Abstract
Cold atmospheric plasma (CAP) has shown promising potential in promoting wound healing. This case report presents the successful application of CAP in a 42-year-old female patient with extensive wound healing disorders and superinfections following the excision of an abscess in the left thoracic region. After several failed split skin graft attempts, the implementation of CAP led to significant improvements in wound healing. This report highlights the wound healing-promoting effects of CAP and discusses its potential mechanisms of action.
1. Introduction
Wound healing disorders and superinfections pose significant challenges in clinical practice. Traditional treatment modalities often fail to achieve optimal outcomes. Cold atmospheric plasma is an ionized gas and can be described as the fourth matter of state. In the last decade, plasma medicine has been established and clinically approved, frequently found to possess antimicrobial properties and stimulate wound healing [1–3]. The mechanisms underlying its therapeutic effects include the generation of reactive oxygen species, which support eradicating the pathogen microbiome [4, 5] and are efficient against Pseudomonas bacteria [6–8]. Moreover, CAP can activate intrinsic wound healing mechanisms by inducing the migration and adhesion of molecules in skin cells [9], promoting VEGF secretion in skin cells [10, 11], and improving microcirculation in wounds [12, 13]. This case report illustrates the successful utilization of CAP in a challenging wound healing scenario, where conventional treatment modalities had failed.
2. Case Presentation
A 42-year-old female patient with a history of nicotine abuse presented with extensive wound healing disorders (covering an area of 12 × 19 cm) and recurrent superinfections following the excision of a 5 cm subcutaneous abscess on the left thoracic region (Figure 1(a), red arrow marks the abscess). The initial abscess formed around a trocar incision site after laparoscopic ovarian cyst resection and was treated by incision and drainage months before. Wound healing was delayed, and healing disorders persisted since. A failed attempt at split skin grafting necessitated patient transfer from a smaller clinic to our university hospital for further management. A computed tomography (CT) scan revealed subcutaneous spread of abscesses. After surgical debridement of infected tissue on the left side of the thorax including the eleventh and twelfth rib, antibiotic therapy followed. Despite persisting wound area with low healing tendency and an express patient's wish to perform another split skin graft to close the wound, we rejected another attempt initially in respect to wound cultures positive for Pseudomonas aeruginosa and E. coli. Subsequent vacuum-assisted closure (VAC) and intraoperative rinsing were performed until negative wound cultures were achieved three times in a row. However, a second split skin graft failed. After additional VAC therapy and intensified wound care, a switch to a regimen of daily disinfections with polyhexanide was made. Noninflamed wound conditions and negative wound cultures were reached. In consideration of the patient's desire, the stable wound conditions, and a lack of options, a third attempt was made and seemed to be successful. However, the graft became infected after two weeks and had to be removed. The patient experienced chronic wound pain and required high doses of analgesics. Despite three weeks of conventional wound treatment carried out by specialized wound care nurses including antiseptic rinsing and daily change of dressings as well as application of topical antibiotics, the wound remained inflamed, reinfected with Pseudomonas aeruginosa, and showed no granulation tendency. Finally, cold atmospheric plasma therapy was initiated.
Figure 1.
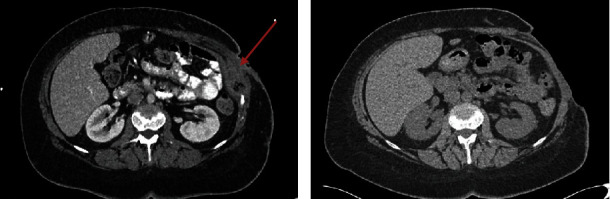
Computed tomography of abdomen (a) pre- and (b) postoperative. (a) Arrow marks the abscess with trapped air. (b) Postoperative lesion of the left abdominal wall.
3. Treatment and Outcome
Cold atmospheric plasma therapy was administered three times a week for a period of four weeks using the “kINPen MED” (neoplas med GmbH, Greifswald, Germany), a cold atmospheric pressure argon plasma jet device operating at an excitation frequency of roughly 1 MHz and generating a nominal output power of around 1 watt. The jet flow was 5 L/min and the wound area was treated for 3 s/cm2. The physical, electrical, and chemical specifications of the device have been extensively documented previously [14]. An antiseptic polyhexanide rinse was applied intermittently. Throughout the treatment course, wound cultures gradually became negative, bacterial biofilm and macroscopic signs of inflammation diminished, and the wound exhibited favorable granulation tendencies (Figure 2). The patient's pain levels decreased significantly and her psychological state improved. Weekly wound assessments were conducted. Wound closure was achieved six weeks after initiation of CAP therapy, allowing for significant reduction of analgesic usage. Regular follow-up examinations in our clinic showed no evidence of new infections or wound healing disorders.
Figure 2.
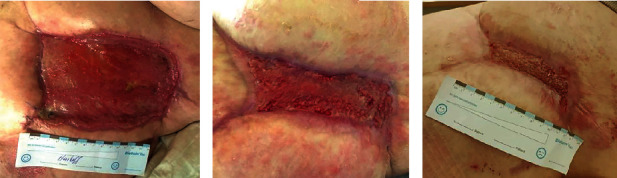
(a) Conventionally treated wound three weeks after failure of split skin graft, (b) wound after three weeks of CAP therapy, and (c) closed wound six weeks after start of CAP therapy.
4. Discussion
Wound infections are known as a crucial risk factor for wound healing disorders that can also appear during skin grafting [15–17]. The success of split skin grafting depends, among other factors, on the presence of Pseudomonas aeruginosa [18]. In view of stagnating wound conditions despite extensive conventional therapies and prompt improvement regarding wound infection and inflammation, it seems that CAP treatment induced wound healing in this challenging case. While the wound healing properties of CAP are widely recognized, it is noteworthy that this is the first reported case of CAP application following the failure of a split skin graft, to the best of our knowledge. Notably, CAP eradicated the persisting biofilm containing Pseudomonas aeruginosa. CAP generates a range of active oxygen and nitrogen products, including O, OH, O2−, NO, and H2O2. These reactive oxygen and nitrogen species (RONS) are capable to eliminate Pseudomonas aeruginosa via oxidative stress [4, 5, 8]. Moreover, CAP operates synergically with antibiotics [7], which could have played a beneficial role in the eradication of the bacterial load in this case. CAP reduced inflammation and facilitated granulation tissue formation as well, which have been reported before [2, 3]. Chronic inflammation is associated with elevated levels of interleukins and cytokines, which hinder wound closure [19]. CAP has the potential to regulate the inflammatory response by reducing cytokine production and facilitates the release of anti-inflammatory mediators like IL-10, TGFβ, and IL-8 [20]. The patient experienced significant pain relief, leading to improved quality of life. The treatment of chronic wounds that are resistant to therapy over a long period of time is expensive and can sometimes take years. Consequently, investing in short-term cold plasma treatment may be a financially more advantageous option in the long term. This can lead to cost savings for the healthcare system, especially when using high-precision devices such as kINPen MED. For this reason, the central German Healthcare Board (G-BA) is currently preparing a comparative clinical trial to assess the benefits of including medical gas plasma treatment into the standard of care catalogue for wound treatment. Furthermore, it is conceivable that applying CAP to the wound before a split skin graft could eliminate bacteria and enhance the success rate of the skin graft. To prove this thesis, clinical studies are needed. A limitation of this case report is the lack of knowledge about potential synergies between polyhexanide and CAP, making it difficult to assess the efficacy of CAP in isolation. However, it is worth noting that prior treatment with antiseptic rinses and antibiotics alone had proven to be insufficient.
5. Conclusion
This case report illustrates the successful implementation of cold atmospheric plasma therapy as a sufficient method to eradicate Pseudomonas aeruginosa and promote wound healing after multiple failures of split skin graft in a complex surgical case. Further research illuminating synergy of CAP with antibiotics and antiseptic rinses in infections and larger-scale studies is warranted to explore the full potential of CAP in diverse clinical scenarios.
Acknowledgments
We owe much gratitude to the wound nurses Sandra Eutin and Sabine Wagner, whose dedication to excellent care made this ultimately successful course of treatment possible. Open Access funding was enabled and organized by Projekt DEAL.
Data Availability
CT images and pictures of wound development are extracted from IT System “Meyerhofer” of University Hospital of Greifswald and can be requested with exact dates. Laboratory parameters representing inflammation could be supplemented. In case of request, please correspond to aydar.khabipov@med.uni-greifswald.de.
Conflicts of Interest
There are no conflicts of interest to declare.
References
- 1.Bekeschus S., von Woedtke T., Emmert S., Schmidt A. Medical gas plasma-stimulated wound healing: evidence and mechanisms. Redox Biology . 2021;46, article 102116 doi: 10.1016/j.redox.2021.102116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samsavar S., Mahmoudi H., Shakouri R., et al. The evaluation of efficacy of atmospheric pressure plasma in diabetic ulcers healing: a randomized clinical trial. Dermatologic Therapy . 2021;34(6, article e15169) doi: 10.1111/dth.15169. [DOI] [PubMed] [Google Scholar]
- 3.Samsavar S., Mahmoudi H., Khani M. R., Daneshpazhooh M., Shokri B. Treatment of chronic venous ulcer with cold atmospheric plasma jet. Case Reports in Dermatology . 2022;14(3):344–349. doi: 10.1159/000527018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lackmann J. W., Bandow J. E. Inactivation of microbes and macromolecules by atmospheric-pressure plasma jets. Applied Microbiology and Biotechnology . 2014;98(14):6205–6213. doi: 10.1007/s00253-014-5781-9. [DOI] [PubMed] [Google Scholar]
- 5.Wiegand C., Elsner P. Plasmamedizin – Kaltes plasma zur Behandlung von Hautinfektionen. Aktuelle Dermatologie . 2017;43(8/9):339–345. doi: 10.1055/s-0043-112681. [DOI] [Google Scholar]
- 6.Alkawareek M. Y., Algwari Q. T., Laverty G., et al. Eradication of Pseudomonas aeruginosa biofilms by atmospheric pressure non-thermal plasma. PLoS One . 2012;7(8, article e44289) doi: 10.1371/journal.pone.0044289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maybin J. A., Thompson T. P., Flynn P. B., et al. Cold atmospheric pressure plasma-antibiotic synergy in Pseudomonas aeruginosa biofilms is mediated via oxidative stress response. Biofilms . 2023;5, article 100122 doi: 10.1016/j.bioflm.2023.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Y., Shao L., Jia L., et al. Subcellular inactivation mechanisms of Pseudomonas aeruginosa treated by cold atmospheric plasma and application on chicken breasts. Food Research International . 2022;160, article 111720 doi: 10.1016/j.foodres.2022.111720. [DOI] [PubMed] [Google Scholar]
- 9.Lee H. R., Kang S. U., Kim H. J., et al. Liquid plasma as a treatment for cutaneous wound healing through regulation of redox metabolism. Cell Death & Disease . 2023;14(2):p. 119. doi: 10.1038/s41419-023-05610-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee H. Y., Lee H. J., Kim G. C., Choi J. H., Hong J. W. Plasma cupping induces VEGF expression in skin cells through nitric oxide-mediated activation of hypoxia inducible factor 1. Scientific Reports . 2019;9(1):p. 3821. doi: 10.1038/s41598-019-40086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arndt S., Unger P., Berneburg M., Bosserhoff A.-K., Karrer S. Cold atmospheric plasma (CAP) activates angiogenesis-related molecules in skin keratinocytes, fibroblasts and endothelial cells and improves wound angiogenesis in an autocrine and paracrine mode. Journal of Dermatological Science . 2018;89(2):181–190. doi: 10.1016/j.jdermsci.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Jensen J. O., Schulz L., Schleusser S., et al. The repetitive application of cold atmospheric plasma (CAP) improves microcirculation parameters in chronic wounds. Microvascular Research . 2021;138, article 104220 doi: 10.1016/j.mvr.2021.104220. [DOI] [PubMed] [Google Scholar]
- 13.Matzkeit N., Schulz L., Schleusser S., et al. Cold atmospheric plasma improves cutaneous microcirculation in standardized acute wounds: results of a controlled, prospective cohort study. Microvascular Research . 2021;138, article 104211 doi: 10.1016/j.mvr.2021.104211. [DOI] [PubMed] [Google Scholar]
- 14.Reuter S., von Woedtke T., Weltmann K.-D. The kINPen – a review on physics and chemistry of the atmospheric pressure plasma jet and its applications. Journal of Physics D: Applied Physics . 2018;51(23, article 233001) doi: 10.1088/1361-6463/aab3ad. [DOI] [Google Scholar]
- 15.Caldwell M. D. Bacteria and antibiotics in wound healing. Surgical Clinics of North America . 2020;100(4):757–776. doi: 10.1016/j.suc.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Xu L., McLennan S. V., Lo L., et al. Bacterial load predicts healing rate in neuropathic diabetic foot ulcers. Diabetes Care . 2007;30(2):378–380. doi: 10.2337/dc06-1383. [DOI] [PubMed] [Google Scholar]
- 17.Xu Z., Hsia H. C. The impact of microbial communities on wound healing: a review. Annals of Plastic Surgery . 2018;81(1):113–123. doi: 10.1097/SAP.0000000000001450. [DOI] [PubMed] [Google Scholar]
- 18.Høgsberg T., Bjarnsholt T., Thomsen J. S., Kirketerp-Møller K. Success rate of split-thickness skin grafting of chronic venous leg ulcers depends on the presence of Pseudomonas aeruginosa: a retrospective study. PLoS One . 2011;6(5, article e20492) doi: 10.1371/journal.pone.0020492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Portou M. J., Baker D., Abraham D., Tsui J. The innate immune system, toll-like receptors and dermal wound healing: a review. Vascular Pharmacology . 2015;71:31–36. doi: 10.1016/j.vph.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Arndt S., Unger P., Wacker E., et al. Cold atmospheric plasma (CAP) changes gene expression of key molecules of the wound healing machinery and improves wound healing in vitro and in vivo. PLoS One . 2013;8(11, article e79325) doi: 10.1371/journal.pone.0079325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
CT images and pictures of wound development are extracted from IT System “Meyerhofer” of University Hospital of Greifswald and can be requested with exact dates. Laboratory parameters representing inflammation could be supplemented. In case of request, please correspond to aydar.khabipov@med.uni-greifswald.de.


