Abstract
Oncolytic viruses (OVs) possess the unique ability to selectively replicate within tumor cells, leading to their destruction, while also reversing the immunosuppression within the tumor microenvironment and triggering an antitumor immune response. As a result, OVs have emerged as one of the most promising approaches in cancer therapy. However, the effective delivery of intravenously administered OVs faces significant challenges imposed by various immune cells within the peripheral blood, hindering their access to tumor sites. Notably, neutrophils, the predominant white blood cell population comprising approximately 50%–70% of circulating white cells in humans, show phagocytic properties. Our investigation revealed that the majority of oncolytic vaccinia viruses (VV) are engulfed and degraded by neutrophils in the bloodstream. The depletion of neutrophils using the anti‐LY6G Ab (1‐A8) resulted in an increased accumulation of circulating oncolytic VV in the peripheral blood and enhanced deposition at the tumor site, consequently amplifying the antitumor effect. Neutrophils heavily rely on PI3K signaling to sustain their phagocytic process. Additionally, our study determined that the inhibition of the PI3Kinase delta isoform by idelalisib (CAL‐101) suppressed the uptake of oncolytic VV by neutrophils. This inhibition led to a greater presence of oncolytic VV in both the peripheral blood and at the tumor site, resulting in improved efficacy against the tumor. In conclusion, our study showed that inhibiting neutrophil functions can significantly enhance the antitumor efficacy of intravenous oncolytic VV.
Keywords: drug delivery, intravenous delivery, neutrophil, oncolytic vaccinia virus, PI3Kinase delta inhibitor
Neutrophils present in the peripheral blood play a crucial role in the uptake and degradation of oncolytic vaccinia viruses (VV) following intravenous administration. This process leads to a reduction in the circulating levels of oncolytic VV, ultimately impacting the availability of the virus to penetrate tumor tissues and exert its antitumor effects. Our research has determined that the depletion of neutrophils through Ab‐mediated elimination or the inhibition of neutrophil activity using CAL‐101, a specific inhibitor targeting PI3Kinase delta isoform, results in decreased uptake of oncolytic VV by neutrophils. Consequently, this leads to an increase in the viral load within peripheral plasma and tumor tissues, thereby enhancing the antitumor effect of intravenously administered oncolytic VV.
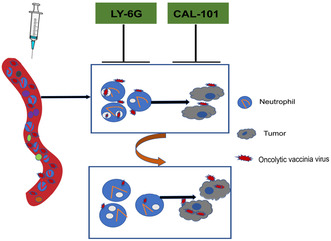
1. INTRODUCTION
In the last decade, oncolytic viruses (OVs) have emerged as a promising approach in cancer immunotherapy, demonstrating notable success. 1 , 2 Clinically tested or approved OVs have shown the ability to target diverse tumor types, including head and neck cancer, 3 , 4 metastatic melanoma, 5 , 6 glioblastoma, 7 , 8 and other malignancies. However, the conventional method of administering OVs through intratumoral injection, despite being effective to some extent, poses limitations in treating tumors that are challenging to access. Therefore, there is an urgent need to develop intravenous injection as an alternative route. 9 , 10 Among the various types of OVs, vaccinia virus (VV) has garnered significant attention in clinical treatment development due to its wide tumor tropism, capacity to accommodate numerous foreign gene sequences, replication within the host cell's cytoplasm, and resistance to neutralization. 11 , 12 , 13 Intravenous injection of oncolytic VV has undergone clinical testing for the treatment of various tumors, including advanced soft tissue sarcoma, pancreatic neuroendocrine and metastatic tumors, and advanced breast cancer. 14 , 15 , 16 However, before oncolytic VV can reach the intended tumor site, it must surmount the obstacles presented by various immune cells in the peripheral blood. Neutrophils, the most abundant immune cells in human peripheral blood, 17 , 18 possess the capability to engulf pathogens and act as antigen‐presenting cells. 19 , 20 Although only a few studies have investigated the association between neutrophils and the efficacy of OVs in the past, notable findings have emerged. In the context of oncolytic adenovirus therapy, patients with a low neutrophil to lymphocyte ratio showed significantly prolonged overall survival rates. 21 In an animal model involving the transplantation of a specific B cell malignant tumor cell line, the antitumor effect of oncolytic measles virus was impacted by neutrophil depletion. 22 Moreover, the administration of recombinant VV in subcutaneous pancreatic tumors in mice and clinical patients with malignant mesothelioma revealed an abundance of neutrophils within tumor tissues. 23 , 24 However, the impact of neutrophils on the efficacy of intravenously delivered oncolytic VV in tumor treatment remains poorly understood. In this study, we observed that the majority of oncolytic VV particles were phagocytosed and degraded by neutrophils in the bloodstream. Notably, the depletion of neutrophils using anti‐LY6G Ab (1‐A8) or the inhibition of PI3Kinase delta with idelalisib (CAL‐101) resulted in increased viral load of oncolytic VV both in the peripheral blood and at the tumor site, thereby enhancing the antitumor effect. These findings shed light on the intricate interplay between neutrophils and oncolytic VV, providing potential avenues for optimizing therapeutic outcomes.
2. MATERIALS AND METHODS
2.1. Cell lines and cell culture
Detailed descriptions of the information of cell lines and cell culture can be found in Appendix S1.
2.2. Viruses
The original virus was a gift from Shenzhen Huayao Kangming Biopharmaceutical Co., Ltd. It was constructed by deleting the TK region, and inserting the GFP gene into the A46R region of VV under the control of the early late stage vaccinia promoter H5 (Figure S1A).
2.3. Acquisition of human primary leukocyte subsets
A detailed description of the method is provided in Appendix S1.
2.4. DNA extraction and quantitative PCR
The analysis method and primer sequence are shown in Appendix S1.
2.5. Western blot analysis
The analysis method and Abs are shown in Appendix S1.
2.6. Animal studies
The research scheme was approved by the Ethics Supervision Committee of Zhengzhou University, and animal care was in accordance with Zhengzhou University guidelines (License ZZU‐LAC20211210[06]). A detailed description of animal experiments can be found in Appendix S1.
2.7. Immunohistochemistry
The analysis method is shown in Appendix S1.
2.8. Live viral replication assay (TCID50)
The analysis method is shown in Appendix S1.
2.9. Transmission electron microscopy
The analysis method is shown in Appendix S1.
2.10. Statistical analysis
A detailed description of the method is provided in Appendix S1.
3. RESULTS
3.1. Neutrophils uptake oncolytic VV in vitro and in vivo
To investigate the correlation between neutrophils and oncolytic VV, we undertook experiments using whole‐blood samples obtained from healthy donors. These samples were incubated with oncolytic VV, and subsequent quantitative PCR (qPCR) analysis with a specific primer targeting the virus confirmed a decrease in the number of oncolytic VV in the culture medium compared to the total amount of virus initially added (Figure 1A). Additionally, we examined the viral load within neutrophils isolated from the peripheral blood of three healthy individuals. The findings revealed the presence of oncolytic VV genetic material in neutrophils. This suggests that neutrophils in the bloodstream are capable of absorbing the oncolytic VV, as shown in Figure 1A. These findings are consistent with previous research outcomes. 25 To investigate whether this phenomenon occurs in patients with surgically challenging tumors, we collected neutrophils from the peripheral blood of patients with pancreatic ductal adenocarcinoma using the same methodology. Comparable to the findings in healthy subjects, there was a reduction in the quantity of oncolytic VV in the group where the virus and cells were coincubated, relative to the group that received the full dose of the virus. In the group where the virus and cells were coincubated, the genomic DNA of the oncolytic VV was detected, unlike in the neutrophil‐only group, as shown in Figure 1B. Considering that neutrophil activation may occur during in vitro experiments, leading to the uptake of oncolytic VV by neutrophils, we undertook in vivo experiments to determine whether neutrophils can take up oncolytic VV under homeostatic conditions (Figure 1C). We explored the relationship between neutrophils and VV using C57BL/6 mice. Consistent with the in vitro tests, genomic DNA of oncolytic VV was detected within neutrophils from C57BL/6 mice (Figure 1D). The sorting efficiency of mouse neutrophils is depicted in Figure S1B. Moreover, we utilized NCG (NOD‐Prkdcem26Cd52Il2rgem26Cd22/Nju) mice to establish a human peripheral blood immune environment. Flow cytometry analysis showed the successful reconstitution of NCG mice with the human peripheral blood milieu within a short time (Figure 1E). Notably, human neutrophils in NCG mice showed uptake of oncolytic VV following intravenous injection of the virus (Figure 1F). Collectively, these findings confirm that neutrophils can take up oncolytic VV both in vitro and in vivo.
FIGURE 1.
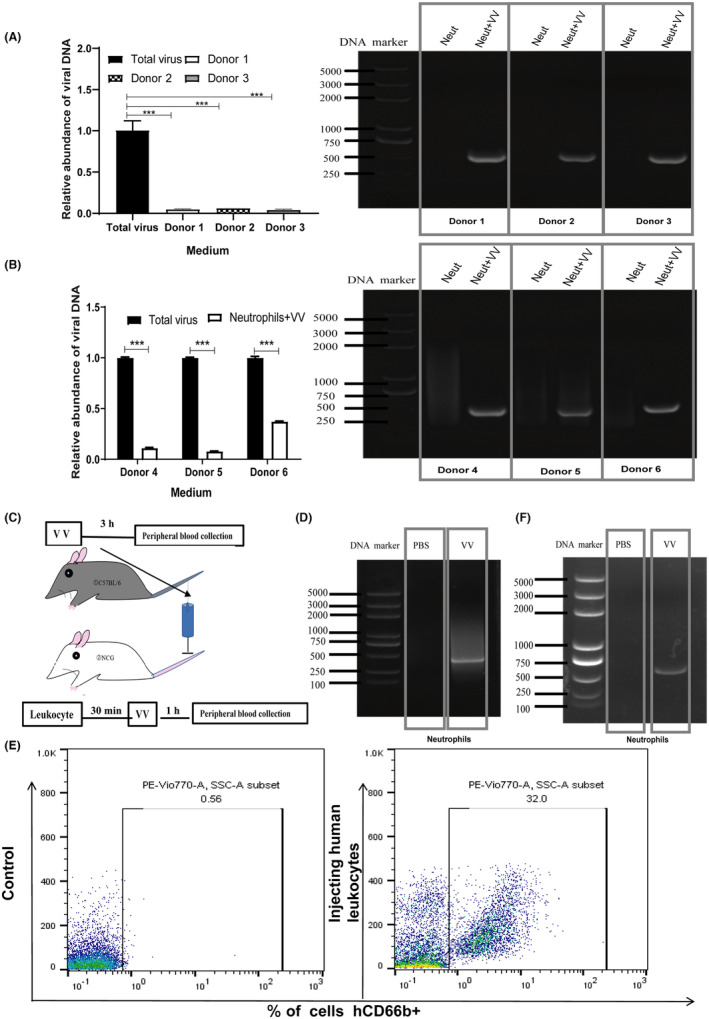
Neutrophil uptake of oncolytic vaccinia virus (VV) in vitro and in vivo. (A, B) Peripheral blood samples were obtained from (A) healthy individuals and (B) patients diagnosed with pancreatic ductal adenocarcinoma. The collected samples were then incubated with oncolytic VV at an MOI of 1 for a duration of 3 h. Neutrophils (Neut) were isolated from the samples using a neutrophil sorting kit. Quantitative PCR and PCR analyses were carried out to assess the viral load in the culture medium and to detect the presence of viral genomic DNA in neutrophils. Data are presented as mean ± SD. Statistical analysis was undertaken using an unpaired Student's t‐test. ***p < 0.001. (C) Schematic representation illustrating the experimental design for the in vivo study. (D) C57BL/6 mice were intravenously injected with 1 × 108 pfu oncolytic VV through the tail vein. After 3 h, neutrophils were purified from the collected blood, and genomic DNA was extracted for PCR analysis to detect the presence of the oncolytic VV DNA. n = 6/group. (E) The percentage of neutrophils was assessed in NCG mice that were reconstituted with human peripheral blood immune cells, simulating the human peripheral environment. The analysis included three mice per group. (F) NCG mice were reconstituted with human peripheral white blood cells. Thirty minutes following reconstitution, the mice received an intravenous injection of oncolytic VV through the tail vein. Blood samples were collected from the orbital venous plexus 1 h after injection, and neutrophils were isolated from the blood for subsequent PCR analysis to detect the presence of the oncolytic VV DNA. n = 5/group.
3.2. Neutrophils take up more oncolytic VV than other subsets of immune cells
We have successfully shown that neutrophils can take up oncolytic VV both in vitro and in vivo. However, in the peripheral blood, there exist various immune cell subsets such as monocytes, T cells, B cells, and natural killer (NK) cells, some of which also possess phagocytic capabilities. To explore whether these immune cells can also take up oncolytic VV, we undertook experiments involving the incubation of monocytes, T cells, B cells, and NK cells with oncolytic VV. The total DNA extracted from the cells underwent PCR analysis to determine the presence of oncolytic VV. This analysis identified measurable amounts of the virus in various cell types, including monocytes, T cells, B cells, and NK cells (Figure 2B), aligning with previous studies. 26 Moreover, the level of VV in the culture medium decreased in the group incubated with the cells and oncolytic VV compared to the total amount of virus initially added (Figure 2A). These findings suggest that all major subsets of immune cells in human peripheral blood are capable of taking up oncolytic VV.
FIGURE 2.

Neutrophils take up more oncolytic vaccinia virus (VV) than other subsets of immune cells. (A, B) Monocytes, T cells, B cells, and natural killer (NK) cells were isolated from the peripheral blood of healthy individuals (1 × 106 cells each). The isolated immune cells were incubated with oncolytic VV, and after 3 h of incubation, the genomic DNA was extracted from both the culture medium and the specific immune cell subsets. Quantitative PCR (qPCR) and PCR analyses were carried out to evaluate the amount of virus in the culture medium and to identify the presence of viral genomic DNA within neutrophils. Data are presented as mean ± SD. Statistical analysis was carried out using an unpaired Student's t‐test. (C, D) White blood cells (4 × 106) were obtained from the peripheral blood of healthy individuals and incubated with oncolytic VV in a 6‐well plate for a duration of 3 h. Following the incubation, the immune cells were separated into neutrophils (CD66b+) and non‐neutrophilic (CD66b−) granulocytes using a neutrophil sorting kit. The viral load of the oncolytic VV in both the culture medium and the immune cells was quantified using qPCR. Data are presented as mean ± SD. Statistical analysis was carried out using an unpaired Student's t‐test. ***p < 0.001; ****p < 0.0001.
To identify which immune cell subset in human peripheral blood shows a greater uptake of oncolytic VV, we collected peripheral blood samples from healthy donors and incubated them with oncolytic VV. Subsequently, we used a neutrophil sorting kit to separate the neutrophil population from the non‐neutrophil population (monocytes, T cells, B cells, and NK cells) and extracted total cellular DNA for qPCR analysis to detect the presence of oncolytic VV. The results showed a reduction in the virus within the culture medium compared to the total amount of virus initially added (Figure 2C). Additionally, it was observed that the total neutrophil population showed a greater uptake of oncolytic VV in comparison to the non‐neutrophil cells (Figure 2D).
3.3. Degradation of oncolytic VV in neutrophils
Our study has provided evidence that all major subsets of immune cells in human peripheral blood are capable of taking up oncolytic VV, with neutrophils showing a higher uptake rate. However, it is crucial to investigate the fate of VV within neutrophils. To address this, we incubated neutrophils with oncolytic VV for 48 h, with CV1 cells serving as controls. Under the fluorescence microscope, we observed GFP fluorescence, indicating replication of oncolytic VV. 13 Interestingly, flow cytometry analysis revealed no detectable GFP signal in neutrophils, suggesting that VV did not replicate within neutrophils (Figure 3A). To gain further insights, we performed transmission electron microscopy on neutrophils incubated with oncolytic VV for 1 h. The images revealed the presence of oncolytic VV within phagocytic vesicles of neutrophils (Figure 3B).
FIGURE 3.
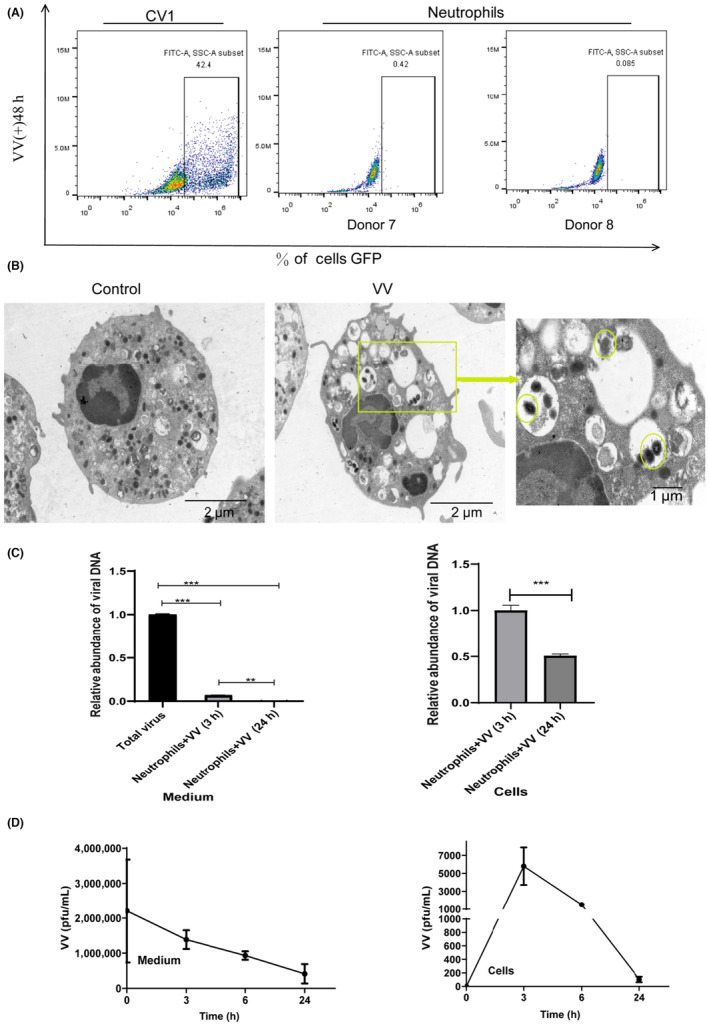
Degradation of oncolytic vaccinia virus (VV) in neutrophils. (A) Neutrophils and CV1 cells were cultured in the presence of oncolytic VV for 48 h, followed by the detection of virus replication using flow cytometry. (B) Transmission electron microscopy was used to visualize the presence of oncolytic VV within neutrophils. (C) Neutrophils were incubated with oncolytic VV for varying durations, and the viral genome DNA load in both the culture medium and neutrophils was quantified using quantitative PCR. Data are presented as mean ± SD, and statistical significance was determined using an unpaired Student's t‐test. (D) Oncolytic VV and neutrophils were coincubated for different time intervals, after which the culture medium and neutrophils were collected for viral titer assays using CV1 cells. ***p < 0.001.
In order to explore the kinetics of oncolytic VV within neutrophils, we incubated neutrophils with oncolytic VV for different time intervals. Subsequently, we collected the culture medium and neutrophils to extract their total DNA for viral detection using qPCR. The findings showed a decrease in the concentration of oncolytic VV in the culture medium as time progressed. Additionally, the viral load inside the neutrophils also diminished with extended incubation periods (Figure 3C). To assess whether the number of live viruses was also reduced, we used the TCID50 assay (tissue culture inhibitory dose 50%). The findings revealed a decrease in the amount of oncolytic VV within neutrophils with increasing incubation time (Figure 3D), indicating that neutrophils can take up and subsequently degrade oncolytic VV. Detection of human neutrophil activity at different time points in vitro experiments by flow cytometry (Figure S2).
3.4. Depletion of neutrophils enhances antitumor effect of oncolytic VV
Our findings indicate that neutrophils have the ability to phagocytose and degrade oncolytic VV, potentially compromising its antitumor efficacy when delivered intravenously. To assess the impact of neutrophils on the survival of intravenously injected oncolytic VV, we used 1‐A8, a neutrophil‐specific Ab, for neutrophil depletion. 27 The efficiency of neutrophil depletion was assessed using a previously described detection method. 28 Figure S1C illustrates the depletion efficiency of neutrophils. Animal experiments were designed to investigate the implications of neutrophil depletion (Figure 4A), confirming an increase in circulating virus load in the peripheral plasma of neutrophil‐depleted animals (Figure 4B).
FIGURE 4.
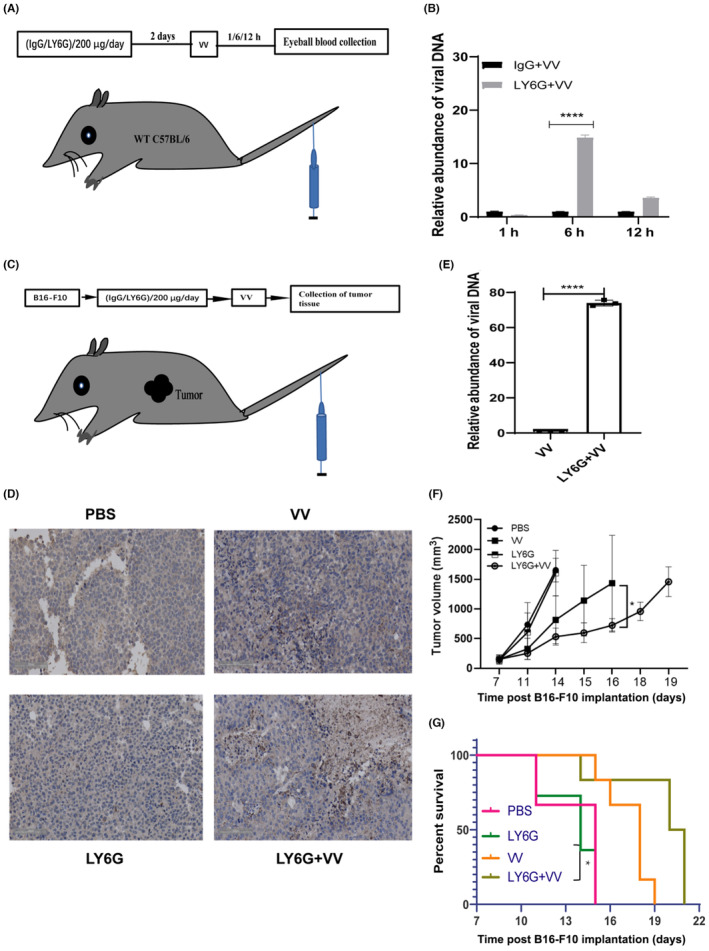
Depletion of neutrophils enhances the antitumor effect of oncolytic vaccinia virus (VV). (A) C57BL/6 mice were intraperitoneally injected with anti‐LY6G Ab at a dose of 200 μg/day/mouse for two consecutive days prior to receiving an intravenous injection of 1 × 108 pfu oncolytic VV into the tail vein every other day. (B) Viral load of oncolytic vaccinia in the peripheral plasma circulation was quantified using quantitative PCR (qPCR). n = 5/group. Data are presented as mean ± SD. Statistical analysis was carried out using one‐way ANOVA with the Brown–Forsythe test. (C) A subcutaneous melanoma tumor model was established by injecting 1 × 106 B16‐F10 cells into the right flank of C57BL/6 mice. Mice received an intraperitoneal injection of anti‐LY6G Ab at a dose of 200 μg/day/mouse for two consecutive days prior to receiving an intravenous injection of 1 × 108 pfu oncolytic VV into the tail vein. The group size is n = 7/group. (D) Immunohistochemistry was carried out to detect the presence of the oncolytic VV coat protein in tumor tissues; n = 7/group. Images were captured at an original magnification of ×20. (E) Oncolytic virus load in tumor tissues was quantified using qPCR, n = 7/group, and data are presented as mean ± SD. Statistical analysis was carried out using two‐way ANOVA with Bonferroni multiple test correction. (F) Tumor growth curve depicting the mean ± SD of tumor size over time, n = 7/group. (G) Kaplan–Meier survival curve representing the survival analysis data of the animal tumor model. n = 7/group. Significance was assessed using the log rank (Mantel–Cox) test. Tumor volume and survival curve are derived from the same treated mice. *p < 0.05; ****p < 0.0001.
To evaluate the benefits of increased circulating virus load in neutrophil‐depleted animals, we utilized a subcutaneous melanoma B16‐F10 tumor model (Figure 4C). Immunohistochemistry analysis of tumor tissue was carried out using an Ab against the VV coat protein. The results revealed the presence of VV in both the control VV group and the anti‐LY6G + VV treatment group (Figure 4D). Genomic DNA was extracted from tumor tissues for qPCR analysis of VV, which showed an increased viral load in the tumor tissue of the anti‐LY6G + VV treatment group compared to the control VV group (Figure 4E). Additionally, reduced tumor burden (Figure 4F) and prolonged survival rate were observed in the anti‐LY6G + VV group (Figure 4G). These experimental results indicate that neutrophil depletion can enhance the antitumor effect of oncolytic VV.
However, it is important to acknowledge that neutrophils play diverse roles in vivo, and one of the crucial mechanisms of oncolytic viruses' antitumor activity is their ability to modulate the immune system within the tumor microenvironment (TME). Irreversible neutrophil depletion using Abs could disrupt their beneficial functions in the TME. Therefore, the clinical implementation of neutrophil‐depleted oncolytic VV treatment has certain limitations.
3.5. CAL‐101 enhances antitumor effect of oncolytic VV by inhibiting viral uptake by neutrophils
CAL‐101, an approved clinical treatment, is an inhibitor of the PI3Kinase delta isoform. 29 It has primarily been utilized in the management of indolent B‐cell tumors, including relapsed small lymphocytic lymphoma, relapsed follicular lymphoma, and relapsed/refractory chronic lymphocytic leukemia (CLL). 29 , 30 , 31 , 32 , 33 The combination of CAL‐101 with rituximab has shown significant therapeutic efficacy in CLL. 29 Notably, CAL‐101 has been shown to inhibit macrophage adhesion to oncolytic VV and enhance its antitumor effect. 34 Additionally, CAL‐101 has been reported to inhibit neutrophil phagocytosis. 35
To investigate whether CAL‐101 can inhibit the uptake of oncolytic VV by neutrophils, we treated neutrophils with varying concentrations of CAL‐101. Our results indicated that CAL‐101 effectively inhibited AKT phosphorylation, a downstream target of PI3Kinase delta, in neutrophils (Figure 5A). Furthermore, we collected neutrophil granulocytes from healthy individuals and treated them with CAL‐101. The findings revealed that CAL‐101 reduced the uptake of oncolytic VV by neutrophils (Figure 5C) and increased the viral load in the culture medium (Figure 5B). To confirm the inhibitory effect of CAL‐101 on the uptake of oncolytic VV by neutrophils in an in vivo setting, we used C57BL/6 and NCG mouse models. CAL‐101 was given orally at a dosage of 10 mg/kg prior to intravenous injection of oncolytic VV (Figure 5D). The results indicated that CAL‐101 inhibited the uptake of oncolytic VV by neutrophils in vivo, resulting in an increased circulating viral load in peripheral blood plasma (Figure 5E,F) and higher viral load in tumor tissues (Figure 5G,H). Ultimately, this enhanced the antitumor effect of oncolytic VV (Figure 5I,J).
FIGURE 5.
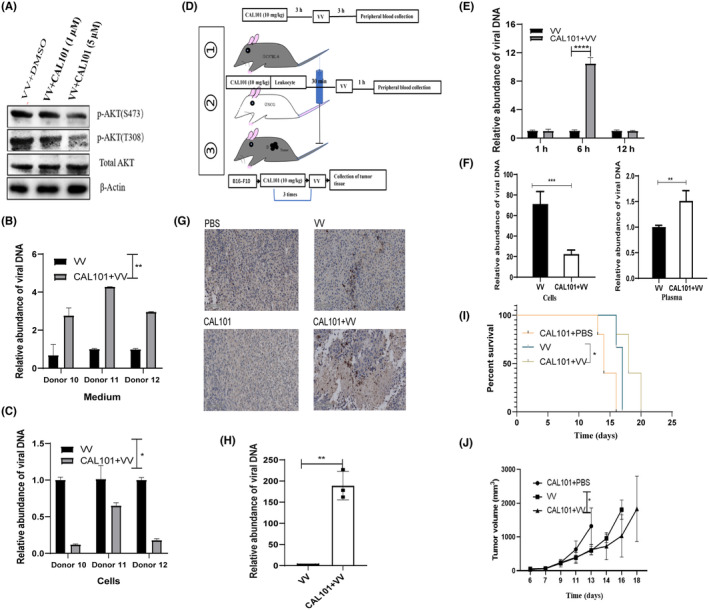
CAL‐101 enhances the antitumor effect of oncolytic vaccinia virus (VV) by inhibiting neutrophil viral uptake. (A) Peripheral blood samples were collected from healthy individuals and treated with different concentrations of CAL‐101 for 3 h. Samples were then incubated with oncolytic VV for 1 h. Neutrophils were isolated for the detection of the signaling molecule AKT through western blot analysis. (B, C) Genomic DNA of oncolytic VV was extracted from (B) culture medium and (C) neutrophils to analyze oncolytic VV DNA expression using quantitative PCR (qPCR). Data are presented as mean ± SD. Statistical analysis was carried out using the unpaired Student's t‐test. (D) Diagram indicating the experimental setup for in vivo experiments. (E, F) CAL‐101 was given orally to (E) C57BL/6 mice (n = 6/group) and (F) NCG mice (n = 5/group) at a dose of 10 mg/kg before intravenous injection of oncolytic VV. Peripheral plasma samples were collected to evaluate the circulating viral load of oncolytic VV. Data are presented as mean ± SD. Statistical analysis was carried out using the unpaired Student's t‐test. (G–J) A subcutaneous melanoma tumor model was established by injecting 1 × 106 B16‐F10 cells into the right flank of C57BL/6 mice. CAL‐101 treatment was given for 3 h prior to intravenous infusion of oncolytic VV. (G) Immunohistochemistry was carried out to detect the presence of the VV coat protein in tumor tissues, n = 7/group. Images were captured at an original magnification of ×20. (H) Viral load of oncolytic vaccinia in different tumor tissues was determined using qPCR, n = 7/group. Data are presented as mean ± SD. Statistical analysis was carried out using two‐way ANOVA with Bonferroni multiple test correction. (I) Kaplan–Meier survival analysis was undertaken using the animal tumor model, n = 6/group. Significance was assessed using the log rank (Mantel–Cox) test. (J) Tumor growth curves represent the mean ± SD of tumor size over time, n = 6/group. Statistical analysis was carried out using two‐way ANOVA with Bonferroni multiple test correction. Tumor volume and survival curve are derived from the same treated mice. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
4. DISCUSSION
In this study, we provide evidence that neutrophils can actively take up and degrade oncolytic VV both in vitro and in vivo. To investigate the impact of neutrophils on the therapeutic efficacy of oncolytic VV, we used specific Abs for neutrophil depletion or a selective inhibitor targeting the PI3Kinase delta isoform. Our findings reveal that neutrophil depletion or inhibition leads to an increased viral load in both the circulation and at tumor sites, ultimately enhancing the antitumor function and improving the survival rate of tumor‐bearing animals (Figure 6).
FIGURE 6.
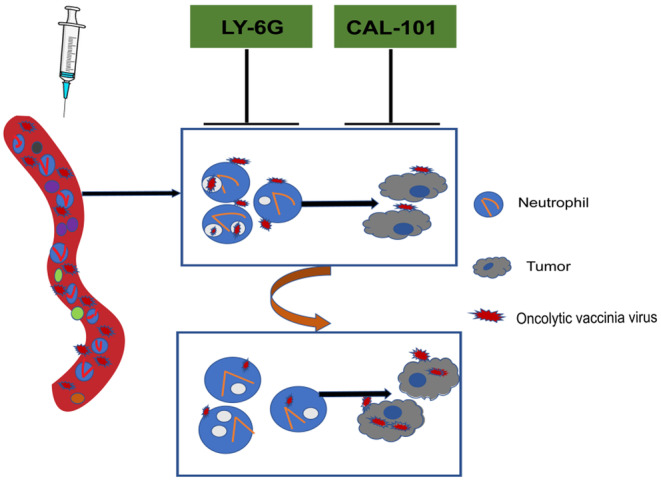
Summary chart of article content. Neutrophils present in the peripheral blood play a crucial role in the uptake and degradation of oncolytic vaccinia virus (VV) following intravenous injection. This process leads to a reduction in the circulating levels of oncolytic VV, ultimately impacting the availability of the virus to penetrate tumor tissues and exert its antitumor effects. Our research has shown that the depletion of neutrophils through Ab‐mediated elimination or the inhibition of neutrophil activity using CAL‐101, a specific inhibitor targeting PI3Kinase delta isoform, results in decreased uptake of oncolytic VV by neutrophils. Consequently, this leads to an increase in the viral load within peripheral plasma and tumor tissues, thereby enhancing the antitumor effect of intravenously administered oncolytic VV.
Previous reports have indicated that VV has the ability to bind to various subtypes of immune cells in peripheral blood, including monocytes, neutrophils, T cells, B cells, and NK cells. 26 , 36 , 37 Notably, studies have highlighted the enhanced binding and infection capabilities of VV in monocytes. 26 , 38 Considering the phagocytic properties of neutrophils and their abundant presence (50%–70%) in human peripheral blood, we focused our investigation on the influence of neutrophils on oncolytic VV both in vitro and in vivo. 17 , 18
Our in vitro experiments showed that neutrophils can effectively take up oncolytic VV, which aligns with previous findings. 25 Moreover, we observed that neutrophils showed a greater capacity for VV uptake due to their numerical superiority compared to other immune cells. When neutrophils were coincubated with oncolytic VV, they engulfed and degraded the virus, indicating that the uptake mechanism by neutrophils likely involved phagocytosis rather than infection. It is worth noting that VV is sensitive to neutrophil binding but not necessarily to infection. 26 Considering that oncolytic VV is commonly delivered intravenously in clinical trials, we undertook a series of in vivo experiments to explore the relationship between neutrophils and oncolytic VV.
Our investigations using C57BL/6 and NCG mice, which were reconstituted with human peripheral blood to simulate clinical scenarios, confirmed that neutrophils can take up oncolytic VV. To further elucidate the impact of neutrophils during intravenous injection of oncolytic VV, we undertook a neutrophil depletion experiment. Antibodies such as anti‐Gr‐1 (RB6‐8C5) and anti‐Ly6G (1‐A8) have been widely used for neutrophil depletion, with anti‐LY6G Ab being the most specific. 27 , 39 , 40 We discovered that depletion of neutrophils resulted in an increased viral load in the bloodstream, leading to higher levels of viruses in tumor tissues. This outcome significantly enhanced the antitumor effect of intravenously administered oncolytic VV. These findings underscore the crucial role played by neutrophils in the treatment of cancers using intravenous oncolytic VV, highlighting their potential as therapeutic targets.
The functionality of neutrophils relies on the signaling pathways involving PI3Kinase. Our study revealed that CAL‐101, an inhibitor of PI3Kinase delta isoform, effectively suppressed AKT phosphorylation, a downstream target of PI3Kinase delta, in neutrophils. Consequently, CAL‐101 treatment reduced the phagocytosis of oncolytic VV by neutrophils, resulting in an increased presence of oncolytic VV in the bloodstream and higher virus levels in tumor tissues. These findings align with previous reports demonstrating the inhibitory effect of CAL‐101 on neutrophil phagocytosis. 35 , 41 Moreover, our study showed that CAL‐101 enhances the antitumor effect of intravenously injected oncolytic VV.
Apart from neutrophils, CAL‐101 has also been reported to inhibit macrophage functions in tissues, leading to an enhanced antitumor effect upon intravenous administration of oncolytic VV. Macrophages are distributed within tissues and can be either monocyte‐derived or tissue‐resident. Due to the challenges associated with depleting tissue‐resident macrophages, our study did not assess their involvement in the intravenous delivery of oncolytic VV. However, CAL‐101's ability to inhibit both neutrophils and macrophages effectively dampens their functions, leading to an improved antitumor effect. It is important to note that this study does not address the specific contributions of these two phagocytic immune cell types to the survival and antitumor activity of oncolytic VV in vivo. Neutrophils were chosen as the focus due to their abundant presence in human peripheral blood, while the contribution of macrophages warrants further investigation.
It is worth noting that the in vivo experimental effects are modest overall, which could be related to the low number of neutrophils in the peripheral blood of mice; the proportion of neutrophils in human peripheral blood is much higher than that of mice. 42 Inhibiting neutrophil function can better improve the effect of intravenous injection of VV. To simulate the human peripheral environment, we utilized NCG mice reconstituted with human peripheral blood. These models successfully demonstrated the significant role of neutrophils in clearing oncolytic VV from the bloodstream. In addition to immune cells, studies have also concentrated on a new type of VV that carries the human CD55 protein on its membrane. This modification enhances the virus's endurance by shielding it from complement‐mediated destruction and helping it avoid neutralization by Abs specific to VV. 43 These findings provide vital theoretical support for future clinical interventions aimed at improving the efficacy of intravenous oncolytic VV delivery.
AUTHOR CONTRIBUTIONS
Danya Zhou: Conceptualization; data curation; methodology; writing – original draft. Wei Xu: Methodology; resources. Xuping Ding: Methodology. Haoran Guo: Methodology. Jianyao Wang: Methodology. Guanghao Zhao: Methodology. Chenglin Zhang: Methodology. Zhongxian Zhang: Methodology. Zhimin Wang: Conceptualization; writing – review and editing. Pengju Wang: Resources; writing – review and editing. Liming Lu: Conceptualization; writing – review and editing. Ming Yuan: Conceptualization; data curation; funding acquisition; methodology; project administration; resources; writing – original draft; writing – review and editing.
FUNDING INFORMATION
This study was supported by the National Natural Science Foundation of China (81773035).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Approval of the research protocol by an institutional review board: The research scheme was approved by the Ethics Supervision Committee of Zhengzhou University.
Informed consent: All participants provided informed consent for blood collection.
Registry and the registration no. of the study/trial: N/A.
Animal studies: Animal care was in accordance with Zhengzhou University guidelines (license ZZU‐LAC20211210[06]).
Supporting information
Appendix S1
Figure S1
ACKNOWLEDGMENTS
We would like to thank Shenzhen Huayao Kangming Biopharmaceutical Co., Ltd for providing oncolytic VV, Ruijin Hospital affiliated with Shanghai Jiaotong University for providing blood from patients with pancreatic duct adenocarcinoma, and healthy blood donors for providing blood.
Zhou D, Xu W, Ding X, et al. Transient inhibition of neutrophil functions enhances the antitumor effect of intravenously delivered oncolytic vaccinia virus. Cancer Sci. 2024;115:1129‐1140. doi: 10.1111/cas.16105
REFERENCES
- 1. Russell SJ, Barber GN. Oncolytic viruses as antigen‐agnostic cancer vaccines. Cancer Cell. 2018;33:599‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alvarez‐Breckenridge CA, Choi BD, Suryadevara CM, Chiocca EA. Potentiating oncolytic viral therapy through an understanding of the initial immune responses to oncolytic viral infection. Curr Opin Virol. 2015;13:25‐32. [DOI] [PubMed] [Google Scholar]
- 3. Huang PI, Chang JF, Kirn DH, Liu TC. Targeted genetic and viral therapy for advanced head and neck cancers. Drug Discov Today. 2009;14:570‐578. [DOI] [PubMed] [Google Scholar]
- 4. Garber K. China approves world's first oncolytic virus therapy for cancer treatment. J Natl Cancer Inst. 2006;98:298‐300. [DOI] [PubMed] [Google Scholar]
- 5. Hamid O, Ismail R, Puzanov I. Intratumoral immunotherapy‐update 2019. Oncologist. 2020;25:e423‐e438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ylösmäki E, Cerullo V. Design and application of oncolytic viruses for cancer immunotherapy. Curr Opin Biotechnol. 2020;65:25‐36. [DOI] [PubMed] [Google Scholar]
- 7. Fukuhara H, Ino Y, Todo T. Oncolytic virus therapy: a new era of cancer treatment at dawn. Cancer Sci. 2016;107:1373‐1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Todo T, Ito H, Ino Y, et al. Intratumoral oncolytic herpes virus G47∆ for residual or recurrent glioblastoma: a phase 2 trial. Nat Med. 2022;28:1630‐1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cai J, Zhu W, Lin Y, et al. Systematic characterization of the biodistribution of the oncolytic virus M1. Hum Gene Ther. 2020;31:1203‐1213. [DOI] [PubMed] [Google Scholar]
- 10. Ferguson MS, Lemoine NR, Wang Y. Systemic delivery of oncolytic viruses: hopes and hurdles. Adv Virol. 2012;2012:805629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Breitbach CJ, Burke J, Jonker D, et al. Intravenous delivery of a multi‐mechanistic cancer‐targeted oncolytic poxvirus in humans. Nature. 2012;477:99‐102. [DOI] [PubMed] [Google Scholar]
- 12. Vanderplasschen A, Hollinshead M, Smith GL. Antibodies against vaccinia virus do not neutralize extracellular enveloped virus but prevent virus release from infected cells and comet formation. J Gen Virol. 1997;78:2041‐2048. [DOI] [PubMed] [Google Scholar]
- 13. Yuan M, Wang P, Chard LS, Lemoine NR, Wang Y. A simple and efficient approach to construct mutant vaccinia virus vectors. J Vis Exp. 2016;116:54171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cousin S, Toulmonde M, Kind M, et al. Phase 2 trial of intravenous oncolytic virus JX‐594 combined with low‐dose cyclophosphamide in patients with advanced breast cancer. Exp Hematol Oncol. 2022;11:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Inoue M, Kim M, Inoue T, et al. Oncolytic vaccinia virus injected intravenously sensitizes pancreatic neuroendocrine tumors and metastases to immune checkpoint blockade. Mol Ther Oncolytics. 2021;24:299‐318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Toulmonde M, Cousin S, Kind M, et al. Randomized phase 2 trial of intravenous oncolytic virus JX‐594 combined with low‐dose cyclophosphamide in patients with advanced soft‐tissue sarcoma. J Hematol Oncol. 2022;15:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brinkmann V, Laube B, Abu Abed U, Goosmann C, Zychlinsky A. Neutrophil extracellular traps: how to generate and visualize them. J Vis Exp. 2010;36:1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dinh HQ, Eggert T, Meyer MA, et al. Coexpression of CD71 and CD117 identifies an early unipotent neutrophil progenitor population in human bone marrow. Immunity. 2020;53(2):319‐334.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hashimoto Y, Moki T, Takizawa T, Shiratsuchi A, Nakanishi Y. Evidence for phagocytosis of influenza virus‐infected, apoptotic cells by neutrophils and macrophages in mice. J Immunol. 2007;178:2448‐2457. [DOI] [PubMed] [Google Scholar]
- 20. Matsushima H, Geng S, Lu R, et al. Neutrophil differentiation into a unique hybrid population exhibiting dual phenotype and functionality of neutrophils and dendritic cells. Blood. 2013;121:1677‐1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taipale K, Liikanen I, Koski A, et al. Predictive and prognostic clinical variables in cancer patients treated with adenoviral oncolytic immunotherapy. Mol Ther. 2016;24:1323‐1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dey A, Zhang Y, Castleton AZ, et al. The role of neutrophils in measles virus get ‐ mediated oncolysis differs between B ‐ cell malignancies and is not always enhanced by GCSF. J Mol Ther. 2016;24:184‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peplinski GR, Tsung AK, Casey MJ, et al. In vivo murine tumor gene delivery and expression by systemic recombinant vaccinia virus encoding interleukin‐1beta. Cancer J Sci Am. 1996;2:21‐27. [PubMed] [Google Scholar]
- 24. Robinson BW, Mukherjee SA, Davidson A, et al. Cytokine gene therapy or infusion as treatment for solid human cancer. J Immunother. 1998;21:211‐217. [DOI] [PubMed] [Google Scholar]
- 25. West BC, Escheté ML, Cox ME, King JW. Neutrophil uptake of vaccinia virus in vitro . J Infect Dis. 1987;156:597‐606. [DOI] [PubMed] [Google Scholar]
- 26. Byrd D, Amet T, Hu N, Lan J, Hu S, Yu Q. Primary human leukocyte subsets differentially express vaccinia virus receptors enriched in lipid rafts. J Virol. 2013;87:9301‐9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G‐specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64‐70. [DOI] [PubMed] [Google Scholar]
- 28. Hasenberg A, Hasenberg M, Männ L, et al. Catchup: a mouse model for imaging‐based tracking and modulation of neutrophil granulocytes. Nat Methods. 2015;12:445‐452. [DOI] [PubMed] [Google Scholar]
- 29. Yang Q, Modi P, Newcomb T, Queva C, Gandhi V. Idelalisib: first‐in‐class PI3K delta inhibitor for the treatment of chronic lymphocytic leukemia, small lymphocytic leukemia, and follicular lymphoma. Clin Cancer Res. 2015;21:1537‐1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ramanathan S, Jin F, Sharma S, Kearney BP. Clinical pharmacokinetic and pharmacodynamic profile of idelalisib. Clin Pharmacokinet. 2016;55:33‐45. [DOI] [PubMed] [Google Scholar]
- 31. Lannutti BJ, Meadows SA, Herman SE, et al. CAL‐101, a p110delta selective phosphatidylinositol‐3‐kinase inhibitor for the treatment of B‐cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117:591‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Herman SE, Gordon AL, Wagner AJ, et al. Phosphatidylinositol 3‐kinase‐δ inhibitor CAL‐101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116:2078‐2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ferguson MS, Chard Dunmall LS, Gangeswaran R, et al. Transient inhibition of PI3Kdelta enhances the therapeutic effect of intravenous delivery of oncolytic oncolytic vaccinia virus. Mol Ther. 2020;28:1263‐1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Da Roit F, Engelberts PJ, Taylor RP, et al. Ibrutinib interferes with the cell‐mediated antitumor activities of therapeutic CD20 antibodies: implications for combination therapy. Haematologica. 2015;100:77‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yu Q, Jones B, Hu N, et al. Comparative analysis of tropism between canarypox (ALVAC) and vaccinia viruses reveals a more restricted and preferential tropism of ALVAC for human cells of the monocytic lineage. Vaccine. 2006;24:6376‐6391. [DOI] [PubMed] [Google Scholar]
- 37. Shepherd N, Lan J, Li W, Rane S, Yu Q. Primary human B cells at different differentiation and maturation stages exhibit distinct susceptibilities to vaccinia virus binding and infection. J Virol. 2019;93:e00973‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chahroudi A, Chavan R, Kozyr N, Waller EK, Silvestri G, Feinberg MB. Vaccinia virus tropism for primary hematolymphoid cells is determined by restricted expression of a unique virus receptor. J Virol. 2005;79:10397‐10407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boivin G, Faget J, Ancey PB, et al. Durable and controlled depletion of neutrophils in mice. Nat Commun. 2020;11:2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pollenus E, Malengier‐Devlies B, Vandermosten L, et al. Limitations of neutrophil depletion by anti‐Ly6G antibodies in two heterogenic immunological models. Immunol Lett. 2019;212:30‐36. [DOI] [PubMed] [Google Scholar]
- 41. Alflen A, Stadler N, Aranda Lopez P, et al. Idelalisib impairs TREM‐1 mediated neutrophil inflammatory responses. Sci Rep. 2018;8:5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731‐2738. [DOI] [PubMed] [Google Scholar]
- 43. Lee N, Jeon YH, Yoo J, et al. Generation of novel oncolytic vaccinia virus with improved intravenous efficacy through protection against complement‐mediated lysis and evasion of neutralization by vaccinia virus‐specific antibodies. J Immunother Cancer. 2023;11:e006024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Figure S1


