Abstract
Background
Total proctocolectomy with ileal pouch anal anastomosis (IPAA) for medically refractory ulcerative colitis or dysplasia may be associated with structural and inflammatory complications. However, even in their absence, defecatory symptoms secondary to dyssynergic defecation or fecal incontinence may occur. Although anorectal manometry is well established as the diagnostic test of choice for defecatory symptoms, its utility in the assessment of patients with IPAA is less established. In this systematic review, we critically evaluate the existing evidence for anopouch manometry (APM).
Methods
A total of 393 studies were identified, of which 6 studies met all inclusion criteria. Studies were not pooled given different modalities of testing with varying outcome measures.
Results
Overall, less than 10% of symptomatic patients post-IPAA were referred to APM. The prevalence of dyssynergic defecation as defined by the Rome IV criteria in symptomatic patients with IPAA ranged from 47.0% to 100%. Fecal incontinence in patients with IPAA was characterized by decreased mean and maximal resting anal pressure on APM, as well as pouch hyposensitivity. The recto-anal inhibitory reflex was absent in most patients with and without incontinence.
Conclusion
Manometry alone is an imperfect assessment of pouch function in patients with defecatory symptoms, and confirmatory testing may need to be performed with dynamic imaging.
Keywords: ileal pouch anal anastomosis, manometry
Key Messages.
What is already known?
Postileal pouch anal anastomosis (IPAA) structural and inflammatory disorders are common, and functional disorders such as dyssynergic defecation (DD) and fecal incontinence (FI) are increasingly being recognized in symptomatic patients.
What is new here?
Less than 10% of patients post-IPAA are referred to anopouch manometry (APM). Normal APM parameters are not well established, and results are difficult to interpret due to different testing strategies.
How can this study help patient care?
Manometry alone is an imperfect assessment of pouch function in patients with defecatory symptoms, and confirmatory testing may need to be performed with dynamic imaging.
Introduction
Despite pharmacological advances in the treatment of ulcerative colitis (UC), 10% to 15% of patients will require a total proctocolectomy (TPC) with ileal pouch anal anastomosis (IPAA) for medically refractory disease or dysplasia.1,2 Although the TPC with IPAA is an excellent alternative to a permanent end ileostomy, it is not without its complications. Post-IPAA structural and inflammatory disorders are common; and functional disorders such as dyssynergic defecation (DD) and fecal incontinence (FI) are increasingly being recognized in symptomatic patients.3
According to the International Ileal Pouch Consortium,3 DD in patients with IPAA is characterized by symptoms of excessive straining and incomplete evacuation in the absence of structural outlet obstruction. Dyssynergic defecation may also include symptoms of abdominal/pelvic pain and bloating and occurs in approximately 75% of patients with IPAA and defecatory symptoms.4 Dyssynergic defecation is commonly referred to in the literature interchangeably as functional evacuatory difficulty, dyschezia, or anismus. Fecal incontinence is characterized by the chronic passive passage of solid or liquid stool due to functional and structural causes.5 Approximately 25% to 30% of patients with IPAA experience FI or minor seepage due to structural changes, both of which significantly impact quality of life.6 Dyssynergic defecation and FI are typically evaluated via anorectal manometry (ARM), balloon expulsion testing (BET), electromyography (EMG), and defecography. However, these tests were first developed to evaluate rectal function and recto-anal coordination in patients with intact rectums, and their utility in the characterization of defecatory symptoms in patients with IPAA is unclear. In a retrospective review of our institutional TPC with IPAA database to be presented at the American College of Gastroenterology’s annual meeting, only 19 patients completed manometry for symptoms of DD or FI. Results were mixed—paradoxical contraction during attempted defecation was seen in 47.3% of patients with symptoms of DD, whereas decreased rest and squeeze pressures and hyposensitivity of the pouch were observed in patients with FI.7
Herein, we critically evaluate the available data on manometry in symptomatic patients with IPAA to better understand its utility in characterizing DD and FI. For clarity, we propose the term anopouch manometry (APM) to delineate testing in patients with IPAA.
Methods
A comprehensive search comprising both index terms and keywords was executed in the MEDLINE, EMBASE, and Cochrane Central databases to identify studies that described the prevalence, diagnosis, and/or management of DD or FI in patients with IPAA. Inclusion criteria required that English abstracts were available for screening and included adult subjects with a history of IBD who underwent IPAA with J-pouch construction and anorectal physiology studies with either APM or EMG to evaluate defecatory symptoms. Studies that included patients with S- or W-pouches were excluded. There were no restrictions on number of subjects or types of studies. This review was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.
Search terms are described in the Supplement Appendix 1. All articles were screened for relevance to the study question, and potentially relevant articles were reviewed in more detail. The reference lists of eligible articles were reviewed to identify additional potentially relevant publications. Two investigators performed independent eligibility and quality/risk of bias assessments of relevant publications using the previously mentioned eligibility criteria.
Search results were imported into the Covidence online systematic review software and screened according to predefined eligibility criteria. Initial screening was conducted based on article titles and abstracts. Results that met the eligibility or could not be conclusively excluded were screened based on full text. Both rounds of screening were conducted independently by 2 reviewers (Y.L. and M.K.), and any discrepancy was settled by a third reviewer (B.J.). Screening results are shown in Figure 1.
Figure 1.
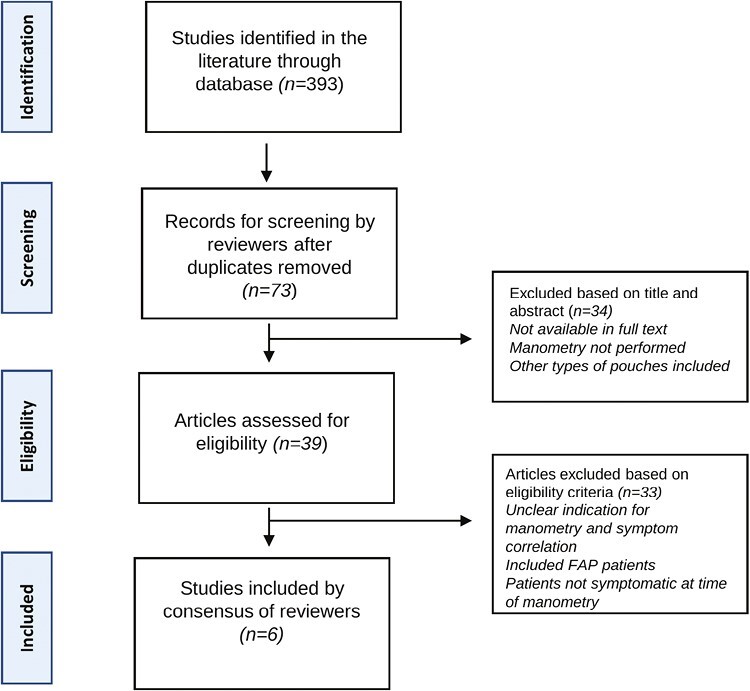
Flowchart of study selection.
Results including manometric patterns (ie, paradoxical or nonparadoxical) on APM or EMG, abnormalities (ie, resting pressures, presence or absence recto-anal inhibitory reflex, sensitivity thresholds) on APM, balloon expulsion testing (BET), barium results, or MRI (magnetic resonance imaging) defecography results were extracted into tables. The proportions of patients who had a diagnosis of DD or fecal incontinence as defined by each study were also collected. Meta-analysis was not performed due to significant methodological heterogeneity. Two reviewers assessed quality of studies independently using the NIH Quality Assessment Tool for Case Series Studies8 and risk of bias using Risk of Bias in Nonrandomized Studies of Interventions (ROBINS-I).9 Disagreements were resolved by a third reviewer (B.J.).
Results
A total of 393 studies were identified. After screening of titles, abstracts and full texts, 6 studies met all inclusion criteria (Figure 1). The few studies on the use of APM for the evaluation of defecatory symptoms in patients post-IPAA have been limited by their size, scope, and methodology (Tables 1 and 2). Studies were not pooled given different modalities of testing with varying outcome measures.
Table 1.
Studies utilizing APM to assess for dyssynergic defecation in patients with IPAA.
| Author and Year [Reference] | No. Patients | Pattern of APM Abnormality | Balloon Expulsion Test | MRI or Barium Defecography | Risk of Biasb | Quality Assessmentc |
|---|---|---|---|---|---|---|
| Hull et al. (1993)10 | n = 6 | 6/6 (10.00%) paradoxical puborectalis contraction on EMG | NA | NA | High risk | Poor |
| Khanna et al. (2013)11 | n = 35 with inflammatory/structural pouch disorder | 6/35 (17.1%) paradoxical contraction on APM | 7/35 (20.0%) failed BET | 6/35 (20.7%) paradoxical | Some concerns | Fair |
| n = 10 with functional pouch disorder | 5/10 (50.0%) paradoxical contraction on APM | 6/10 (60.0%) failed BET | 5/10 (50.0%) paradoxical | |||
| Quinn et al. (2017)12 | n = 66 with chronic pouchitis | 55/66 (83.3%) nonrelaxing pelvic floor disordera on APM | 43/55 (78.2%) failed BET | 14/55 (25.5%) abnormal | Some concerns | Good |
| n = 45 without chronic pouchitis | 28/45 (62.2%) non relaxing pelvic floor disorder on APM | 22/28 (78.6%) failed BET | 7/28 (25.0%) abnormal | |||
| The Mount Sinai Experience (2022)7 | n = 10 with inflammatory/structural pouch disorder | 4/10 (40.0%) paradoxical contraction on APM | 3/3 (100.0%) failed BET | 1/2 (50.0%) abnormal | N/A | N/A |
| n = 7 with functional pouch disorder | 4/7 (57.1%) paradoxical contraction on APM | 3/6 (50.0%) failed BET | 2/6 (33.3%) abnormal |
Abbreviations: APM, anopouch manometry; BET, balloon expulsion test; EMG, electromyography; IPAA, ileo-anal pouch anastomosis.
aNonrelaxing pelvic floor disorder defined in text.
bROBINS-E Risk of Bias.
cNIH Quality Assessment Tool for Case Series Study.
Table 2.
Studies utilizing APM to assess for fecal incontinence in patients with IPAA.
| Author and Year (Reference) | No. Patients | Pattern of APM Abnormalitya | RAIR | Risk of Biasb | Quality Assessmentc |
|---|---|---|---|---|---|
| Braun et al. (1991)13 | n = 8 with incontinence | Decrease in maximum resting anal pressure | 7/8 (87.5%) with absent RAIR | Some concerns | Fair |
| n = 25 with continence | Slight decrease in maximum squeeze pressure | 19/25 (76.0%) with absent RAIR | |||
| Reduced positive pouch anal pressure gradient | |||||
| Leblanc et al. (1994)14 | n = 4 with incontinence | Decrease in the mean resting anal pressure | Absent in all patients | High risk | Poor |
| n = 9 with continence | Hyposensitive pouch | ||||
| Sarmiento et al. (1997)15 | n = 22 with nocturnal incontinence | Decrease in mean anal pressure | Absent in all patients | Some concerns | Fair |
| n = 22 with complete incontinence | |||||
| The Mount Sinai Experience (2022)7 | n = 2 with incontinence | Decreased rest and squeeze pressures | 1/1 (100.0%) with absent RAIR | N/A | N/A |
| Hyposensitive pouch |
Abbreviations: APM, anopouch manometry; IPAA, ileo-anal pouch anastomosis; RAIR, recto-anal inhibitory reflex.
aIn patients with incontinence.
bROBINS-E Risk of Bias.
cNIH Quality Assessment Tool for Case Series Study.
Dyssynergic Defecation
The first study to evaluate DD in patients with IPAA was published in 1995 by Hull et al and included 6 J-pouch patients with defecatory difficulties (and 7 S-pouch patients).10 High-resolution manometry was not available at the time. On EMG, all 6 J-pouch patients demonstrated paradoxical puborectalis contraction during attempted defecation. The authors also performed EMG on 25 asymptomatic patients with IPAA, and none demonstrated paradoxical puborectalis contraction.
The second study to evaluate symptoms of DD with IPAA was published in 2013 by Khanna et al and included 45 patients11 who all underwent APM. Patients were divided into 2 groups: the inflammatory/structural pouch disorder (ISPD) group (n = 35), and the functional pouch disorder (FPD) group (n = 10). On manometry, the authors found that paradoxical contraction and failure of balloon expulsion occurred in 60.0% and 50.0%, respectively, in the FPD group and in 20.0% and 17.1% of the IPSD group.
The third and largest study to describe APM patterns in patients with IPAA and DD was published in 2017 by Quinn et al and included 111 patients (66 chronic pouchitis, 45 no pouchitis).12 The included group represented only 9.0% of the 1233 patients who had underwent IPAA at this high-volume institution over 15 years. All patients underwent APM. Of the 111 patients, 78.4% (83.3% of patients with chronic pouchitis vs 62.2% of patients without chronic pouchitis) met criteria for nonrelaxing pelvic floor dysfunction (N-RPFD). Nonrelaxing pelvic floor dysfunction was broadly defined as one or more of the following parameters: abnormal BET, 2 abnormal ARM parameters (including elevated mean resting pressure, reduced pouch-anal gradient, reduced pouch pressure, anal relaxation <20%, elevated residual anal pressure), abnormal MRI, barium defecography with >50% retained contrast, or EMG demonstrating elevated baseline activity with paradoxical increase or failure to appropriately relax during simulated defecation.
Fecal Incontinence
The first study to evaluate FI in patients with IPAA was published in 1991 and included 25 patients with continence and 8 patients without.13 In patients with FI, the study noted (1) decreased maximum resting anal pressure, (2) decreased maximum squeeze anal pressure, and (3) reduced positive pouch anal pressure gradient. More patients with FI had an absent recto-anal inhibitory reflex (RAIR) compared with patients without (87.5% vs 76.0%).
The second study to evaluate FI in patients with IPAA was published in 1994 and included 9 patients with continence and 4 patients with incontinence.14 In patients with FI, a marked decrease in the resting anal pressure and an increase in the volume necessary to onset of threshold sensation were observed. There was no difference in squeeze pressure, and the RAIR was absent in all patients.
The third and largest study to date of patients with IPAA and FI was published in 1997 and included 22 patients with nocturnal incontinence and 22 patients with continence.15 A low basal anal canal pressure was seen in patients with nocturnal incontinence. Squeezing anal canal pressures did not differ; and defecating anorectal angle, percentage of pouch evacuation, and perineal descent—all measured scintigraphically—did not differ between groups. The RAIR was absent in all patients.
Discussion
The true prevalence of defecatory disorders in patients post-IPAA is unknown, in part due to the overlap of symptoms with inflammatory and structural pouch disorders, and also in part due to infrequent diagnostic testing. Overall, less than 10% of patients post-IPAA are being referred to APM, and even then, results are difficult to interpret due to different testing strategies and metrics. Normal APM parameters are not well established, and their definition has been hindered by the prevalence of structural and/or functional pouch disorders even in asymptomatic patients. In a recent study published by Quinn et al, 6 of the 20 patients with self-reported “healthy” pouch function were excluded from final analysis due to symptoms suggestive of pouch evacuation disorder and/or structural abnormality on MR imaging.16
According to the Rome IV criteria, the diagnosis of DD requires (1) constipation; (2) demonstration of DD on ARM, EMG, or defecography; and (3) an abnormal BET or inability to evacuate/retention of barium on defecography.17 As reported in our results, the prevalence of DD as defined by the Rome IV criteria and diagnosed via EMG or manometry in symptomatic patients with IPAA ranged widely from 47.0% to 100%, which may be related to underlying differences in mechanism, accounting for symptoms (inflammatory vs structural). However, there are several considerations whether the Rome criteria for DD applies to patients with IPAA. First, patients with IPAA tend to have stools corresponding with Bristol scores >4 and increased frequency at baseline.3 Even with a “dyssynergic pattern” on APM, patients may not fulfill clinical criteria for constipation. Second, the qualification of adequate propulsive force in patients with IPAA is not established. In patients without IPAA, adequate propulsive force during defecation is defined as an increase in intrarectal pressure ≥40 mmHg.17 However, a decrease in intrapouch pressure during defecation has been observed in patients with self-reported normal pouch function, suggesting adequate propulsive force in the pouch is different than that in the rectum.16 Third, an abnormal BET can occur even in patients with self-reported normal pouch function (and may not necessarily indicate DD), with more than one-third of patients in the recent paper by Quinn et al unable to expel the balloon in 60 seconds.16 The authors speculate this may be the result of a relatively fixed surgical anastomosis or inadequate intrapouch propulsive force.
The Rome IV criteria for FI is based on symptoms, recognizing that there are multiple etiologies for FI ranging from functional and structural causes. As noted in our results, FI in patients with IPAA was characterized by decreased mean and maximal resting anal pressure on APM, as well as pouch hyposensitivity. However, these parameters are not all-encompassing, may not necessarily delineate all etiologies of FI, and may be affected by anatomical changes post-IPAA. There may be differences in sensitivity testing (eg, volume for first sensation, urge, and discomfort), given that J-pouches are less distensible than normal rectums3,16 and may intrinsically be more sensitive given the previous pelvic inflammation.18 Furthermore, many patients with IPAA receive opioids or antidiarrheal agents that can variably affect squeeze pressures and sensitivity.19,20 Previously, studies have linked the absence of RAIR with the likelihood of developing incontinence21; however, the RAIR was absent in most patients with and without incontinence in our review. This may be an expected finding in patients with hand-sewn pouches where there is no residual rectal cuff or in patients with a very short rectal cuff.
A standardized consensus for manometry protocol and parameters should be developed for patients with IPAA. Recently, the International Anorectal Physiology Working Group (IAWPG) published the London Classification with standardized methods for ARM, rectal sensory testing, BET, and nomenclature describing alterations in anorectal motor and sensory function.22 Although very comprehensive, these classifications may not apply to patients with IPAA. The RAIR is commonly absent or is abnormal in patients post-IPAA with “normal” pouch function possibly because of the short residual rectal cuff. Thus, consideration should be made as to whether BET or RAIR should even be performed. Furthermore, the protocols corresponding to each testing indication need to be clearly delineated; for example in cases of FI, thorough assessment of cough reflex, squeeze amplitude and duration, and sensitivity testing should be carefully evaluated, in addition to presence of dyssynergia. Finally, normal APM parameters should be established and delineated by age and sex and potentially compared with a control cohort of patients with IPAA for the indication of familial adenomatous polyposis syndrome.
Consensus guidelines are also needed for the positioning of manometry, defecography, and BET in the assessment of defecatory disorders in patients with IPAA who have no evidence of inflammatory or structural disorders. Manometry parameters alone may not capture the full spectrum of the underlying pathophysiology contributing to defecatory disorders. Electromyography can be a useful adjunct to identifying dyssynergic defecatory patterns in symptomatic patients (and for delivering biofeedback23), but it may be less useful in settings where sensitivity testing is needed, such as patients who report fecal incontinence. The relative positioning of manometry to MRI or barium defecography will likely depend on clinical context and availability of each test at a specific institution. However, given that “abnormal” BET can be seen in patients with self-reported normal function, a confirmatory testing with defecography may need to be performed if a dyssynergic pattern is seen on APM. Several studies have also assessed the utility of defecography or scintigraphy to evaluate pouch emptying efficiency and have found that a slow or incomplete pattern of evacuation correlates with symptoms of straining, anal pain, incontinence, or pelvic floor descent.24,25 The role of defecography is less established for the indication of FI. For high-volume centers performing IPAAs, it is critical to have access to multiple modalities including dynamic imaging to evaluate patients with defecatory disorders. We propose an algorithm to evaluate patients with IPAA presenting with defecatory symptoms in Figure 2.
Figure 2.
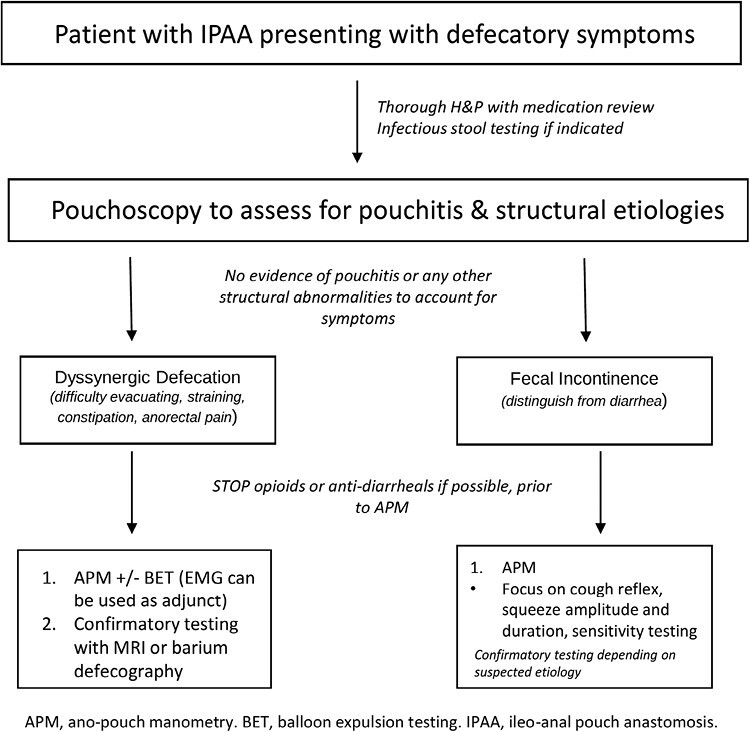
Proposed algorithm for patients with IPAA presenting with defecatory symptoms.
Our systematic review is limited by the small number of eligible studies and their heterogeneous methodology, which precluded pooling of results. We did not evaluate outcomes of biofeedback—the cornerstone of treatment of DD for patients without an IPAA—because of limited available data. The strength of our study is the comprehensive evaluation and critique of the data available for the use of APM or EMG in evaluating defecatory symptoms in patients with IPAA and the addition of our institutional experience. This review emphasizes that manometry alone is an imperfect assessment of pouch function in patients with defecatory symptoms and that confirmatory testing may need to be performed with dynamic imaging. Some APM parameters and BET may be “abnormal” even in patients with self-reported normal pouch function, and larger studies are needed to establish normal parameters. Validated metrics are also needed to assess defecatory symptoms in patients with IPAA for initial evaluation and therapeutic outcome assessments such as biofeedback.
Supplementary Material
Contributor Information
Yuying Luo, Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Natalia Schmidt, Department of Internal Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Marla C Dubinsky, Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Barry Jaffin, Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Maia Kayal, Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Author Contribution
Y.L., B.J., M.K.—conception/design of study. Y.L., N.S.—data collection. Y.L., B.J.—interpreting data. Y.L., N.S.—drafting manuscript. Y.L., B.J., M.C.D., M.K.—editing manuscript.
Funding
Funding was not provided for this project.
Conflicts of Interest
Y.L. has received consulting fees from Mahana Therapeutics. M.K. has served as a consultant for GoodRx and receives grant support from NIH-DK127241-01A1. M.C.D. has served as a consultant for Abbvie, Arena Pharmaceuticals, Boehringer Ingelheim International, Bristol-Myers Squibb, Celgene, Eli Lilly, F. Hoffman-La Roche, Genentech, Gilead, Janssen, Pfizer, Prometheus, Takeda, UCB, has research grants from Pfizer, Abbvie, Janssen, and Prometheus, has ownership interest in Trellus and licensing fees from Takeda. S.M. reports receiving research grants from Genentech and Takeda; receiving payment for lectures from Takeda, Genentech, Morphic; and receiving consulting fees from Takeda, Morphic, Ferring, and Arena Pharmaceuticals.
References
- 1. Hahnloser D, Pemberton JH, Wolff BG, Larson DR, Crownhart BS, Dozois RR. Results at up to 20 years after ileal pouch–anal anastomosis for chronic ulcerative colitis. Br J Surg. 2007;94(3):333–340. doi: 10.1002/bjs.5464 [DOI] [PubMed] [Google Scholar]
- 2. Parragi L, Fournier N, Zeitz J, et al. ; Swiss IBD Cohort Study Group. Colectomy rates in ulcerative colitis are low and decreasing: 10-year follow-up data from the Swiss IBD Cohort Study. J Crohns Colitis. 2018;12(7):811–818. doi: 10.1093/ecco-jcc/jjy040 [DOI] [PubMed] [Google Scholar]
- 3. Shen B, Kochhar GS, Kariv R, et al. Diagnosis and classification of ileal pouch disorders: consensus guidelines from the International Ileal Pouch Consortium. Lancet Gastroenterol Hepatol. 2021;6(10):826–849. doi: 10.1016/S2468-1253(21)00101-1 [DOI] [PubMed] [Google Scholar]
- 4. Rezaie A, Gu P, Kaplan GG, Pimentel M, Al-Darmaki AK. Dyssynergic defecation in inflammatory bowel disease: a systematic review and meta-analysis. Inflamm Bowel Dis. 2018;24(5):1065–1073. doi: 10.1093/ibd/izx095 [DOI] [PubMed] [Google Scholar]
- 5. Simren M, Palsson OS, Whitehead WE. Update on Rome IV Criteria for colorectal disorders: implications for clinical practice. Curr Gastroenterol Rep. 2017;19(4):15. doi: 10.1007/s11894-017-0554-0. PMID: 28374308CIDPMC5378729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chang S, Shen B, Remzi F. When not to pouch: important considerations for patient selection for ileal pouch-anal anastomosis. Gastroenterol Hepatol (N Y). 2017;13(8):466–475. PMID: 28867978CIDPMC5572960. [PMC free article] [PubMed] [Google Scholar]
- 7. Schmidt N, Luo Y, Jaffin B, Kayal M. Anorectal manometry protocols and biofeedback outcomes vary for patients with ileal pouch-anal anastomosis. American College of Gastroenterology Annual Conference; October 2022, Charlotte.
- 8. National Institute of Health. Quality Assessment Tool for Case Series Studies. Accessed September 15, 2022. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- 9. Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of exposures. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hull TL, Fazio VW, Schroeder T. Paradoxical puborectalis contraction in patients after pelvic pouch construction. Dis Colon Rectum. 1995;38(11):1144–1146. doi: 10.1007/BF02048329 [DOI] [PubMed] [Google Scholar]
- 11. Khanna R, Li Y, Schroeder T, et al. Manometric evaluation of evacuatory difficulty (dyschezia) in ileal pouch patients. Inflamm Bowel Dis. 2013;19(3):569–575. doi: 10.1097/MIB.0b013e31827e78d6 [DOI] [PubMed] [Google Scholar]
- 12. Quinn KP, Tse CS, Lightner AL, Pendegraft RS, Enders FT, Raffals LE. Nonrelaxing pelvic floor dysfunction is an underestimated complication of ileal pouch-anal anastomosis. Clin Gastroenterol Hepatol. 2017;15(8):1242–1247. doi: 10.1016/j.cgh.2017.02.024 [DOI] [PubMed] [Google Scholar]
- 13. Braun J, Treutner KH, Harder M, Lerch MM, Töns C, Schumpelick V. Anal sphincter function after intersphincteric resection and stapled ileal pouch-anal anastomosis. Dis Colon Rectum. 1991;34(1):8–16. doi: 10.1007/BF02050200 [DOI] [PubMed] [Google Scholar]
- 14. Le Blanc I, Mihot F, Duparc F, et al. Anorectal manometry and ileo-anal anastomosis: pre- and postoperative manometric comparison. Ann Chir. 1994;48(2):183–187. [PubMed] [Google Scholar]
- 15. Sarmiento JM, Pemberton JH, Reilly WT. Physiologic determinants of nocturnal incontinence after ileal pouch-anal anastomosis. J Gastrointest Surg. 1997;1(4):324–330. doi: 10.1016/s1091-255x(97)80052-2 [DOI] [PubMed] [Google Scholar]
- 16. Quinn KP, Busciglio IA, Burton DD, et al. Defining normal pouch function in patients with ileal pouch-anal anastomosis: a pilot study. Aliment Pharmacol Ther. 2022;55(12):1560–1568. doi: 10.1111/apt.16859 [DOI] [PubMed] [Google Scholar]
- 17. Rao SSC, Patcharatrakul T. Diagnosis and treatment of dyssynergic defecation. J Neurogastroenterol Motil. 2016;22(3):423–435. doi: 10.5056/jnm16060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shen B, Sanmiguel C, Bennett AE, et al. Irritable pouch syndrome is characterized by visceral hypersensitivity. Inflamm Bowel Dis. 2011;17(4):994–1002. doi: 10.1002/ibd.21412 [DOI] [PubMed] [Google Scholar]
- 19. Hallgren T, Fasth S, Delbro DS, Nordgren S, Oresland T, Hultén L. Loperamide improves anal sphincter function and continence after restorative proctocolectomy. Dig Dis Sci. 1994;39(12):2612–2618. doi: 10.1007/BF02087698 [DOI] [PubMed] [Google Scholar]
- 20. Lodhia NA, Horton L, Thapa N, Goldin AH, Chan WW. Opioid-associated anorectal dysfunction in chronic constipation. Dig Dis Sci. 2022;67(8):3904–3910. doi: 10.1007/s10620-021-07288-5 [DOI] [PubMed] [Google Scholar]
- 21. Saigusa N, Belin BM, Choi HJ, et al. Recovery of the rectoanal inhibitory reflex after restorative proctocolectomy: does it correlate with nocturnal continence? Dis Colon Rectum. 2003;46(2):168–172. doi: 10.1007/s10350-004-6519-z [DOI] [PubMed] [Google Scholar]
- 22. Carrington EV, Heinrich H, Knowles CH, et al. ; All members of the International Anorectal Physiology Working Group. The international anorectal physiology working group (IAPWG) recommendations: standardized testing protocol and the London classification for disorders of anorectal function. Neurogastroenterol Motil. 2020;32(1):e13679. doi: 10.1111/nmo.13679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Skardoon GR, Khera AJ, Emmanuel AV, Burgell RE. Review article: dyssynergic defaecation and biofeedback therapy in the pathophysiology and management of functional constipation. Aliment Pharmacol Ther. 2017;46(4):410–423. [DOI] [PubMed] [Google Scholar]
- 24. Selvaggi F, Cuocolo A, Giuliani A, et al. The role of scintigraphic defecography in the assessment of bowel function after restorative proctocolectomy for ulcerative colitis. Int J Colorectal Dis. 2006;21(5):448–452. doi: 10.1007/s00384-005-0036-y [DOI] [PubMed] [Google Scholar]
- 25. Stellingwerf ME, Maeda Y, Patel U, et al. The role of the defaecating pouchogram in the assessment of evacuation difficulty after restorative proctocolectomy and pouch-anal anastomosis. Colorectal Dis. 2016;18(8):O292–O300. doi: 10.1111/codi.13431 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


