Abstract
Papillary thyroid carcinoma (PTC) is the most common endocrine malignancy, and its incidence has increased rapidly in recent years. The BRAF inhibitor vemurafenib is effective against BRAFV600E-positive PTC; however, acquired resistance to single agent therapy frequently leads to tumor recurrence and metastasis, underscoring the need to develop tailored treatment strategies. We previously showed that the oncogenic kinase PIM1 was associated with the malignant phenotype and prognosis of PTC. In this study, we showed that sustained expression of the PIM1 protein in PTC was affected by the BRAFV600E mutation. Based on this regulatory mechanism, we tested the synergistic effects of inhibitors of BRAF (BRAFi) and PIM1 in BRAFV600E-positive PTC cell lines and xenograft tumors. LC-MS metabolomics analyses suggested that BRAFi/PIMi therapy acted by restricting the amounts of critical amino acids and nucleotides required by cancer cells as well as modulating DNA methylation. This study elucidates the role of BRAFV600E in the regulation of PIM1 in PTC and demonstrates the synergistic effect of a novel combination, BRAFi/PIMi, for the treatment of PTC. This discovery, along with the pathways that may be involved in the powerful efficacy of BRAFi/PIMi strategy from the perspective of cell metabolism, provides insight into the molecular basis of PTC progression and offers new perspectives for BRAF-resistant PTC treatment.
Keywords: Thyroid carcinoma, BRAFV600E, PIM1, Synergism, Metabolomics
Introduction
The constitutively active serine/threonine kinase PIM1 is upregulated in many tumor types [1]. It is implicated in multiple oncogenic signaling pathways, and contributes to cell cycle progression, cell growth, cell survival, and resistance to therapy. PIM1 is thus considered as a potent oncogenic driver [2], [3], [4], [5]. Previous work from our group demonstrated a significant correlation between PIM1 expression levels and the malignant phenotype and prognosis of papillary thyroid carcinoma (PTC) [6]. However, the precise mechanisms underlying the role of PIM1 in carcinogenesis remain unknown, and the presence of the BRAFV600E mutation may be relevant in this context.
The BRAFV600E mutation, which occurs in approximately 60 % of PTC cases [7], is a key driver of thyroid tumorigenesis through its involvement in the activation of the mitogen-activated protein kinase (MAPK)/extracellular signal–regulated kinase (ERK) pathway [8,9]. This mutation is strongly associated with invasive characteristics, including metastasis, recurrence, and resistance to radioactive iodine therapy, making it an important target for PTC treatment [10], [11], [12]. Despite the introduction of selective BRAF inhibitors (BRAFi) such as vemurafenib, PTC patients often develop resistance to therapeutic drugs [13,14]. To overcome this resistance, it is necessary to identify alternative approaches [15], [16], [17].
In this study, we showed that PIM1 expression depended on the mutant status of the BRAF gene, thereby elucidating the mechanism by which PIM1 exerts its oncogenic effect. Increasing evidence supports that PIM1, which serves as a negative prognostic factor in various tumors, plays a crucial role in conferring tumor cells resistance to targeted therapy [2,18,19]. Our previous findings on the involvement of PIM1 in the aggressiveness and unfavorable prognosis of PTC, together with the present findings showing a strong correlation between PIM1 and the BRAFV600E mutation, led us to propose and validate the following hypothesis: dual blocking of PIM1 and the BRAFV600E mutation can improve the efficacy of PTC treatment.
Cancer is characterized by metabolic changes, and tumor cells frequently modify their metabolic patterns to accommodate a faster growth rate [20]. Tumor metabolic reprogramming is involved in decreasing the sensitivity to therapeutic drugs and in increasing the rate of metastasis [21,22]. Therefore, clarifying the metabolic regulatory pathways activated in response to targeted therapy will disclose the metabolic requirements for metastasis and growth, thereby contributing to our understanding of the mechanisms underlying the resistance to vemurafenib therapy.
Based on the involvement of the BRAFV600E mutation in regulating PTC protein levels, the aim of this study was to determine whether the combination of PIM1 inhibitor and BRAFi was effective in PTC treatment, and to explain the antitumor effects of combination therapy from the perspective of tumor cell metabolism.
Materials & methods
Cell culture and drug treatment
The human thyroid cancer cell lines TPC-1, BCPAP, CAL-62, 8505C and KTC-1 were incubated in RPMI-1640 (Gibco, USA) containing 10 % fetal bovine serum (FBS) (Gibco, USA). Cells were maintained at 37°C in 5 % CO2. For in vitro and in vivo experiments, cells were treated with the BRAF kinase inhibitor vemurafenib (TagerMol, USA) and the PIM1 inhibitor SGI-1776 (TagerMol, USA) individually or in combination at the indicated concentrations and times.
Clinical tissues, immunohistochemistry (IHC), and hematoxylin-eosin (HE) staining
Clinical tumor tissues and matched adjacent nontumor tissues were collected from patients with primary PTC. The cancer tissues were sequenced to determine the BRAF genotype and tissue microarrays were constructed. Fifty-seven tissue samples were selected and embedded in paraffin. The paraffin-embedded tissue sections were deparaffinized with xylene and rehydrated with graded ethanol following the standard procedure. Samples were stained with HE, and sections were incubated with 3 % hydrogen peroxide before antigen retrieval in citrate buffer at 100f, followed by incubation with PIM1 antibodies (1:200; Huabio, China) at 4°C overnight. Nuclei were counterstained with hematoxylin, and the slides were dehydrated and mounted for further analysis. Slides were visualized using the Olympus Image Viewer (Olympus, Japan), and ImageJ software was used to analyze the mean optical density (IOD/area) of each tissue[23].
Cell transfection
PTC cells were transfected at 60 % confluency using the Lipofectamine 3000 Transfection Reagent (Invitrogen, USA) following the manufacturer's instructions. Plasmids used for transfections were pEnCMV-BRAF-3xFLAG and PEnCMV-BRAFV600E-3xFLAG, which were constructed by GENE Company (Hong Kong). Transfection efficiency was evaluated by western blotting.
Western blotting
Total protein was extracted from cells using RIPA lysis buffer containing protease inhibitors (Beyotime, China), and quantified using the BCA protein assay (Beyotime, China). Cell lysates were separated by 12 % SDS-PAGE, transferred to PVDF membranes, blocked with 5 % skim milk at room temperature for 2 h, and then incubated with primary antibody at 4°C overnight. The membranes were washed and incubated with HRP-conjugated secondary antibody (Huabio, China), and the protein bands were visualized by ECL Plus (Amersham Pharmacia Biotech, UK). The primary antibodies used were as follows: anti-PIM1 (1:1,000; Huabio, China), anti-β-actin (1:4000; Proteintech, China), anti-Flag (1:1,000; Sigma-Aldrich, USA).
Cell proliferation assay
BCPAP (4 × 103) and KTC-1 (3 × 103) cells were seeded in 96-well plates at a density of 100 μL/well. After 24 h, the original medium was replaced with medium containing vemurafenib and SGI-1776 individually or in combination at the indicated concentrations and times. Then, cells were incubated with culture medium containing 10 % CCK8 (BestBio, China) and the absorbance was measured at 450 nm. Each experiment was repeated at least three times. Cell survival rate was calculated as (treatment-blank)/(control-blank). The IC50 values and coefficient of drug in interaction (CI) (CI, CI <1, indicates a synergistic effect) were calculated to determine the antiproliferative effect of SGI-1776 and vemurafenib as single agents or in combination [24].
Colony formation assay
Cells were seeded in 6-well (BCPAP) or 12-well (KTC-1) plates at a density of 1 × 103 cells/well. After 24 h, cells were treated with different drugs and cultured in an incubator for 7–14 days. Finally, cells were stained with 0.5 % Crystal Violet (Dawen Biotec, China). Colonies were counted using ImageJ software and the experiment was performed in triplicate.
Xenograft models
Male BALB/c nu-mice (4–6-weeks-old) were maintained in the animal husbandry facility of the SPF laboratory. All experiments were approved by the Experimental Animal Ethics Committee of Zhejiang Cancer Hospital. BCPAP cell suspensions (8 × 106 cells/150 μL) were injected in the right subcutaneous armpit of each mouse. After 1 month, tumors were inoculated into the second generation. When tumor volumes reached 30–50 mm3, the mice were randomly divided into four groups. Each group of mice (n = 5/group) was intraperitoneally injected with PBS, SGI-1776 (25 mg/kg), vemurafenib (20 mg/kg), or a combination of both drugs every other day. Tumor growth was monitored every 2 days by caliper, and tumor volume was calculated as V = 1/2 (width2 × length). Nude mice were executed and tumors were dissected 18 days post treatment. Tumors were weighed and recorded, and then flash frozen in liquid nitrogen for metabolomics studies.
Extraction of metabolites and LC-MS analysis
An appropriate amount of sample was weighed and transferred to a 2 mL EP tube, and treated with tissue extraction solution (1 mL) containing 75 % methanol: chloroform in a 9:1 ratio and 25 % H2O. After samples were ground and sonicated, they were incubated on ice for 30 min and centrifuged at 12,000 rpm at 4°C for 10 min. The supernatants were collected, concentrated and dried. Samples were redissolved in 2-chloro-L-phenylalanine solution prepared with 200 μL of 50 % acetonitrile solution. The samples were stored at 4°C for liquid chromatography–mass spectrometry (LC-MS) analysis. LC analysis was performed using the Thermo Vanquish LC system (Thermo Fisher Scientific, USA) with an ACQUITY UPLC® HSS T3 column (2.1 × 150 mm, 8 µm; Acquity, USA). The mobile phase was 0.1 % formic acid acetonitrile (C) and 0.1 % formic acid water (D) in the positive ion mode, and acetonitrile (A) and 5 mM ammonium formate water (B) in the negative ion mode. The following gradient elution conditions were used: 0–1 min, 2 % (buffers A/C) A/C; 1–9 min, 2 %–50 % A/C; 9–12 min, 50 %–98 % A/C; 12–13.5 min, 98 % A/C; 13.5–14 min, 98 %–2 % A/C; and 14–20 min, 2 % A/C.
Metabolomics data processing
Raw data were formatted (to. mzXML) with the ProteoWizard package (v3.0.8789)1, and processed with the XCMS R package2 to obtain a quantitative list of substances, including peak picking, peak alignment, and peak alignment processing. Orthogonal partial least squares discriminant analysis (OPLS-DA) and dimensionality reduction on the sample data were performed to visualize the metabolic shift among groups using the Ropls R package9. Multiple parameters including P values, variable influence on projection (VIP) obtained from dimensionality reduction, and fold change between groups were used to evaluate the influence and interpretation of each metabolite component in classifying and distinguishing samples, thus assisting in the identification of marker metabolites. Finally, features with VIP >1.0 and P <0.05 were defined as differential metabolites. Metabolic pathway analysis was performed using MetaboAnalyst10 software, and results were visualized using KEGG Mapper.
Statistical analysis
GraphPad Prism version 9.0 was used for statistical analyses. For all experiments, Student's t-tests and one-way analysis of variance (ANOVA) test were used to compare mean values between test and control groups. N represents repeats of the experiment. P < 0.05 was considered significant.
Results
The expression of PIM1 in PTC is closely associated with the BRAF mutation
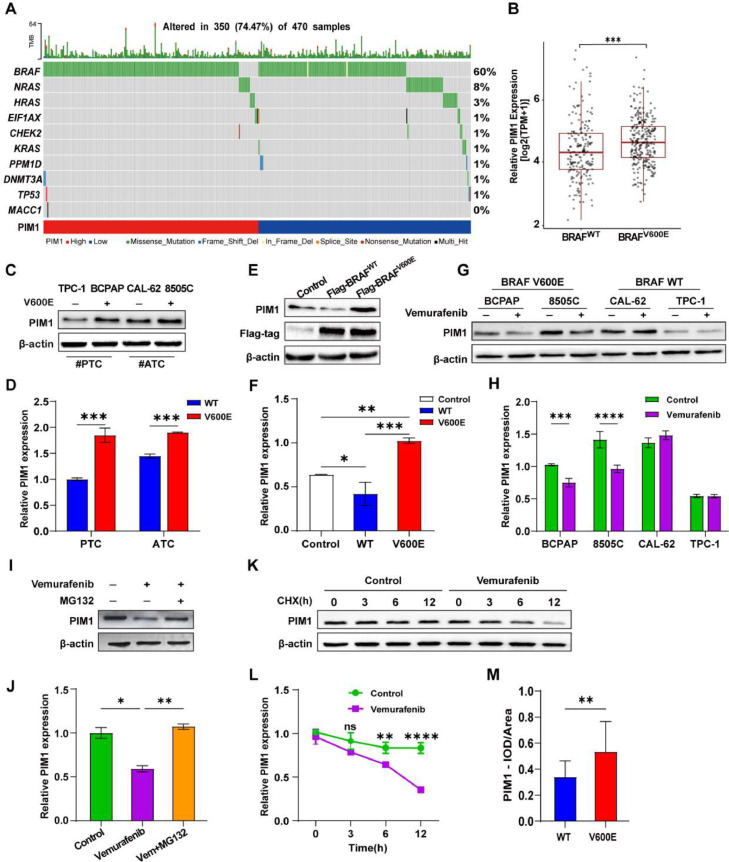
Differential analysis of PIM1 expression in 470 PTC clinical samples from The Cancer Genome Atlas (TCGA) showed that the presence of BRAF mutation was associated with high PIM1 expression, and PIM1 expression was higher in PTC patients harboring the BRAFV600E mutation (n=277) than in those with BRAF WT (n=189) (Fig. 1A and B).
Fig. 1.
The BRAFV600E oncoprotein regulates PIM1 expression and increases its stability in thyroid cancer (TC). A Mutations in the top 10 genes in PTC patients with high and low PIM1 expression. B Differential analysis of PIM1 expression in 470 thyroid papillary carcinoma tissues with BRAF WT and BRAFV600E mutation. TPM: reads per million mapped reads. C, D Expression of PIM1 in thyroid papillary carcinoma (PTC) and anaplastic cancer (ATC) with or without the BRAFV600E mutation. E, F Immunoblot of PIM1 protein from extracts of TPC-1 transfected with BRAFV600E or BRAF WT plasmid. G, H Expression level of PIM1 in TCs treated with 10 μM vemurafenib or DMSO for 48 h. I, J BRAFV600E PTC cells were treated with vemurafenib, MG-132, or a combination for 6 h. Lysates were harvested for western blotting. K, L Half-life analysis of PIM1 in PTC treated with vemurafenib. PTC cells carrying the BRAFV600E mutation in the control or vemurafenib group were treated with 100 μg/mL cycloheximide (CHX) and collected at the indicated times, followed by western blot analysis of PIM1 protein expression. Experiments were performed three times. (mean ± SD, N = 3) (*P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001, Student's t-test). M Quantitative data of the BRAF WT or V600E mutant PTC tissues and the corresponding analyses, which were performed by measuring the optical density (IOD) and all area with Image J and calculation of the average optical density values (IOD/area).
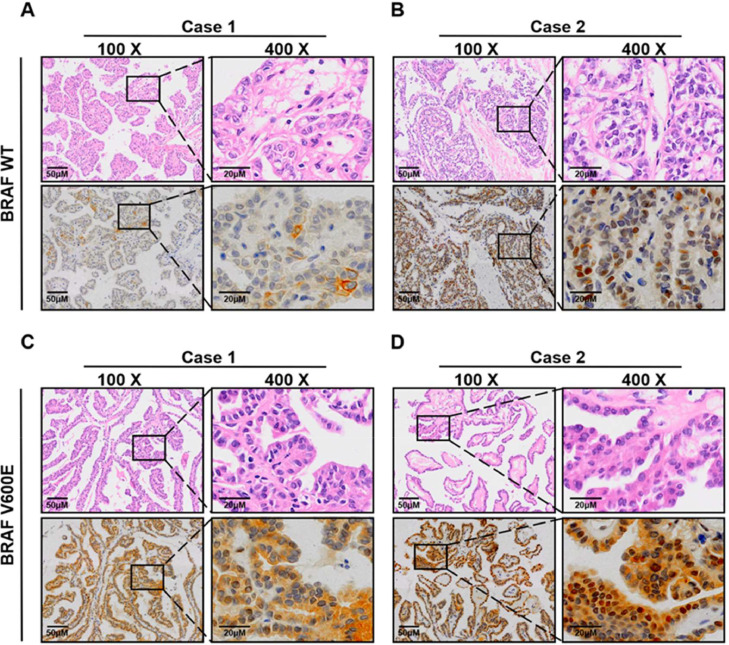
Analysis of PIM1 expression in TC cell lines showed that PIM1 expression levels were higher in BCPAP and 8505C cells (carrying the BRAFV600E mutation) than in TPC-1 and CAL-62 cells (carrying BRAF WT) (Fig. 1C and D). IHC analysis of PIM1 expression in BRAFV600E mutant (n=42) and BRAF WT (n=14) PTC samples demonstrated that PTC patients with the BRAFV600E mutation had higher levels of PIM1 than BRAF WT patients (Fig. 2A-D; Supplementary Table 1).
Fig. 2.
Correlation between BRAF mutation and PIM1 level in PTC patients. D Representative images (100 ×, 400 ×) of HE and IHC staining of PIM1 protein level from PTC patients harboring BRAF WT (A and B) and BRAFV600E mutation (C and D).
The BRAFV600E mutant regulates the expression of PIM1 in PTC
The results suggesting a link between PIM1 and BRAF mutations led us to hypothesize that the BRAFV600E oncoprotein might upregulate PIM1 expression in PTC. To validate this hypothesis, we constructed BRAF WT-Flag and BRAFV600E-Flag overexpression plasmids using pcDNA3.1 as a vector for cell transfection. PIM1 expression was significantly higher in cells transfected with the V600E plasmid than in BRAF WT cells (Fig. 1E and F). Treatment with vemurafenib (BRAFV600E inhibitor) downregulated PIM1 only in BRAFV600E-transfected cells (Fig. 1G and H). We further detected the stability of the PIM1 protein in PTC cells carrying BRAFV600E. In the presence of MG132, PIM1 protein levels were not affected by BRAFi, indicating that BRAFV600E may prevent PIM1 degradation via the proteasome pathway (Fig. 1I and J). To confirm the effect of BRAFV600E on the stability of PIM1, BCPAP cells were treated with cycloheximide (CHX) for 0, 3, 6, and 12 h. PIM1 protein levels remained high and stable in the presence of BRAFV600E, whereas treatment with vemurafenib markedly decreased PIM1 protein levels and accelerated its degradation (Fig. 1K and L).
Vemurafenib combined with PIM1 inhibitors enhanced the inhibitory effect on PTC in vitro and in vivo
The efficacy of the combination of BRAF and PIM1 inhibitors for the treatment of PTC harboring BRAFV600E was investigated in this study, considering the role of BRAFV600E in regulating PIM1. First, we performed dose-response studies of vemurafenib and SGI-1776 as single agents, which showed that both inhibitors decreased the proliferation of PTC cells containing BRAFV600E in a dose- and time-dependent manner(Figure S1A, B, E and F). The IC50 values for vemurafenib and SGI-1776 after 48 h of treatment were 3.68 μM and 3.81 μM in BCPAP cells(Figure S1C, and D), and 8.30 μM and 5.88 μM in KTC-1 cells(Figure S1G and H), respectively.
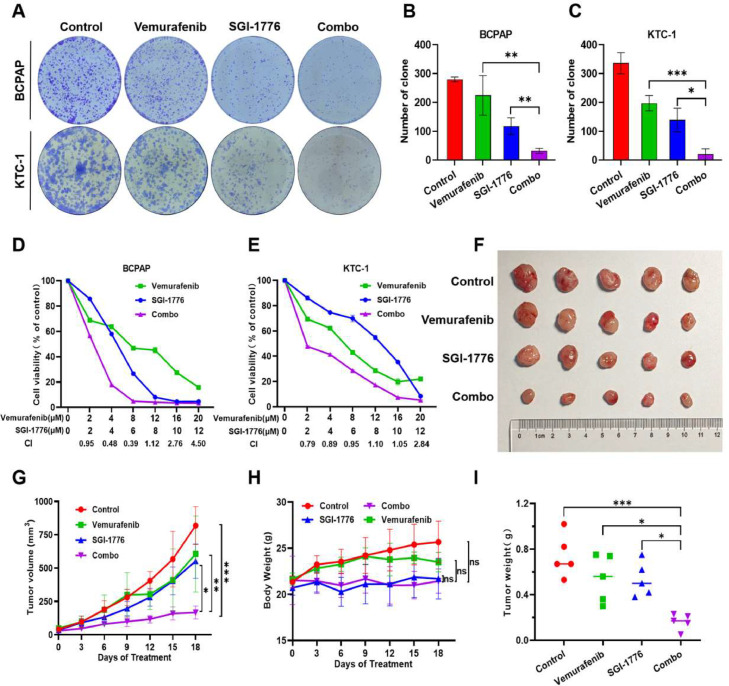
The effect of combination treatment was evaluated by calculating the CI. The data showed that the combination of drugs increased the inhibitory effect on KTC-1 cells (CI < 1), with a more substantial synergistic effect on BCPAP cells (CI < 0.5) (Fig. 3D and E). In addition, combination treatment markedly inhibited the colony forming ability of PTC cells (Fig. 3A and C). Furthermore, we opted for a novel PIM1 kinase inhibitor combined with Vemurafenib to treat PTC cells, and our outcomes further substantiate that the concomitant application of BRAFi and PIM1 inhibitor (AZD1208) imparts a synergistic suppression of proliferation and clonogenic capacity in BRAF-mutated PTC cells, as depicted in Fig S2A-E. Moreover, distinct PIM1 shRNAs were employed to knock down PIM1 in KTC-1 cells (Fig S3A,B). The ensuing proliferation assays elucidated that PIM1 KD synergizes with BRAFi in PTC (Fig S3C-F).
Fig. 3.
Synergistic effects of SGI-1776 and vemurafenib in BRAFV600E mutant PTC cells and a xenograft model. A-C BCPAP and KTC-1 cells were treated with different concentrations of vemurafenib and SGI-1776 alone or in combination for the indicated time points. The resulting cells were fixed and stained, and cell colony numbers were counted. (mean ± SD, N = 3) (*P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001, Student's t-test). D, E Combination effect curves and coefficient of drug in interaction (CI) of BCPAP and KTC-1 cells treated with vemurafenib and SGI-1776 alone or in combination for 48 h. F-I Subcutaneous xenografts generated by injecting BCPAP cells were treated with SGI-1776 (25 mg/kg), vemurafenib (20 mg/kg), or both drugs for 18 days. Tumor size and weight were monitored (mean ± SD, N = 5 mice per group) (*P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001, ANOVA test) (F, G and I). Change in body weight of nude mice in each group. (mean ± SD, N = 5 mice per group)(P > 0.05, ANOVA test) (H).
In vivo experiments showed a considerable reduction in tumor volume and weight in the combination treatment group compared with the single therapy groups (Fig. 3F, G, and I), demonstrating a significant inhibition of tumor growth. No significant variation in body weight was observed in the different treatment groups, indicating that toxicity did not increase (Fig. 3H).
Metabolite differences and identification in the combination and single treatment groups
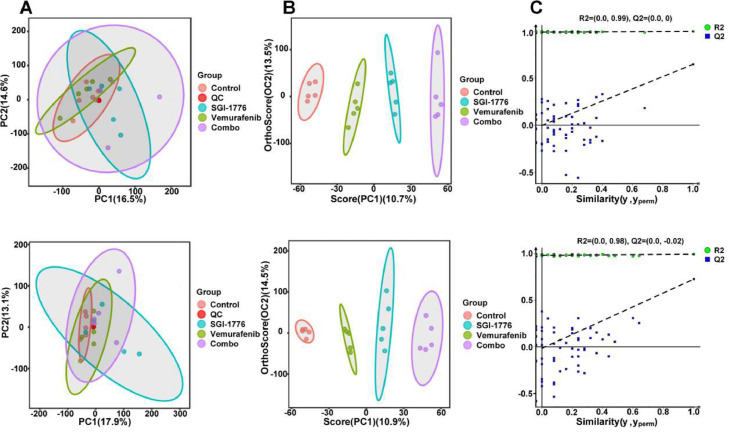
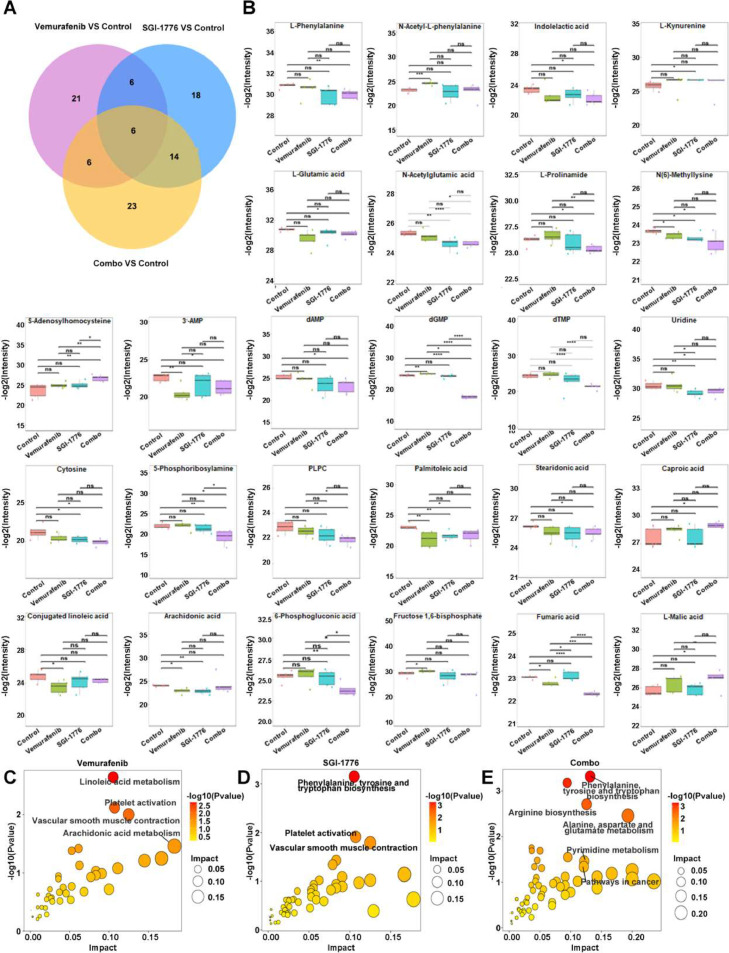
To clarify the mechanism underlying the effect of combination therapy on reducing PTC carcinogenesis, endogenous metabolites in mouse tumor tissues from each group were examined. The principal component analysis plot demonstrated clustering of Quality Control (QC) samples, suggesting the stability and reproducibility of the analytical system. Comparison of each treatment group with the control group showed distinct clustering in both positive and negative ion modes, which were evaluated using OPLS-DA to identify sample differences (Fig. 4A and B). Permutation tests also showed satisfactory explanatory and predictive ability (the regression line at a blue Q2 point crosses from the abscissa) (Fig. 4C). After treatment with vemurafenib, SGI-1776, and their combination, 39, 44, and 49 differential metabolites were detected, respectively, using the criteria of fold change >1.5, VIP >1.0, and P <0.05 (Fig. 5A). Table 1 lists the relative changes in major metabolites related to amino acids, nucleotides, fatty acids, and glucose metabolism after single and combination therapies.
Fig. 4.
Metabolomics analysis shows differences among control, vemurafenib, SGI-1776, and combination groups. A Score plots of QA-PCA models. Each point represents a sample; pink: control, red: QC, green: vemurafenib, blue: SGI-1776, purple: vemurafenib and SGI-1776. B Score plots of OPLS-DA models. Each point represents a sample, red: control, green: vemurafenib, blue: SGI-1776, purple: combination. C Permutation test results.
Fig. 5.
Metabolite changes as a result of treatment with vemurafenib, SGI-1776 and combination. A Venn diagrams comparing the drug responsive metabolites identified in PTC in the indicated comparisons. B Differential expression of the metabolites in Table 1 between the vemurafenib, SGI-1776, and combination groups. *P < 0.5⁎⁎P < 0.01⁎⁎⁎P < 0.001⁎⁎⁎⁎P < 0.0001 C-E Visualization of results from Joint pathway enrichment analyses (P < 0.05, impact > 0.1), using MetaboAnalyst, of the metabolites that were significantly altered in PTC cells treated with (C) vemurafenib, (D) SGI-1776, and (E) combination. The x-axis represents the pathway impact score, which is the relative degree to which the overall pathway is expected to be altered given the relative (positional) importance of altered metabolites in the pathway. The y-axis represents the -log10-transformed P values for pathway enrichment. Nodes are colored according to P value and sized according to pathway impact score.
Table 1.
Major metabolites significantly dysregulated after single and combination therapies in PTC xenografts.
| Vemurafenib |
SGI-1776 |
Combination |
|||||
|---|---|---|---|---|---|---|---|
| Metabolites name | Fold Change | P value | Fold Change | P value | Fold Change | P value | Trend |
| Amino acids | |||||||
| L-Phenylalanine | 0.58 | 0.005 | −− | ||||
| N-Acetyl-L-phenylalanine | 2.79 | <0.001 | |||||
| Indolelactic acid | 0.46 | 0.016 | − | ||||
| L-Kynurenine | 1.71 | 0.034 | |||||
| L-Glutamic acid | 0.66 | 0.018 | − | ||||
| N-Acetylglutamic acid | 0.62 | 0.005 | 0.60 | <0.001 | −−− | ||
| L-Prolinamide | 0.55 | 0.014 | − | ||||
| N(6)-Methyllysine | 0.67 | 0.039 | − | ||||
| N-Acetylmethionine | 1.61 | 0.024 | + | ||||
| S-Adenosylhomocysteine | 6.33 | 0.005 | ++ | ||||
| Nucleotide Metabolism | |||||||
| 3′-AMP | 0.29 | 0.003 | 0.44 | 0.018 | − | ||
| dAMP | 0.36 | 0.029 | − | ||||
| dGMP | 0.01 | <0.001 | −−− | ||||
| dTMP | 0.13 | <0.001 | −−− | ||||
| Uridine | 0.40 | 0.008 | 0.48 | 0.030 | − | ||
| Cytosine | 0.45 | 0.046 | 0.36 | 0.015 | − | ||
| 5-Phosphoribosylamine | 0.21 | 0.009 | −− | ||||
| Inosine | 1.68 | 0.013 | |||||
| Xanthosine | 0.63 | 0.011 | |||||
| Fatty Acids | |||||||
| PLPC | 0.5 | 0.006 | −− | ||||
| Palmitoleic acid | 0.29 | 0.007 | 0.39 | 0.004 | 0.40 | 0.019 | − |
| Stearidonic acid | 0.63 | 0.034 | − | ||||
| Caproic acid | 2.17 | 0.039 | + | ||||
| Conjugated linoleic acid | 0.41 | 0.022 | |||||
| Arachidonic acid | 0.49 | 0.010 | 0.46 | 0.001 | |||
| TCA cycle | |||||||
| 6-Phosphogluconic acid | 0.36 | 0.009 | −− | ||||
| Fructose 16-bisphosphate | 2.04 | 0.042 | |||||
| Fumaric acid | 0.60 | <0.001 | −−− | ||||
| L-Malic acid | 2.53 | 0.046 | + | ||||
Values represent fold change (dosed/control). Unpaired two-tailed t-test after setting the p value to 0.05 for statistical significance. Trend is indicated for the combined treatment as follows: + (fold >1.5, P < 0.05), ++ (fold >1.5, P < 0.01), − (fold < 0.67, P < 0.05), −− (fold < 0.67, P < 0.01), and −−− (fold < 0.67, P < 0.001). Blank: not significant.
The present data suggested that combination therapy caused a marked decrease in the levels of various amino acids and nucleotides, whereas single agent treatment affected only a few lipids and amino acids or their derivatives. Combination treatment significantly decreased the levels of many amino acids, including phenylalanine, glutamic acid, prolinamide, and methyllysine, and almost all nucleotides, such as dAMP, dGMP, and dTMP, as well as uridine and cytosine. S-homocysteine levels increased >5-fold after combination therapy compared with controls (Fig. 5B). Pathway enrichment of these metabolites using the KEGG database revealed that vemurafenib or SGI-1776 alone affected linoleic acid metabolism and the phenylalanine, tyrosine, and tryptophan pathway, whereas combination treatment primarily affected various amino acid and purine metabolic pathways, including arginine biosynthesis, alanine, aspartate, and glutamate metabolism; phenylalanine, tyrosine, and tryptophan biosynthesis; and purine metabolism (Fig. 5C-D). We accordingly assessed the expression levels of the critical metabolic enzyme ASS1 in the arginine biosynthesis pathway. TPC (BRAF WT) and KTC-1 (BRAF V600E) cells were administered different single drugs, or their combination for a duration of 48 hours. Subsequent Western Blotting analysis showed that the combinatory treatment exerted a more pronounced effect on BRAF-mutant PTC cells than monotherapy, as presented in Fig S2F, an effect not paralleled in BRAF WT cells.
Discussion
PIM1 kinases have been shown to be involved in tumorigenesis, to enhance tumor growth and to induce chemo-resistance, which is why they have become an attractive therapeutic target for cancer therapy [3,18]. Despite promoting tumorigenesis and protecting cells from apoptotic signals, PIM1 has also been identified as a metabolic modulator that affects the tumor microenvironment [25]. In previous work, we proved that PIM-1 plays a carcinogenic role in PTC; however, the detailed mechanism is not clear; therefore, we carried out more in-dep th research.
The publicly accessible TCGA database was used to analyze data from 470 patients, which showed that high PIM1 expression was associated with a higher frequency of BRAFV600E mutation. Subsequent experiments confirmed that BRAFV600E stably maintained PIM1 expression, whereas BRAF inhibitors suppressed PIM1 expression, potentially by increasing the half-life of the PIM1 protein. Consistent with previous research, the present results indicated that PIM1 could potentially act as a facilitator in the malignant progression of PTC caused by BRAF mutations [26,27]. BRAFV600E is present in the majority of PTC cases, and inhibition of BRAF mutations can delay the malignant progression of thyroid carcinoma [28]. Therefore, targeting BRAFV600E has emerged as an effective therapy for suppressing the progression of PTC. PIM kinases are linked to carcinogenesis and tumor progression. BRAFi treatment upregulates the expression of anti-apoptotic B-cell lymphoma 2 (BCL-2) family members, thereby preventing cell apoptosis in PTC with the BRAFV600E mutation. Combination treatment with vemurafenib and the BCL-2 inhibitor navitoclax increases apoptosis and suppresses growth [29]. Therefore, combination therapy appeared to be the optimal strategy to decrease BRAF inhibitor resistance. Given that BRAFV600E stabilized PIM1 protein expression, we believe that the BRAFi/PIMi combination strategy would be effective in PTC.
The present results suggested that although monotherapies could limit the proliferation of cancer cells in vitro (Supplementary Figure 1), none of them could completely inhibit the progression of PTC tumors in vivo. In contrast, the combination of vemurafenib and SGI-1776 demonstrated considerable synergistic antitumor effects both in vivo and in vitro. This synergistic effect was not promising when high doses (>10 µM) of single agents were combined to inhibit the proliferation of PTC cells, which may be due to the fact that most tumor cells die when treated with high concentrations of single agents. In addition, considering that SGI-1776 had dose limiting cardio toxicity in previous clinical studys, we incorporated a new PIM1 kinase inhibitor, AZD1208, into our research. Data from two escalating-dose clinical trials indicate broad tolerance of AZD1208 among patients with Acute Myeloid Leukemia (AML) and those with advanced-stage solid malignancies. [30] The outcomes further substantiate that the concomitant application of BRAF and PIM1 inhibitor imparts a synergistic suppression in BRAF-mutated PTC cells. Crucially, to validate the specificity of the biological effects to the PIM1 target, we treated PTC cells with PIM1 shRNA plus Vemurafenib, and the findings were aligns with the synergistic outcomes observed in our combinatory drug application experiment. In conclusion, targeting PIM1 and the BRAFV600E mutation could be a promising therapeutic approach for patients with refractory PTC.
We used LC-MS analysis to explore the characteristic metabolic alterations and to elucidate the potential therapeutic mechanisms involved in the effect of treatment with the two drugs alone or in combination in PTC. Within the criteria used, a wide range of essential amino acids such as L-Phe, pro, Glu, Lys, Met, or their derivatives, as well as nearly all forms of raw materials for nucleotide synthesis were downregulated in the combination treatment group. In general, pathways that promote protein and nucleotide synthesis are indicators of cell proliferation [31]. The present results suggested that the antitumor effects of combination treatment were markedly increased by targeting amino acid and nucleotide metabolism compared with the effects of single agents. Pathway analysis showed that phenylalanine, tyrosine, and tryptophan pathways were enriched in both SGI-1776 and combination groups. Dietary restriction of Phe and Tyr has anticancer activity in several tumor models in vivo, which is consistent with the synergistic effects observed in this study [32]. Furthermore, the combination group showed a significant enrichment in arginine biosynthesis, as well as alanine, aspartate, and glutamate pathways. We accordingly examined the levels of ASS1, a key rate-limiting enzyme in arginine biosynthesis pathway. Interestingly, our results indicated that combinatory treatment exerted a more pronounced effect on BRAF-mutant PTC cells rather BRAF WT cells, which corroborate our metabolic data.
The level of arginine is commonly increased in cancers, including PTC [33], providing a metabolic advantage for rapid cell proliferation and evasion of apoptosis [34]. Pro, a nonessential amino acid, is crucial for the synthesis of arginine, and clonal generation of tumor cells is impaired by Pro deprivation or inhibition of its biosynthetic enzymes [35,36]. In the metabolomics analysis, Pro levels were considerably lower in the combination group, which was consistent with the cell growth inhibitory effect in vitro. Cancer cells break down Pro to produce Glu and α-ketoglutarate, which are essential nitrogen sources for nucleotide biosynthesis, and their subsequent catabolism supplies fuel for the TCA cycle [37,38]. In this study, the level of Pro was dramatically reduced after co-targeting of BRAF and PIM1, as well as dAMP, dGMP, dTMP, uracil, and cytosine. These findings indicated that combination therapy targeted the unique vulnerability of cancer cells to certain amino acids, thereby reducing the increased energy requirements for cell proliferation. Meanwhile, nucleotide synthesis, which is essential for the formation of tumors, was greatly suppressed in the combination group, accounting for the antitumor effect in vivo.
Notably, in this study, combination therapy had a distinct effect on cancer epigenetics. Most malignant tumors exhibit dysregulation of DNA methyltransferases (DNMTs), which use S-adenosylmethionine as the principal methyl donor and catalyze its conversion into S-adenosylhomocysteine (SAH). Subsequently, SAH undergoes further metabolic processes, resulting in the production of adenosine and homocysteine, thereby participating in nucleotide metabolism. The accumulation of SAH in cells can result in the inhibition of DNMTs through a feedback mechanism [39,40]. Although DNMTs are downregulated in response to BRAFi treatment in melanoma, they are upregulated upon the development of resistance, both in vitro and in vivo [41]. In this study, SAH levels were >5-fold higher in the combination group than in the control group. This not only supported the idea that nucleotide synthesis was diminished, but it also raised the possibility that DNMT enzyme activity was suppressed, suggesting a potential mechanism for the synergistic effect of combination strategy.
Conclusion
In summary, this study clarified how BRAFV600E regulated PIM1 expression in PTC, which led us to examine the potential effects of BRAFi plus PIMi as an alternative therapy for recalcitrant PTC. We then confirmed the synergistic effects of this combination. Analysis of the underlying pathways showed that the combination strategy acted primarily by limiting the availability of essential amino acids (e.g., phenylalanine, arginine, and glutamate) necessary for cell growth and proliferation, as well as by restricting nucleotide synthesis pathways and modulating DNA methylation levels. Overall, the proposed approach has the potential to become a clinically feasible substitute treatment for overcoming resistance against BRAF inhibitors in PTC patients.
CRediT authorship contribution statement
Qianqian Xu: Writing – original draft, Investigation, Formal analysis. Jiaqi Wang: Writing – review & editing, Validation, Investigation. Yuting Mao: Validation. Ziyang Xuan: Validation. Ke Yang: Investigation. Xi Tang: Investigation. Xin Zhu: Supervision, Resources, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Ethics approval and consent to participate
All animal procedures were performed in compliance with relevant laws and institutional guidelines and have been approved by Medical Ethics Committee of Zhejiang Cancer Hospital (IRB-2023-438).
Funding
This research was supported by National Natural Science Foundation of China (No. 82072950).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neo.2024.100996.
Appendix. Supplementary materials
References
- 1.Shah N., et al. Potential roles for the PIM1 kinase in human cancer - a molecular and therapeutic appraisal. Eur. J. Cancer (Oxford, Engl.: 1990) 2008;44(15):2144–2151. doi: 10.1016/j.ejca.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 2.Wińska P., et al. Synthesis and Anticancer Activity of Novel Dual Inhibitors of Human Protein Kinases CK2 and PIM-1. Pharmaceutics. 2023;15(7):1991. doi: 10.3390/pharmaceutics15071991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao L., et al. PIM1 kinase promotes cell proliferation, metastasis and tumor growth of lung adenocarcinoma by potentiating the c-MET signaling pathway. Cancer Lett. 2019;444:116–126. doi: 10.1016/j.canlet.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 4.Brasó-Maristany F., et al. PIM1 kinase regulates cell death, tumor growth and chemotherapy response in triple-negative breast cancer. Nat. Med. Nov. 2016;22(11):1303–1313. doi: 10.1038/nm.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tursynbay Y., et al. Pim-1 kinase as cancer drug target: An update. Biomed. Rep. 2016;4(2):140–146. doi: 10.3892/br.2015.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wen Q.-L., et al. Role of oncogene PIM-1 in the development and progression of papillary thyroid carcinoma: Involvement of oxidative stress. Mol. Cell. Endocrinol. .2021;523 doi: 10.1016/j.mce.2020.111144. [DOI] [PubMed] [Google Scholar]
- 7.Xia Y., et al. Construction of a tumor immune microenvironment-related prognostic Model in BRAF-mutated papillary thyroid cancer. Front. Endocrinol. 2022;13 doi: 10.3389/fendo.2022.895428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poulikakos P.I., et al. Molecular pathways and mechanisms of BRAF in cancer therapy. Clin Cancer Res. 2022;28(21):4618–4628. doi: 10.1158/1078-0432.CCR-21-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lj L.-G., L I. Mechanistic Insights of Thyroid Cancer Progression. Endocrinology. 2023;164(9) doi: 10.1210/endocr/bqad118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nam M., et al. Papillary thyroid cancer immune phenotypes via tumor-infiltrating lymphocyte spatial analysis. Endocr. Relat. Cancer. 2023;30(9) doi: 10.1530/ERC-23-0110. [DOI] [PubMed] [Google Scholar]
- 11.Sato A., et al. Circulating Tumor DNA Harboring the BRAFV600E mutation may predict poor outcomes of primary papillary thyroid cancer patients. Thyroid. 2021;31(12):1822–1828. doi: 10.1089/thy.2021.0267. [DOI] [PubMed] [Google Scholar]
- 12.Schubert L., et al. MAPK Pathway inhibitors in thyroid cancer: preclinical and clinical data. Cancers. 2023;15(3):710. doi: 10.3390/cancers15030710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu L., et al. Pharmacological inhibition of Ref-1 enhances the therapeutic sensitivity of papillary thyroid carcinoma to vemurafenib. Cell Death Dis. 2022;13(2):124. doi: 10.1038/s41419-022-04550-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fallahi P., et al. Molecular targets of tyrosine kinase inhibitors in thyroid cancer. Semin. Cancer Biol. 2022;79:180–196. doi: 10.1016/j.semcancer.2020.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Aprile M., et al. Targeting metabolism by B-raf inhibitors and diclofenac restrains the viability of BRAF-mutated thyroid carcinomas with Hif-1α-mediated glycolytic phenotype. Br. J. Cancer. 2023;129(2):249–265. doi: 10.1038/s41416-023-02282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luckett K.A., et al. Co-inhibition of SMAD and MAPK signaling enhances 124I uptake in BRAF-mutant thyroid cancers. Endocr. Relat. Cancer. 2021;28(6):391–402. doi: 10.1530/ERC-21-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hicks H.M., et al. Fibronectin contributes to a braf inhibitor-driven invasive phenotype in thyroid cancer through EGR1, which can be blocked by inhibition of ERK1/2. Mol. Cancer Res. 2023;21(9):867–880. doi: 10.1158/1541-7786.MCR-22-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J., et al. PIM Kinases in Multiple Myeloma. Cancers. 2021;13(17):4304. doi: 10.3390/cancers13174304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang B.-W., et al. Pim1 kinase inhibitors exert anti-cancer activity against HER2-positive breast cancer cells through downregulation of HER2. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.614673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pavlova N.N., et al. The hallmarks of cancer metabolism: Still emerging. Cell Metab. 2022;34(3):355–377. doi: 10.1016/j.cmet.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faubert B., et al. Metabolic reprogramming and cancer progression. Science. 2020;368(6487):eaaw5473. doi: 10.1126/science.aaw5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bao M.H.-R., Wong C.C.-L. Hypoxia, metabolic reprogramming, and drug resistance in liver cancer. Cells. 2021;10(7):1715. doi: 10.3390/cells10071715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan W., et al. Oncogenic BRAF noncanonically promotes tumor metastasis by mediating VASP phosphorylation and filopodia formation. Oncogene. Oct. 2023;42(43):3194–3205. doi: 10.1038/s41388-023-02829-w. [DOI] [PubMed] [Google Scholar]
- 24.Tang Q., et al. Tubeimoside-I sensitizes temozolomide-resistant glioblastoma cells to chemotherapy by reducing MGMT expression and suppressing EGFR induced PI3K/Akt/mTOR/NF-κB-mediated signaling pathway. Phytomedicine. 2022;99 doi: 10.1016/j.phymed.2022.154016. [DOI] [PubMed] [Google Scholar]
- 25.Xin G., et al. Targeting PIM1-mediated metabolism in myeloid suppressor cells to treat Cancer. Cancer Immunol. Res. 2021;9(4):454–469. doi: 10.1158/2326-6066.CIR-20-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanland L.J., et al. CBF-Beta Mitigates PI3K-Alpha-specific inhibitor killing through PIM1 in PIK3CA-Mutant Gastric Cancer. Mol. Cancer Res. 2023;21(11):1148–1162. doi: 10.1158/1541-7786.MCR-23-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim K.-T., et al. Constitutively activated FLT3 phosphorylates BAD partially through pim-1. Br. J. Haematol. 2006;134(5):500–509. doi: 10.1111/j.1365-2141.2006.06225.x. [DOI] [PubMed] [Google Scholar]
- 28.Zhi J., et al. Inhibition of BRAF Sensitizes Thyroid Carcinoma to Immunotherapy by Enhancing tsMHCII-mediated Immune Recognition. J. Clin. Endocr. Metab. 2021;106(1):91–107. doi: 10.1210/clinem/dgaa656. [DOI] [PubMed] [Google Scholar]
- 29.Jeong J.H., et al. Combination Treatment with the BRAFV600E Inhibitor Vemurafenib and the BH3 Mimetic Navitoclax for BRAF -Mutant Thyroid Carcinoma. Thyroid. 2019;29(4):540–548. doi: 10.1089/thy.2018.0511. [DOI] [PubMed] [Google Scholar]
- 30.Cortes J., et al. Phase I studies of AZD1208, a proviral integration Moloney virus kinase inhibitor in solid and haematological cancers. Br. J. Cancer. 2018;118(11):1425–1433. doi: 10.1038/s41416-018-0082-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diehl F.F., et al. Nucleotide imbalance decouples cell growth from cell proliferation. Nat. Cell Biol. 2022;24(8):1252–1264. doi: 10.1038/s41556-022-00965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uhlenkott C.E., et al. Attachment, invasion, chemotaxis, and proteinase expression of B16-BL6 melanoma cells exhibiting a low metastatic phenotype after exposure to dietary restriction of tyrosine and phenylalanine. Clin. Exp. Metastas. 1996;14(2):125–137. doi: 10.1007/BF00121209. [DOI] [PubMed] [Google Scholar]
- 33.Du Y., et al. Serum Metabolomics study of papillary thyroid carcinoma based on HPLC-Q-TOF-MS/MS. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.593510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alapati S., et al. Evaluation of metabolomics as diagnostic targets in oral squamous cell carcinoma: a systematic review. Metabolites. 2023;13(8):890. doi: 10.3390/metabo13080890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kay E.J., et al. Cancer-associated fibroblasts require proline synthesis by PYCR1 for the deposition of pro-tumorigenic extracellular matrix. Nat. Metab. 2022;4(6):693–710. doi: 10.1038/s42255-022-00582-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Westbrook R.L., et al. Proline synthesis through PYCR1 is required to support cancer cell proliferation and survival in oxygen-limiting conditions. Cell Rep. 2022;38(5) doi: 10.1016/j.celrep.2022.110320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pandhare J., et al. Regulation and function of proline oxidase under nutrient stress. J. Cell Biochem. 2009;107(4):759–768. doi: 10.1002/jcb.22174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fang K., et al. Targeting IGF1R signaling enhances the sensitivity of cisplatin by inhibiting proline and arginine metabolism in oesophageal squamous cell carcinoma under hypoxia. J. Exp. Clin. Cancer Res. 2023;42(1):73. doi: 10.1186/s13046-023-02623-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hermes M., et al. Alterations in S-adenosylhomocysteine metabolism decrease O6-methylguanine DNA methyltransferase gene expression without affecting promoter methylation. Biochem. Pharmacol. 2008;75(11):2100–2111. doi: 10.1016/j.bcp.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 40.Wang Z., et al. Methionine is a metabolic dependency of tumor-initiating cells. Nat. Med. 2019;25(5):825–837. doi: 10.1038/s41591-019-0423-5. [DOI] [PubMed] [Google Scholar]
- 41.Gassenmaier M., et al. Expression of DNA Methyltransferase 1 Is a hallmark of melanoma, correlating with proliferation and response to b-raf and mitogen-activated protein kinase inhibition in melanocytic tumors. Am. J. Pathol. 2020;190(10):2155–2164. doi: 10.1016/j.ajpath.2020.07.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.