Summary
Background
Hospital-acquired infections (HAI) are a leading cause of morbidity and mortality globally. These infections are diverse, but the majority are lower respiratory tract infection (LRTI), surgical site infection (SSI), bloodstream infection (BSI), and urinary tract infection (UTI). For most sub-Saharan African countries, studies revealing the burden and impact of HAI are scarce, and few systematic reviews and meta-analysis have been attempted. We sought to fill this gap by reporting recent trends in HAI in sub-Saharan Africa (SSA) with attention to key patient populations, geographic variation, and associated mortality.
Methods
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, we conducted a literature search of six electronic databases (Web of Science, Pubmed, APA PsycInfo, CINAHL, Embase, and the Cochrane Library) to identify studies assessing the prevalence of HAI in SSA countries. Studies published between 01 January 2014 and 31 December 2023 were included. We applied no language or publication restrictions. Record screening and data extractions were independently conducted by teams of two or more reviewers. Using the R software (version 4.3.1) meta and metafor packages, we calculated the pooled prevalence estimates from random-effect meta-analysis, and further explored sources of heterogeneity through subgroup analyses and meta-regression. This study is registered with PROSPERO, CRD42023433271.
Findings
Forty-one relevant studies were identified for analysis, consisting of 15 from West Africa (n = 2107), 12 from Southern Africa (n = 2963), 11 from East Africa (n = 2142), and 3 from Central Africa (n = 124). A total of 59.4% of the patient population were associated with paediatric admissions. The pooled prevalence of HAI was estimated at 12.9% (95% CI: 8.9–17.4; n = 7336; number of included estimates [k] = 41, p < 0.001). By subregions, the pooled current prevalence of HAI in the West Africa, Southern Africa, East Africa and Central Africa were estimated at 15.5% (95% CI: 8.3–24.4; n = 2107; k = 15), 6.5% (95% CI: 3.3–10.7; n = 2963; k = 12), 19.7% (95% CI: 10.8–30.5; n = 2142; k = 11) and 10.3% (95% CI: 1.1–27.0; n = 124; k = 3) of the patient populations respectively. We estimated mortality resulting from HAI in SSA at 22.2% (95% CI: 14.2–31.4; n = 1118; k = 9).
Interpretation
Our estimates reveal a high burden of HAI in SSA with significant heterogeneity between regions. Variations in HAI distribution highlight the need for infection prevention and surveillance strategies specifically tailored to enhance prevention and management with special focus on West and East Africa, as part of the broader global control effort.
Funding
No funding was received for this study.
Keywords: Hospital-acquired infections, Nosocomial, Trends, Healthcare, Burden, Sub-Saharan Africa
Research in context.
Evidence before this study
We searched Web of Science, Pubmed, APA PsycInfo, CINAHL, Embase, and the Cochrane Library databases, from 01 January 2014 to 31 December 2023, for papers with no language restrictions, using search terms “Hospital-acquired infection” OR “Healthcare-associated infection” OR “Nosocomial infection” AND “Burden” OR “prevalence” AND “sub-Saharan Africa”. Our search yielded 41 articles that were included in the meta-analysis. There are few studies estimating the prevalence of HAI in SSA but those that do, raise concern for excess burden in this part of the world. In measuring the burden of SSI in SSA and progress associated with key efforts such as investments in infection prevention and international campaigns (for example, the World Health Organizations Campaign: “Save Lives: Clean Your Hands”), surveillance systems are still not in place in most SSA countries. In their absence, prevalence studies are useful to estimate the current HAI burden. Understanding the size and distribution of HAI is also critical in designing effective new interventions and targeting regions that continue to see a disproportionate burden.
Added value of this study
A comprehensive analysis of HAI from SSA describing the trend, burden, associated mortality, as well as prevalence in neonatal, paediatric, and intensive care units was conducted. This study quantifies the burden of HAI along subregional lines bringing attention to region-specific differences. The overall trend from this review reveals a low burden of HAI during the peak years of COVID-19 (2020–2022). Our analyses revealed a high overall burden of HAI in SSA associated with an in-hospital mortality rate of 22%. Although data from 16 countries contributed to this review, most reports were from a small number of countries suggesting the need for additional research in high-burden nations. This study also highlights the lack of adult HAI data from SSA and the urgent need for this to be addressed.
Implications of all the available evidence
Patients entering hospitals in West and East Africa are at greatest risk for HAI in SSA and these infections are frequently deadly. More broadly, the high overall burden of HAI and low number of studies from SSA highlights the need for greater surveillance and resource distribution to address the HAI burden. Enhanced data generation from SSA will allow healthcare decisionmakers and donors to move ahead with new policy implementation and target enhanced responses towards the highest risk contexts.
Introduction
Hospital-acquired infections (HAI) are a leading cause of morbidity and mortality globally1 and are mainly attributable to central-line–associated bloodstream infections (CLABSIs), catheter-associated urinary tract infections (CAUTIs), ventilator-associated pneumonia (VAP), and surgical site infections (SSIs).2 Hospital-acquired infections are acquired by patients after 48 hours of admission to a hospital3 and were neither present nor incubating at the time of admission.1,2 Hospital-acquired infections develop in approximately 1 in 31 hospital patients4 with the intensive care units contributing 9–20% of these infections.5
Pooled data estimates that the burden of HAI in the African region is twice that of developed settings.6 In sub-Saharan Africa (SSA), 22.1% of HAI have been reported among neonatal populations.7 There is a dearth of HAI data involving adult populations in SSA. In South Africa, the impact of HAI within the public health system and across different patient populations has not been well quantified.8 Hospital-acquired infections contribute significantly to hospital morbidity and mortality, cost of healthcare, and reduce health-related quality of life.6,9
Although a few systematic reviews reporting on the burden of HAI have been conducted in Africa,6,10 no systematic review extensively interrogating HAI in SSA and its subregions has been performed. In addition, no systematic review has quantified the burden of HAI in neonatal and paediatric populations, as well as temporal HAI trends in SSA. In this systematic review and meta-analysis, we aimed to estimate the current regional and subregional prevalence of HAI in the overall in-patient population, as well as among ICU patients, adults, paediatric, and neonatal admissions in SSA. We further estimated the prevalence of major HAI and associated mortality in SSA between 2014 and 2023 since comprehensive data for this region and its subregions were lacking.
Methods
Search strategy and selection criteria
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) reporting guidelines.11 Our review protocol was prospectively registered with PROSPERO CRD42023433271. A comprehensive search of six electronic databases including Web of Science, Pubmed, APA PsycInfo, CINAHL, Embase, and the Cochrane Library for studies published between 01 January 2014 and 31 December 2023 was carried out. A manual search was conducted on reference lists of review articles. Field experts from within and beyond the review team were consulted to identify potentially relevant articles missing in the databases. Although we did not explicitly search the grey literature,12 we retrieved conference abstracts as well as unpublished data. We contacted authors of studies to provide us with missing data where there was insufficient data in the publication. We conducted quality appraisal using the JBI manual of Evidence synthesis–for systematic reviews of prevalence and incidence.13 Full details of our electronic search strategy have been included as (Supplementary File S1). Title, abstract, and full text screenings were independently conducted by paired reviewers. Discrepancies were resolved by discussion at all stages. The eligibility of included studies was independently verified by other reviewers.
We included prevalence studies that defined HAI as infection(s) acquired during the process of receiving health care in patient(s) admitted to hospital for 48 h or more, and such infection(s) were not present during the time of admission. Prevalence studies involving all patient populations or subpopulations where HAI were not overestimated were eligible for inclusion regardless of age and gender. Such studies must be conducted in any hospital unit or ward, including neonatal, paediatrics and intensive care units. In this review, neonates and paediatrics are separately addressed, with a neonate defined as a child below 28 days and paediatric being a child equal to, or above 28 days, but less than 18 years of age. We included studies that estimated the prevalence of HAI alongside their associated risk factors, and mortality. Such studies must have been conducted in any of the SSA sub-regions. In this review, SSA subregions were based on the United Nations Development Programme (UNDP) classification.14 There were no language restrictions. We used a web-based tool (Google Translate) to translate studies conducted in languages other than English. Studies were excluded if they involved only data from specimen culture results and did not involve patient populations. Studies conducted outside of SSA were excluded. We excluded very restrictive studies (subset of a subpopulation) where HAI prevalences were incorrectly over- or underestimated. Studies where sample size and cases were unknown and such data could not be retrieved from the authors were excluded. Finally, we excluded reviews, commentaries, books, and letters to editors.
To ensure retrieval of as many studies as possible, we searched chosen databases, using keywords with names of SSA countries: (Hospital-acquired infection) OR (Healthcare-associated infection)) OR (Nosocomial infection)) AND (Burden)) OR (prevalence)) AND (sub-Saharan Africa))) AND (Cameroon OR Central African OR Republic Chad OR Congo OR Equatorial Guinea OR Gabon OR Sao Tome and Principe OR Burundi OR Comoros OR Democratic Republic of the Congo OR Djibouti OR Eritrea OR Ethiopia OR Kenya OR Madagascar OR Rwanda OR Somalia OR South Sudan OR Uganda OR United Republic of Tanzania OR Angola OR Botswana OR Eswatini OR Lesotho OR Malawi OR Mauritius OR Mozambique OR Namibia OR South Africa OR Zambia OR Zimbabwe OR Benin OR Burkina Faso OR Cabo Verde OR Côte d’Ivoire OR Gambia OR Ghana OR Guinea OR Guinea-Bissau OR Liberia OR Mali OR Niger OR Nigeria OR Senegal OR Sierra Leone OR Togo.
We focused on the number of patients with HAI rather than the number of specimens obtained when abstracting data from laboratory-based investigations.
Quality appraisal
We conducted quality appraisal using the JBI critical appraisal checklist for studies reporting prevalence data (Supplementary File S2.2). With the JBI appraisal tool, studies were classified as low, medium, or high quality. Low quality studies were defined as those whose appraisal scores were 49% or less. Studies were classified as medium quality if they scored between 50 and 69%. Studies scoring 70% and above were rated as high-quality studies.
Data extraction
Three reviewers independently extracted data from the included studies. Data were extracted onto a standardized data extraction form which was customized to our proposed review. Extracted data were further verified by other co-authors. Extracted information from eligible studies included names and year of study, country where study was conducted, study sample size, number of HAI cases, mortality, and associated risk factors. No ethical approval was obtained because this systematic review and meta-analysis was exclusively based on published evidence.
Outcomes
Our primary outcomes were the prevalences of HAI in the overall population, neonates, paediatrics, ICU, as well as the different types of HAI reported in SSA. The major HAI included surgical site infections (SSI), urinary tract infections (UTI), lower respiratory tract infections (LRTI), and blood stream infections (BSI). Our secondary outcomes included the trend of HAI over the period under consideration (2014–2023) and mortality resulting from HAI. Also included in our secondary outcome were the identified risk factors for HAI.
Data analysis
The prevalence of HAI for each study was calculated by dividing the number of cases with HAI by the number of individuals examined. For the meta-analysis, individual sample proportion estimates with their 95% confidence intervals (CIs) were calculated with stabilized variances via the Freeman-Tukey double arcsine transformation15 to approximate normal distribution. Random-effects models were fitted via the restricted maximum-likelihood estimation method to estimate variance heterogeneity and the 95% CIs of summary measures were calculated with the Knapp-Hartung variance estimator. Inconsistency was quantified with the I2 statistic to describe the percentage of variation attributed to between-sample heterogeneity, with values higher than 75% indicating considerable heterogeneity. All statistical data analyses were performed in the R software (version 4.3.1) using the meta and metafor packages.
Ethics statement
The data used in this study are publicly available as published articles and do not require ethical assessment.
Role of the funding source
There was no funding source for this study.
Results
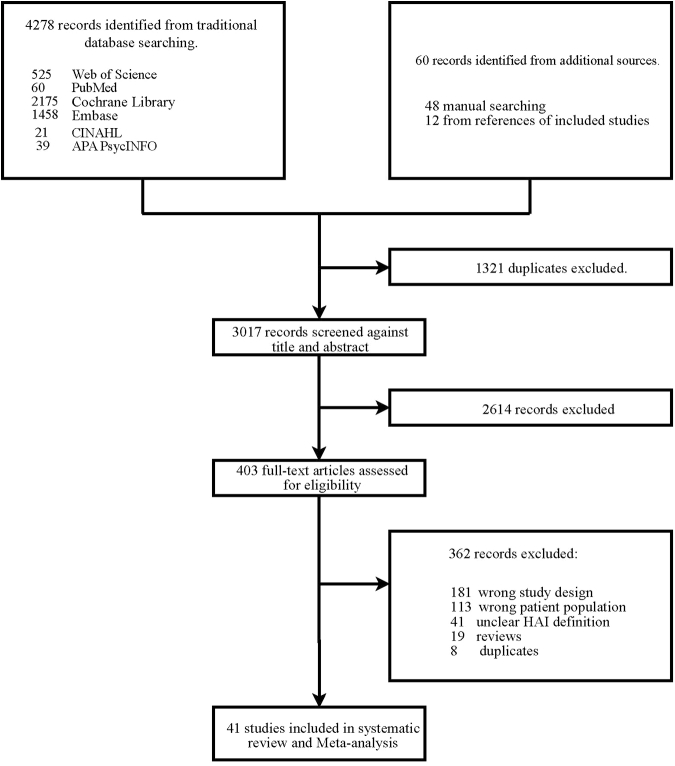
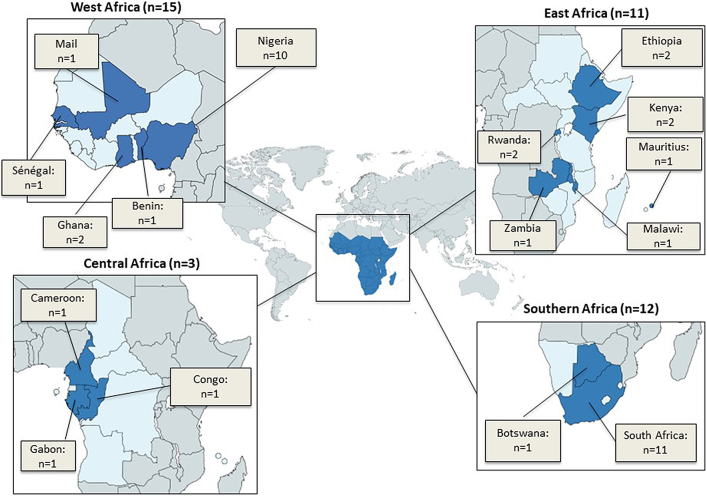
Of 4338 citations identified, 41 prevalence studies,16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56 which included 6821 patients, met our eligibility criteria (Fig. 1). The included studies were selected from all four subregions of SSA: fifteen studies from West Africa,17,21,22,25,27,28,32,36,37,43,46,47,49,50,55 twelve from Southern Africa,16,19,23,29,30,33,38,40,45,52, 53, 54 eleven from East Africa,20,24,26,31,34,35,41,42,44,48,51 and three from Central Africa18,39,56 (Fig. 2). Eligible studies were from the following SSA countries: Benin, Botswana, Cameroon, Congo, Ethiopia, Gabon, Ghana, Kenya, Malawi, Mali, Mauritius, Nigeria, Rwanda, Senegal, South Africa, and Zambia. Table S2 of Supplementary File S2 shows the study characteristics of the 41 included studies from 2014 to 2023. Of the total in-patient population included in this study, 59.4% were from paediatric (children ≥28 days, but <18 years) admissions only Table 1.
Fig. 1.
Flowchart for search and selection strategy.
Fig. 2.
Map showing geographical distribution of included Hospital-acquired Infections (HAI) studies in sub-Saharan Africa from 2014 to 2023.
Table 1.
Summary prevalence of characteristic dimensions of hospital-acquired infections in sub-Saharan Africa from 2014 to 2023.
| Items | N | n | P (%) | EP | 95% CI | p-value |
|---|---|---|---|---|---|---|
| Overall HAI prevalence | 223,199 | 7336 | 3.3 | 12.9 | 8.9–17.4 | <0.001 |
| Overall HAI prevalence in Intensive Care Units (ICU) | 11,000 | 1755 | 16.0 | 25.0 | 11.0–42.2 | <0.001 |
| Overall HAI prevalence among paediatrics | 132,514 | 1616 | 1.2 | 9.5 | 3.9–17.1 | <0.001 |
| Overall HAI mortality | 4215 | 1118 | 26.5 | 22.2 | 14.2–31.4 | <0.001 |
| Overall HAI prevalence among neonates | 60,205 | 2735 | 4.5 | 13.2 | 3.9–26.6 | <0.001 |
| Region-specific | ||||||
| HAI Prevalence in West Africa | 22,283 | 2107 | 9.5 | 15.5 | 8.3–24.4 | <0.001 |
| HAI Prevalence in Southern Africa | 169,424 | 2963 | 1.7 | 6.5 | 3.3–10.7 | <0.001 |
| HAI Prevalence in East Africa | 12,212 | 2142 | 17.5 | 19.7 | 10.8–30.5 | <0.001 |
| HAI Prevalence in Central Africa | 3403 | 124 | 3.6 | 10.3 | 1.1–27.0 | <0.001 |
| Major types of HAI | ||||||
| Bloodstream infection, prevalence | 4500 | 2671 | 59.4 | 36.8 | 19.5–56.0 | <0.001 |
| Urinary tract infection, prevalence | 2514 | 973 | 38.7 | 30.4 | 22.3–39.1 | <0.001 |
| Surgical site infection, prevalence | 2437 | 692 | 28.4 | 43.7 | 25.4–62.9 | <0.001 |
| Lower respiratory tract infection, prevalence | 1943 | 332 | 17.1 | 24.5 | 11.0–41.1 | <0.001 |
HAI, Hospital-acquired Infections; ICU, Intensive Care Units; N, Sample size; n, number confirmed HAI; P (%), proportion in percentages; EP, Estimated prevalence in percentages from meta-analysis; CI, Confidence Interval, Neonates, children <28 days; Paediatrics, children ≥28 days, but <18 years.
Of the included studies, 11 reported on the prevalence of HAI in intensive care units (ICU), 17 reported on the prevalence of HAI among paediatric admissions, 8 reported on the prevalence of HAI among neonatal admissions, 20 reported on the prevalence of blood stream infections (BSI), 19 reported on the prevalence of urinary tract infections (UTI), 18 reported on the prevalence of surgical site infections (SSI), while 17 reported on the prevalence of lower respiratory tract infection (LRTI) among HAI patients. Only nine studies reported on the mortality from HAI. (Supplementary File S2). Tables S3–S16 of Supplementary File S2 show the HAI distributions of all the studies included in this review.
Overall prevalence of HAI in sub-Saharan Africa
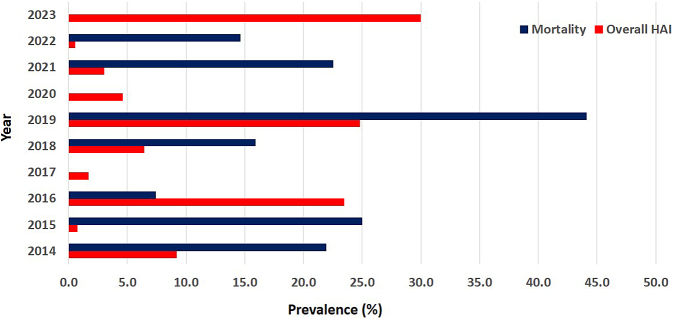
Of the overall pooled patient population (223,199 patients), 7336 (3.3%) were diagnosed with HAI. The highest proportion of infections was observed in 2023 (30.0%), followed by 2019 (24.8%) and 2016 (23.5%) (Table S3 of Supplementary File S2). The lowest proportion of 0.5% was recorded in 2022. The trend in HAI prevalence and mortality over the study period is shown in Fig. 3, with a cycle pattern observed from 2014 to 2023. The pooled prevalence of HAI was estimated at 12.9% (95% CI: 8.9–17.4; n = 7336; number of included estimates [k] = 41, p < 0.001).
Fig. 3.
Trends in overall HAI and mortality in sub-Saharan Africa between 2014 and 2023. HAI, Hospital-acquired infection; blank spaces show absent data of required variable in a particular year.
Prevalence of HAI in SSA subregions
The pooled current prevalence of HAI in East Africa, West Africa, Central Africa, and Southern Africa were estimated at 19.7% (95% CI: 10.8–30.5; n = 2142; k = 11), 15.5% (95% CI: 8.3–24.4; n = 2107; k = 15), 10.3% (95% CI: 1.1–27.0; n = 124; k = 3), and 6.5% (95% CI: 3.3–10.7; n = 2963; k = 12) of the patient populations respectively.
For the random effects model, the diamonds representing the estimated prevalence and 95% confidence limits in Fig. S1a–d of Supplementary File S3 did not cross the line of no prevalence. The tests of heterogeneity (all p < 0.001) suggested the presence of heterogeneous results, with the heterogeneity statistics (I2 = 98.7%, 99.5%, 99.4%, 98.9%) indicating high between-sample heterogeneity and their 95% CI (98.4%–99.0%, 99.4%–99.6%, 99.3%–99.5%, 98.2%–99.3%) indicative of a potentially important to substantial heterogeneity in the effect sizes.
Overall prevalence of HAI in intensive care units in SSA
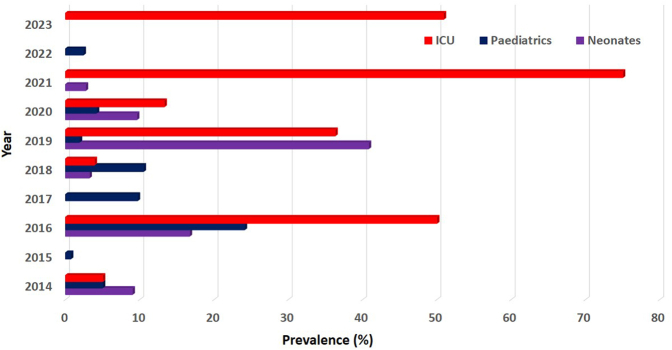
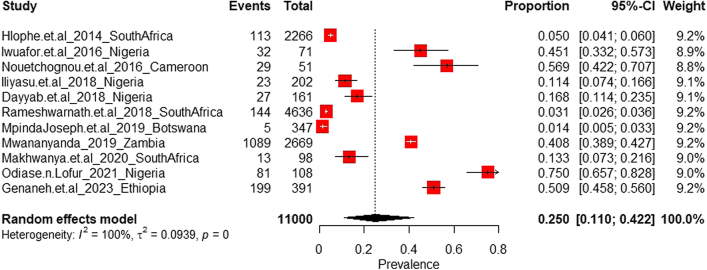
A total of 11 studies reporting on HAI in ICU in SSA were identified (Table S9 of Supplementary File S2). Of the pooled total study population, a proportion of 16.0% (1755 of 11,000 cases) was recorded across six countries. The pooled prevalence of HAI in ICU was estimated at 25.0% (95% CI: 11.0–42.2; n = 1755; k = 11). Fig. 4 shows the trend of HAI among neonatal, paediatric and ICU admissions in SSA between 2014 and 2023.
Fig. 4.
Trend of Hospital-Acquired Infections among neonatal, paediatric and ICU admissions in SSA between 2014 and 2023; ICU, Intensive Care Unit; SSA, sub-Saharan Africa. Blank spaces show absent data for variable.
For the random effects model, the diamonds representing the estimated prevalence and 95% confidence limits in Fig. 5 did not cross the line of no prevalence (at origin 0). The test of heterogeneity (p < 0.001) suggested the presence of heterogeneous results, with the heterogeneity statistic (I2 = 99.6%) indicating a high between-sample heterogeneity and its 95% CI (99.5%–99.7%) indicative of a potentially important to substantial heterogeneity in the effect sizes.
Fig. 5.
Current prevalence of Hospital-acquired Infections in Intensive Care Units in sub-Saharan Africa.
Prevalence of HAI among neonates in SSA
A total of eight studies reporting on HAI among neonates in SSA were identified (Table S16 of Supplementary File S2). Of the pooled total study population, a proportion of 4.5% (2735 of 60,205 cases) was recorded across five countries.
The pooled current prevalence of HAI among neonates was estimated at 13.2% (95% CI: 3.9–26.6; n = 2735; k = 8). For the random effects model, the diamonds representing the estimated prevalence and 95% confidence limits in Fig. S3 of Supplementary File S3 did not cross the line of no prevalence. Our analysis suggests the existence of heterogeneous results [I2 = 99.8%; 95% CI (99.7%–99.9%)] with significant heterogeneity in the effect sizes (p < 0.001).
Prevalence of HAI among paediatric admissions in SSA
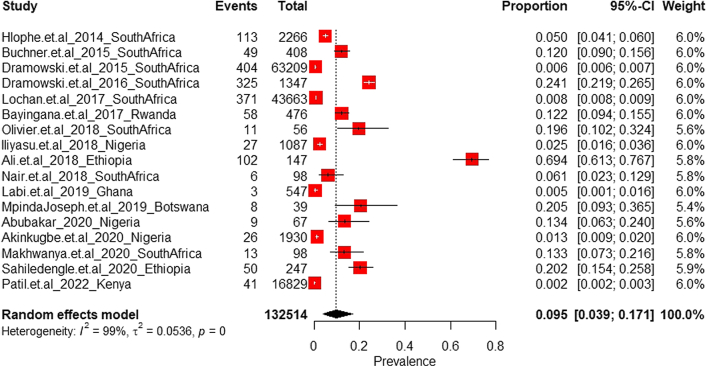
A total of 17 studies reporting on HAI among paediatric admissions in SSA were identified (Table S10 of Supplementary File S2). Of the pooled total study population, a proportion of 1.2% (1616 of 132,514 cases) was recorded across seven countries.
The pooled current prevalence of HAI among paediatric admissions was estimated at 9.5% (95% CI: 3.9–17.1; n = 1616; k = 17). For the random effects model, the diamonds representing the estimated prevalence and 95% confidence limits in Fig. 6 did not cross the line of no prevalence. Our analysis suggests the existence of heterogeneous results [I2 = 99.3%; 95% CI (99.2%–99.4%)] with significant heterogeneity in the effect sizes (p < 0.001).
Fig. 6.
Current prevalence of Hospital-acquired Infections among paediatrics admissions in sub-Saharan Africa.
Overall mortality from HAI in sub-Saharan Africa
A total of nine studies reported on mortality associated with HAI in SSA (Table S15 of Supplementary File S2). Of the pooled total study population, a proportion of 26.5% (1118 of 4215 cases) was recorded across seven countries. The pooled prevalence of mortality from HAI was estimated at 22.2% (95% CI: 14.2–31.4; n = 1118; k = 9). For the random effects model, the diamonds representing the estimated prevalence and 95% confidence limits in Fig. 7 did not cross the line of no prevalence.
Fig. 7.
Current mortality from Hospital-acquired Infections in sub-Saharan Africa.
The test of heterogeneity (p < 0.001) suggested the presence of heterogeneous results, with the heterogeneity statistic (I2 = 97.5%) indicating a high between-sample heterogeneity and its 95% CI (96.5%–98.2%) indicative of a potentially important to substantial heterogeneity in the effect sizes.
Prevalence of major hospital-acquired infections
The following number of studies in SSA, per specific type of HAI, were identified: 20 BSI, 19 UTI, 18 SSI and 17 LRTI (Tables S11–S14 of Supplementary File S2). Of the pooled total study population, a proportion of 59.4% (2671 of 4500 cases) was recorded for BSI across 10 countries, 38.7% (973 of 2514 cases) was recorded for UTI across 11 countries, 28.4% (692 of 2437 cases) was recorded for SSI across 10 countries and 17.1% (332 of 1943 cases) was recorded for LRTI across nine countries. Of all patients with HAI, the pooled prevalence for BSI, UTI, SSI and LRTI were estimated at 36.8% (95% CI: 19.5–56.0; n = 2671; k = 20), 30.4% (95% CI: 22.3–39.1; n = 973; k = 19), 43.7% (95% CI: 25.4–62.9; n = 692; k = 18) and 24.5% (95% CI: 11.0–41.1; n = 332; k = 17) of the patient populations respectively.
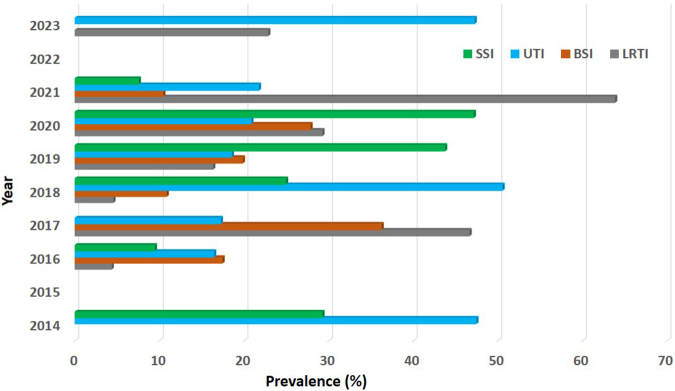
For the random effects model, the diamonds representing the estimated prevalence and 95% confidence limits in Fig. S2a–d of Supplementary File S3 did not cross the line of no prevalence. The tests of heterogeneity (all p < 0.001) suggested the presence of heterogeneous results, with the heterogeneity statistics (I2 = 99.7%, 94.7%, 98.1%, 97.7%) indicating high between-sample heterogeneity and their 95% CI (99.6%–99.9%, 93.0%–96.0%, 97.7%–98.5%, 97.1%–98.2%) indicative of a potentially important to substantial heterogeneity in the effect sizes. Fig. 8 shows the trend of different HAI in SSA between 2014 and 2023.
Fig. 8.
Trend of different Hospital-acquired Infections in sub-Saharan Africa between 2014 and 2023; SSI, Surgical site infections; UTI, Urinary tract infections; BSI, Blood stream infections; LRTI, Lower respiratory tract infections.
Quality appraisal, publication bias, and sensitivity analysis
Using the JBI critical appraisal tool, 46.3% each of the included studies were of high and medium qualities respectively. Low quality studies comprised 7.3%. Of the high-quality studies, 41.2% were from Southern Africa, 31.6% from West Africa, 21.1% and 5.3% were from East Africa and Central Africa respectively. Thirty-six point eight percent, 31.6%, 21.1% and 10.5% of the medium quality studies were from West Africa, East Africa, Southern Africa and Central Africa respectively. The Low-quality studies were from West Africa (66.7%) and East Africa (33.3%) (Table S1b in Supplementary File S2).
To assess the potential publication bias in the conducted meta-analysis in this study, the funnel plots (Fig. 9), (Kendall's tau [τ]) rank correlation test and Egger's test for funnel plot asymmetry were performed. Figs. S1–S6 of Supplementary File S3 shows the obtained funnel plots for the overall and sub-unit prevalences of HAI, HAI prevalence in ICU, among paediatric admissions, neonates, region-specific, infections and mortality from HAI. In the HAI prevalence, the data points showed an asymmetrical pattern in the funnel plot that might be indicative of publication bias and the presence of small-study effects, although the effect is not very evident. Similar deductions can be observed for the HAI prevalence in ICU, among paediatric admissions, neonates, region-specific, infections and mortality from HAI.
Fig. 9.
Funnel plot for overall prevalence of Hospital-acquired Infections in sub-Saharan Africa.
From the rank correlation tests for funnel plot asymmetry performed (Table S1 of Supplementary File S3), it can be concluded at a 5% level of significance that there is a significant relationship between the sample size and the observed effect size of each study for the overall HAI prevalence, among paediatric admissions, ICU, mortality, Western Africa, Southern Africa, Eastern Africa, BSI, UTI, SSI, and LRTI. However, the rank correlation test fails to find a significant relationship between sample size and effect size of each study for HAI in neonates and Central Africa. Similarly, from the Egger's regression tests for funnel plot asymmetry performed (Table S1 of Supplementary File S3), it can be concluded at a 5% level of significance that small-study effects truly exist in the meta-analysis study for the overall HAI prevalence, among paediatric admissions, mortality, Western Africa, Southern Africa, Eastern Africa, Central Africa, BSI, UTI and SSI. Thus, their resulting funnel plots are significantly asymmetrical, indicating that the data points in the funnel plot are indeed asymmetrical. Overall, this corroborates with the initial findings from the funnel plots that there were small-study effects. However, the Egger's test was not significant for possible HAI in ICU, neonates and LRTI.
Furthermore, using the trim-and-fill method to adjust for the effect estimate for the presence of asymmetry in the funnel plots, this method added 20 imputed missing studies to the meta-analysis of HAI prevalence, leading to an adjusted random effects prevalence of 2.5% (95% CI: 0.4–6.1), with a 99.7% heterogeneity statistic (I2 = 99.7%; 95% CI: 99.1–99.9; k = 61 (with 20 added studies)). Since there is no difference between the heterogeneity for HAI prevalence before using the trim-and-fill method (99.7%) and after applying it (99.7%), the validity of the estimated summary effect size can be said to be robust. In addition, the heterogeneity results obtained after applying the trim-and-fill method to the meta-analysis were slightly similar compared to those obtained before applying the method.
Influence analysis
Influence analysis was further done in the meta-analysis to identify the outlying and influencing studies. The impact of influencing studies with (very) high effect sizes on the heterogeneity of the overall effect in the meta-analysis was also identified. This was done via the leave-one-out method whereby we re-ran the meta-analysis, iteratively removing studies and checking how the overall effect estimate changes when different studies were removed.
Overall prevalence of HAI in sub-Saharan Africa
Two studies35,45 were detected as outliers and found to be adding more to the heterogeneity of the meta-analysis. After excluding them from the meta-analysis, the prevalence of HAI stood at 12.6% (I2 = 99.5%; n = 4812; k = 39) with a slight shrinkage of the I2 heterogeneity from 99.7% to 99.5%. Using the trim-and-fill method to adjust for the effect estimate for the presence of asymmetry in the funnel plot, this method added 20 imputed missing studies to the meta-analysis, leading to an adjusted random effects prevalence of 2.0% (I2 = 99.6%; k = 59 (with 20 added studies)) with a negligible growth of the I2 heterogeneity from 99.5% to 99.6%.
Overall prevalence of HAI in intensive care units in sub-Saharan Africa
One study35 was detected as an outlier and found to be adding more to the heterogeneity of the meta-analysis. After excluding it from the meta-analysis, the prevalence of HAI in ICU stood at 23.5% (I2 = 99.1%; n = 666; k = 10) with a negligible shrinkage of the I2 heterogeneity from 99.6% to 99.1%. Using the trim-and-fill method to adjust for the effect estimate for the presence of asymmetry in the funnel plot, this method added five imputed missing studies to the meta-analysis, leading to an adjusted random effects prevalence of 4.2% (I2 = 99.4%; k = 15 (with 5 added studies)) with a negligible growth of the I2 heterogeneity from 99.1% to 99.4%.
Prevalence of HAI among paediatric admissions in sub-Saharan Africa
One study54 was detected as an outlier and found to be adding more to the heterogeneity of the meta-analysis. After excluding it from the meta-analysis, the prevalence of HAI among paediatric admissions stood at 8.8% (I2 = 98.9%; n = 1291; k = 16) with a negligible shrinkage of the I2 heterogeneity from 99.3% to 98.9%. Using the trim-and-fill method to adjust for the effect estimate for the presence of asymmetry in the funnel plot, this method added eight imputed missing studies to the meta-analysis, leading to an adjusted random effects prevalence of 0.7% (I2 = 99.0%; k = 24 (with 8 added studies)) with a negligible growth of the I2 heterogeneity from 98.9% to 99.0%.
Overall mortality from HAI in sub-Saharan Africa
One study35 was detected as an outlier which influenced the heterogeneity of the meta-analysis. On exclusion of the said study from the meta-analysis, the mortality from HAI stood at 19.4% (I2 = 92.6%; n = 638; k = 8) with a shrinkage of the I2 heterogeneity from 97.5% to 92.6%. However, using the trim-and-fill method to adjust for the effect estimate for the presence of asymmetry in the funnel plot, no imputed missing studies were added to the meta-analysis.
Prevalence of HAI in SSA sub-regions
Similarly, the following outlying studies from West Africa,50 Southern Africa,45 and East Africa35 added to the respective heterogeneity of the meta-analysis. After excluding them from the meta-analysis, the prevalence of HAI in West Africa, Southern Africa and East Africa stood at 15.3% (I2 = 98.3%; n = 1510; k = 14), 6.9% (I2 = 99.4%; n = 1528; k = 11) and 17.8% (I2 = 98.6%; n = 1053; k = 10) respectively with a negligible change of the I2 heterogeneity from 98.7% to 98.3%, 99.5%–99.4% and 99.4%–98.6%. Using the trim-and-fill method to adjust for the effect estimate for the presence of asymmetry in the funnel plot, this method added six imputed missing studies to the meta-analysis for the Western Africa, six imputed missing studies to the meta-analysis for the Southern Africa and five imputed missing studies to the meta-analysis for the Eastern Africa, leading to an adjusted random effects prevalence of 6.5% (I2 = 98.6%; k = 20 (with 6 added studies)), 1.0% (I2 = 99.5%; k = 17 (with 6 added studies)) and 7.5% (I2 = 99.0%; k = 15 (with 5 added studies)) with a negligible growth of the I2 heterogeneity from 98.3% to 98.6%, 99.4%–99.5% and 98.6%–99.0% respectively. However, no outlier was found within the Central Africa studies.
Prevalence of different HAI in SSA
Outlying studies adding to heterogeneity of BSI,45 UTI,50 SSI,21 and LRTI51 were identified. After excluding them from the meta-analysis, the prevalence of BSI, UTI, SSI and LRTI in HAI stood at 32.1% (I2 = 99.4%; n = 1236; k = 19), 29.2% (I2 = 94.5%; n = 685; k = 18), 38.8% (I2 = 96.4%; n = 576; k = 17), and 23.3% (I2 = 97.4%; n = 243; k = 16) with a negligible change of the I2 heterogeneity from 99.7% to 99.4%, 94.7%–94.5%, 98.1%–96.4% and 97.7%–97.4% respectively. Using the trim-and-fill method to adjust for the effect estimate for the presence of asymmetry in the funnel plot, this method added eight imputed missing studies to the meta-analysis for the BSI, eight imputed missing studies to the meta-analysis for the UTI, six imputed missing studies to the meta-analysis for the SSI and seven imputed missing studies to the meta-analysis for the LRTI leading to an adjusted random effects prevalence of 63.3% (I2 = 99.6%; k = 27 (with 8 added studies)), 44.6% I2 = 96.7%; k = 26 (with 8 added studies)), 19.3% (I2 = 97.4%; k = 23 (with 6 added studies)) and 4.1% (I2 = 98.4%; k = 23 (with 7 added studies)) with a slight growth of the I2 heterogeneity from 99.4% to 99.6%, 94.5% to 96.7%, 96.4% to 97.4% and 97.4% to 98.4% respectively.
Prevalence of HAI among neonates in sub-Saharan Africa
The study of Reddy et al. (2021) was detected as an outlier which influenced the heterogeneity of the meta-analysis. On exclusion of the said study from the meta-analysis, the prevalence of HAI among neonates stood at 15.4% (I2 = 99.7%; n = 1300; k = 7) with a negligible shrinkage of the I2 heterogeneity from 99.8% to 99.7%. However, using the trim-and-fill method to adjust for the effect estimate for the presence of asymmetry in the funnel plot, no imputed missing studies were added to the meta-analysis.
Risk factors for HAI
Meta-analysis could not be conducted for risk factors as there was no uniformity in the reporting of same in the original studies. Among neonates, preterm delivery28,30,45 and indwelling medical devices28,30,33 as risk factors were supported by three studies (9%) each. Other influencing risk factors for HAI among neonates included low birth weight,28,30 antibiotic usage,30 multiple medical conditions,30 overcrowding,57 birth asphyxia,30 central lines,30 mechanical ventilation,30 and maternal conditions–multiple delivery,30 Wassermann's reaction,30 and prolonged rupture of membrane.30 Risk factors predisposing to HAI in children included indwelling medical device,16,38,48,54 prolonged hospitalization,53,54 and multiple medical conditions.38,53 Use of antibiotics,38,48 blood transfusion48 and age less than 12 years48 were also reported. Associated risk factors for HAI in adults were indwelling device,18,20,24,26,29,42,44,55,56 prolonged hospital stay,22,24,26,34,42,47 surgery,29,38,42,46,47,55,56 antibiotic use,47,55,56 pre-existing medical conditions,22,26,27,29,43 blood transfusion34,46 and male sex.21
Discussion
This review reveals a high burden of HAI in SSA with significant subregional variations. The overall prevalence (12.9%, 95% CI 8.9–17.4) of HAI in SSA is high compared to the prevalence rate of 3.2% in the United States,5 6.5% in Europe,58 and 9.0% in South East Asia.59 Prior systematic reviews of HAI in African countries, which included North Africa, reported a similar overall prevalence rate of 12.8%, with rates estimated between 2.5% and 14.8%, but this study adds important elements by revealing a high HAI-associated rate of mortality and a disproportionate burden of HAI in West and East Africa.6,10
Our results indicate that studies contributing to HAI prevalence in SSA have largely focused on the paediatric population which encompassed close to 60% of the study population. Although adult studies were included in our analysis, sample sizes were relatively small compared to paediatric studies included. Children are a particularly vulnerable population at increased risk for HAI due to extended hospital stays and frequent comorbid severe malnutrition.41 Child malnutrition is particularly high within countries in East and West Africa.60 HAI exert a considerable burden on the SSA health care system and pose a major threat to patient safety. Africa is home to more than half of the world's extremely poor and has a rapidly growing population.61 Globally, healthcare associated expenditure in low-income countries averaged US$ 41 per person in 2017, compared with US$ 2937 in high-income countries, a more than 70-fold difference.62 The high prevalence of HAI in African countries, including SSA, is likely attributable to several factors including weak infection prevention and control programs, overcrowding in hospitals and deficiencies in both hand hygiene and infrastructure.63, 64, 65 Other important factors include poverty66 and a higher rate of maternal and child malnutrition predisposing to infection.67 In addition, the region has the highest rate of HIV infection in the world accounting for more than 70% of global infections68 and this—as well as other immunocompromising states such as malnutrition—may contribute to a heightened risk of infection acquisition.69 Finally, poor access to clean water and sanitation is common and has been strongly linked to poorer hospital outcomes.70 The combination of poor healthcare expenditure, a high burden of disease and low numbers of doctors and nurses in SSA (0.18 doctors per 1000 population versus 1.34 in the world)71 creates a healthcare environment where best practices related to infection prevention and control have been difficult to achieve.
The prevalence of HAI varied across the SSA subregions. Numerically, we found the highest prevalence in East Africa at 19.7% (95% CI 10.8–30.5), followed by West Africa at 15.5% (95% CI 8.3–24.4). Lower rates were observed in Central Africa at 10.3% (95% CI 1.1–27.0) and in Southern Africa at 6.5% (95% CI 3.3–10.7). According the World Health Organization, the lowest government healthcare spending is in Central, East and West Africa62 where most countries are also classified as low-income countries.72
The prevalence of HAI among ICU admissions was high at 25.0% (95% CI 11.0–42.2), whereas in neonatal and paediatric admissions, lower prevalence rates of 13.2% (95% CI 3.9–26.6) and 9.5% (95% CI 3.9–17.1), respectively were seen. Similar findings have been obtained in a systematic review conducted in African countries6 and a prevalence of 20.6% was previously reported in a large European study.73 The lower prevalence in the paediatric setting may reflect an inclusion bias since 91.8% of paediatric patients included were from the Southern African region where the overall HAI rate was lower. The higher HAI prevalence among the ICU population is likely due to heightened risk for infection due to increased severity of illness, increased age, comorbidity, and the frequent necessity for invasive devices as well as longer duration of stay.74 The use of specific bundles pertaining to the care of invasive devices such as intravascular catheters, urinary catheters and endotracheal intubation has been shown to significantly reduce infection rates and should be promoted in healthcare settings along with education around surveillance, audit and hand hygiene.75 In a large Zambian neonatal ICU study, a low cost infection prevention and control bundle focusing on hand hygiene, education and the availability of locally produced hand sanitizing alcohol solution reduced the nosocomial infection rate by approximately 50%.35
The most common type of HAI in this study was SSI, followed by BSI, a finding that corroborates with previous prevalence surveys36,76 and a systematic review.9 These findings differ from the epidemiology of HAI in higher income countries where respiratory tract infections are the most common type of infection seen.73,75 This may relate to differences in patient profile including the lower number of patients requiring, or offered, ventilatory support in included studies, the high prevalence of HIV infection in SSA, poor availability of specialized wound dressings and local challenges with wound management. Surgical site infections is usually the case following child birth or caesarean section which accounts for 80% of the surgical operations carried out in SSA.77
In this review, a high mortality rate of 22.2% (95% CI: 14.2–31.4; n = 1118; k = 9). was observed in those with HAI. This was higher than that reported in a mini review, which reported global rates between 2.3% and 14.4%.78 This high mortality rate also speaks to the vital importance of reporting on and reducing HAI in this region where the confluence of (often difficult to treat) HAI and increased patient vulnerability makes hospitalization itself a risky event for African patients. However, it also suggests a vital opportunity—with deliberate and sustained efforts to reduce HAI—to reduce morbidity and mortality with the interventions outlined above.
Our review identified preterm birth as a major risk factor for HAI among neonates. In South Africa, preterm births and low-birth weight are disproportionally affected by HAI and HAI-related deaths.79,80 While the sequence of events predisposing preterm babies to HAI is multifactorial, the impact of interrupting the developing immune system that starts in utero has strongly been emphasized.81 Similarly, poorly developed mucosal (respiratory and gastrointestinal) barriers in preterm babies further increase neonatal exposures to microbial invasion by infective organisms. Overcrowding was also identified as a neonatal risk factor for HAI, and this may be related to the huge demographic shift from home-based to hospital-based deliveries in the last two decades in SSA, without the concomitant expansion of infrastructure or investment in infection prevention and control (IPC) to cope with it.82 For both paediatric and adult population groups, we found length of hospital stay an important risk factor for HAI. This finding, which is consistent with other studies,83, 84, 85, 86 may be related to patients having multiple conditions warranting increased use of invasive interventions, and in turn increases their risks of contracting HAI.87
This review had several important strengths. First, it was a comprehensive meta-analysis that included key databases and conference abstracts. Second, our search did not apply any language restrictions which enabled us to include studies reported in languages other than English. Third, a large and experienced team of researchers were involved in the screening and data extraction processes with discrepancies resolved through consensus. Likewise, this review had several limitations. First, there is a limited availability of studies on the prevalence of HAI in SSA. For example, although Southern Africa recorded a lower HAI prevalence, all the included studies in this subregion were from South Africa and Botswana, both of which are upper-middle income countries.88 This is likely at least in part due to the unavailability of adequate microbiology facilities in many parts of SSA.89 Secondly, data were not available for most SSA countries. However, by analyzing along subregional lines, we were able to reduce the impact of this limitation. A further limitation was our inability to conduct a meta-analysis on the risk factors for HAI. We were, however, able to list the studies in which the risk factors were identified. Significant heterogeneity of the studies made pooling of findings difficult. The presence of small study effects and publication bias may have skewed some of our results in the overall prevalence analyses as well as for mortality, subregional differences, and paediatric admission. Finally, in our meta-analysis, asymmetry in funnel plots were detected. Since funnel plots seek to display the relationship between study effect size and its precision, the asymmetry may indicate a publication bias as small studies with non-significant or favorable results may have been left unpublished in favour of larger studies.90 To validate our finding, we have in addition, used the rank's correlation test,91 Egger's regression,92 and Trim-and-Fill method93,94 to quantify the detected asymmetry. From the rank correlation tests performed, the presence of a significant relationship between sample size and effect size provided evidence for asymmetry in the funnel plot and suggested the possibility of publication bias. However, a non-significant test results should not be taken as evidence of a lack of publication bias when the meta-analysis involves small study sizes, as is the case in this study due to the limited numbers of HAI research conducted in most of SSA. With the detection of asymmetry in funnel plots, we used the Trim-and-Fill method to conduct sensitivity analysis to adjust for the effect estimate for this bias. Heterogeneity results obtained after applying the trim-and-fill method to the meta-analysis were slightly higher (although negligible) compared to those obtained before applying the method. However, there are no guarantees that the adjusted random effects obtained will match what would have been observed in the absence of publication bias. In addition, this method does not consider reasons for the presence of funnel plot asymmetry. Therefore, any conclusions regarding the presence of publication bias based on these methods should be drawn with caution.
There is a high overall burden of HAI in SSA which is associated with a substantial mortality rate. The most common HAI reported is surgical site infection although significant regional variation was noted. There is an urgent need to improve infection prevention and control, and to improve patient safety, and our results suggest that there is a particular need to target these efforts on West Africa and East Africa. This will require political will,95 improved human resource and data collection, adequate healthcare budget allocation and training, and capacity building for healthcare workers96 in SSA. This study further revealed an overall paucity of adult data in SSA which may suggest that the current (high) estimates are still unrepresentative of the true burden of HAI in the region and this need to be urgently addressed.
Contributors
Conceptualization: HM, RF, EVM, PEM; Study design: HM, OO, PEM.
Literature search: HM; Quality appraisal & Risk of bias assessment: HM, OO.
Data extraction: HM, CC, WTB; Data verification: EVM, RAM, WTB, RF, PEM, MOA.
Formal analysis: OO; HM; Investigation: HM, RF, EVM, CC, OO, MOA, PE, SO, RAM, WTB, PEM.
Writing—original draft: HM, OO; RF, WTB Writing—review and editing: HM, RF, EVM, CC, OO, MOA, PE, SO, RAM, WTB, PEM.
All authors accessed and verified the data and are responsible for decision to submit the manuscript.
Data sharing statement
All data used for the study has been included in the manuscript and supplementary material.
Declaration of interests
The authors declare no competing interests.
Acknowledgements
The authors acknowledge Patrick Sibulawa and Nompumelelo Mayi of the Medical Library, Port Elizabeth Provincial Hospital for library support services.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102571.
Appendix A. Supplementary data
References
- 1.Liu X., Long Y., Greenhalgh C., et al. A systematic review and meta-analysis of risk factors associated with healthcare-associated infections among hospitalized patients in Chinese general hospitals from 2001 to 2022. J Hosp Infect. 2023;135:37–49. doi: 10.1016/j.jhin.2023.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Alrebish S.A., Yusufoglu H.S., Alotibi R.F., Abdulkhal ik N.S., Ahmed N.J., Khan A H, et al. Epidemiology of healthcare-associated infections and adherence to the HAI prevention strategies. Healthcare. 2023;11:1–9. doi: 10.3390/healthcare11010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eggers R. Overview of the IPC situation worldwide: Highlights of achievements and gaps. World Health Organization; 2022. Infection prevention and control; pp. 3–54. [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Healthcare-associated infections (HAIs) 2018. https://www.cdc.gov/hai/data/index.html#:∼:text=Onanygivenday%2Cabout,targetareasthatneedassistance
- 5.Magill S.S., Edwards J.R., Bamberg W, et al. Changes in prevalence of health care–associated infections in U.S. Hospitals. N Engl J Med. 2018;379:1732–1744. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abubakar U., Amir O., Rodríguez-Baño J. Healthcare-associated infections in Africa: a systematic review and meta-analysis of point prevalence studies. J Pharm Policy Pract. 2022;15:1–16. doi: 10.1186/s40545-022-00500-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lloyd L.G., Bekker A., Van Weissenbruch M.M., Dramowski A. Healthcare-associated infections in very low birth-weight infants in a South African neonatal unit: disease burden, associated factors and short-term outcomes. Pediatr Infect Dis J. 2022;41:911–916. doi: 10.1097/INF.0000000000003666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahomed S., Mahomed O., Sturm A.W., Knight S., Moodley P. Challenges with Surveillance of Healthcare-Associated Infections in Intensive Care Units in South Africa. Crit Care Res Pract. 2017 doi: 10.1155/2017/7296317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allegranzi B., Nejad S.B., Combescure C, et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet. 2011;377:228–241. doi: 10.1016/S0140-6736(10)61458-4. [DOI] [PubMed] [Google Scholar]
- 10.Nejad S.B., Allegranzi B., Syed S.B., Ellisc B., Pittetd D. Systematic reviews ystematic reviews Health-care-associated infection in Africa: a systematic review. Bull World Health Organ. 2011;89:757–765. doi: 10.2471/BLT.11.088179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The prisma 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paez A. Gray literature: an important resource in systematic reviews. J Evid Base Med. 2017;10:233–240. doi: 10.1111/jebm.12266. [DOI] [PubMed] [Google Scholar]
- 13.Munn Z., Moola S., Lisy K., Riitano D., Tufanaru C. JBI; 2021. JBI Manual of evidence synthesis. [DOI] [Google Scholar]
- 14.United Nations . 2023. Standard country or area codes for statistical use (M49). Statistics Division; pp. 1–25.https://unstats.un.org/unsd/methodology/m49/overview/ [Google Scholar]
- 15.Chen Y., Chen D., Wang Y., Han Y. Using freeman-Tukey double arcsine transformation in meta-analysis of single proportions. Aesthetic Plast Surg. 2023;47:83–84. doi: 10.1007/s00266-022-02977-6. [DOI] [PubMed] [Google Scholar]
- 16.Hlophe S.T., Mc-Kerrow N.H. Hospital-acquired Klebsiella pneumoniae infections in a paediatric intensive care unit. SAJCH South African J. Child Heal. 2014;8:125–128. [Google Scholar]
- 17.Dalhatu A., et al. Bacterial agents of abdominal surgical site infections in general hospital Funtua, Katsina State, North-Western Nigeria. J Dent Med Sci. 2014;13:48–52. [Google Scholar]
- 18.Nouetchognou J.S., Ateudjieu J., Jemea B., Mesumbe E.N., Mbanya D. Surveillance of nosocomial infections in the Yaounde university Teaching Hospital, Cameroon. BMC Res Notes. 2016;9:1–8. doi: 10.1186/s13104-016-2310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lochan H., Pillay V., Bamford C., Nuttall J., Eley B. Bloodstream infections at a tertiary level paediatric hospital in South Africa. BMC Infect Dis. 2017;17:1–9. doi: 10.1186/s12879-017-2862-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bayingana C., Gahutu J.B., Sendegeya A, et al. Prevalence of bacterial species involved in nosocomial infections in pediatrics unit at Butare University Teaching Hospital (Chub) Int J Curr Microbiol Appl Sci. 2017;6:3227–3232. doi: 10.20546/ijcmas.2017.606.380. [DOI] [Google Scholar]
- 21.Tabiri S., Yenli E., Kyere M., Anyomih T.T.K. Surgical site infections in emergency abdominal surgery at Tamale Teaching Hospital, Ghana. World J Surg. 2017;42:916–922. doi: 10.1007/s00268-017-4241-y. [DOI] [PubMed] [Google Scholar]
- 22.Olowo-okere A., Ibrahim Y., Sani A., Atata R., Olayinka B. Prevalence of surgical site infection in a Nigerian University Teaching Hospital. J Pharm Allied Sci. 2017;14:2430–2438. [Google Scholar]
- 23.Olivier C., Kunneke H., O’Connell N., von Delft E, Wates M, Dramowski MA. Healthcare-associated infections in paediatric and neonatal wards: a point prevalence survey at four South African hospitals. South African Med. J. 2018;108:418–422. doi: 10.7196/SAMJ.2018.v108i5.12862. [DOI] [PubMed] [Google Scholar]
- 24.Sattar F.A.A., Quadros D.R.S., Olang P., Chokwe T. Incidence of ventilator-associated pneumonia in the critical care unit at Kenyatta National Hospital, a public tertiary care hospital. East Afr Med J. 2018;95:1613–1623. [Google Scholar]
- 25.Iliyasu G., Dayyab F.M., Abubakar S, et al. Laboratory-confirmed hospital-acquired infections: an analysis of a hospital’s surveillance data in Nigeria. Heliyon. 2018;4 doi: 10.1016/j.heliyon.2018.e00720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ali S., Birhane M., Bekele S, et al. Healthcare associated infection and its risk factors among patients admitted to a tertiary hospital in Ethiopia: longitudinal study. Antimicrob Resist Infect Control. 2018;7:1–9. doi: 10.1186/s13756-017-0298-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dayyab F.M., Iliyasu G, Aminu A, et al. A prospective study of hospital-acquired infections among adults in a tertiary hospital in north-western Nigeria. Trans R Soc Trop Med Hyg. 2018;112:36–42. doi: 10.1093/trstmh/try020. [DOI] [PubMed] [Google Scholar]
- 28.Osinupebi O., Ogunlesi T., Fetuga M. Pattern of nosocomial infections in the special care baby unit of the Olabisi Onabanjo University Teaching Hospital, Sagamu, Nigeria. Niger J Paediatr. 2014;41:54–58. [Google Scholar]
- 29.Nair A., Steinberg W.J., Habib T., Saeed H., Raubenheimer J.E. Prevalence of healthcare-associated infection at a tertiary hospital in the Northern Cape Province, South Africa. S Afr Fam Pract. 2018;60:162–167. [Google Scholar]
- 30.Rameshwarnath S., Naidoo S. Risk factors associated with nosocomial infections in the neonatal intensive care unit at Mahatma Gandhi Memorial hospital between 2014 and 2015. S Afr J Infect Dis. 2018;33:93–100. [Google Scholar]
- 31.Tolera M., Abate D., Dheresa M., Marami D. Bacterial nosocomial infections and antimicrobial susceptibility pattern among patients admitted at hiwot fana specialized University Hospital, Eastern Ethiopia. Adv Met Med. 2018:1–7. doi: 10.1155/2018/2127814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Labi A.K., Obeng-Nkrumah N., Owusu E, et al. Multi-centre point-prevalence survey of hospital-acquired infections in Ghana. J Hosp Infect. 2019;101:60–68. doi: 10.1016/j.jhin.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 33.Mpinda-Joseph P., Paramadhas B.D.A., Reyes G, et al. Healthcare-associated infections including neonatal bloodstream infections in a leading tertiary hospital in Botswana. Hosp Pract. 2019;47:203–210. doi: 10.1080/21548331.2019.1650608. [DOI] [PubMed] [Google Scholar]
- 34.Mukagendaneza M.J., Munyaneza E., Muhawenayo E, et al. Incidence, root causes, and outcomes of surgical site infections in a tertiary care hospital in Rwanda: a prospective observational cohort study. Patient Saf Surg. 2019;13:4–11. doi: 10.1186/s13037-019-0190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mwananyanda L., Pierre C., Mwansa J, et al. Preventing bloodstream infections and death in Zambian neonates: impact of a low-cost infection control bundle. Clin Infect Dis. 2019;69:1360–1367. doi: 10.1093/cid/ciy1114. [DOI] [PubMed] [Google Scholar]
- 36.Abubakar U. Point-prevalence survey of hospital acquired infections in three acute care hospitals in Northern Nigeria. Antimicrob Resist Infect Control. 2020;9:1–7. doi: 10.1186/s13756-020-00722-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akinkugbe O., Cooke F.J., Pathan N. Healthcare-Associated bacterial infections in the paediatric ICU. JAC Antimicrob Resist. 2020;2:1–7. doi: 10.1093/jacamr/dlaa066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Makhwanya Tshimangadzo Mildred . 2020. Prevalence of nosocomial infection in paediatric intensive care unit at Pietersburg Hospital in Limpopo. South Africa. [Google Scholar]
- 39.Scherbaum M., Kösters K, Mürbeth RE, et al. Incidence, pathogens and resistance patterns of nosocomial infections at a rural hospital in Gabon. BMC Infect Dis. 2014;14:13–15. doi: 10.1186/1471-2334-14-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dell A.J., Navsaria P.H., Gray S., Kloppers J.C. Nosocomial infections: a further assault on patients in a high-volume urban trauma centre in South Africa. South African Med. J. 2020;110:123–125. doi: 10.7196/SAMJ.2020.v110i2.14243. [DOI] [PubMed] [Google Scholar]
- 41.Sahiledengle B., Seyoum F, Abebe D, et al. Incidence and risk factors for hospital-acquired infection among paediatric patients in a teaching hospital: a prospective study in southeast Ethiopia. BMJ Open. 2020;10:1–10. doi: 10.1136/bmjopen-2020-037997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bunduki G.K., Feasey N., Henrion M.Y.R., Noah P., Musaya J. Healthcare-associated infections and antimicrobial use in surgical wards of a large urban central hospital in Blantyre, Malawi: a point prevalence survey. Infect Prev Pract. 2021;3 doi: 10.1016/j.infpip.2021.100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Odiase F., Lofor P. Pathogens and antimicrobial resistance amongst stroke patients in the intensive care unit: a five years review from Benin City, Nigeria. Ann Clin Biomed Res. 2021;2:53–58. [Google Scholar]
- 44.Nuckchady D.C. Incidence, risk factors, and mortality from hospital-acquired infections at a hospital in Mauritius. Cureus. 2021;13 doi: 10.7759/cureus.19962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reddy K., Bekker A., Whitelaw A.C., Esterhuizen T.M., Dramowski A. A retrospective analysis of pathogen profile, antimicrobial resistance and mortality in neonatal hospital-acquired bloodstream infections from 2009–2018 at Tygerberg Hospital, South Africa. PLoS One. 2021;16:1–14. doi: 10.1371/journal.pone.0245089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rokhaya D., Codou N.N., Mbar W.T.M., Aminata S. Surgical site infections in general surgery at the regional hospital of Thies (Snegal): epidemiological, bacterial aspects and risk factors. Microbiol Infect Dis. 2021;5:5–10. doi: 10.33425/2639-9458.1117. [DOI] [Google Scholar]
- 47.Adeyanju A., Schaumburg F., Onayade A., et al. Local epidemiology of nosocomial Staphylococcus aureus infection in a Nigerian University Teaching Hospital. Antibiotics. 2022;11 doi: 10.3390/antibiotics11101372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patil R.K., Kabera B., Muia C.K., Ale B.M. Hospital acquired infections in a private paediatric hospital in Kenya: a retrospective cross-sectional study. Pan Afr Med J. 2022;41 doi: 10.11604/pamj.2022.41.28.25820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aminata M., Alioune B.S., Yacouba C., et al. Vol. 15. University Teaching Hospital of Point-G; Bamako, Mali: 2023. pp. 1–7. (Multidrug resistant bacteria isolated from nosocomial infections at). [Google Scholar]
- 50.Ahoyo T.A., Bankolé H.S., Adéoti F.M., et al. Prevalence of nosocomial infections and anti-infective therapy in Benin: results of the first nationwide survey in 2012. Antimicrob Resist Infect Control. 2014;3:2–7. doi: 10.1186/2047-2994-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Genaneh W., Sibhat M., Techane T., Legesse T.G. Health care-associated infections and associated factors among adult patients admitted to intensive care units of selected public hospitals, Addis Ababa, Ethiopia. Int J Afr Nurs Sci. 2023;18 [Google Scholar]
- 52.Buchner A., Du Plessis N.M., Reynders DT, et al. Nosocomial outbreak of hepatitis B virus infection in a pediatric hematology and oncology unit in South Africa: epidemiological investigation and measures to prevent. Pediatr Blood Cancer. 2015;62:1914–1919. doi: 10.1002/pbc.25605. [DOI] [PubMed] [Google Scholar]
- 53.Dramowski A., Cotton M.F., Rabie H., Whitelaw A. Trends in paediatric bloodstream infections at a South African referral hospital. BMC Pediatr. 2015;15:1–11. doi: 10.1186/s12887-015-0354-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dramowski A., Whitelaw A., Cotton M. Burden, spectrum, and impact of healthcare-associated infection at a South African children's hospital. J Hosp Infect. 2016;94:364–372. doi: 10.1016/j.jhin.2016.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iwuafor A.A., Ogunsola F.T., Oladele R.O., et al. Incidence, clinical outcome and risk factors of intensive care unit infections in the lagos university teaching hospital (LUTH), Lagos, Nigeria. PLoS One. 2016;11:1–15. doi: 10.1371/journal.pone.0165242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kakupa D.K., Muenze P.K., Byl B., Wilmet M.D. Etude de la prévalence des infections nosocomiales et des facteurs associes dans les deux hopitaux universitaires de Lubumbashi, République Démocratique du Congo: Cas des Cliniques Universitaires de Lubumbashi et l’Hôpital Janson Sendwe. Pan Afr Med J. 2016;24:1–6. doi: 10.11604/pamj.2016.24.275.7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fischer D., Schlößer R.L., Kempf VAJ, et al. Overcrowding in a neonatal intermediate care unit : impact on the incidence of organisms. BMC Infect Dis. 2019;19:357. doi: 10.1186/s12879-019-3981-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suetens C., Latour K., Kärk T, et al. Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: results from two European point prevalence surveys, 2016 to 2017. Euro Surveill. 2018;23:1–18. doi: 10.2807/1560-7917.ES.2018.23.46.1800516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ling M.L., Apisarnthanarak A., Madriaga G. The burden of healthcare-associated infections in southeast Asia: a systematic literature review and meta-analysis. Clin Infect Dis. 2015;60:1690–1699. doi: 10.1093/cid/civ095. [DOI] [PubMed] [Google Scholar]
- 60.Akombi B., Agho K., Merom D., Renzaho A., Hall J. Child malnutrition in sub-Saharan Africa: a meta-analysis of demographic and health surveys (2006-2016) PLoS One. 2017;12:35–63. doi: 10.1371/journal.pone.0177338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baloch M.A., Danish Khan S.U.D., Ulucak Z.Ş., Ahmad A. Analyzing the relationship between poverty, income inequality, and CO2 emission in Sub-Saharan African countries. Sci Total Environ. 2020;740 doi: 10.1016/j.scitotenv.2020.139867. [DOI] [PubMed] [Google Scholar]
- 62.World Health Organization (WHO) World Health Organizationation; 2019. Global spending on health: a world transition. [Google Scholar]
- 63.Ataiyero Y., Dyson J., Graham M. Barriers to hand hygiene practices among health care workers in sub-Saharan African countries: a narrative review. Am J Infect Control. 2019;47:565–573. doi: 10.1016/j.ajic.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 64.Holmen I.C., Seneza C., Nyiranzayisaba B, et al. Improving hand hygiene practices in a rural hospital in sub-Saharan Africa. Infect Control Hosp Epidemiol. 2016;37:834–839. doi: 10.1017/ice.2016.71. [DOI] [PubMed] [Google Scholar]
- 65.Rothe C., Schlaich C., Thompson S. Healthcare-associated infections in sub-Saharan Africa. J Hosp Infect. 2013;85:257–267. doi: 10.1016/j.jhin.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 66.Marmot M. Social determinants of health inequalities. Lancet. 2005;365(94):1099–1104. doi: 10.1016/S0140-6736(05)71146-6. [DOI] [PubMed] [Google Scholar]
- 67.Scott V., Schaay N., Schneider H., Sanders D. Addressing social determinants of health in South Africa: the journey continues. SAHR 20th Ed. 2017;86:5–6. [Google Scholar]
- 68.Kharsany A.B.M., Karim Q.A. HIV infection and AIDS in sub-Saharan Africa: current status, challenges and opportunities. Open AIDS J. 2016;10:34–48. doi: 10.2174/1874613601610010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bertagnolio S., Donati K.D.G., Tacconelli DE, et al. Hospital-acquired candidemia in HIV-infected patients, incidence, risk factors and predictors of outcome. J Chemother. 2004;16:172–178. doi: 10.1179/joc.2004.16.2.172. [DOI] [PubMed] [Google Scholar]
- 70.World Health Organization [WHO] World Heal. Organ.; 2020. Achieving quality health services for all, through better water, sanitation and hygiene: lessons from three African Countries Ethiopia, Ghana and Rwanda. [Google Scholar]
- 71.Crisp N., Chen L. Global supply of health professionals. N Engl J Med. 2014;370:950–957. doi: 10.1056/NEJMra1111610. [DOI] [PubMed] [Google Scholar]
- 72.Fantom N., Serajuddin U. World Bank’s Classif. Ctries. by Income; 2016. The world bank's classification of countries by income. [DOI] [Google Scholar]
- 73.Vincent J.L., Bihari D.J., Suter P.M., et al. The prevalence of nosocomial infection in intensive care units in Europe: results of the European prevalence of infection in intensive care (EPIC) study. JAMA. 1995;274:639–644. doi: 10.1001/jama.1995.03530080055041. [DOI] [PubMed] [Google Scholar]
- 74.Blot S., Ruppé E., Harbarth S., et al. Healthcare-associated infections in adult intensive care unit patients: changes in epidemiology, diagnosis, prevention and contributions of new technologies. Intensive Crit Care Nurs. 2022:70. doi: 10.1016/j.iccn.2022.103227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Edwardson S., Cairns C. Nosocomial infections in the ICU. Anaesth Intensive Care Med. 2019;20:14–18. [Google Scholar]
- 76.Loftus M.J., Curtis SJ, Naidu R., et al. Prevalence of healthcare-associated infections and antimicrobial use among inpatients in a tertiary hospital in Fiji: a point prevalence survey. Antimicrob Resist Infect Control. 2020;9:1–8. doi: 10.1186/s13756-020-00807-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Seale A.C., Mwaniki M., Newton C.R., Berkley J.A. Maternal and early onset neonatal bacterial sepsis: burden and strategies for prevention in sub-Saharan Africa. Lancet Infect Dis. 2009;9:428–438. doi: 10.1016/S1473-3099(09)70172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Al-Tawfiq J.A., Tambyah P.A. Healthcare associated infections (HAI) perspectives. J Infect Public Health. 2014;7:339–344. doi: 10.1016/j.jiph.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 79.Dramowski A., Velaphi S., Reubenson G., et al. National Neonatal Sepsis Task Force launch: supporting infection prevention and surveillance, outbreak investigation and antimicrobial stewardship in neonatal units in South Africa. South African Med J. 2020;110:360–363. doi: 10.7196/SAMJ.2020.v110i5.14564. [DOI] [PubMed] [Google Scholar]
- 80.Madhi S.A., Pathirana J., Baillie V., et al. Unraveling specific causes of neonatal mortality using minimally invasive tissue sampling: an observational study. Clin Infect Dis. 2019;69:S351–S360. doi: 10.1093/cid/ciz574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nilsen S.M., Valand J., Rogne T., et al. Gestational age at birth and hospitalisations for infections among individuals aged 0–50 years in Norway: a longitudinal, register-based, cohort study. eClinicalMedicine. 2023;62:102108. doi: 10.1016/j.eclinm.2023.102108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ndirima Z., Neuhann F., Beiersmann C. Listening to their voices: understanding rural women's perceptions of good delivery care at the Mibilizi District Hospital in Rwanda. BMC Wom Health. 2018;18:1–11. doi: 10.1186/s12905-018-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Silber J.H., Bellini L.M., Shea J A, et al. Patient safety outcomes under flexible and standard resident duty-hour rules. N Engl J Med. 2019;380:905–914. doi: 10.1056/NEJMoa1810642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ofori-Asenso R., Liew D., Mårtensson J., Jones D. The frequency of, and factors associated with prolonged hospitalization: a multicentre study in Victoria, Australia. J Clin Med. 2020;9:1–14. doi: 10.3390/jcm9093055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barnett A.G., Page K., Campbell M., et al. The increased risks of death and extra lengths of hospital and ICU stay from hospital-acquired bloodstream infections: a case-control study. BMJ Open. 2013;3:1–6. doi: 10.1136/bmjopen-2013-003587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khan H.A., Baig F.K., Mehboob R. Nosocomial infections: epidemiology, prevention, control and surveillance. Asian Pac J Trop Biomed. 2017;7:478–482. [Google Scholar]
- 87.Jeon C.Y., Neidell M., Jia H., Sinisi M., Larson E. On the role of length of stay in healthcare-associated bloodstream infection. Infect Control Hosp Epidemiol. 2012;33:1213–1218. doi: 10.1086/668422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.World Bank . World Bank Data; 2016. Countries and economies. [Google Scholar]
- 89.Amenyogbe E., Ayisi C.L., Droepenu EK, et al. Challenges and opportunities in advancing microbiology research in Africa: a review. African J Bacteriol Res. 2023;15:36–49. [Google Scholar]
- 90.Ioannidis J.P.A., Trikalinos T.A. The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. C Can Med Assoc J. 2007;176:1091–1096. doi: 10.1503/cmaj.060410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Forthofer R.N., Lee E.S., Hernandez M. 2nd ed. 2007. Biostatistics; pp. 21–69. [DOI] [Google Scholar]
- 92.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maier M., VanderWeele T.J., Mathur M.B. Using selection models to assess sensitivity to publication bias: a tutorial and call for more routine use. Campbell Syst Rev. 2022;18:1–7. doi: 10.1002/cl2.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mavridis D., Salanti G. How to assess publication bias: funnel plot, trim-and-fill method and selection models. Evid Base Ment Health. 2014;17:30. doi: 10.1136/eb-2013-101699. [DOI] [PubMed] [Google Scholar]
- 95.Thusi X., Matyana M., Jili N.N. Lack of political will: a barrier to public service delivery in South Africa and a high cost for citizens. J Stud Soc Sci Humanit. 2023;9:137–147. [Google Scholar]
- 96.Oleribe O.O., Momoh J., Uzochukwu B.S.C., et al. Identifying key challenges facing healthcare systems In Africa and potential solutions. Int J Gen Med. 2019:395–403. doi: 10.2147/IJGM.S223882. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.