Abstract
Selected glucagon-like peptide-1 receptor agonists (GLP-1RAs) and sodium glucose cotransporter-2 inhibitors (SGLT-2i) have cardioprotective effects in patients with type 2 diabetes (T2D) and elevated cardiovascular risk. Prescription and consistent use of these medications are essential to realize their benefits. In a nationwide de-identified US administrative claims database of adults with T2D, the prescription practices of GLP-1RAs and SGLT-2i were evaluated across guideline-directed comorbidity indications in 2018–2020. The monthly fill rates were assessed for 12 months following initiation of therapy by calculating the proportion of days with consistent medication use. Among 587,657 individuals with T2D, 80,196 (13.6%) were prescribed GLP-1RAs and 68,149 (11.5%) SGLT-2i during 2018–2020, representing 12.9% and 11.6% of individuals with indications for each medication, respectively. Among new initiators, one-year fill rate was 52.5% for GLP-1RAs and 52.9% for SGLT-2i, which was higher for patients with commercial insurance than those with Medicare Advantage plans for both GLP-1RAs (59.3% vs 51.0%, p-value<0.001) and SGLT-2i (63.4% vs 50.3%, p-value<0.001). After adjusting for comorbidities, there were higher rates of prescription fills for patients with commercial insurance (OR=1.17, 95%CI [1.06–1.29] for GLP-1RAs, and 1.59 [1.42–1.77] for SGLT-2i); and higher income (OR=1.09 [1.06–1.12] for GLP-1RAs, and 1.06 [1.03–1.1] for SGLT-2i). In 2018–2020, use of GLP-1RAs and SGLT-2i remained limited to fewer than 1 in 8 individuals with T2D and indications, with one-year fill rates around 50%. The low and inconsistent use of these medications compromises their longitudinal health outcomes benefits in a period of expanding indications for their use.
Keywords: Glucagon-like peptide-1 receptor agonists, Sodium glucose cotransporter-2 inhibitors, Prescription fill, Insurance
Introduction
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) and sodium glucose cotransporter-2 inhibitors (SGLT-2i) are recommended in clinical practice guidelines for treatment of patients with type 2 diabetes and compelling cardiovascular and kidney indications, independent of glucose control.1–5 This is underpinned by the results from seminal, large outcome trials in patients with type 2 diabetes reported between 2015 and 2019.6,7 Guidelines and professional society recommendations began to endorse their use as early as in 2017, with consistent Class I recommendations beginning in 2018, with omission of all glucose control considerations harmonious across guidelines and society recommendations by 2020. Both drug classes confer protection against major atherosclerosis-based adverse cardiovascular events in patients with atherosclerotic cardiovascular disease (ASVCD).8 SGLT-2i also lower the risk of hospitalization for heart failure and prevent worsening kidney function in patients with type 2 diabetes and ASCVD risk or with diabetic kidney disease.6 Despite the expanding indications for GLP-1 RAs and SGLT-2i in clinical guidelines and society recommendations for cardiovascular and kidney benefits, the overall prescription rates of these medications remain low.9–12 Furthermore, the clinical benefits of GLP-1 RAs and SGLT-2i are only achieved if patients continually take these medications after prescription, underscoring the importance of both prescription and consistent use. In the absence of high-quality data on the evolving use of GLP-1 RAs and SGLT-2i in the real world, assessment of their consistent use after initiation of therapy has been challenging. In addition, affordability of these agents remains a major concern, potentially affecting both their initiation and continuation of use. In this US national study, we evaluated the contemporary patterns of prescription for GLP-1 RAs and SGLT-2i among individuals with type 2 diabetes meeting evidence-based indication for their use and assessed the actual use of these agents by measuring their monthly fill rates after starting treatment. The patterns of use of these medications were evaluated across key patient subgroups defined by guideline- and professional-society recommended clinical indications for their use and the insurance coverage of treated individuals.
Methods
We used Optum Labs’ de-identified administrative claims data, which contains longitudinal enrollment records, medical claims, and pharmacy claims for Medicare Advantage and commercially insured beneficiaries and represents a diverse population across the US.13 Optum Labs is the research and development arm of the UnitedHealth Group. Data is sourced from covered entities that permit de-identification of their data under applicable Business Associate Agreements (BAA). The administrative claims data include information on patient demographics, type of insurance plan (Medicare Advantage vs commercial), healthcare conditions, and their treatments captured as standard health insurance claims.
The study population included individuals 18 years of age or older with 36 months of continuous enrollment in Medicare Advantage with Part D coverage or commercial insurance with pharmacy coverage between January 2018 through December 2020 who had two or more claims more than 90 days apart with a principal or secondary diagnosis of type 2 diabetes (Supplementary Table 1).
The diagnosis of type 2 diabetes was defined based on International Classification of Disease tenth revision (ICD-10) codes. Antihyperglycemic treatment was defined as the receipt of one or more agents included in Standards of Medical Care in Diabetes by the American Diabetes Association.14 These included GLP-1 RAs, SGLT-2i, metformin, sulfonylureas, thiazolidinediones, dipeptidyl peptidase-4 (DPP-4) inhibitors, and insulins. The individual agents included in each drug class are presented in Supplementary Table 2. Fixed-dose drug combinations were considered equivalent to taking the individual component medications separately. Drug information was obtained from pharmacy claims corresponding to a cumulative supply >30 days between January 1, 2018, and December 31, 2020. The Medicare Advantage and commercially insured enrollees in the study represented all individuals with available claims in the database who met the inclusion/exclusion criteria.
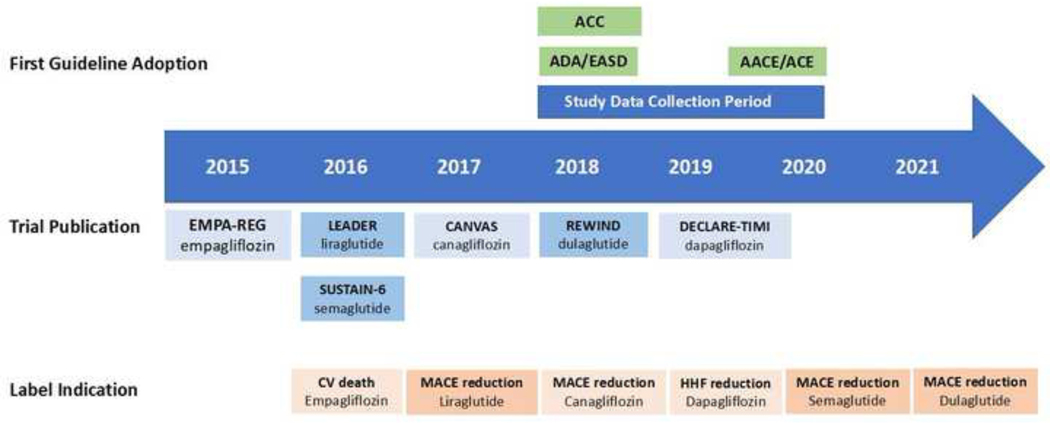
We used clinical practice recommendations of the American Diabetes Association (ADA), American College of Cardiology (ACC), and American Association of Clinical Endocrinologists and American College of Endocrinology (AACE/ACE) for management of type 2 diabetes to define indications for GLP-1 RAs and SGLT-2i. These indications included heart failure and diabetic nephropathy for SGLT-2i, and ASCVD for both GLP-1 RAs and SGLT-2i.2,14,15 The criteria for identifying these indications were consistent with prior studies and are detailed in the Supplementary Table 3.6,7 Figure 1 represents the timeline of data collection in the context of clinical trials and approval of cardiovascular and kidney indications in product labels for individual agents within each drug class.
Figure 1. Timeline of data collection, approval of drug labels, and uptake by clinical practice guidelines for GLP-1 RAs and SGLT-2i.
Figure represents the timeline of FDA approval for GLP-1 RA and SGLT-2i drug labels, adoption by clinical practice guidelines, and data collection in this study. Abbreviations: AACE, American Association of Clinical Endocrinologists; ACC, American College of Cardiology; ACE, American College of Endocrinology; ADA, American Diabetes Association; CV, cardiovascular; EASD, European Association of the Study of Diabetes; HHF, hospitalization for heart failure; MACE, major adverse cardiovascular event.
Individuals with claim-based evidence of contraindications for each medication based on the US Food and Drug Administration (FDA) product information were considered non-eligible. These conditions included medullary thyroid carcinoma and multiple endocrine neoplasia syndrome type 2 for GLP-1 RAs, and chronic kidney disease (CKD) stage IV-V, end stage kidney disease (ESKD), and dialysis for SGLT-2i (Supplementary Table 3).
We included demographic characteristics of the study population, including age, sex, type of health insurance plan, neighborhood income (as identified by zip code), and comorbid conditions included in the Diabetes Complications and Severity Index (DCSI) and Charlson Comorbidity Index (CCI).16,17 In addition, the use of other cardiovascular therapies was identified within pharmacy claims, which included statins, beta blockers, angiotensin converting enzyme inhibitors (ACEI), angiotensin II receptor blocker (ARB), and oral anticoagulants (including warfarin and direct-acting oral anticoagulants). The characteristics of the study population and medication use were obtained from insurance claims in 2018 (the baseline study year).
The study focused on two key outcomes: 1) proportionate rates of prescription for GLP-1 RAs and SGLT-2i among individuals with type 2 diabetes, overall and among those with compelling indications; and 2) monthly fill rates for GLP-1 RAs and SGLT-2i for 12 months after initial prescription of the therapy.
The proportionate use of GLP-1 RAs and SGLT-2i were defined as the proportion of individuals with any prescription for these agents during the 3-year window from January 2018 through December 2020. We also assessed trends in the counts of new prescriptions for each drug class among eligible individuals between January 2019 through December 2020. New initiators were defined as individuals who were initially prescribed a GLP-1 RA and/or an SGLT-2i after at least a 12-month period without any prescriptions for the respective medications. Identification of new initiators was irrespective of prior treatments with other anti-hyperglycemic drug classes, including previous treatment with a GLP-1 RA among SGLT-2i initiators and vice versa. Data from January through December 2018 were used to ensure no prescription prior to starting the treatment in new initiators.
The monthly fill rates for GLP-1 RAs and SGLT-2i were assessed by calculating the proportion of days covered (PDC) among new initiators. PDC is defined as the proportion of days in a certain period of time with evidence of medication dispense based on pharmacy claims and is a measure of consistent fill of the medication.18 To ensure that each person had at least 12 months of claims to provide fill information, these analyses were restricted to new initiators in 2019, with the first fill occurring between January through December 2019, and the subsequent fill data derived from pharmacy claims through December 2020. Monthly fill rates for metformin and sulfonylureas were similarly assessed in new initiators of each drug class in the similar period to compare with GLP-1 RAs and SGLT-2i.
Categorical variables are presented as frequency and percentages, and continuous variables as mean and standard deviations or median and interquartile ranges, as appropriate. Differences in characteristics across categories of individuals based on indications for GLP-1 RAs and SGLT-2i were compared using chi square for categorical variables and analysis of variance for continuous variables.
Independent predictors of consistent fills for each drug class were tested using logistic regression. PDC for GLP-1 RAs and SGLT-2i were averaged among new initiators of each medication over 12 months after initiation of treatment and then dichotomized into a binary outcome variable, comparing the highest tertile with the lower two tertiles of PDC. Neighborhood income was also dichotomized into a binary variable, comparing top quartile with the lower three quartiles before inclusion in the regression model. Age, sex, neighborhood income, type of insurance plan, DCSI score, CCS score, ASCVD, heart failure, and diabetic nephropathy were considered as independent factors in the model.
Analyses were performed using R 3.4.0 (CRAN). All hypothesis tests were 2-sided, with a level of significance set at 0.05. The UnitedHealth Group Office of Human Research and the Yale Institutional Review Board exempted this study from review and waived informed consent as the study was limited to retrospective analyses of de-identified data and compliant with the Health Insurance Portability and Accountability Act (HIPAA) of 1996.
Results
We identified 587,657 individuals with type 2 diabetes and 36 months of continuous enrollment. Demographic and clinical characteristics of study population across eligibility groups are represented in Table 1. The mean age of the study population was 72.9 (9.2) years, 52.9% were women, median neighborhood income was $53,317 [IQR $46,338-$62,553], 89% had Medicare Advantage coverage and 11% were commercially insured. The study population included 209,983 individuals with ASCVD (35.7%), 68,506 with heart failure (11.6%), and 159,436 with diabetic nephropathy (27.1%).
Table 1.
Demographic and clinical characteristics of study population across eligibility for GLP-1 RAs and SGLT-2i
| Overall | ASCVD | Heart Failure | Diabetic Nephropathy | |
|---|---|---|---|---|
| Overall | 587,657 | 209,983 | 68,506 | 159,436 |
| Age (year) | 72.89 (9.2) | 74.93 (7.9) | 74.79 (8.5) | 75.13 (8.1) |
| Female Sex | 310,842(52.9) | 96,869 (46.1) | 35,469 (51.8) | 81,870 (51.3) |
| Health Insurance | ||||
| Commercial | 64,740(11) | 13,518 (6.4) | 3,456 (5.0) | 9,643 (6.0) |
| Medicare Advantage | 522,917(89) | 196,465(93.6) | 65,050(95.0) | 149,793(94.0) |
| Neighborhood Income ($) | 53,317 [46,338–62,553] | 52,782 [46,315–62,000] | 52,389 [45,551–61,695] | 53,063 [46,338–62,036] |
| Medications | ||||
| Metformin | 356,215(60.6) | 114,838 (54.7) | 30,749 (44.9) | 72,530 (45.5) |
| Sulfonylureas | 167,669(28.5) | 59,835 (28.5) | 19,085 (27.9) | 50,809 (31.9) |
| Thiazolidinediones | 36,069(6.1) | 11,220 (5.3) | 2,781 (4.1) | 10,928 (6.9) |
| DPP-4 Inhibitors | 76,923(13.1) | 28,189 (13.4) | 8,976 (13.1) | 24,506 (15.4) |
| Meglitinides | 5,441(0.9) | 2,340 (1.1) | 738 (1.1) | 2,158 (1.4) |
| Insulin Long-acting | 115,333(19.6) | 49,925 (23.8) | 20,619 (30.1) | 44,944 (28.2) |
| Insulin Short-acting | 70,871(12.1) | 33,328 (15.9) | 14,976 (21.9) | 30,526 (19.1) |
| Statin | 435,118(74.0) | 169,353 (80.7) | 53,567 (78.2) | 124,619 (78.2) |
| ACEI | 247,882(42.2) | 88,085 (41.9) | 27,585 (40.3) | 66,292 (41.6) |
| ARB | 191,602(32.6) | 73,101 (34.8) | 25,548 (37.3) | 58,944 (37.0) |
| Beta Blocker | 251,746(42.8) | 129,870 (61.8) | 51,161 (74.7) | 87,119 (54.6) |
| Oral Anticoagulant | 58,703(10.0) | 33,663 (16.0) | 19,959 (29.1) | 21,999 (13.8) |
| DCSI Score | 1.88 (1.83) | 3.34 (1.77) | 4.13 (1.78) | 3.30 (1.91) |
| Cardiovascular Disease | 229,516(39.1) | 181,179 (86.3) | 66,469 (97.0) | 82,556 (51.8) |
| Cerebrovascular Disease | 58,244(9.9) | 55,380 (26.4) | 13,262 (19.4) | 21,308 (13.4) |
| Metabolic Disease | 18,031(3.1) | 9,039 (4.3) | 4,307 (6.3) | 7,661 (4.8) |
| Neuropathy | 169,555(28.9) | 78,429 (37.4) | 28,340 (41.4) | 58,205 (36.5) |
| Peripheral Vascular Disease | 105,680(18.0) | 74,753 (35.6) | 22,744 (33.2) | 40,126 (25.2) |
| Retinopathy | 100,014(17.0) | 42,574 (20.3) | 15,280 (22.3) | 36,180 (22.7) |
| Nephropathy | 159,436(27.1) | 74,585(35.5) | 34,234(50.0) | 159,436(100) |
Data represent mean (SD) for age, median (IQR) for neighborhood income, and number (percent) for other variables. Abbreviations: ASCVD, atherosclerotic cardiovascular disease; DPP-4, dipeptidyl peptidase-4; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor II blocker; DCSI, diabetes complications and severity index.
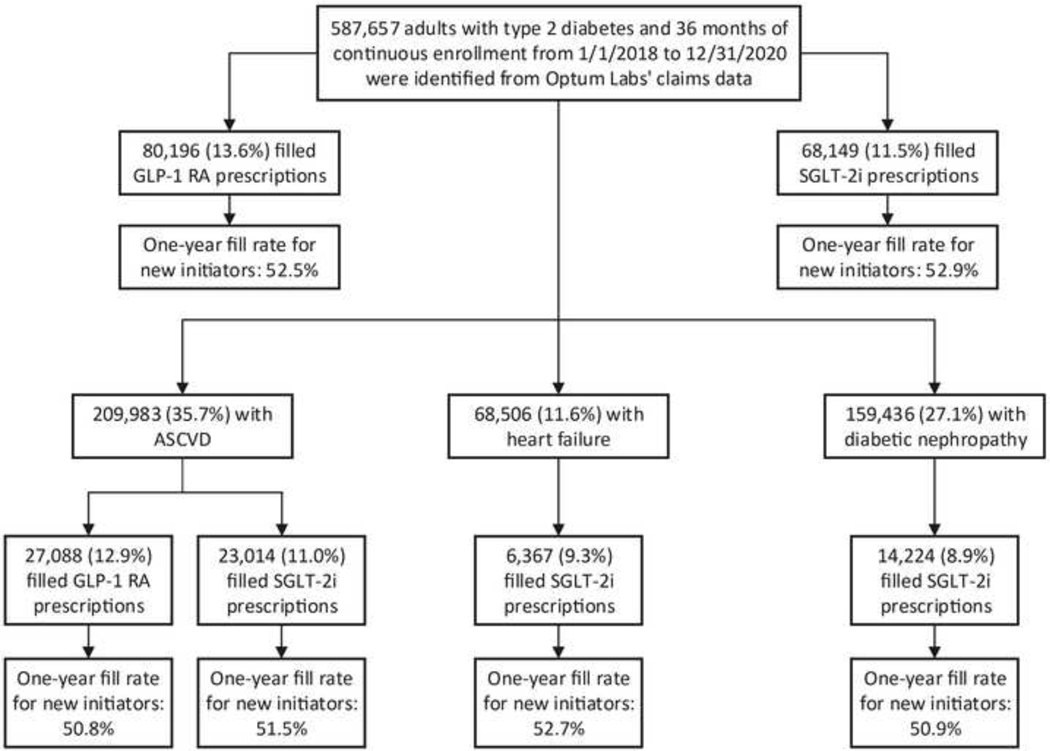
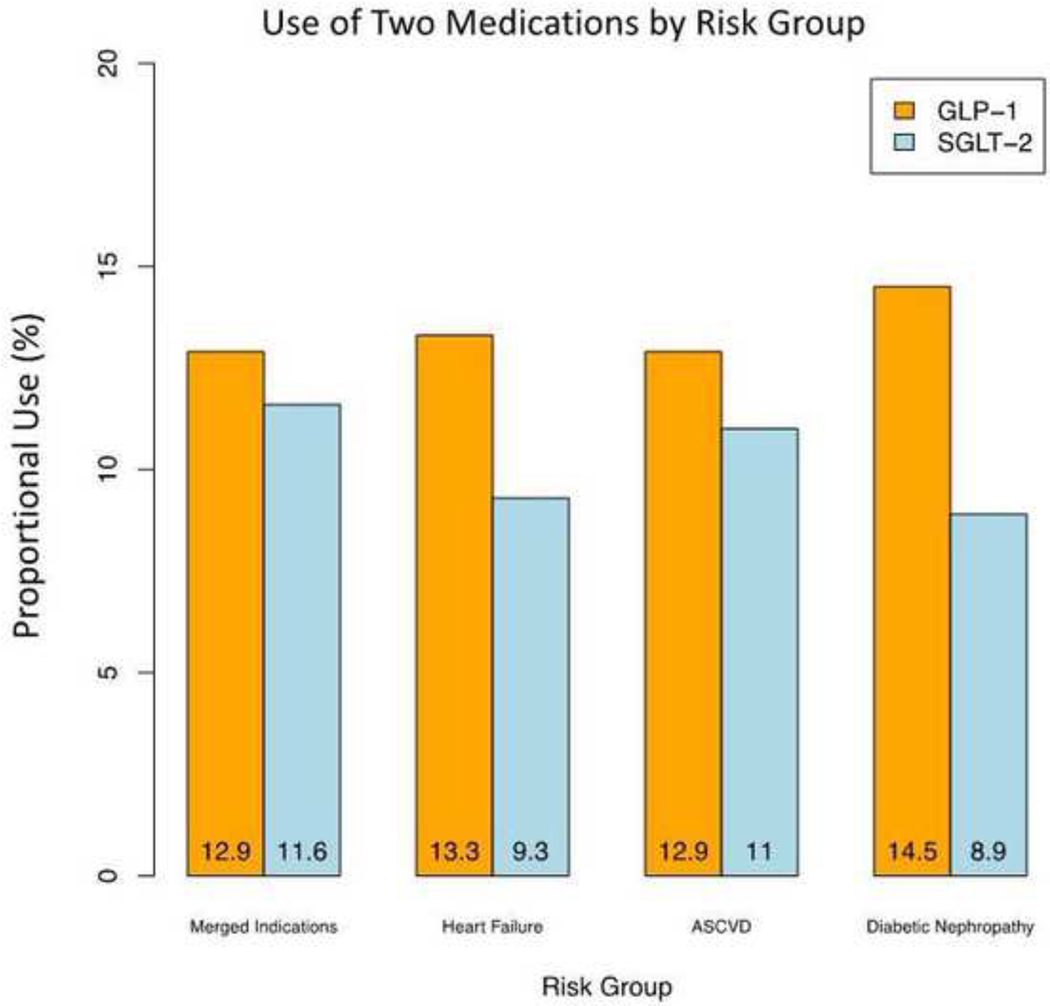
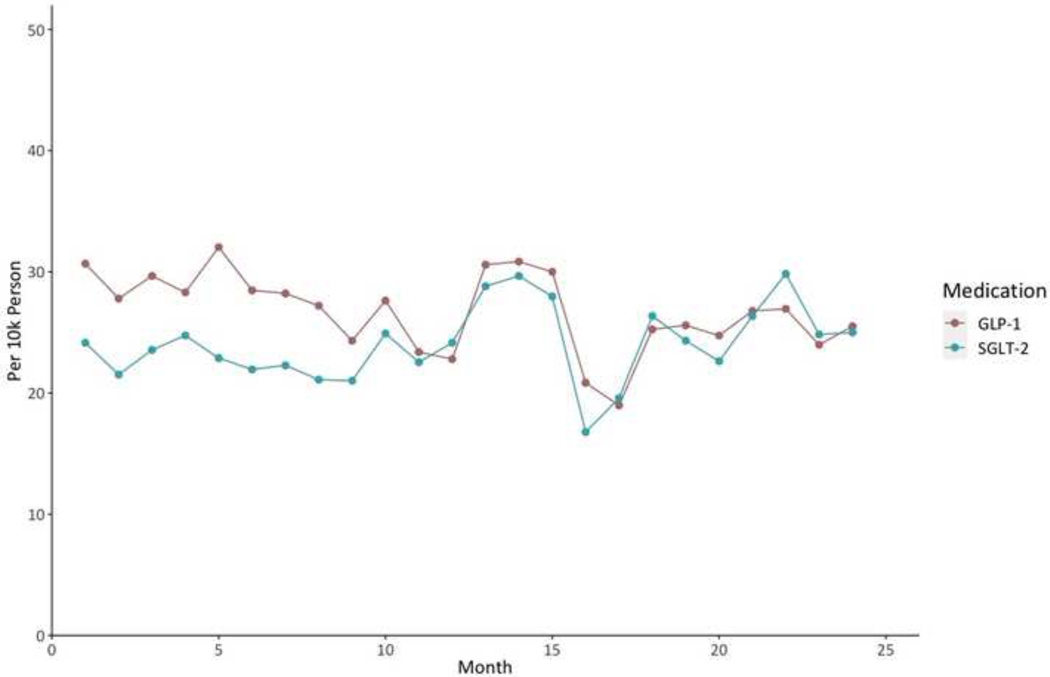
Overall, 80,196 individuals filled GLP-1 RA prescriptions in 2018–2020, representing 13.6% of individuals with type 2 diabetes and 12.9% of those with type 2 diabetes and coexisting established ASCVD (Figure 2, Figure 3). For SGLT-2i, a total of 68,149 individuals filled a prescription in this drug class, representing 11.5% of individuals with type 2 diabetes and 11.6% of those with type 2 diabetes and any indication, including 23,014 individuals with ASCVD (11%), 6,367 with heart failure (9.3%), and 14,224 with diabetic nephropathy (8.9%) (Figure 2, Figure 3). During 2019–2020, there was no significant trend in the counts of new prescriptions for SGLT-2i (slope 0.13 per 10k person [95% confidence interval (CI): −0.05_0.32]); however, the counts of new prescriptions for GLP-1 RAs significantly decreased (slope −0.22 per 10k person [95% CI: −0.40_−0.04]) among eligible patients during this period (Figure 4). Demographic and clinical characteristics of individuals filling GLP-1 RAs and SGLT-2i are presented in Supplementary Table 4.
Figure 2. Study flow diagram.
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; GLP-1 RA; glucagon-like peptide-1 receptor agonist; SGLT-2i, sodium glucose cotransporter 2 inhibitor.
Figure 3. Use of GLP-1 RAs and SGLT-2i among eligible patients.
Data represent percentage of individuals filling a prescription for each medication overall and in selected subgroups by indication. Indications for each drug class were evaluated independently based on guideline and professional society recommendations and included ASCVD for GLP-1 RAs, and ASCVD, heart failure, and diabetic nephropathy for SGLT-2i. Abbreviations: ASCVD, atherosclerotic cardiovascular disease; GLP-1; glucagon-like peptide-1 receptor agonist; SGLT-2, sodium glucose cotransporter 2 inhibitor.
Figure 4. Trends of the counts of new prescriptions for GLP-1 RAs and SGLT-2i during 2019–2020.
Data represent number of new prescriptions for GLP-1 RAs and SGLT-2i per 10k eligible individuals during the study period from 2019 through 2020. Abbreviations: GLP-1; glucagon-like peptide-1 receptor agonist; SGLT-2, sodium glucose cotransporter 2 inhibitor.
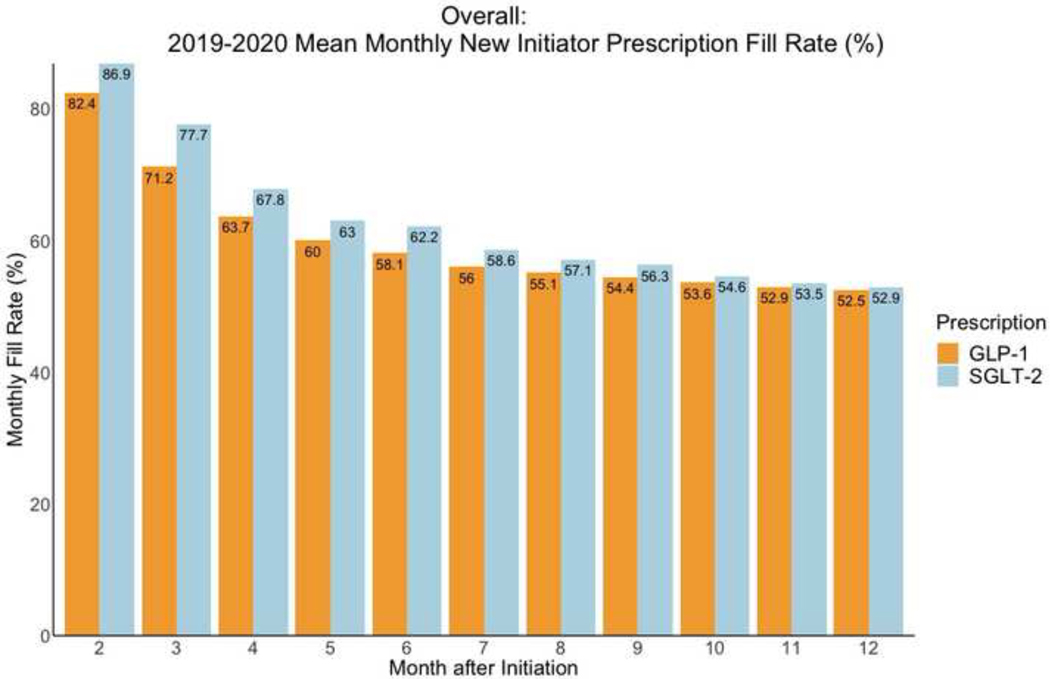
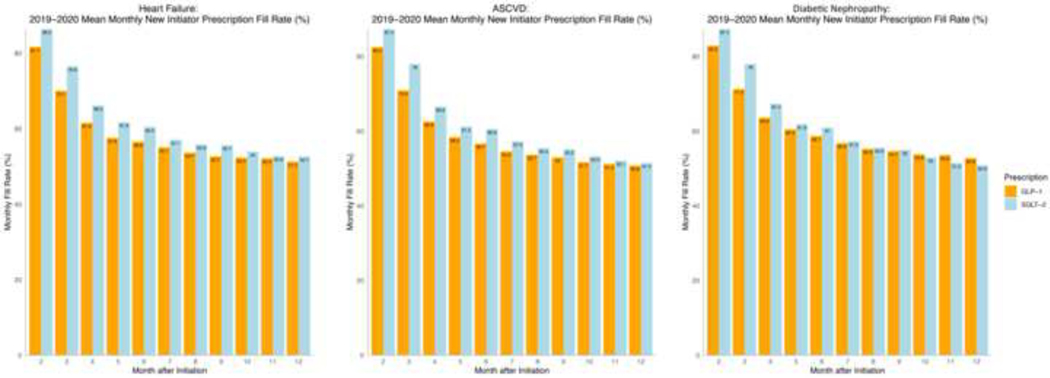
Among new initiators, monthly fill rates for GLP-1 RA and SGLT-2i prescriptions steadily decreased in the year after the initial prescription (Figure 5). Fill rates were 63.7% for GLP-1 RAs and 67.8% for SGLT-2i 3 months after initiation of therapy. One-year fill rates among new initiators were 52.5% for GLP-1 RAs and 52.9% for SGLT-2i, compared with 55.8% for metformin and 62% for sulfonylureas (Supplementary Figure 1). For GLP-1 RAs, one-year fill rate was 50.8% for patients with ASCVD. Across patient subgroups, one-year fill rates for SGLT-2i were 51.5% for individuals with ASCVD, 52.7% for individuals with heart failure, and 50.9% for those with diabetic nephropathy (Figure 6).
Figure 5. Monthly fill rates for GLP-1 RA and SGLT-2i prescriptions among new initiators in 2019–2020.
Data represent monthly fill rates based on the proportion of days covered among new initiators of each drug class for 12 months after starting treatment. Abbreviations: GLP-1; glucagon-like peptide-1 receptor agonist; SGLT-2, sodium glucose cotransporter-2 inhibitor.
Figure 6. Monthly fill rates for GLP-1 RA and SGLT-2i prescriptions among new initiators across patient subgroups in 2019–2020.
Data represent monthly fill rates based on the proportion of days covered among new initiators of each drug class for 12 months after initiating treatment. Abbreviations: ASCVD, atherosclerotic cardiovascular disease; GLP-1; glucagon-like peptide-1 receptor agonist; SGLT-2, sodium glucose cotransporter-2 inhibitor.
Mean monthly fill rates for GLP-1 RA and SGLT-2i prescriptions consistently decreased in the year following the initial prescription among new initiators across commercially insured and Medicare Advantage beneficiaries (Figure 7). One-year fill rates were higher for individuals with commercial insurance than those with Medicare Advantage plans for GLP-1 RAs (59.3% vs 51.0%, p-value<0.001) and SGLT-2i (63.4% vs 50.3%, p-value<0.001).
Figure 7. Monthly fill rates for GLP-1 RA and SGLT-2i prescriptions among new initiators with Medicare Advantage and commercial insurance plans.
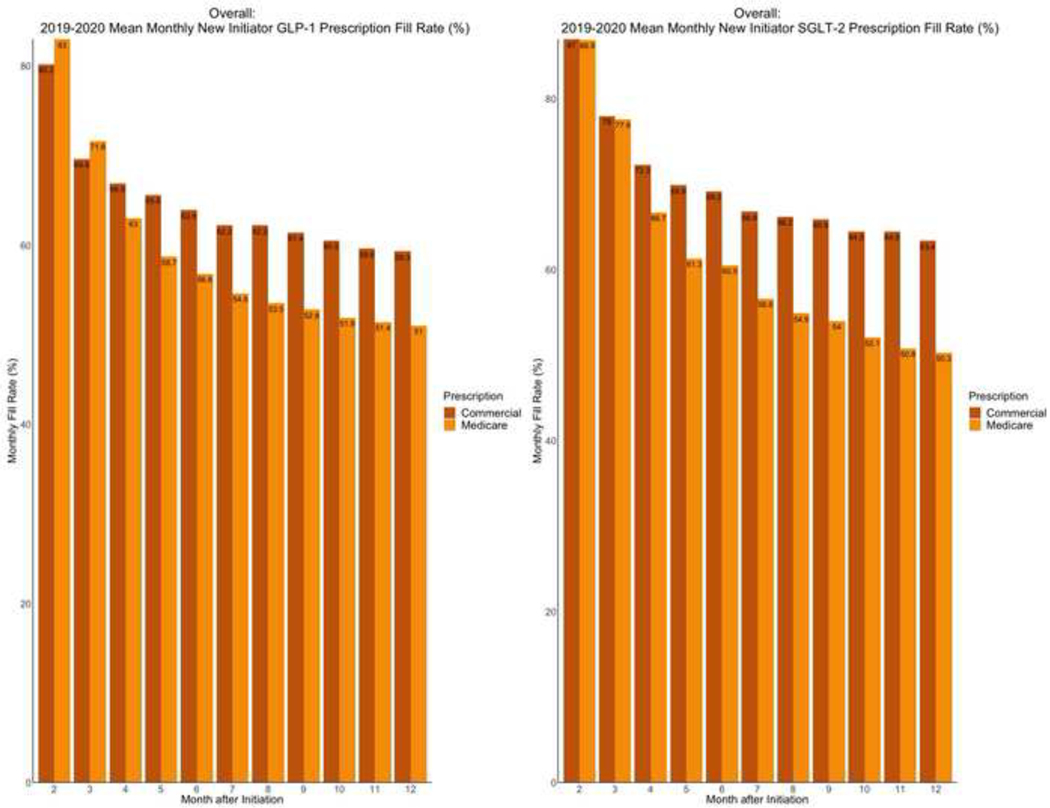
Data represent monthly fill rates based on the proportion of days covered among new initiators of each drug class for 12 months after initiating treatment. Abbreviations: GLP-1; glucagon-like peptide-1 receptor agonist; SGLT-2, sodium glucose cotransporter-2 inhibitor.
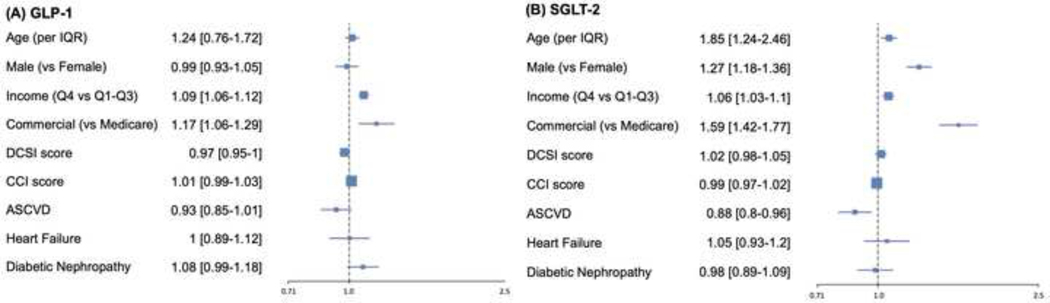
In multivariable model with one-year fill as the outcome, higher neighborhood income (comparing top income quartile with the other quartiles combined: OR 1.09 [95% CI: 1.06–1.12] for GLP-1 RAs; and 1.06 [1.03–1.1] for SGLT-2i) and commercial insurance (compared with Medicare Advantage: OR 1.17 [1.06–1.29] for GLP-1 RAs and 1.59 [1.42–1.77] for SGLT-2i) were associated with higher rates of prescription fills. History of heart failure and diabetic nephropathy were not associated with fill rates for either GLP-1 RAs or SGLT-2i. Established ASCVD was associated with lower rates of prescription fills for SGLT-2i (OR 0.88 [0.8–0.96]) (Figure 8).
Figure 8. Multivariable predictors of one-year fill rates for GLP-1 RA and SGLT-2i prescriptions.
Data represent odds ratio (95% CI) in multivariable model with one-year prescription fill rates for GLP-1 RA and SGLT-2i prescriptions as outcome. Abbreviations: ASCVD, atherosclerotic cardiovascular disease; CCI, Charlson comorbidity index; DCSI, diabetes complications and severity index; GLP-1; glucagon-like peptide-1 receptor agonist; IQR, interquartile range; SGLT-2, sodium glucose cotransporter-2 inhibitor.
Discussion
In this nationwide study analyzing administrative data from the United States, GLP-1 RAs and SGLT-2i were used in 11 to 13 percent of adults with type 2 diabetes in 2018–2020, with only 1 in 8 individuals with clear clinical indications receiving prescriptions for these medications. While the uptake of SGLT-2i remained unchanged in 2019–2020, the counts of new prescriptions for GLP-1 RAs significantly decreased in this period. Furthermore, among patients who started these medications, only two-thirds were consistently taking these medications at 3 months and only half one year after starting the treatment. Patients with guideline-directed indications for the use of these medications had similarly low fill rates, with high income and coverage under commercial insurance associated with higher fills one year after initiation of therapy.
The uptake of the novel antihyperglycemic medications among patients with type 2 diabetes remains low through 2020 despite multiple clinical trials reporting cardiovascular and kidney protective effects of these agents since 2016, with endorsement in clinical practice guidelines and society recommendations since 2018. The fill rates of these medications are similarly low among patients with guideline-directed indications, with no significant increase in the counts of new prescriptions during 2019–2020; nevertheless, this limited period of time might not be sufficient for capturing a meaningful trend. The present results support the observations of prior studies suggesting low rates of prescriptions of these medications,9–11 with this study reporting the latest data on the real-world use of GLP-1 RAs and SGLT-2i in a large and diverse population across the US. The adoption rates of GLP-1 RAs and SGLT-2i are relatively low compared with other cardioprotective medications in a similar policy position, such as sacubitril-valsartan for treatment of heart failure. Analysis of US national trends in the uptake of sacubitril-valsartan suggests almost 50% use of this medication among eligible individuals within 3 years after FDA approval, which is remarkably higher than the utilization rates of GLP-1 RAs and SGLT-2i in our analysis.19 The constellation of these findings suggests a guideline-discordant prescription pattern that does not adequately incorporate trial-proven non-glycemic benefits of these two drug classes.
We found a month-to-month decline in the number of patients who actively fill these agents in the year following their initial prescription, such that at 12 months after initiation only half of the prescriptions were filled. Variations in the refill rates of GLP-1 RAs and SGLT-2i are potentially multifactorial, with individuals’ compliance, discontinuation of therapy by the provider, adverse events associated with the medication, and long-term affordability as some of the potential contributing factors. The pattern of underfilling of prescriptions for GLP-1 RAs and SGLT-2i did not differ among patients with and without risk for cardiovascular and kidney disease, suggesting a lack of selectivity or emphasis on their importance in clinical use. We also found evidence of a larger drop off in the rates of prescription fills for these agents compared with the older and less expensive medications, such as metformin and sulfonylureas, which may reflect the financial burden of novel antihyperglycemic medications or the familiarity of prescribers and patients with well-established therapies as potential barriers to the consistent use of GLP-1 RAs and SGLT-2i. The use of these drug classes in individuals with type 2 diabetes and established ASCVD has been suggested as a quality measure by Pharmacy Quality Alliance.20 The large attrition and inconsistent use reported in the present study has major implications on the practical definition of such quality measures, as initial prescription of these medications may not necessarily translate into their actual use by patients in the long term. As these medications become available for broader indications, such as obesity pharmacotherapy for GLP-1 RAs21,22 and heart failure with preserved ejection fraction and CKD for SGLT-2i,23–26 addressing the utilization and persistence obstacles for their established indications grows increasingly important.
There are certain additional insights from these results that merit discussion. On average, higher income was associated with higher rates of prescription fills of both medications among new initiators. Moreover, patients with commercial health insurance plans were more likely than Medicare Advantage beneficiaries to fill prescriptions after starting treatment. Notably, the difference between commercially insured and Medicare Advantage beneficiaries remained significant after adjusting for income, suggesting challenges with the Medicare Advantage plan design to facilitate affordable drug coverage. In 2019, median out-of-pocket costs for GLP-1 RAs and SGLT-2i in individuals with Medicare Advantage and Part D medication coverage were estimated to range from $1,000 to $2,500 per year, depending on the prescribed agent and plan details;27,28 nevertheless, the out-of-pocket costs for these agents are not reported for commercial insurances. Hence, we cannot compare out-of-pocket costs, quantitively, for GLP-1 RAs and SGLT-2i across plan types; however, our findings may reflect challenges with affordability with these agents, especially with Medicare Advantage plans, which continues to be a major obstacle to expand their use even among insured individuals. Future studies are warranted to compare out-of-pocket costs for these novel therapies across different insurance plans.
Our study has limitations that merit consideration. First, our data do not represent all payers, limiting the generalizability of the present observations to the general population. Compared with a US national sample of individuals with type 2 diabetes, this study population is older, and comprises predominantly Medicare Advantage beneficiaries, with higher prevalence of ASCVD and heart failure, and lower prevalence of CKD.9 Nevertheless, Optum Labs’ de-identified administrative claims data represents a diverse mixture of sociodemographic subgroups and geographic regions across the US. Second, we were unable to account for insurance coverage differences for individual patients, manifested in plan-specific deductible thresholds, copay tiers, and cost sharing expectations, which may have implications for medication prescription and long-term adherence. Third, our dataset did not include uninsured individuals who are less likely to have access to these agents. Therefore, both the initial prescription and longitudinal fill rates for these agents in the general population are likely to be overestimated in our study. Fourth, we did not have access to the measures of glycemic control. However, we accounted for the severity of diabetes by including DCSI and CCI scores in our models. Fifth, we were limited in elucidating the reasons of discontinuation of therapies after initiation of GLP-1 RAs and SGLT-2i. Nevertheless, the drop-out rates in our analysis were much higher than the previously reported rates in the landmark clinical trials.29–32 Sixth, all comorbidities were determined using claim-based indicators during the baseline period, which may underrepresent the true prevalence of comorbidities (e.g., diabetic nephropathy) in comparison with lab-based diagnostic methods; our assessment of ASCVD was limited to established disease, and not high ASCVD risk, which depending on the specific guideline or society recommendation, may be considered another indication for GLP-1 RAs and SGLT-2i. Finally, our proxies of income were derived at the zip-code level, not the individual level, and we were unable to account for other key social determinants of health such as occupation or education level.
In this large nationwide US study on the real-world use of GLP-1 RAs and SGLT-2i, these medications were used in only 1 in 8 individuals with type 2 diabetes and indications in 2018–2020, with 50% drop off in the rates of prescription fills one year after initiation of therapy. The low frequency and inconsistent use of these evidence-based medications represent a challenge to realizing their longitudinal health outcomes benefits in a period of expanding indications of their use.
Supplementary Material
Funding:
The study was funded by Research & Development at UnitedHealth Group, and the authors Callahan Clark, Abraham Reddy, and Samuel Amodeo are full-time employees of the UnitedHealth Group. These authors played an active role in all aspects of the development of the study, including design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The study was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award, 1K23HL153775 to Dr. Khera. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosures
Callahan Clark, Abraham Reddy, and Samuel Amodeo are full time employees of UnitedHealth Group and own stock in the company. Marc Suchard reports grants from the National Science Foundation and the National Institutes of Health during the conduct of the study and grants from Janssen Research and Development outside the submitted work. Darren McGuire reports personal fees from Boehringer Ingelheim, Sanofi US, Merck & Co., Merck Sharp and Dohme Corp., Eli Lilly and Company, NovoNordisk, AstraZeneca, Lexicon Pharmaceuticals, Eisai, Pfizer, Metavant, Applied Therapeutics, Afimmune, Bayer, CSL Behring and Esperion. Zhenqiu Lin works under contract with the Centers for Medicare & Medicaid Services to develop quality measures. Silvio Inzucchi has received honoraria or consultancy fees from AstraZeneca, Boehringer Ingelheim, Novo Nordisk, Lexicon, Merck, vTv Therapeutics, Esperion, and Abbott. Evangelos Oikonomou and Rohan Khera are coinventors of US Provisional Patent Application No. 63/177,117, Methods for neighborhood phenomapping for clinical trials and are co-founders of Evidence2Health, a precision health and digital health analytics platform. The remaining authors report no potential conflicts of interest.
Footnotes
Data statement
The data are proprietary and not available for open sharing due to restrictions on our data use agreement.
References
- 1.Addendum. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021;44(Suppl. 1):S125–S150. Diabetes Care 2021;44:2183–2185. [DOI] [PubMed] [Google Scholar]
- 2.Das SR, Everett BM, Birtcher KK, Brown JM, Januzzi JL Jr., Kalyani RR, Kosiborod M, Magwire M, Morris PB, Neumiller JJ, Sperling LS. 2020 Expert Consensus Decision Pathway on Novel Therapies for Cardiovascular Risk Reduction in Patients With Type 2 Diabetes: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol 2020;76:1117–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, Huikuri HV, Johansson I, Juni P, Lettino M, Marx N, Mellbin LG, Ostgren CJ, Rocca B, Roffi M, Sattar N, Seferovic PM, Sousa-Uva M, Valensi P, Wheeler DC, Group ESCSD. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2020;41:255–323. [DOI] [PubMed] [Google Scholar]
- 4.Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C, D’Alessio DA, Davies MJ. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020;43:487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Back M, Benetos A, Biffi A, Boavida JM, Capodanno D, Cosyns B, Crawford C, Davos CH, Desormais I, Di Angelantonio E, Franco OH, Halvorsen S, Hobbs FDR, Hollander M, Jankowska EA, Michal M, Sacco S, Sattar N, Tokgozoglu L, Tonstad S, Tsioufis KP, van Dis I, van Gelder IC, Wanner C, Williams B, Societies ESCNC, Group ESCSD. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2021;42:3227–3337. [DOI] [PubMed] [Google Scholar]
- 6.McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney DZI, Dagogo-Jack S, Pratley R, Greenberg M, Wang S, Huyck S, Gantz I, Terra SG, Masiukiewicz U, Cannon CP. Association of SGLT2 Inhibitors With Cardiovascular and Kidney Outcomes in Patients With Type 2 Diabetes: A Meta-analysis. JAMA Cardiol 2021;6:148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sattar N, Lee MMY, Kristensen SL, Branch KRH, Del Prato S, Khurmi NS, Lam CSP, Lopes RD, McMurray JJV, Pratley RE, Rosenstock J, Gerstein HC. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol 2021;9:653–662. [DOI] [PubMed] [Google Scholar]
- 8.Brown E, Heerspink HJL, Cuthbertson DJ, Wilding JPH. SGLT2 inhibitors and GLP-1 receptor agonists: established and emerging indications. Lancet 2021;398:262–276. [DOI] [PubMed] [Google Scholar]
- 9.Nargesi AA, Jeyashanmugaraja GP, Desai N, Lipska K, Krumholz H, Khera R. Contemporary National Patterns of Eligibility and Use of Novel Cardioprotective Antihyperglycemic Agents in Type 2 Diabetes Mellitus. J Am Heart Assoc 2021;10:e021084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sangha V, Lipska K, Lin Z, Inzucchi SE, McGuire DK, Krumholz HM, Khera R. Patterns of Prescribing Sodium-Glucose Cotransporter-2 Inhibitors for Medicare Beneficiaries in the United States. Circ Cardiovasc Qual Outcomes 2021. [DOI] [PMC free article] [PubMed]
- 11.McCoy RG, Van Houten HK, Karaca-Mandic P, Ross JS, Montori VM, Shah ND. Second-Line Therapy for Type 2 Diabetes Management: The Treatment/Benefit Paradox of Cardiovascular and Kidney Comorbidities. Diabetes Care 2021. [DOI] [PMC free article] [PubMed]
- 12.McCoy RG, Van Houten HK, Deng Y, Mandic PK, Ross JS, Montori VM, Shah ND. Comparison of Diabetes Medications Used by Adults With Commercial Insurance vs Medicare Advantage, 2016 to 2019. JAMA Netw Open 2021;4:e2035792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallace PJ, Shah ND, Dennen T, Bleicher PA, Crown WH. Optum Labs: building a novel node in the learning health care system. Health Aff (Millwood) 2014;33:1187–1194. [DOI] [PubMed] [Google Scholar]
- 14.Introduction: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021;44:S1–S2. [DOI] [PubMed] [Google Scholar]
- 15.Garber AJ, Handelsman Y, Grunberger G, Einhorn D, Abrahamson MJ, Barzilay JI, Blonde L, Bush MA, DeFronzo RA, Garber JR, Garvey WT, Hirsch IB, Jellinger PS, McGill JB, Mechanick JI, Perreault L, Rosenblit PD, Samson S, Umpierrez GE. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm - 2020 Executive Summary. Endocr Pract 2020;26:107–139. [DOI] [PubMed] [Google Scholar]
- 16.Glasheen WP, Renda A, Dong Y. Diabetes Complications Severity Index (DCSI)-Update and ICD-10 translation. J Diabetes Complications 2017;31:1007–1013. [DOI] [PubMed] [Google Scholar]
- 17.Glasheen WP, Cordier T, Gumpina R, Haugh G, Davis J, Renda A. Charlson Comorbidity Index: ICD-9 Update and ICD-10 Translation. Am Health Drug Benefits 2019;12:188–197. [PMC free article] [PubMed] [Google Scholar]
- 18.Loucks J, Zuckerman AD, Berni A, Saulles A, Thomas G, Alonzo A. Proportion of days covered as a measure of medication adherence. Am J Health Syst Pharm 2021. [DOI] [PubMed]
- 19.Ozaki AF, Krumholz HM, Mody FV, Jackevicius CA. National Trends in the Use of Sacubitril/Valsartan. Journal of Cardiac Failure 2021;27:839–847. [DOI] [PubMed] [Google Scholar]
- 20. https://www.pqaalliance.org/pqa-endorses-two-new-plan-measures.
- 21.Srivastava G, Kumar RB. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med 2021;385:e4. [DOI] [PubMed] [Google Scholar]
- 22.Rubino D, Abrahamsson N, Davies M, Hesse D, Greenway FL, Jensen C, Lingvay I, Mosenzon O, Rosenstock J, Rubio MA, Rudofsky G, Tadayon S, Wadden TA, Dicker D, Investigators S. Effect of Continued Weekly Subcutaneous Semaglutide vs Placebo on Weight Loss Maintenance in Adults With Overweight or Obesity: The STEP 4 Randomized Clinical Trial. JAMA 2021;325:1414–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Belohlavek J, Bohm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukat A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O’Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjostrand M, Langkilde AM, Committees D-HT, Investigators. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 24.Packer M, Butler J, Zannad F, Filippatos G, Ferreira JP, Pocock SJ, Carson P, Anand I, Doehner W, Haass M, Komajda M, Miller A, Pehrson S, Teerlink JR, Schnaidt S, Zeller C, Schnee JM, Anker SD, Group EM-PTS. Effect of Empagliflozin on Worsening Heart Failure Events in Patients with Heart Failure and a Preserved Ejection Fraction: The EMPEROR-Preserved Trial. Circulation 2021. [DOI] [PMC free article] [PubMed]
- 25.Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, Giannetti N, Gomez-Mesa JE, Janssens S, Januzzi JL, Gonzalez-Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Pina IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M, Investigators EM-PT. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med 2021.
- 26.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Bohm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F, Investigators EM-RT. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med 2020;383:1413–1424. [DOI] [PubMed] [Google Scholar]
- 27.Luo J, Feldman R, Rothenberger SD, Hernandez I, Gellad WF. Coverage, Formulary Restrictions, and Out-of-Pocket Costs for Sodium-Glucose Cotransporter 2 Inhibitors and Glucagon-Like Peptide 1 Receptor Agonists in the Medicare Part D Program. JAMA Netw Open 2020;3:e2020969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeJong C, Masuda C, Chen R, Kazi DS, Dudley RA, Tseng C-W. Out-of-Pocket Costs for Novel Guideline-Directed Diabetes Therapies Under Medicare Part D. JAMA Internal Medicine 2020;180:1696–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. New England Journal of Medicine 2016;375:1834–1844. [DOI] [PubMed] [Google Scholar]
- 30.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JFE, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. New England Journal of Medicine 2016;375:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde A-M, Sabatine MS. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. New England Journal of Medicine 2018;380:347–357. [DOI] [PubMed] [Google Scholar]
- 32.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. New England Journal of Medicine 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.