1997;19:471–480. doi: 10.1038/sj.bmt.1700685. [DOI] [PubMed] [Google Scholar]
- 45.Williams D E, de Vries P, Namen A E, Widmer M B, Lyman S D. The Steel factor. Dev Biol. 1992;151:368–376. doi: 10.1016/0012-1606(92)90176-h. [DOI] [PubMed] [Google Scholar]