Abstract
Herpes simplex virus types 1 and 2 (HSV-1 and HSV-2) express an immediate-early protein, ICP47, that effectively inhibits the human transporter associated with antigen presentation (TAP), blocking major histocompatibility complex (MHC) class I antigen presentation to CD8+ T cells. Previous work indicated that the mouse TAP is relatively resistant to inhibition by the HSV-1 and HSV-2 ICP47 proteins (ICP47-1 and ICP47-2) and that mouse cells infected with HSV-1 are lysed by anti-HSV CD8+ cytotoxic T lymphocytes (CTL). Therefore, mice are apparently not suitable animals in which to study the in vivo effects of ICP47. In order to find an animal model, we introduced ICP47-1 and ICP47-2 into cells from various animal species—mice, rats, guinea pigs, rabbits, dogs, pigs, cows, monkeys, and humans—and measured TAP activity in the cells. Both proteins were unable to inhibit TAP in mouse, rat, guinea pig, and rabbit cells. In contrast, ICP47-1 and ICP47-2 inhibited TAP in pig, dog, cow, and monkey cells, and the TAP in pig and dog fibroblasts was often more sensitive to both proteins than TAP in human fibroblasts. These results were extended by measuring CD8+-T-cell recognition (CTL lysis) of cells from various species. Cells were infected with recombinant HSV-1 constructed to express murine MHC class I proteins so that the cells would be recognized and lysed by well-characterized murine anti-HSV CTL unless antigen presentation was blocked by ICP47. Anti-HSV CD8+ CTL effectively lysed pig and primate cells infected with a recombinant HSV-1 ICP47− mutant but were unable to lyse pig or primate cells infected with a recombinant HSV-1 that expressed ICP47. Therefore, pigs, dogs, and monkeys may be useful animal models in which to test the effects of ICP47 on HSV pathogenesis or the use of ICP47 as a selective immunosuppressive agent.
Herpes simplex virus (HSV) infection of human fibroblasts leads to inhibition of antigen presentation to CD8+ T cells so that the virus-infected fibroblasts are not lysed by cytotoxic T lymphocytes (CTL) (10, 12, 14). The principal reason for this resistance to CTL appears to be the expression of an HSV immediate-early protein, ICP47, which causes major histocompatibility complex (MHC) class I proteins to accumulate in infected cells in a peptide-empty form (19). ICP47 was subsequently shown to inhibit the transporter associated with antigen presentation (TAP), which functions to translocate antigenic peptides across the membrane of the endoplasmic reticulum (ER) (3, 8), and without antigenic peptides, MHC class I proteins accumulate in the ER. More recent results demonstrated that ICP47 blocks peptide binding to TAP by binding with high affinity to a domain of TAP that includes the peptide binding site (1, 15).
Although HSV type 1 (HSV-1) ICP47 (ICP47-1) effectively blocks TAP in human fibroblasts, it inhibits TAP little, if at all, in a variety of mouse cells unless applied in high concentrations (1, 3, 15, 19). Similarly, HSV-2 ICP47 (ICP47-2), which has only 42% amino acid identity with ICP47-1 (4), effectively blocks human TAP but inhibits murine TAP less effectively (16). Inhibition of murine TAP with these proteins occurs at ICP47-1 and ICP47-2 concentrations 50- to 100-fold higher than those required to inhibit human TAP. ICP47-1 and ICP47-2 bind poorly to mouse TAP (15, 16), which explains their inability to block peptide transport and antigen presentation in mouse cells.
We were interested in extending the study of the species specificity of ICP47 for several reasons. Firstly, we wanted to find an animal model with which to assess the effects of ICP47 in vivo, both to assess its role in virus-host interactions and to provide a model for the use of ICP47 in autoimmunity, in transplantation, and in gene therapy vectors. Secondly, we wanted to determine whether ICP47 was functional in the species currently widely used for HSV pathogenesis and vaccine studies—mice, rabbits, and guinea pigs. Thirdly, we were interested in the mechanism of the extraordinary virulence of HSV in owl monkeys (aotus), speculating that the TAP in this New World primate might be exceptionally susceptible to ICP47.
In order to assess the effects of ICP47 on the TAPs of various species, cells were permeabilized, recombinant ICP47-1 and ICP47-2 were introduced into the cells, and assays of TAP activity were performed. To examine the effects of ICP47 on antigen presentation and recognition by CD8+ T cells, fibroblasts were infected with recombinant HSV-1 that expresses mouse class I proteins and not ICP47, and lysis of the cells by mouse anti-HSV CTL was tested. We found that ICP47-1 and ICP47-2 did not block TAP in mouse, rat, guinea pig, or rabbit skin fibroblasts but effectively inhibited TAP and antigen presentation in pig, dog, cow, and monkey fibroblasts. Therefore, pigs, dogs, and monkeys can be used to study the in vivo effects of ICP47, though for several reasons, the use of pigs might be a practical starting point.
MATERIALS AND METHODS
Cells.
Human fibroblasts were derived from foreskins of neonates. Tissues were minced with scissors and subjected to five or six 10-min treatments with trypsin-EDTA (GIBCO) and vigorous stirring. Cells released from the tissues were washed in alpha-minimal essential medium (alpha-MEM) containing 10% fetal bovine serum (FBS), penicillin-streptomycin (GIBCO), and gentamicin (GIBCO). The cells were plated in plastic flasks, and after 2 h the plastic was washed extensively with medium to remove nonadherent cells. The cells were then grown for 10 to 15 passages in alpha-MEM supplemented with 10% FBS and penicillin-streptomycin. Mouse fibroblasts were derived from the skin of 6-week-old BALB/c or C57BL/6 mice in the same manner as the human foreskin fibroblasts (four different preparations behaved similarly in TAP assays). Rabbit, guinea pig, and rat fibroblasts were derived from skin biopsy samples from animals in the same manner as the human fibroblasts. Chimpanzee skin fibroblasts were obtained from two different animals, F503 and F435, and were kindly supplied by Chris Walker at Chiron. Rhesus macaque fibroblasts were derived by direct outgrowth from dorsal skin biopsy samples from two different adults housed at the Oregon Regional Primate Center. Owl monkey kidney (OMK) cells were obtained from the American Type Culture Collection (ATCC). Bovine fibroblasts were derived from a testicle taken from a calf at the time of slaughter. Pig skin fibroblasts were derived from a skin biopsy of an adult, female pig at the time of slaughter. Canine fibroblasts were derived from epithelial tissue of an embryo (ATCC CRL 6226; CF31A). PK15 porcine kidney cells were obtained from ATCC. The cells were all passaged in alpha-MEM supplemented with 10 or 15% FBS and penicillin-streptomycin. Monkey Vero cells and human R970 cells were passaged in Dulbecco’s modified MEM containing 5 to 7% FBS and penicillin-streptomycin.
Viruses.
Wild-type HSV-1 strain F and recombinants, F-US5MHC (19), F-US5MHC/ICP47Δ, and F-US5MHC/US11ICP47Δ, as well as HSV-1 mutants F-ICP47Δ (5) and R3631 (11), which was kindly provided by Bernard Roizman (University of Chicago), were all propagated and titers were determined on Vero cells.
ICP47-1 and ICP47-2.
Purification of ICP47-1 and ICP47-2 from bacteria as glutathione S-transferase (GST) fusion proteins and removal of the GST domains has been described in detail previously (15, 16). In the present experiments, thrombin was supplied by Pharmacia and used to cleave GST–ICP47-1 at 2.5 U/ml for 40 to 60 min and to cleave GST–ICP47-2 at 5.0 U/ml for 60 to 90 min. ICP47-2 was found to be more resistant to thrombin digestion than ICP47-1.
TAP assays.
Cells were removed from dishes using trypsin-EDTA and washed twice in Dulbecco’s modified MEM with 10% FBS and once in transport buffer (8) at 4°C. The cells (approximately 106 per assay) were pelleted and suspended in transport buffer containing 1 to 2 U/ml of streptolysin O (a partially purified preparation derived from culture filtrates of hemolytic streptococcus and reduced before lyophilization; Murex, Dartmouth, England) for 10 min at 37°C in order to permeabilize approximately 60 to 90% of the cells, as measured by trypan blue exclusion. Trypan blue exclusion was assessed for each cell line during each assay. The cells were washed with transport buffer and incubated for 5 min with transport buffer containing ICP47-1 or ICP47-2 and lacking ATP. A library of peptides containing the N-linked glycosylation motif NXT and a tyrosine residue (7) was labelled with 125I as described previously (2, 8). The radiolabelled peptide library was incubated with the permeabilized cells in the presence of ICP47-1 or ICP47-2 for a further 10 min at 37°C. Transport was terminated by addition of a large excess of cold buffer containing 10 mM EDTA, and the cells were pelleted and then lysed with lysis buffer (Tris-HCl, pH 7.5; 5 mM MgCl2; 0.5% Nonidet P-40 [NP-40]). The nuclei were pelleted, and concanavalin A-Sepharose (100 μl) was added for 90 min at 4°C. The concanavalin A-Sepharose was washed three times in lysis buffer, and 125I-labelled glycopeptides were quantified with a gamma counter. For each assay, human fibroblasts were incubated with ICP47 at three concentrations to ensure that the 50% inhibitory concentration (IC50) was in the range of 0.2 to 0.5 μM.
Immunoprecipitation of ICP47 and MHC class I proteins.
Human R970 cells were infected with F-US5MHC, F-ICP47Δ, R-3631, or recombinant viruses derived from these viruses. Three hours after infection, the cells were washed in medium lacking cysteine and methionine and then incubated with medium lacking cysteine and methionine and supplemented with 50 μCi of [35S]methionine-cysteine (NEN-Dupont) per ml for a further 5 h. The cells were lysed in NP40-DOC buffer (50 mM Tris-HCl [pH 7.5], 100 mM NaCl, 1% NP-40, and 0.5% sodium deoxycholate) containing 2 mg of bovine serum albumin per ml and 0.5 mM phenylmethylsulfonyl fluoride. Cell extracts were mixed with rabbit anti-ICP47 serum (15) and simultaneously with serum specific for murine MHC class I (K locus), anti-peptide 8 (13), and antibody-antigen complexes adsorbed to protein A-Sepharose (Pharmacia). The protein A-Sepharose was washed with NP40-DOC buffer, and the proteins were eluted by boiling in buffer containing 2% sodium dodecyl sulfate and subjected to electrophoresis with 14% polyacrylamide gels as previously described (19).
CTL assays.
C57BL/6 mice (6 to 9 weeks of age) were inoculated in both hind footpads with HSV-1 strain F at 5 × 106 to 2 × 107 PFU/footpad in 50 μl. Five days later, the animals were euthanized, their popliteal lymph nodes were removed, and a single-cell suspension of lymphocytes was prepared. The lymphocytes (4 × 106 cells/ml) were cultured for 3 days in RPMI 1640 medium supplemented with 10% FBS and 50 μM β-mercaptoethanol and, in some experiments, murine interleukin-2 (5 U/ml). 51Cr release assays were performed as described (6, 19). Target cells were plated in round-bottom, 96-well dishes so that the cells were subconfluent (5,000 to 10,000 cells per well). The cells were labelled with 51Cr (NEZ 030, 40 to 90 μCi/ml; New England Nuclear) for 9 to 14 h, washed, and infected with F-US5MHC, F-US5MHC/ICP47Δ, or F-US5MHC/US11ICP47Δ for 3 to 4 h. Effector cells (lymphocytes from HSV-infected mice) were added to the wells at various effector/target cell ratios in triplicate. After 4 h, half of the cell culture supernatant was removed and radioactivity was counted. Specific 51Cr release was calculated as described previously (17); maximum release was calculated by adding 2% NP-40 to the wells, and spontaneous release was measured by using wells to which no effector cells were added. Spontaneous release was, in every case, less than 23% of the maximum release.
RESULTS
ICP47-1 and ICP47-2 inhibit human and monkey TAP.
In order to characterize the effects of ICP47-1 and ICP47-2 in cells of different species, the proteins were produced in bacteria as GST fusion proteins with the GST sequences removed by thrombin (15, 16). The ICP47-1 and ICP47-2 proteins were introduced into cells after permeabilization of the cells with streptolysin O as described previously (8, 15). To measure TAP activity, permeabilized cells were incubated with 125I-labelled peptides that gain access to the cytoplasm through the streptolysin pores. The peptides could then be transported into the lumen of the ER by TAP and be modified with N-linked oligosaccharides. Thus, binding of glycosylated peptides to concanavalin A-Sepharose could be used as an indirect measure of TAP activity.
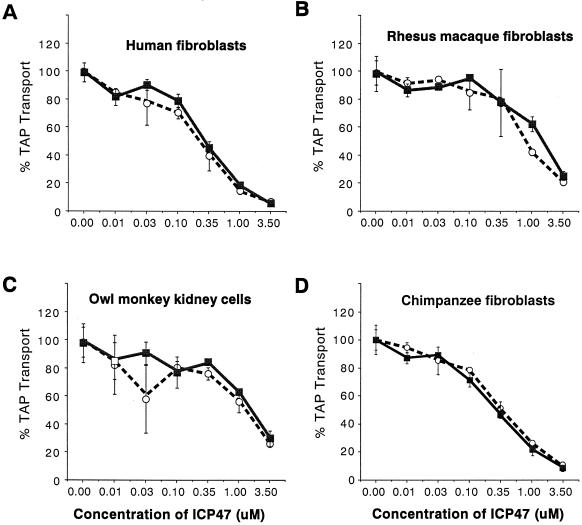
The steady-state levels of TAP can vary greatly in different cell types. Lymphocytes and professional antigen-presenting cells, for example, express high levels of TAP, which may explain poor inhibition of MHC class I antigen presentation in HSV-infected lymphocytes (reviewed in reference 19). By contrast, there are lower levels of TAP in human fibroblasts and antigen presentation is more effectively blocked after HSV infection. Furthermore, it may be dangerous to compare different cell types from various species because the degradation of radiolabelled peptides by cytosolic proteases (8a) can vary between cell types. With these considerations in mind, we chose to examine the effects of ICP47 in different species by using dermal fibroblasts in each case except for that of the owl monkey, where a kidney-derived cell line was used. In human fibroblasts, ICP47-1 and ICP47-2 inhibited TAP at an IC50 of approximately 0.3 μM (Fig. 1A). There was also inhibition of TAP in skin fibroblasts derived from rhesus macaques, but ICP47-1 and ICP47-2 were required at moderately higher concentrations; IC50 values were approximately 1.0 μM (Fig. 1B). Similarly, the TAP activity in OMK cells was inhibited by both proteins, but again the IC50 values were approximately 1.0 μM (Fig. 1C). In chimpanzee fibroblasts, ICP47-1 and ICP47-2 inhibited TAP at lower concentrations; IC50 values were approximately 0.3 μM, similar to those in human fibroblasts. Therefore, the TAP molecules produced in various monkey cells are sensitive to both ICP47-1 and ICP47-2.
FIG. 1.
Effects of ICP47-1 and ICP47-2 on TAP-mediated transport in human and monkey cells. Streptolysin-permeabilized cells were incubated with ICP47-1 (open circles) and ICP47-2 (filled squares) for 5 min at 4°C, after which a 125I-labelled peptide library was added for a further 10 min at 37°C. The transport assay was terminated, and peptides that had been glycosylated after transport into the lumen of the ER were quantified by measuring binding to concanavalin A. The level of transport measured in the absence of ICP47 was arbitrarily set at 100%.
ICP47-1 and ICP47-2 do not inhibit TAP in rodent fibroblasts.
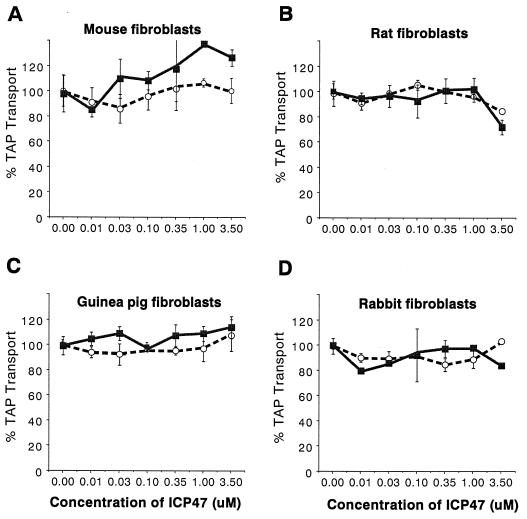
Previously, TAP assays performed using murine fibroblasts had indicated that neither ICP47-1 nor ICP47-2 was effective in inhibiting TAP until protein concentrations reached 10 to 35 μM, and even at high concentrations there was only partial inhibition (16). It appears that inhibition at these protein concentrations may not be biologically relevant because mouse cells infected with HSV-1 can be lysed by anti-HSV CTL (18). We attempted to determine whether ICP47-1 and ICP47-2 could inhibit TAP in fibroblasts from other rodents, animals that would make convenient animal models. ICP47-1 and ICP47-2 did not inhibit TAP activity in mouse fibroblasts (Fig. 2A), as was expected. There was also little inhibition of the rat TAP by either ICP47-1 or ICP47-2, although at 3.5 μM ICP47-2 there was a reproducible 20% inhibition (Fig. 2B). Similarly, there was little or no inhibition of TAP in guinea pig and rabbit fibroblasts (Fig. 2C and D). Therefore, rats, guinea pigs, and rabbits would not be appropriate models for testing the effects of ICP47.
FIG. 2.
Effects of ICP47-1 and ICP47-2 on TAP in rodent cells. Permeabilized skin fibroblasts were incubated with ICP47-1 (open circles) and ICP47-2 (filled squares), after which assays of TAP-mediated peptide transport were performed as described for Fig. 1.
Inhibition of TAP in bovine, porcine, and canine fibroblasts.
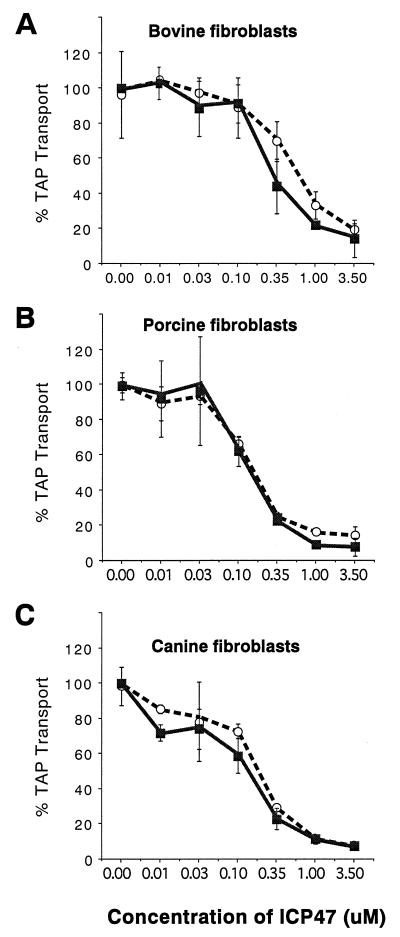
To examine animal species that are more closely related to primates, skin fibroblasts were derived from cows, pigs, and dogs. ICP47-2 inhibited TAP in bovine fibroblasts with IC50 values that ranged from 0.35 to 0.5 μM (Fig. 3A), whereas inhibition of the bovine TAP required moderately more ICP47-1 (IC50 values ranged from 0.5 to 0.8 μM). This difference between ICP47-1 and ICP47-2 was reproducible and was the only substantial difference between the two proteins in this study. Inhibition of porcine fibroblasts required lower concentrations of both ICP47-1 and ICP47-2 (IC50 values were approximately 0.15 to 0.2 μM [Fig. 3B]). Similarly, ICP47-1 and ICP47-2 caused 50% inhibition of TAP in canine fibroblasts at concentrations ranging from 0.15 to 0.25 μM (Fig. 3C). The results with pig and dog fibroblasts were surprising because the inhibition of porcine and canine TAP by both ICP47-1 and ICP47-2 occurred at lower concentrations than those necessary to inhibit human and monkey TAP.
FIG. 3.
Inhibition of TAP by ICP47-1 and ICP47-2 in bovine, porcine, and canine cells. Permeabilized skin fibroblasts were incubated with ICP47-1 (open circles) and ICP47-2 (filled squares), after which TAP transport assays were performed as described for Fig. 1.
Construction of recombinant HSV-1 expressing murine class I proteins and unable to express ICP47.
Previously, we utilized a recombinant HSV-1, F-US5MHC, which can express mouse MHC class I heavy-chain and β2-microglobulin proteins, in order to examine lysis of human and murine target cells by murine anti-HSV CTL (19). One might expect that mouse anti-HSV CTL would lyse the target cells when murine class I molecules and HSV antigens are delivered into heterologous cells. However, if ICP47 is effective in blocking class I antigen presentation, CTL lysis might not occur. In these earlier studies, we found that mouse cells infected with F-US5MHC were lysed by mouse anti-HSV CTL. In contrast, human fibroblasts infected with F-US5MHC were not lysed (19). Thus, it appeared that ICP47 produced resistance to murine, anti-HSV CTL in human fibroblasts but not in mouse fibroblasts. However, the relationship between ICP47 and CTL lysis was not directly tested in these earlier studies. In order to extend our present observations and determine whether ICP47 could cause resistance to CD8+ T lymphocytes in porcine, canine, and monkey cells, we constructed mutant versions of F-US5MHC that did not express ICP47.
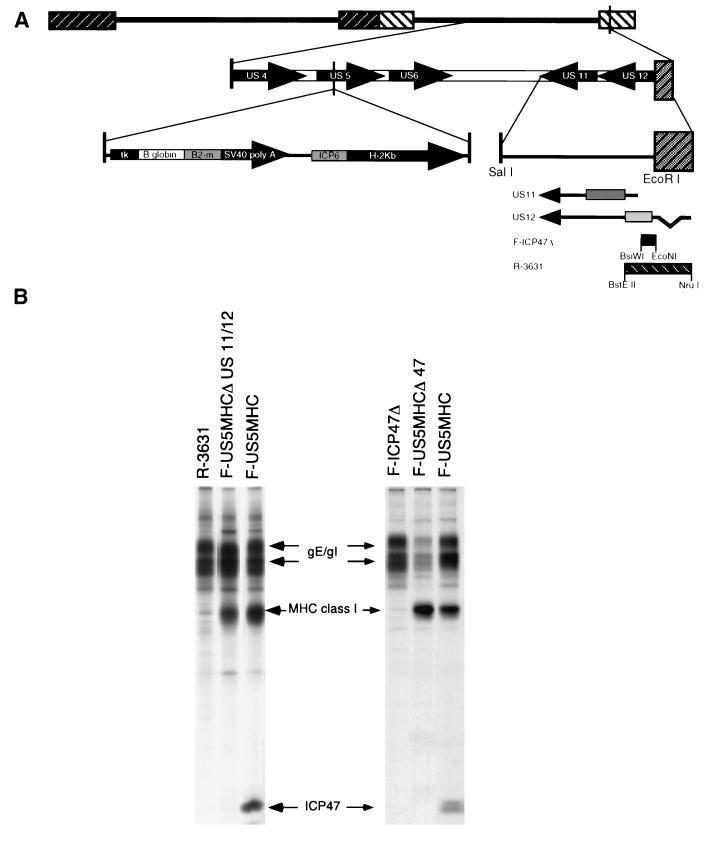
F-US5MHC contains the mouse β2-microglobulin and H-2Kb heavy-chain genes, both coupled to HSV promoters and inserted into the US5 gene, which is not essential for HSV replication (Fig. 4A) (19). Two different HSV-1 recombinants that are unable to express ICP47 were derived from F-US5MHC. The first recombinant virus was produced by coinfecting Vero cells with F-US5MHC and an HSV-1 ICP47− mutant, F-ICP47Δ, which contains a small deletion removing the ICP47 start codon and two downstream ATG codons (5). The neighboring US11 gene is intact in F-ICP47Δ. A second recombinant was derived by coinfecting cells with F-US5MHC and R3631, a mutant that contains a larger deletion affecting both the ICP47 (US12) and the US11 genes (11). Recombination between F-US5MHC and either R3631 or F-ICP47Δ gave rise to viruses able to express MHC class I but not ICP47. Parental and recombinant viruses were isolated from this coinfection and screened for expression of MHC class I (H-2Kb) and ICP47 by infection of R970 cells and immunoprecipitation of radiolabelled proteins. A recombinant, F-US5MHCΔICP47, derived from F-ICP47Δ, expressed murine MHC class I proteins but not ICP47 (Fig. 4B, right panel). This virus expressed US11 (not shown), as did the parent, F-ICP47Δ (5). A second recombinant, F-US5MHCΔUS11/12 (derived from R3631), expressed mouse MHC class I but not ICP47 (Fig. 4B, left panel) and, like R3631, did not express US11 (data not shown).
FIG. 4.
Construction of recombinant HSV-1 able to express murine MHC class I proteins and not ICP47. (A) Diagram depicting the genome of HSV-1. An HSV-1 recombinant, F-US5MHC, able to express the murine MHC class I heavy-chain and β2-microglobulin (β2-m) proteins, was previously described (18). In the US5 gene in F-US5MHC, the murine β2-microglobulin gene is coupled to the HSV-1 thymidine kinase (tk) promoter and the simian virus 40 (SV40) polyadenylation site, and the class I (H-2Kb) heavy-chain gene is coupled to the HSV-1 ICP6 promoter. HSV-1 mutants unable to express ICP47, F-ICP47Δ (5), or ICP47 and the neighboring US11 gene, R3631 (11), have also been described. F-ICP47Δ has a deletion of the N-terminal region of the ICP47 protein, while R3631 has a larger deletion that abolishes expression of ICP47 and US11. Vero cells were coinfected with F-US5MHC and either F-ICP47Δ or R-3631, so that recombination occurred in the infected cells. Viruses able to express murine class I proteins but unable to express ICP47, F-US5MHCΔ47 (derived from F-ICP47Δ), and F-US5MHCΔUS11/12 (derived from R3631) were isolated from these coinfections. (B) Expression of ICP47 and MHC class I proteins was assessed in cells infected with the recombinant viruses by infecting R970 cells, labelling the cells with [35S]methionine, and then simultaneously immunoprecipitating ICP47 and MHC class I proteins from cell extracts. The HSV immunoglobulin G Fc receptor composed of gE and gI was precipitated by the rabbit immunoglobulin G present in the anti-ICP47 and anti-MHC class I serum.
CTL lysis of mouse, porcine, monkey, and human cells infected with F-US5MHC, F-US5MHCΔICP47, and F-US5MHCΔUS11/12.
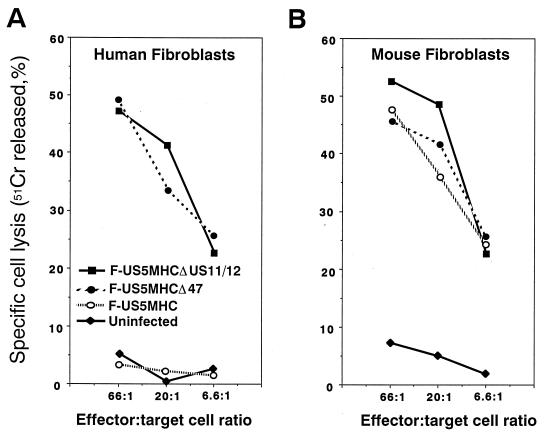
As in previous experiments (19), human fibroblasts infected with F-US5MHC, which expresses ICP47, were not lysed by CTL derived from HSV-infected C57BL/6 mice (Fig. 5A). In contrast, fibroblasts infected with either F-US5MHCΔICP47 or F-US5MHCΔUS11/12, neither of which expresses ICP47, were effectively lysed. This demonstrates that ICP47 is responsible for the resistance of these human fibroblasts to lysis by anti-HSV CTL. The effects of ICP47 were dramatically different when CTL lysis of murine fibroblasts was studied. Mouse fibroblasts infected with either F-US5MHCΔICP47, F-US5MHCΔUS11/US12, or F-US5MHC were all lysed by anti-HSV CTL (Fig. 5B); there was no obvious effect of ICP47 expression. Therefore, in these normal mouse fibroblasts, and previously in transformed mouse cells (19), ICP47 does not block class I antigen presentation and CTL lysis.
FIG. 5.
CTL lysis of human and mouse fibroblasts infected with recombinant HSV-1 expressing murine class I proteins. Fibroblasts were labelled with 51Cr for 8 to 12 h and infected for 3 h either with F-US5MHC, which expresses mouse class I (H-2Kb) proteins and ICP47, or with F-US5MHCΔ47 or F-US5MHCΔUS11/12, both of which express mouse class I proteins but not ICP47, or were left uninfected. Lymphocytes taken from lymph nodes of HSV-infected C57BL/6 (H-2Kb) mice were added to wells containing the 51Cr-labelled target cells for 4 h at 37°C. Release of 51Cr was measured and specific cell lysis was calculated.
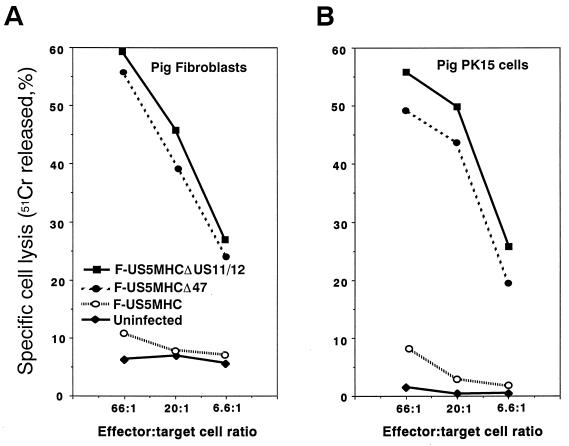
The effects of ICP47 on CTL lysis of pig cells were evaluated using normal pig skin fibroblasts as well as a transformed cell line—PK15 pig kidney cells. Pig fibroblasts and PK15 cells infected with recombinant HSV-1 F-US5MHCΔICP47 or with F-US5MHCΔUS11/12 were efficiently lysed by CTL (Fig. 6). Lysis of pig cells infected with F-US5MHC was low, similarly to that of uninfected cells. There were no obvious differences between the normal fibroblasts and the transformed kidney cells. Therefore, ICP47 effectively inhibits MHC class I antigen presentation in both these pig cells.
FIG. 6.
Lysis of pig cells infected with recombinant HSV-1 expressing murine class I proteins by mouse anti-HSV CTL. Cells were labelled with 51Cr and infected with F-US5MHC, F-US5MHCΔ47, or F-US5MHCΔUS11/12 for 3 h or left uninfected. Lymphocytes were taken from lymph nodes of HSV-infected C57BL/6 mice and were added to wells containing the target cells for 4 h at 37°C. Release of 51Cr was measured and specific cell lysis was calculated as described in Materials and Methods.
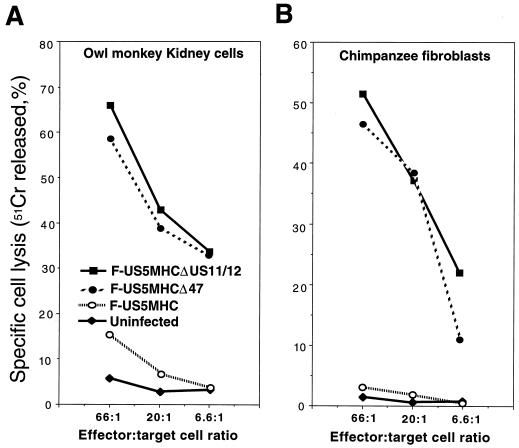
The results were extended to primate cells—OMK cells and chimpanzee skin fibroblasts. OMK cells infected with recombinant HSV-1 lacking ICP47, F-US5MHCΔICP47, and F-US5MHCΔUS11/12 were efficiently lysed by anti-HSV CTL, and cells infected with F-US5MHC were lysed poorly or not at all (Fig. 7A). Unfortunately, we were unable to perform CTL experiments with rhesus macaque fibroblasts. Fibroblasts from two different rhesus macaques did not serve as targets for murine anti-HSV CTL in several experiments. However, chimpanzee fibroblasts infected with F-US5MHCΔICP47 or F-US5MHCΔUS11/12 were lysed by anti-HSV CTL, and there was no lysis of F-US5MHC-infected cells (Fig. 7B). Other experiments in which African green monkey Vero kidney cells were infected with these recombinant viruses suggested that ICP47-1 also effectively blocks class I presentation in these cells (data not shown). Together, these results demonstrate that ICP47 can function to inhibit MHC class I antigen presentation and CTL lysis in pig cells and in nonhuman primate cells.
FIG. 7.
Lysis of monkey cells infected with recombinant HSV-1 expressing murine class I proteins by mouse anti-HSV CTL. Cells were labelled with 51Cr and infected with F-US5MHC, F-US5MHCΔ47, or F-US5MHCΔUS11/12 for 3 h or left uninfected. Lymphocytes taken from lymph nodes of HSV-infected C57BL/6 mice were added to wells containing the target cells for 4 h at 37°C. Release of 51Cr was measured and specific cell lysis was calculated as described in Materials and Methods.
DISCUSSION
The molecular details of how HSV-1 ICP47 blocks the MHC class I antigen presentation pathway by inhibiting human TAP have been described in some detail. However, important questions of how ICP47 functions in vivo have not been addressed. We suspect that ICP47 can function to shield HSV from CD8+-T-lymphocyte responses during virus infection in the epithelium and possibly during acute infection in the nervous system, but this has not been tested (reviewed in reference 9). Beyond the virology, there is substantial interest in the prospect of using ICP47 as a specific and selective immunosuppressive agent to block inappropriate CD8+-T-cell responses, e.g., against adenovirus gene therapy vectors, in transplantation, or in autoimmunity. Our efforts to investigate ICP47’s effects in vivo have been hindered by observations that neither ICP47-1 nor ICP47-2 blocks the murine TAP effectively (1, 8, 15, 16).
It is important to note that the effects of ICP47 during HSV replication in mice are more complicated than might be predicated from previous biochemical studies involving cultured mouse cells. We recently observed that an HSV-1 ICP47− mutant was markedly less neurovirulent (unable to cause neurologic disease and encephalitis) than wild type HSV-1 in mice, yet the mutant replicated normally in the corneal epithelium or in the periocular skin (5). When animals were depleted of CD8+ T cells, the ICP47− mutant caused neurologic disease similar to that caused by wild-type HSV-1. Since MHC class I and TAP are apparently expressed at low or undetectable levels in the nervous system, there may be inhibition of TAP by ICP47 in this tissue. However, effects of ICP47 in mouse epithelial tissues were not observed, and therefore an animal model that reflects the effect of ICP47 on human TAP is highly desirable.
Both ICP47-1 and ICP47-2 inhibited TAP in monkey cells (fibroblasts from rhesus macaques and chimpanzees and OMK cells). Inhibition of the rhesus macaque and owl monkey TAP required concentrations approximately threefold higher (1.0 μM) than those that were required to inhibit TAP in human fibroblasts (0.3 μM). TAP transport in chimpanzee fibroblasts was inhibited at ICP47-1 and ICP47-2 concentrations more similar to those required to inhibit TAP in human fibroblasts. It is not clear whether these differences between different primate cells relate to levels of TAP expressed in the cells or whether certain TAP molecules are less sensitive to ICP47. Related to this point, there is evidence that human fibroblasts and lymphocytes differ in their sensitivity to ICP47-1: TAP in human lymphocytes was inhibited at an ICP47-1 concentration of 1.0 μM, three times the concentration required to inhibit TAP in human fibroblasts (15), and lymphocytes express much higher levels of TAP and other MHC proteins than fibroblasts. These differences between cell types expressing an identical TAP may be functionally important, especially when coupled with poor replication of HSV in lymphocytes. Human lymphocytes infected with HSV-1 can be lysed by anti-HSV CTL (12, 14, 18).
A number of factors can influence the outcome of TAP assays, including entry into the cytoplasm and proteolysis of the protein by cytosolic proteases. Therefore, the concentrations of ICP47 required to inhibit TAP in the TAP assays are probably relative rather than absolute. We performed CTL assays as a second line of evidence for species differences in susceptibility to ICP47. In each case, the CTL assays confirmed the results from TAP assays. CTL assays involving owl monkey and chimpanzee cells demonstrated that ICP47 could inhibit MHC class I antigen presentation, and there was inhibition of TAP by ICP47-1 and ICP47-2 in TAP assays with OMK cells and rhesus macaque and chimpanzee fibroblasts. Owl monkeys succumb to low doses of wild-type HSV, and chimpanzees are endangered; thus, these animals may not be useful as animal models. More likely, rhesus macaques can be used in these studies, since the macaque TAP was sensitive to both ICP47-1 and ICP47-2. Furthermore, cell surface expression of MHC class I in rhesus macaque tissues can be reduced to background levels by infection of the tissues with an adenovirus expressing ICP47-1 (data not shown).
ICP47-1 and ICP47-2 TAP did not inhibit TAP in fibroblasts taken from mice, rats, guinea pigs, and rabbits. Previously, we observed some (20%) inhibition of murine TAP expressed in insect microsomes at 3.5 μM concentrations of ICP47-1 and ICP47-2, and this inhibition increased to over 50% with 30 μM concentrations (16). Here, there was little or no inhibition of TAP in permeabilized mouse fibroblasts incubated with 3.5 μM ICP47-1 or ICP47-2. This difference probably reflects the sensitivity of the two different assays; with permeabilized cells, the protein concentration in the cytosol may not immediately reach that applied to the cells. However, it is unlikely that sufficient ICP47 concentrations can be reached in HSV-infected mouse fibroblasts to inhibit the class I pathway because there is no CTL lysis of mouse fibroblasts (18) (Fig. 5). Based on the TAP assays, the case is the same for rat, guinea pig, and rabbit fibroblasts. These animals will therefore probably not be useful as models.
Given these observations, we were surprised to find that TAP activity was inhibited in bovine, porcine, and canine fibroblasts and, in the case of pig and dog cells, at lower ICP47-1 and ICP47-2 concentrations than those observed with three different monkey cell lines and three different human fibroblast lines. It is possible that pig and dog TAP are more susceptible to ICP47 than even human TAP. Also possible are differences in peptide proteolysis, entry of peptide into the cytoplasm, and reduced levels of TAP in pig and dog cells relative to human cells. However, these possibilities appear to be less likely because the TAP activity (the amount of peptide transported) in pig PK15 cells was similar to that in human fibroblasts. CTL assays confirmed that MHC class I antigen presentation was effectively inhibited by ICP47 in porcine skin fibroblasts and transformed pig PK15.
In summary, TAP and MHC class I antigen presentation was effectively blocked in pig, dog, cow, and nonhuman primate cells by ICP47. There were no differences between ICP47-1 and ICP47-2 except minor ones in bovine cells, though the proteins have only 42% sequence identity. Pigs and dogs have a number of advantages as models, though little is known about how HSV replicates in these animals. Studies of autoimmunity and transplantation frequently utilize pigs and dogs, and there is intense interest in the use of pig tissues in xenotransplantation.
ACKNOWLEDGMENTS
We thank Steven Primorac and Nico van Schoot for excellent technical assistance. Andrew Townsend and Kim Goldsmith helped create the figures. We are indebted to Bernard Roizman for HSV-1 recombinant R3631 and for anti-US11 sera and to Chris Walker, who provided chimpanzee fibroblasts.
This work was supported by NIH grant EY11245 to D.C.J. and NIH grants to H.P.
REFERENCES
- 1.Ahn K, Meyer T H, Uebel S, Sempe P, Djaballah H, Yang Y, Peterson P A, Fruh K, Tampe R. Molecular mechanism and species specificity of TAP inhibition by herpes simplex virus ICP47. EMBO J. 1996;15:3247–3255. [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn K, Gruhler A, Galocha B, Jones T R, Wiertz E J, Ploegh H L, Peterson P, Yang Y, Fruh K. The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity. 1997;6:613–621. doi: 10.1016/s1074-7613(00)80349-0. [DOI] [PubMed] [Google Scholar]
- 3.Fruh K, Ahn K, Djaballah H, Sempe P, van Endert P, Tampe R, Peterson P, Yang Y. A viral inhibitor of peptide transporters for antigen presentation. Nature. 1995;375:415–418. doi: 10.1038/375415a0. [DOI] [PubMed] [Google Scholar]
- 4.Galocha B, Hill A B, Cook R, Barnett B, Nolan A, Raimondi A, McGeoch D, Ploegh H L. Identification of the active site of ICP47, an herpes simplex virus encoded inhibitor of TAP. J Exp Med. 1997;185:1565–1572. doi: 10.1084/jem.185.9.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldsmith K, Chen W, Johnson D C, Hendricks R L. ICP47 enhances herpes simplex virus neurovirulence by blocking the CD8+ T cell response. J Exp Med. 1998;187:341–348. doi: 10.1084/jem.187.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanke T, Graham F L, Rosenthal K L, Johnson D C. Identification of an immunodominant cytotoxic T-lymphocyte recognition site in glycoprotein B of herpes simplex virus by using recombinant adenovirus vectors and synthetic peptides. J Virol. 1991;65:1177–1186. doi: 10.1128/jvi.65.3.1177-1186.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heemels M T, Schumacher T N, Wonigeit K, Ploegh H L. Peptide translocation by variants of the transporter associated with antigen processing. Science. 1993;262:2059–2063. doi: 10.1126/science.8266106. [DOI] [PubMed] [Google Scholar]
- 8.Hill A, Jugovic P, York I, Russ G, Bennink J, Yewdell J, Ploegh H L, Johnson D. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 8a.Hill, A. Unpublished data.
- 9.Johnson, D. C., and A. B. Hill. Herpesvirus evasion of the immune system. Curr. Top. Microbiol. Immunol., in press. [DOI] [PubMed]
- 10.Koelle D M, Tigges M A, Burke R L, Symington F W, Riddell S R, Abbo H, Corey L. Herpes simplex virus infection of human fibroblasts and keratinocytes inhibits recognition by cloned CD8+ cytotoxic T lymphocytes. J Clin Invest. 1993;91:961–968. doi: 10.1172/JCI116317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mavromara-Nazos P, Ackermann M, Roizman B. Construction and properties of a viable herpes simplex virus 1 recombinant lacking coding sequences of the α47 gene. J Virol. 1986;60:807–812. doi: 10.1128/jvi.60.2.807-812.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Posavad C M, Rosenthal K L. Herpes simplex virus-infected human fibroblasts are resistant to and inhibit cytotoxic T-lymphocyte activity. J Virol. 1992;66:6264–6272. doi: 10.1128/jvi.66.11.6264-6272.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith M H, Barber B H. The conformational flexibility of class I H-2 molecules as revealed by anti-peptide antibodies specific for intracytoplasmic domains: differential reactivity of S2-microglobulin “bound” and “free” H-2Kb heavy chains. Mol Immunol. 1990;27:169–180. doi: 10.1016/0161-5890(90)90112-d. [DOI] [PubMed] [Google Scholar]
- 14.Tigges M A, Leng S, Johnson D C, Burke R L. Human herpes simplex virus (HSV)-specific CD8+ CTL clones recognize HSV-2-infected fibroblasts after treatment with IFN-gamma or when virion host shutoff functions are disabled. J Immunol. 1996;156:3901–3910. [PubMed] [Google Scholar]
- 15.Tomazin R, Hill A B, Jugovic P, York I, van Endert P, Ploegh H L, Andrews D W, Johnson D C. Stable binding of the herpes simplex virus ICP47 protein to the peptide binding site of TAP. EMBO J. 1996;15:3256–3266. [PMC free article] [PubMed] [Google Scholar]
- 16.Tomazin R, van Schoot N E G, Goldsmith K, Jugovic P, Sempe P, Fruh K, Johnson D C. Herpes simplex virus type 2 ICP47 inhibits human TAP but not mouse TAP. J Virol. 1998;72:2560–2563. doi: 10.1128/jvi.72.3.2560-2563.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker C M, Paetku V, Rawls W E, Rosenthal K. Abrogation of anti-Pinchinde virus cytotoxic T cell memory by cyclophosphamide and restoration by coinfection and interleukin 2. J Immunol. 1985;135:1401–1407. [PubMed] [Google Scholar]
- 18.York, I., and D. C. Johnson. Unpublished observations.
- 19.York I A, Roop C, Andrews D W, Riddell S R, Graham F L, Johnson D C. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell. 1994;77:525–535. doi: 10.1016/0092-8674(94)90215-1. [DOI] [PubMed] [Google Scholar]