Abstract
Background
Almost 60% of adults with amnestic mild cognitive impairment (aMCI) have obstructive sleep apnea (OSA). Treatment with continuous positive airway pressure (CPAP) may delay cognitive decline, but CPAP adherence is often suboptimal. In this study, we report predictors of CPAP adherence in older adults with aMCI who have increased odds of progressing to dementia, particularly due to Alzheimer’s disease.
Methods
The data are from Memories 2, “Changing the Trajectory of Mild Cognitive Impairment with CPAP Treatment of Obstructive Sleep Apnea.” Participants had moderate to severe OSA, were CPAP naïve, and received a telehealth CPAP adherence intervention. Linear and logistic regression models examined predictors.
Results
The 174 participants (mean age 67.08 years, 80 female, 38 Black persons) had a mean apnea–hypopnea index of 34.78, and 73.6% were adherent, defined as an average of ≥4 hours of CPAP use per night. Only 18 (47.4%) Black persons were CPAP adherent. In linear models, White race, moderate OSA, and participation in the tailored CPAP adherence intervention were significantly associated with higher CPAP use at 3 months. In logistic models, White persons had 9.94 times the odds of adhering to CPAP compared to Black persons. Age, sex, ethnicity, education, body mass index, nighttime sleep duration, daytime sleepiness, and cognitive status were not significant predictors.
Conclusions
Older patients with aMCI have high CPAP adherence, suggesting that age and cognitive impairment should not be a barrier to prescribing CPAP. Research is needed to improve adherence in Black patients, perhaps through culturally tailored interventions.
Keywords: Motivational enhancement, Obstructive sleep apnea, Race, Tailored
Mild cognitive impairment (MCI) is defined as deficits in memory or other domains of cognition that do not significantly impact daily functioning (1). Persons with the amnestic type of MCI (aMCI), defined as MCI with memory complaints and deficits (1), have a high risk of progression to dementia, particularly due to Alzheimer’s disease (2). An estimated 58.7% of older adults with MCI also have obstructive sleep apnea (OSA) (3). OSA is characterized by repeated upper airway collapse and/or narrowing that can result in hypoxia and arousals (brief awakenings) from sleep, and impairments in memory, psychomotor speed, executive function, and other cognitive domains (4). The number of times per hour that the upper airway collapses and/or narrows is described as the apnea–hypopnea index (AHI). The severity of OSA is classified as: none/minimal AHI <5; mild ≥5; moderate ≥15, but <30; or severe >30 (5).
MCI is also prevalent in patients referred to sleep clinics for OSA. Beaudin et al. found that MCI, defined as a Montreal cognitive assessment test score of <26, was present in 47.9% of all patients referred to sleep clinics for OSA (n = 1 084) and >55% of patients with moderate to severe OSA (n = 560) (6). These findings were referenced in the American Thoracic Society’s report on the link between OSA and cognitive decline (7).
Continuous positive airway pressure (CPAP) is the standard of practice for treating OSA (8). Pressurized air through a nasal mask is titrated to reduce the AHI, decrease arousals from sleep, and eliminate oxygen desaturation. Studies have shown that CPAP therapy increases stages N3 nonrapid eye movement sleep and rapid eye movement sleep, reduces daytime sleepiness, and improves blood pressure in hypertensive patients, but the effects on other outcomes such as long-term cardiovascular risk and neurocognitive function are inconsistent (9). If used as prescribed, CPAP is associated with significantly lowered odds of incident diagnosis of Alzheimer’s disease and unspecified dementia (10) and improves 1-year cognitive function in older adults with aMCI (11), but CPAP initial and continued adherence (ie, the majority of patients with OSA require long-term CPAP treatment) are often suboptimal (3).
Factors likely to influence CPAP adherence in patients have been evaluated in a number of studies. In general, disease severity (higher AHI and lower oxygen desaturation) (3,12,13), higher levels of reported daytime sleepiness, and early adoption of CPAP (3,14) are consistently associated with increased long-term adherence to CPAP. Older research on how CPAP adherence varied by age has been mixed, but these past studies were often limited by small samples (15–17). In a recent study of telemonitoring data from 789 260 patients in a CPAP manufacturer database, overall adherence was 72.6%, but adherence varied dramatically by age and sex, ranging from 51.3% in 18- to 30-year-old women to 80.6% in 71- to 80-year-old men (18).
Other factors, such as higher body mass index (19), living as a couple (20,21), White race (22,23), and higher socioeconomic status (24) are often associated with higher CPAP adherence, but the evidence is modest. The most comprehensive evidence is for Black persons residing in the United States. In a systematic review, 16 of 22 studies showed significantly lower CPAP use for Black persons compared with White persons (22).
Patients with OSA may have comorbid insomnia. A systematic review and meta-analysis showed that 38% of patients with OSA meet insomnia diagnostic criteria (25,26). Insomnia is defined as frequent self-reported difficulties initiating sleep, maintaining sleep, and/or undesirable early morning awakenings from sleep that are associated with daytime impairment (27). Patients with both OSA and insomnia have lower average nightly use of CPAP therapy, compared to OSA alone (28,29).
Various interventions, such as education/supportive care, behavioral therapies, and patient engagement applications, have been evaluated in attempts to improve CPAP use. The evidence clearly supports the efficacy of behavioral interventions, such as motivational enhancement and cognitive behavioral therapy, but the efficacy of other interventions is unclear (9). A systematic review conducted by Askland et al. summarized and evaluated the evidence on the efficacy of behavioral interventions to improve CPAP use (30). They assessed the effect of educational, supportive, behavioral, or mixed (combination of 2 or more intervention types) strategies. When compared with usual care, behavioral interventions produce a clinically meaningful increase in device usage by 1.31 h/night (high-certainty evidence).
Although investigators have identified health outcomes associated with CPAP use, factors that affect CPAP use, and interventions that increase CPAP use, to our knowledge no one has prospectively examined predictors of CPAP adherence in a well-characterized, large sample of older adults with aMCI who have memory deficits that may affect their ability to use CPAP. The predictions can then be used by clinicians for planning and implementing therapeutic interventions for those at high risk for CPAP nonadherence, perhaps improving their adherence, and reducing the incidence of dementia. Our objective was to determine if demographics (age, sex, race, ethnicity, and education) and clinical factors (AHI, body mass index, nighttime sleep duration, daytime sleepiness, fidelity to a telehealth motivational enhancement CPAP adherence intervention, and cognitive status) predict 3-month CPAP adherence in older adults with aMCI and moderate to severe OSA.
Method
Study Design and Settings
We are conducting a quasi-experimental multisite study to determine the effects of CPAP adherence on cognitive function in older adults with moderate to severe OSA and aMCI. The primary outcome assessment is at 1 year (Clinical Trials.gov Identifier: NCT03113461). Accrual began in January 2018 and ended in November 2021. The end of follow-up is January 2023.
The University of Pennsylvania Central Institutional Review Board approved the research protocol for all sites—University of Pennsylvania, University of Texas at Austin, Washington University, and University of Virginia (Approval No. 828022). Written informed consent was obtained from all potential participants before any data were collected. We report here baseline data from the trial, fidelity data on the motivational enhancement telehealth CPAP adherence intervention, and CPAP use during the first 3 months of CPAP treatment.
Recruitment and Sample
Participants were recruited from sleep disorder centers, referrals from primary care, geriatric medicine, neurology and neuropsychology clinics, and community organizations or events, such as health fairs. Other sources were advertisements in community newsletters and on local radio, and flyers placed in businesses, such as barber shops. For sleep disorders centers, research staff reviewed sleep study interpretations, and patients who met the inclusion/exclusion criteria and had a documented memory complaint were identified and contacted by research staff. Interested participants were prescreened by telephone using the modified telephone interview for cognitive status (31). If prescreening was positive (telephone interview for cognitive status score ranging from 28 to 37 for English speakers and 26 to 37 for Spanish speakers), participants were invited for informed consent, followed by detailed cognitive testing for aMCI (32) and evaluation of the remaining criteria for study eligibility.
The inclusion criteria for Memories 2 are (a) aged ≥55–85 years; (b) moderate to severe OSA as defined by an AHI ≥15 events per hour, or no apnea as determined by diagnostic polysomnography or home sleep test; (c) clinical dementia rating scale score = 0–0.5 (33); (d) Mini-Mental State Examination score = 23–30 (34); (e) memory impairment as determined by scores on the logical memory II (1.0–1.5 standard deviations [SDs] below normal [adjusted for age and education]) (35); (f) stable for at least 4 weeks on medications (12 weeks for cholinesterase inhibitors/memantine); (g) nondepressed per geriatric depression scale score <6 (36); (h) has a study partner (caregiver/informant able to answer questions about the study participant); (i) adequate visual and auditory acuity for testing; (j) postmenopause or surgically sterile (females); (k) willing and able to complete baseline measures and provide CPAP adherence data; (l) at least 6 grades of education; and (m) fluent in English or Spanish.
Additional criteria for inclusion in the present analysis: (i) willing to try CPAP; (ii) had obtained a CPAP device through one of the following pathways: (a) ordered by the participant’s sleep or primary care physician as part of their regular care, and covered by insurance; or (b) provided with a research Auto-CPAP device (ResMed AirSense AutoSet) by the Memories 2 project if participants did not have insurance or whose insurance copay for CPAP exceeded $250.
Exclusions for Memories 2: (a) significant neurologic disease other than aMCI; (b) psychiatric disorders such as schizophrenia or behavioral problems that could lead to difficulty complying with the protocol; (c) history of alcohol abuse or dependence within 6 months; (d) any current significant systemic illness that could affect testing such as unstable cardiovascular disease, current use of supplemental oxygen, daytime hypoxemia on room air, laboratory abnormalities such as untreated B12 deficiency or thyroid disease, and resident of a skilled nursing facility; (e) participation in clinical studies involving neuropsychological testing more than twice a year; (f) predominant central sleep apnea; and (g) adherence to CPAP or bilevel pressure for OSA within the past 6 months. Participants were not excluded if they reported a history of comorbid insomnia.
Measures
Demographic factors
Age, race, sex, ethnicity, education, and other demographic characteristics were self-reported by study participants.
Diagnostic polysomnography and home sleep tests
To determine their AHI, participants underwent full-night or split-night overnight diagnostic polysomnography as part of a clinical sleep assessment in an accredited sleep laboratory, or a 2-night diagnostic home sleep test, either as part of a clinical sleep assessment or the Memories 2 research protocol (ResMed ApneaLink Air Home Sleep Testing Device). Participants without insurance or whose insurance copay exceeded $250 qualified for a Memories 2 research home sleep test.
CPAP pressure
To determine their pressure settings, participants attended a laboratory titration study or an at-home auto-pressure adjustment using Automatic-Pap (APAP). A registered polysomnography technologist titrated the CPAP pressure during the clinical in-laboratory polysomnography. Settings for participants receiving either a clinical or research APAP machine were determined during unattended at-home use of the APAP unit as per clinical standards of practice (37). The device automatically titrated the pressure in response to changes in airway resistance. A sleep medicine physician reviewed the in-laboratory and home data and ordered the pressure settings, defined as one that normalized the AHI and eliminated snoring, desaturation, and arousals, and restored a normal flow contour.
Body mass index
Study research staff measured participant height and weight using a calibrated scale except during the coronavirus 2019 (COVID-19) pandemic lockdown. The lockdown began in March 2020 and ended when restrictions at each site were lifted in 2021. During the pandemic lockdown, study data were collected using virtual platforms, and height and weight were taken by research staff from medical records (if available), or self-reported by participants.
Nighttime sleep duration
Participants reported the usual minutes slept at night prior to starting CPAP on the study sleep history form.
Epworth Sleepiness Scale
The Epworth Sleepiness Scale is a chronic daytime sleepiness questionnaire containing 8 questions with responses on a 4-point scale (0–3) (38). The scale is reliable and internally consistent and bears relation to the severity of OSA. Scores range from 0 to 24 and a higher score is associated with increased sleepiness. Participants completed the scale prior to starting CPAP.
Fidelity to CPAP adherence intervention
All participants were provided with a tailored telehealth CPAP adherence intervention from trained research staff consisting of: (a) OSA education, treatment expectations, and methods to minimize barriers and facilitate CPAP use; (b) motivational enhancement to reinforce participants’ health-related goals and CPAP self-efficacy (perceived confidence in one’s ability to use CPAP), and to offer anticipatory guidance for common experiences with CPAP; and (c) social support by a study partner. Most support calls lasted 5–15 min, and emphasized an interactive dialogue. The intervention began the day before the first night of CPAP (Day 0) and continued on days 1, 2, 5, and 7; weeks 2, 3, and 4; 3 months; and every 3 months to follow until the study end. For this study, intervention fidelity was defined as the frequency of attendance (0–9). The intervention was adapted from the work of Aloia et al. (39), with tailored modifications for older adults with cognitive impairment such as the inclusion of a study partner and memory coaching on CPAP use processes. If participants decided to stop trying to use CPAP, research staff switched them to an attention control condition with the identical frequency and duration of phone contact.
Clinical Dementia Rating Scale Sum of Boxes
Trained research staff used semistructured interviewing of participants and study partners (informants) to rate participants using the CDR on six domains of cognitive functioning: memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care. They then obtained the Clinical Dementia Rating Scale Sum of Boxes (CDR-SOB) by summing each of the CDR domain scores (33). The CDR-SOB is a widely used, well-validated tool for staging cognitive functioning and tracking cognitive change. Scores range from 0 to 18, with scores 0.5–4.0 indicating questionable cognitive impairment (corresponding to CDR global score 0.5) and scores 16–18 indicating severe dementia (corresponding to CDR global score 3.0) (40).
CPAP adherence
Although CPAP reduces apneas and hypopneas associated with OSA, it must be consistently used for at least 4 h/night for a therapeutic response (41). CPAP systems precisely record and store CPAP use. Project staff downloaded the CPAP use data from the CPAP manufacturer’s online platform or smartcard. Mean hours of CPAP use per night were calculated for each participant. Adherence was defined as mean CPAP usage ≥4 h/night during the first 3 months. Participants averaging <4 hours of CPAP use per night during the first 3 months were designated nonadherent.
Blinding
Those collecting predictors of CPAP adherence data were blind to CPAP adherence group. Cumulative CPAP use was abstracted from the CPAP manufacturer cloud database and double entered to ensure replicability.
Statistical Analysis
Descriptive statistics (means, SDs, medians, interquartile ranges, ranges, frequencies, and percentages) were generated to characterize this sample of 174 older adults with aMCI and moderate to severe OSA. Unadjusted and adjusted linear regression models were used to examine predictors of mean daily hours of CPAP use at 3 months. Similarly, unadjusted and adjusted logistic regression models were used to assess predictors of CPAP adherence versus not at 3 months. Additionally, backward elimination models for each outcome were also generated. According to this method, all variables demonstrating statistical significance at the 0.20 level in the unadjusted models were included in a single model for each outcome. Variables were then removed one at a time based on the largest p value until all predictors in the final model for each outcome demonstrated significance at the .05 level. Predictors of interest included baseline age, sex (male/female), race (White/Black/Other), ethnicity (Hispanic/non-Hispanic), education (>high school, ≤high school), AHI, body mass index, nighttime sleep duration, Epworth sleepiness scale, CPAP adherence intervention fidelity, and CDR-SOB. In unadjusted linear and logistic regression models, both continuous and dichotomous versions of AHI were used as a sole predictor, respectively, while in adjusted linear and logistic regression models, only dichotomous AHI (moderate, severe/very severe) was used. Due to missing data for CDR-SOB in 3 participants and for nighttime sleep duration in 10 participants, the sample size for analyses when including only CDR-SOB and only nighttime sleep duration was 171 and 164, respectively, and was 161 when analyses included both variables. As such, complete-case analysis was used to handle missing data. We did not analyze being an Asian person or another race because of small sample sizes. Collinearity in the adjusted linear model for CPAP use at 3 months was examined using the variance inflation factor (VIF), where a VIF of less than 10 for all variables is acceptable. Statistical significance was taken at the .05 level. All analyses were performed using SAS V9.4 (SAS Institute Inc., Cary, NC).
Sample Size
This is a secondary analysis of an ongoing study. As a general rule in logistic regression, at least 10 events per predictor variable are needed (42). Given our sample size (N = 174) and the number of nonevents (n = 46 CPAP nonadherent), we had sufficient power to detect four predictors of CPAP adherence.
Results
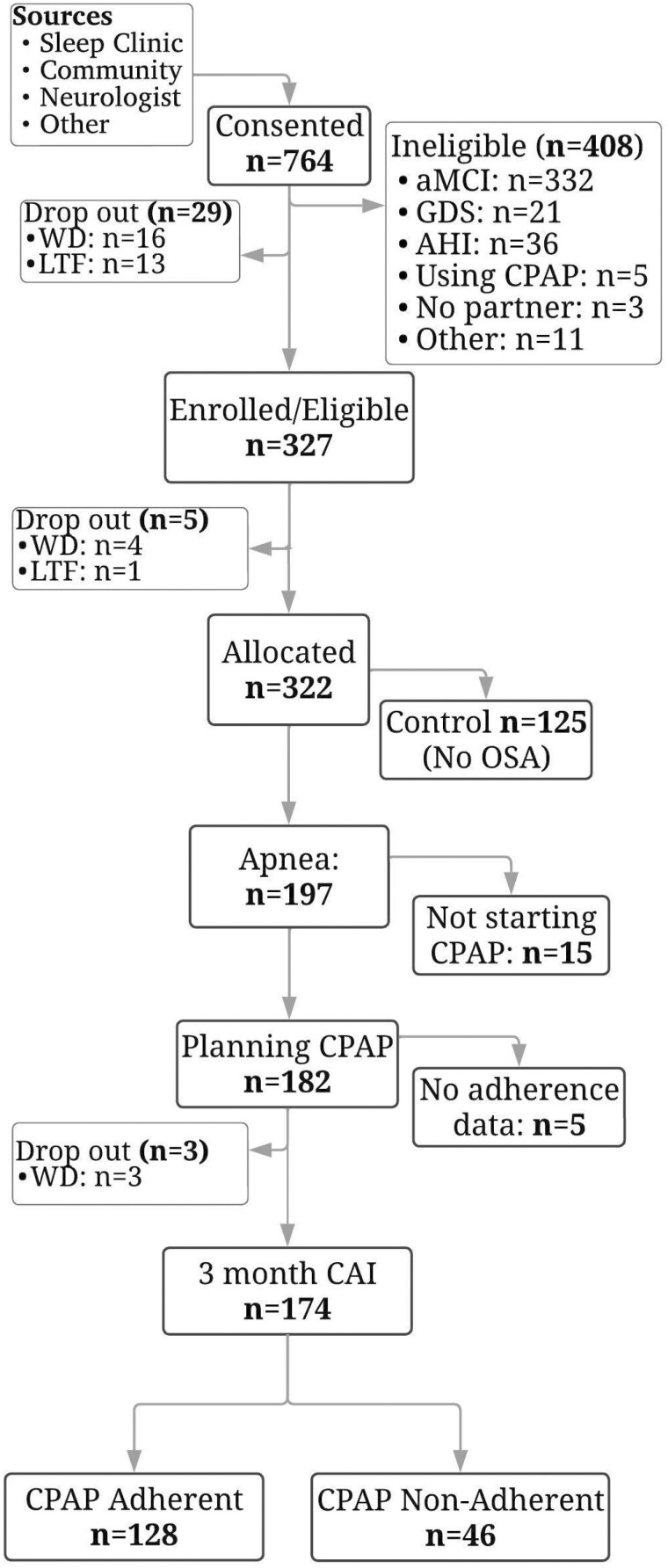
Figure 1 depicts how the sample was obtained from the Memories 2 trial data. Of the 174 participants, most were male (54%), White persons (71.3%), and non-Hispanic persons (92.5%), with a mean age of 67.08 years (SD 7.48) and mean CDR-SOB of 0.81 (SD 0.74). The Other category for race consisted of self-reported Asian, Native Hawaiian/Other Pacific, and Multiracial persons. The mean AHI was in the severe OSA range, with an average of 34.78 (SD 20.82) apneas/hypopneas per hour, and 46% of participants had severe OSA (AHI ≥30). The mean body mass index was 33.10 (SD 7.09), which falls into the obesity range. The average reported nighttime sleep duration was 7.41 hours (444.66 minutes, SD 85.69 minutes). Only 3 participants (2 CPAP-adherent, 1 CPAP nonadherent) reported an average nighttime sleep duration of less than 4 hours. Most did not report excessive daytime sleepiness (mean Epworth Sleepiness Scale = 8.89, SD 4.96), with 117 participants (67.24%) normal (score 0–10) (38). In general, participants attended most of the nine telehealth CPAP adherence intervention sessions that were offered to them. The mean attendance for the telehealth sessions was 6.59 (SD 1.39). For sample characteristics, please see Table 1.
Figure 1.
Consort diagram for Memories 2 Apnea Group. AHI = apnea–hypopnea index, aMCI = amnestic mild cognitive impairment, CAI = CPAP adherence intervention, CPAP = continuous positive airway pressure, GDS = geriatric depression scale, LTF = lost to follow-up, OSA = obstructive sleep apnea, PCP = primary care provider, WD = voluntary withdrawal.
Table 1.
Baseline Demographics and Descriptive Characteristics Memories 2 Apnea Group
| Age (y) | Mean ± SD | 67.08 ± 7.48 |
| Median (Q1, Q3) | 67 (61, 72) | |
| Min, Max | 55, 85 | |
| Sex, n (%) | Male | 94 (54.0%) |
| Race, n (%) | White | 124 (71.3%) |
| Black | 38 (21.8%) | |
| Other | 12 (6.9%) | |
| Ethnicity, n (%) | Hispanic | 13 (7.5%) |
| Education, n (%) | >High school | 138 (79.3%) |
| Apnea–hypopnea index | mean ± SD | 34.78 ± 20.82 |
| median (Q1, Q3) | 28.45 (19.50, 44.70) | |
| Min, Max | 15, 119.4 | |
| Apnea–hypopnea index, n (%) | Moderate (<30) | 94 (54.0%) |
| Severe (≥30) | 80 (46.0%) | |
| Body mass index | mean ± SD | 33.10 ± 7.09 |
| median (Q1, Q3) | 32.02 (27.95, 36.30) | |
| Min, Max | 20.12, 66.12 | |
| Nighttime sleep duration (min) | mean ± SD | 444.66 ± 85.69 |
| median (Q1, Q3) | 450.94 (399.34, 500.73) | |
| Min, Max | 140, 740 | |
| Epworth sleepiness scale | mean ± SD | 8.89 ± 4.96 |
| median (Q1, Q3) | 8 (6, 12) | |
| Min, Max | 0, 22 | |
| CAI fidelity | mean ± SD | 6.59 ± 1.39 |
| median (Q1, Q3) | 7 (6, 7) | |
| Min, Max | 2, 8 | |
| CDR-SOB | mean ± SD | 0.81 ± 0.74 |
| median (Q1, Q3) | 0.5 (0, 1) | |
| Min, Max | 0, 3.5 | |
| Mean daily CPAP use (h) | mean ± SD | 5.15 ± 2.50 |
| median (Q1, Q3) | 5.67 (3.8, 7.05) | |
| Min, Max | 0, 9.3 | |
| 3-Month CPAP use, n (%) | Adherent (mean ≥4 h) | 128 (73.6%) |
| Not adherent (mean <4 h) | 46 (26.4%) |
Notes: CAI = CPAP adherence intervention; CDR-SOB = clinical dementia rating sum of boxes; CPAP = continuous positive airway pressure; SD = standard deviation; Q1 = first quartile; Q3 = third quartile; due to 3 missing values, N = 171 for CDR-SOB. Due to 10 missing values, N = 164 for nighttime sleep duration; except for CDR-SOB and nighttime sleep duration, N = 174 for all other variables.
Mean daily hours of CPAP use at 3 months was 5.15 hours (SD 2.50). A majority of participants, 128 (73.6%), were adherent to CPAP, maintaining an average of 4 or more hours of use per night. However, only 18 (47.4%) of Black persons were adherent compared to 101 (81.5%) of White persons (p < .001), and 9 (75%) of other persons (p < .001). Black persons (n = 38) mean daily hours of CPAP use was 3.87 (SD 2.47), compared to 5.58 (SD 2.39) in White persons (n = 124), p < .001, and 4.75 (SD 2.54) in Other persons (n = 12), p = .30 (Supplementary Table 1). Supplementary Tables 2 and 3 further describe CPAP use by apnea severity and CPAP adherence intervention fidelity by Race. Mean nightly CPAP use in the moderate group (AHI <30) was 5.67 hours (SD 2.39) compared to 4.55 hours (SD 2.52) in the severe group (AHI >30) (p = .001). There were no significant differences among Races in apnea severity or fidelity, although there was a trend for Black persons in the adherent group to attend fewer intervention sessions than Other persons (p = .06).
Adjusted linear model results demonstrated that being a White person (β = 2.01, SE = 0.50, p < .001), moderate (AHI <30) OSA (β = −1.17, SE = 0.36, p = .002), and higher CPAP adherence intervention fidelity (β = 0.38, SE = 0.13, p = .004) were significantly associated with higher CPAP use at 3 months (Table 2). Age, sex, ethnicity, education, body mass index, nighttime sleep duration, Epworth sleepiness scale, and CDR-SOB were not statistically significant predictors. Using backward elimination methods, only being a White person (β = 1.57, SE = 0.43, p < .001), moderate OSA (β = −1.06, SE = 0.35, p = .003), and higher CPAP adherence intervention fidelity (β = 0.39, SE = 0.13, p = .003) were significantly associated with higher CPAP use (data not shown).
Table 2.
Unadjusted and Adjusted Linear Model Results for Mean Daily CPAP Use (h)
| Independent Variable | Unadjusted | Adjusted | ||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | SE | 95% CI | p Value | Estimate | SE | 95% CI | p Value | |
| Age | 0.02 | 0.03 | (−0.03, 0.07) | .44 | −0.03 | 0.03 | (−0.08, 0.02) | .28 |
| Sex | .77 | .39 | ||||||
| Female | 0.11 | 0.38 | (−0.64, 0.87) | 0.33 | 0.38 | (−0.43, 1.09) | ||
| Male | REF | REF | REF | REF | REF | REF | ||
| Race | <.001 | <.001 | ||||||
| White | 1.71 | 0.45 | (0.83, 2.60) | <.001 | 2.01 | 0.50 | (1.02, 2.99) | <.001 |
| Other | 0.88 | 0.80 | (−0.70, 2.46) | .27 | 1.39 | 0.92 | (−0.43, 3.20) | .13 |
| Black | REF | REF | REF | REF | REF | REF | ||
| Ethnicity | .99 | .85 | ||||||
| Hispanic | −0.01 | 0.72 | (−1.44, 1.42) | 0.16 | 0.87 | (−1.56, 1.88) | ||
| Non-Hispanic | REF | REF | REF | REF | REF | REF | ||
| Education | .39 | .87 | ||||||
| > High School | 0.41 | 0.47 | (−0.52, 1.33) | 0.08 | 0.47 | (−0.85, 1.00) | ||
| ≤ High School | REF | REF | REF | REF | REF | REF | ||
| Apnea–hypopnea Index | −0.01 | 0.01 | (−0.03, 0.01) | .31 | ||||
| Apnea–hypopnea Index | .003 | .002 | ||||||
| ≥30 | −1.12 | 0.37 | (−1.85, −0.38) | −1.17 | 0.36 | (−1.89, −0.46) | ||
| <30 | REF | REF | REF | REF | REF | REF | ||
| Body mass index | −0.01 | 0.03 | (−0.06, 0.04) | .67 | 0.02 | 0.03 | (−0.03, 0.08) | .45 |
| Nighttime sleep duration | 0.00 | 0.00 | (−0.00, 0.01) | .45 | 0.00 | 0.00 | (−0.00, 0.01) | .39 |
| Epworth sleepiness scale | −0.02 | 0.04 | (−0.10, 0.05) | .53 | 0.01 | 0.04 | (−0.07, 0.08) | .89 |
| CAI fidelity | 0.45 | 0.13 | (0.19, 0.71) | <.001 | 0.38 | 0.13 | (0.12, 0.64) | .004 |
| CDR-SOB | −0.28 | 0.25 | (−0.79, 0.22) | .27 | −0.24 | 0.26 | (−0.74, 0.27) | .36 |
Notes: CAI = CPAP adherence intervention; CDR-SOB = clinical dementia rating sum of boxes; CI = confidence interval; REF = reference; SE = standard error. For unadjusted model, N = 171 for CDR-SOB, N = 164 for nighttime sleep duration and N = 174 for all other variables; for adjusted model, N = 161.
Unadjusted and adjusted logistic regression model results are summarized in Table 3. Adjusted logistic regression model results demonstrated that White race (p < .001) and higher CPAP adherence intervention fidelity (p = .002) were significantly associated with increased odds of CPAP adherence (≥4 hours mean CPAP use per night) at 3 months. Specifically, for every additional CPAP adherence intervention session attended, the odds of CPAP adherence increased by 58% (odds ratio [OR] = 1.58, 95% confidence interval [CI] = 1.18–2.11). Additionally, White persons compared with Black persons had 9.94-fold increased odds of adhering to CPAP treatment (OR = 9.94, 95% CI = 3.21–30.85). Age, sex, ethnicity, education, AHI, body mass index, nighttime sleep duration, Epworth sleepiness scale, and CDR-SOB were not statistically significant predictors. Using backward elimination methods, White race (OR = 4.66, 95% CI = 2.08–10.45, p < .001) and higher fidelity to the CPAP adherence intervention (OR = 1.46, 95% CI = 1.14–1.87, p = .003) were significantly associated with increased odds of adherence at 3 months (data not shown).
Table 3.
Unadjusted and Adjusted Logistic Model Results for 3-month CPAP Use (Adherent Vs Not Adherent)
| Independent Variable | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | p Value | Odds Ratio | 95% CI | p Value | |
| Age | 1.00 | (0.95, 1.04) | .92 | 0.95 | (0.89, 1.01) | .11 |
| Sex | .77 | .62 | ||||
| Female | 0.90 | (0.46, 1.78) | 1.26 | (0.51, 3.09) | ||
| Male | REF | REF | REF | REF | ||
| Race | <.001 | <.001 | ||||
| White | 4.88 | (2.23, 10.66) | <.001 | 9.94 | (3.21, 30.85) | <.001 |
| Other | 3.33 | (0.78, 14.26) | .11 | 8.34 | (0.99, 69.90) | .05 |
| Black | REF | REF | REF | REF | ||
| Ethnicity | .78 | .54 | ||||
| Hispanic | 1.21 | (0.32, 4.62) | 0.53 | (0.07, 4.01) | ||
| Non-Hispanic | REF | REF | REF | REF | ||
| Education | .29 | .69 | ||||
| >High School | 1.53 | (0.69, 3.38) | 1.24 | (0.43, 3.58) | ||
| ≤High School | REF | REF | REF | REF | ||
| Apnea–hypopnea index | 1.00 | (0.98, 1.01) | .76 | |||
| Apnea–hypopnea index | .10 | .08 | ||||
| ≥30 | 0.56 | (0.28, 1.11) | 0.47 | (0.20, 1.09) | ||
| <30 | REF | REF | REF | REF | ||
| Body mass index | 1.00 | (0.96, 1.05) | .90 | 1.07 | (0.99, 1.16) | .10 |
| Nighttime sleep duration | 1.00 | (1.00, 1.00) | .90 | 1.00 | (1.00, 1.01) | .93 |
| Epworth sleepiness scale | 0.99 | (0.93, 1.06) | .83 | 1.03 | (0.94, 1.12) | .58 |
| CAI fidelity | 1.49 | (1.17, 1.88) | .001 | 1.58 | (1.18, 2.11) | .002 |
| CDR-SOB | 0.77 | (0.49, 1.21) | .25 | 0.86 | (0.48, 1.54) | .61 |
Notes: CAI = CPAP adherence intervention; CDR-SOB = clinical dementia rating sum of boxes; CI = confidence interval; CPAP = continuous positive airway pressure; REF = reference. Adherent = average of 4 or more hours of CPAP use per night; not adherent = average of less than 4 hours of CPAP use per night. For unadjusted model, N = 171 for CDR-SOB, N = 164 for nighttime sleep duration, and N = 174 for all other variables; for adjusted model, N = 161.
Discussion
To our knowledge, Memories 2 is the only large prospective study that has reported predictors of CPAP adherence in older adults with aMCI and moderate to severe OSA. Improving adherence in this population is an important goal, because consistent nightly use of CPAP in individuals with OSA results in improvements in daytime sleepiness, health-related quality of life, and depression, decreases risk for future cardiovascular events, and may affect the progression of cognitive impairment (41). Further, although few studies have been conducted on the outcomes of adherence to CPAP in older adults with aMCI and OSA, there may be a linear relationship between longer CPAP use and physiological benefits, as has been shown in studies of risk reduction for cardiovascular events (11,43).
In the Memories 2 study, almost 75% of this sample, all of whom are at high risk for progression to dementia due to Alzheimer’s disease, adhered to CPAP for 3 months based on a cut-point of ≥4 h/night. Their average CPAP use was 5.15 ± 2.50 hours. This finding is notable, as other behavioral interventions that show evidence for improving cognition in this population, such as exercise, may be challenging to continue over time (43). The 1-year CPAP adherence for participants in the Memories 1 study (n = 54), who received the same telehealth CPAP adherence intervention as the present study, was 53.7%, with average use of 4.92 h/night (11).
In a recent pilot randomized controlled crossover study of CPAP treatment versus no treatment in 29 older adults with clinical MCI and OSA, the average CPAP use for patients in a typical clinical setting was somewhat less, 3.2 h/night (SD 2.8), although benefits in some domains of cognitive function occurred related to CPAP treatment (44). Skiba et al. found no relationship between CPAP therapy and progression to dementia over 2 years in a retrospective chart review of 96 patients (45). The average CPAP use was 3.9 h/night (45). Future trials in older adults with aMCI and OSA might compare CPAP adherence intervention strategies to determine which are most effective in this population of older adults with aMCI and OSA who are at high risk for dementia.
In this study, White race was a significant predictor of CPAP adherence in both linear and logistic models. Although a majority of study participants, 73.6%, were adherent to CPAP, only 18 (47.4%) of Black participants were adherent. Attendance at the adherence intervention sessions did not explain why Black persons were less likely to adhere to CPAP, as there were no significant differences among Black persons, White persons, or Other persons in the number of sessions attended. These findings regarding low CPAP adherence in Black persons are consistent with literature in other populations. In a recent review, 16 of 22 studies showed significantly lower CPAP use in Black persons compared with White persons (22). For example, in a younger sample with no mention of cognitive impairment, Billings et al. found that the mean CPAP use for 3 months was 267 minutes (SD 141) in White persons (n = 119) versus 179 minutes (SD 106) in Black persons (n = 42) (24). Future descriptive studies might focus on better understanding perceptions of Black persons regarding bias in clinical encounters, their health-related goals regarding OSA and memory, and their ideas about more effective adherence interventions. Lower CPAP use in Black persons may reflect health care inequities and distrust of medical professionals. In a nationally representative, probability-based survey of 802 non-Hispanic Black U.S. adults and a comparison group of 902 non-Hispanic White U.S. adults, about one third of Black persons reported experiencing discrimination in clinical encounters (46). These findings suggest a need for more active interventions to address racial implicit bias in clinical practice.
An additional strategy to address low CPAP adherence in Black persons is cultural tailoring of the adherence intervention. For example, Jean-Louis et al. developed and tested a culturally tailored telehealth intervention to increase the evaluation and treatment of OSA in Black persons (47). The investigators culturally tailored the intervention based on findings from focus groups. They then presented the intervention they derived to a subset of patients and their Community Steering Committee to ensure that the messaging was culturally sensitive, feasible, and acceptable. A trained sleep health educator of the same racial background delivered the intervention. In the randomized controlled trial (n = 380), those in the intervention arm were 3.17 times more likely to attend an initial sleep consultation visit, compared to an attention control group, but there was no difference in self-reported CPAP adherence between groups.
Investigators in future studies that aim to evaluate the effect of interventions to improve CPAP adherence in older Black persons with aMCI might consider testing the effect of a culturally tailored version (using the methods to develop the intervention described by Jean-Louis and colleagues) of the telehealth CPAP adherence intervention that we used in this study. Prior to beginning a future trial, it would be important that the interventionists receive training on implicit bias in clinical encounters, and demonstrate self-awareness of any biases and intervention competence during role playing. Also, in future CPAP adherence studies investigators might consider patient perception of discrimination in clinical encounters as an effect modifier.
In general, participants in the present study engaged in the CPAP adherence intervention. They attended more than 75% of the sessions. The telehealth intervention consisted of short phone sessions with trained research staff. The phone calls focused on motivational enhancement, individual goal setting, building self-efficacy, and guidance for any CPAP problems. The goal was that participants would identify the relationship between their personal goals and use of CPAP, and discover ways to overcome any perceived barriers to CPAP use.
In the present study, fidelity to the CPAP adherence intervention, as measured by the number of sessions attended, was significantly associated with CPAP use in both linear and logistic models. A recent systematic review identified 2 important mechanisms by which motivational enhancement could influence health behaviors: (1) motivation, defined as a client behavior that initiates, guides, and maintains goal-directed behavior, and (2) motivational interviewing spirit, defined as the key therapist behavior of collaboration and evoking client ideas about change and autonomy (48). These mechanisms have implications for clinicians. Our results add to the evidence that provider collaboration with patients and use of behavioral strategies to increase patient motivation are important for CPAP adherence (41). Perhaps patient motivation and self-efficacy beliefs moderate the relationship between treatment benefits deemed to be important by patients and CPAP adherence.
Those with moderate apnea (AHI 15–30) were significantly more likely to use CPAP longer each night than those with severe apnea (AHI ≥30). Other research has reported that a higher AHI is associated with longer CPAP use (3,12,13). Our findings may reflect dichotomizing the sample of older adults with aMCI into only 2 groups (moderate or severe apnea), or the homogeneity of the sample (eg, those with mild apnea were excluded).
The finding that age was not significantly associated with CPAP adherence provides evidence that older adults with aMCI can successfully use CPAP into their 80s, a positive finding. There were only 13 Hispanic persons in the study, which may have been an insufficient sample to show relationships between ethnicity and CPAP adherence. Similarly, there were only 12 participants of Other race which limited the ability to identify relationships between Other race and CPAP adherence. Due to participants choosing not to report their incomes, education (high school or higher vs less than high school) was used as a proxy for socioeconomic status. More comprehensive measures of socioeconomic status might yield different results.
There was no relationship between CPAP use and cognition as measured by CDR-SOB. Although our results may reflect the lack of cognitive variability in the sample, Ancoli-Israel et al. also found similar CPAP use (5.8 h/night) in a sample of older adults with probable mild AD (49). Taken together, our findings and those of Ancoli-Israel and colleagues are encouraging because they indicate that both older adults with aMCI and mild dementia are cognitively able to use CPAP.
Strengths of this study include a large, diverse sample of females, Black persons, and Hispanic persons; objective CPAP adherence data; a comprehensive telehealth motivational enhancement CPAP adherence intervention; and both linear and logistic regression modeling. There are also limitations. Conclusions regarding factors not statistically associated with adherence may reflect the relatively small sample size. There may have been insufficient power to observe more modest associations. Although the Memories 2 study MCI diagnostic criteria mirrored those of the Alzheimer’s Disease Neuroimaging Initiative 2 (35) and were similar to other large studies on MCI (50), we recognize that using a single measure of episodic memory to diagnose MCI, and including participants with early MCI may increase susceptibility to false positive diagnostic errors.
Conclusions and Relevance
Older adults with aMCI can have high levels of CPAP adherence, suggesting that neither age nor cognitive impairment should be a barrier to prescribing CPAP. Research is needed to improve CPAP adherence in Black patients. Motivational enhancement interventions that are developed with the help of the population of interest, and delivered by trained educators of the same racial background may improve CPAP adherence and health in older Black persons with aMCI and apnea.
Supplementary Material
Acknowledgments
The Memories 2 Study Research Team: Vanessa Aguilar, Matthew James Barrett, Tammie Benzinger, Nick R. Bryan, Patricia Carter, KerCheng Chen, Cynthia Cheng, Luqi Chi, Jesse Chittams, Sandhitsu Das, Eric M. Davis, John A. Detre, Karl Doghramji, Elyse A. Everett, Jamie L. Fuentecilla, Lora Gallagher, Nalaka Gooneratne, Alexandra Hanlon, Wenyan Ji, Dahlia Kamel, Sharon Klein, Sneha Khan, Matthew Loftspring, Alicia Lozano, Carol A. Manning, Brandye Mazdra, Stephen Moelter, Jennifer Morris, Janet Morrison, Manasa Mula, Grace Nathanson, Ilya Nasrallah, Hilary L.P. Orlowski, Elena Park, Yaretzi Campos Polanco, Mark Stephen Quigg, Cyrus A. Raji, Sneha Rangu, Joseph Rhodes, Kathy C. Richards, Barbara J. Snider, Vani Vallabhaneni, Colleen Webber, David Wolk, and Paul Yushkevich. We thank the Memories 2 study participants and their partners for the generous gift of their time and the student volunteers for their efforts. We also thank sleep centers and community organizations for their support: Austin ENT Clinic, Austin Neuropsychology, Comprehensive Sleep Medicine Associates, Georgetown Sleep Center, Latino Healthcare Forum, Pulmonary and Critical Care Specialists of Northern Virginia, REM Sleep Center, Sleep 360 Diagnostic Center, Sleep Centers of Austin, Sleep Medicine Consultants, South Austin Medical Clinic, and Texas Sleep Medicine.
Contributor Information
Kathy C Richards, School of Nursing, University of Texas at Austin, Austin, Texas, USA.
Alicia J Lozano, Department of Statistics, Center for Biostatistics and Health Data Science, Virginia Tech, Roanoke, Virginia, USA.
Jennifer Morris, Department of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, USA.
Stephen T Moelter, Department of Psychology, Saint Joseph’s University, Philadelphia, Pennsylvania, USA.
Wenyan Ji, Department of Statistics, Center for Biostatistics and Health Data Science, Virginia Tech, Roanoke, Virginia, USA.
Vani Vallabhaneni, Sleep 360, Austin, Texas, USA.
Yanyan Wang, National Clinical Research Center for Geriatrics & Nursing Key Laboratory of Sichuan Province, West China Hospital & West China School of Medicine, Sichuan University, Chengdu, China.
Luqi Chi, Department of Neurology, Washington University, St. Louis, Missouri, USA; Department of Sleep Medicine, Washington University, St. Louis, Missouri, USA.
Eric M Davis, Division of Pulmonary and Critical Care, Department of Medicine, University of Virginia, Charlottesville, Virginia, USA.
Cindy Cheng, Department of Family and Community Medicine, Thomas Jefferson University, Philadelphia, Pennsylvania, USA.
Vanessa Aguilar, School of Nursing, University of Texas at Austin, Austin, Texas, USA.
Sneha Khan, Department of Osteopathic Medicine, Arkansas State University, Jonesboro, Arkansas, USA.
Mira Sankhavaram, School of Nursing, University of Texas at Austin, Austin, Texas, USA.
Alexandra L Hanlon, Department of Statistics, Center for Biostatistics and Health Data Science, Virginia Tech, Roanoke, Virginia, USA.
David A Wolk, Department of Neurology, University of Pennsylvania, Philadelphia, Pennsylvania, USA.
Nalaka Gooneratne, Department of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, USA.
Funding
This work was supported by the National Institute on Aging at the National Institutes of Health (R01AG054435 to N.G., K.C.R., and D.W. and R01AG034682 to K.C.R.).
Conflict of Interest
K.C. R. has received Horizant and placebo from Arbor Pharmaceuticals, and consulting fees from Merck and Woolsey Pharmaceuticals, Inc. D.A.W. has received grant support from Biogen and Merck, serves on the Data Safety Monitoring Board for Functional Neuromodulation, and has received consulting fees from Eli Lilly, GE Healthcare, and Qynapse.
Data Availability
Data will be shared with others upon request through the Memories 2 website https://www.med.upenn.edu/memories2/researcher-welcome.html no later than July 1, 2026.
References
- 1. Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet. 2006;367(9518):1262–1270. doi: 10.1016/S0140-6736(06)68542-5 [DOI] [PubMed] [Google Scholar]
- 2. Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical Review. JAMA. 2014;312(23):2551–2561. doi: 10.1001/jama.2014.13806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15(6):343–356. doi: 10.1016/j.smrv.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leng Y, McEvoy CT, Allen IE, Yaffe K. Association of sleep-disordered breathing with cognitive function and risk of cognitive impairment: a systematic review and meta-analysis. JAMA Neurol. 2017;74(10):1237–1245. doi: 10.1001/jamaneurol.2017.2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gupta M. Obstructive sleep apnea phenotypes and positive airway therapy adherence. In: Shapiro CM, Gupta M, Zalai D, eds. CPAP Adherence: Factors and Perspectives. Springer International Publishing; 2022:141–151. doi: 10.1007/978-3-030-93146-9_13 [DOI] [Google Scholar]
- 6. Beaudin AE, Raneri JK, Ayas NT, et al. Cognitive function in a sleep clinic cohort of patients with obstructive sleep apnea. Ann Am Thorac Soc. 2021;18(5):865–875. doi: 10.1513/AnnalsATS.202004-313OC [DOI] [PubMed] [Google Scholar]
- 7. Lal C, Ayappa I, Ayas N, et al. The link between obstructive sleep apnea and neurocognitive impairment: an official American thoracic society workshop report. Ann Am Thorac Soc. 2022;19(8):1245–1256. doi: 10.1513/AnnalsATS.202205-380ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG. Treatment of adult obstructive sleep apnea with positive airway pressure: an American academy of sleep medicine clinical practice guideline. J Clin Sleep Med. 2019;15(02):335–343. doi: 10.5664/jcsm.7640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Freedman N, Johnson K. Chapter 132: positive airway pressure treatment for obstructive sleep apnea. In: Kryger M, Roth T, Goldstein CA, Dement WC, eds. Principles and Practice of Sleep Medicine. 7th ed. Elsevier; 2022:1260–1283. [Google Scholar]
- 10. Dunietz GL, Chervin RD, Burke JF, Conceicao AS, Braley TJ. Obstructive sleep apnea treatment and dementia risk in older adults. Sleep. 2021;44(9):zsab076. doi: 10.1093/sleep/zsab076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Richards KC, Gooneratne N, Dicicco B, et al. CPAP adherence may slow 1-year cognitive decline in older adults with mild cognitive impairment and apnea. J Am Geriatr Soc. 2019;67(3):558–564. doi: 10.1111/jgs.15758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Campos-Rodriguez F, Martinez-Alonso M, Sanchez-de-la-Torre M, Barbe F; Spanish Sleep Network. Long-term adherence to continuous positive airway pressure therapy in non-sleepy sleep apnea patients. Sleep Med. 2016;17:1–6. doi: 10.1016/j.sleep.2015.07.038 [DOI] [PubMed] [Google Scholar]
- 13. Kohler M, Smith D, Tippett V, Stradling JR. Predictors of long-term compliance with continuous positive airway pressure. Thorax. 2010;65(9):829–832. doi: 10.1136/thx.2010.135848 [DOI] [PubMed] [Google Scholar]
- 14. Budhiraja R, Parthasarathy S, Drake CL, et al. Early CPAP use identifies subsequent adherence to CPAP therapy. Sleep. 2007;30(3):320–324. doi: 10.1093/sleep/30.3.320 [DOI] [PubMed] [Google Scholar]
- 15. Sin DD, Mayers I, Man GC, Pawluk L. Long-term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population-based study. Chest. 2002;121(2):430–435. doi: 10.1378/chest.121.2.430 [DOI] [PubMed] [Google Scholar]
- 16. Pelletier-Fleury N, Rakotonanahary D, Fleury B. The age and other factors in the evaluation of compliance with nasal continuous positive airway pressure for obstructive sleep apnea syndrome: a Cox’s proportional hazard analysis. Sleep Med. 2001;2(3):225–232. doi: 10.1016/s1389-9457(00)00063-0 [DOI] [PubMed] [Google Scholar]
- 17. Woehrle H, Graml A, Weinreich G. Age- and gender-dependent adherence with continuous positive airway pressure therapy. Sleep Med. 2011;12(10):1034–1036. doi: 10.1016/j.sleep.2011.05.008 [DOI] [PubMed] [Google Scholar]
- 18. Patel SR, Bakker JP, Stitt CJ, Aloia MS, Nouraie SM. Age and sex disparities in adherence to CPAP. Chest. 2021;159(1):382–389. doi: 10.1016/j.chest.2020.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Myllylä M, Kurki S, Anttalainen U, Saaresranta T, Laitinen T. High adherence to CPAP treatment does not prevent the continuation of weight gain among severely obese OSAS Patients. J Clin Sleep Med. 2016;12(4):519–528. doi: 10.5664/jcsm.5680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luyster FS, Buysse DJ. The impact of partner and family support in PAP therapy. In: Shapiro CM, Gupta M, Zalai D, eds. CPAP Adherence: Factors and Perspectives. Springer International Publishing; 2022:109–116. doi: 10.1007/978-3-030-93146-9_10 [DOI] [Google Scholar]
- 21. Gagnadoux F, Le Vaillant M, Goupil F, et al. Influence of marital status and employment status on long-term adherence with continuous positive airway pressure in sleep apnea patients. PLoS One. 2011;6(8):e22503. doi: 10.1371/journal.pone.0022503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wallace DM, Williams NJ, Sawyer AM, et al. Adherence to positive airway pressure treatment among minority populations in the US: a scoping review. Sleep Med Rev. 2018;38:56–69. doi: 10.1016/j.smrv.2017.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Means MK, Ulmer CS, Edinger JD. Ethnic differences in continuous positive airway pressure (CPAP) adherence in veterans with and without psychiatric disorders. Behav Sleep Med. 2010;8(4):260–273. doi: 10.1080/15402002.2010.509255 [DOI] [PubMed] [Google Scholar]
- 24. Billings ME, Auckley D, Benca R, et al. Race and residential socioeconomics as predictors of CPAP adherence. Sleep. 2011;34(12):1653–1658. doi: 10.5665/sleep.1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang Y, Ren R, Lei F, et al. Worldwide and regional prevalence rates of co-occurrence of insomnia and insomnia symptoms with obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev. 2019;45:1–17. doi: 10.1016/j.smrv.2019.01.004 [DOI] [PubMed] [Google Scholar]
- 26. Sweetman A, Lack L, McEvoy RD, et al. Bi-directional relationships between co-morbid insomnia and sleep apnea (COMISA). Sleep Med Rev. 2021;60:101519. doi: 10.1016/j.smrv.2021.101519 [DOI] [PubMed] [Google Scholar]
- 27. Sateia MJ. International classification of sleep disorders-third edition. Chest. 2014;146(5):1387–1394. doi: 10.1378/chest.14-0970 [DOI] [PubMed] [Google Scholar]
- 28. Pieh C, Bach M, Popp R, et al. Insomnia symptoms influence CPAP compliance. Sleep Breath. 2013;17(1):99–104. doi: 10.1007/s11325-012-0655-9 [DOI] [PubMed] [Google Scholar]
- 29. Philip P, Bioulac S, Altena E, et al. Specific insomnia symptoms and self-efficacy explain CPAP compliance in a sample of OSAS patients. PLoS One. 2018;13(4):e0195343. doi: 10.1371/journal.pone.0195343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Askland K, Wright L, Wozniak DR, Emmanuel T, Caston J, Smith I. Educational, supportive and behavioural interventions to improve usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database Syst Rev. 2020;(4):CD007736. doi: 10.1002/14651858.CD007736.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Knopman DS, Roberts RO, Geda YE, et al. Validation of the telephone interview for cognitive status-modified in subjects with normal cognition, mild cognitive impairment, or dementia. Neuroepidemiology. 2010;34(1):34–42. doi: 10.1159/000255464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566 [DOI] [PubMed] [Google Scholar]
- 34. Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 35. Petersen RC, Aisen PS, Beckett LA, et al. Alzheimer disease neuroimaging initiative (ADNI). Neurology. 2010;74(3):201–209. doi: 10.1212/WNL.0b013e3181cb3e25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4 [DOI] [PubMed] [Google Scholar]
- 37. Morgenthaler TI, Aurora RN, Brown T, et al. Practice parameters for the use of autotitrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome: an update for 2007. An American Academy of Sleep Medicine report. Sleep. 2008;31(1):141–147. doi: 10.1093/sleep/31.1.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(5):540–545. doi: 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 39. Aloia MS, Smith K, Arnedt JT, et al. Brief behavioral therapies reduce early positive airway pressure discontinuation rates in sleep apnea syndrome: preliminary findings. Behav Sleep Med. 2007;5(2):89–104. doi: 10.1080/15402000701190549 [DOI] [PubMed] [Google Scholar]
- 40. O’Bryant SE, Waring SC, Cullum CM, et al. Staging dementia using clinical dementia rating scale sum of boxes scores: a Texas Alzheimer’s research consortium study. Arch Neurol. 2008;65(8):1091–1095. doi: 10.1001/archneur.65.8.1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dzierzewski JM, Wallace DM, Wohlgemuth WK. Adherence to continuous positive airway pressure in existing users: self-efficacy enhances the association between continuous positive airway pressure and adherence. J Clin Sleep Med. 2016;12(02):169–176. doi: 10.5664/jcsm.5478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and cox regression. Am J Epidemiol. 2007;165(6):710–718. doi: 10.1093/aje/kwk052 [DOI] [PubMed] [Google Scholar]
- 43. Rivera-Torres S, Fahey TD, Rivera MA. Adherence to exercise programs in older adults: informative report. Gerontol Geriatr Med. 2019;5. doi: 10.1177/2333721418823604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hoyos CM, Cross NE, Terpening Z, et al. Continuous positive airway pressure for cognition in sleep apnea and mild cognitive impairment: a pilot randomized crossover clinical trial [correspondence]. Am J Respir Crit Care Med. 2022;205(12):1479–1482. doi: 10.1164/rccm.202111-2646LE [DOI] [PubMed] [Google Scholar]
- 45. Skiba V, Novikova M, Suneja A, McLellan B, Schultz L. Use of positive airway pressure in mild cognitive impairment to delay progression to dementia. J Clin Sleep Med. 2020;16(6):863–870. doi: 10.5664/jcsm.8346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bleich SN, Findling MG, Casey LS, Blendon RJ, Benson JM, SteelFisher GK, Sayde JM, Miller C. Discrimination in the United States: experiences of Black Americans. Health Serv Res. 2019;54(S2):1399–1408. doi: 10.1111/1475-6773.13220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jean-Louis G, Newsome V, Williams NJ, Zizi F, Ravenell J, Ogedegbe G. Tailored behavioral intervention among blacks with metabolic syndrome and sleep apnea: results of the MetSO Trial. Sleep. 2017;40(1). doi: 10.1093/sleep/zsw008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Copeland L, McNamara R, Kelson M, Simpson S. Mechanisms of change within motivational interviewing in relation to health behaviors outcomes: a systematic review. Patient Educ Couns. 2015;98(4):401–411. doi: 10.1016/j.pec.2014.11.022 [DOI] [PubMed] [Google Scholar]
- 49. Ancoli-Israel S, Palmer BW, Cooke JR, et al. Cognitive effects of treating obstructive sleep apnea in Alzheimer’s disease: a randomized controlled study. J Am Geriatr Soc. 2008;56(11):2076–2081. doi: 10.1111/j.1532-5415.2008.01934.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lu Y, Liu C, Yu D, et al. Prevalence of mild cognitive impairment in community-dwelling Chinese populations aged over 55 years: a meta-analysis and systematic review. BMC Geriatr. 2021;21(1):10. doi: 10.1186/s12877-020-01948-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be shared with others upon request through the Memories 2 website https://www.med.upenn.edu/memories2/researcher-welcome.html no later than July 1, 2026.