Abstract
Skeletal muscle injury is known to predispose its sufferers to neurological complications of concurrent poliovirus infections. This phenomenon, labeled “provocation poliomyelitis,” continues to cause numerous cases of childhood paralysis due to the administration of unnecessary injections to children in areas where poliovirus is endemic. Recently, it has been reported that intramuscular injections may also increase the likelihood of vaccine-associated paralytic poliomyelitis in recipients of live attenuated poliovirus vaccines. We have studied this important risk factor for paralytic polio in an animal system for poliomyelitis and have determined the pathogenic mechanism linking intramuscular injections and provocation poliomyelitis. Skeletal muscle injury induces retrograde axonal transport of poliovirus and thereby facilitates viral invasion of the central nervous system and the progression of spinal cord damage. The pathogenic mechanism of provocation poliomyelitis may differ from that of polio acquired in the absence of predisposing factors.
Provocation poliomyelitis (PPM) is the phenomenon of poliomyelitis resulting from physical trauma during infection with poliovirus (PV) (15, 17). Typically, PV infects the gastrointestinal tract, causing mild symptoms or no symptoms at all. In 1 to 2% of infections, PV invades the central nervous system (CNS), where it uniquely targets motor neurons for destruction, resulting in flaccid paralysis (15, 17). Poliomyelitis can be controlled by vaccination with inactivated or orally administered, attenuated PV types 1 to 3 (OPV) (15, 16, 25). Indeed, the World Health Organization has targeted PV for global eradication with predominantly OPV by the year 2000 (26). Although considered effective and safe (6), OPV may cause, on very rare occasions, neurological complications (vaccine-associated paralytic poliomyelitis) (2). This has generally been attributed to the emergence of neurovirulent PV variants in OPV recipients (7, 16, 25). However, an accumulation of cases of vaccine-associated paralytic poliomyelitis following vaccination with OPV in Romania has been linked to multiple intramuscular (i.m.) injections of various therapeutic or preventive agents administered to OPV recipients (22). These recent observations have greatly renewed the interest in PPM, a phenomenon that was first recognized in infections with wild-type PVs (1, 10, 11, 14). Occurrences of PPM remain a health problem in developing countries and countries with a preference for i.m. administration in pediatric practice (10, 27). Although a provoking effect of injections could be reproduced in nonhuman primates previously (4), no pathogenic mechanism leading to PPM has been determined.
The worldwide application of OPV involves the administration of hundreds of millions of doses per year, an effort being accelerated by the World Health Organization’s action plan to eradicate PV. The scope of the dissemination of live PV vaccine makes studies of the risk factors for neuroinvasion by this agent necessary. Here, we present experiments with mice transgenic for the human PV receptor (hPVR-tg mice) that have allowed us to identify a risk factor for neurological complications associated with multiple i.m. injections of wild-type-PV-infected animals. We found that the pathogenic spread of PV to the CNS in PPM may be fundamentally different from neuropathogenesis in the absence of skeletal muscle trauma. Determining the pathogenic mechanism for PPM may help in the development of delivery systems that target the CNS and may enhance our understanding of the adverse effects of i.m. vaccine administration in general.
MATERIALS AND METHODS
Sciatic nerve transection.
All survival surgical procedures were performed in accordance with institutional guidelines on animal welfare. Mice were anesthetized with an intraperitoneal injection of 0.8 mg of xylazine per g of body weight and 0.01 mg of ketamine per g of body weight. The left flanks of sedated animals were shaved in preparation for surgery. A transcutaneous incision of approximately 1/2 in. was made to expose the fascia overlying the biceps femoris and caudofemoralis muscles. A small incision into the fascia served to access the sciatic nerve in the recess between the muscles. An ∼1/4-in. segment of the sciatic nerve was removed. The cutaneous defect was treated with three to five sutures with Prolene 0-5 (Ethicon). Mice were treated with an analgesic, buprenorphine hydrochloride, administered intraperitoneally (in 0.012-mg doses) every 8 h. All 36 mice subjected to the procedure survived without any sequelae apart from the neurological defects stemming from the nerve transection. Discontinuity of the sciatic nerve was confirmed by autopsy in all surgically treated mice at the time of sacrifice.
Virus propagation, virus inoculation, and i.m. injection of experimental animals.
PV type 1 (Mahoney) [PV1(M)] was propagated and purified as described elsewhere (8). Defined amounts of virus as determined by plaque assay were suspended in sterile phosphate-buffered saline and inoculated into one of the tail veins of a sedated mouse by using a 27-gauge 1/2-in. hypodermic needle. i.m. injections were performed as follows. A 27-gauge 1/2-in. hypodermic needle was used to inject approximately 10 μl of phosphate-buffered saline into the left gastrocnemius muscle of a sedated mouse. Three times, the needle was pulled back and pushed forward to penetrate a wider area of the gastrocnemius muscle.
Determination of virus load and neuropathology.
Tissues of interest (from various spinal cord regions and skeletal muscle) were removed from sacrificed hPVR-tg mice. Each sample was weighed, placed in 1 ml of minimal essential medium, and homogenized in a Dounce homogenizer (Wheaton). Each homogenate was serially diluted, and the amount of infectious particles per 3 mg of tissue was analyzed in a plaque assay. Tissue specimens from the spinal cords of sacrificed mice were removed and subjected to fixation and paraffin embedding as described previously (9). They were cut at a thickness of 10 μm on a rotary microtome and mounted for histological staining. Histological staining with luxol fast blue, periodic acid-Schiff stain, and hematoxylin was performed according to standard protocols (9).
RESULTS
Upon PV infection, hPVR-tg mice develop a paralytic condition clinically and pathologically reminiscent of primate poliomyelitis (9, 12, 20). Four experimental groups of hPVR-tg mice were used: group I, untreated hPVR-tg mice; group II, mice that were treated with a left sciatic nerve transection alone (Fig. 1); group III, sham-operated mice that received multiple i.m. injections at intervals indicated in the legend to Fig. 2; and group IV, mice subjected to a left-sciatic-nerve transection that were treated with the same regimen of multiple i.m. injections as group III (Fig. 2).
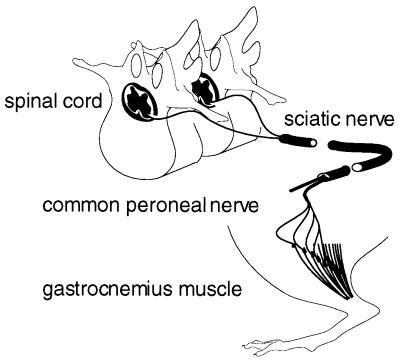
FIG. 1.
Implementation of the neural block. The sciatic nerve was severed, disconnecting its peripheral branches, the common peroneal and tibial nerves, from the spinal cord. Motoric and sensory innervation of the gastrocnemius muscle in the limb treated was thus interrupted.
FIG. 2.
Clinical course of PPM in hPVR-tg mice infected with PV1(M). hPVR-tg mice of four experimental groups were infected with 5 × 106 PFU of PV1(M) by the intravenous route. At defined intervals following virus inoculation (2 h, 6 h, 24 h, and 48 h p.i., indicated by vertical broken lines), animals of groups III and IV were treated with i.m. injections into the left gastrocnemius muscle (see Materials and Methods). All mice were observed for the appearance of neurological symptoms and clinically assessed according to the following scheme: 0, no symptoms; 1, general symptoms (ruffled fur and reduced activity); 2, paraparesis; 3, paraplegia and/or involvement of the upper extremities; 4, respiratory involvement; and 5, death. The average clinical scores for four mice are indicated. Mice that were treated with repeated i.m. injections developed more severe neurological symptoms earlier than animals of all other experimental groups. A sciatic nerve block prevented the accelerated course of PPM in mice treated with multiple i.m. injections. □, group I; ○, group II; ▪, group III; •, group IV.
Sciatic nerve transections were performed in such a manner as to minimize muscle trauma interfering with the experiment (Fig. 1 and Materials and Methods). Sham-operated mice were subjected to the identical procedure, only the nerve transection itself was left out. Seven days after surgery, all mice were inoculated intravenously with 5 × 106 PFU of PV1(M). Mice from groups III and IV were subsequently given i.m. injections into the left gastrocnemius muscle at regular intervals (Fig. 2). At 48, 72, and 96 h postinfection (p.i.), four animals from each experimental group were sacrificed, and the following analyses were carried out. (i) At the time of sacrifice, a clinical neurological status was established. (ii) Tissues from the cerebral cortex, cervical spinal cord, lumbosacral spinal cord, injected gastrocnemius muscle (where applicable), uninjected skeletal muscle, and serum were obtained. All tissues were homogenized and the virus load in the homogenate was quantified in a plaque assay. (iii) Tissue specimens from four areas of the spinal cord were obtained and analyzed histopathologically.
Clinical evaluation revealed an increased susceptibility to PV-induced neurological complications due to muscle injury inflicted by multiple i.m. injections (Fig. 2). Group III mice developed more severe signs of poliomyelitis earlier than their peers from group I (Fig. 2). The onset of paralysis in animals of group III occurred preferentially in the injected lower extremity, whereas animals of groups I and II as well as from group IV generally developed paraparesis initially (Table 1). Interruption of the peripheral nerve connection between the site of i.m. injections (gastrocnemius muscle) and the ipsilateral spinal cord protected mice from aggravation of the clinical course of poliomyelitis induced by multiple i.m. injections (group IV [Fig. 2]). This was evidenced by the slow course of clinical progression in infected animals that had been treated with a sciatic nerve transection prior to the administration of i.m. injections. The nerve transection itself did not influence the course of progression of poliomyelitis, since untreated control mice showed progression of neurological deterioration at the same rate as mice from group II (Fig. 2).
TABLE 1.
Sites of initial paralysis in PV-infected hPVR-tg micea
| Exptl group | No. of mice with paralysis
|
|||
|---|---|---|---|---|
| Left lower extremity | Right lower extremity | Paraparesis | Upper extremities | |
| I | 1 | 0 | 13 | 2 |
| II | 0 | 2 | 13 | 1 |
| III | 13 | 0 | 3 | 0 |
| IV | 0 | 1 | 15 | 0 |
Members of groups of experimental animals comprising 16 mice each were infected by the intravenous route with 5 × 106 PFU of PV1(M). They were treated according to the experimental protocol outlined in the legend to Fig. 2 and observed clinically twice daily, and it was recorded which limb showed initial signs of weakness.
Analyses of tissues of hPVR-tg mice revealed two factors involved in the provocation-induced, aggravated clinical course of poliomyelitis. Firstly, whereas skeletal muscle scarcely produces PV progeny in viremic animals (8), virus growth was stimulated in muscle tissue that had been injured by repeated i.m. injections (Fig. 3A). Secondly, in sham-operated mice treated with multiple i.m. injections, virus replication in lower segments of the spinal cord was significantly accelerated and elevated over propagation levels in the same area in nerve-transected animals (Fig. 3B). In contrast, replication rates in different experimental groups were less divergent among the same cervical segments of the spinal cord (Fig. 3C). No viral replication beyond the load of the original inoculum was detected in homogenates of tissues from the cerebral cortex and in serum.
FIG. 3.
Replication profiles of PV1(M) in tissues of hPVR-tg mice. Mice were sacrificed at the indicated time points following intravenous virus administration. (A) Replication rates in gastrocnemius muscle. Virus replication in uninjured skeletal muscle from all experimental groups was negligible (only values for groups I and II are shown). In animals that had received multiple i.m. injections injury of gastrocnemius muscle led to elevated titers of virus. (B) Virus growth in lumbosacral segments of the spinal cords of mice from group III was accelerated and significantly enhanced compared to virus propagation in controls and nerve-transected animals that had also been treated with multiple i.m. injections. (C) Viral replication in cervical segments was less divergent, with a slight lead in efficiency of virus replication in group III mice over their nerve-transected peers. ▪, groups I and II; •, group III; ○, group IV.
Enhanced virus replication within traumatized muscle occurred independently of sciatic nerve transection as evidenced by its occurrence to the same extent in animals derived from groups III and IV (Fig. 3A). This differs from the nature of viral propagation within the lower spinal cord in animals with sciatic nerve transection and mice with intact sciatic nerves (Fig. 3B). In the former, replication rates in the lumbosacral spinal cord were much lower than in the same region of the spinal cords of mice that were sham operated only.
From these observations it is apparent that the provocation effect of muscle injury is based on the induction of PV entry into the peripheral nerve and retrograde axonal transport to the CNS. Facilitated access to the CNS by retrograde axonal transport induced accidentally by muscle injury may shorten the incubation period, localize the initial paralytic symptoms, and hasten the progress of virus replication during PPM. This is strongly supported by the observation that peripheral nerve transection interrupting the neural connection between injured muscle and the spinal cord protects mice from PPM (Fig. 2). We observed an increase in viral replication within injured skeletal muscle; in this muscle only small quantities of virus were produced, but the increase may have enhanced the efficiency of PV uptake into the peripheral nerve. Although muscle injury in animals from group IV caused an increase in viral replication, the enhancement of proliferation did not extend to the spinal cord (Fig. 3B).
The conclusions based on clinical observations and quantitation of viral replication in specific tissues were confirmed by histopathological analysis. Spinal cord tissue specimens were harvested from sacrificed mice at given time points and processed for histopathological examination (Fig. 4). The sequence of images demonstrates the progression of poliomyelitic lesions within the lumbosacral spinal anterior horn in hPVR-tg mice. Mice from experimental groups I and II developed lesions typically seen in poliomyelitis only after 96 h p.i. (Fig. 4, panel 1C). Treatment of mice with multiple i.m. injections accelerated the progression of spinal cord lesions induced by PV. In mice from experimental group III, complete destruction of the motor neuron population was already achieved by 72 h p.i. (Fig. 4, panel 2B). At this time, no histopathological damage was evident in the control group (Fig. 4, panel 1B). The increase in the rate of appearance of histological lesions due to repeated i.m. injections covaried with the aggravated clinical course as well as the elevation of virus titers in tissues targeted by PV in mice of group III. As is evident, sciatic nerve transection protected hPVR-tg mice from the aggravated progression of poliomyelitic lesions. In mice from group IV, the pace of advance of poliomyelitic pathology was synchronous with the rate of progression in the control group (Fig. 4; compare panels 1A to C with panels 3A to C). Sections obtained from thoracocervical spinal segments showed a less evident advance of lesion progression in mice from group III (data not shown). This corresponded to the minor increase in viral replication in cervical regions of the spinal cords of mice from group III (Fig. 3C).
FIG. 4.
Histopathological evaluation of PV-induced damage within the lumbosacral segments of the spinal cords of mice from experimental groups I (panels 1A to C), III (panels 2A to C), and IV (panels 3A to C). The results of histopathological analysis of tissue from mice of group II have been omitted because the pace of progression of spinal lesions was indistinguishable from that in group I. Spinal cord tissue specimens were obtained from mice sacrificed 48 h (panels A), 72 h (panels B), and 96 h (panels C) following virus inoculation and were processed as described previously (9). Mice that had not been treated with multiple i.m. injections developed minor signs of virus-induced lesions within 96 h p.i. (panel 1C; arrow indicates motor neurons with characteristic cytopathic changes). In contrast, the spinal cords of animals that had been treated with the regimen of i.m. injections displayed complete destruction of the motor neuron population only 72 h p.i. (panel 2B). The acceleration of the progression of spinal lesions caused by multiple i.m. injections could be prevented by implementing a neural block through monolateral sciatic nerve transection (compare panels 2A to C with panels 3A to C). Bar, 250 μm.
DISCUSSION
Muscle injury due to injection of vaccines or therapeutic agents is common in medical practice. It has been observed that, if concurrent with PV infection, such injury may increase the risk of neurological complications (13, 22). Using a mouse model developed for the study of poliomyelitis, we have shown that muscular trauma induced by multiple injections can lead to rapid progression of PV-induced paralysis, upregulation of viral replication in certain tissues, and acceleration of the progression of histopathological lesions. Thus, our data provide direct experimental evidence for the concept of PPM.
What is the mechanism by which PPM is induced? Two different routes of PV invasion of the CNS have been observed in experimental infections: traversal of the blood-brain barrier (28) and retrograde axonal transport (3, 21). In 1954, Bodian (4) described experiments with monkeys to mimic PPM where the outcome of provocation in the form of i.m. injections was measured by clinical observations alone. It was speculated that entry of PV into the CNS may be facilitated by localized alterations of vascular permeability in the CNS induced by muscle trauma (4, 23).
It has been repeatedly observed in experimental infections of nonhuman primates and transgenic mice inoculated i.m. with PV that the onset of paralytic symptoms appears in the injected limb. i.m. administration of improperly prepared inactivated polio vaccine in the Cutter incident of 1955 tragically confirmed these observations; afflicted children developed initial signs of paralysis in the injected arm (19). These circumstantial reports as well as experimental evidence (21) suggested that retrograde axonal transport is responsible for PV invasion of the CNS after i.m. administration of virus.
In this study, we show that retrograde axonal transport of PV can be induced in viremic animals through skeletal muscle injury. Thereby, we simulated the natural course of PPM in humans, since the onset of neurological disease in PV-infected patients is regularly preceded by viremia (3). The augmentation associated with PPM of viral replication rates in the spinal cord could be prevented by sciatic nerve dissection ipsilateral to the site of i.m. injection prior to infection. Localization of initial paralytic symptoms to the limb treated with multiple i.m. injections was reversed by the sciatic nerve block. These observations indicated a pathogenic mechanism of PPM involving stimulation of retrograde axonal transport of PV through skeletal muscle injury. Indeed, it was observed previously that peripheral nerve trauma enhances the pace of retrograde axonal transport in general (5).
We have thus provided direct evidence that retrograde axonal transport accounts for facilitated access of PV to the CNS via peripheral nerves, resulting in PPM. As in experimental animals treated with i.m. injections, a shortened incubation period and initial paralysis localized to the injected limb were described for victims of the Cutter incident (19). Thus, the pathogenic mechanism for PPM determined in this study may underlie the Cutter incident, which never could be adequately explained (19). The pronounced influence of i.m. injections on localization of paralysis in transgenic mice was not observed in primates (18), probably reflecting the significant extent of extraneural proliferation of PV in the latter.
Upon the induction of muscle trauma, we observed an upregulation of PV propagation within injured muscle tissue that may indicate a pathogenic link between i.m. injections and the induction of retrograde axonal transport of PV. The stimulation of PV replication may rely on the upregulation of the cellular binding site for PV or viral replication within invading inflammatory cells. We did not detect upregulation of the human PV receptor by Northern blotting or immunohistochemical analysis of skeletal muscle tissue. However, the extremely low levels of extraneural PV production, even after stimulation through injury, hinder the evaluation of the role of local extraneural replication during pathogenesis.
Several human enteroviruses other than PV have the propensity to cause poliomyelitis, although the mechanism by which these viruses reach and enter motor neurons is currently unknown (15, 17). It is appealing to speculate that provoking factors may also play a role in the etiology of non-PV-induced poliomyelitis.
ACKNOWLEDGMENTS
Transgenic mice were a generous gift from A. Nomoto, the University of Tokyo, Tokyo, Japan. We are grateful to R. Sutter and P. Strebel, Polio Eradication Activity, National Immunization Program, Centers for Disease Control and Prevention, Atlanta, Ga., for critical reviews of the manuscript.
This work was supported by contract 95-200-0948 with the CDC and NIH grant RO1AI39485-01.
REFERENCES
- 1.Anderson G, Skaar A. Poliomyelitis occurring after antigen injections. Pediatrics. 1962;7:741–759. [PubMed] [Google Scholar]
- 2.Asaad F, Cockburn W F. The relation between acute persisting spinal paralysis and poliomyelitis vaccine—results of a ten-year enquiry. Bull W H O. 1982;60:231–233. [PMC free article] [PubMed] [Google Scholar]
- 3.Bodian D. Viremia, invasiveness, and the influence of injections. Ann NY Acad Sci. 1955;61:877–882. doi: 10.1111/j.1749-6632.1955.tb42545.x. [DOI] [PubMed] [Google Scholar]
- 4.Bodian D. Viremia in experimental poliomyelitis. II. Viremia and the mechanism of the “provoking effect” of injections or trauma. Am J Hyg. 1954;60:358–370. [PubMed] [Google Scholar]
- 5.Curtis R, Adryan K M, Zhu Y, Harkness P J, Lindsay R M, DiStefano P S. Retrograde axonal transport of ciliary neurotrophic factor is increased by peripheral nerve injury. Nature. 1993;365:253–255. doi: 10.1038/365253a0. [DOI] [PubMed] [Google Scholar]
- 6.Esteves K. Safety of oral poliomyelitis vaccine: results of a WHO enquiry. Bull W H O. 1988;66:739–746. [PMC free article] [PubMed] [Google Scholar]
- 7.Georgescu M-M, Delpeyroux F, Tardy-Panit M, Balanant J, Combiescu M, Combiescu A A, Guillot S, Crainic R. High diversity of poliovirus strains isolated from the central nervous system from patients with vaccine-associated paralytic poliomyelitis. J Virol. 1994;68:8089–8101. doi: 10.1128/jvi.68.12.8089-8101.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gromeier M, Alexander L, Wimmer E. Internal ribosomal entry site substitution eliminates neurovirulence in intergeneric poliovirus recombinants. Proc Natl Acad Sci USA. 1996;93:2370–2375. doi: 10.1073/pnas.93.6.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gromeier M, Lu H H, Wimmer E. Mouse neuropathogenic poliovirus strains cause damage in the central nervous system distinct from poliomyelitis. Microb Pathog. 1995;18:253–268. doi: 10.1016/S0882-4010(05)80002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guyer B, Bisong A A E, Gould J, Brigaud M, Aymard M. Injections and paralytic poliomyelitis in tropical Africa. Bull W H O. 1980;58:285–291. [PMC free article] [PubMed] [Google Scholar]
- 11.Hill A B, Knowelden J. Inoculation and poliomyelitis: a statistical investigation in England and Wales in 1949. Br Med J. 1955;II:1–6. doi: 10.1136/bmj.2.4669.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koike S, Taya C, Kurata T, Abe S, Ise I, Yonekawa Y, Nomoto A. Transgenic mice susceptible to poliovirus. Proc Natl Acad Sci USA. 1991;88:951–955. doi: 10.1073/pnas.88.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lancet. Provocation poliomyelitis. Lancet. 1992;340:1005–1006. . (Editorial.) [Google Scholar]
- 14.McCloskey B P. The relation of prophylactic inoculations to the onset of poliomyelitis. Lancet. 1950;i:659–663. doi: 10.1002/(sici)1099-1654(199910/12)9:4<219::aid-rmv249>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 15.Melnick J L. Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses. In: Fields B N, et al., editors. Virology. N.Y: Raven Press; 1995. pp. 549–605. [Google Scholar]
- 16.Minor P D. The molecular biology of polio vaccines. J Gen Virol. 1992;73:3065–3077. doi: 10.1099/0022-1317-73-12-3065. [DOI] [PubMed] [Google Scholar]
- 17.Modlin J F. Poliomyelitis and poliovirus immunization. In: Rotbart H A, editor. Human enterovirus infections. Washington, D.C: American Society for Microbiology; 1995. pp. 195–220. [Google Scholar]
- 18.Nathanson N, Bodian D. Experimental poliomyelitis following intramuscular virus injection. I. The effect of neural block on a neurotropic and a pantropic strain. II. Viremia and the effect of antibody. Bull Johns Hopkins Hosp. 1961;108:308–333. [PubMed] [Google Scholar]
- 19.Nathanson N, Langmuir A D. The Cutter incident. Am J Hyg. 1963;78:16–81. doi: 10.1093/oxfordjournals.aje.a120327. [DOI] [PubMed] [Google Scholar]
- 20.Ren R, Costantini R, Gorgacz E J, Lee J J, Racaniello V R. Transgenic mice expressing a human poliovirus receptor: a new model for poliomyelitis. Cell. 1990;63:353–362. doi: 10.1016/0092-8674(90)90168-e. [DOI] [PubMed] [Google Scholar]
- 21.Ren R, Racaniello V R. Poliovirus spreads from muscle to the central nervous system by neural pathways. J Infect Dis. 1992;166:747–752. doi: 10.1093/infdis/166.4.747. [DOI] [PubMed] [Google Scholar]
- 22.Strebel P M, Ion-Nedelcu N, Baughman A L, Sutter R M, Cochi S L. Intramuscular injections within 30 days of immunization with oral poliovirus vaccine—a risk factor for vaccine-associated paralytic poliomyelitis. N Engl J Med. 1995;332:500–506. doi: 10.1056/NEJM199502233320804. [DOI] [PubMed] [Google Scholar]
- 23.Sutter R W, Patriarca P A, Suleiman A J M, Brogan S, Malankar P G, Cochi S L, Al-Ghassani A A, el-Bualy M S. Attributable risk of DTP (diphtheria and tetanus toxoids and pertussis vaccine) injection in provoking paralytic poliomyelitis during a large outbreak in Oman. J Infect Dis. 1992;165:444–449. doi: 10.1093/infdis/165.3.444. [DOI] [PubMed] [Google Scholar]
- 24.Trueta J, Hodes R. Provoking and localizing factors in poliomyelitis. An experimental study. Lancet. 1954;i:998. doi: 10.1016/s0140-6736(54)92396-x. [DOI] [PubMed] [Google Scholar]
- 25.Wimmer E, Hellen C U T, Cao X M. Genetics of poliovirus. Annu Rev Genet. 1993;27:353–436. doi: 10.1146/annurev.ge.27.120193.002033. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. Expanded programme on immunization, global poliomyelitis eradication by the year 2000: manual for managers of immunization programmes on activities related to polio eradication. Geneva, Switzerland: World Health Organization; 1989. pp. 35–64. [Google Scholar]
- 27.Wyatt H V. Provocation of poliomyelitis by multiple injections. Trans R Soc Trop Med Hyg. 1985;79:355–358. doi: 10.1016/0035-9203(85)90379-7. [DOI] [PubMed] [Google Scholar]
- 28.Yang W X, Terasaki T, Shiroki K, Ohka S, Aoki J, Tanabe S, Nomura T, Terada E, Sugiyama Y, Nomoto A. Efficient delivery of circulating poliovirus to the central nervous system independently of poliovirus receptor. Virology. 1997;229:421–428. doi: 10.1006/viro.1997.8450. [DOI] [PubMed] [Google Scholar]