ABSTRACT
BACKGROUND
In real-world clinical practice, treatments selected for patients with autosomal dominant polycystic kidney disease (ADPKD) in the chronic kidney disease (CKD) without kidney replacement therapy (KRT) have not been reported. This study investigated the oral treatments used in these patients and the changes in their use in recent years. Additionally, we studied the factors affecting tolvaptan dose reduction or discontinuation.
METHODS
This retrospective cohort study was conducted using the medical records of 160 hospitals in Japan. Patients with ADPKD or polycystic kidney disease registered on the database between January 2014 and December 2020 were selected. Changes in prescription proportions over time were assessed using the Cochran–Armitage test. We focused on patients prescribed with >15 mg of tolvaptan daily to identify the factors related to its dose reduction or discontinuation and used Multivariate Cox regression analysis to evaluate them.
RESULTS
Tolvaptan use in patients with ADPKD in the CKD without KRT stage has increased. As of 2020, 25% of patients were treated with tolvaptan. Overall, 3639 patients with ADPKD were enrolled in the database, of whom 156 were treated with tolvaptan. Of these, 64 patients (41%) reduced or discontinued tolvaptan during the observation period. The presence of an estimated glomerular filtration rate <60 mL/min/1.73 m2 at the beginning of the treatment was associated with a higher risk of tolvaptan dose reduction or discontinuation.
CONCLUSION
The proportion of patients with ADPKD treated with high-dose tolvaptan is increasing. However, patients with late-stage CKD tended to reduce or discontinue tolvaptan.
Keywords: Autosomal dominant polycystic kidney disease (ADPKD), chronic kidney disease (CKD), tolvaptan
INTRODUCTION
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited renal disease and is generally caused by polycystic kidney disease (PKD) 1 or PKD2 mutations, with PKD1 mutations resulting in a faster progression to kidney failure [1, 2]. Accumulation of cysts in the kidney parenchyma is the dominant phenotype of ADPKD [3]. ADPKD is an incurable disease where compensatory mechanisms function until approximately the age of 40 years, after which kidney function gradually declines [4]. Almost half of the patients with ADPKD develop kidney failure at approximately 60 years of age [5]. ADPKD is characterised by a younger age of dialysis induction compared to other diseases [6], accounts for approximately 3.6% of patients undergoing dialysis in Japan, and is the fifth most common primary disease among these patients [6].
The efficacy of tolvaptan, a vasopressin V2 receptor antagonist, was initially reported in 2012 [7]. Tolvaptan suppresses ADPKD progression and has been approved in Japan since March 2014 for patients with ADPKD in the chronic kidney disease (CKD) without kidney replacement therapy (KRT). Interestingly, tolvaptan’s efficacy in patients with ADPKD in the later stages of CKD was reported in 2017 [8] and it was approved in the United States in April 2018. The daily oral dose of tolvaptan for ADPKD is higher than that for other diseases, such as congestive heart failure, and the major adverse effects include polyuria, dry mouth, liver dysfunction, and acute kidney injury (AKI) [8]. Since tolvaptan has been approved for ADPKD treatment, several cohort studies have supported its efficacy in patients with ADPKD [9, 10]. However, there are few reports focusing on therapies performed in patients in the CKD without KRT because it is a rare disease. Approximately 15–23% of patients with ADPKD discontinued tolvaptan during the observation period [7, 8], although the factors leading to its dose reduction or discontinuation remain unknown. Therefore, this study aimed to reveal the medical treatments prescribed to patients with ADPKD in the CKD without KRT in Japan and to examine how this changed following the approval of tolvaptan in 2014. Furthermore, we investigated the factors leading to the dose reduction or discontinuation of tolvaptan.
MATERIALS AND METHODS
DATA SOURCE
This retrospective cohort study used the RWD database, which is maintained by the Health, Clinic, and Education Information Evaluation Institute (HCEI, Kyoto, Japan) with support from the Real World Data Co., Ltd. (Kyoto, Japan) [11, 12]. This database contains the records of approximately 20 million patients from over 160 medical institutions in Japan as of 2020. The database does not include information regarding hospital type (academic hospital or not) and number of beds.
Specifically, the stored information includes demographic data; diagnostic information using the International Classification of Diseases, 10th revision (ICD-10) codes; prescriptions; procedures; and laboratory results from both outpatient and inpatient services. Data were automatically extracted from the electronic medical records of each medical institution. Furthermore, patient records were maintained by allocating unique identifiers to each valid individual within the same institution.
STUDY POPULATION
In this study, we defined patients with ADPKD as those with a diagnosis of ADPKD or PKD. We extracted data from patients diagnosed with ADPKD (ICD-10 code Q612) or PKD (ICD-10 code Q613) between January 2014 and December 2020 from the RWD database. The ICD-10 coding algorithm had a sensitivity and specificity of 33.7% (95% confidence interval [CI]: 30.0–37.7) and 86.2% (95% CI: 75.7–92.5), respectively, for identifying ADPKD [13].
Study population to describe changes in the oral treatment of ADPKD
Patients with ADPKD in the CKD without KRT were selected based on the following exclusion criteria (Fig. 1): Patients receiving KRT (procedure codes in the administrative claims data: C102, J038, J038-2, J039, J041, J042, and K780-2) or those with missing procedure codes, including dialysis information, were excluded. Patients with autosomal recessive polycystic kidney disease were excluded by eliminating those aged <20 years in each year between 2014 and 2020. To calculate the estimated glomerular filtration rate (eGFR) and determine their CKD stage, patients with missing birth year or serum creatinine (sCre) data 30 days before or after the last prescription date were excluded. We also excluded patients with any prescription after death from the demographic information in the administrative claims data. Fig. 1 presents this study’s exclusion criteria.
Fig. 1 . Flow diagram for selecting patients with ADPKD in the CKD without KRT.
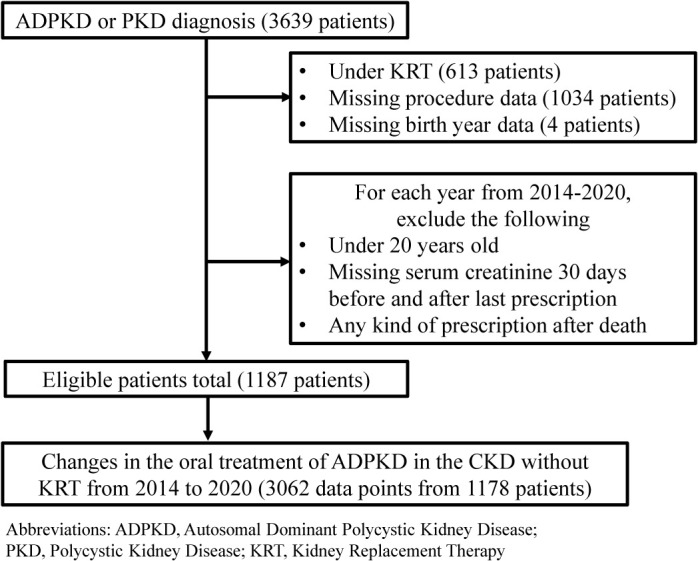
Abbreviations: ADPKD, Autosomal Dominant Polycystic Kidney Disease; PKD, Polycystic Kidney Disease; KRT, Kidney Replacement Therapy
We extracted the data of patients with ADPKD in the CKD without KRT in each year. The last prescription date for each year and prescription details, including drug name, date of prescription, drug dose, and prescription days, within 90 days were extracted. Using the Anatomical Therapeutic Chemical Classification System (ATC) code, we extracted diuretics, antihypertensive drugs, and lipid-lowering drugs frequently used in treating CKD. Finally, the percentage of patients prescribed these drugs each year was calculated.
Study population to evaluate the dose reduction or discontinuation of tolvaptan
We selected patients who were treated with tolvaptan at >15 mg/day. The standard dose of tolvaptan for ADPKD is 60–120 mg/day; however, it is frequently reduced because many patients cannot tolerate higher doses. The maximum tolvaptan dose for other situations, such as fluid retention for heart failure and liver cirrhosis, is 15 mg/day. Therefore, we considered that patients who were treated with tolvaptan of >15 mg/day were treated for ADPKD. Patients undergoing KRT were included because dialysis may begin after completing tolvaptan treatment. Tolvaptan is used only for patients in the CKD without KRT; therefore, all selected patients were considered to have CKD without KRT.
Tolvaptan discontinuation was defined as an interruption in drug therapy for >30 days, whereas dose reduction was defined as a decrease in dose during the observation period. Additionally, the discontinuation date was defined as the last prescription date plus the number of prescription days (Supplementary Fig. S1).
The eGFR was calculated using the following equation using the sCre value: eGFR (mL/min/1.73 m2) = 194 × sCre−1.094 (mg/dL) × age−0.287 (years) (×0.739 for women). This formula was modified for the body composition of the Japanese population from the original Modification of Diet in Renal Disease equation [14]. After calculating the patients’ eGFR, they were classified into CKD stages as proposed by the Kidney Disease Improving Global Outcomes (KDIGO) guidelines [15].
OUTCOMES AND VARIABLES
The primary outcome was the dose reduction or discontinuation of tolvaptan, whereas the secondary outcome was the discontinuation of tolvaptan. Additionally, we defined the grace period as 30 days and considered prescription interruption within 30 days as the continuation of tolvaptan.
To describe the major adverse effects, such as acute kidney injury (AKI), acute liver injury, and hypernatremia, we initially identified the date when the dose of tolvaptan was reduced or discontinued. Second, we extracted the maximum sCre, aspartate transaminase (AST), alanine transaminase (ALT), and serum sodium levels for 14 days before and after tolvaptan dose reduction or discontinuation.
AKI was described according to the KDIGO guidelines [16]. Acute liver injury has been described in previous studies based on AST and ALT levels [7, 8]. Hypernatremia has various definitions; however, our study used three levels: >145 mEq/L, >150 mEq/L, and >160 mEq/L. The baseline sCre level used to identify AKI was measured using the average sCre level from 30 to 90 days before its occurrence (Supplementary Fig. S2).
STATISTICAL ANALYSIS
The study population characteristics were expressed as proportions for categorical variables and median and interquartile range (IQR) for continuous variables. Changes in the prescription rate and trends over time were assessed using the Cochran–Armitage test, and P-values were calculated. Additionally, the trend of tolvaptan usage over time was assessed the same analysis.
We performed multivariate Cox regression analysis of the primary outcome and defined censoring as death, or no outcome occurring within the observation period. In multivariate Cox analysis, age, sex, presence of CKD, diabetes, and hypertension before starting tolvaptan were used as confounding variables potentially affecting the occurrence of adverse effects. Adjusted hazard ratios (aHRs), 95% CIs, and P-values were calculated for each variable. We treated eGFR was as a continuous variable in the sensitivity analysis. During the secondary outcome analysis, the model was adjusted for the presence of CKD and hypertension because of the low occurrence of outcomes. All P-values were two-sided, and the alpha level was set at 0.05. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Overall, 3,639 patients with ADPKD or PKD were enrolled in the database between 2014 and 2020.
CHANGES IN THE ORAL TREATMENT OF ADPKD IN THE CKD WITHOUT KRT BETWEEN 2014 AND 2020
Medical data of patients in the CKD without KRT were extracted from the database for each year, and the changes in medical prescriptions over time are summarised in Table 1. As of 2020, approximately 25% of patients with ADPKD in the CKD without KRT were receiving tolvaptan, and 67% were treated with antihypertensive medication. The percentage of tolvaptan use increased after 2014, the year of tolvaptan approval in Japan, with a significant increase in patients taking >15 mg/day of tolvaptan. The proportions of lipid-lowering and antihypertensive drugs, other than loop diuretics, did not change significantly during the observation period.
Table 1 . Changes in the oral treatment of ADPKD in the CKD without KRT.
| Variables, n (%) | Year | P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | ||
| Overall ADPKD cases in the CKD without KRT (3062 data points) | 412 (100) | 448 (100) | 478 (100) | 494 (100) | 470 (100) | 423 (100) | 337 (100) | |
| Diuretics | 97 (24) | 102 (23) | 141 (29) | 152 (31) | 151 (32) | 150 (35) | 143 (42) | <0.0001 |
| Tolvaptan (>15 mg/day) | 8 (1.9) | 21 (4.7) | 54 (11) | 66 (13) | 78 (17) | 85 (20) | 83 (25) | <0.0001 |
| Tolvaptan (≤15 mg/day) | 5 (1.2) | 7 (1.6) | 13 (2.7) | 17 (3.4) | 17 (3.6) | 15 (3.5) | 18 (5.3) | 0.0002 |
| Loop | 62 (15) | 64 (14) | 67 (14) | 60 (12) | 47 (10) | 42 (10) | 39 (12) | 0.005 |
| Thiazide | 30 (7.3) | 27 (6) | 34 (7.1) | 30 (6.1) | 31 (6.6) | 22 (5.2) | 24 (7.1) | 0.63 |
| Anti-aldosterone | 18 (4.4) | 18 (4.0) | 21 (4.4) | 23 (4.7) | 17 (3.6) | 16 (3.8) | 13 (3.9) | 0.59 |
| Antihypertensives | 267 (65) | 265 (59) | 284 (59) | 297 (60) | 298 (63) | 265 (63) | 227 (67) | 0.17 |
| ARB | 193 (47) | 191 (43) | 194 (41) | 190 (38) | 196 (42) | 175 (41) | 149 (44) | 0.41 |
| ACEI | 22 (5.3) | 22 (4.9) | 27 (5.6) | 32 (6.5) | 21 (4.5) | 20 (4.7) | 14 (4.2) | 0.41 |
| Renin blocker | 2 (0.49) | 5 (1.1) | 3 (0.63) | 2 (0.40) | 3 (0.64) | 2 (0.47) | 2 (0.59) | 0.61 |
| CCB | 190 (46) | 182 (41) | 185 (39) | 206 (42) | 199 (42) | 176 (42) | 147 (44) | 0.15 |
| β-blocker | 55 (13) | 54 (12) | 52 (11) | 72 (15) | 69 (15) | 59 (14) | 48 (14) | 0.22 |
| Other | 51 (12) | 48 (11) | 36 (7.5) | 39 (7.9) | 42 (8.9) | 39 (9.2) | 41 (12) | 0.64 |
| Lipid-lowering drug | 91 (22) | 96 (21) | 113 (24) | 114 (23) | 100 (21) | 93 (22) | 77 (23) | 0.96 |
| Statin | 79 (19) | 86 (19) | 105 (22) | 108 (22) | 95 (20) | 88 (21) | 73 (22) | 0.42 |
| Fibrate | 4 (1.0) | 1 (0.22) | 3 (0.63) | 3 (0.60) | 3 (0.64) | 3 (0.71) | 3 (0.89) | 0.74 |
| Other | 10 (2.4) | 11 (2.5) | 7 (1.5) | 8 (1.6) | 7 (1.5) | 5 (1.2) | 5 (1.5) | 0.11 |
Abbreviations: ADPKD, Autosomal Dominant Polycystic Kidney Disease; CKD, Chronic Kidney Disease; KRT, Kidney Replacement Therapy; ARB, Angiotensin II Receptor Blocker; ACEI, Angiotensin-Converting Enzyme Inhibitor; CCB, Calcium Channel Blocker
DOSE REDUCTION OR DISCONTINUATION OF TOLVAPTAN
Overall, 206 patients started treatment with high-dose tolvaptan (Fig. 2). In total, 156 patients met the inclusion criteria, and their characteristics at the time of tolvaptan administration are summarised in Table 2. The median (IQR) observation period was 547 days (range, 101–994 days), and the eGFR was 37.9 mL/min/1.73 m2 (24.4–54.3).
Fig. 2 . Patient flow when considering tolvaptan dose reduction or discontinuation.
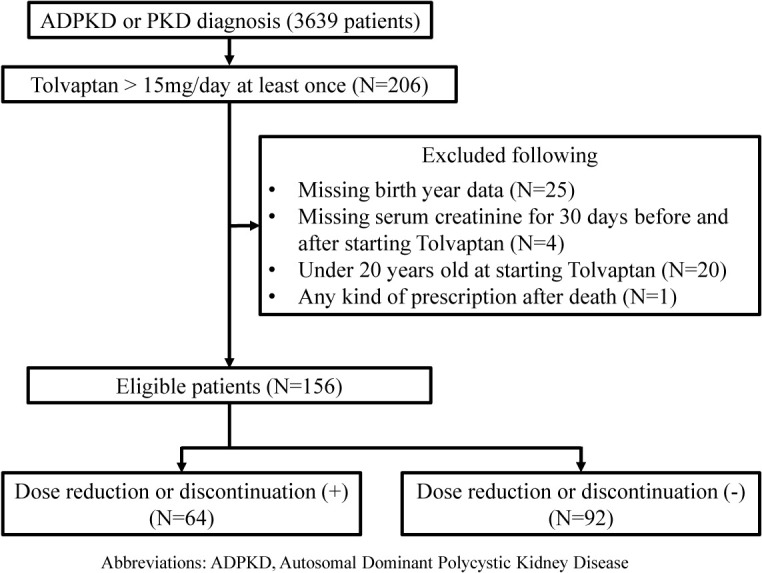
Abbreviations: ADPKD, Autosomal Dominant Polycystic Kidney Disease; sCre, serum creatinine
Table 2 . Patient characteristics at the start of tolvaptan treatment.
| Variables Overall | Overall n = 156 |
|---|---|
| Age (year), median (IQR) | 69.5 (52.0–82.0) |
| Male, n (%) | 44 (28) |
| BMI (kg/m2), median (IQR) | 22.6 (21.0–25.4) |
| Oral period of tolvaptan (days), median (IQR) | 547 (101–994) |
| eGFR (mL/min/1.73 m2), median (IQR) | 37.9 (24.4–54.3) |
| CKD Stage, n (%) | |
| G1 + G2 | 25 (16) |
| G3a | 32 (21) |
| G3b | 49 (31) |
| G4 | 44 (28) |
| G5 | 6 (4) |
| Co-morbidity, n (%) | |
| Diabetes mellitus | 20 (13) |
| Hypertension | 110 (71) |
| Hyperuricemia | 33 (21) |
Data are presented as unweighted numbers (percentages) of patients unless otherwise indicated.
All values, excluding BMI, were expressed at the start of the tolvaptan treatment.
BMI was expressed at the time the patients entered the database.
Abbreviations BMI: Body Mass Index; eGFR: estimated Glomerular Filtration Rate; CKD, Chronic Kidney Disease; G, Glomerular Filtration Rate; IQR: Interquartile Range
Of the 156 patients, 64 (41%) reduced the dose or discontinued tolvaptan, and 21 (13%) discontinued it. Fig. 3 summarises the factors associated with tolvaptan dose reduction or discontinuation. The presence of an eGFR of <60 mL/min/1.73 m2 at the time the patients started treatment with tolvaptan was associated with a higher risk of tolvaptan dose reduction or discontinuation (aHR: 3.29, 95% CI: 1.19–9.15). We obtained similar results in the sensitivity analysis (Supplementary Fig. S3); the higher eGFR was associated with a lower risk of tolvaptan dose reduction or discontinuation. However, the factors related to the discontinuation of tolvaptan showed no statistically significant differences (Supplementary Fig. S4). Adverse effects, such as severe liver function abnormalities and stage 3 or higher AKI, were found in 2.6% and 1.3% of patients, respectively (Supplementary Table S1).
Fig. 3 . Primary outcome: factors associated with tolvaptan dose reduction or discontinuation.
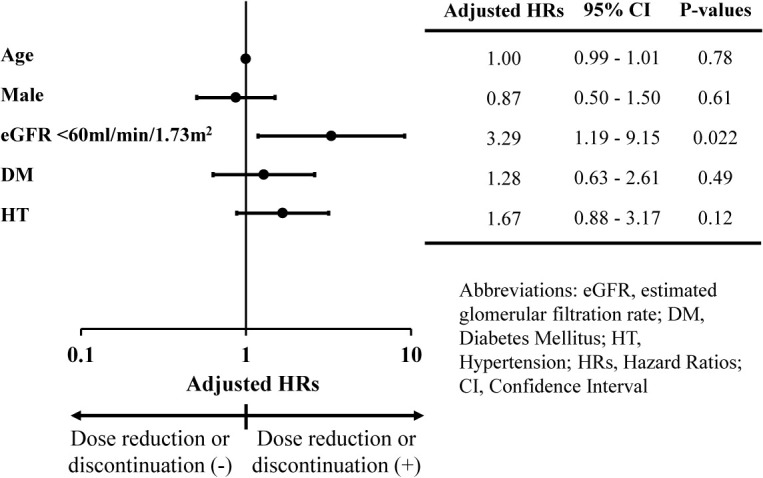
Abbreviations: eGFR, estimated Glomerular Filtration Rate; DM, Diabetes Mellitus; HT, Hypertension; HRs, Hazard Ratios; CI, Confidence Interval; CKD, Chronic Kidney Disease
Table 3 presents the percentages of patients with ADPKD treated with tolvaptan at each CKD stage. Tolvaptan is mainly administered in the early stages of CKD. Fig. 4 shows the daily dose of tolvaptan for each CKD stage as of 2020. Although no significant differences were found, patients with advanced CKD stages tended to be treated with lower doses of tolvaptan (<60 mg/day; P = 0.10).
Table 3 . CKD stages of patients with ADPKD taking tolvaptan at >15 mg/day.
| Variables, n (%) | Year | P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | ||
| Patient overall | 8 (100) | 21 (100) | 54 (100) | 66 (100) | 78 (100) | 85 (100) | 83 (100) | |
| CKD stage | ||||||||
| G1 + G2 | 0 (0) | 2 (9.5) | 5 (9.3) | 5 (7.6) | 11 (14) | 11 (13) | 11 (13) | 0.17 |
| G3a | 2 (25) | 3 (14) | 9 (17) | 16 (24) | 15 (19) | 19 (22) | 20 (24) | 0.36 |
| G3b | 2 (25) | 5 (24) | 16 (30) | 16 (24) | 22 (28) | 26 (31) | 22 (27) | 0.78 |
| G4 | 2 (25) | 7 (33) | 17 (31) | 20 (30) | 21 (27) | 19 (22) | 17 (20) | 0.07 |
| G5 | 2 (25) | 4 (19) | 7 (13) | 9 (14) | 9 (12) | 10 (12) | 13 (16) | 0.68 |
Abbreviations: CKD, Chronic Kidney Disease; G, Glomerular Filtration Rate; ADPKD, Autosomal Dominant Polycystic Kidney Disease
Fig. 4 . Daily dose of tolvaptan for each CKD stage as of 2020.
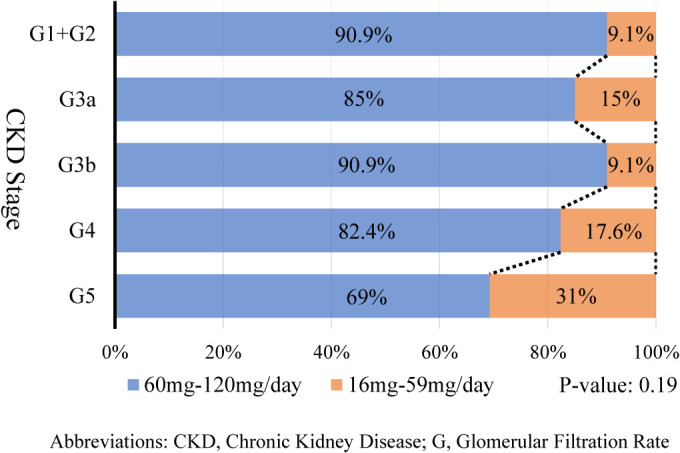
Abbreviations: CKD, Chronic Kidney Disease; G, glomerular filtration rate
DISCUSSION
This study showed the current treatments used for patients with ADPKD in the CKD without KRT and trends in tolvaptan use. During the observation period, 41% of patients with ADPKD discontinued or reduced the dose of tolvaptan, whereas 13% discontinued it. Previous clinical trials have reported that approximately 15–23% of patients discontinued tolvaptan [7, 8], whereas another report showed that half of patients who discontinued the drug did so due to side effects [17]. In previous clinical trials, patients had a body mass index (BMI) of 26.4–28.0 kg/m2 [7, 8], whereas in our study the BMI was 22.6 kg/m2 (Table 2). Differences in body size between individuals in the United States and Japan may have resulted in more dose reductions or discontinuations. However, no previous reports have discussed the relationship between tolerance of high-dose tolvaptan and renal function. Our study demonstrated that the presence of an eGFR of <60 mL/min/1.73 m2 was related to the dose reduction or discontinuation of tolvaptan. This result implies the importance of introducing high-dose tolvaptan for patients with ADPKD in the early stage of CKD to prevent its progression. Conversely, no statistically significant differences were found between tolvaptan discontinuation and an eGFR of <60 mL/min/1.73 m2; however, this may be because of the small sample size (of the 156 patients, 64 [41%] reduced their dose or discontinued tolvaptan, and 21 [13%] discontinued tolvaptan). Therefore, we focused not only on the discontinuation but also dose reduction of tolvaptan. This is because, in many cases, when some adverse effects occur, clinicians may reduce the drug dose before discontinuing.
Here, we describe treating patients with ADPKD in the CKD without KRT. In the United States, hypertension occurs in 50–80% of patients with ADPKD, and >60% develop hypertension while the kidney function is maintained [18]. Our study showed that approximately 67% of patients with ADPKD were taking some antihypertensive medication, and this result was similar to a previous study [18]. In 2020, the number of patients meeting the inclusion criteria decreased slightly. This may be due to the impact of the novel coronavirus disease epidemic.
Several reports are available on ADPKD prevalence; in Japan, approximately 1 in 1000–4000 people are considered to have ADPKD [5, 19]. Additionally, since the population of Japan was 126 million in 2020 [20], it is estimated that there will be 31,500–120,000 patients with ADPKD in Japan. The number of patients with ADPKD already receiving dialysis in 2020 was estimated as 12,000 [21]; therefore, it was estimated that there would be approximately 19,500–108,000 patients with ADPKD in the CKD without KRT. In 2020, 5426 patients with ADPKD in the CKD without KRT were reportedly taking tolvaptan [17], which suggested that, in Japan, 5–28% of patients with ADPKD in the CKD without KRT are taking tolvaptan. Here, we defined patients taking >15 mg/day of tolvaptan as those taking tolvaptan for ADPKD and found that 25% of patients with ADPKD in the CKD without KRT were taking tolvaptan for ADPKD. These results suggest that the definition used in our case was reasonable.
This study has three strengths. First, although ADPKD is a rare disease, we clarified the therapies used for ADPKD in the CKD without KRT using this database. Several cohort studies have reported only patients with ADPKD taking tolvaptan [9, 10]; however, no cohort studies have included the overall patients with ADPKD in the CKD without KRT. Second, unlike other observational studies, the database used included laboratory values such as serum sodium, sCre, ALT, and AST levels. This enabled us to calculate eGFR, determine CKD stage, reveal AKI and hypernatremia, and assess liver function. Third, we considered the discontinuation and dose reduction of tolvaptan and the reduction in its dosage. Tolvaptan is the only drug with strong evidence of suppressing ADPKD progression. Therefore, we considered that when the adverse effect was recognised, patients may prefer to continue tolvaptan at a reduced dose rather than discontinue it. Considering the dose reduction, we conducted a study based on actual clinical practice.
This study had some limitations. First, total kidney volume, the most important prognostic factor in ADPKD, was not included in the database. We used eGFR as a surrogate marker and revealed that the presence of CKD at the start of tolvaptan treatment was associated with dose reduction or discontinuation; however, total kidney volume may be a confounding factor. Second, there are issues specific to observational studies using administrative databases, such as hospital transfers, the validity of disease names, and the generalisability of results. Tolvaptan induction for ADPKD is frequently performed during hospitalisation to manage adverse effects; however, patients may be transferred to another hospital when their condition stabilizes. We could not follow up any transferred patients, which may have resulted in underestimating the number of tolvaptan dose reductions or discontinuations. Additionally, the validity study of the diagnosis of ADPKD was performed in the United States, rather than in Japan. Furthermore, the ICD-10 algorithm used for select patients with ADPKD had a low sensitivity of 33.7% [13]. This may lead to the underdiagnosis of patients with ADPKD. The RWD database does not cover all hospitals in Japan; therefore, our results cannot be generalized to Japan as a whole. Third, adverse effects not represented in the database, such as dry mouth, could not be captured. Additionally, it was unclear whether the adverse effects were the reason for the tolvaptan dose reduction or discontinuation. Fourth, we could not distinguish patients taking tolvaptan for ADPKD from those taking it for syndrome of inappropriate secretion of antidiuretic hormone. The maximum dose of tolvaptan for it may be up to 60 mg/day, and as of June 2020, it became eligible for insurance coverage. Although no patients in this database were diagnosed with the syndrome of inappropriate secretion of antidiuretic hormone, as we included patients with ADPKD from January 2014 to December 2020 in this study, it is possible that there are patients with ADPKD who are using tolvaptan for syndrome of inappropriate secretion of antidiuretic hormone.
CONCLUSION
Although ADPKD is a rare disease, we clarified real-world data for tolvaptan use for patients with ADPKD in the CKD without KRT using this database. As of 2020, 25% of patients with ADPKD were receiving tolvaptan. Additionally, the number of patients taking tolvaptan for ADPKD increased slightly each year and may increase further. Among patients with ADPKD, those with an eGFR of <60 mL/min/1.73 m2 tended to discontinue or reduce their dose. Future studies should focus on improving the tolerability of tolvaptan in late stages of ADPKD.
COMPLIANCE WITH ETHICAL STANDARDS
CONFLICT OF INTEREST STATEMENT
Kayoko Mizuno has been employed by the Department of Digital Health and Epidemiology with support from Eisai Co., Ltd. and Kyowa Kirin Co., Ltd.; and has received grants from Japan Society for the Promotion of Science (JSPS). Motoko Yanagita received research grants from Mitsubishi Tanabe Pharma and Boehringer Ingelheim. Koji Kawakami has received research funds from Eisai Co., Ltd., Kyowa Kirin Co., Ltd., Mitsubishi Corporation, OMRON Corporation, Real World Data Co., Ltd., Sumitomo Pharma Co., Ltd., and Toppan Inc.; consulting fees from Advanced Medical Care Inc., JMDC Inc., LEBER Inc., and Shin Nippon Biomedical Laboratories Ltd.; executive compensation from Cancer Intelligence Care Systems, Inc.; honoraria from Chugai Pharmaceutical Co., Ltd., Kaken Pharmaceutical Co., Ltd., Mitsubishi Chemical Holdings Corporation, Mitsubishi Corporation, Pharma Business Academy, and Toppan Inc.; and held stock in Real World Data Co., Ltd.
Kazunori Sakoda, Kayoko Mizuno, Tomotsugu Seki, Kanna Shinkawa, Yuriko Ohara, Ayano Hayashi, Satomi Yoshida, and Masato Takeuchi have no conflict of interest to declare.
ETHICAL APPROVAL
This article does not contain any studies with human participants or animals performed by any of the authors. This study was approved by the Kyoto University Graduate School and Faculty of Medicine Ethics Committee (Approval number: R2877), and the STROBE guidelines were followed [22]. However, the requirement for informed consent was waived because of the anonymised patient data used in this study.
FUNDING
No funding was received for this study.
ACKNOWLEDGMENTS
The authors thank Real World Data for providing and allowing us to use the data.
Supplementary Material
Supplementary Material
REFERENCES
- 1.Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet 2007;369:1287–1301. [DOI] [PubMed] [Google Scholar]
- 2.Cornec-Le Gall E, Alam A, Perrone RD. Autosomal dominant polycystic kidney disease. Lancet 2019;393:919–935. [DOI] [PubMed] [Google Scholar]
- 3.Testa F, Magistroni R. ADPKD current management and ongoing trials. J Nephrol 2020;33:223–237. [DOI] [PubMed] [Google Scholar]
- 4.Grantham JJ, Chapman AB, Torres VE. Volume progression in autosomal dominant polycystic kidney disease: the major factor determining clinical outcomes. Clin J Am Soc Nephrol 2006;1:148–157. [DOI] [PubMed] [Google Scholar]
- 5.Higashihara E, Nutahara K, Kojima M, Tamakoshi A, Yoshiyuki O, Sakai H, et al. Prevalence and renal prognosis of diagnosed autosomal dominant polycystic kidney disease in Japan. Nephron 1998;80:421–427. [DOI] [PubMed] [Google Scholar]
- 6.Masakane I, Taniguchi M, Nakai S, Tsuchida K, Wada S, Ogata S, et al. Annual Dialysis Data Report 2016, JSDT Renal Data Registry. Ren Replace Ther 2018;4:1–45. [Google Scholar]
- 7.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 2012;367:2407–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Koch G, et al. Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med 2017;377:1930–1942. [DOI] [PubMed] [Google Scholar]
- 9.Mochizuki T, Muto S, Miyake M, Tanaka T, Wang W. Safety and efficacy of Tolvaptan in real-world patients with autosomal dominant polycystic kidney disease- interim results of SLOW-PKD surveillance. Clin Exp Nephrol 2021;25:1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higashihara E, Nutahara K, Itoh M, Okegawa T, Tambo M, Yamaguchi T, et al. Long-term outcomes of longitudinal efficacy study with tolvaptan in ADPKD. Kidney Int Reports 2022;7:270–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takeuchi M, Ogura M, Minoura T, Inagaki N, Kawakami K. Comparative effectiveness of sodium-glucose cotransporter-2 inhibitors versus other classes of glucose-lowering medications on renal outcome in type 2 diabetes. Mayo Clin Proc 2020;95:265–273. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto H, Takeuchi M, Kawakami K. Association between biopsies for anti-neutrophil cytoplasmic antibody-associated vasculitis and prognosis: a retrospective cohort study. Clin Rheumatol 2022;41:541–548. [DOI] [PubMed] [Google Scholar]
- 13.Kalatharan V, McArthur E, Nash DM, Welk B, Sarma S, Garg AX, et al. Diagnostic accuracy of administrative codes for autosomal dominant polycystic kidney disease in clinic patients with cystic kidney disease. Clin Kidney J 2020;14:612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009;53:982–992. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, de Jong PE, Coresh J, Nahas ME, Astor BC, Matsushita K, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int 2011;80:17–28. [DOI] [PubMed] [Google Scholar]
- 16.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012;120:c179–c184. [DOI] [PubMed] [Google Scholar]
- 17.Otsuka Pharmaceutical. Use-Results Survey for SAMSCA (All case study). Available at:https://www.otsuka-elibrary.jp/product/samsca/investigation/index.html. Accessed Feb 14, 2021.
- 18.Rizk D, Jurkovitz C, Veledar E, Bagby S, Baumgarten DA, Rahbari-Oskoui F, et al. Quality of life in autosomal dominant polycystic kidney disease patients not yet on dialysis. Clin J Am Soc Nephrol 2009;4:560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshimoto T, Takaya N, Akanuma M, Arai K, Morita Y, Watanabe Y, et al. Epidemiological features of and screening for polycystic kidney disease in Japan. Ningen Dock Int 2019;6:62–68. [Google Scholar]
- 20.Basic Complete Tabulation on Population and Households (Statistics Bureau of Japan). Available at: https://www.stat.go.jp/data/kokusei/2020/kekka/pdf/summary_01.pdf. Accessed Feb 14, 2021.
- 21.Hanafusa N, Abe M, Joki N, Ogawa T, Kanda E, Kikuchi K, er al. Annual Dialysis Data Report 2020, JSDT Renal Data Registry. Ren Replace Ther 2021;54:611–657. [Google Scholar]
- 22.STROBE Statement. Available at: https://www.strobe-statement.org/. Accessed Feb 14, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


