Abstract
The current study was conducted on the inhabitants living in the area adjacent to the Hudiara drain using bore water and vegetables adjacent to the Hudiara drain. Toxic heavy metals badly affect human health because of industrial environmental contamination. Particularly hundreds of millions of individuals globally have faced the consequences of consuming water and food tainted with pollutants. Concentrations of heavy metals in human blood were elevated in Hudiara drainings in Lahore city, Pakistan, due to highly polluted industrial effluents. The study determined the health effects of high levels of heavy metals (Cd, Cu, Zn, Fe, Pb, Ni, Hg, Cr) on residents of the Hudiara draining area, including serum MDA, 8-Isoprostane, 8-hydroxyguanosine, and creatinine levels. An absorption spectrophotometer was used to determine heavy metals in wate water, drinking water, soil, plants and human beings blood sampleas and ELISA kits were used to assess the level of 8-hydroxyguanosine, MDA, 8-Isoprostane in plasma serum creatinine level. Waste water samples, irrigation water samples, drinking water samples, Soil samples, Plants samples and blood specimens of adult of different weights and ages were collected from the polluted area of the Hudiara drain (Laloo and Mohanwal), and control samples were obtained from the unpolluted site Sheiikhpura, 60 km away from the site. Toxic heavy metals in blood damage the cell membrane and DNA structures, increasing the 8-hydroxyguanosine, MDA, creatinine, and 8-Isoprostane. Toxic metals contaminated bore water and vegetables, resulting in increased levels of creatinine, MDA, Isoprostane, and 8-hydroxy-2-guanosine in the blood of inhabitants from the adjacent area Hudiara drain compared to the control group. In addition,. This study also investigated heavy metal concentrations in meat and milk samples from buffaloes, cows, and goats. In meat, cow samples showed the highest Cd, Cu, Fe and Mn concentrations. In milk also, cows exhibited elevated Cu and Fe levels compared to goats. The results highlight species-specific variations in heavy metal accumulation, emphasizing the need for targeted monitoring to address potential health risks. The significant difference between the two groups i.e., the control group and the affected group, in all traits of the respondents (weight, age, heavy metal values MDA, 8-Isoprostane, 8-hydroxyguaniosine, and serum creatinine level). Pearson’s correlation coefficient was calculated. The study has shown that the level of serum MDA, 8-Isoprostane, 8-hydroxyguaniosine, or creatinine has not significantly correlated with age, so it is independent of age. This study has proved that in Pakistan, the selected area of Lahore in the villages of Laloo and Mohanwal, excess of heavy metals in the human body damages the DNA and increases the level of 8-Isoprostane, MDA, creatinine, and 8-hydroxyguaniosine. As a result, National and international cooperation must take major steps to control exposure to heavy metals.
Keywords: DNA damage, Heavy metals, 8-hydroxyguanosine, MDA, 8-Isoprostane
Subject terms: Ecology, Environmental sciences
Introduction
Heavy metals play vital role in biochemical and physiological functions of plants and animals. It is indicated that heavy metals have severe ramifications on cellular organelles and other components1. Scientific studies show sewage water causes soil pollution, harming humans and animals. The heavy metals contaminate the drinking water due to improper sewage disposal and municipal waste management. A high quantity of heavy metals is present in industrial wastewater2. Heavy metals affect plant growth, easily accumulating in soil and entering the food chain. Essential and non-essential metals exist in our environment3. Using wastewater in agriculture results in toxic metal contamination in vegetables4,5. The absorption of heavy metals is harmful to our bodies6. The Hudiara drain starts from Batala in the Gurdaspur district of India and enters Pakistan from the Laloo village of India to the Hudiara village of Pakistan. The length of the drain in India is 55 km, and 63 km inside Pakistan7.
The end junction in Pakistan is the river Ravi. The water is rich in pollutants due to hundreds of industries near the Hudiara drain8. The farmers use this wastewater for irrigation purposes, and because of this, there is an excess of heavy metals in vegetables and crops. The heavy metals enter animal bodies (cow, buffalo, goat) through feed and drinking water; when humans eat meat and drink the milk of animals (cow, buffalo, goat), these heavy metals enter the human body9. The level of heavy metals in milk (cow, buffalo, goat) is higher than the permissible limit compared to normal in many countries like Pakistan10, Egypt10, and Nigeria11. Meat is the source of protein used for the formation, growth, and repair of tissues12. Heavy metals toxicity in meat causes toxic effects on human health10.
The bioaccumulation of heavy metals in the food chain causes a serious health threat to human beings13. Metals directly affect on mental development and cause cancer13. Drinking polluted water and using wastewater for irrigation of vegetables and crops transfer heavy metals to the meat of animals and contaminate human feed14 Trace elements may cause oxidative DNA damage in living organisms, particularly humans. Biomarkers are considered a vital tool for exposure identification, and their use may provide important information15. In terms of Biochemistry, Biomarkers are observable endpoints in the continuity of events leading from exposure of environmental agents to diseases16–18. Once validated through laboratory tests and studies, biomarkers can provide direct measures of the actual effect of chemicals upon living organisms in the field, thereby overcoming large areas of uncertainty expressed in standard risk assessment19. 8-OHdG serum level measurement may be a valuable marker for biomonitoring in the case of mixed occupational exposure to toxic metals and increased cancer risk20.
The present study aimed to determine the concentrations of heavy metals like iron, zinc, copper, cadmium, arsenic, lead, and mercury in irrigation water, drinking water, soil, vegetables, milk, beef, goat meat, and human serum samples. This study selected and also used three biomarkers 8-hydroxy-2-deoxyguanosine, malondialdehyde (MDA), and 8-isoprostane, as three bioindicators to demonstrate the effect of heavy metals on human health in the vicinity of Hudiara drain.
Material and method
The experimental data collection followed all the rules and regulations at the Institute of Molecular Biology and Biotechnology, The University of Lahore, Lahore, Pakistan. Permissions from the authorities were obtained as required. It has been confirmed that the plant samples were collected and used while complied with relevant institutional, national, and international guidelines and legislation with appropriate permissions from authorities of the Local Government authorities of District Lahore and Sheikpura, and the Institute of Molecular Biology and Biotechnology, The University of Lahore, Lahore, Pakistan. Ethical Review Committee of the Institute of Molecular Biology and Biotechnology, University of Lahore, Lahore, Pakistan, gave the ethical approval, with reference number 2018010120. It has also confirmed that informed consent was obtained from all subjects and/or their legal guardian(s). It has also been confirmed that the study was reported following ARRIVE guidelines.
Study area
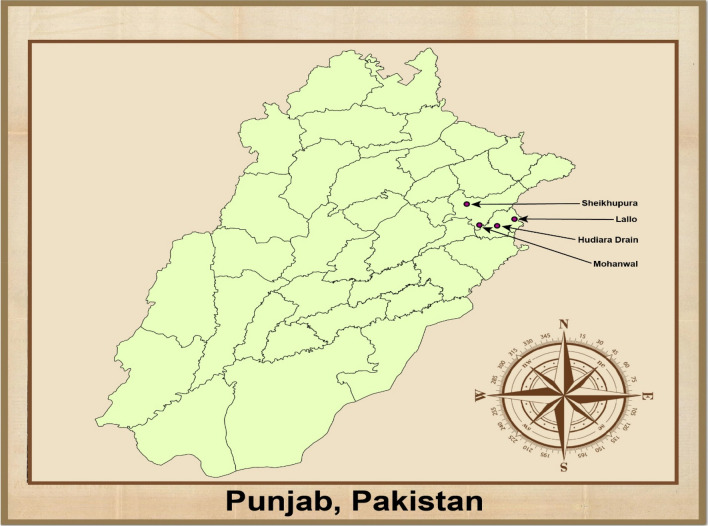
The study area is Hudiara, located in the Lahore area in Punjab of Pakistan, with latitude and longitude 310 2327 N 740 1427 E. The control area (Sheikpura) was located 60 km away from Hudiara Drain Lahore, Punjab, Pakistan, with latitude and longitude 310766 N 730984 E (Figure 1). Herbaceous are present for grazing animals. The main grain crops of the area are wheat, rice, and vegetables: carrots, cauliflower, turnip, Mustard, spinach, beet, cabbage, garlic, and onion. The study area of this research project was the Laloo and Mohanwal villages of Lahore.
Figure 1.
Location map for collection of samples.
We followed the rules when collecting plant samples in Laloo and Mohanwal villages of Lahore. We got the green light from the local authorities and the Institute of Molecular Biology and Biotechnology at the University of Lahore, Pakistan. Our methods align with ARRIVE guidelines, and the Ethical Review Committee at the institute approved our work with reference number 2018010120. It has also confirmed that informed consent was obtained from all subjects and/or their legal guardian(s).
Samples collection
Wastewater samples were collected from the surface of stagnant water in the Hudiara drain. Samples of irrigation water were collected from the channels used by farmers in the fields after pumping water. The vegetables were collected from the same field irrigating Hudiara drain water. The polyethylene bottle container was washed with detergent, rinsed with distilled water, and pre-treated with 20% nitric acid (HNO3), then finally rinsed through with deionized water, followed by drying in an oven. Ten samples of each vegetable and cereal were collected from the adjacent area of the Hudiara drain (Laloo and Mohanwal). Ten samples of each plant's edible parts (cereals and vegetables) were collected. The rice samples (Oryza sativa) and leaves of fodder crop samples were collected in the summer of 2017. The Wheat (Triticum aestivum), spinach (Spinacia oleracea), onion (Allium cepa), potato (Solanum tuberosum), cabbage (Brassica oleracea var. capitata), cauliflower (Brassica oleracea var. botrytis), mustard (Brassica campestris), turnip (Brassica rapa subsp. Rapa), beet (Beta vulgaris subsp), garlic (Allium sativum) and carrot (Daucus carota) in 2018 winter.
Fifty drinking water samples (250 ml) were collected randomly from tape and Laloo and Mohanwal village wells adjacent to Hudiara drain. Fifty soil samples (250 g) were collected randomly from different places adjacent to Hudiara drain and agriculture fields with a 5-10 cm depth. Ten samples of milk (Goat, Cow, and Buffalo) and ten samples of (buffalo, cow, and goat) meat (buffalo, cow, and goat) were collected from the Laloo and Mohanwal adjacent to the Hudiara drain. Three hundred human blood samples were collected from the Median Cubital vein of adults who are inhabitants of Hudiara drain in 2017–2018. These samples were analyzed in the laboratory of Institute of Molecular Biology and Biotechnology, University of Lahore, Pakistan to determine heavy metals and again verified at Comsat University Abbottabad. The same sample size of control samples of water used for irrigating, soil, vegetables, cereals, meat, milk, and human blood was collected 60 km from Hudiara drain. Anti-coagulant (EDTA) was added for whole blood analysis and placed in the refrigerator. The blood and serum were separated by using a centrifugation process. The samples were digested for further analysis of metals. This study has two groups: a control group of drinking water, irrigation water, plants, animals, and human beings; the second is the affected heavy metals group. One group is the affected inhabitants (Demographic data: supplementary material table 1) near Hudiara Drain, and the second group is the control group 60 km (Sheikhpura) away from Hudiara Drain.
Methods for standard solution preparation
Heavy metal analysis used standard solutions for drinking water, wastewater, plants, soil, milk, meat, and blood samples. The stock of different heavy metals in nitrate and sulfate form has a concentration of 1000 ppm21. The standard solutions for all the heavy metals under study were prepared in three to five different concentrations to obtain a calibration curve by diluting the stock standard solution of a concentration of 1000 ppm22.
Digestion methods
Digestion is necessary before applying the solution to the atomic absorption instrument. Drinking water is digested for application on an atomic absorption spectrophotometer23. Different acid mixtures were used ( ,, HCl, HF in the ratio of 2:1:1:1) for digestion24. The digestion of wastewater samples is carried out by adding aqua regia25. The plant samples were dried and digested26. The raw milk samples were collected from buffalo, goats, and cows of the adjacent inhabitants of Hudiara drain in washed polyethylene containers and digested27. Fresh meat samples of cow (beef), buffalo (beef), and goat (mutton) were collected from the adjacent local meat shops of Hudiara drain, digested, and applied for further analysis of heavy metals28. The digestion of blood samples was carried out by taking of blood (3000 rpm for 10 min)29. The EIA standard 8-Isoprostane ( Cat# EK7123) was used for quantitative determination30. The 8-hydroxy-2’-deoxyguanosine (Cat # MBS 267,161) was quantitatively determined in serum by using ELISA kit by Glory Science Co. Ltd. USA. For MDA (Cat# 90357) centrifuged at the speed of 3000 rpm for 600 s. After centrifugation, it separates the top layer and measures the absorbance of the solution at 532 nm31. The Cayman Chemical USA creatinine kit (Cat# 700460) is used by using the Jaffe Method for the determination of creatinine in blood serum levels in adult males. The absorbance was measured at 490 nm31.
Statistical analysis
Statistical paired comparison analysis is used for the comparison of selected heavy metals in stagnant and running water of Huidara Drain, heavy metals in drinking water are shown by t-statistic and p-value for significant difference where ANOVA F-test is used for a statistical difference of control and affected group. Concentration of heavy metals in vegetables, meat and milk is represented in graphical form. The statistical analysis in tabulated and graphical form is done by using SPSS Statistic 29.
Ethical approval
The ethical approval was given by Ethical Review Committee of Institute of Molecular Biology and Biotechnology, University of Lahore, Lahore, Pakistan with references number 2018010120. It has also confirming that informed consent was obtained from all subjects and/or their legal guardian(s).
Results
Heavy metal concentrations in hudiara drain
In this study, Table 1 provides a comparative analysis of the concentrations of selected heavy metals (Fe, Cu, Mn, Ni, Cr, Cd, Pb, Zn) in both stagnant and running water within the Hudiara Drain. Understanding the variations in heavy metal levels in different water conditions is essential for assessing this important waterway's environmental impact and potential risks. Table 1,2,3 offers insights concentration of heavy metals in running (irrigation) and stagnant water and shows the concentration of heavy metals high in stagnant water as compare to running water.
Table 1.
Quantity of selected heavy metals (Fe, Cu, Mn, Ni, Cr, Cd, Pb, Zn) in stagnant and running water of Hudiara drain in vicinity of Lahore.
| Heavy metals in stagnant water of hudiara drain water | Mean | Std. deviation | Heavy metals inirrigating hudiara drain water | Mean | Std. deviation |
|---|---|---|---|---|---|
| Fe (mg/l) | 818.46 | 84.458 | Fe (mg/l) | 423.4100 | 108.25304 |
| Cu (mg/l) | 810.68 | 108.475 | Cu (mg/l) | 399.4976 | 79.35818 |
| Mn (mg/l) | 83.32 | 13.367 | Mn (mg/l) | 28.44 | 8.825 |
| Ni (mg/l) | 215.04 | 29.802 | Ni (mg/l) | 134.38 | 43.130 |
| Cr (mg/l) | 337.96 | 77.448 | Cr (mg/l) | 161.40 | 76.648 |
| Cd (mg/l) | 428.78 | 94.477 | Cd (mg/l) | 264.06 | 86.145 |
| Pb (mg/l) | 586.50 | 69.740 | Pb (mg/l) | 367.18 | 50.180 |
| Zn (mg/l) | 396.46 | 51.393 | Zn (mg/l) | 280.82 | 40.724 |
Table 2.
Comparison of concentration of selected heavy metals (Fe, Cu, Mn, Ni, Cr, Cd, Pb, Zn) in stagnant and running water of Hudiara drain in vicinity of Lahore.
| Paired comparison analysis | N | Mean | Std. deviation | t-Statistics | P-Values | |
|---|---|---|---|---|---|---|
| Pair 1 | Fe mg/l in stagnant water–Fe mg/l in hudiara drain water | 50 | 395.05000 | 131.72349 | 21.207 | 0.000 |
| Pair 2 | Cu mg/l in stagnant water–Cu mg/l in hudiara drain water | 50 | 411.18240 | 137.07649 | 21.211 | 0.0000 |
| Pair 3 | Mn mg/l in stagnant water–Mn mg/l in hudiara drain water | 50 | 54.880 | 16.473 | 23.557 | 0.000 |
| Pair 4 | Ni mg/l in stagnant water–Ni mg/l in hudiara drain water | 50 | 80.660 | 49.933 | 11.422 | 0.000 |
| Pair 5 | Cr mg/l in stagnant water–Cr mg/l in hudiara drain water | 50 | 176.560 | 93.018 | 13.422 | 0.000 |
| Pair 6 | Cd mg/l in stagnant water–Cd mg/l in hudiara drain water | 50 | 164.720 | 102.243 | 11.392 | 0.000 |
| Pair 7 | Pb mg/l in stagnant water–Pb mg/l in hudiara drain water | 50 | 219.320 | 83.392 | 18.597 | 0.000 |
| Pair 8 | Zn mg/l in stagnant water–Zn mg/l in hudiara drain water | 50 | 115.640 | 65.568 | 12.471 | 0.000 |
Table 3.
Quantity of selected heavy metals in soil (Fe, Cu, Zn, Pb, Cd, Cr, Ni and Mn) in control and affected soil samples in vicinity of Hudiara drain, Lahore.
| Heavy Metals in soil (mg/kg) | Group | N | Mean | Std. deviation | F-statistics | P-values |
|---|---|---|---|---|---|---|
| Fe | Control | 50 | 46.0120 | 8.10481 | 275.163 | 0.000 |
| Affected | 50 | 696.2400 | 277.05754 | |||
| Cu | Control | 50 | 6.8260 | 1.16668 | 182.411 | 0.000 |
| Affected | 50 | 10.6380 | 1.61926 | |||
| Zn | Control | 50 | 3.0660 | 1.00663 | 732.818 | 0.000 |
| Affected | 50 | 22.8826 | 5.07744 | |||
| Pb | Control | 50 | 0.9094 | 0.16935 | 116.942 | 0.000 |
| Affected | 50 | 5.5438 | 3.02562 | |||
| Cd | Control | 50 | 0.0051 | 0.00188 | 519.831 | 0.000 |
| Affected | 50 | 1.0972 | 0.33868 | |||
| Cr | Control | 50 | 0.0678 | 0.01389 | 65.677 | 0.000 |
| Affected | 50 | 2.6236 | 2.22995 | |||
| Ni | Control | 50 | 0.4118 | 0.12586 | 1272.874 | 0.000 |
| Affected | 50 | 1.0580 | 0.02373 | |||
| Mn | Control | 50 | 0.6660 | 0.35584 | 397.128 | 0.000 |
| Affected | 50 | 5.8184 | 1.79326 |
Quantity of selected heavy metals in soil
Table 3 offers insights into the contamination levels of these heavy metals in the soil, shedding light on the environmental impact and potential risks associated with soil quality near the drain.
Heavy metals in fodder crop leaves and wastewater of Hudiara drain
Table 3 provides valuable information on the concentrations of selected heavy metals in the leaves of fodder crops and wastewater samples collected from Hudiara Drain. This data is critical for assessing potential contamination and risks associated with the consumption of crops grown in this area, as well as the environmental impact of heavy metal presence in the wastewater of Hudiara Drain.
Concentration of heavy metals in drinking water
Table 4 presents crucial information regarding the concentrations of various heavy metals in the drinking water of the areas adjacent to the Hudiara Drain. Understanding the levels of these heavy metals in the drinking water is vital for assessing the potential health and environmental impacts in the vicinity of Hudiara Drain.
Table 4.
Concentration of selected heavy metals (Fe, Cu, Mn, Ni, Cr, Cd, Pb, Zn) in fodder crop leaves and wastewater of Hudiara drain.
| Paired comparison analysis | N | Mean | Std. deviation | t-Statistics | P-Values | |
|---|---|---|---|---|---|---|
| Pair 1 | Fe mg/l in stagnant water–Fe mg/l in hudiara drain water | 10 | 395.05000 | 131.72349 | 21.207 | 0.000 |
| Pair 2 | Cu mg/l in stagnant water–Cu mg/l in hudiara drain water | 10 | 411.18240 | 137.07649 | 21.211 | 0.000 |
| Pair 3 | Mn mg/l in stagnant water–Mn mg/l in hudiara drain water | 10 | 54.880 | 16.473 | 23.557 | 0.000 |
| Pair 4 | Ni mg/l in stagnant water–Ni mg/l in hudiara drain water | 10 | 80.660 | 49.933 | 11.422 | 0.000 |
| Pair 5 | Cr mg/l in Stagnant Water–Cr mg/l in hudiara drain water | 10 | 176.560 | 93.018 | 13.422 | 0.000 |
| Pair 6 | Cd mg/l in stagnant water–Cd mg/l in hudiara drain Water | 10 | 164.720 | 102.243 | 11.392 | 0.000 |
| Pair 7 | Pb mg/l in stagnant water–Pb mg/l in hudiara drain water | 10 | 219.320 | 83.392 | 18.597 | 0.000 |
| Pair 8 | Zn mg/l in stagnant water–Zn mg/l in hudiara drain water | 10 | 115.640 | 65.568 | 12.471 | 0.000 |
Level of heavy metals and biomarkers in blood serum of males
Table 5 provides comprehensive data on the levels of various heavy metals along with selected biomarkers such as 8-Isoprostane, 8-Hydroxy-2-Deoxyguanosine, Malondialdehyde (MDA), and Creatinine, in the blood serum of two groups—a control group and an affected group of males. This table is a crucial resource for assessing the potential health impacts and biomarker variations in individuals residing near the source of heavy metal exposure.
Table 5.
Concentration of heavy metals (Cd. Co, Cu, Cr, Fe, Hg, Mn, Ni, Pb, Zn) in drinking water of adjoining area of Hudiara drain.
| Heavy metals | N | Mean | Std. deviation | Test value | Mean difference | t-statistics | P-value |
|---|---|---|---|---|---|---|---|
| Zn (mg/l) | 50 | 0.7996 | 0.53207 | 0 | 0.79960 | 10.626 | 0.000 |
| Fe (mg/l) | 50 | 1.4662 | 1.51325 | 0 | 1.46620 | 6.851 | 0.000 |
| Cu (mg/l) | 50 | 0.9578 | 0.86190 | 0 | 0.95780 | 7.858 | 0.000 |
| Mn (mg/l) | 50 | 0.3084 | 0.18019 | 0 | 0.30840 | 12.103 | 0.000 |
| Cd (mg/l) | 50 | 0.0061 | 0.00211 | 0.005 | 0.00110 | 3.683 | 0.001 |
| Pb (mg/l) | 50 | 0.0318 | 0.01804 | 0.01 | 0.02180 | 8.547 | 0.000 |
| Cr (mg/)l | 50 | 0.2780 | 0.16449 | 0.1 | 0.17800 | 7.652 | 0.000 |
| Ni (mg/l) | 50 | 0.0712 | 0.01452 | 0.07 | 0.00120 | 0.584 | 0.562 |
Concentration of heavy metals in cereals and vegetables
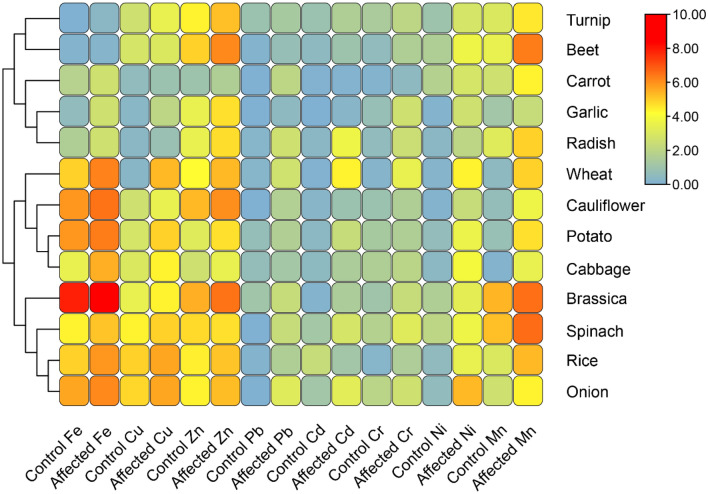
Our research and investigation have revealed significant differences in the heavy metal content of various vegetables and cereals between the control and affected groups (Fig. 2). In the case of iron (Fe), concentrations in affected wheat, spinach, rice, onion, and potato were notably higher than their respective controls, with values ranging from 0.16 to 340 in the simulated samples. Similarly, affected cabbage, Brassica, Turnip, Beet, Garlic, Carrot, and Radish also exhibited elevated iron concentrations. Copper (Cu) levels significantly increased in affected wheat, spinach, rice, onion, and several other samples. Zinc (Zn) content saw substantial elevation in affected wheat, spinach, rice, onion, and different samples, with concentrations ranging from 2 to 90 Table 6.
Figure 2.
Concentration of Fe, Cu, Mn, Ni, Cr, Cd, Pb and Zn in Cereals and Vegetables of control and affected group of Hudiara drain vicinity in Lahore.
Table 6.
Level of heavy metals (Cd. Co, Cu, Cr, Fe, Hg, Mn, Ni, Pb, Zn) and biomarkers (8- isoprostane, 8-Hydroxy-2-Deoxyguaniosine, MDA, Creatinine) in blood serum of control and affected group of males.
| Variables | Mean ± Std. deviation | ANOVA F-statistics | (P-value) | |
|---|---|---|---|---|
| Weight Kg | Control group | 52.07 ± 6.703 | 150.226 | 0.000 |
| Affected group | 62.16 ± 12.585 | |||
| Age Years | Control group | 41.59 ± 8.173 | 1.017 | 0.314 |
| Affected group | 42.56 ± 14.519 | |||
| 8-Isoprostane pg/ml | Control group | 1.2045 ± 0.49960 | 27,380.978 | 0.000 |
| Affected group | 49.6800 ± 5.04943 | |||
| 8-hydroxy-2-deoxyguaniosine mg/ml | Control group | 0.2099 ± 0.02475 | 20,957.601 | 0.000 |
| Affected group | 1.3893 ± 0.13891 | |||
| MDA nmol/ml | Control group | 1.8011 ± 0.60081 | 763.537 | 0.000 |
| Affected group | 3.8609 ± 1.14285 | |||
| Creatinine mg/dl | Control group | 0.6481 ± 0.11428 | 3571.839 | 0.000 |
| Affected group | 1.7653 ± 0.30292 | |||
| Concentration of Cd in human blood µg/l | Control group | 33.8128 ± 26.23737 | 300.700 | 0.000 |
| Affected group | 372.8357 ± 337.60983 | |||
| Co µg/l | Control group | 243.30 ± 143.807 | 1045.855 | 0.000 |
| Affected group | 536.30 ± 62.810 | |||
| Cu µg/l | Control group | 1650.30 ± 893.352 | 96.204 | 0.000 |
| Affected group | 1108.40 ± 343.00 | |||
| Cr µg/l | Control group | 0.1385 ± 0.10129 | 2168.731 | 0.000 |
| Affected group | 2.7732 ± 0.97467 | |||
| Fe µg/l | Control group | 0.07805 ± 0.052758 | 3.089 | 0.079 |
| Affected group | 0.22379 ± 1.435382 | |||
| Hg µg/l | Control group | 0.4394 ± 0.34586 | 1294.195 | 0.000 |
| affected group | 4.5963 ± 1.97130 | |||
| Cd µg/l | Control group | 608.37 ± 102.580 | 3369.421 | 0.000 |
| affected group | 1172.10 ± 133.314 | |||
| Ni µg/l | Control group | 0.2277 ± 0.10639 | 310.909 | 0.000 |
| affected group | 1.4972 ± 1.24251 | |||
| Pb µg/l | Control group | 48.92 ± 29.598 | 785.124 | 0.000 |
| Affected group | 1315.21 ± 782.195 | |||
| Zn µg/l | Control group | 156.09 ± 48.918 | 401.651 | 0.000 |
| Affected group | 282.77 ± 97.942 | |||
Lead (Pb) concentrations were notably increased in several affected samples, with values reaching as high as 8 in onion. Cadmium (Cd) levels also showed a considerable increase in some affected samples, with values up to 20 in wheat. Chromium (Cr) was elevated in affected wheat (10), spinach (8), onion (5) and garlic (5) samples. Nickel (Ni) was significantly higher in various affected samples, reaching 40 in onion. Finally, manganese (Mn) levels were elevated in affected spinach, with values as high as 98 others were Brassica (94), beet (80) and rice (40). These results indicate the substantial impact of heavy metal contamination on the composition of these vegetables and cereals, raising significant concerns about their safety for consumption.
Concentration of heavy metal (mg/kg) in buffalo, cow and goat meat
During the investigation of the concentration of heavy metals, the results revealed varying levels of heavy metals across the three livestock species meat (Figure 3). Specifically, the highest concentration of Cd was observed in cow meat (0.43 mg/kg). For Cu, cows exhibited the highest concentration (6.7 mg/kg), followed by buffaloes (4 mg/kg). Among the heavy metals tested, Fe demonstrated the most pronounced differences, with cows exhibiting the highest concentration (200 mg/kg) compared to buffaloes (90 mg/kg) and goats (12.07 mg/kg). Similar trends were observed for Mn, Ni, Pb, and Zn, with varying concentrations across the three species. These findings shed light on the differential accumulation of heavy metals in the meat of buffaloes, cows, and goats, emphasizing the importance of species-specific monitoring in mitigating potential health risks associated with heavy metal exposure through dairy consumption.
Figure 3.
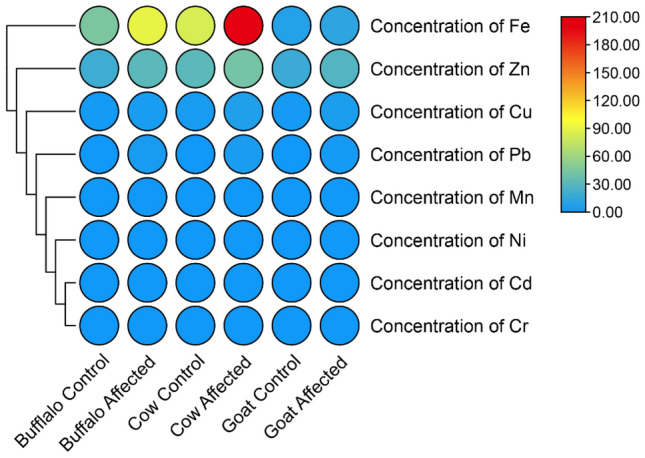
Concentration of Fe, Cu, Mn, Ni, Cr, Cd, Pb, and Zn mg/kg in buffalo, cow, and goat meat of control and affected group.
Concentration of heavy metal (mg/kg) in buffalo, cow and goat milk
During the investigation, the concentration of heavy metals revealed varying levels across the three livestock species' milk (Figure 4). Affected animals had significantly higher heavy metal concentrations compared to the control groups. For instance, in the case of Copper (Cu), cows and goats had much higher concentrations (3 and 1.6 mg/kg, respectively). Similarly, in the case of Iron (Fe), cows had a much higher concentration (180 mg/kg) of heavy metal than all other species. These results suggested potential heavy metal contamination in the affected animal groups, which could have had adverse health implications.
Figure 4.
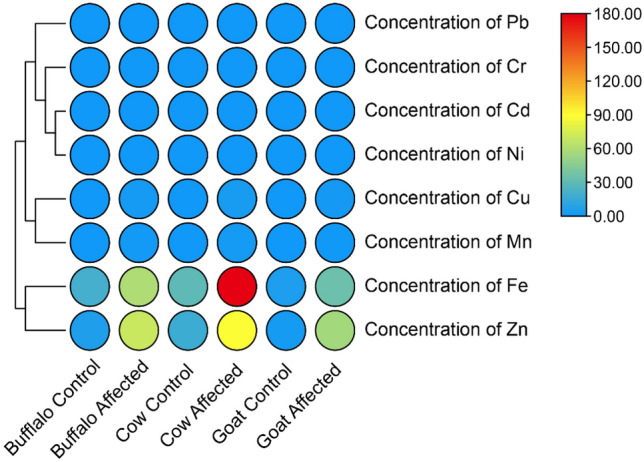
Concentration of Fe, Cu, Mn, Ni, Cr, Cd, Pb and Zn mg/kg in buffalo, cow and goat milk of control and affected group.
Discussion
The present study revealed that soil is contaminated with some heavy metals Fe, Cd, which have higher mean concentrations 6.96, 1.09 mg kg−1 and crossed the threshold32 value set by FAO/WHO 2007, respectively. The higher content in affected soil compared to control was most likely due to wastewater containing a higher concentration of metals. Waste water application builds up the metal concentration Fe, Pb, Zn in surface soil 0-15 cm. Soil irrigated with sewage from the city had higher content in some metals Fe, Cd, Cr. The level of Cd in soil irrigated with city effluent exceeded the standard guidelines set by FAO/WHO 200033. Cereals, fruits, and vegetables are the main sources of heavy metals in the human body. The concentration of heavy metals in food depends on both the mobility and bioavailability of heavy metals in soil. Trace elements are vital in humans, plants, and animals' chemical, biological, and biochemical reactions. The concentration of heavy metals in food increases due to androgenic sources34,35. The higher concentration of analyzed metal in different cereals, wheat, and rice grown near the Hudiara drain might be due to irrigation of untreated industrial and domestic water that significantly affects the metal content in crops. Most metals' concentrations were above the international organizations' threshold limits. The highest concentration of Cu and Cd was reported in wheat and rice in most of the studies on soil receiving a high load of sewage sludge or wastewater36. Ni in sampled wheat grains irrigated through the Hudiara drain of the current study was higher than the permissible limits of FAO and WHO 200037. The higher content of Ni in wheat grain might be due to different inputs of waste from different industries, especially glass, paint, and textile38. Cr content was higher than the established permissible limits from the different international organizations and potentially threatened people using affected wheat grains39. The observed level of Cd in cabbage is higher than the permissible limits of FAO, 2007. This might be due to the area's higher continuous wastewater usage40.
Leafy vegetables irrigated through industrial sewage water showed higher levels than the permissible limits41,42. Fe has significant importance for all crops regarding photosynthesis and other metabolites. The results revealed the Fe level is higher in leafy vegetables i.e., Brassica, cauliflower cabbage, cauliflower, and beets grown on affected/polluted soil than control/unpolluted soil. Our findings align with studies conducted on effluent industries43. Similarly, the mean concentration of Mn is in the range of previous studies conducted in Pakistan43. Pb concentration in the sampled vegetables aligns with studies conducted on effluent industries43. The findings of the conducted study regarding Zn concentration treated with Hudiara drain water and underground water are within permissible limits established by FAO and WHO, 200044.
In this study, the leafy vegetables i.e., Cabbage, cauliflower, spinach, brassica, and turnip were studied for metal acquisition under irrigation through the Hudiara drain and underground water. The results revealed that bio-available concentrations of heavy metals were higher in vegetables irrigated with sewage water than underground water. The metals Pb, Cd, and Cr accumulated in leafy vegetables brassica, cabbage, cauliflower, and turnip have a higher value than permissible limits 0.3, 0.3, and 1.3 mg kg−1. This might be due to the continuous irrigation of sewage wastewater from the Hudiara drain, the main root of heavy metals accumulation in soils5,45–47. Beside this, the higher concentration of Lead, Cadmium, and Chromium in leafy vegetables from the permissible limits might be due to the presence of various industries installed on the way of Hudiara drain, Steels, and Iron foundries, rolling mills, and continuous broken steel coated with Cd and Pb in the wastewater. Furthermore, it has been reported that soil had a higher content of heavy metals irrigated with wastewater48.
Heavy metals in different vegetables cultivated on wastewater in Bahawalpur that have a higher concentration of Pb, Cr, and Cd; and exceeded the permissible limits. However, the present research showed that higher concentrations of Ni, Zn, Fe, and Cu in leafy vegetables grown near Hudiara drain did not exceed the threshold value established by WHO, 200749. The plants/vegetables treated with waste water have a higher content of heavy metals than plants treated with fresh water. The variation in heavy metals content in leafy and root vegetables directly depends upon the nature of plants, physicochemical properties of soil, as well as the environment and anthropogenic activities49.
In the present research study, metals analyzed in drinking water were examined as per standard guidelines of WHO/ FAO, 2007. The concentration of metals Zn, Fe, Mn, and Cu was within tolerable limits according to WHO, 2007, and Pakistan EPA standards50. However, drinking water quality exhibited elevated levels in metals of Pb, Cr, Ni, and Cd from WHO's standard guidelines. The high level of Ni in the drinking water might be due to ultramafic industrial activities and continuous seepage of sewage water from the Hudiara drain in the study area. Lead in drinking water exceeded the permissible limits accomplished by WHO. The raised lead concentration in the study area might be due to the usage of pesticides on agricultural land, and continuous application of Hudiara drains' sewage water may lead to metal leaching51. Previous work has also illustrated the elevated lead levels in Lahore, Jhang, and Vehari52. The present study revealed the variation in metal concentration in the meat of buffalo, cow, and goat in affected/polluted and controlled/non-polluted areas of Hudiara drain. The higher concentration of Cd in raw meat of buffalo grazed in affected/polluted areas compared to controlled/unpolluted. Areas and Cd concentrations in raw buffalo and cow meat exceeded the permissible limit set by the International Dairy Federation 1979. It is the most acceptable limit for Cd in raw meat53.
The Cd level in cow’s meat watering from the industrial effluent and drinking from the uncontaminated river water. They concluded that Cd level was twice as high in cow’s meat collected from the contaminated area than in the non-contaminated area. Iftikhar and his colleagues collected raw meat from urban and rural areas, illustrating that Cd level was higher in the urban areas than rural areas54. Lead in meat is one of the most toxic metals affecting human health. It is the most common industrial metal that can contaminate the soil, water, air, forage, and food55.
In the present study, the maximum mean lead level in cow’s meat was observed in the affected polluted areas, followed by buffalo and goat at 0.19, 0.16, and 0.12 mg kg−1, respectively. However, the minimum Pb concentration in Goat meat was recorded in the controlled area 0.0003 mg kg−1. The higher metal level in meat could be due to the grazing of animals in polluted areas. The present study revealed lead concentration exceeded the permissible limits established by WHO37. The present study's findings align with the previous study, which reported a higher Pb level in the industrial polluted areas than in non-polluted areas. Similarly, the highest concentration was 23.24 mg L−1 of lead in cow’s meat; cows were drinking untreated sewage water in Faisalabad, Pakistan56.
In the present study, the highest mean level of nickel was observed in cows, followed by buffalo and goat 0.023, 0.018, and 0.012 mg L−1, respectively, reared in the contaminated areas; however, the low level was detected in the control or unpolluted area. Fe is an essential trace element that stimulates several metabolic reactions in the human body, although its higher level may produce toxicity and cause health issues. In the present study, a higher level of Fe was recorded in cow’s meat, followed by buffalo and goat meat, respectively, compared to the controlled area. It exceeded the permissible limits furnished by IDF 1979. A higher level of Fe in cow’s meat near the industrial polluted areas compared to the non-polluted areas in Egypt, and a significant level was also reported in India52. Copper has an essential role in human health; however, its higher level may cause various toxic effects on the human body. The level of Cu was recorded in the meat of cows followed by buffalo and goat, respectively, in polluted compared to non-polluted areas. The cow’s meat ranging from 0.0136 to 36 mg L−1, and exceeds the permissible level 0.01 mg L−1 set by IDF, 1979 world., reported in various studies53. The current work revealed a higher level of Cr in raw meat of buffalo, cows, and goats grazing in the polluted areas compared to the controlled areas, an exceeded the permissible value set by IDF. However, its value is below what was detected in this study and reported in Codex Alimentarius Commission, 2011. All the detected values of Zn were within permissible limits of WHO 121 mg kg−1. The Zn concentration in camel, cattle, buffalo and goat meat from various regions of KPK. They reported the Zn concentration was within permissible limits and far below that of current work. The contaminated heavy metals in fodder crops are used as feed and contaminate animal feed53.
Excessive Cu causes disturbance in the immunity system, dermatitis, nervous system, and gastrointestinal diseases52 . The permissible concentration of Cu in meat is .01 µg/g IDF 1979. According to Puls 1994, the normal median value of Cu in meat is 0.5 µg/g. The daily intake of Co is 4.33 µg/kg/day and Cd 0.011 µg/kg/day. In the current research, the higher level of heavy metals in the raw meat of examined animals might be attributed to the use of sewage water of Hudiara drain for agricultural purposes and direct access to polluted water for drinking. Heavy metal toxicity caused by animal meat ingestion has created human health issues for many years13.
Overall, the findings of the present work showed a higher level of heavy metal contents in meat samples of affected/polluted areas compared to control/non-polluted areas. The result of the present study showed a higher level of Pb in cows 4.3 mg kg−1 followed by buffalo and goats 3.2 and 2 mg kg−1, respectively; its level was above the permissible limits of 0.1 mg kg−1 set by WHO13. The prescribed level in the present work revealed that cadmium concentration in cow, goat, and buffalo is higher than the permissible limits.
In Iran, a similar trend was observed for Cd levels in meat, as its concentration exceeded the tolerable limits set by WHO in 200713. Besides this, levels of Zn and Cu in the prescribed study revealed a difference in the level of metals detected in the affected as compared to the control; however, their level did not cross the acceptable limits set by WHO. The content of Zn and Cu was analyzed in meat samples collected from the Lahore Market. Cr and Ni concentrations57 in the meat of analyzed samples collected from the polluted and non-polluted areas showed variation in its level; although the higher level detected in the collected samples; was lower than the acceptable limits USDA, 2006. Fe concentration in collected samples of buffalo and goat are below the tolerable limits, however, the concentration in cow crossed the permissible limits. The variation in the level of Fe in meat might be due to the feeding behavior of respective animals57. The variation of Fe concentration was examined in the meat of both goat and cow. The results of the present study illustrated the significant variation in analyzed metals samples in human blood. The higher concentration was detected in samples collected from the vicinity of the Hudiara drain; however, the lower metal concentration was recorded in samples taken far away from the locality of the Hudiara drain. The levels of most metals content in human blood were higher than the permissible limits. The significant level of metals in the human population living near the sewage effluent58. Detected a potential threat of Pb, Ni, and Cd in human blood. The accumulation of heavy metals in the human body generates ROS and RNS, which are directly involved in damaging lipids, proteins, and DNA. The biochemical properties alter due to cross-linking of DNA. The adduct of MDA and 4-HNE also affects the synthesis of protein. It promotes cell death and activates the signaling pathway.
The excess formation of free radicals weak the antioxidant system, which causes oxidative stress and as result increases the process of lipid peroxidation, malondialdehyde MDA, Isoprostane Iso, and an adduct of DNA59. Studies have proved that 8-OHdG increases with exposure of toxic metals, as the association between 8-OHdG and cadmium is very strong. Malondialdehyde MDA can measure the damaging of free radicals. The malondialdehyde MDA can be measured by reaction with thiobarbituric acid using a spectrophotometer. In our study, heavy metals cause ROS and enhance MDA60.
The more effective marker of lipid peroxidation is Isoprostane, which arises from unsaturated fatty acids. The areas of high-level heavy metals will result in severe complications in the future. National and international cooperation should take major steps to control exposure to heavy metals by providing safe guidelines on using chemicals and industries. There should be proper channel lining of sewerage channels to avoid seepage of waste water and toxic chemicals in the soil of the adjacent area.
Supplementary Information
Acknowledgements
The authors have acknowledged the Institute of Molecular Biology and Biotechnology, University of Lahore, Lahore, Pakistan, for providing lab support to conduct this research work. The manuscript is from PhD research work of author Saima Jadoon.
Author contributions
SJ and QA wrote the initial manuscript, AS and MZH performed the analysis. MAJ, MA, MAK, QA, provided the technical guidance regarding statistics and interpretation. QA supervised the project. All the authors reviewed and finalized the manuscript. The Ethical Review Committee of the Institute of Molecular Biology and Biotechnology, University of Lahore, Lahore, Pakistan, participants and the authors gave the proper consent for publication.
Funding
The research funding was provided by the Institute of Molecular Biology and Biotechnology, University of Lahore, Lahore, Pakistan, from student research funds.
Data availability
The produced, collected, or generated during the study has been given in the manuscript file and its supplementary file.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Saima Jadoon, Email: Saima.jadoon95@gmail.com.
Qurban Ali, Email: saim1692@gmail.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-58655-x.
References
- 1.Perveen S, et al. Study on accumulation of heavy metals in vegetables receiving sewage water. J. Chem. Soc. Pakistan. 2011;33(220):227. [Google Scholar]
- 2.Zige T, Izah SC. Microbial load and heavy metals properties of leachates from solid wastes dumpsites in the Niger Delta Nigeria. J. Environ. Treat. Tech. 2015;3:148–153. [Google Scholar]
- 3.Ali H, Khan E. Trophic transfer, bioaccumulation, and biomagnification of non-essential hazardous heavy metals and metalloids in food chains/webs—Concepts and implications for wildlife and human health. Hum. Ecol. Risk Assess. Int. J. 2019;25:1353–1376. doi: 10.1080/10807039.2018.1469398. [DOI] [Google Scholar]
- 4.Nayek S, Gupta S, Saha R. Metal accumulation and its effects in relation to biochemical response of vegetables irrigated with metal contaminated water and wastewater. J. Hazard. Mater. 2010;178:588–595. doi: 10.1016/j.jhazmat.2010.01.126. [DOI] [PubMed] [Google Scholar]
- 5.Abbas A, Rashad A, Rehman AU, Bukhari MS. Exploring the response mechanisms of rice to salinity stress. Bull. Biol. Allied Sci. Res. 2024;2024:58. doi: 10.54112/bbasr.v2024i1.58. [DOI] [Google Scholar]
- 6.Vila M, Sánchez-Salcedo S, Vallet-Regí M. Hydroxyapatite foams for the immobilization of heavy metals: From waters to the human body. Inorg. Chim. Acta. 2012;393:24–35. doi: 10.1016/j.ica.2012.06.027. [DOI] [Google Scholar]
- 7.Nisar N, Aamir K. Phytoavailability of chromium in Triticum Aestivum in natural and synthetically fertilized soil irrigated with hudiara drain wastewater. Lahore IJAAR. 2014;4:34. [Google Scholar]
- 8.Qadir A, Malik RN, Husain SZ. Spatio-temporal variations in water quality of Nullah Aik-tributary of the river Chenab Pakistan. Environ. Monitor. Assess. 2008;140:43–59. doi: 10.1007/s10661-007-9846-4. [DOI] [PubMed] [Google Scholar]
- 9.Yu H, et al. Comparative evaluation of groundwater, wastewater and canal water for irrigation on toxic metal accumulation in soil and vegetable: Pollution load and health risk assessment. Agric. Water Manag. 2022;264:107515. doi: 10.1016/j.agwat.2022.107515. [DOI] [Google Scholar]
- 10.Emami MH, et al. 2023 A review of heavy metals accumulation in red meat and meat products in the Middle East. J. Food Prot. 2023;86:100048. doi: 10.1016/j.jfp.2023.100048. [DOI] [PubMed] [Google Scholar]
- 11.Dibie N, Asuelimen P. Periodic study with selected mineral elements levels of cow and goat milk samples obtained from two animal markets in Benin City and estimated daily intake of studied heavy metals. Niger. J. Technol. 2022;41:613–624. doi: 10.4314/njt.v41i3.22. [DOI] [Google Scholar]
- 12.Trisyani N. The role of mineral content in bamboo shell meat (shell meal) and calm flour (meat meal) and formation process on the health of living-being in Indonesia. AgBioforum. 2022;24:95–105. [Google Scholar]
- 13.Zainab N, et al. Health risk assessment and bioaccumulation of potentially toxic metals from water, soil, and forages near coal mines of district Chakwal, Punjab, Pakistan. Environ. Geochem. Health. 2023 doi: 10.1007/s10653-023-01531-w. [DOI] [PubMed] [Google Scholar]
- 14.Engwa GA, Ferdinand PU, Nwalo FN, Unachukwu MN. Mechanism and health effects of heavy metal toxicity in humans. Poison. Modern world-N. Tricks Old Dog. 2019;10:70–90. [Google Scholar]
- 15.Jenkins JA, Musgrove M, White SJO. Outlining potential biomarkers of exposure and effect to critical minerals: Nutritionally essential trace elements and the rare earth elements. Toxics. 2023;11:188. doi: 10.3390/toxics11020188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldstein SL, et al. Consensus-based recommendations on priority activities to address acute kidney injury in children: A modified Delphi consensus statement. JAMA Netw. Open. 2022;5:e2229442–e2229442. doi: 10.1001/jamanetworkopen.2022.29442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ullah I, et al. Prevalence and risk factors of helicobacter pylori infection among individuals with tobacco consumption habits in district Peshawar: a cross-sectional study. Bull. Biol. Allied Sci. Res. 2023;2023:42–42. doi: 10.54112/bbasr.v2023i1.42. [DOI] [Google Scholar]
- 18.Hassan N, et al. Antiviral response of drugs used against hbv patients of Khyber Pakhtunkhwa, Pakistan. Bull. Biol. Allied Sci. Res. 2023;2023:49–49. doi: 10.54112/bbasr.v2023i1.49. [DOI] [Google Scholar]
- 19.Castelli FA, et al. Metabolomics for personalized medicine: the input of analytical chemistry from biomarker discovery to point-of-care tests. Anal. Bioanal. Chem. 2022;414:759–789. doi: 10.1007/s00216-021-03586-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung C-J, et al. Determination of potential sources of heavy metals in patients with urothelial carcinoma in central Taiwan: A biomonitoring case–control study. Environ. Geochem. Health. 2023;45:1–14. doi: 10.1007/s10653-023-01481-3. [DOI] [PubMed] [Google Scholar]
- 21.Atty SA, Shallan AI, Abdel-Hakim A, Hammad MA, Abou El-Alamin MM. Fabrication of ultra-sensitive modified electrode for eco-friendly determination of neurotoxic heavy metals in beef, dairy products and biological samples. J. Electrochem. Soc. 2022;169:096502. doi: 10.1149/1945-7111/ac8bae. [DOI] [Google Scholar]
- 22.Kassim NA, Ghazali S, Bohari FL, Abidin N. Assessment of heavy metals in wastewater plant effluent and lake water by using atomic absorption spectrophotometry. Mater. Today Proc. 2022;66:3961–3964. doi: 10.1016/j.matpr.2022.04.671. [DOI] [Google Scholar]
- 23.Adolfo FR, do Nascimento PC, Brudi L, Bohrer D, do Carvalho LM. Simultaneous determination of Ba Co, Fe, and Ni in nuts by high-resolution continuum source atomic absorption spectrometry after extraction induced by solid-oil-water emulsion breaking. Food Chem. 2021;345:128766. doi: 10.1016/j.foodchem.2020.128766. [DOI] [PubMed] [Google Scholar]
- 24.Bai J-H, Ma J-L, Wei G-J, Zhang L, Zhong S-X. Ce and Nd stable isotope purification and determination of geological samples by MC-ICP-MS. J. Anal. At. Spectrom. 2022;37:1618–1628. doi: 10.1039/D2JA00082B. [DOI] [Google Scholar]
- 25.Sichler TC, et al. Determination of the phosphorus content in sewage sludge: comparison of different aqua regia digestion methods and ICP-OES, ICP-MS, and photometric determination. Environ. Sci. Eur. 2022;34:1–14. doi: 10.1186/s12302-022-00677-1. [DOI] [Google Scholar]
- 26.Lejwoda P, Świnder H, Thomas M. Evaluation of the stability of heavy metal-containing sediments obtained in the wastewater treatment processes with the use of various precipitating agents. Environ. Monit. Assess. 2023;195:442. doi: 10.1007/s10661-023-11036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hussain, M. et al. (s Note: MDPI stays neu-tral with regard to jurisdictional claims in …, (2021).
- 28.Ugya A, Ahmad A, Adamu H, Giwa S, Imam T. Phytoextraction of heavy metals and risk associated with vegetables grown from soil irrigated with refinery wastewater. J. Applied Biol. Biotechnol. 2019;7:14–19. doi: 10.7324/JABB.2019.70203. [DOI] [Google Scholar]
- 29.Sampath V, Han K, Kim IH. Influence of yeast hydrolysate supplement on growth performance, nutrient digestibility, microflora, gas emission, blood profile, and meat quality in broilers. J. Animal Sci. Technol. 2021;63:563. doi: 10.5187/jast.2021.e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graille M, et al. Urinary 8-isoprostane as a biomarker for oxidative stress. A systematic review and meta-analysis. Toxicology letters. 2020;328:19–27. doi: 10.1016/j.toxlet.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 31.El-Gendy ZA, et al. Hepatoprotective effect of Omega-3 PUFAs against acute paracetamol-induced hepatic injury confirmed by FTIR. Hum. Exp. Toxicol. 2021;40:526–537. doi: 10.1177/0960327120954522. [DOI] [PubMed] [Google Scholar]
- 32.Nduka JK, et al. Ecological and health risk assessment of heavy metals in roadside soil, dust and water of three economic zone in Enugu Nigeria. Urban Climate. 2023;51:101627. doi: 10.1016/j.uclim.2023.101627. [DOI] [Google Scholar]
- 33.Ahmad K, et al. Evaluation of potential toxic metals accumulation in wheat irrigated with wastewater. Bull. Environ. Contam. Toxicol. 2019;102:822–828. doi: 10.1007/s00128-019-02605-1. [DOI] [PubMed] [Google Scholar]
- 34.Sonone SS, Jadhav S, Sankhla MS, Kumar R. Water contamination by heavy metals and their toxic effect on aquaculture and human health through food Chain. Lett. Appl. NanoBioSci. 2020;10:2148–2166. doi: 10.33263/LIANBS102.21482166. [DOI] [Google Scholar]
- 35.Rasheed M, Malik A. Mechanism of drought stress tolerance in wheat. Bull. Biol.Allied Sci. Res. 2022;2022:23–23. doi: 10.54112/bbasr.v2022i1.23. [DOI] [Google Scholar]
- 36.Achkir A, et al. Implication of sewage sludge increased application rates on soil fertility and heavy metals contamination risk. Emerg. Contam. 2023;9:100200. doi: 10.1016/j.emcon.2022.100200. [DOI] [Google Scholar]
- 37.Hussain MI, et al. Blood, hair and feces as an indicator of environmental exposure of sheep, cow and buffalo to cobalt: A health risk perspectives. Sustainability. 2021;13:7873. doi: 10.3390/su13147873. [DOI] [Google Scholar]
- 38.Peydayesh M, Bagnani M, Soon WL, Mezzenga R. Turning food protein waste into sustainable technologies. Chem. Rev. 2022;123:2112–2154. doi: 10.1021/acs.chemrev.2c00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ugulu I, et al. Potentially toxic metal accumulation in grains of wheat variety Galaxy-2013 irrigated with sugar industry wastewater and human health risk assessment. Euro-Mediterr. J. Environ. Integr. 2021;6:1–11. doi: 10.1007/s41207-020-00203-w. [DOI] [Google Scholar]
- 40.Gemeda FT, Guta DD, Wakjira FS, Gebresenbet G. Occurrence of heavy metal in water, soil, and plants in fields irrigated with industrial wastewater in Sabata town Ethiopia. Environ. Sci. Pollut. Res. 2021;28:12382–12396. doi: 10.1007/s11356-020-10621-6. [DOI] [PubMed] [Google Scholar]
- 41.Gupta N, et al. Investigation of heavy metal accumulation in vegetables and health risk to humans from their consumption. Front. Environ. Sci. 2022;10:791052. doi: 10.3389/fenvs.2022.791052. [DOI] [Google Scholar]
- 42.Fatima S, et al. The genome-wide bioinformatics analysis of 1-aminocyclopropane-1-carboxylate synthase (acs), 1-aminocyclopropane-1-carboxylate oxidase (aco) and ethylene overproducer 1 (eto1) gene family of fragaria vesca (woodland strawberry) Bull. Biol. Allied Sci. Res. 2023;2023:38–38. doi: 10.54112/bbasr.v2023i1.38. [DOI] [Google Scholar]
- 43.Jehan S, Muhammad S, Ali W, Hussain ML. Potential risks assessment of heavy metal (loid) s contaminated vegetables in Pakistan: A review. Geocarto Int. 2022;37:7287–7302. doi: 10.1080/10106049.2021.1969449. [DOI] [Google Scholar]
- 44.Yang, H.-H. et al. Wastewater Driven Heavy Metal Transfer Up the Food Chain in Peri-Urban Agricultural Lands of Lahore. Available at SSRN 4421418.
- 45.Ullah H, et al. Health risk assessment and multivariate statistical analysis of heavy metals in vegetables of Khyber Pakhtunkhwa region Pakistan. Biol. Trace Element Res. 2022;200:3023–3038. doi: 10.1007/s12011-021-02892-y. [DOI] [PubMed] [Google Scholar]
- 46.Junaid MD, Gokce AF. Global agricultural losses and their causes. Bull. Biol. Allied Sci. Res. 2024;2024:66. doi: 10.54112/bbasr.v2024i1.66. [DOI] [Google Scholar]
- 47.Javed MM, et al. The contribution of transgenic rice to enhance grain yield. Bull. Biol. Allied Sci. Res. 2024;2024:65. doi: 10.54112/bbasr.v2024i1.65. [DOI] [Google Scholar]
- 48.Kasi WA, Panezai S, Saqib SE. Wastewater irrigation and associated health and environmental factors in Pakistan: A scoping review. Resourc. Environ. 2022;12:76–87. [Google Scholar]
- 49.Kumari S, Mishra A. Soil Contamination-Threats and Sustainable Solutions. IntechOpen; 2021. [Google Scholar]
- 50.Din IU, Muhammad S, Faisal S, Rehman IU, Ali W. Heavy metal (loid) s contamination and potential risk assessment via groundwater consumption in the district of Hangu Pakistan. Environ. Sci. Pollut. Res. 2023;30:33808–33818. doi: 10.1007/s11356-022-24562-9. [DOI] [PubMed] [Google Scholar]
- 51.Rehman HU, Rahman MU, Ahmed S. Depthwise evaluation of total dissolved solids and arsenic from a drilled borehole near River Ravi, Lahore, Pakistan. J. Water Climate Change. 2023 doi: 10.2166/wcc.2023.170. [DOI] [Google Scholar]
- 52.Cheema SM, et al. IoAT Enabled smart farming: Urdu language-based solution for low-literate farmers. Agriculture. 2022;12:1277. doi: 10.3390/agriculture12081277. [DOI] [Google Scholar]
- 53.Boudebbouz A, et al. Determination of heavy metal levels and health risk assessment of raw cow milk in Guelma Region Algeria. Biol. Trace Element Res. 2023;201:1704–1716. doi: 10.1007/s12011-022-03308-1. [DOI] [PubMed] [Google Scholar]
- 54.Karahan F, et al. Heavy metal levels and mineral nutrient status in different parts of various medicinal plants collected from eastern Mediterranean region of Turkey. Biol. Trace Element Res. 2020;197:316–329. doi: 10.1007/s12011-019-01974-2. [DOI] [PubMed] [Google Scholar]
- 55.Năstăsescu V, et al. Heavy metal and pesticide levels in dairy products: Evaluation of human health risk. Food Chem. Toxicol. 2020;146:111844. doi: 10.1016/j.fct.2020.111844. [DOI] [PubMed] [Google Scholar]
- 56.Tedesco R, et al. Trace and rare earth elements determination in milk whey from the Veneto region Italy. Food Control. 2021;121:107595. doi: 10.1016/j.foodcont.2020.107595. [DOI] [Google Scholar]
- 57.Hannan, E. J. & Deistler, M. The statistical theory of linear systems. In Society for Industrial and Applied Mathematics S. No. 70 (Siam, 2012).
- 58.Ahmed MS, et al. Risk assessment and evaluation of heavy metals concentrations in blood samples of plastic industry workers in Dhaka Bangladesh. Toxicol. Rep. 2020;7:1373–1380. doi: 10.1016/j.toxrep.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shabalala SC, et al. Detrimental effects of lipid peroxidation in type 2 diabetes: Exploring the neutralizing influence of antioxidants. Antioxidants. 2022;11:2071. doi: 10.3390/antiox11102071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Doğan M, Çavuşoğlu K, Yalçin E, Acar A. Comprehensive toxicity screening of Pazarsuyu stream water containing heavy metals and protective role of lycopene. Sci. Rep. 2022;12:16615. doi: 10.1038/s41598-022-21081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The produced, collected, or generated during the study has been given in the manuscript file and its supplementary file.