Abstract
We have evaluated the in vivo distribution of the major human immunodeficiency virus/simian immunodeficiency virus (HIV/SIV) coreceptors, CXCR4, CCR3, and CCR5, in both rhesus macaques and humans. T lymphocytes and macrophages in both lymphoid and nonlymphoid tissues are the major cell populations expressing HIV/SIV coreceptors, reaffirming that these cells are the major targets of HIV/SIV infection in vivo. In lymphoid tissues such as the lymph node and the thymus, approximately 1 to 10% of the T lymphocytes and macrophages are coreceptor positive. However, coreceptor expression was not detected on follicular dendritic cells (FDC) in lymph nodes, suggesting that the ability of FDC to trap extracellular virions is unlikely to be mediated by a coreceptor-specific mechanism. In the thymus, a large number of immature and mature T lymphocytes express CXCR4, which may render these cells susceptible to infection by syncytium-inducing viral variants that use this coreceptor for entry. In addition, various degrees of coreceptor expression are found among different tissues and also among different cells within the same tissues. Coreceptor-positive cells are more frequently identified in the colon than in the rectum and more frequently identified in the cervix than in the vagina, suggesting that the expression levels of coreceptors are differentially regulated at different anatomic sites. Furthermore, extremely high levels of CXCR4 and CCR3 expression are found on the neurons from both the central and peripheral nervous systems. These findings may be helpful in understanding certain aspects of HIV and SIV pathogenesis and transmission.
Viral entry into target cells is mediated through a complex interaction between the viral envelope proteins and specific cellular receptors. The CD4 glycoprotein is the primary receptor for human immunodeficiency virus types 1 and 2 (HIV-1 and HIV-2) and simian immunodeficiency virus (SIV) (12, 32). However, expression of the CD4 molecule alone is not sufficient for viral entry, suggesting the need for one or more additional cellular coreceptors (4, 38). The recent identification of multiple chemokine receptors as the coreceptors for HIV/SIV entry has greatly assisted our understanding of HIV/SIV pathogenesis, transmission, and tropism. Currently, eight coreceptors, CXCR4, CCR2b, CCR3, CCR5, Bonzo (STRL33), BOB (GPR15), GPR1, and US28, have been found to play an essential role in HIV/SIV entry (1, 7, 9, 14–17, 19, 20, 36). Among these coreceptors, CXCR4, CCR3, and CCR5 are the major ones used by HIV isolates for efficient entry (1, 7, 16, 17, 20). Additionally, phenotypic characteristics dictate the specific coreceptor(s) used by viral isolates (11, 49, 58). Non-syncytium-inducing (NSI) or macrophage (M)-tropic primary viruses predominantly use CCR5, whereas syncytium-inducing (SI) T-cell line (T)-tropic viruses preferentially use CXCR4 (11, 49, 58). Some NSI and SI isolates also use CCR3, although the number is extremely small. M-tropic viruses are preferentially transmitted through sexual contact and predominate in the majority of infected individuals, whereas T-tropic viruses, which emerge during the late stages of infection, are more virulent and are associated with higher rates of CD4+-T-cell decline (10, 48, 51, 59, 60).
The ability of viruses to infect target cells is dependent not only on the viral phenotype but also on the availability and expression levels of the appropriate primary receptors and coreceptors. The primary receptor CD4 is differentially expressed on different cell subsets, with significantly lower levels of expression on macrophages than on T lymphocytes. The lower level of CD4 expression has rendered macrophages relatively resistant to infection by many of the primary viruses, especially those that are heavily dependent on a high level of CD4 expression for entry (33, 45). The coreceptors CXCR4 and CCR5 are also differentially regulated on different cell subsets. In freshly isolated peripheral blood lymphocytes, CXCR4 is found predominantly on naive (CD26lowCD45RA+CD45RO−) T lymphocytes whereas CCR5 is expressed on memory T lymphocyte subsets (CD26highCD45RA−CD45RO+) (3, 56). The reciprocal pattern of coreceptor expression may partially explain the previous observations that CD4+ memory T lymphocytes are preferentially infected by HIV-1 in vitro (8, 47).
The complex pattern of primary-receptor and coreceptor expression is probably reflected by an equally complex pattern of HIV/SIV infection in vivo. Many of the stromal and lymphoid cells in the bone marrow, thymus, lymph nodes, and spleen are infected by HIV/SIV (2, 6, 21, 29, 53). In the early stages of HIV/SIV infection of lymph nodes, the majority of viral replication occurs primarily in macrophages and lymphocytes, whereas in the later stages, viral RNA is concentrated primarily in the germinal center, colocalizing with the follicular dendritic cells (FDC) (22, 44). HIV/SIV infection of the thymus has shown another level of complexity. SI viruses replicate faster, generate higher viral titers, and cause a more severe depletion of CD4+ thymocytes than do NSI isolates (30, 50), which suggests that the phenotypic switch from NSI to SI during the course of infection is likely to trigger more severe cytopathic effects on thymocytes. In addition, the mucosa-associated lymphoid tissue is a target for HIV/SIV infection. Infectious viral particles have been successfully isolated directly from tissues of the gastrointestinal (GI) and genitourinary (GU) tracts (25, 26, 37, 40, 42). The primary infected cells in the mucosa-associated lymphoid tissue have been identified as tissue macrophages, dendritic cells, and T lymphocytes located in the lamina propria adjacent to the surface epithelium (26, 27, 40–42).
Although significant advancement has been made recently in identifying and characterizing multiple novel coreceptors for HIV/SIV infection, the majority of these studies have been performed in vitro. Due to the complex pattern of coreceptor regulation and signaling by the chemokines, it is difficult to assess the significance of a particular coreceptor without understanding the pattern and level of its expression in vivo. For these reasons, we sought to investigate the in vivo expression pattern of the three major coreceptors used by HIV/SIV, CXCR4, CCR3, and CCR5, in human and rhesus monkey tissues.
MATERIALS AND METHODS
Animals.
Tissue samples were collected from four SIV-infected (5685, 1204, 1216, and 1208) and two uninfected (1360 and 1366) rhesus macaques (Table 1). Of these four SIV-infected animals, one (5685) was at the terminal stage of SIV infection, with clinical symptoms consistent with simian AIDS, and the remaining three (1204, 1216, and 1208) were clinically asymptomatic at the time of necropsy. The last three animals were included in our previous studies on the effects of progesterone implants on SIV vaginal transmission (39). These animals were sacrificed 3 or 4 days after vaginal inoculation with SIVmac251 (39). The uninfected animals (1360 and 1366) were healthy at the time of necropsy. These animals were multiparous adult females and were maintained at the Laboratory for Experimental Medicine and Surgery in Primates (Tuxedo, N.Y.) in accordance with American Association for Accreditation of Laboratory Animal Care standards.
TABLE 1.
Clinical and virological characterization of the study subjects
| Study subjecta | Status of infectionb | Status of disease | Duration of infection | Viral culturec | Tissues testedd |
|---|---|---|---|---|---|
| Rh5685 | Yes | Simian AIDS | 1.5 yr | Positive | LN, TM, GI, GU, NS |
| Rh1204 | Yes | Asymptomatic | 3–4 days | Negative | LN, GI, GU |
| Rh1208 | Yes | Asymptomatic | 3–4 days | Negative | LN, GI, GU |
| Rh1216 | Yes | Asymptomatic | 3–4 days | Negative | LN, GI, GU |
| Rh1360 | No | Healthy | NAe | NA | LN, TM, GI, GU, NS |
| Rh1366 | No | Healthy | NA | NA | LN, GI, GU, NS |
| Hu0009 | Yes | Asymptomatic | 2 yr | Positive | LN |
| Hu1004 | Yes | Asymptomatic | 4 yr | Positive | LN |
| HuVM | No | Healthy | NA | NA | GU |
Rh, rhesus macaque; Hu, human.
Determined by the presence or absence of proviral DNA in the peripheral blood mononuclear cells (PBMC).
Determined by in vitro coculture and the production of p27/p24 in the culture supernatant; the detection threshold for p27 is 62.5 pg/ml (Cellular Products Inc., Buffalo, N.Y.), and that for p24 is 30 pg/ml (Abbott Laboratories, Chicago, Ill.).
LN, lymph node; TM, thymus; NS, nervous system.
NA, not applicable.
Tissue collection and processing.
Samples were obtained from the thymus, lymph nodes, brain, and GI (rectum and colon) and GU (vagina and cervix) tracts of the rhesus monkeys. An axillary and a cervical lymph node were obtained from two HIV-1-infected humans on combination antiretroviral therapy (Table 1). Multiple fixed paraffin-embedded human vaginal mucosal samples were obtained from the pathology department archive at Georgetown University Hospital. The tissues were completely deidentified. The tissue samples were sectioned into pieces approximately 0.3 by 0.3 by 0.3 cm, washed in phosphate-buffered saline, and fixed in Streck’s tissue fixative (Streck Laboratories, Inc., Omaha, Nebr.) for 1 week. The tissue samples were then embedded into paraffin with an automated tissue processor under heat and vacuum pressure.
Immunohistochemistry.
Immunohistochemistry was performed with the LSAB2 kit (Dako Diagnostics, Carpinteria, Calif.) as specified by the manufacturer. Sections approximately 8 μm thick were fixed onto positively charged slides coated with 3-aminopropylethoxysilane. The sections were then deparaffinized in xylene and rehydrated through graded alcohol concentrations (100, 95, and 70%). Citra solution (BioGenex, San Ramon, Calif.) was applied to the sections at 90°C for 6 min to retrieve the antigens from the fixed specimens. Immunostaining was performed for 60 min at room temperature and was developed by the avidin-biotin-peroxidase complex technique with the chromogen aminoethylcarbazole (AEC). The following dilutions and antibodies (all from Dako Diagnostics), which were found to cross-react with macaque antigens, were used: major histocompatibility complex class II, mouse anti-human-HLA-DR (clone TAL.1B5), 1:50; an anti-lysosomal membrane antibody which is highly expressed on monocytes/macrophages and some dendritic cells, mouse anti-human CD68 (clone KP1), 1:33; T lymphocytes, rabbit anti-human CD3 (polyclonal), 1:33; and dendritic cells, rabbit anti-cow S-100 (polyclonal), 1:100. Coreceptor-specific monoclonal antibodies were used in the following dilutions: 1:800 for mouse anti-human CXCR4 antibody 12G5; 1:50 for mouse anti-human CCR3 antibody 7B11, and 1:20 for mouse anti-human CCR5 antibody 2D7. The coreceptor-specific antibodies have been extensively characterized and were found to stain strongly both fresh and activated peripheral blood mononuclear cells (3, 18, 24, 55). They are also capable of blocking HIV-1 or HIV-2 infection of peripheral blood mononuclear cells in vitro (18, 23, 55). 12G5 detects both naive and memory T lymphocytes (3), whereas 2D7 readily stains activated T lymphocytes (55). 7B11 binds strongly to eosinophils and blocks HIV-1 infection of neuronal microglia (23, 24). All tissue sections were counterstained with Mayer’s hematoxylin and mounted with Crystal/Mount (Biomeda, Culver City, Calif.), and a coverslip was applied for microscopic examination. Nonreactive mouse and rabbit antibodies of a similar isotype were used as negative controls. Appropriate controls were run on all sections.
RESULTS
Using immunohistochemistry, we have detected numerous coreceptor-positive cells in both lymphoid and nonlymphoid tissues. The majority of the coreceptor-positive cells are T lymphocytes and tissue macrophages. Although we studied samples from six animals (Table 1), we did not observe significant differences in coreceptor expression between SIV-infected and uninfected subjects. Therefore, we decided to illustrate the expression pattern of these coreceptors in vivo by using one uninfected animal (1360). Additionally, human specimens from the lymph nodes and vagina were studied. To quantify the differences between various tissues, we measured the number of coreceptor-positive cells per square millimeter of tissue (Table 2). As shown in Table 2, significant differences were noted between different tissues and between different cell types within the same tissue. Approximately 51 and 176 coreceptor-positive cells mm−2 were found in the lymph node and the thymus, respectively. This is equivalent to 1 to 10% of the T lymphocytes and the macrophages in the lymphoid tissue. In the GI and GU tracts, however, the coreceptor-positive cells were identified more frequently in the colon (51 mm−2) than in the rectum (12 mm−2) and more frequently in the cervix (19 mm−2) than in the vagina (<6 mm−2).
TABLE 2.
Quantitative analysis of the number of coreceptor-positive cells per square millimeter in various tissues from rhesus macaques
| Coreceptor | Cell typea | No. of positive cells/mm2b of:
|
||||||
|---|---|---|---|---|---|---|---|---|
| Lymphoid tissue
|
GI tract
|
GU tract
|
Others (CNS) | |||||
| LN | TM | RT | CL | VG | CV | |||
| CXCR4 | M | 10 | 2 | <1 | 16 | <1 | 9 | 1 |
| T | 11 | 160 | <1 | <1 | <1 | <1 | NAc | |
| CCR3 | M | 8 | 5 | 4 | 17 | <1 | 6 | 1 |
| T | 4 | <1 | <1 | 5 | <1 | 2 | NA | |
| CCR5 | M | <1 | <1 | <1 | 3 | <1 | 1 | <1 |
| T | 18 | 9 | 8 | 10 | <1 | 1 | NA | |
| Total | 51 | 176 | 12 | 51 | <6 | 19 | 2 | |
M, macrophages; T, T lymphocytes.
Measured as the number of positive cells per square millimeter of tissue section. Multiple sections were examined. LN, lymph node; TM, thymus; RT, rectum; CL, colon; VG, vagina; CV, cervix.
NA, not applicable.
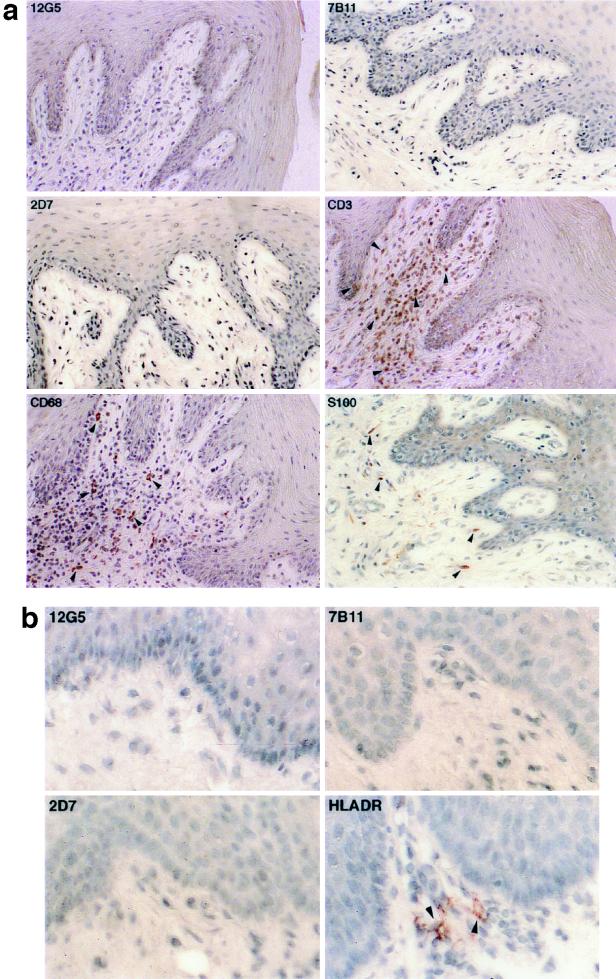
Abundant expression of coreceptors in lymphoid tissues.
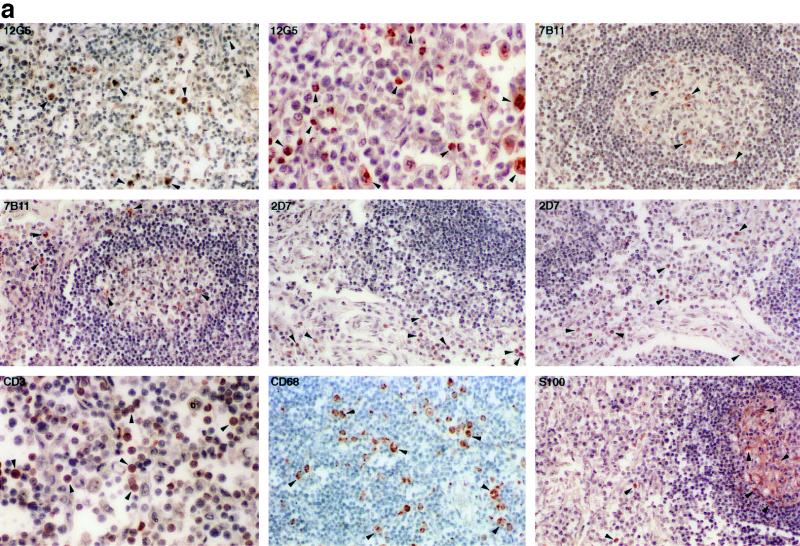
Figure 1a demonstrates the expression pattern of the coreceptors in an ileal lymph node. The CXCR4- and CCR3-positive cells were located predominantly in the medulla and inside the germinal center. Based on their morphological appearances, the majority of the cells are macrophages and T lymphocytes. However, there appeared to be variable expression of both coreceptors. The CXCR4- and CCR3-positive cells constituted only a small proportion of the entire cell population, suggesting that the expression of coreceptors may be regulated. In a previous report, tissue macrophages were also CCR3 positive (24). Therefore, the coreceptor CCR3, although originally isolated and cloned from eosinophils (13, 46), is unlikely to be eosinophil specific.
FIG. 1.
(a) Immunohistochemical localization and phenotypes of coreceptor-positive cells in an ileal lymph node from rhesus macaque 1360 (AEC with hematoxylin counterstain; magnification, ×156 except as otherwise indicated). Some of the representative positive cells are indicated by arrowheads. The CXCR4-positive cells in the medulla are shown at two magnifications (×156 and ×468). The CCR3-positive cells are inside and surrounding the two germinal centers. The CCR5-positive cells are surrounding rather than inside the germinal centers. T lymphocytes and macrophages in the medulla are identified by the anti-CD3 and the anti-CD68 antibodies, respectively. No detectable levels of coreceptors were identified on the FDC, despite the presence of an intact FDC network in the germinal center. (b) Immunohistochemical localization and phenotypes of coreceptor-positive cells in an axillary lymph node from HIV-1-infected individual Hu1004 (AEC with hematoxylin counterstain; magnification, ×600). Some of the representative positive cells are indicated by arrowheads. The CXCR4- and CCR3-positive cells in the medulla are morphologically similar to macrophages identified by anti-CD68 antibody. A large number of T lymphocytes were identified by anti-CD3 antibody, but the CCR5-positive cells were not visualized. No detectable levels of CXCR4, CCR3, or CCR5 on the FDC were demonstrated despite the presence of an intact FDC network.
In contrast, the expression pattern of CCR5 in the lymph nodes was quite distinct. The majority of the CCR5-positive cells were found in the medulla and the cortex surrounding, rather than inside, the germinal centers (Fig. 1a). Almost all of these cells had a typical lymphocyte morphology; however, only a minority of the lymphocytes expressed CCR5. In addition, FDC did not express any of the three coreceptors.
The coreceptor expression pattern in a human axillary lymph node was not significantly different from that observed in rhesus macaques (Fig. 1b). The CXCR4- and CCR3-positive cells were found primarily in the medulla and in the germinal centers. Morphologically, these cells appeared to be macrophages. However, we could not detect CCR5-positive cells from this specimen despite the existence of a substantial number of T lymphocytes (Fig. 1b), suggesting that the expression of CCR5 in this node is quite limited. Furthermore, similar to rhesus macaques, the FDC in the human lymph node did not express CXCR4, CCR3, or CCR5 (Fig. 1b).
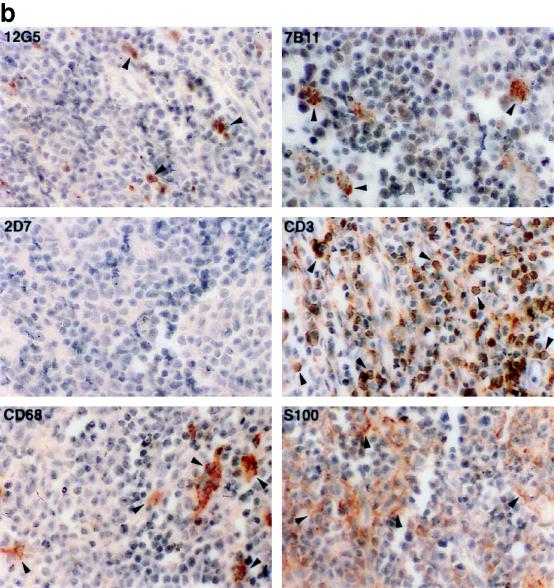
In the thymus, the coreceptor expression pattern was dramatically different from that observed in the lymph nodes. Most of the cells in the cortex, predominantly immature T lymphocytes, and in the central portion of the medulla, mostly mature T lymphocytes, were positive for CXCR4 (Fig. 2). The mature T lymphocytes, in the central portion of the medulla, were heavily stained with anti-CD3 antibody (Fig. 2). In contrast, the CCR3-positive cells were rarely demonstrated in either the cortex or the medulla. Based on the staining pattern with S-100 and CD68 antibodies, these cells were either dendritic cells or macrophages. Furthermore, the CCR5-positive cells populated two distinct regions of the thymus: the outer cortex and the central region of medulla. The cells in the outer cortex were invariably larger than those in the deep medulla (Fig. 2), consistent with the morphological appearance of lymphoblasts (5). The smaller cells in the deep medulla, believed to be the daughter cells of lymphoblast mitosis, are likely to be mature T lymphocytes (5). As observed in the lymph node and the spleen, CCR5 was expressed on a minority of the mature and immature T lymphocytes in the thymus.
FIG. 2.
Immunohistochemical localization and phenotypes of coreceptor-positive cells in the thymus of rhesus macaque 1360 (AEC with hematoxylin counterstain; magnification, ×100). Some of the representative positive cells are indicated by arrowheads. Due to the high density, the CXCR4-positive cells in the cortex are not as obvious as in the lymph node. The CCR3-positive cells are large and irregular compared to their neighboring cells and are believed to be macrophages identified by the anti-CD68 antibody. The majority of the CCR5-positive cells are located in the outer cortex and the central region of medulla, although a few are also identified in the interlobular septa. The epithelial framework of the thymus is stained strongly with anti-HLA-DR antibody.
Differential expression of coreceptors in different proportions of the GI tract.
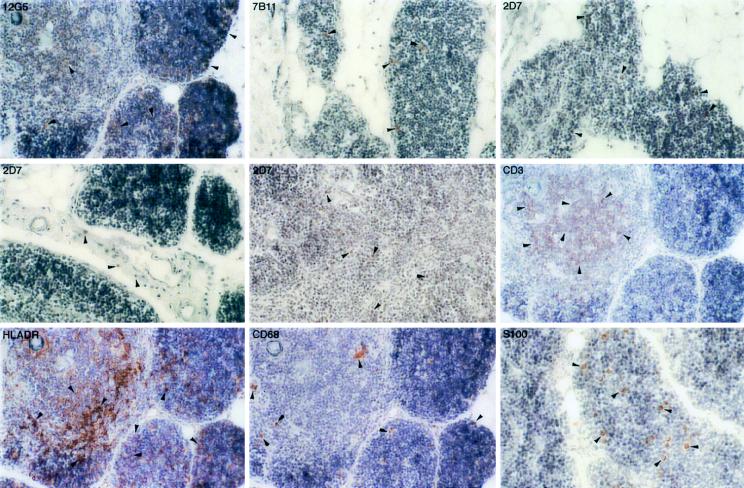
In the rectum, the CXCR4-positive cells were infrequent, had a morphology similar to the macrophages or dendritic cells stained by anti-CD68 and anti-HLA-DR antibodies, and were located primarily in the lamina propria (Fig. 3). In contrast, the CCR3-positive cells were readily detected in the lamina propria and were morphologically similar to macrophages and dendritic cells stained by anti-CD68 and anti-S-100 antibodies, respectively. Many of the eosinophils located in the lamina propria were also CCR3 positive (data not shown). A large number of CCR5-positive cells were identified in the rectum, had a morphological appearance similar to T lymphocytes (Fig. 3), and were randomly distributed in the lamina propria. As noted above for the lymphoid tissues, the coreceptor-positive cells constituted only a small proportion of the entire lymphocyte and macrophage population.
FIG. 3.
Immunohistochemical localization and phenotypes of coreceptor-positive cells in the rectum of rhesus macaque 1360 (AEC with hematoxylin counterstain; magnification, ×154 except as otherwise indicated). Some of the representative positive cells are indicated by arrowheads. The CXCR4-positive cells are minimal and are barely visible at high magnification (×462). The CCR3- and CCR5-positive cells are located primarily in the lamina propria. A large number of T lymphocytes, macrophages, HLA-DR-positive cells, and dendritic cells are readily detected.
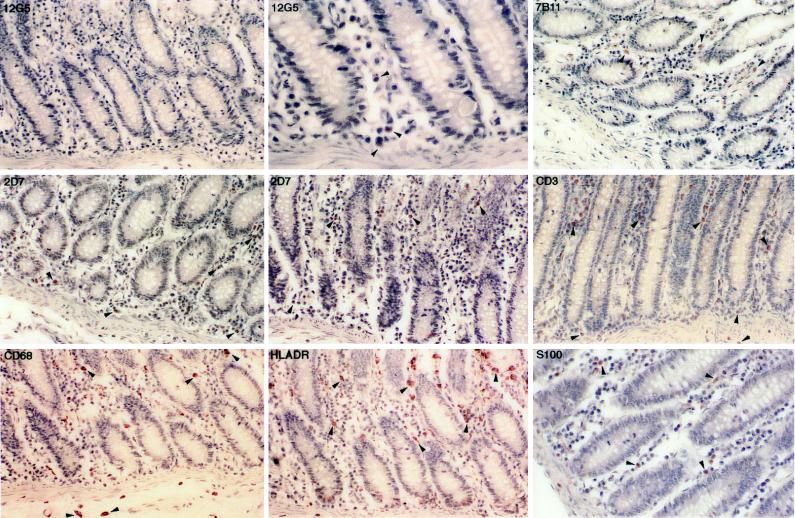
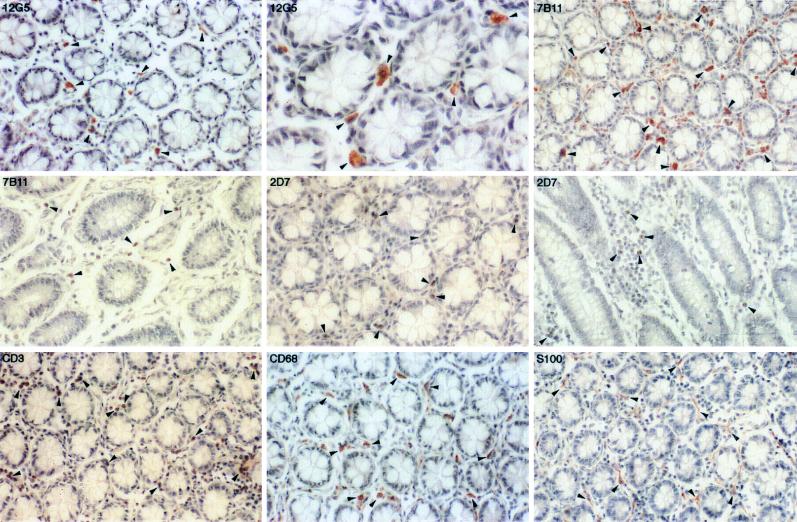
The expression of CXCR4 was higher in the colon than in the rectum (Fig. 4; Table 2), although the location, morphological appearance, and staining characteristics of the CXCR4-positive cells in the colon were similar to those observed in the rectum (Fig. 4). The CCR3-positive cells were also abundant in the colon and were morphologically similar to macrophages, dendritic cells, or T lymphocytes. The lamina propria eosinophils were also positive for CCR3 (data not shown). The CCR5-positive cells were frequently identified in the lamina propria and had a morphological appearance typical of either macrophages or lymphocytes (Fig. 4). The coreceptor distribution is consistent with previous studies in which virus-infected cells are identified predominantly as T lymphocytes and macrophages in the lamina propria adjacent to the surface epithelium (26, 27).
FIG. 4.
Immunohistochemical localization and phenotypes of coreceptor-positive cells in the colon of rhesus macaque 1360 (AEC with hematoxylin counterstain; magnification, ×164 except as otherwise indicated). Some of the representative positive cells are indicated by arrowheads. The CXCR4-positive cells are demonstrated in the lamina propria (magnifications, ×164 and ×492). Both the CCR3- and CCR5-positive cells are shown in cross section and longitudinal section. Numerous T lymphocytes and macrophages are detected in the lamina propria. S-100 antibody avidly stains the dendritic cells in the lamina propria as well as the nerve fibers from the unnamed plexus.
The cervical mucosa expresses high levels of coreceptors in the GU tract.
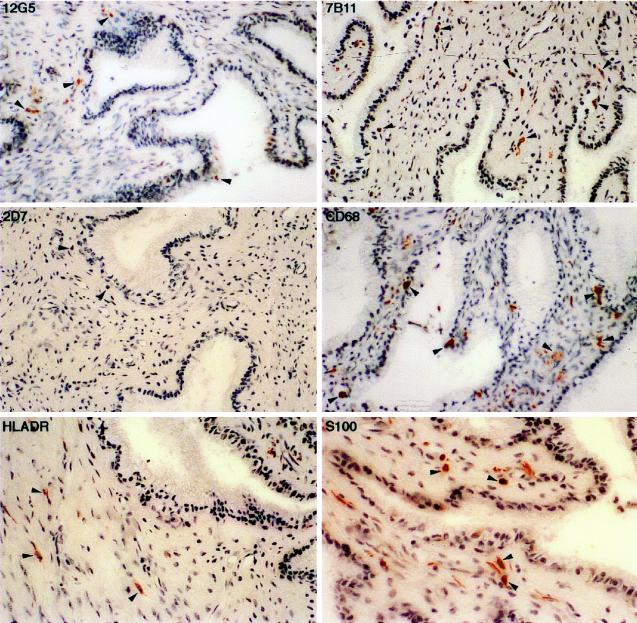
We could not demonstrate coreceptor expression in the vagina in multiple samples from either infected or uninfected rhesus macaques (Fig. 5a) or from an uninfected human (Fig. 5b), despite the presence of a large number of T lymphocytes, macrophages, and HLA-DR-positive cells in the submucosa.
FIG. 5.
(a) Immunohistochemical localization and phenotypes of coreceptor-positive cells in the vagina of rhesus macaque 1360 (AEC with hematoxylin counterstain; magnification, ×400). Some of the representative positive cells are indicated by arrowheads. No CXCR4-, CCR3-, or CCR5-positive cells were identified in the lamina propria of the vagina, despite the presence of a large number of T lymphocytes, macrophages, and dendritic cells, stained by anti-CD3, anti-CD68, and anti-S-100 antibodies, respectively. (b) Immunohistochemical localization and phenotypes of coreceptor-positive cells in the vagina of human HuVM. The arrowheads indicate some of the HLA-DR-positive cells (AEC with hematoxylin counterstain; magnification, ×600). As in rhesus macaque 1360, no CXCR4-, CCR3-, or CCR5-positive cells were identified in the lamina propria of the vagina.
In the cervix, many coreceptor-positive cells were detected. The CXCR4-positive cells, which appeared morphologically as tissue macrophages or dendritic cells, were located in the lamina propria beneath the columnar epithelium, whereas a few positive cells were also found in the epithelial lining (Fig. 6). The CCR3-positive cells, either macrophages or dendritic cells, were also prevalent in the lamina propria (Fig. 6). However, only a few CCR5-positive cells were identified in this tissue. These cells were located in the lamina propria adjacent to the epithelial layer and appeared morphologically as T lymphocytes (Fig. 6).
FIG. 6.
Immunohistochemical localization and phenotypes of coreceptor-positive cells in the cervix of rhesus macaque 1360 (AEC with hematoxylin counterstain; magnification, ×400). Some of the representative positive cells are indicated by arrowheads. The majority of the CXCR4- and CCR3-positive cells are believed to be macrophages or dendritic cells and are located primarily in either the lamina propria or the epithelial lining. The CCR5-positive cells are located adjacent to the epithelium. A few HLA-DR-positive cells are also located below the epithelium.
High levels of CXCR4 and CCR3 are expressed in the central and peripheral nervous systems.
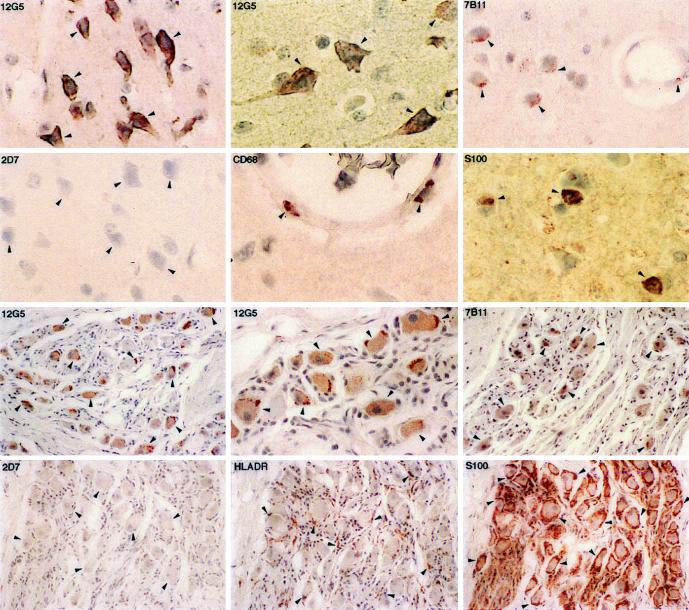
In the brain and regional ganglia of the rhesus macaque, neurons expressed extremely high levels of CXCR4 and CCR3, especially in the cell body (Fig. 7). In addition, macrophages located primarily in the walls of small blood vessels expressed CXCR4 (data not shown) and CCR3, which had previously been found to be the major targets for HIV/SIV infection in the central nervous system (CNS) (34). In contrast to CXCR4 and CCR3, CCR5 could not be demonstrated in the neurons or the regional ganglia. Oligodendrocytes in the CNS, which stained strongly with S-100 antibody, were negative for all three coreceptors (Fig. 7). The Schwann cells in the regional ganglia, although highly positive for HLA-DR and S-100, were also negative for all three coreceptors (Fig. 7).
FIG. 7.
Immunohistochemical localization and phenotypes of coreceptor-positive cells in the brain (upper two panels) and in the regional ganglia (lower two panels) of rhesus macaque 1360 (AEC with hematoxylin counterstain; magnification, ×200 except as otherwise indicated). Some of the representative positive cells are indicated by arrowheads. Neurons in the brain and regional ganglia express high levels of CXCR4 (magnifications, ×400 and ×600) and CCR3 (magnification, ×400) but no detectable level of CCR5 (magnification, ×400). Macrophages located on the wall of small blood vessels (magnification, ×600) are also positive for CXCR4 (data not shown) and CCR3 (magnification, ×400). S-100 antibody stains oligodendrocytes in the CNS (magnification, ×600) and Schwann cells in the regional ganglia. Schwann cells are also positive for HLA-DR.
DISCUSSION
We have studied the in vivo distribution of the chemokine receptors CXCR4, CCR3, and CCR5, which are the major coreceptors for HIV/SIV infection. Although we did not evaluate the distribution pattern of other chemokine receptors in this study, the future availability of reagents such as high-quality antibodies specific for other chemokine receptors will certainly make this type of study possible. It should be emphasized that other chemokine receptors such as CCR1, CCR2b, Bonzo (STRL33), BOB (GPR15), GPR1, and US28 may also play some roles in HIV/SIV pathogenesis.
In this study, we found that T lymphocytes and macrophages in both lymphoid and nonlymphoid tissues represent the majority of the coreceptor-positive cells, reaffirming that these cells are the major targets for HIV/SIV infection in vivo and therefore are potentially subject to viral cytopathic consequences. The high level of coreceptor expression may also explain the initial and persistent viral (HIV/SIV) infection of lymphoid tissues throughout the course of infection (21, 22, 29, 44). In addition, the high level of CXCR4 expression on both immature and mature thymocytes is likely to render these cells particularly susceptible to the SI isolates. Our finding is consistent with reports that SI isolates are relatively thymocyte tropic and replicate faster than NSI isolates both in vitro and in SCID-hu mice (30, 31, 50). Therefore, HIV/SIV can mediate lymphocyte depletion by directly infecting and replicating in thymocytes in vivo during all stages of T-lymphocyte maturation. The phenotypic switch from NSI to SI during infection is also likely to trigger more severe cytopathic effects in the thymus. Furthermore, the failure to detect coreceptor expression on FDC despite the presence of an intact FDC network suggests that the trapping and presentation of viral particles to target T lymphocytes in germinal centers is independent of coreceptors.
Coreceptor-positive cells are frequently identified in both the GI and GU tracts. However, the level of expression is generally higher in the proximal than the distal parts of the GI and GU tracts, suggesting that the expression may be differentially regulated. Since only a small proportion of the macrophages and the T lymphocytes express the coreceptors, a strict regulatory mechanism may govern the timing, the location, and the level of coreceptor expression. Although the exact mechanism is currently unknown, we believe that the degree of cellular maturation, the level of activation, and the concentration of the coreceptor-specific chemokines (e.g., SDF-1 and β-chemokine within the tissue environment are all likely to affect the level of coreceptor expression.
The coreceptor-positive T lymphocytes and macrophages in the distal portion of the GI and GU tracts may serve as a portal of viral entry as well as a source of viral dissemination. Based on our current knowledge, NSI rather than SI viruses are predominantly transmitted through the GI and GU tracts (59, 60), which suggests the existence of selective pressure in favor of NSI isolates during sexual contact. Is this selective pressure imposed on the viral replication rate, the preferential usage of certain coreceptors, or the degree of cytopathicity? In the rectum, since numerous CCR5- and CCR3-positive cells are readily detected in the lamina propria whereas the CXCR4-positive cells are barely visible, the coreceptor expression pattern may serve as one selective pressure for initial infection by NSI isolates. In contrast, the expression pattern of coreceptors in the GU tract is quite different. Although we did not detect coreceptor expression in the vagina, a large number of CXCR4- and CCR3-positive, but not CCR5-positive, cells are frequently identified in the cervix. Coreceptor expression in the GU tract is therefore not necessarily in favor of CCR5-using or NSI viruses. One intriguing question raised from this observation is whether the coreceptor CXCR4 can be utilized initially if SI isolates are present in the inoculum. That individuals who have homozygous defective CCR5 are indeed infectable by an SI isolate may indicate that CXCR4 can actually be used during the sexual transmission (43, 52). A simian/human immunodeficiency virus strain which uses exclusively CXCR4 in vitro can also penetrate the GU mucosal barrier and establish infection in a rhesus macaque (6a). Collectively, these findings suggest that not only CCR5 but also CXCR4 can be used during sexual transmission. Both host factors and the phenotypic composition of the infecting virus will influence coreceptor selection during this process. The delineation of the selection process will require further investigation.
Consistent with previous reports, a high level of CXCR4 was found on the neurons in both the CNS and the peripheral nervous system (28, 35, 54, 57). In addition, we found a high level of CCR3 in neuronal tissues. Other chemokine receptors such as CXCR2 and the Duffy antigen are also expressed in neurons, but the biological function of these chemokine receptors in neuronal development, function, and disease remains to be determined (28). The level of expression may also promote the entry of CD4-independent viral isolates, found primarily among HIV-2 strains (18). An intriguing finding, however, is that the macrophages located primarily in the walls of small blood vessels are also positive for CXCR4 and CCR3. These perivascular macrophages or microglia, previously shown to be the targets of HIV/SIV infection (34), can therefore serve as a mechanism for viral dissemination to the CNS.
ACKNOWLEDGMENTS
This work was supported by grants from the NIH (AI35168, AI45218, and AI40387), GCRC support to The Rockefeller University, the Glaxo Wellcome Institute for Digestive Health, and the Aaron Diamond Foundation.
We thank Preston A. Marx, Agegnehu Gettie, and Christian Hangen for providing animal and human tissue specimens, and we thank Charles R. Mackay and James A. Hoxie for providing the coreceptor-specific antibodies. We also thank J. Moore and C. Cheng-Mayer for helpful discussions.
REFERENCES
- 1.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP1-alpha, MIP1-beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Bahner I, Kearns K, Coutinho S, Leonard E H, Kohn D B. Infection of human marrow stroma by human immunodeficiency virus-1 (HIV-1) is both required and sufficient for HIV-1-induced hematopoietic suppression in vitro: demonstration by gene modification of primary human stroma. Blood. 1997;90:1787–1798. [PubMed] [Google Scholar]
- 3.Bleul C C, Wu L, Hoxie J A, Springer T A, Mackay C R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broder C C, Dimitrov D S, Blumenthal R, Berger E A. The block of HIV-1 envelope glycoprotein-mediated membrane fusion in animal cells expressing human CD4 can be overcome by a human cell component(s) Virology. 1993;193:483–491. doi: 10.1006/viro.1993.1151. [DOI] [PubMed] [Google Scholar]
- 5.Burkitt H G, Young B, Heath J W. Functional histology. 3rd ed. New York, N.Y: Churchill Livingstone; 1994. [Google Scholar]
- 6.Canque B, Marandin A, Rosenzwajg M, Louache F, Vainchenker W, Gluckman J C. Susceptibility of human bone marrow stromal cells to human immunodeficiency virus (HIV) Virology. 1995;208:779–783. doi: 10.1006/viro.1995.1211. [DOI] [PubMed] [Google Scholar]
- 6a.Cheng-Mayer, C. Personal communication.
- 7.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The b-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 8.Chun T-W, Chadwick K, Margolick J, Siliciano R F. Differential susceptibility of naive and memory CD4+ T cells to the cytopathic effects of infection with human immunodeficiency virus type 1 strain LAI. J Virol. 1997;71:4436–4444. doi: 10.1128/jvi.71.6.4436-4444.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1a, and MIP-1b as the major HIV suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 10.Connor R, Ho D D. Human immunodeficiency virus type 1 variants with increased replicative capacity develop during the asymptomatic stage before disease progression. J Virol. 1994;68:4400–4408. doi: 10.1128/jvi.68.7.4400-4408.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1–infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalgleish A G, Beverley P C, Clapham P R, Crawford D H, Greaves M F, Weiss R A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 13.Daugherty B L, Siciliano S J, DeMartino J A, Malkowitz L, Sirotina A, Springer M S. Cloning, expression, and characterization of the human eosinophil eotaxin receptor. J Exp Med. 1996;183:2349–2354. doi: 10.1084/jem.183.5.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz S, Burkhart M, DiMarzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major coreceptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 15.Deng H K, Unutmaz D, KewalRamani V N, Littman D R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 16.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual tropic primary HIV-1 isolate that uses fusion and the b-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 17.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 18.Endres M J, Clapham P R, Marsh M, Ahuja M, Turner J D, McKnight A, Thomas J F, Stoebenau-Haggarty B, Choe S, Vance P J, Wells T N C, Power C A, Sutterwala S S, Doms R W, Landau N R, Hoxie J A. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996;87:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 19.Farzan M, Choe H, Martin K, Marcon L, Hofmann W, Karlsson G, Sun Y, Barrett P, Marchand N, Sullivan N, Gerard N, Gerard C, Sodroski J. Two orphan seven-transmembrane segment receptors which are expressed in CD4-positive cells support simian immunodeficiency virus infection. J Exp Med. 1997;186:405–411. doi: 10.1084/jem.186.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of seven-transmembrane domain, G-protein coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 21.Frankel S S, Tenner-Racz K, Racz P, Wenig B M, Hansen C H, Heffner D, Nelson A M, Pope M, Steinman R M. Active replication of HIV-1 at the lymphoepithelial surface of the tonsil. Am J Pathol. 1997;151:89–96. [PMC free article] [PubMed] [Google Scholar]
- 22.Haase A T, Henry K, Zupancic M, Sedgewick G, Faust R A, Melroe H, Cavert W, Gebhard K, Staskus K, Zhang Z Q, Dailey P J, Balfour H H, Jr, Erice A, Perelson A S. Quantitative image analysis of HIV-1 infection in lymphoid tissue. Science. 1996;274:985–989. doi: 10.1126/science.274.5289.985. [DOI] [PubMed] [Google Scholar]
- 23.He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay C R, Sodroski J, Gabuzda D. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 24.Heath H, Qin S, Rao P, Wu L, LaRosa G, Kassam N, Ponath P D, Mackay C R. Chemokine receptor usage by human eosinophils. The importance of CCR3 demonstrated using an antagonistic monoclonal antibody. J Clin Invest. 1997;99:178–184. doi: 10.1172/JCI119145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heise C, Miller C J, Lackner A, Dandekar S. Primary acute simian immunodeficiency virus infection of intestinal lymphoid tissue is associated with gastrointestinal dysfunction. J Infect Dis. 1994;169:1116–1120. doi: 10.1093/infdis/169.5.1116. [DOI] [PubMed] [Google Scholar]
- 26.Heise C, Vogel P, Miller C J, Halsted C H, Dandekar S. Simian immunodeficiency virus infection of the gastrointestinal tract of rhesus macaques. Functional, pathological, and morphological changes. Am J Pathol. 1993;142:1759–1771. [PMC free article] [PubMed] [Google Scholar]
- 27.Heise C, Vogel P, Miller C J, Lackner A, Dandekar S. Distribution of SIV infection in the gastrointestinal tract of rhesus macaques at early and terminal stages of AIDS. J Med Primatol. 1993;22:187–193. [PubMed] [Google Scholar]
- 28.Hesselgesser J, Halks-Miller M, DelVecchio V, Peiper S C, Hoxie J, Kolson D L, Taub D, Horuk R. CD4-independent association between HIV-1 gp120 and CXCR4: functional chemokine receptors are expressed in human neurons. Curr Biol. 1997;7:112–121. doi: 10.1016/s0960-9822(06)00055-8. [DOI] [PubMed] [Google Scholar]
- 29.Hufert F T, van Lunzen J, Janossy G, Bertram S, Schmitz J, Haller O, Racz P, von Laer D. Germinal centre CD4+ T cells are an important site of HIV replication in vivo. AIDS. 1997;11:849–857. doi: 10.1097/00002030-199707000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Kaneshima H, Su L, Bonyhadi M L, Connor R I, Ho D D, McCune J M. Rapid-high, syncytium-inducing isolates of human immunodeficiency virus type 1 induce cytopathicity in the human thymus of the SCID-hu mouse. J Virol. 1994;68:8188–8192. doi: 10.1128/jvi.68.12.8188-8192.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitchen S G, Zack J A. CXCR4 expression during lymphopoiesis: implications for human immunodeficiency virus type 1 infection of the thymus. J Virol. 1997;71:6928–6934. doi: 10.1128/jvi.71.9.6928-6934.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klatzmann D, Champagne E, Chamaret S, Gruest J, Guetard D, Hercend T, Gluckman J C, Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984;312:767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- 33.Kozak S L, Platt E J, Madani N, Ferro F E, Jr, Peden K, Kabat D. CD4, CXCR-4, and CCR-5 dependencies for infections by primary patient and laboratory-adapted isolates of human immunodeficiency virus type 1. J Virol. 1997;71:873–882. doi: 10.1128/jvi.71.2.873-882.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lackner A A, Smith M O, Munn R J, Martfeld D J, Gardner M B, Marx P A, Dandekar S. Localization of simian immunodeficiency virus in the central nervous system of rhesus monkeys. Am J Pathol. 1991;139:609–621. [PMC free article] [PubMed] [Google Scholar]
- 35.Lavi E, Strizki J M, Ulrich A M, Zhang W, Fu L, Wang Q, Hoxie J A, Gonzalez-Scarano F. CXCR-4 (Fusin), a co-receptor for the type 1 human immunodeficiency virus (HIV-1), is expressed in the human brain in a variety of cell types, including microglia and neurons. Am J Pathol. 1997;151:1035–1042. [PMC free article] [PubMed] [Google Scholar]
- 36.Liao F, Alkhatib G, Peden K W, Sharma G, Berger E A, Farber J M. STRL33, a novel chemokine receptor-like protein, functions as a fusion cofactor for both macrophage-tropic and T cell line-tropic HIV-1. J Exp Med. 1997;185:2015–2023. doi: 10.1084/jem.185.11.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu Y, Brosio P, Lafaile M, Li J, Collman R G, Sodroski J, Miller C J. Vaginal transmission of chimeric simian/human immunodeficiency viruses in rhesus macaques. J Virol. 1996;70:3045–3050. doi: 10.1128/jvi.70.5.3045-3050.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maddon P J, Dalgleish A G, McDougal J S, Clapham P R, Weiss R A, Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986;47:333–385. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- 39.Marx P A, Spira A I, Gettie A, Dailey P J, Veazey R S, Lackner A A, Mahoney C J, Miller C J, Claypool L E, Ho D D, Alexander N J. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med. 1996;2:1084–1089. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 40.Miller C J, Alexander N J, Sutjipto S, Lackner A A, Gettie A, Hendrickx A G, Lowenstine L J, Jennings M, Marx P A. Genital mucosal transmission of simian immunodeficiency virus: animal model for heterosexual transmission of human immunodeficiency virus. J Virol. 1989;63:4277–4284. doi: 10.1128/jvi.63.10.4277-4284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller C J, Vogel P, Alexander N J, Dandekar S, Hendrickx A G, Marx P A. Pathology and localization of simian immunodeficiency virus in the reproductive tract of chronically infected male rhesus macaques. Lab Invest. 1994;70:255–262. [PubMed] [Google Scholar]
- 42.Miller C J, Vogel P, Alexander N J, Sutjipto S, Hendrickx A G, Marx P A. Localization of SIV in the genital tract of chronically infected female rhesus macaques. Am J Pathol. 1992;141:655–660. [PMC free article] [PubMed] [Google Scholar]
- 43.O’Brien T, Winkler C, Dean M, Nelson J A, Carrington M, Michael N L, White G C., II HIV-1 infection in a man homozygous for CCR5 delta 32. Lancet. 1997;349:1219. doi: 10.1016/s0140-6736(97)24017-1. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 44.Pantaleo G, Graziosi C, Demarest J F, Butini L, Montroni M, Fox C H, Orenstein J M, Kotler D P, Fauci A S. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993;362:355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- 45.Platt E J, Madani N, Kozak S L, Kabat D. Infectious properties of human immunodeficiency virus type 1 mutants with distinct affinities for the CD4 receptor. J Virol. 1997;71:883–890. doi: 10.1128/jvi.71.2.883-890.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ponath P D, Qin S, Post T W, Wang J, Wu L, Gerard N P, Newman W, Gerard C, Mackay C R. Molecular cloning and characterization of a human eotaxin receptor expressed selectively on eosinophils. J Exp Med. 1996;183:2437–2448. doi: 10.1084/jem.183.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schnittman S M, Lane H C, Greenhouse J, Justement J S, Baseler M, Fauci A S. Preferential infection of CD4+ memory T cells by human immunodeficiency virus type 1: evidence for a role in the selective T-cell functional defects observed in infected individuals. Proc Natl Acad Sci USA. 1990;87:6058–6062. doi: 10.1073/pnas.87.16.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schuitemaker H, Koot M, Kootstra N A, Dercksen M W, Goede R E, Steenwijk R P, Lange J M A, Schattenkerk J K M, Miedeman F, Tersmette M. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell tropic populations. J Virol. 1992;66:1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simonds G, Wilkinson D, Reeves J D, Dittmar M T, Beddows S, Weber J, Carnegie G, Desselberger U, Gray P W, Weiss R A, Clapham P R. Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either LESTR or CCR5 as coreceptors for virus entry. J Virol. 1996;70:8355–8360. doi: 10.1128/jvi.70.12.8355-8360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stanley S K, McCune J M, Kaneshima H, Justement J S, Sullivan M, Boone E, Baseler M, Adelsberger J, Bonyhadi M, Orenstein J, et al. Human immunodeficiency virus infection of the human thymus and disruption of the thymic microenvironment in the SCID-hu mouse. J Exp Med. 1993;178:1151–1163. doi: 10.1084/jem.178.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tersmette M, Goede R E Y, Ai B J M, Winkel I N, Gruters R A, Cuypers H T, Huisman H G, Miedema F. Differential syncytium-inducing capacity of human immunodeficiency virus isolates: frequent detection of syncytium-inducing isolates in patients with acquired immunodeficiency virus syndrome (AIDS) and AIDS-related complex. J Virol. 1988;62:2026–2032. doi: 10.1128/jvi.62.6.2026-2032.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Theodorou I, Meyer L, Magierowska M, Katlama C, Rouzioux C. HIV-1 infection in an individual homozygous for CCR5 delta 32. Seroco Study Group. Lancet. 1997;349:1219–1220. . (Letter.) [PubMed] [Google Scholar]
- 53.Valentin H, Nugeyre M T, Vuillier F, Boumsell L, Schmid M, Barre-Sinoussi F, Pereira R A. Two subpopulations of human triple-negative thymic cells are susceptible to infection by human immunodeficiency virus type 1 in vitro. J Virol. 1994;68:3041–3050. doi: 10.1128/jvi.68.5.3041-3050.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vallat A V, De Girolami U, He J, Mhashilkar A, Marasco W, Shi B, Gray F, Bell J, Keohane C, Smith T W, Gabuzda D. Localization of HIV-1 co-receptors CCR5 and CXCR4 in the brain of children with AIDS. Am J Pathol. 1998;152:167–178. [PMC free article] [PubMed] [Google Scholar]
- 55.Wu L, LaRosa G, Kassam N, Heath H, Ruffing N, Gordon C, Chen H, Humblias J, Samson M, Parmentier M, Moore J, Mackay C R. Inhibition of chemokine and HIV-1 binding to CCR5 using monoclonal antibodies to different extracellular domain of CCR5. J Exp Med. 1997;185:1681–1691. [Google Scholar]
- 56.Wu L, Paxton W A, Kassam N, Ruffing N, Rottman J B, Sullivan N, Choe H, Sodroski J, Newman W, Koup R A, Mackay C R. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zaitseva M, Blauvelt A, Lee S, Lapham C K, Klaus-Kovtun V, Mostowski H, Manischewitz J, Golding H. Expression and function of CCR5 and CXCR4 on human Langerhans cells and macrophages: implications for HIV primary infection. Nat Med. 1997;3:1369–1375. doi: 10.1038/nm1297-1369. [DOI] [PubMed] [Google Scholar]
- 58.Zhang L, Huang Y, He T, Cao Y, Ho D D. HIV-1 subtype and second-receptor use. Nature. 1996;383:768. doi: 10.1038/383768a0. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 59.Zhang L Q, MacKenzie P, Cleland A, Holmes E C, Brown A J, Simmonds P. Selection for specific sequences in the external envelope protein of human immunodeficiency virus type 1 upon primary infection. J Virol. 1993;67:3345–3356. doi: 10.1128/jvi.67.6.3345-3356.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu T, Mo H, Wang N, Nam D S, Cao Y, Koup R A, Ho D D. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]