Abstract
Solitary fibrous tumor (SFT) of the lung is a rare mesenchymal neoplasm of uncertain histogenesis, unknown molecular features, and unpredictable clinical behavior, characterized by NAB2-STAT6 fusion. Hypoglycemia accompanying SFT (Doege-Potter syndrome) is an uncommon presentation. We present the cytomorphological features on biopsy imprint smears of a histopathologically confirmed case of SFT of the lung with an uncommon presentation. A 76-year-old non-smoker, non-alcoholic, and non-diabetic man presented with complaints of intermittent episodes of confusion with syncopal attacks (>10 episodes) for six months. The patient had no respiratory complaints and no history of weight loss. Laboratory investigations revealed fasting blood sugar of 38 mg/dl with low serum insulin and C-peptide levels. Physical examination revealed reduced air entry on the left side of the chest. Chest X-ray showed left-sided homogenous opacity. High-resolution computed tomography (HRCT) of the chest showed a large left-sided lung mass. A biopsy was performed. Biopsy imprint smears were cellular and showed tumor cells arranged in clusters and fragments with traversing capillaries displaying monomorphic pump to oval nuclei, fine granular evenly dispersed chromatin, regular nuclear membrane, inconspicuous nucleoli, and a moderate amount of wispy cytoplasm. Foci of intercellular hyaline stromal material were noted. A cytodiagnosis of low-grade mesenchymal neoplasm was made. Histopathology revealed a cellular tumor comprising tightly packed round to fusiform cells arranged around blood vessels with intervening thick collagen, positive for CD99, vimentin, BCL2, CD34, and STAT6 and negative for EMA, CK AE1/AE3, S100, TLE1, and SMA. Familiarity with cytomorphology plays a pivotal role in clinching an early diagnosis of this rare neoplasm of the lung, particularly in the setting of presentation with hypoglycemia.
Keywords: paraneoplastic syndromes, hypoglycemia, cytomorphology, solitary fibrous tumor, mesenchymal neoplasm
Introduction
Solitary fibrous tumor (SFT) associated with non-islet cell tumor hypoglycemia (NICTH) is referred to as Doege-Potter syndrome [1,2]. It is a rare paraneoplastic syndrome presenting as hypoinsulinemic hypoglycemia from the ectopic production of incompletely processed form or prohormone of insulin-like growth factor 2 (IGF-2) by the SFT of the lung [3,4]. It is seen in about 4% of SFT cases. SFT of the lung is a rare mesenchymal neoplasm of uncertain histogenesis, unknown molecular features, and unpredictable clinical behavior. It is characterized by NAB2-STAT6 fusion [5]. We present the cytomorphological features on imprint smears of a histopathologically confirmed case of SFT of the lung with an uncommon presentation.
This article was previously presented as a poster at CYTOCON 2021, Delhi Chapter, held on November 19-21, 2021.
Case presentation
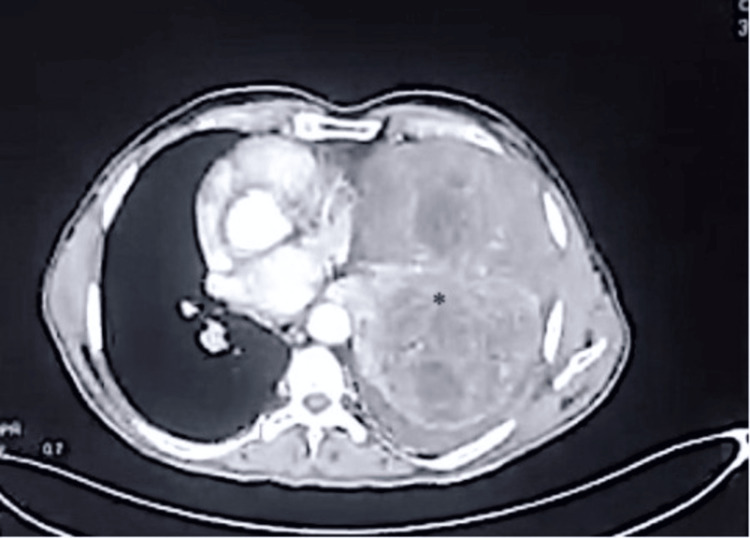
A 76-year-old man presented with intermittent episodes of confusion and syncopal attacks (>10 episodes) for six months. The patient was a non-smoker, a non-alcoholic, and a farmer by profession. There was no history of fever, cough, shortness of breath, hemoptysis, chest pain, weight loss, or loss of appetite. There was no known history of diabetes mellitus, hypertension, or oral hypoglycemic medication. Auscultation revealed decreased left-sided air entry. His cardiovascular, abdominal, and central nervous system examination was unremarkable. At the time of presentation, his fasting blood sugar level was 38 mg/dl, his serum insulin level was <1 µU/mL (normal range: 4-16 µU/mL), and his C-peptide level was 0.11 ng/mL (normal range: 1.07-3.51 ng/mL). Serum IGF-2 level was not performed in this case. Chest X-ray revealed a left-sided homogenous opacity. Contrast-enhanced computed tomography (CECT) of the thorax showed a heterogeneously enhancing mass lesion in the left lung parenchyma measuring 16x15.2x15.9 cm in its maximum dimension, almost completely replacing the left lung parenchyma. There was no calcification or air foci within the lesion (Figure 1).
Figure 1. Contrast-enhanced computed tomography of the thorax shows a heterogeneously enhancing mass lesion in the left lung parenchyma, almost entirely replacing the left lung parenchyma (highlighted by asterisk).
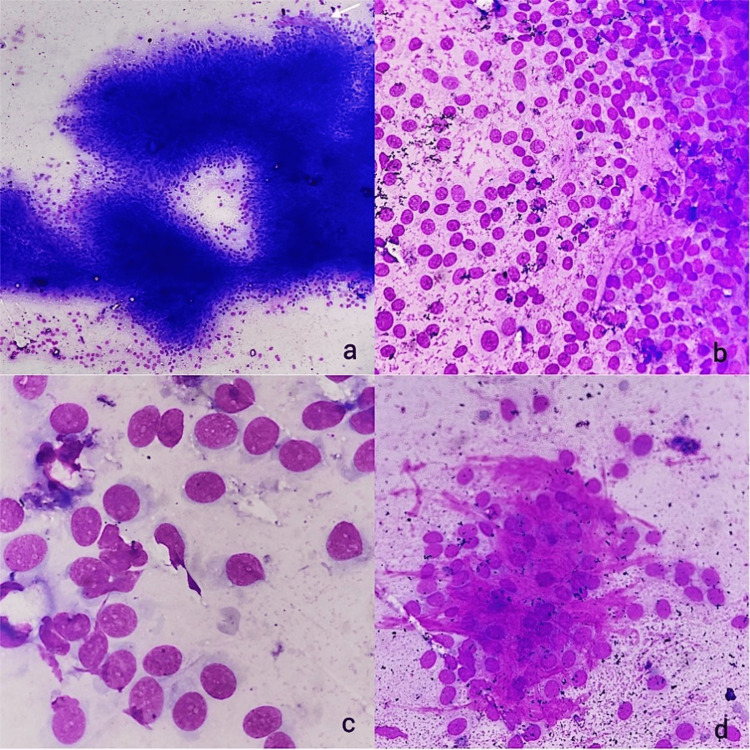
A biopsy was performed, and imprint smears were submitted for cytology. The imprint smears were moderately cellular and showed tumor cells with monomorphic plump to oval nuclei arranged in clusters and fragments with traversing capillaries. Tumor cells had monomorphic plump to oval nuclei, fine granular evenly dispersed chromatin, regular nuclear membrane, and inconspicuous nucleoli with a moderate amount of focally wispy cytoplasm. Single cells and a few stripped nuclei were seen being smeared out from the fragments. No mitosis or necrosis was seen on the cytosmears. Intercellular collagenous matrix was seen in many of the fragments (Figure 2). A cytodiagnosis of low-grade mesenchymal neoplasm was made.
Figure 2. (a) Imprint smears showing fragments of tumor cells with traversing capillaries (white arrow) (10× May-Grünwald Giemsa stain). (b) Single cells and a few stripped nuclei smeared out from the fragments (20× May-Grünwald Giemsa stain). (c) Tumor cells showing monomorphic plump to oval nuclei, fine granular evenly dispersed chromatin, regular nuclear membranes, and inconspicuous nucleoli with a moderate amount of cytoplasm (40× May-Grünwald Giemsa stain). (d) Intercellular ropy collagenous matrix (20× May-Grünwald Giemsa stain).
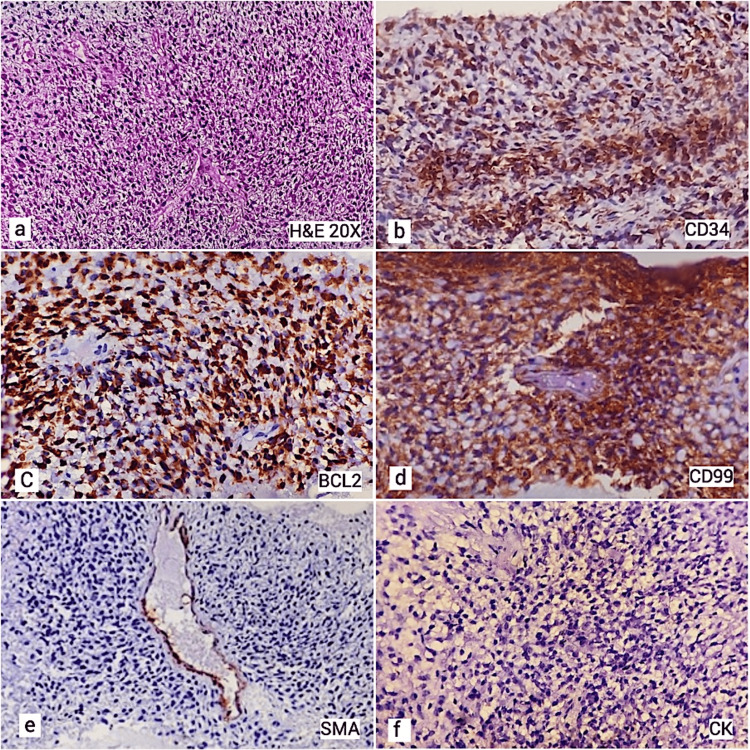
Biopsy showed a cellular tumor comprising round to fusiform cells, arranged around blood vessels with intervening thick ropy collagen. The cells displayed fusiform to spindled nuclei with mild pleomorphism. No mitosis or necrosis was seen. The tumor cells were positive for immunohistochemistry (IHC) for CD99, vimentin, BCL2, CD34, and STAT6 and negative for EMA, CK AE1/AE3, S100, TLE1, and SMA (Figure 3).
Figure 3. (a) Sections showing cellular tumor comprising of round to fusiform cells, arranged around blood vessels with intervening collagen (20× hematoxylin and eosin). (b) Immunohistochemistry showing positivity for CD34. (c) Immunohistochemistry showing positivity for BCL2. (d) Immunohistochemistry showing positivity for CD99. (e) Negative immunohistochemistry for SMA. (f) Negative immunohistochemistry for cytokeratin.
A diagnosis of SFT with NICTH (Doege-Potter syndrome) was made. During hospitalization, the patient's hypoglycemia was initially managed with 25 g of 50% intravenous dextrose. The patient continued to experience further episodes of spontaneous hypoglycemia requiring intravenous infusions of 10% dextrose. He was started on 60 mg of oral prednisone once daily. The patient was advised and counselled for surgery and further medical therapy, which the patient declined. He was discharged with an advice for regular glucose monitoring, caloric intake modification, and follow-up. The patient eventually succumbed to complications.
Discussion
SFT is a rare fibroblastic neoplasm. Initially thought to be originating from the pleural mesothelium, it is now proved to be of mesenchymal origin. It usually arises from the visceral pleura but may occur within the lung parenchyma, pericardium, mediastinum, meninges, deep soft tissues, and other extrapulmonary locations. Pleuropulmonary SFT cases may remain clinically asymptomatic or may present with chest pain, cough, and shortness of breath.
Patients with SFT may occasionally present with paraneoplastic syndrome, most commonly hypoglycemic episodes, which may be the only presenting symptom of SFT, as was seen in the present case. Doege-Potter syndrome is characterized by refractory NICTH. It is seen in less than 5% of SFT cases, is particularly associated with large tumors, and is caused by the tumor secretion of IGF-2 [3,4]. In contrast to hyperinsulinemic hypoglycemia, patients with NICTH have low serum insulin and C-peptide concentrations during hypoglycemia. Other biochemical findings include low beta-hydroxybutyrate, elevated IGF-2/IGF-1 ratio (>3), and negative oral hypoglycemic agent screening [6]. NICTH can be seen associated with various other benign or malignant tumors as well [6]. Initial management of hypoglycemia is oral glucose and/or intravenous glucose or dextrose-containing fluids. The mainstay of therapy for NICTH is surgical resection of the underlying tumor. Complete removal of the tumor is curative for hypoglycemia in most cases. Increased caloric intake and medical management with glucocorticoids, glucagon, or recombinant human growth hormone (rhGH) are modes of treatment in cases where the tumor cannot be resected [6].
Radiographically, SFT appears as a well-circumscribed, usually homogenous soft tissue mass, with homogenous or heterogenous contrast enhancement. Large tumors may show cystic areas, calcifications, myxoid degeneration, or hemorrhage [7].
Cytomorphological features of SFT on fine needle aspiration cytology (FNAC) have been described in several studies. In this report, we present the cytomorphological features of SFT on imprint smears. In the present case, we found cells with round to oval bland nuclei, delicate capillaries within tissue fragments, and ropy collagen as important cytological features of SFT. Similar features have been highlighted in other case reports and series, including low to high cellularity, spindled to oval cells in clusters and fragments, with bland uniform chromatin, scant cytoplasm, and the presence of collagenous matrix [5,8-12]. Several authors have reported the presence of inconspicuous nucleoli in some cases, as was seen in the present case [5,8]. Traversing capillaries, as observed in the present case, have been described as an uncommon occurrence in most studies, though a few have reported similar findings. The presence of capillaries provides an important clue to diagnosis, replicating the histological appearance of hemangiopericytomatous vasculature [5,9,10].
Malignant features of SFT have been described in a few studies. Ali et al. described hypercellular smears, single cells with nuclear pleomorphism, and prominent nucleoli as important clues to malignancy [7]. Cho et al. reported that malignant SFTs showed a greater number of cells in clusters, and displayed mitotic activity, without significant cytological atypia [13]. Okada et al. described highly atypical epithelioid cells and mitotic figures in malignant cases of SFT [14]. In another study, Bishop et al. reviewed and studied cytological features in 13 cases of malignant SFT and reported hypercellularity, occasional prominent nucleoli, lack of single cells, focal pleomorphism, occasional mitosis, and rare necrosis as salient characteristics in their cases [15].
Differential diagnoses on cytology, particularly in the lung, may include small cell carcinoma, carcinoid, synovial sarcoma, sarcomatoid mesothelioma, myoepithelioma, inflammatory myofibroblastic tumor, fibromatosis, and malignant peripheral nerve sheath tumor (MPNST) [16-20]. In addition to differentiating cytomorphological features, preparation of cell blocks, immunocytochemistry (ICC), and immunohistochemistry (IHC) can aid in diagnosis (Table 1) [16-20].
Table 1. Table comparing the cytomorphological features and useful markers for the differential diagnosis of SFT.
| Differential diagnosis | Cytomorphological differentiating features | Useful Immunocytochemistry/immunohistochemistry markers |
| Small cell carcinoma | Round to oval cells with high N:C ratio, hyperchromasia, finely dispersed chromatin, no distinct nucleoli, and minimal cytoplasm. Molding, crushing, high mitotic rate. | Synaptophysin, chromogranin, ki-67>30% |
| Carcinoid | Monomorphic small round/elongated or plasmacytoid tumor cells arranged in loose groups or singly dispersed, sometimes around branching capillaries, rosette-like structures. Smooth nuclear contour, with salt and pepper chromatin and a small nucleolus; no or rare mitoses. Absence of molding, necrosis, or nuclear crushing. | Synaptophysin, chromogranin, CK AE1/AE3 |
| Monophasic synovial sarcoma | Cellular and shows a pericapillary arrangement of oval to round cells along with the presence of mast cells. The cells are highly pleomorphic compared to SFT. | EMA/cytokeratin, TLE1 |
| SFT | Low to moderate cellularity. Oval to elongate, rounded, or stellate cells with wispy cytoplasm. Intercellular ropy collagenous matrix. | STAT 6 (nuclear positivity), CD34, Bcl2 |
| Sarcomatoid mesothelioma | Loosely cohesive sheets of obviously malignant spindle cells. Poorly cellular smears of atypical spindle cells with a few fragments of collagen strands. | D2-20, calretinin, CK AE1/AE3 |
| Inflammatory myofibroblastic tumor | Hypocellular smears with several small clusters of spindle cells with bland nuclei, small nucleoli, and occasional myxoid matrix. Prominent inflammatory component composed of lymphocytes and plasma cells. | ALK |
| Malignant peripheral nerve sheath tumor | Highly cellular, single as well as syncytial and three-dimensional clusters. | S100 |
| Fibromatosis | Bland spindle cells with elongated nuclei embedded in metachromatic matrix material. | SMA, beta-catenin (nuclear expression), cyclin D1 |
Conclusions
Doege-Potter syndrome is often incidentally diagnosed during the workup of hypoglycemia of uncertain etiology. Patients presenting with hypoglycemia without any prior history of diabetes mellitus should be evaluated for possible underlying neoplasm. Although the cytological diagnosis of an SFT is challenging, the presence of distinctive features like cells with round to oval bland nuclei, delicate capillaries within tissue fragments, and ropy collagen may provide clues to clinch the diagnosis in an appropriate clinical setting.
The authors have declared that no competing interests exist.
Author Contributions
Concept and design: Tanya Sharma, Mahadev Meena, Garima Goel, Abhishek Goyal
Acquisition, analysis, or interpretation of data: Tanya Sharma, Ingitha Pulikkal, Garima Goel, Abhishek Goyal
Drafting of the manuscript: Tanya Sharma, Ingitha Pulikkal, Mahadev Meena
Critical review of the manuscript for important intellectual content: Tanya Sharma, Garima Goel, Abhishek Goyal
Human Ethics
Consent was obtained or waived by all participants in this study
References
- 1.Fibro-sarcoma of the mediastinum. Doege KW. https://pubmed.ncbi.nlm.nih.gov/17866430/ Ann Surg. 1930;92:955–960. [PMC free article] [PubMed] [Google Scholar]
- 2.Hypoglycemia with neoplasia (Doege-Potter syndrome) Baldwin RS. https://pubmed.ncbi.nlm.nih.gov/14282806/ Wis Med J. 1965;64:185–189. [PubMed] [Google Scholar]
- 3.A case of solitary fibrous pleura tumor associated with severe hypoglycemia: Doege-Potter syndrome. Jang JG, Chung JH, Hong KS, et al. Tuberc Respir Dis (Seoul) 2015;78:120–124. doi: 10.4046/trd.2015.78.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doege-Potter syndrome: a review of the literature including a new case report. Han G, Zhang Z, Shen X, et al. Medicine (Baltimore) 2017;96:0. doi: 10.1097/MD.0000000000007417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cytopathology of solitary fibrous tumor: a series of 34 cases. Wakely PE Jr, Rekhi B. J Am Soc Cytopathol. 2021;10:382–390. doi: 10.1016/j.jasc.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Management of non-islet-cell tumor hypoglycemia: a clinical review. Bodnar TW, Acevedo MJ, Pietropaolo M. J Clin Endocrinol Metab. 2014;99:713–722. doi: 10.1210/jc.2013-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solitary fibrous tumor. A cytologic-histologic study with clinical, radiologic, and immunohistochemical correlations. Ali SZ, Hoon V, Hoda S, Heelan R, Zakowski MF. Cancer. 1997;81:116–121. doi: 10.1002/(sici)1097-0142(19970425)81:2<116::aid-cncr5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 8.Cytomorphological spectrum of solitary fibrous tumour: revisited. Shastri M, Gupta N, Dey P, Srinivasan R, Radotra B. Cytopathology. 2022;33:688–695. doi: 10.1111/cyt.13163. [DOI] [PubMed] [Google Scholar]
- 9.Solitary fibrous tumour: a diagnostic challenge for the cytopathologist. Gupta N, Barwad A, Katamuthu K, Rajwanshi A, Radotra BD, Nijhawan R, Dey P. Cytopathology. 2012;23:250–255. doi: 10.1111/j.1365-2303.2011.00880.x. [DOI] [PubMed] [Google Scholar]
- 10.Cytopathological features of solitary fibrous tumor of the pleura: a study of 5 cases. Weynand B, Collard P, Galant C. Diagn Cytopathol. 1998;18:118–124. doi: 10.1002/(sici)1097-0339(199802)18:2<118::aid-dc7>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 11.FNA cytology of solitary fibrous tumors and the diagnostic value of STAT6 immunocytochemistry. Tani E, Wejde J, Åström K, Wingmo IL, Larsson O, Haglund F. Cancer Cytopathol. 2018;126:36–43. doi: 10.1002/cncy.21923. [DOI] [PubMed] [Google Scholar]
- 12.Solitary fibrous tumor: a study of cytologic features of six cases diagnosed by fine-needle aspiration. Clayton AC, Salomão DR, Keeney GL, Nascimento AG. Diagn Cytopathol. 2001;25:172–176. doi: 10.1002/dc.2032. [DOI] [PubMed] [Google Scholar]
- 13.Fine needle aspiration cytology of solitary fibrous tumours of the pleura. Cho EY, Han JJ, Han J, Oh YL. Cytopathology. 2007;18:20–27. doi: 10.1111/j.1365-2303.2007.00378.x. [DOI] [PubMed] [Google Scholar]
- 14.Scratch cytologic findings on surgically resected solitary fibrous tumors of the pleura. Okada S, Ebihara Y, Kudo M, Serizawa H, Shimizu T, Otani M, Tsuji K. Acta Cytol. 2001;45:372–380. doi: 10.1159/000327633. [DOI] [PubMed] [Google Scholar]
- 15.Malignant solitary fibrous tumor: cytopathologic findings and differential diagnosis. Bishop JA, Rekhtman N, Chun J, Wakely PE Jr, Ali SZ. Cancer Cytopathol. 2010;118:83–89. doi: 10.1002/cncy.20069. [DOI] [PubMed] [Google Scholar]
- 16.Cytologic features of synovial sarcoma with emphasis on the monophasic fibrous variant: a morphologic and immunocytochemical analysis of bcl-2 protein expression. Viguer JM, Jiménez-Heffernan JA, Vicandi B, López-Ferrer P, Gamallo C. https://pubmed.ncbi.nlm.nih.gov/9500652/ Cancer. 1998;84:50–56. [PubMed] [Google Scholar]
- 17.The cytopathology of malignant peripheral nerve sheath tumor: a report of 55 fine-needle aspiration cases. Wakely PE Jr, Ali SZ, Bishop JA. Cancer Cytopathol. 2012;120:334–341. doi: 10.1002/cncy.21195. [DOI] [PubMed] [Google Scholar]
- 18.Cytology of fine-needle aspiration of inflammatory myofibroblastic tumor. Stoll LM, Li QK. Diagn Cytopathol. 2011;39:663–672. doi: 10.1002/dc.21444. [DOI] [PubMed] [Google Scholar]
- 19.Sarcomatoid mesothelioma: unusual findings and literature review. Clopton B, Long W, Santos M, Asarian A, Genato R, Xiao P. J Surg Case Rep. 2022;2022:0. doi: 10.1093/jscr/rjac512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deep fibromatosis (desmoid tumor): cytopathologic characteristics, clinicoradiologic features, and immunohistochemical findings on fine-needle aspiration. Owens CL, Sharma R, Ali SZ. Cancer. 2007;111:166–172. doi: 10.1002/cncr.22689. [DOI] [PubMed] [Google Scholar]