Abstract
Tepotinib is a highly selective MET tyrosine kinase inhibitor (TKI) that has demonstrated robust and durable clinical activity in patients with MET exon 14 (METex14) skipping non–small‐cell lung cancer (NSCLC). In the Phase II VISION study, patients received oral tepotinib 500 mg once daily. The primary endpoint was an objective response by an independent review committee (IRC) according to RECIST v1.1 criteria. The secondary endpoints included duration of response (DOR), progression‐free survival (PFS), overall survival (OS), and safety. Here we report the analysis of the efficacy and safety of tepotinib in all Japanese patients with advanced METex14 skipping NSCLC from VISION (n = 38) with >18 months' follow‐up. The median age of the Japanese patients was 73 years (range 63–88), 39.5% of patients were ≥75 years old, 68.4% were male, 55.3% had a history of smoking, 76.3% had adenocarcinoma, and 10.5% of patients had known brain metastases at baseline. Overall, the objective response rate (ORR) was 60.5% (95% confidence interval (CI): 43.4, 76.0) with a median DOR of 18.5 months (95% CI: 8.3, not estimable). ORR in treatment‐naïve patients (n = 18) was 77.8% (95% CI: 52.4, 93.6), and in patients aged ≥75 years (n = 15), ORR was 73.3% (95% CI: 44.9, 92.2). The most common treatment‐related adverse event (AE) with any grade was blood creatinine increase (65.8%), which resolved following tepotinib discontinuation. Other common treatment‐related AEs were peripheral edema (60.5%), hypoalbuminemia (34.2%), diarrhea (28.9%), and nausea (15.8%). In summary, tepotinib demonstrated robust and durable clinical activity irrespective of age or therapy line, with a manageable safety profile in Japanese patients with METex14 skipping NSCLC enrolled in VISION.
Keywords: MET receptor tyrosine kinase, METex14 skipping, molecular targeting therapy, non–small‐cell lung cancer, tepotinib
This manuscript presents an extended analysis of efficacy and safety of tepotinib in all Japanese patients from the VISION study with >18 months’ follow‐up. Tepotinib provides robust and durable clinical activity, irrespective of age or therapy line. In addition, tepotinib was well tolerated with mostly mild‐to‐moderate adverse events and a low rate of treatment discontinuations, confirming the manageable safety profile of tepotinib in this population, with no new safety signals.

Abbreviations
- AE
adverse event
- ALK
anaplastic lymphoma kinase
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BM
brain metastases
- CI
confidence interval
- CNS
central nervous system
- CSF
cerebrospinal fluid
- DOR
duration of response
- ECOG PS
Eastern Cooperative Oncology Group performance status
- EGFR
epidermal growth factor receptor
- ILD
interstitial lung disease
- IRC
independent review committee
- LBx
liquid biopsy
- m
median
- MedDRA
Medical Dictionary for Regulatory Activities
- MET
mesenchymal–epithelial transition factor
- METex14
MET exon 14
- ne
not estimable
- NSCLC
non–small‐cell lung cancer
- ORR
objective response rate
- OS
overall survival
- PFS
progression‐free survival
- RECIST
Response Evaluation Criteria in Solid Tumors
- SOLD
sum of longest diameters
- TBx
tissue biopsy
- TKI
tyrosine kinase inhibitor
1. INTRODUCTION
MET exon 14 skipping is an oncogenic driver mutation in NSCLC that occurs in 1–4% of East Asian patients, 1 , 2 , 3 , 4 , 5 and can be treated with selective MET TKIs. One such agent is tepotinib, a highly selective and blood–brain barrier‐penetrating TKI, whose approval in Japan in March 2020 was the first worldwide of a MET inhibitor in unresectable advanced or recurrent NSCLC with METex14 skipping. 6 , 7 , 8 Approval was based on data from an early analysis of the VISION study that included 15 Japanese patients. VISION is a global, multicohort Phase II trial of tepotinib that enrolled patients with METex14 skipping NSCLC. 9 , 10 , 11 In VISION (N = 313; data cut‐off: February 20, 2022), tepotinib had robust and durable clinical activity in patients with METex14 skipping NSCLC, with an ORR of 50.8% (95% confidence interval [CI]: 45.1, 56.5) and median DOR of 18.0 months (95% CI: 12.4, not estimable [ne]). 12 Previous subgroup analyses of Japanese patients in VISION (data cut‐off: January 1, 2020) demonstrated robust and durable clinical efficacy of tepotinib. 6 In Japanese patients (n = 15) with ≥9 months of follow‐up, ORR was 60.0% (95% CI: 32.3, 83.7), and median DOR was not reached (95% CI: 6.9, ne). Tepotinib was well tolerated with mostly mild‐to‐moderate AEs and low rate of treatment discontinuations. An analysis of post‐marketing surveillance conducted in Japan investigating the occurrence of interstitial lung disease, fluid retention, hepatic function disorder, renal impairment, and QT interval prolongation, confirmed the manageable safety profile of tepotinib in this population, with no new safety signals. 13 , 14 , 15 Based on data from the VISION study, the Japanese Lung Cancer Society strongly recommended tepotinib for the treatment of NSCLC with METex14 skipping. 16
Here, we report the extended analysis of efficacy and safety of tepotinib in all Japanese patients from VISION, having a follow‐up of at least 18 months.
2. MATERIALS AND METHODS
The full methodology of the Phase II VISION study has been published previously and the protocol is available online. 6 , 9 , 11
2.1. Study design and objectives
VISION (NCT02864992) is a Phase II, single‐arm, open‐label, multicenter trial conducted in 149 sites from 11 countries, including 13 sites in Japan. The study aimed to assess the antitumor activity and tolerability of tepotinib in patients with advanced NSCLC harboring METex14 skipping.
2.2. Patients
Eligible patients in Japan were ≥20 years of age with histologically or cytologically confirmed advanced (locally advanced or metastatic) NSCLC, measurable disease per RECIST v1.1, and ECOG PS of 0 to 1. METex14 skipping was tested centrally either by TBx by collecting RNA from tumor‐biopsy tissue, or LBx by collecting circulating tumor DNA from plasma. Next‐generation sequencing using the Oncomine™ Focus Assay (52 genes, Thermo Fisher Scientific, Waltham, MA, USA) or the Archer®MET diagnostic assay was carried out to analyze TBx samples, and the Guardant360® assay (73 genes, Guardant Health, Redwood City, CA, USA) or the Archer®MET diagnostic assay (ArcherDX, Boulder, CO, USA) were used for LBx samples. Patients could also be enrolled based on TBx results by LC‐SCRUM 17 (a nationwide cancer genomic screening project for the application of personalized medicine to advanced NSCLC in Japan) and central confirmation was not required to enroll these patients. Patients could have had up to two lines of prior therapy for advanced/metastatic disease (immunotherapy was allowed but prior MET inhibitors were not permitted). Patients with tumors harboring activating EGFR mutations or ALK rearrangements were excluded. Patients with asymptomatic or neurologically stable brain metastases were eligible; however, patients were excluded if they had symptomatic brain metastases, were neurologically unstable, required an increase in steroid dose within 2 weeks, received prior stereotactic radiosurgery/gamma knife within 2 weeks, received other prior treatment for brain metastases within 4 weeks, and/or had leptomeningeal disease.
2.3. Treatment administration
Patients received tepotinib 500 mg once daily with food until disease progression (as assessed according to RECIST v1.1), intolerable toxicity, or withdrawal of consent.
2.4. Study endpoints and assessments
The primary endpoint was an objective response (defined as confirmed complete or partial response) by IRC according to RECIST v1.1 criteria. Confirmation was obtained by a tumor assessment at least 4 weeks (28 days) after an initial tumor assessment, indicating a complete or partial response. Secondary endpoints included DOR, PFS, OS, and safety. Safety was evaluated through physical examinations and clinical laboratory tests. AEs were coded according to the Medical Dictionary for Regulatory Activities (MedDRA) v23.0, and the severity of AEs was graded by the investigator using the National Cancer Institute Common Terminology Criteria for Adverse Events v4.03. Treatment‐emergent AEs were defined as those events that emerged during treatment and were absent at pretreatment or worsened relative to the pretreatment state and occurred within ≥1 day of trial treatment until 30 days after the last dose of trial treatment. AEs related to trial treatment were defined as any AE considered by the investigator as reasonably related to tepotinib.
The assessments for efficacy were conducted every 6 weeks within the first 9 months of treatment (and every 12 weeks thereafter). Tumors were assessed by computed tomography or magnetic resonance imaging of the chest, abdomen, and pelvis. Additional anatomic areas were investigated based on individual signs or symptoms. Subgroup analysis in patients enrolled in Japan was predefined.
2.5. Statistical analysis
Formal statistical comparisons were not conducted; data were analyzed in a descriptive manner. Predefined analysis sets for all endpoints included patients with METex14 skipping detection by TBx, LBx, and either biopsy method (TBx and/or LBx). DOR, PFS, and OS were analyzed using Kaplan–Meier methods. Qualitative variables and rates were summarized by counts and percentages along with two‐sided exact Clopper–Pearson 95% CIs. The efficacy and safety populations included all patients who had enrolled in the study and received at least one dose of tepotinib.
3. RESULTS
3.1. Patients
The median age of Japanese patients (n = 38) was 73 years (range 63–88 years), 39.5% of these patients were ≥75 years old, 68.4% of patients were male, 55.3% had smoking history, adenocarcinoma was the most common histologic subtype (76.3%), and 10.5% of patients had known brain metastases at baseline. Altogether, 18 patients (47.4%) were treatment naïve and 20 patients (52.6%) received tepotinib as second‐or‐later line therapy, of which 12 patients (31.6%) received tepotinib as second line therapy (Table 1). Among patients who received prior therapies, 16 patients (42.1%) received platinum‐based chemotherapy, 13 patients (34.2%) received immunotherapy and three patients (7.9%) received a combination of immunotherapy and chemotherapy. Of 34 patients (89.5%) enrolled by TBx, 21 patients (55.3% of the total) were enrolled through LC‐SCRUM. There were 14 patients (36.8%) who had METex14 skipping detected by LBx, ten of whom also had METex14 skipping detected by TBx. Overall, 22 patients had positive TBx and negative LBx results. There were no patients with a positive LBx and a negative TBx result; TBx results were not available for four patients. Full baseline characteristics are shown in Table 1. The median and mean duration of tepotinib treatment was 6.5 months (range 0.3–50.5 months) and 11.6 months (standard deviation 12.4 months), where five patients had received treatment for more than 2 years.
TABLE 1.
Baseline characteristics of Japanese patients with METex14 skipping NSCLC in VISION.
| Baseline characteristics | Japanese patients (n = 38) |
|---|---|
| Age | |
| Median, years (range) | 73 years (63–88) |
| <65 years, n (%) | 2 (5.3) |
| ≥65 years, n (%) | 36 (94.7) |
| <75 years, n (%) | 23 (60.5) |
| ≥75 years, n, (%) | 15 (39.5) |
| Sex, n (%) | |
| Male | 26 (68.4) |
| Female | 12 (31.6) |
| Smoking history, n (%) | |
| Yes | 21 (55.3) |
| No | 16 (42.1) |
| Histology, n (%) | |
| Adenocarcinoma | 29 (76.3) |
| Squamous | 2 (5.3) |
| Adenosquamous | 1 (2.6) |
| Sarcomatoid | 0 |
| Others a | 6 (15.8) |
| ECOG PS, n (%) | |
| 0 | 14 (36.8) |
| 1 | 24 (63.2) |
| Line of therapy, n (%) | |
| Treatment naïve | 18 (47.4) |
| Previously treated | 20 (52.6) |
| METex14 skipping detection, n (%) b | |
| T+ | 34 (89.5) |
| L+ | 14 (36.8) |
| Brain metastases at baseline, c n (%) | 4 (10.5) |
Other histology included: large cell, adenocarcinoma with sarcomatoid, NSCLC/NSCLC‐NOS, and missing/cannot classify.
In 10 patients, METex14 skipping was detected by both liquid and tissue biopsy.
Identified by IRC or investigator.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; IRC, independent review; committee; L+, METex14 skipping detected in liquid biopsy; METex14, MET exon 14; NOS, not otherwise specified; T+, METex14 skipping detected in tissue biopsies.
3.2. Efficacy
The ORR was 60.5% (95% CI: 43.4, 76.0; Table 2, Figure 1) and the disease control rate was 78.9% (95% CI: 62.7, 90.4). The median DOR for patients with an objective response (n = 23) was 18.5 months (95% CI: 8.3, ne; Table 2, Figure 2). The median PFS was 14.8 months (95% CI: 6.9, ne), and the median OS was 25.5 months (95% CI: 17.7, ne; Table 2, Figure 2). Four patients (10.5%) were still receiving tepotinib at the data cut, 34 patients (89.5%) discontinued tepotinib and, of these, 21 patients (55.3%) received subsequent treatment. Among patients who received subsequent treatment, 13 received one line of subsequent therapy, six received two lines and one patient each received three and four lines of subsequent therapies. Subsequent therapies included chemotherapy (n = 16), immunotherapy (n = 11), anti‐angiogenic monoclonal antibodies (n = 4; three patients received ramucirumab plus docetaxel and one patient received bevacizumab plus pemetrexed), MET‐TKI (n = 2; both patients received tepotinib), and some of the patients also received the combination of these agents (Table S1). The two patients who received subsequent tepotinib had shown partial response as the best overall response to initial tepotinib treatment in the VISION trial before discontinuing due to progressive disease. Thereafter, both patients received chemo‐immunotherapy combination treatment and then again received tepotinib for durations of 2.7 and 10.1 months. Among the 13 patients who did not receive subsequent treatments, four patients discontinued tepotinib due to AEs, all of whom remained alive for several months without further treatment. Other reasons for not receiving subsequent treatments were death, disease progression, withdrawal of consent, and patient choice.
TABLE 2.
Efficacy of tepotinib in Japanese patients with METex14 skipping NSCLC.
| Efficacy | Japanese patients (n = 38) |
|---|---|
| BOR, n (%) | |
| CR | 0 |
| PR | 23 (60.5) |
| SD | 7 (18.4) |
| PD | 6 (15.8) |
| NE | 2 (5.3) |
| ORR, % (95% CI) | 60.5 (43.4, 76.0) |
| DCR, % (95% CI) | 78.9 (62.7, 90.4) |
| mDOR, months (95% CI) | 18.5 (8.3, ne) |
| mPFS, months (95% CI) | 14.8 (6.9, ne) |
| mOS, months (95% CI) | 25.5 (17.7, ne) |
Abbreviations: BOR, best objective response; CI, confidence interval; CR, complete response; DCR, disease control rate; mDOR, median duration of response; mOS, median overall survival; mPFS, median progression‐free survival; NE, not evaluable; ne, not estimable; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease.
FIGURE 1.
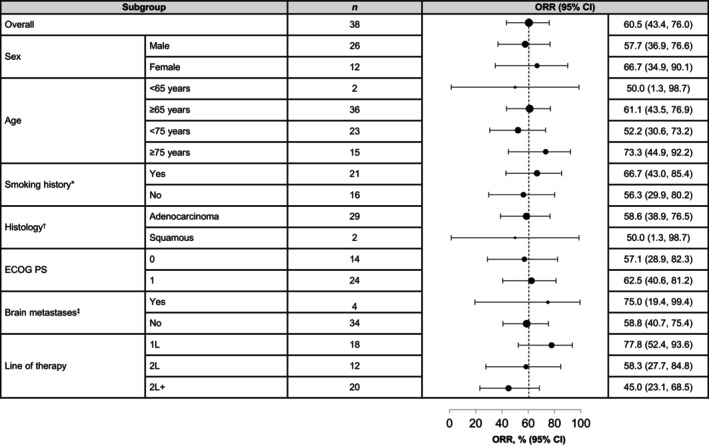
ORR of tepotinib in Japanese patients according to baseline characteristics. *Smoking history was missing for one patient. †Seven patients had histology other than squamous and adenocarcinoma. ‡Identified at baseline (investigator or independent review). 1L, first line; 2L, second line; 2L+, second‐or‐later line; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; METex14, MET exon 14; ORR, objective response rate.
FIGURE 2.
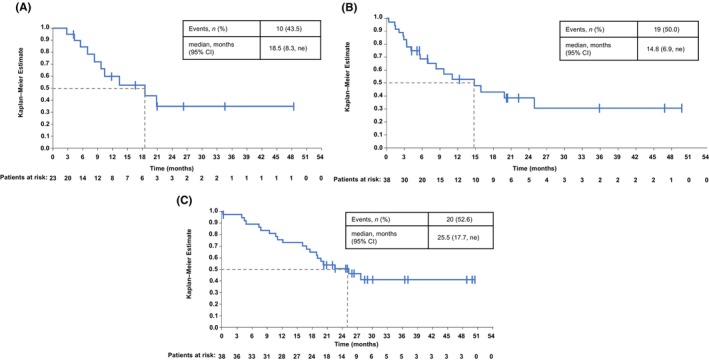
Efficacy of tepotinib in Japanese patients. (A) Duration of response. (B) Progression‐free survival. (C) Overall survival. CI, confidence interval; DOR, duration of response; m, median; ne, not estimable; OS, overall survival; PFS, progression‐free survival.
3.3. Efficacy in subgroups
Although sample sizes were small, ORR was comparable across subgroups according to baseline characteristics (Figure 1). ORR in patients aged <75 years (n = 23) was 52.2% (95% CI: 30.6, 73.2), and was 73.3% (95% CI: 44.9, 92.2) in patients aged ≥75 years (n = 15; Figure 1). The ORR for patients who received tepotinib in the first‐line setting (n = 18) was 77.8% (95% CI: 52.4, 93.6) and patients who received tepotinib as second line (n = 12) and second‐or‐later line therapy (n = 20) had an ORR of 58.3% (95% CI: 27.7, 84.8) and 45.0% (95% CI: 23.1, 68.5), respectively (Figure 1). Almost all patients in the treatment‐naïve subgroup and the majority of patients (84.2%) in the previously treated subgroup had tumor shrinkage (Figure 3). The duration of treatment, as illustrated in the swimmer plot (Figure 4A), was >12 months in 12 patients, including eight treatment‐naïve patients and four previously treated patients. Among these 12 patients with long treatment duration (>12 months), four patients were aged ≥75 years, eight were aged <75 years, nine had adenocarcinoma, and one had squamous cell histology. Of four patients with ongoing treatment, all four patients were treatment naïve and, of these, one patient was ≥75 years of age and three were <75 years of age. At the data cut‐off (February 20, 2022), the treatment duration for the ongoing patients was 30.3, 37.2, 49.0, and 50.5 months. ORR in patients who had brain metastases at baseline (n = 4) was 75.0% (95% CI: 19.4, 99.4). In patients without brain metastases (n = 34), the ORR was 58.8% (95% CI: 40.7, 75.4). [Correction added on 23 March 2024, after first online publication: In section 3.3, the lower 95% CI value of the ORR (objective response rate) of the patients who received tepotinib as second line therapy has been corrected from 27.2 to 27.7 for accuracy with the data presented in Figure 1.]
FIGURE 3.
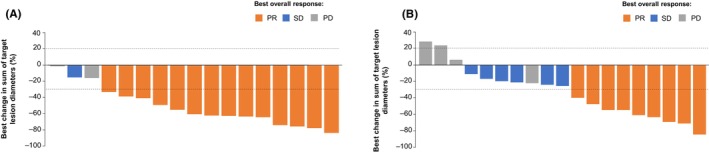
Best percentage change in sum of longest diameters in Japanese patients with METex14 skipping NSCLC*. (A) Treatment‐naïve patients, (B) Previously treated patients. *One treatment‐naïve and one previously treated patient are not shown due to baseline/on‐treatment measurements not being available. PD, progressive disease; PR, partial response; SD, stable disease.
FIGURE 4.
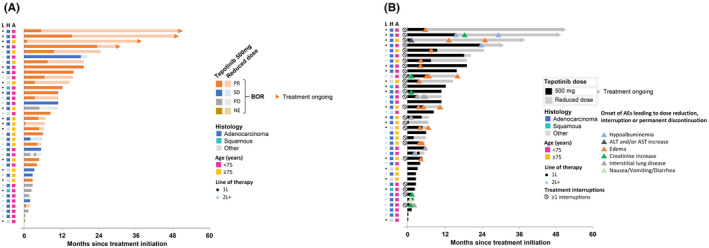
Swimmer plot showing (A) Duration of tepotinib treatment and best overall response and (B) duration of tepotinib treatment and timing of adverse events in Japanese patients. 1L, first line; 2L+, second‐or‐later line; A, age; AE, adverse event; ALT, alanine transaminase; AST, aspartate transaminase; BOR, best overall response; H, histology; L, line of therapy; NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease.
3.4. Efficacy by METex14 skipping detection method
In patients who were enrolled by TBx (n = 34), ORR was 58.8% (95% CI: 40.7, 75.4) with a median DOR of 13.4 months (95% CI: 8.3, ne), a median PFS of 14.8 months (95% CI: 5.6, ne), and a median OS of 25.5 months (95% CI: 17.7, ne; Table S2, Figures S1–S3). In patients who were enrolled by LBx (n = 14), ORR was 71.4% (95% CI: 41.9, 91.6) with a median DOR of 9.7 months (95% CI: 2.8, ne), a median PFS of 9.6 months (95% CI: 3.3, ne), and a median OS of 19.6 months (95% CI: 10.9, ne; Table S2, Figures S1–S3). Patients who were enrolled by TBx had lower median SOLD of target lesions by IRC (45.7 mm [range: 14.3–180.2 mm]), which may indicate lower overall tumor load compared with patients who were enrolled by LBx (59.9 mm [range: 14.3–180.2 mm]) at baseline.
3.5. Safety
Treatment‐related AEs with any grade were observed in 97.4% of patients and 50.0% of patients had Grade ≥3 treatment‐related AEs (Table 3). The most common treatment‐related AEs were blood creatinine increase, peripheral edema, hypoalbuminemia, and diarrhea (Table 3). Changes in creatinine levels from baseline to the end of treatment and follow‐up are shown in Figure 5. The figure demonstrates a trend for creatinine levels to increase while on treatment with tepotinib, which returned to baseline values after treatment discontinuation. The majority of patients had at least one dose reduction or interruption, as shown in Figure 4B. Adverse events of particular interest (hypoalbuminemia, ALT and/or AST increase, edema, creatinine increase, ILD, nausea/vomiting/diarrhea) led to dose reduction, treatment interruptions or permanent treatment discontinuation in 25 patients. Of 25 patients who had a blood creatinine increase, 20 patients (80%) continued tepotinib without dose reduction or interruption. The other five patients (20%) continued tepotinib with dose reduction or interruption and, of these, none permanently discontinued tepotinib due to creatinine increase (Figure 5C). Among the 23 patients who developed edema, 11 patients (48%) continued treatment with tepotinib without dose reduction or interruption; whereas 12 patients (52%) who developed edema continued tepotinib with dose reduction or interruption and, of these, one patient discontinued tepotinib due to edema. Permanent treatment discontinuation was also reported due to ILD (n = 2). As illustrated in the swimmer plot, 10 patients did not have any treatment interruption or dose reduction, six of these patients were < 75 years of age, whereas four were ≥ 75 years of age. Overall, most AEs were manageable and did not lead to discontinuation.
TABLE 3.
Safety of tepotinib in Japanese patients.
| AEs in Japanese patients (n = 38) | All‐cause AEs, n (%) | Treatment‐related AEs, n (%) |
|---|---|---|
| Any grade | 38 (100.0) | 37 (97.4) |
| Grade ≥3 | 23 (60.5) | 19 (50.0) |
| Leading to death | 3 a (7.9) | 0 |
| Treatment‐related AEs (any grade) occurring in ≥10% of patients, n (%) | ||
|---|---|---|
| Any grade | Grade ≥ 3 | |
| Blood creatinine increased | 25 (65.8) | 0 |
| Peripheral edema | 23 (60.5) | 5 (13.2) |
| Hypoalbuminemia | 13 (34.2) | 4 (10.5) |
| Diarrhea | 11 (28.9) | 1 (2.6) |
| Nausea | 6 (15.8) | 0 |
| Alanine aminotransferase increased | 6 (15.8) | 0 |
| Amylase increased | 6 (15.8) | 1 (2.6) |
| Aspartate aminotransferase increased | 5 (13.2) | 0 |
| Dysgeusia | 5 (13.2) | 0 |
| Lipase increased | 5 (13.2) | 1 (2.6) |
| Edema | 5 (13.2) | 1 (2.6) |
| Anemia | 4 (10.5) | 1 (2.6) |
| White blood cell count decreased | 4 (10.5) | 0 |
| Decreased appetite | 4 (10.5) | 1 (2.6) |
| Interstitial lung disease | 4 (10.5) | 0 |
| Pruritus | 4 (10.5) | 0 |
Abbreviation: AEs, adverse events.
Two patients died due to disease progression and one patient died due to pulmonary hemorrhage.
FIGURE 5.
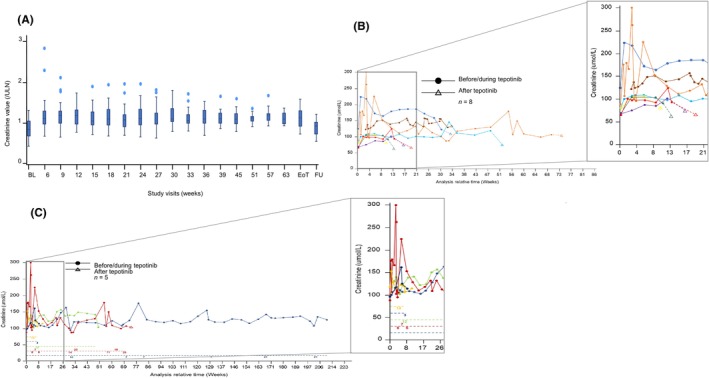
Creatinine levels after initiating and discontinuation of tepotinib in Japanese patients. (A) Box plots* for creatinine values over time; (B) Line graph showing creatinine values before and after tepotinib in patients with post‐treatment data available; (C) Line graph showing creatinine value before and after tepotinib in patients who had treatment interruptions† due to blood creatinine increase. *Time points with data from 10 or fewer patients are not presented, with the exception of baseline, end of treatment and 30‐day safety follow‐up visits. †In (C), the positioning of numbers along the dashed line reflects the timings of treatment interruptions, with values corresponding to the number of interruption days at each instance. 1L, first line; 2L+, second‐or‐later line, EoT, end of treatment; FU, 30‐day safety follow‐up.
4. DISCUSSION
In this extended analysis of Japanese patients (n = 38) with METex14 skipping NSCLC in VISION, with a follow‐up of at least 18 months, tepotinib demonstrated robust and durable clinical efficacy, overall and across clinical subgroups defined by age, line of therapy, METex14 skipping detection method or other variables, with a manageable safety profile.
Efficacy was clinically meaningful in treatment naïve (n = 18, ORR: 77.8%) and in previously treated patients (n = 20; ORR: 45.0%). Efficacy outcomes in this extended Japanese population with longer follow‐up confirmed previous results and further supported the approval and use of tepotinib in clinical practice. 9 , 10 Another MET‐TKI, capmatinib, is approved in Japan for METex14 skipping NSCLC. The GEOMETRY mono‐1 study of capmatinib reported efficacy in both treatment naïve (n = 2, ORR: 50.0%) and previously treated (n = 11, ORR: 36.4%) Japanese patients. 18
The outcomes from VISION are noteworthy as the study involves an elderly population with a median age of 73 years. 10 , 13 Older patients can be more challenging to treat owing to age‐related frailty, comorbid conditions, and simultaneous use of multiple medications for treating comorbidities. In addition, the Japanese guidelines for NSCLC provide only weak evidence for the use of chemotherapy. 18 , 19 However, the guidelines strongly recommend monotherapy with tepotinib or capmatinib for patients with METex14 skipping NSCLC. 19 In the present analysis of tepotinib, patients aged ≥75 years attained a high ORR of 73.3%, which may partly reflect the greater proportion of patients in this subgroup who received tepotinib as first‐line therapy (60.0%, compared with 39.1% of patients aged <75 years). The data demonstrate clinical benefit in patients aged ≥75 years and support the use of tepotinib in older patients.
In line with the overall VISION study population, most of the Japanese patients were enrolled by TBx. Although both LBx and TBx can be used for detecting METex14 skipping, the analysis derived from TBx may be more commonly used and accepted. 20 Our results in Japanese patients demonstrate that patients enrolled by either TBx or LBx had clinically meaningful outcomes with tepotinib, which were particularly durable in patients enrolled with TBx. A higher tumor load in the LBx population may be the reason behind shorter time‐dependent endpoints in the LBx population. 11 However, the small sample size in this population limited the comparison.
In the overall VISION analysis (N = 313), tepotinib demonstrated clinically meaningful systemic efficacy and intracranial activity. 12 The results from the subgroup analysis here also demonstrate encouraging systemic efficacy in Japanese patients with brain metastases, although intracranial efficacy was not investigated due to the small subgroup size. The clinical benefit of tepotinib in Japanese patients with central nervous system (CNS) metastasis is further supported by case reports from four Japanese patients with METex14 skipping NSCLC with brain metastases or leptomeningeal disease, who exhibited intracranial radiographic responses to tepotinib treatment, some of which were dramatic. 21 , 22 , 23 , 24 Cerebrospinal fluid (CSF) analyses in two of these patients demonstrated relevant blood–brain barrier penetration of tepotinib, with CSF‐to‐plasma ratios of 1.19–1.83%. 23 , 24 Taken together, these data indicated that tepotinib can be of benefit to Japanese patients with advanced NSCLC harboring METex14 skipping having brain metastases.
In the present analysis from VISION with an extended follow‐up of at least 18 months, tepotinib demonstrated a tolerable safety profile. Blood creatinine increase was the most common treatment‐related AE of any grade (65.8%) but was mild to moderate with no Grade 3 events being reported. The frequent occurrence of blood creatinine increase was in agreement with the previous analysis in Japanese patients with tepotinib, 6 and the GEOMETRY mono‐1 study of capmatinib. 18 The higher frequency of AE reports of blood creatinine increase in Japanese patients (65.8%) compared with the global population (21.7%) may be a result of different medical practices to report laboratory abnormalities as AEs in Japan. 25 However, there was no evidence that creatinine increase was associated with renal impairment in the safety analysis of the overall VISION population. 26 , 27 This is also supported by the creatinine level changes in Japanese patients, demonstrating the reversibility of creatinine increases with tepotinib treatment. There were also no treatment discontinuations in Japanese patients due to creatinine increase. A series of case studies has distinguished asymptomatic creatinine increase with tepotinib or capmatinib from acute kidney injury using non‐creatinine‐based methods to estimate glomerular filtration rate. 28 , 29 , 30 A potential explanation for observed creatinine increases could be that serum creatinine is increased by inhibition of renal transporters. 27 , 28 , 29 , 31 , 32 Studies suggest that tepotinib or its main metabolite inhibit the renal tubular transporter proteins, organic cation transporter 2 and multidrug and toxin extrusion transporters 1 and 2 and, as a result, creatinine, being the substrate of these inhibitors, increases. 33
Peripheral edema considered a class effect of MET inhibitors, 26 was the second most common treatment‐related AE (of any grade) with an occurrence of 60.5% in Japanese patients in VISION. A recent post‐marketing surveillance analysis of tepotinib in 147 patients reported peripheral edema in 37.4% of patients, reflecting the occurrence of edema with tepotinib in real‐world practice. 14 , 15 An exposure–response analysis of tepotinib identified an association between advanced age and increased risk of edema, independent of tepotinib exposure, while reporting a flat relationship within the observed exposure range (500 mg daily) between tepotinib exposure and edema. 27 Treatment interruptions (65.8%) and dose reductions (52.6%) enabled patients with AEs to remain on treatment, including five patients for more than 2 years.
The main limitation of the study is that VISION is an open‐label, non‐randomized study, with no direct comparison with other available therapies. The overall sample size in this study is a strength, given the rarity of patients with advanced NSCLC harboring METex14 skipping and the scarcity of the data for this population. Outcomes in subgroups of Japanese patients according to clinical characteristics should, however, be interpreted cautiously considering the limited sizes.
In conclusion, tepotinib demonstrated robust and durable clinical activity, with a manageable safety profile, in Japanese patients with METex14 skipping NSCLC enrolled in VISION. Notably, clinical benefits were observed across clinical subgroups including age, treatment line, and METex14 skipping detection method.
AUTHOR CONTRIBUTIONS
Masahiro Morise: Conceptualization; investigation; validation; writing – review and editing. Terufumi Kato: Conceptualization; investigation; validation; writing – review and editing. Shingo Matsumoto: Investigaton; validation; writing – review and editing. Takako Inoue: Investigation; validation; writing – review and editing. Tomohiro Sakamoto: Investigation; validation; writing – review and editing. Takaaki Tokito: Investigation; validation; writing – review and editing. Shinji Atagi: Investigation; validation; writing – review and editing. Toshiyuki Kozuki: Conceptualization; investigation; validation; writing – review and editing. Hiroaki Takeoka: Investigation; validation; writing – review and editing. Kenichi Chikamori: Investigation; validation; writing – review and editing. Naofumi Shinagawa: Investigation; validation; writing – review and editing. Hiroshi Tanaka: Investigation; validation; writing – review and editing. Eisuke Horii: Conceptualization; methodology; validation; writing – review and editing. Svenja Adrian: Conceptualization; data curation; methodology; validation; writing – review and editing. Rolf Bruns: Conceptualization; data curation; formal analysis; methodology; validation; writing – review and editing. Andreas Johne: Conceptualization; methodology; validation; writing – review and editing. Paul K. Paik: Conceptualization; investigation; validation; writing – review and editing. Hiroshi Sakai: Conceptualization; investigation; validation; writing – review and editing.
CONFLICT OF INTEREST STATEMENT
MM: Chugai, MSD, ONO, AstraZeneca; T Kato: Merck Biopharma Co., Ltd., Tokyo, Japan, an affiliate of Merck KGaA, Regeneron, AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Chugai, Eli Lilly, MSD, Novartis, Ono, Pfizer, Taiho, Boehringer Ingelheim, Daiichi‐Sankyo, Nippon Kayaku, Takeda; SM: AstraZeneca, Pfizer, Novartis, Chugai Pharma, MSD, Chugai Pharma, Novartis, Lilly, Merck Biopharma Co., Ltd., Tokyo, Japan, an affiliate of Merck KGaA, MSD; TI: AstraZeneca, Bristol Myers Squibb, Chugai, Daiichi‐Sankyo, MSD, Ono, and Takeda; TS: Nothing to disclose; TT: Nothing to disclose; S Atagi: Nothing to disclose; T Kozuki: Chugai Pharmaceutical Co., AstraZeneca, Eli Lilly Japan, Taiho Pharmaceutical Co., Bristol Myers Squibb, MSD, Kyowa Hakko Kirin, Ono Pharmaceutical Co., Pfizer Japan, Nippon Boehringer Ingelheim, Nippon Kayaku, Novartis, Daiichi‐Sankyo, Merck Biopharma Co., Ltd., Tokyo, Japan, an affiliate of Merck KGaA; HT: Nothing to disclose; KC: Nothing to disclose; NS: Boehringer Ingelheim Japan, Olympus, and AstraZeneca; HT: Chugai Pharmaceutical Co., AstraZeneca; EH: Employee of Merck Biopharma Co., Ltd., Tokyo, Japan, an affiliate of Merck KGaA; S Adrian: Employee of Merck; RB: Employee of Merck. Mr Bruns holds stock in Merck; AJ: Employee of Merck. Mr Johne holds stock in Merck; PP: IDEOlogy, Touch IME, Excerpta Medica, ACE Oncology, Physicians Education Resource, Medscape, Agile, Axis Medical Education, PeerVoice, Aptitude Health, MJH, Annenberg Center, Cardinal Health, Novartis, Mirati, Janssen, Bicara, EMD Serono, Inc., Rockland, MA, USA, an affiliate of Merck KGaA; HS: BMS, Ono Pharmaceutical, MSD K.K., AstraZeneca, Chugai Pharmaceutical Co., Taiho Pharmaceutical, Boehringer Ingelheim.
FUNDING INFORMATION
This study was sponsored by Merck (CrossRef Funder ID: 10.13039/100009945).
ETHICS STATEMENT
Approval of the research protocol by an Institutional Reviewer Board: The study was approved by the institutional review board or independent ethics committee of each center.
Informed Consent: The study was conducted in accordance with the Declaration of Helsinki, International Conference on Harmonization Good Clinical Practice, local laws, and applicable regulatory requirements. All patients provided written informed consent for participation.
Registry and the Registration No. of the study/trial: NCT02864992; https://clinicaltrials.gov/ct2/show/NCT02864992.
Animal Studies: N/A.
Supporting information
Table S1.
Table S2.
Figure S1.
Figure S2.
Figure S3.
ACKNOWLEDGMENTS
The authors would like to thank patients and their families, investigators, co‐investigators, and the study teams at all participating centers, as well as Merck. Medical writing assistance (funded by Merck) was provided by Bhartendu K Srivastava, PhD, of Syneos Health, UK.
Morise M, Kato T, Matsumoto S, et al. Long‐term experience with tepotinib in Japanese patients with MET exon 14 skipping NSCLC from the Phase II VISION study. Cancer Sci. 2024;115:1296‐1305. doi: 10.1111/cas.16107
DATA AVAILABILITY STATEMENT
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to Merck's (CrossRef Funder ID: 10.13039/100009945) Data Sharing Policy. All requests should be submitted in writing to Merck's data‐sharing portal (https://www.merckgroup.com/en/research/our‐approach‐to‐research‐and‐development/healthcare/clinical‐trials/commitment‐responsible‐data‐sharing.html). When Merck has a co‐research, co‐development, or co‐marketing or co‐promotion agreement, or when the product has been out‐licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, Merck will endeavor to gain agreement to share data in response to requests.
REFERENCES
- 1. Lee GD, Lee SE, Oh DY, et al. MET exon 14 skipping mutations in lung adenocarcinoma: Clinicopathologic implications and prognostic values. J Thorac Oncol. 2017;12(8):1233‐1246. doi: 10.1016/j.jtho.2017.04.031 [DOI] [PubMed] [Google Scholar]
- 2. Gow CH, Hsieh MS, Wu SG, Shih JY. A comprehensive analysis of clinical outcomes in lung cancer patients harboring a MET exon 14 skipping mutation compared to other driver mutations in an East Asian population. Lung Cancer. 2017;103:82‐89. doi: 10.1016/j.lungcan.2016.12.001 [DOI] [PubMed] [Google Scholar]
- 3. Liu SY, Gou LY, Li AN, et al. The unique characteristics of MET exon 14 mutation in Chinese patients with NSCLC. J Thorac Oncol. 2016;11(9):1503‐1510. doi: 10.1016/j.jtho.2016.05.016 [DOI] [PubMed] [Google Scholar]
- 4. Sugimoto A, Matsumoto S, Udagawa H, et al. A large‐scale prospective concordance study of plasma‐ and tissue‐based next‐generation targeted sequencing for advanced non–small cell lung cancer (LC‐SCRUM‐Liquid). Clin Cancer Res. 2023;29(8):1506‐1514. doi: 10.1158/1078-0432.CCR-22-1749 [DOI] [PubMed] [Google Scholar]
- 5. Sunami K, Furuta K, Tsuta K, et al. Multiplex diagnosis of oncogenic fusion and MET exon skipping by molecular counting using formalin‐fixed paraffin embedded lung adenocarcinoma tissues. J Thorac Oncol. 2016;11(2):203‐212. doi: 10.1016/j.jtho.2015.10.005 [DOI] [PubMed] [Google Scholar]
- 6. Sakai H, Morise M, Kato T, et al. Tepotinib in patients with NSCLC harboring MET exon 14 skipping: Japanese subset analysis from the phase II VISION study. Jpn J Clin Oncol. 2021;52(8):1‐9. doi: 10.1093/jjco/hyab072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shitara K, Yamazaki K, Tsushima T, et al. Phase I trial of the MET inhibitor tepotinib in Japanese patients with solid tumors. Jpn J Clin Oncol. 2020;50(8):859‐866. doi: 10.1093/jjco/hyaa042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tanaka M, Idei M, Sakaguchi H, et al. Achievements and challenges of the Sakigake designation system in Japan. Br J Clin Pharmacol. 2021;87(10):4027‐4035. doi: 10.1111/bcp.14807 [DOI] [PubMed] [Google Scholar]
- 9. Paik P, Felip E, Veillon R, et al. Tepotinib in non‐small‐cell lung cancer with MET exon 14 skipping mutations. N Engl J Med. 2020;383(10):931‐943. doi: 10.1056/NEJMoa2004407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Le X, Sakai H, Felip E, et al. Tepotinib efficacy and safety in patients with MET exon 14 skipping NSCLC: outcomes in patient subgroups from the VISION study with relevance for clinical practice. Clin Cancer Res. 2022;28(6):1117‐1126. doi: 10.1158/1078-0432.CCR-21-2733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mazieres J, Paik PK, Garassino MC, et al. Tepotinib treatment in patients with MET exon 14–skipping non–small cell lung cancer: long‐term follow‐up of the VISION phase 2 nonrandomized clinical trial. JAMA Oncol. 2023;9(9):1260‐1266. doi: 10.1001/JAMAONCOL.2023.1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thomas M, Garassino MC, Felip E, et al. Tepotinib in patients with MET exon 14 (METex14) skipping NSCLC: primary analysis of the confirmatory VISION cohort C. J Thorac Oncol. 2022;17(9):S9‐S10. doi: 10.1016/j.jtho.2022.07.024 [DOI] [Google Scholar]
- 13. Kato T, Ogura T, Sato M, et al. Post marketing surveillance of Tepotinib (kinase inhibitor indicated for the treatment of non‐small cell lung cancer harboring MET exon 14 skipping alterations)_ interim analysis. The 62nd Annual Meeting of the Japan Lung Cancer Society. JLCS; 2021. [Google Scholar]
- 14. Kato T, Ogura T, Sato M, et al. Post marketing surveillance of Tepotinib (kinase inhibitor indicated for the treatment of non‐small cell lung cancer harboring MET exon 14 skipping alterations)_ 2nd interim analysis. The 63rd Annual Meeting of the Japan Lung Cancer Society. JLCS; 2022. [Google Scholar]
- 15. Kato T, Ogura T, Sato M, et al. Post marketing surveillance of Tepotinib (kinase inhibitor for unresectable, advanced or recurrent NSCLC with METex14 skipping alterations)_ final analysis. In: 2023 the Japanese Society of Medical Oncology Annual Meeting (JSMO2023); 2023.
- 16. Yatabe Y, Goto K, Matsumoto S, et al. METex14 skipping testing guidance for lung cancer patients: the guidance from the biomarker committee, the Japan Lung Cancer Society. Lung Cancer. 2021;61(5):361‐370. doi: 10.2482/haigan.61.361 [DOI] [Google Scholar]
- 17. Takeda M, Sakai K, Takahama T, Fukuoka K, Nakagawa K, Nishio K. New era for next‐generation sequencing in Japan. Cancers (Basel). 2019;11(6):742. doi: 10.3390/cancers11060742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seto T, Ohashi K, Sugawara S, et al. A phase 2 study of capmatinib in patients with METex14‐mutated advanced NSCLC (GEOMETRY mono‐1): Japanese subgroup analysis. Jpn J Lung Cancer. 2019;59(6):538. [Google Scholar]
- 19. The Japan Lung Cancer Society . [Guidelines for the treatment of lung cancer 2020 edition]. Available at: https://www.haigan.gr.jp/modules/guideline/index.php?content_id=3
- 20. Ahn MJ, Mendoza MJL, Pavlakis N, et al. Asian Thoracic Oncology Research Group (ATORG) expert consensus statement on MET alterations in NSCLC: diagnostic and therapeutic considerations. Clin Lung Cancer. 2022;23(8):670‐685. doi: 10.1016/j.cllc.2022.07.012 [DOI] [PubMed] [Google Scholar]
- 21. Takamori S, Matsubara T, Fujishita T, et al. Dramatic intracranial response to tepotinib in a patient with lung adenocarcinoma harboring MET exon 14 skipping mutation. Thorac Cancer. 2021;12:978‐980. doi: 10.1111/1759-7714.13871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hashiguchi MH, Sato T, Yamamoto H, et al. Successful tepotinib challenge after capmatinib‐induced interstitial lung disease in a patient with lung adenocarcinoma harboring MET exon 14 skipping mutation: case report. JTO Clin Res Rep. 2022;3(2):100271. doi: 10.1016/j.jtocrr.2021.100271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ninomaru T, Okada H, Fujishima M, Irie K, Fukushima S, Hata A. Lazarus response to tepotinib for leptomeningeal metastases in a patient with MET exon 14 skipping mutation–positive lung adenocarcinoma: case report. JTO Clin Res Rep. 2021;2(3):100145. doi: 10.1016/j.jtocrr.2021.100145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tanaka H, Taima K, Makiguchi T, Nakagawa J, Niioka T, Tasaka S. Activity and bioavailability of tepotinib for leptomeningeal metastasis of NSCLC with MET exon 14 skipping mutation. Cancer Commun. 2021;41(1):83‐87. doi: 10.1002/cac2.12124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wakao R, Taavola H, Sandberg L, et al. Data‐driven identification of adverse event reporting patterns for Japan in VigiBase, the WHO global database of individual case safety reports. Drug Saf. 2019;42(12):1487‐1498. doi: 10.1007/s40264-019-00861-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Veillon R, Sakai H, Le X, et al. Safety of tepotinib in patients with MET exon 14 skipping NSCLC and recommendations for management. Clin Lung Cancer. 2022;23(4):320‐332. doi: 10.1016/j.cllc.2022.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xiong W, Hietala SF, Nyberg J, et al. Exposure‐response analyses for the MET inhibitor tepotinib including patients in the pivotal VISION trial: support for dosage recommendations. Cancer Chemother Pharmacol. 2022;90(1):53‐69. doi: 10.1007/S00280-022-04441-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mohan A, Herrmann S. Capmatinib‐induced pseudo‐acute kidney injury: a case report. Am J Kidney Dis. 2021;79(1):120‐124. doi: 10.1053/J.AJKD.2021.04.009 [DOI] [PubMed] [Google Scholar]
- 29. Wijtvliet V, Roosens L, De Bondt C, Janssens A, Abramowicz D. Pseudo‐acute kidney injury secondary to tepotinib. Clin Kidney J. 2022;16(4):760‐761. doi: 10.1093/ckj/sfac180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sandoval L, Radhakrishnan Y, Vaughan LE, Potter A, Mansfield AS, Herrmann SM. Capmatinib‐associated pseudoacute kidney injury in nonsmall cell lung cancer. Kidney Int Rep. 2023;8(11):2482‐2485. doi: 10.1016/J.EKIR.2023.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mathialagan S, Rodrigues AD, Feng B. Evaluation of renal transporter inhibition using creatinine as a substrate in vitro to assess the clinical risk of elevated serum creatinine. J Pharm Sci. 2017;106(9):2535‐2541. doi: 10.1016/J.XPHS.2017.04.009 [DOI] [PubMed] [Google Scholar]
- 32. Omote S, Matsuoka N, Arakawa H, Nakanishi T, Tamai I. Effect of tyrosine kinase inhibitors on renal handling of creatinine by MATE1. Sci Rep. 2018;8(1):9237. doi: 10.1038/s41598-018-27672-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. European Medicines Agency . TEPMETKO (Tepotinib) Product Information. 2022. https://www.ema.europa.eu/en/documents/product‐information/tepmetko‐epar‐product‐information_en.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Table S2.
Figure S1.
Figure S2.
Figure S3.
Data Availability Statement
Any requests for data by qualified scientific and medical researchers for legitimate research purposes will be subject to Merck's (CrossRef Funder ID: 10.13039/100009945) Data Sharing Policy. All requests should be submitted in writing to Merck's data‐sharing portal (https://www.merckgroup.com/en/research/our‐approach‐to‐research‐and‐development/healthcare/clinical‐trials/commitment‐responsible‐data‐sharing.html). When Merck has a co‐research, co‐development, or co‐marketing or co‐promotion agreement, or when the product has been out‐licensed, the responsibility for disclosure might be dependent on the agreement between parties. Under these circumstances, Merck will endeavor to gain agreement to share data in response to requests.


