Keywords: cyst, Dientamoeba fragilis, electron microscopy, transmission
Abstract
Little is known about the life cycle and mode of transmission of Dientamoeba fragilis. Recently it was suggested that fecal–oral transmission of cysts may play a role in the transmission of D. fragilis. In order to establish an infection, D. fragilis is required to remain viable when exposed to the pH of the stomach. In this study, we investigated the ability of cultured trophozoites to withstand the extremes of pH. We provide evidence that trophozoites of D. fragilis are vulnerable to highly acidic conditions. We also investigated further the ultrastructure of D. fragilis cysts obtained from mice and rats by transmission electron microscopy. These studies of cysts showed a clear cyst wall surrounding an encysted parasite. The cyst wall was double layered with an outer fibrillar layer and an inner layer enclosing the parasite. Hydrogenosomes, endoplasmic reticulum and nuclei were present in the cysts. Pelta-axostyle structures, costa and axonemes were identifiable and internal flagellar axonemes were present. This study therefore provides additional novel details and knowledge of the ultrastructure of the cyst stage of D. fragilis.
Introduction
Dientamoeba fragilis is a pathogenic protozoan parasite which causes diarrhoea and gastrointestinal disease in humans (Barratt et al., 2011; Stark et al., 2016). It belongs to the family Trichomonadidae and close relatives are Histomonas and Trichomonas species. This organism was discovered a century ago but its life cycle is yet to be elucidated. Until recently, trophozoites were the recognized D. fragilis life cycle stage, and it degenerates quickly once passed from the host in stool. Several theories suggest how D. fragilis could survive outside the host for a short period of time to allow transmission. One possibility is that Dientamoeba is transmitted via a helminth vector, or through a resistant pseudocyst or a cyst stage (Barratt et al., 2011; Stark et al., 2006).
Jepps and Dobell (1918) described the fragile nature of the trophozoite stage of D. fragilis but were unable to find a cyst stage in human stool samples. It was suggested that D. fragilis may form cysts in an unidentified animal species (Jepps and Dobell, 1918) indicating the potential for zoonotic transmission. Dientamoeba fragilis has been reported in several animal species including non-human primates, budgerigars, rats, cattle, sheep and pigs (Chan et al., 2016; Galán-Puchades et al., 2021; Stark et al., 2008; Yetismis et al., 2022; Yildiz and Erdem Aynur, 2022). Knowles and Das Gupta (1936) detected Dientamoeba in stool of a captive macaques using iron haematoxylin staining methods (Knowles and Gupta, 1936). Another study reported Dientamoeba infections in wild monkeys in the Philippines (Hegner and Chu, 1930). Two studies showed the presence of Dientamoeba in the stools of western lowland gorilla by microscopy with further confirmation by polymerase chain reaction (PCR) (Lankester et al., 2010; Stark et al., 2008).
Evidence for a cyst stage has not only been elusive, but also controversial. However, several authors described long ago what appears to be a cyst-like or a pre-cyst stage of D. fragilis (Knoll and Howell, 1946; Piekarski, 1948; Silard et al., 1979). A pseudocyst stage was described as a small individual with a finely granular cytoplasm with distinct endosomes (Wenrich, 1936). A more recent report dismissed these studies and suggested that these forms were degenerating trophozoites (Johnson et al., 2004). Therefore, the general perception until recently was that a cyst stage was absent in D. fragilis (Barratt et al., 2011). However, recent studies found cyst forms of the parasite in clinical samples, albeit at very low levels (Abd, 2021; Garcia, 2016; Stark et al., 2014a).
There have been several attempts to induce experimental infections in a range of animals which have been largely unsuccessful (Dobell, 1940; Kean and Malloch, 1966; Knoll and Howell, 1946; Lankester et al., 2010; Wenrich, 1944). Wenrich (1944) tried to infect laboratory rats with cultured D. fragilis trophozoites orally and via rectal inoculation; however, these attempts were unsuccessful (Wenrich, 1944). Latter attempts to infect laboratory rats mention that there was damage to the underlying cells where D. fragilis attached to the caecal mucosa in which oedema was evident, however no actual ulceration was detected (Kean and Malloch, 1966). The failure to develop a reproducible animal model of infection with D. fragilis has hindered research into many aspects of this parasite.
Recently, it was reported that Balb/C mice can be infected orally with culture derived from trophozoites (Munasinghe et al., 2013). Cysts were detectable in stool samples by light microscopy and cross-transmission studies with Sprague Dawley rats showed them to be also susceptible to infection by D. fragilis (Munasinghe et al., 2013). Dientamoeba fragilis got its name because of the fragile nature of the trophozoite when outside their host. This fragile nature leads us to hypothesize that human-to-human transmission of the trophozoite would be impractical. Thus, we hypothesize that cysts play some role in D. fragilis transmission. As such, excystation and encystation must be features of the D. fragilis life cycle. This process must involve oral ingestion and transversal of the parasites across the hostile environment of the mammalian stomach and other parts of the gastrointestinal track since trophozoites are commonly found in stool samples. In this study, we investigated the ability of cultured trophozoites to withstand the extremes of pH and we provide evidence that trophozoites of D. fragilis are susceptible to highly acidic conditions. We also investigated further the ultrastructure of D. fragilis cysts obtained from mice and rats by transmission electron microscopy (TEM).
Materials and methods
Ethics statement
All animal-based research was approved by the Animal Care and Ethics Committee (ACEC) at the University of Technology, Sydney. Use of clinical samples derived from humans was approved by the Human Research Ethics Committee at St Vincent's Hospital Sydney.
Parasite culture
Strain F of D. fragilis was isolated and cultured from a symptomatic patient at St. Vincent's Hospital Sydney. The trophozoites were grown microaerophilically at 37 °C in McCartney Bottles containing Loeffler's slopes overlaid with phosphate-buffered saline (PBS) as described (Barratt et al., 2010). The presence of D. fragilis was initially determined in the symptomatic patient and the parasite culture using the EasyScreenTM Enteric Protozoan Detection Kits (Genetic Signatures, Australia) according to the manufacturer's instructions (Stark et al., 2014b). Identification of parasites in the culture was confirmed by Sanger Sequencing a region of the small subunit ribosomal DNA (SSU rDNA) at the Australian Genome Research Facility using the novel primers SSUF3 5′-CCG GGT AGT TCC TAC CTT AAA C-3′ and SSUR3 5′-GGA CAT CAC GGA CCT GTT ATT-3′ (Genbank: OR826038). Cycling conditions were denaturation at 95 °C, annealing at 60 °C and extension at 72 °C each for 1 min for a total of 40 cycles.
Resistance of trophozoites to pH conditions
Six solutions of PBS were adjusted using concentrated HCl to cover a pH range of 1–6. PBS (pH 7.0) was used as the negative control with and without a Loeffler's slope. Dientamoeba fragilis trophozoites from the log phase culture (48 h post inoculation of cultures) were centrifuged at 500 g for 10 min. The supernatant was removed and the cell pellet containing the trophozoites was resuspended in the pH-adjusted PBS (pH 1–7). The trophozoite (pH 1–7) suspensions were sampled at time intervals: 0, 2, 4 and 6 h and at each sampling time, the trophozoites resistance to treatment was determined using cell counts with a viability stain (1% trypan blue). These data were used to calculate a total cell count relative to time point 0 (T0), viability (percentage) and viable cell count relative to T0.
Animal experiments
Six-week-old specific pathogen-free Balb/C female mice and Sprague Dawley rats were obtained from the Animal Resource Centre (Perth, WA) and housed in filter top cages in separate rooms. The mice were given sterile rodent chow as standard diet and sterile water to drink ad libitum. The cage bedding was changed every day to avoid any possible cross-contamination. Prior to infection with D. fragilis trophozoites, the animals were surveyed for the presence of intestinal parasites. Two methods were used. Feces was tested daily for 7 days by light microscopy using a modified iron haematoxylin stain as previously described (Stark et al., 2010) to identify any enteric parasites. PCR was also undertaken using D. fragilis-specific primers as previously described (Stark et al., 2010). A tandem multiplex RT-PCR for the detection of Cryptosporidium spp., D. fragilis, Entamoeba histolytica and Giardia intestinalis (Stark et al., 2011) was also used as a final screen to confirm these animals were free of D. fragilis (along with other protozoa) prior to experimental infection. All these methods showed that the animals were free of any protozoal parasites, including D. fragilis, prior to study.
Mice and rats were infected with approximately100 μL PBS (pH7.0) containing 103–1010 trophozoites orally using a 1 mL syringe. Fecal pellets were collected every day from each mouse into vials containing sodium acetic acid formalin and the presence of D. fragilis was investigated by light microscopy of iron haematoxylin stained fecal smears. The weight, stool consistency and the data on the pattern of shedding were recorded every day during the study (summarized in Supplementary information).
Sprague Dawley rats were given (by a 1 mL syringe) a fecal suspension containing D. fragilis cysts obtained from infected Balb/C mice. The fecal suspension was prepared from feces of infected Balb/C mice where the presence of cysts was confirmed via iron haematoxylin staining. Fecal samples containing D. fragilis cysts were suspended in PBS and thoroughly mixed into a suspension that was administered orally to rats. Fecal samples of infected rats were collected every day post infection, pooled and used for TEM (described below).
Transmission electron microscopy
The methods used are as follows (Munasinghe et al., 2013). Pooled fecal pellets from infected mice or rats were collected and resuspended in PBS. Fecal debris was removed by centrifugation at 500 g for 10 min. The supernatant containing cysts was centrifuged at 1500 g for 10 min to pellet the cysts which were washed 3 times with PBS by centrifugation and resuspension in PBS. The pellets containing cysts were fixed in 3% glutaraldehyde in PBS (pH 7.2) at room temperature overnight from all animals where shedding of D. fragilis could be confirmed by light microscopy of iron haematoxylin stained fecal smears. The fixed fecal specimens containing cysts were centrifuged at 500 g for 10 min. The fixatives were removed and PBS added. This washing step with PBS was repeated 3 times for 15 min for each wash. Cell pellets were then embedded in 2% low melt agarose. The agar blocks were cut into 1 mm cubes and post fixed in 1% osmium tetroxide in PBS for 1 h at room temperature. Following post fixation, the samples were washed in distilled water 3 times for 15 min each and subsequently immersed in 2% aqueous uranyl acetate for 30 min. The pellets were dehydrated through a graded series of ethanols (50–100%), infiltrated in mixtures of ethanol and L.R. White Resin (London Resin) and subsequently embedded in LR White Resin. Semi-thin and ultra-thin sections were cut using a Reichert Ultracut S ultramicrotome (Leica Microsystems, North Ryde, NSW, Australia). Semi-thin sections (1 μm thickness) were stained with 1% methylene blue and observed by transmitted light microscopy (Olympus BH2, Olympus, Japan). Ultra-thin sections (70 nm) were mounted onto pioloform coated, 300-mesh, thin-bar copper grids, stained with saturated aqueous uranyl acetate (7.7%) for 30 min and Reynold's lead citrate (Reynolds, 1963) for 4 min, rinsed with milliQ H2O after each stain. Ultrathin sections were examined using a Philips CM10 transmission electron microscope (Eindhoven, Netherlands).
Results
Resistance of trophozoites to acid pH
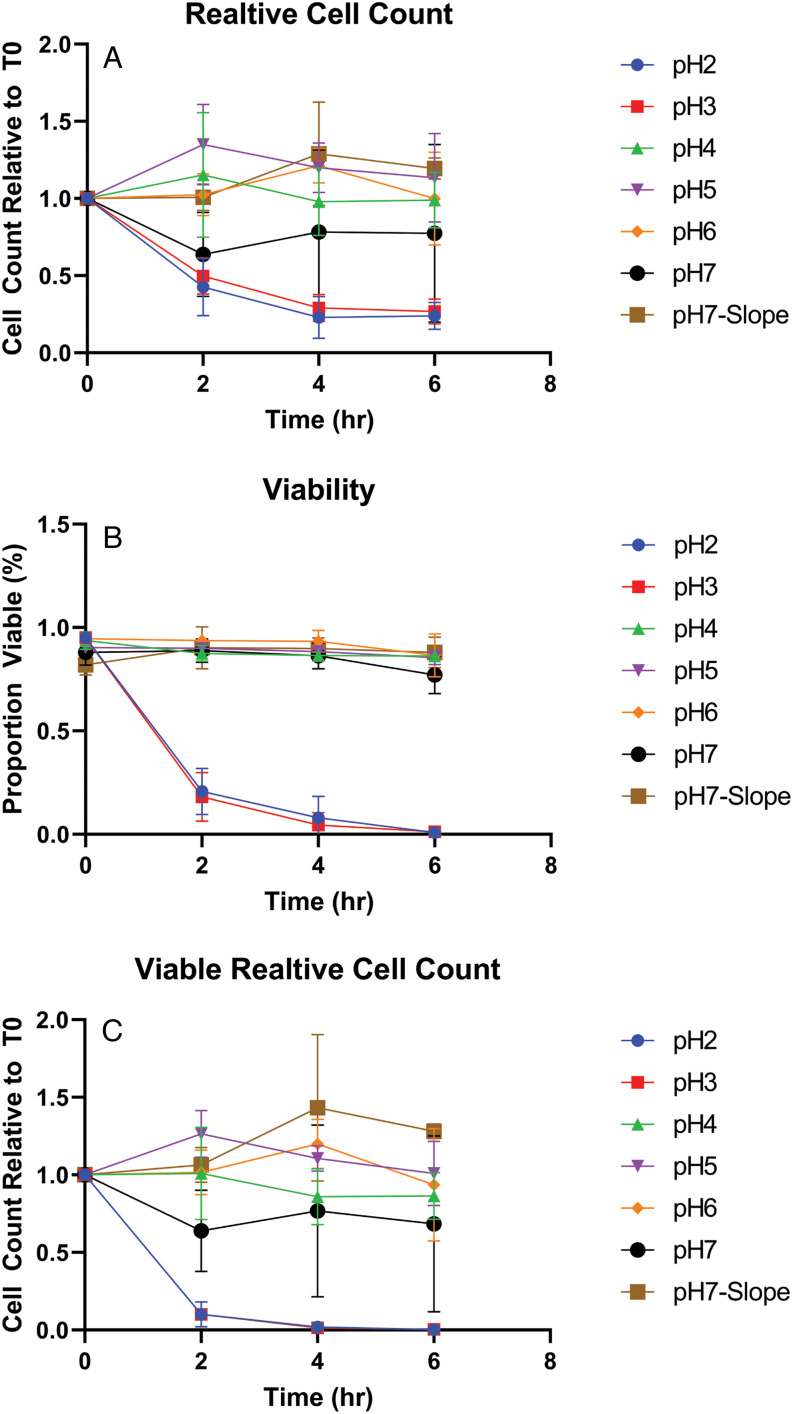
Infection of mice with D. fragilis trophozoites led to the presence of cysts in stool. These observations suggest that cultured trophozoites could survive the pH conditions found in the mouse stomach which is typically pH 3 (fed) and pH 4 (fasted) and higher than that found in the human stomach (McConnell et al., 2008). In order to investigate the ability of trophozoites to withstand exposure to extremes of pH, cultured trophozoites were resuspended in PBS adjusted to different pH levels and incubated for periods of time up to 6 h. After incubation, the viability of the cells was assessed by cell counts with a trypan blue viability stain. Our main conclusion was that trophozoites were unable to withstand acidic pH conditions below pH 3 for 2 h, as judged by viable cell counts (Fig. 1). The susceptibility of trophozoites to extremes of pH suggests that D. fragilis cannot easily survive the pH conditions found in the human which typically range from 1 to 3 (Hong et al., 2005).
Figure 1.
Viability of D. fragilis in-vitro following incubation at acid pH. Dientamoeba fragilis trophozoites were incubated in PBS at different pH levels (2–7) and negative control condition with a Loeffler slope (pH7-Slope) for 0–6 h. The graphs show the resulting total cell counts relative to T0 (a), viable proportion (b) and relative viable cell counts (c). The error bars represent standard error of mean cell counts, performed in triplicate.
Transmission electron microscopy of cysts
The ultrastructure of the cell wall and other organelles including nuclei and flagellar axonemes was examined. Dientamoeba fragilis cysts had a well-developed cyst wall and the parasite was enclosed within. Twenty D. fragilis cysts from the rats were measured and the cyst diameter fell within range 4–6 μm. The cyst diameter of the 5 cysts from the mice was 4–5 μm, with the range found in the rat model.
Nuclei
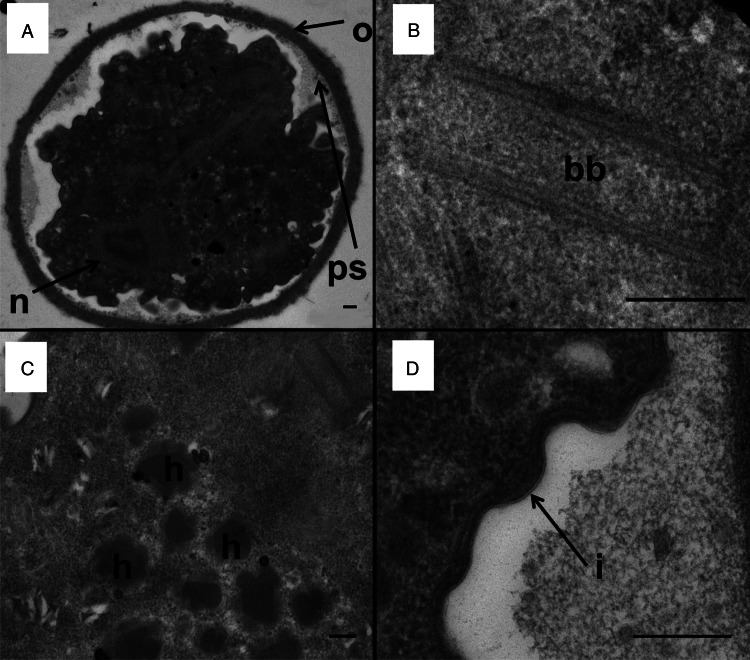
Cysts contained either 1 (Fig. 2A; 80% of cysts seen) or 2 nuclei (Fig. 2A; 20% of cysts seen) in the TEM planes analysed. The nuclear membrane was not visible in some cysts (Fig. 2A). The nucleolus was located centrally in the nucleus (Figs 2A and 3A).
Figure 2.
Transmission electron micrographs of a D. fragilis cyst showing the cyst wall and other organelles. (A) Transverse section across a mononucleated cyst showing the outer fibrillar cyst wall (o), peritrophic space (ps) and the nucleus of the encysted parasite (n). (B) Basal body structure (bb). (C) Developing hydrogenosomes which contain a dense core and a less electron-dense outer layer (h). (D) The inner membrane of the cyst wall (i). Scale bar represents 200 nm.
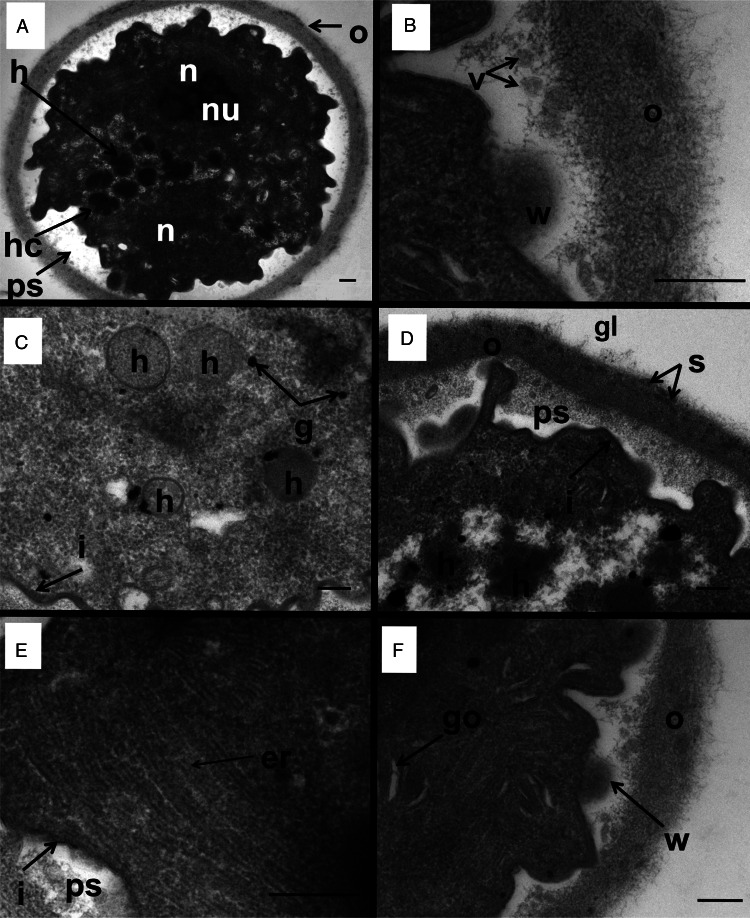
Figure 3.
Transmission electron micrographs of a D. fragilis cyst showing the whole cyst and other organelles and structures. (A) Transverse section across a binucleated cyst showing the outer cyst wall (o), the peritrophic space (ps), the 2 nuclei (n) nucleolus (nu). Note the hydrogenosomes (h) and the ‘cauliflower-like’ hydrogenosomes (hc) that lie in the space between the margins of the 2 nuclei. (B) A closer view of the outer cyst wall (o). Note the weakened area of the inner membrane (w) lying directly next to the ESV-like structures (v). (C) Hydrogenosomes (h), glycogen granules (g) and the inner membrane of the cyst (i). (D) Electron micrograph of the outer cyst wall (o) showing the dark striations (s) and coated in a glycocalyx (gl), the inner membrane (i), the peritrophic space (ps). Note the developing hydrogenosomes with the darker inner core and less electron-dense outer layer (h). (E) Smooth endoplasmic reticulum (er), inner cyst membrane (i) and peritrophic space (ps). (F) Golgi complex of D. fragilis cyst: weakening of the inner membrane (w): outer cyst wall (o).
The nucleus as far as it existed in the cutting plane showed an ovoid or an irregular shape. In all cysts with a single nucleus observed, no nuclear membrane was visible (Fig. 2A) which could be due to the difficulty in distinguishing the membrane from the often dense cytoplasmic contents. In contrast, cysts with 2 observed nuclei contained a nuclear membrane that was visible as described previously (Munasinghe et al., 2013). A clear nucleolus was seen which is electron dense, fragmented, irregular in shape and lacks a membrane.
Cyst wall
The cyst wall consisted of an outer layer and an inner layer (Munasinghe et al., 2013). The outer cyst wall contained fibrillar elements which appeared to be dense and had a uniform thickness (approximately 0.2 μm) (Figs 2A and 3A). The outer cyst wall was coated by a glycocalyx (Fig. 3D). There were dark striations along the outer fibrillar cyst wall (Fig. 3D). The inner cyst wall consists of 2 layers which collectively appear as a dark margin outlining the parasite (Fig. 2D).
Encystation specific vesicles (ESVs)
The presence of ESV-like structures in the cyst was described previously (Munasinghe et al., 2013). A weakening in the inner membrane of the encysted parasite occurs which is surrounded by vesicles in the peritrophic space (Fig. 3B, F). These weakened areas of the inner cyst membrane resemble areas which liberate the ESV-like structures from the encysted parasite into the peritrophic space. This is similar to that process which occurs in Giardia cysts where the ESVs are exported from inside the cyst to the periphery of the cell for deposition (Carpenter et al., 2012).
Cytoplasm
Other organelles were not as visible in the cyst as in the trophozoite stage of D. fragilis, although the endoplasmic reticulum (ER) and structures that are morphologically similar to Golgi bodies were easily identifiable (Fig. 3E, F). Electron-dense particles that measure 0.1–0.2 μm could be seen scattered across the cytoplasm which are similar in appearance to glycogen granules reported in the trophozoites of D. fragilis (Banik et al., 2012) and other trichomonads (Munsch et al., 2009) (Fig. 3C). ER was scattered around the cytoplasm (Fig. 3E).
Hydrogenosomes
Hydrogenosomes are double layered membrane bound, electron-dense organelles located in the cytoplasm of the D. fragilis cyst. They ranged in size from 0.3 to 0.4 μm and the number present in cysts ranged from 10 to 12 (Fig. 3C). The hydrogenosomes in some cysts were spherical in shape, bound with a double membrane, electron dense and located in the cytoplasm. In some cysts, the hydrogenosomes had an appearance of a ‘cauliflower shape’, the size ranging from 0.33 to 0.38 μm (n = 6) and the number present varied from 4 to 20 (Fig. 2C). These ‘cauliflower-type’ hydrogenosomes possessed an outer layer which was less electron dense and an inner core which was more electron dense. Some of these had an outer membrane (Fig. 2C). The hydrogenosomes in some cysts contained a homogeneous matrix (Fig. 3C). Glycogen granules were also often observed near both types of hydrogenosomes.
Basal body structure
A structure that looks similar to a basal body was present in D. fragilis cysts (Fig. 2B). The cysts also possessed an axostyle, flagellar axonemes, pelta and a costa (Munasinghe et al., 2013).
Discussion
To our knowledge, our report of a cyst stage in the life cycle of D. fragilis and the successful establishment of an animal model to study infection and transmission was important in providing evidence that transmission of D. fragilis may occur through a fecal–oral process via a cyst stage (Munasinghe et al., 2013). In this study, we extend these observations by showing that trophozoites are susceptible to acid exposure, and so are unlikely to be able to withstand the extremes of pH found in the human gastrointestinal tract (discussed below). We also provide a description of the cyst stage as studied by TEM. These studies also complement and extend those of the trophozoite stage of D. fragilis (Banik et al., 2012).
The treatment of trophozoites with PBS of pH less than 7 was carried out to determine the effect of extreme acid pH on the survival of D. fragilis trophozoites. The results showed that D. fragilis trophozoites were unable to survive for 2 h in pH 3 or less. This represents an important finding since it demonstrates that D. fragilis is not tolerant of environmental extremes, supporting the long-held dogma of the ‘fragile’ nature of this organism. After ingestion via the fecal–oral route, D. fragilis must travel through the stomach into the gut where it has to survive an acidic pH which is usually 1.8–2.0 in a healthy human (Hong et al., 2005) for 1–6 h depending on stomach content (Feldman et al., 1984; Meyer et al., 1988; Pal et al., 2007). Trophozoites were infrequently found in the feces of infected mice and rats compared to the relative abundance of cysts, suggesting that D. fragilis cysts may play a role in zoonotic transmission of this organism. Alternatively, this could indicate that the development of cysts is upregulated when conditions are not favourable for the multiplication of D. fragilis, such as finding itself in the wrong host. In contrast, in humans the major morphological forms found are trophozoites (Stark et al., 2010). Precystic forms are rarer and cyst forms almost never seen (Stark et al., 2014a). Recent studies confirmed the identification of cysts from human clinical samples which are morphologically similar to the cysts that were found in this study (Abd, 2021; Garcia, 2016; Stark et al., 2014a). The frequency in which cysts are reliably found in human clinical samples leads us to believe that human-to-human transmission may not be the dominant form of transmission for D. fragilis and that transmission from animals may be a significant contributor to human infection.
The observation that the trophozoite of D. fragilis is unable to resist acidic conditions might appear to be in contradiction to the establishment of a D. fragilis model in mice using cultured trophozoites (Munasinghe et al., 2013). It is important to note, that cultured trophozoites were unable to establish infections in rats; in contrast, a suspension of mouse feces containing cysts was able to result in infection. After the establishment of the mouse model, a study determined that a dose of 105 trophozoites per mouse was required for a 37% chance to establish infections in mice using the oral route and showed a 100% infection with a dose of 4 × 106 trophozoites per mouse (El-Gayar et al., 2016). This is higher than in our experiments as the minimum infectious dose was 105 and 103 in our second experiment. This impact could be a result of how D. fragilis adapts to growing in culture. The isolate that we selected for this study was the one that we were culturing which grew most readily (data not shown). Compared to the infective dose of other gastrointestinal protozoa, this is potentially higher: greater than 1 cyst for Entamoeba coli (Rendtorff, 1954), greater than 1000 cysts for E. histolytica (Ryan et al., 2004), 30–1000 oocysts for Cryptosporidium parvum (DuPont et al., 1995) and 10–10 000 cysts for G. intestinalis (Schaefer et al., 1991). Compared to the healthy pH range of 1.8–2.0 in the stomach of humans (Hong et al., 2005), the pH range of the stomach in a mouse is 3.0–4.0 (McConnell et al., 2008). It is important to note that the pH range of the rat stomach is 3.2–3.9, which is similar to a mouse, so the pH of the stomach cannot solely explain the failure to establish an infection in a rat using trophozoites (McConnell et al., 2008). Mice have a shorter gut transit time than rats which may limit the exposure of D. fragilis trophozoites to acid in their stomach in comparison (Giron et al., 2016). We speculate these differences have allowed for the establishment of the mouse model.
Our observations that trophozoites cannot withstand acid pH treatments are consistent with the mechanisms of transmission involving either the pre-cyst, cyst stage or E. vermincularis vector. Evidence for the role of E. vermincularis as a vector of D. fragilis infection includes detecting D. fragilis DNA inside pinworm ova (Clark et al., 2014; Ögren et al., 2013; Roser et al., 2013). While these results are certainly compelling, this still does not indicate that the organisms within are viable or transmissible, as previously suggested (Clark et al., 2014). In previous studies on the use of hypochlorite to destroy DNA, a 2.5–3% solution (w/v) was recommended for at least 15–40 min (Greenstone et al., 2012; Kemp and Smith, 2005). It is likely that this sterilization procedure employed in those studies on D. fragilis may have been insufficient to remove DNA (Ögren et al., 2013; Roser et al., 2013). A final step of DNAase treatment appears preferable to ensure that all exogenous DNA had been completely removed. Indeed, a similar experiment which employed DNAse treatment found that DNA of D. fragilis was not detected within E. vermicularis ova (Menghi et al., 2005). Additionally, E. vermicularis is a human-specific Oxyurid nematode, with rare identifications in captive chimpanzees (Murata et al., 2002). The identification of D. fragilis in a variety of non-human hosts (Chan et al., 2016; Galán-Puchades et al., 2021; Hegner and Chu, 1930; Knowles and Gupta, 1936; Lankester et al., 2010; Stark et al., 2008; Yetismis et al., 2022; Yildiz and Erdem Aynur, 2022) indicates that a model of transmission using E. vermilcularis as a vector responsible for its transmission is unlikely. In our study, rodents were not infected with pinworm. Notably, rodent pinworms belong either to the Syphacia and Aspiculuris genera, not Enterobius like the human pinworm (Meade and Watson, 2014). For the helminth vector model to remain, a non-species-specific helminth must be involved which has yet to be identified, or the mechanism by which D. fragilis enter the ova must not be species-specific. The variety of D. fragilis hosts supports the importance of the cyst model of transmission. Further studies are required to ascertain the exact life cycle and mode of transmission of this parasite.
TEM of the D. fragilis cysts showed they had a spherical to ovoid shape with a thick cyst wall that is filamentous in appearance. The overall structure of the cysts observed in this paper and our previous study shows remarkable similarity to the cysts of Trichomitus batrachorum and Trichomonitus sanguisugae including the presence of a peritrophic space and axoneme (Brugerolle, 1973; Munasinghe et al., 2013). The cyst wall contained 2 layers: an outer dense fibrillar layer and a double-membrane bilayer enclosing the encysted parasite. Thus, the cyst wall of D. fragilis is similar to that of T. batrachorum, T. sanguisugae, Honigbergiella sp. and Giardia which also has an outer fibrillar layer and an inner double layer which is continuous with the plasma membrane of the trophozoite (Brugerolle, 1973; Coggins and Schaefer, 1986; Hampl et al., 2007). The outer cyst wall consisted of a meshwork of fibrillar elements whereas the inner bilayer encloses the parasite. There is a space between the cyst wall and the encysted parasite which is similar to the peritrophic space reported in Giardia cysts (Coggins and Schaefer, 1986). Similar to the cyst of Honigbergiella sp., the outer cyst wall is coated with a glycocalyx (Hampl et al., 2007). This space shrinks in mature D. fragilis cysts where the plasma membrane is closely attached to the cyst wall as seen in Entamoeba species (García-Zapién et al., 1999).
Despite these similarities, there were some structural differences in the cyst wall of Giardia and D. fragilis. The cyst wall of Giardia contains a filamentous outer layer and 2 cell membranes separated from the plasma membrane of the parasite by a peritrophic space (Chávez-Munguía et al., 2004). In D. fragilis, the cyst wall contains an outer filamentous portion and an inner bilayer membrane enclosing the parasite. The peritrophic space was present between the outer layer and the inner bilayer. Fibrillar material is also present in the cyst wall of Entamoeba species (García-Zapién et al., 1999). In Entamoeba invadens, the cyst wall is a single continuous fibrillar layer which is closely associated with the plasma membrane (Chávez-Munguía et al., 2007). In Acanthamoeba castellanii which is a free-living amoeba, the cyst wall is a double-layered structure which includes the ectocyst and endocyst (Chávez-Munguía et al., 2007) which is not seen in D. fragilis. The cyst wall of D. fragilis was different to the precyst stage described in Histomonas meleagridis which is a close relative of D. fragilis (Barratt et al., 2011) where a fibrillar-dense cyst wall was absent instead it contained only an outer amorphous zone (Zaragatzki et al., 2010).
Immediately beneath the cyst wall as well as inside the peritrophic space, there were numerous double-membrane vesicles which are similar to the ESVs described in Giardia cysts (Chávez-Munguía et al., 2007). Some of these vesicles were also seen embedded in the cyst wall. It has been suggested that in Giardia cysts, these ESVs contain material that forms the fibrillar component of the cyst wall (Reiner et al., 1990). These ESVs were described as spherical and electron dense in Giardia which contain condensed material that goes on to form the cyst wall (Hehl and Marti, 2004). Formation and maturation of large ESVs (up to 1 μm in diameter) is functionally linked to the regulation of cyst wall proteins and evidence suggests that ESVs represent a novel Golgi equivalent in Giardia (Hehl et al., 2000; Marti et al., 2003). In Entamoeba species, similar forms of small vesicles are present close to the plasma membrane which is involved in a process of depositing granular material to the cell wall (Chávez-Munguía and Martínez-Palomo, 2011). In Acanthamoeba, vesicles with a similar appearance are present in the cytoplasm of mature cysts and the fibrillar content inside these vesicles resembles the appearance of the endocyst and ectocyst wall (Chávez-Munguía et al., 2005). Overall, we speculate that ESVs are present in the D. fragilis cyst. We also speculate that the mechanisms of cyst wall formation are probably Giardia-like, mainly because of the morphological similarities seen by TEM between this species and D. fragilis.
The enclosed parasite within the cyst wall was amoeboid in appearance. There were small spherical particles that appeared across the cytoplasm which are deemed to be free ribosomes. These are also apparent in Histomonas pseudocysts (Mielewczik et al., 2008). The ER was scattered throughout the cytoplasm and around the nuclear region and glycogen granules were present which has also been described in H. meleagridis (Munsch et al., 2009). Although the nuclear membrane was often not visible, a clear nucleolus was present at the centre of the nucleus.
Hydrogenosomes are found in several trichomonad species and other anaerobic protozoa living in oxygen-poor environments. The hydrogenosome contains enzymes that participate in pyruvate metabolism and formation of ATP (Benchimol, 2009). The gene for pyruvate : ferredoxin oxidoreductase was identified in the transcriptome of D. fragilis along with other hydrogenosomal ATP synthases (Barratt et al., 2015). Therefore, the hydrogenosomes are the main energy-producing organelles in D. fragilis cysts. Hydrogenosomes were present in all the cysts observed. Some of these were spherical in shape which was similar to those seen in the trophozoite stage (Banik et al., 2012). In some cysts, hydrogenosomes were irregular in shape (‘cauliflower-like’) with irregular inclusions. These irregular-shaped hydrogenosomes do not appear to have been reported before in any other protozoa. Other irregular hydrogenosomes have been identified in Tritrichomonas foetus, having a ‘heart’-shaped morphology (Benchimol, 2009). We suggest the unusual appearance of the hydrogenosomes we describe is linked to a reorganization of membranous structures that occur during cyst development or a division process similar to that described in Benchimol (2009).
A basal body structure containing an axostyle and costa was observed in D. fragilis trophozoites. Additionally, the cyst of D. fragilis contains a pelta and flagella which were not found in the trophozoite (Banik et al., 2012). Previous phylogenetic studies resulted in the inclusion of D. fragilis in the Parabasalia purely on the presence of hydrogenosomes (Cepicka et al., 2010; Silberman et al., 1996). The loss of the pelta axostyle complex was seen as a derived trait. The results presented here and in Munasinghe et al. (2013) of the presence of internal flagella and pelta in the encysted parasite are therefore important. The pelta is a crescent-shaped sheet which could be found along the periphery of the nucleus (Munasinghe et al., 2013). The pelta apparently supports the periflagellar canal from which the flagella emerge in the trichomonads (Benchimol et al., 2000). The costa is found only in trichomonads which possess an undulating membrane and it is assumed that its function is to provide mechanical support to the undulating membrane (Benchimol, 2010). Although the costa and pelta structure are visible in D. fragilis, no external flagella, undulating membrane or axonemes are seen in the peritrophic space. Therefore, although these structures may be remnants derived from an evolutionary ancestor, they do provide further ultrastructural evidence for D. fragilis to be classified as a trichomonad. In the H. meleagridis life cycle, however, an amoeboid stage, cyst-like stage and a flagellated stage have been identified (Munsch et al., 2009). As D. fragilis is a close relative of Histomonas sp., we wonder whether there is a further life cycle stage of D. fragilis waiting to be discovered.
During encystment, many biochemical and cellular changes occur in the cell including the production of a cyst wall (Chávez-Munguía et al., 2007). In E. invadens, Giardia and Acanthamoeba species that have been studied extensively, encystation can be divided into several phases. The observations reported here suggest that D. fragilis undergoes similar reorganization of its internal structures. For example, the loss of the nuclear membranes and the presence of hydrogenosomes with unusual shape and structure suggest that cyst formation involves extensive cellular remodelling, which includes reorganization of membrane-bound organelles. In Giardia cysts, a similar process of remodelling occurs where the nuclei membranes fuse and disappear and exchange genetic information during encystation (Carpenter et al., 2012). We presume similar processes may be occurring in D. fragilis.
The identification of cysts containing 1 or 2 nuclei suggests the cyst probably contains a single cell. The most prominent change that occurs during the encystation of protozoan parasites is the synthesis of cyst wall components and their transport to the cell membrane via ESVs which release their contents to the cell surface through exocytosis (Chávez-Munguía et al., 2003). ESVs do not occur in non-encysting trophozoites of Giardia (Benchimol, 2002). In Giardia, ESVs fuse with the plasma membrane and release their contents to the exterior leaving behind empty vesicles that are found in the peritrophic space (Hehl and Marti, 2004). In D. fragilis, numerous ESV-like structures were present in the peritrophic space. These preliminary observations of a cyst wall and the presence of ESV-like structures suggest a mechanism for cyst wall formation in D. fragilis similar to that in Giardia.
Conclusion
The current study demonstrated that D. fragilis trophozoites cannot withstand extreme pH conditions present in the human stomach, showing that fecal–oral transmission via the trophozoite to humans is possible but improbable. This highlights the need for further studies into both the cyst stage and Enterobius vermicularis ova vector models of transmission. Cyst forms are the dominant morphological form found in experimentally infected rodent feces; it is possible that the cyst form plays an important role in the zoonotic transmission. The transmission electron microscopic studies revealed the close similarity of the cysts ultrastructure of D. fragilis and other trichomonads, especially Histomonas which further highlights the presence of a cyst stage in the life cycle. All these findings have important implications for dientamoebiasis and advance our knowledge on the recurrent nature and transmission of the disease.
Supporting information
Hall et al. supplementary material
Hall et al. supplementary material
Acknowledgements
We thank Debra Birch (Macquarie University) for her advice and assistance in specimen processing and image interpretation. This work was supported by Sydpath, St. Vincent Hospital, Darlinghurst and the University of Technology Sydney. This research was performed by L. M. H. and V. S. M. in partial fulfilment of a PhD at UTS. The graphical abstract was created in BioRender under agreement number ED26486CAW.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182024000076
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Author contributions
L. M. H.: methodology, data curation, investigation, writing – original draft, review and editing. V. S. M.: conceptualization, investigation, writing – original draft. N. G. F. V.: investigation, supervision. J. T. E.: conceptualization, methodology, supervision, writing – review and editing. D. S.: conceptualization, supervision, writing, resources
Financial support
The research was supported by St Vincent's Hospital, SydPath, Department of Microbiology.
Competing interest
None.
Ethical standards
These experiments were completed to the standards of the Australian Research Council Codes and Guidelines. HREC approval was obtained through the HREC at St Vincent's Hospital Sydney for the establishment of cultures from clinical specimens. ACEC approval was obtained through UTS.
References
- Abd H (2021) Dientamoeba fragilis trophozoites undergoing encystation, apoptosis and necrosis as human parasitic amoeba in clinical stool samples. European Academic Research IX, 4633–4649. [Google Scholar]
- Banik GR, Birch D, Stark D and Ellis JT (2012) A microscopic description and ultrastructural characterisation of Dientamoeba fragilis: an emerging cause of human enteric disease. International Journal for Parasitology 42, 139–153. [DOI] [PubMed] [Google Scholar]
- Barratt JLN, Banik GR, Harkness J, Marriott D, Ellis JT and Stark D (2010) Newly defined conditions for the in vitro cultivation and cryopreservation of Dientamoeba fragilis: new techniques set to fast track molecular studies on this organism. Parasitology 137, 1867–1878. [DOI] [PubMed] [Google Scholar]
- Barratt JL, Harkness J, Marriott D, Ellis JT and Stark D (2011) The ambiguous life of Dientamoeba fragilis: the need to investigate current hypotheses on transmission [review]. Parasitology 138, 557–572. [DOI] [PubMed] [Google Scholar]
- Barratt JL, Cao M, Stark DJ and Ellis JT (2015) The transcriptome sequence of Dientamoeba fragilis offers new biological insights on its metabolism, kinome, degradome and potential mechanisms of pathogenicity. Protist 166, 389–408. [DOI] [PubMed] [Google Scholar]
- Benchimol M (2002) A new set of vesicles in Giardia lamblia. Experimental Parasitology 102, 30–37. [DOI] [PubMed] [Google Scholar]
- Benchimol M (2009) Hydrogenosomes under microscopy. Tissue & Cell 41, 151–168. [DOI] [PubMed] [Google Scholar]
- Benchimol M (2010) The mastigont system in trichomonads. In de Souza W (ed.), Structures and Organelles in Pathogenic Protists, vol. 17. Berlin: Springer Berlin Heidelberg, pp. 1–26. [Google Scholar]
- Benchimol M, Diniz JA and Ribeiro K (2000) The fine structure of the axostyle and its associations with organelles in trichomonads. Tissue & Cell 32, 178–187. [DOI] [PubMed] [Google Scholar]
- Brugerolle G (1973) Sur ‘existence de vrais kystes chez les trichomonadines intestinales. Ultrastructure des kystes de Trichomitus batrachorum Perty 1852, Trichomitus sanguisugae Alexeleff 1911, et Monocercomonas tipulae Mackinnon 1910. Comptes Rendus de l'Académie des Sciences. Paris 277, 2193–2196. [Google Scholar]
- Carpenter ML, Assaf ZJ, Gourguechon S and Cande WZ (2012) Nuclear inheritance and genetic exchange without meiosis in the binucleate parasite Giardia intestinalis. Journal of Cell Science 125, 2523–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepicka I, Hampl V and Kulda J (2010) Critical taxonomic revision of parabasalids with description of one new genus and three new species. Protist 161, 400–433. [DOI] [PubMed] [Google Scholar]
- Chan D, Barratt J, Roberts T, Phillips O, Šlapeta J, Ryan U, Marriott D, Harkness J, Ellis J and Stark D (2016) Detection of Dientamoeba fragilis in animal faeces using species specific real time PCR assay. Veterinary Parasitology 227, 42–47. [DOI] [PubMed] [Google Scholar]
- Chávez-Munguía B and Martínez-Palomo A (2011) High-resolution electron microscopical study of cyst walls of Entamoeba spp. Journal of Eukaryotic Microbiology 58, 480–486. [DOI] [PubMed] [Google Scholar]
- Chávez-Munguía B, Cristóbal-Ramos AR, González-Robles A, Tsutsumi V and Martínez-Palomo A (2003) Ultrastructural study of Entamoeba invadens encystation and excystation. Journal of Submicroscopic Cytology and Pathology 35, 235–243. [PubMed] [Google Scholar]
- Chávez-Munguía B, Cedillo-Rivera R and Martínez-Palomo A (2004) The ultrastructure of the cyst wall of Giardia lamblia. Journal of Eukaryotic Microbiology 51, 220–226. [DOI] [PubMed] [Google Scholar]
- Chávez-Munguía B, Omaña-Molina M, González-Lázaro M, González-Robles A, Bonilla P and Martínez-Palomo A (2005) Ultrastructural study of encystation and excystation in Acanthamoeba castellanii. Journal of Eukaryotic Microbiology 52, 153–158. [DOI] [PubMed] [Google Scholar]
- Chávez-Munguía B, Omaña-Molina M, González-Lázaro M, González-Robles A, Cedillo-Rivera R, Bonilla P and Martínez-Palomo A (2007) Ultrastructure of cyst differentiation in parasitic protozoa. Parasitology Research 100, 1169–1175. [DOI] [PubMed] [Google Scholar]
- Clark CG, Röser D and Stensvold CR (2014) Transmission of Dientamoeba fragilis: pinworm or cysts? Trends in Parasitology 30, 136–140. [DOI] [PubMed] [Google Scholar]
- Coggins JR and Schaefer FW III (1986) Giardia muris: ultrastructural analysis of in vitro excystation. Experimental Parasitology 61, 219–228. [DOI] [PubMed] [Google Scholar]
- Dobell C (1940) Researches on the intestinal protozoa of monkeys and man: X. The life-history of Dientamoeba fragilis: observations, experiments, and speculations. Parasitology 32, 417–461. [Google Scholar]
- DuPont HL, Chappell CL, Sterling CR, Okhuysen PC, Rose JB and Jakubowski W (1995) The infectivity of Cryptosporidium parvum in healthy volunteers. New England Journal of Medicine 332, 855–859. [DOI] [PubMed] [Google Scholar]
- El-Gayar EK, Mokhtar AB and Hassan WA (2016) Study of the pathogenic potential of Dientamoeba fragilis in experimentally infected mice. Parasite Epidemiology Control 1, 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman M, Smith HJ and Simon TR (1984) Gastric emptying of solid radiopaque markers: studies in healthy subjects and diabetic patients. Gastroenterology 87, 895–902. [PubMed] [Google Scholar]
- Galán-Puchades MT, Trelis M, Sáez-Durán S, Cifre S, Gosálvez C, Sanxis-Furió J, Pascual J, Bueno-Marí R, Franco S, Peracho V, Montalvo T and Fuentes MV (2021) One health approach to zoonotic parasites: molecular detection of intestinal protozoans in an urban population of Norway rats, Rattus norvegicus, in Barcelona, Spain. Pathogens 10, 311. 10.3390/pathogens10030311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Zapién AG, González-Robles A and Mora-Galindo J (1999) Congo red effect on cyst viability and cell wall structure of encysting. Entamoeba invadens. Archives of Medical Research 30, 106–115. [DOI] [PubMed] [Google Scholar]
- Garcia LS (2016) Dientamoeba fragilis, one of the neglected intestinal protozoa [review]. Journal of Clinical Microbiology 54, 2243–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giron R, Perez-Garcia I and Abalo R (2016) X-ray analysis of gastrointestinal motility in conscious mice. Effects of morphine and comparison with rats. Neurogastroenterology and Motility 28, 74–84. [DOI] [PubMed] [Google Scholar]
- Greenstone MH, Weber DC, Coudron TA, Payton ME and Hu JS (2012) Removing external DNA contamination from arthropod predators destined for molecular gut-content analysis. Molecular Ecology Resources 12, 464–469. [DOI] [PubMed] [Google Scholar]
- Hampl V, Cepicka I, Flegr J, Tachezy J and Kulda J (2007) Morphological and molecular diversity of the Monocercomonadid genera Monocercomonas, Hexamastix, and Honigbergiella gen. nov. Protist 158, 365–383. [DOI] [PubMed] [Google Scholar]
- Hegner R and Chu HJ (1930) A comparative study of the intestinal protozoa of wild monkeys and man*. American Journal of Epidemiology 12, 62–108. [Google Scholar]
- Hehl AB and Marti M (2004) Secretory protein trafficking in Giardia intestinalis. Molecular Microbiology 53, 19–28. [DOI] [PubMed] [Google Scholar]
- Hehl AB, Marti M and Köhler P (2000) Stage-specific expression and targeting of cyst wall protein-green fluorescent protein chimeras in Giardia. Molecular Biology of the Cell 11, 1789–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W, Jiao W, Hu J, Zhang J, Liu C, Fu X, Shen D, Xia B and Chang Z (2005) Periplasmic protein HdeA exhibits chaperone-like activity exclusively within stomach pH range by transforming into disordered conformation. Journal of Biological Chemistry 280, 27029–27034. [DOI] [PubMed] [Google Scholar]
- Jepps MW and Dobell C (1918) Dientamoeba fragilis. A new intestinal amoeba from man. Parasitology 10, 352–367. [Google Scholar]
- Johnson EH, Windsor JJ and Clark CG (2004) Emerging from obscurity: biological, clinical, and diagnostic aspects of Dientamoeba fragilis [review]. Clinical Microbiology Reviews 17, 553–570, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kean BH and Malloch CL (1966) The neglected ameba: Dientamoeba fragilis. A report of 100 ‘pure’ infections. The American Journal of Digestive Diseases 11, 735–746. [DOI] [PubMed] [Google Scholar]
- Kemp BM and Smith DG (2005) Use of bleach to eliminate contaminating DNA from the surface of bones and teeth. Forensic Science International 154, 53–61. [DOI] [PubMed] [Google Scholar]
- Knoll EW and Howell KM (1946) Studies on Dientamoeba fragilis; its incidence and possible pathogenicity. Med 53, 40–46. [PubMed] [Google Scholar]
- Knowles R and Gupta B (1936) Some observations on the intestinal protozoa of macaques. The Indian Journal of Medical Research 24, 547–556. [Google Scholar]
- Lankester F, Kiyang JA, Bailey W and Unwin S (2010) Dientamoeba fragilis: initial evidence of pathogenicity in the western lowland gorilla (Gorilla gorilla gorilla). Journal of Zoo and Wildlife Medicine 41, 350–352. [DOI] [PubMed] [Google Scholar]
- Marti M, Li Y, Schraner EM, Wild P, Köhler P and Hehl AB (2003) The secretory apparatus of an ancient eukaryote: protein sorting to separate export pathways occurs before formation of transient Golgi-like compartments. Molecular Biology of the Cell 14, 1433–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell EL, Basit AW and Murdan S (2008) Measurements of rat and mouse gastrointestinal pH, fluid and lymphoid tissue, and implications for in-vivo experiments. Journal of Pharmacy and Pharmacology 60, 63–70. [DOI] [PubMed] [Google Scholar]
- Meade TM and Watson J (2014) Characterization of rat pinworm (Syphacia muris) epidemiology as a means to increase detection and elimination. Journal of the American Association for Laboratory Animal Science 53, 661–667. [PMC free article] [PubMed] [Google Scholar]
- Menghi C, Makiya R, Gatta C and Méndez OC (2005) Dientamoeba fragilis: molecular biology techniques for the elucidation of its mode of transmission. Parasitologia Latinoamericana 60, 25–31. [Google Scholar]
- Meyer JH, Elashoff J, Porter-Fink V, Dressman J and Amidon GL (1988) Human postprandial gastric emptying of 1-3-millimeter spheres. Gastroenterology 94, 1315–1325. [DOI] [PubMed] [Google Scholar]
- Mielewczik M, Mehlhorn H, Al-Quraishy S, Grabensteiner E and Hess M (2008) Transmission electron microscopic studies of stages of Histomonas meleagridis from clonal cultures. Parasitology Research 103, 745–750. [DOI] [PubMed] [Google Scholar]
- Munasinghe VS, Vella NG, Ellis JT, Windsor PA and Stark D (2013) Cyst formation and faecal-oral transmission of Dientamoeba fragilis – the missing link in the life cycle of an emerging pathogen. International Journal for Parasitology 43, 879–883. [DOI] [PubMed] [Google Scholar]
- Munsch M, Lotfi A, Hafez HM, Al-Quraishy S and Mehlhorn H (2009) Light and transmission electron microscopic studies on trophozoites and cyst-like stages of Histomonas meleagridis from cultures. Parasitology Research 104, 683–689. [DOI] [PubMed] [Google Scholar]
- Murata K, Hasegawa H, Nakano T, Noda A and Yanai T (2002) Fatal infection with human pinworm, Enterobius vermicularis, in a captive chimpanzee. Journal of Medical Primatology 31, 104–108. [DOI] [PubMed] [Google Scholar]
- Ögren J, Dienus O, Löfgren S, Iveroth P and Matussek A (2013) Dientamoeba fragilis DNA detection in Enterobius vermicularis eggs. Pathogens and Disease 69, 157–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal A, Brasseur JG and Abrahamsson B (2007) A stomach road or ‘Magenstrasse’ for gastric emptying. Journal of Biomechanics 40, 1202–1210. [DOI] [PubMed] [Google Scholar]
- Piekarski G (1948) Zur Frage der Cystenbildung bei Dientamoeba fragilis. Medical Microbiology and Immunology 127, 496–500. [PubMed] [Google Scholar]
- Reiner DS, McCaffery M and Gillin FD (1990) Sorting of cyst wall proteins to a regulated secretory pathway during differentiation of the primitive eukaryote, Giardia lamblia. European Journal of Cell Biology 53, 142–153. [PubMed] [Google Scholar]
- Rendtorff RC (1954) The experimental transmission of human intestinal protozoan parasites. I. Endamoeba coli cysts given in capsules. American Journal of Hygiene 59, 196–208. [DOI] [PubMed] [Google Scholar]
- Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. Journal of Cell Biology 17, 208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roser D, Nejsum P, Carlsgart AJ, Nielsen HV and Stensvold CR (2013) DNA of Dientamoeba fragilis detected within surface-sterilized eggs of Enterobius vermicularis. Experimental Parasitology 133, 57–61. [DOI] [PubMed] [Google Scholar]
- Ryan K, Kenneth J, Ray C and Sherris J (2004) Sherris Medical Microbiology: An Introduction to Infectious Diseases, 4th Edn. United States: McGraw-Hill. 10.1036/0838585299. [DOI] [Google Scholar]
- Schaefer FW, Johnson CH, Hsu CH and Rice EW (1991) Determination of Giardia lamblia cyst infective dose for the Mongolian gerbil (Meriones unguiculatus). Applied and Environmental Microbiology 57, 2408–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silard R, Colea A, Panaitescu D, Florescu P and Roman N (1979) Studies on Dientamoeba fragilis in Romania. I. Dientamoeba fragilis isolated from clinical cases. Problems of diagnosis, incidence, clinical aspects. Archives Roumaines de Pathologie Experimentales et de Microbiologie 38, 359–372. [PubMed] [Google Scholar]
- Silberman JD, Clark CG and Sogin ML (1996) Dientamoeba fragilis shares a recent common evolutionary history with the trichomonads. Molecular and Biochemical Parasitology 76, 311–314. [DOI] [PubMed] [Google Scholar]
- Stark DJ, Beebe N, Marriott D, Ellis JT and Harkness J (2006) Dientamoebiasis: clinical importance and recent advances. Trends in Parasitology 22, 92–96. [DOI] [PubMed] [Google Scholar]
- Stark D, Phillips O, Peckett D, Munro U, Marriott D, Harkness J and Ellis J (2008) Gorillas are a host for Dientamoeba fragilis: an update on the life cycle and host distribution. Veterinary Parasitology 151, 21–26. [DOI] [PubMed] [Google Scholar]
- Stark D, Barratt J, Roberts T, Marriott D, Harkness J and Ellis J (2010) Comparison of microscopy, two xenic culture techniques, conventional and real-time PCR for the detection of Dientamoeba fragilis in clinical stool samples. European Journal of Clinical Microbiology & Infectious Diseases 29, 411–416. [DOI] [PubMed] [Google Scholar]
- Stark D, Al-Qassab SE, Barratt JLN, Stanley K, Roberts T, Marriott D, Harkness J and Ellis JT (2011) Evaluation of multiplex tandem real-time PCR for detection of Cryptosporidium spp., Dientamoeba fragilis, Entamoeba histolytica, and Giardia intestinalis in clinical stool samples. Journal of Clinical Microbiology 49, 257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark D, Garcia LS, Barratt JLN, Phillips O, Roberts T, Marriott D, Harkness J and Ellis JT (2014a) Description of Dientamoeba fragilis cyst and precystic forms from human samples. Journal of Clinical Microbiology 52, 2680–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark D, Roberts T, Ellis JT, Marriott D and Harkness J (2014b) Evaluation of the EasyScreen™ enteric parasite detection kit for the detection of Blastocystis spp., Cryptosporidium spp., Dientamoeba fragilis, Entamoeba complex, and Giardia intestinalis from clinical stool samples. Diagnostic Microbiology and Infectious Disease 78, 149–152. [DOI] [PubMed] [Google Scholar]
- Stark D, Barratt J, Chan D and Ellis JT (2016) Dientamoeba fragilis, the neglected trichomonad of the human bowel. Clinical Microbiology Reviews 29, 553–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenrich D (1936) Studies on Dientamoeba fragilis (Protozoa). I. Observations with special reference to nuclear structure. The Journal of Parasitology 22, 76–83. [Google Scholar]
- Wenrich DH (1944) Studies on Dientamoeba fragilis (Protozoa). IV. Further observations, with an outline of present-day knowledge of this species. The Journal of Parasitology 30, 322–338. [Google Scholar]
- Yetismis G, Yildirim A, Pekmezci D, Duzlu O, Ciloglu A, Onder Z, Simsek E, Ercan N, Pekmezci GZ and Inci A (2022) First report and genotyping of Dientamoeba fragilis in pet budgerigars (Melopsittacus undulatus), with zoonotic importance. Zoonoses and Public Health 69, 572–578. [DOI] [PubMed] [Google Scholar]
- Yildiz İ and Erdem Aynur Z (2022) First detection and molecular characterization of Dientamoeba fragilis in cattle. Zoonoses and Public Health 69, 897–903. [DOI] [PubMed] [Google Scholar]
- Zaragatzki E, Hess M, Grabensteiner E, Abdel-Ghaffar F, Al-Rasheid KA and Mehlhorn H (2010) Light and transmission electron microscopic studies on the encystation of Histomonas meleagridis. Parasitology Research 106, 977–983. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hall et al. supplementary material
Hall et al. supplementary material
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.