Abstract
We have previously demonstrated an increase in proapoptotic caspase-3 in the kidney of Han:SPRD rats with polycystic kidney disease (PKD). The aim of the present study was to determine the effect of caspase inhibition on tubular cell apoptosis and proliferation, cyst formation, and renal failure in the Han:SPRD rat model of PKD. Heterozygous (Cy/+) and littermate control (+/+) male rats were weaned at 3 weeks of age and then treated with the caspase inhibitor IDN-8050 (10 mg/kg per day) by means of an Alzet (Palo Alto, CA) minipump or vehicle [polyethylene glycol (PEG 300)] for 5 weeks. The two-kidney/total body weight ratio more than doubled in Cy/+ rats compared with +/+ rats. IDN-8050 significantly reduced the kidney enlargement by 44% and the cyst volume density by 29% in Cy/+ rats. Cy/+ rats with PKD have kidney failure as indicated by a significant increase in blood urea nitrogen. IDN-8050 significantly reduced the increase in blood urea nitrogen in the Cy/+ rats. The number of proliferating cell nuclear antigen-positive tubular cells and apoptotic tubular cells in non-cystic and cystic tubules was significantly reduced in IDN-8050-treated Cy/+ rats compared with vehicle-treated Cy/+ rats. On immunoblot, the active form of caspase-3 (20 kDa) was significantly decreased in IDN-8050-treated Cy/+ rats compared with vehicle-treated Cy/+ rats. In summary, in a rat model of PKD, caspase inhibition with IDN-8050 (i) decreases apoptosis and proliferation in cystic and noncystic tubules; (ii) inhibits renal enlargement and cystogenesis, and (iii) attenuates the loss of kidney function.
Keywords: caspase inhibitor, IDN-8050, caspase-3
Autosomal dominant polycystic kidney disease (ADPKD) is a systemic hereditary disorder transmitted in an autosomal-dominant manner. It occurs throughout the world, and the prevalence varies between 1 in 400 and 1 in 1,000. More than 600,000 Americans have the disease, making it one of the most common hereditary diseases in the United States. ADPKD is an important cause of end-stage renal failure (1).
The heterozygous Han:SPRD rat exhibits many of the features of ADPKD in humans (2), including autosomal dominant inheritance and relatively slow progression to end-stage renal failure with uremia, hypertension, and anemia. The Han:SPRD rat is a suitable and well documented animal model of polycystic kidney disease (PKD) even though the disease is not caused by a defect in the PKD1 or PKD2 genes (3–5) (6).
The precisely controlled balance between cellular proliferation and apoptosis is disturbed in polycystic kidneys (7). There is increased proliferation in both noncystic and cystic tubules in the Han:SPRD rat (6, 8). Based on our published data that both caspase activity and apoptosis are increased in Han:SPRD polycystic kidneys (9, 10), as well as the protective effect of caspase inhibition on apoptotic injury in other organs, we developed the hypothesis that caspase inhibition would reduce cyst formation and disease progression in PKD by means of inhibition of tubular cell apoptosis and proliferation. This hypothesis was tested in the present study.
Methods
Animals. The study was conducted in heterozygous (Cy/+) and normal littermate control (+/+) Han:SPRD rats. All of the normal rats and Cy/+ rats studied were males. The Cy/+ Han:SPRD rat develops clinically detectable PKD by 8 weeks of age as evidenced by a doubling of the two-kidney/total body weight ratio (2K/TBW) compared with +/+ rats (2, 4). A colony of Han:SPRD rats was established in our animal care facility from a litter that was obtained from the Polycystic Kidney Program at the University of Kansas Medical Center. The study protocol was approved by the University of Colorado Health Sciences Center Animal Care and Use Committee. Rats had free access to tap water and standard rat chow.
Experimental Protocol. Male Cy/+ and +/+ rats were weaned at 3 weeks of age and then treated with IDN-8050 (10 mg/kg per day) or vehicle [polyethylene glycol (PEG)-300] for 5 weeks. IDN-8050 or vehicle was delivered by an AlzetR (Palo Alto, CA) miniosmotic pump (model 2001) placed in the peritoneum and replaced weekly under light anesthesia. IDN-8050 was a kind gift from Idun Pharmaceuticals (San Diego, CA). At the end of the 8th week of age, rats were anesthetized by i.p. injection of pentobarbital sodium (50 mg/kg body weight), and kidneys were removed and weighed. The left kidney was fixed in 4% paraformaldehyde in PBS for 120 min and then put into 70% ethanol and embedded in paraffin for histological examinations. The right kidney was immediately frozen in liquid N2 and then stored at –80°C.
Cyst Volume Density. Hematoxylin/eosin-stained sections were used to determine the cyst volume density. This measurement was performed by a reviewer, blinded to the identity of the treatment modality, by using point counting stereology (11). Areas of the cortex at 90°, 180°, and 270° from the hilum of each section were selected to guard against field selection variation.
Proliferating Cell Nuclear Antigen (PCNA) Staining. Immunohistochemical detection of PCNA staining was performed by using an anti-PCNA antibody (sc-7907, Santa Cruz Biotechnology, 1:50). The sections were incubated with horseradish peroxidaselabeled polymer (DAKO EnVision System, Cat no. K1395), and the antigen site was visualized by incubating the sections with the substrate 3,3′-diaminobenzidine (DAB) chromogen. The tissues were counterstained with hematoxylin. Negative control sections showed no staining with DAB.
In Situ Detection of DNA Fragmentation. The TUNEL method was used to detect in situ DNA strand breaks. TACS 2 terminal deoxynucleotidyltransferase (TdT)-blue label in situ apoptosis detection kit (Trevigen, Gaithersburg, MD) was used. Paraffin blocks were sectioned at 4-μm thickness on slides. Paraffin sections were deparaffinized in xylene and rehydrated in graded ethanols and PBS. The tissue sections were incubated with proteinase K for 1 h at 37°C for permeabilization. Endogenous peroxidase activity was quenched by incubating the tissue sections with 3% hydrogen peroxide in methanol for 5 min. The sections were then treated with biotinylated nucleotide, manganese cation, and TdT enzyme for 1 h at 37°C. After treating with streptavidin-horseradish peroxidase for 3 min at room temperature, the sections were stained with blue label for 7 min. The tissues were counterstained with nuclear fast red.
Positive and negative controls for TUNEL stain were performed. A histological section of a wild-type +/+ rat kidney was treated with TACS-nuclease before the TUNEL stain was performed. The vast majority of the cells exhibited nuclear staining. This TACS-nuclease-positive control confirmed that the permeabilization and the labeling reaction had worked. In contrast, a histological section of kidney stained with the TUNEL stain in the absence of terminal deoxynucleotidyltransferase enzyme demonstrated the absence of nuclear staining and the presence of the counterstain in all of the cells.
All cells with apoptotic morphology (cellular rounding and shrinkage, pyknotic nuclei, and formation of apoptotic bodies) that stained positive with the TUNEL assay were counted.
Quantitation of Tubular Cell Proliferation and Apoptosis. The number of PCNA-positive cells or TUNEL-positive cells per tubule was counted by using a Nikon Eclipse E400 microscope equipped with a digital camera connected to spot advanced 3.5 imaging software by an observer blinded to the treatment modality. Noncystic tubules were defined as tubules <50 μm in diameter. At least 15 areas in the cortex per sample were randomly selected.
To avoid confusion between noncystic tubules and small cysts as well as potential changes in tubular cells lining massive cysts, PCNA-positive tubular cells and TUNEL-positive cells were counted in “medium sized cysts” of ≈250-μm diameter. At least 15 areas per sample in the cortex were randomly selected.
Chemistry. Serum urea nitrogen levels were measured by using a Beckman autoanalyzer.
Immunoblotting. Immunoblot analysis was performed as we have described (12). Renal cortex was homogenized in lysis buffer (5 mM Na2HPO4/5 mM NaH2PO4/150 mM NaCl/1 mM EDTA/0.1% Triton X-100/50 mM NaF/0.2 mM Na3VO4/0.1% 2-mercaptoethanol, pH 7.2) plus the following proteinase inhibitors: 1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF), 15 μM pepstatin A, 14 μM l-transepoxysuccinyl-leucylamide-(4-guanido)-butane (E-64), 40 μM bestatin, 22 μM leupeptin, and 0.8 μM aprotonin. The homogenates were centrifuged (10,000 × g at 4°C for 10 min) to remove unbroken cells and debris. Supernatants were mixed with sample buffer containing 50 mM Tris base (pH 6.8)/0.5% glycerol/0.01% bromophenol blue/0.75% SDS and heated at 95°C for 5 min. Equal amounts of protein (100 μg per lane) were fractionated by Tris/glycine/SDS/12.5% PAGE. The electrophoretically separated proteins were then transferred to a nitrocellulose membrane (Millipore) by wet electroblotting for 55 min. The membranes were blocked with 5% nonfat dry milk in TBST buffer (50 mM Tris, pH 7.5/150 mM NaCl/0.1% Tween 20) at pH 7.5 overnight at 4°C. Immunoblot analyses were performed with a rabbit polyclonal antibody raised against a recombinant protein corresponding to amino acids 1–277 representing the full-length precursor form of caspase-3 of human origin (1:1,000) (Catalog no. sc-7148)(Santa Cruz Biotechnology). The antibody has species reactivity to human, mouse, and rat. The antibody reacts with the processed form of caspase-3 (20 kDa). Purified recombinant caspase-3 (Upstate Group, Lake Placid, NY) containing the pro and active form of caspase-3 was used as a positive control. The membranes were incubated with primary antibodies for 1 h at room temperature, washed in TBST buffer, and further incubated with donkey anti-rabbit IgG coupled to horseradish peroxidase (Amersham Pharmacia) (for caspase-3 immunoblot) at 1:1,000 dilution in TBST buffer for 1 h at room temperature. Subsequent detection was carried out by enhanced chemiluminescence (Amersham Pharmacia), according to the manufacturer's instructions. Prestained protein markers (Bio-Rad) were used for molecular mass determination.
Concentration of IDN-8050 That Inhibits the Activity of Caspases, Calpain, and Cathepsin B by 50% (IC50). The IC50 for caspases was determined as described (13) (14). To confirm the specificity of IDN-8050 for caspases, we also determined the IC50 for two other cysteine proteases, calpain, and cathepsin B. Briefly, increasing concentrations of IDN-8050 were added to purified μ-calpain (2.5–5 μg) from porcine erythrocytes (Calbiochem) or purified cathepsin B (0.3–0.6 μg) from human liver tissue (Biomol, Plymouth Meeting, PA). Calpain and cathepsin B activity were determined by use of fluorescent substrates as we have described (15). N-succinyl-Leu-Tyr-7-amido-4-methyl coumarin (Sigma) in DMSO was used as a substrate for calpain. Z-Phe-Arg-AMC (Peptide Institute, Osaka) in DMSO was used as a substrate for cathepsin B.
Statistical Analysis. Non-normally distributed data were analyzed by the unpaired Student t test. Multiple group comparisons were performed by using a two-way ANOVA with posttest according to Tukey. A P value of <0.05 was considered statistically significant. Values are expressed as means ± SE.
Results
Effect of IDN-8050 on Body Weight, 2K/TBW, Cyst Volume Density (CVD), and Blood Urea Nitrogen (BUN). The effect of IDN-8050 on body weight, 2K/TBW, CVD, and BUN is given in Table 1. The 2K/TBW more than doubled in Cy/+ rats compared with +/+ rats. IDN-8050 reduced the kidney enlargement by 44%. IDN-8050 reduced the CVD by 29%. IDN-8050 significantly reduced the increase in BUN in the Cy/+ rats.
Table 1. Effect of IDN-8050 in PKD.
| Mice | Body weight, g | 2K/TBW, % | CVD, % | BUN, mg/dl |
|---|---|---|---|---|
| +/+ | 267 ± 13 | 0.9 ± 0.04 | 0.4 ± 0.1 | 23 ± 1 |
| n = 8 | n = 8 | n = 4 | n = 4 | |
| +/+ IDN-8050 | 249 ± 28 | 0.8 ± 0.02 | 0.5 ± 0.2 | 19 ± 2 |
| n = 4 | n = 3 | n = 3 | n = 3 | |
| Cy/+ | 259 ± 7 | 2.1 ± 0.1* | 43 ± 2* | 38 ± 1* |
| n = 7 | n = 8 | n = 8 | n = 7 | |
| Cy/+ IDN-8050 | 257 ± 17 | 1.6 ± 0.1** | 30 ± 3** | 26 ± 1** |
| n = 7 | n = 6 | n = 6 | n = 3 |
CVD, cyst volume density. *, P < 0.001 vs. +/+ mice; **, P < 0.001 vs. Cy/+ mice. The P value for the interaction between IDN-8050 and genotype was 0.04 for 2K/TBW (%), 0.03 for CVD, and 0.02 for BUN.
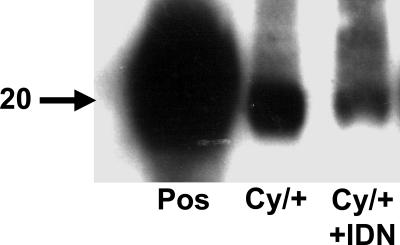
We have previously demonstrated an increase in caspase-3 activity in 3-, 6-, and 8-week-old Han:SPRD polycystic kidneys (9, 10). Immunoblot analysis was performed to confirm that the IDN-8050 was inhibiting caspases in the polycystic kidneys. The processed form of caspase-3 (≈20 kDa) was decreased in Cy/+ polycystic kidneys treated with the caspase inhibitor IDN-8050. IDN-8050 is a pan-caspase inhibitor that also inhibits caspase-8 and -9, which are known to cleave and activate procaspase-3. It is likely that the decrease of the processed form of caspase-3 in the kidney in Cy/+ rats treated with IDN-8050 (Fig. 1) is due to a decrease of activation by caspase-8 and -9.
Fig. 1.
Effects of IDN-8050 (IDN) on caspase-3 protein in Han:SPRD rats. On immunoblot analysis, the processed form of caspase-3 (20 kDa) in the whole kidney was significantly reduced in the rats treated with IDN-8050. Pos, positive control (recombinant caspase-3). Representative immunoblot of six separate experiments.
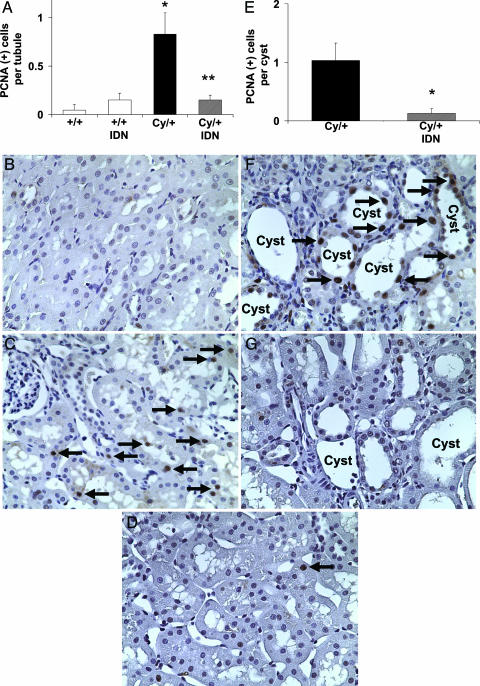
Tubular Cell Proliferation. The number of PCNA-positive cells per tubule cross section in noncystic tubules in the cortex was 0.04 ± 0.04 in vehicle-treated +/+ rats, 0.15 ± 0.08 in IDN-8050-treated +/+ rats, 0.8 ± 0.2 in vehicle-treated Cy/+ rats (P < 0.01 vs. vehicle +/+, n = 7), and 0.15 ± 0.05 in IDN-8050-treated Cy/+ rats (P < 0.05 vs. vehicle-treated Cy/+ rats, not significant vs. +/+, n = 4) (Fig. 2A). Representative pictures are shown in Fig. 2 B, C, and D. The number of PCNA-positive cells per cyst in the cortex was 1.0 ± 0.3 in vehicle-treated Cy/+ rats and 0.12 ± 0.1 in IDN-8050-treated Cy/+ rats (P < 0.05 vs. vehicle-treated) (Fig. 2E). Representative pictures are shown in Fig. 2 F and G.
Fig. 2.
Tubular cell proliferation. (A) The number of PCNA-positive cells (brown staining) per tubule in noncystic tubules in the cortex was increased in vehicle-treated Cy/+ rats compared with vehicle-treated and IDN-8050-treated +/+ rats. IDN-8050-treated Cy/+ rats had significantly less PCNA-positive cells per tubule compared with vehicle-treated Cy/+ rats. *, P < 0.01 vs. +/+; **, P < 0.05 vs. vehicle-treated Cy/+, not significant vs. +/+. P = 0.025 for the interaction between IDN-8050 and genotype on PCNA-positive cells per tubule. (B) Vehicle-treated +/+ rats had relatively few PCNA-positive cells per tubule compared with (C) vehicle-treated Cy/+ rats (arrows). (D) IDN-8050 decreased the number of PCNA-positive cells per tubule in Cy/+ rats (arrows). (E) The number PCNA-positive cells in tubular epithelial cells lining the cysts was significantly decreased by IDN-8050. *, P < 0.05 vs. vehicle-treated Cy/+.(F and G) Shown are representative pictures of the PCNA staining (arrows) in cysts of vehicle-treated Cy/+ (F) and IDN-8050-treated Cy/+ (G) rats.
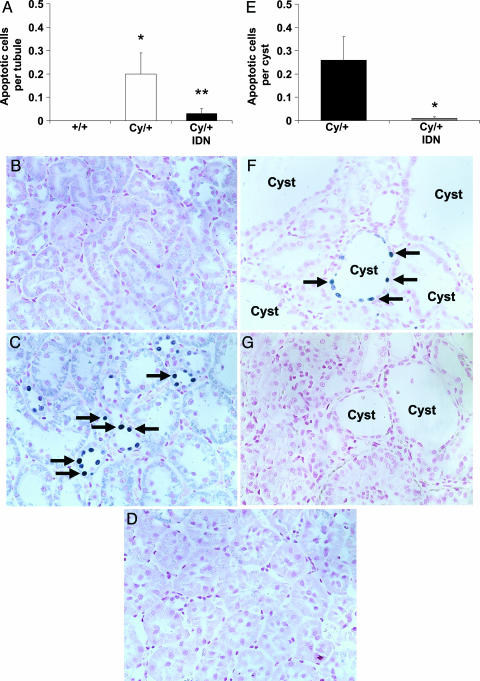
The number of TUNEL-positive apoptotic tubular cells per tubule in noncystic tubules in the cortex was 0 in vehicle-treated and IDN-8050-treated +/+ rats, 0.2 ± 0.1 in vehicle-treated Cy/+ rats (P < 0.05 vs. vehicle +/+, n = 5), and 0.03 ± 0.02 in IDN-8050-treated Cy/+ rats (P < 0.05 vs. vehicle-treated, not significant vs. +/+, n = 5) (Fig. 3A). Representative pictures are shown in Fig. 3 B, C, and D. The number of TUNEL-positive cells per cyst in the cortex was 0.26 ± 0.1 in vehicle-treated Cy/+ rats and 0.01 ± 0.01 in IDN-8050-treated Cy/+ rats (P < 0.05 vs. vehicle-treated) (Fig. 3E). Representative pictures are shown in Fig. 3 F and G.
Fig. 3.
Tubular cell apoptosis. (A) The number of TUNEL-positive cells (blue staining) per tubule in noncystic tubules in the cortex was increased in vehicle-treated Cy/+ rats compared with vehicle-treated +/+ rats. IDN-8050-treated Cy/+ rats had significantly fewer TUNEL-positive cells per tubule compared with vehicle-treated Cy/+ rats. *, P = 0.05 vs. +/+; **, P < 0.05 vs. vehicle-treated Cy/+, not significant vs. +/+.(B) Vehicle-treated +/+ rats had no TUNEL-positive cells per tubule compared with (C) vehicle-treated Cy/+ rats (arrows). (D) IDN-8050 decreased the number of TUNEL-positive cells per tubule in Cy/+ rats. (E) The number of TUNEL-positive cells in tubular epithelial cells lining the cysts was significantly decreased by IDN-8050. *, P = 0.02 vs. vehicle-treated Cy/+.(F and G) Representative picture of the TUNEL staining (arrows) in cysts of vehicle-treated Cy/+ (F) and IDN-8050-treated Cy/+ (G) rats.
IC50 of IDN-8050 for Caspases, Calpain, and Cathepsin B. IDN-8050 is designed to be an affinity irreversible pan-caspase inhibitor that inhibits all caspases potently and has little effect on non-caspase proteases (16). IDN-8050 inhibits caspases by means of a two-step kinetic process: a reversible binding of the inhibitor to the caspase active site followed by a slow in situ covalent nucleophylic substitution (SN) reaction (13). IDN-8050 inhibits all tested caspases with an IC50 in the low nM range. The IC50 for caspases was as follows: 4.5 ± 2.0 nM for caspase-3, 9.0 ± 5.0 nM for caspase-7, 4.0 ± 2.0 nM for caspase-8, and 2.9 ± 1.0 nM for caspase-9. The IC50 was >1,000 μM for calpain and was 469 ± 8 μM for cathepsin B. Thus, the IC50 of IDN-8050 for calpain and cathepsin B is >10,000-fold higher than for caspases.
Discussion
Apoptosis is a pathological feature of PKD (17). Increased levels of tubular cell apoptosis and proliferation are observed in human ADPKD (17–19), the cpk model of autosomal recessive PKD (19), the newly developed pck model of ADPKD (20), and dysplastic renal disease (21). Increased tubular cell apoptosis and proliferation are features of transgenic mice overexpressing the protooncogene c-myc (SBM mice) (22), mice lacking the transcription factor AP-2β (23), and Bcl-2-deficient mice (24). We have described in detail the increases in caspases and apoptosis in 2-, 6-, and 8-week-old Han:SPRD rats with PKD (9, 10). To our knowledge, the effect of caspase inhibition on apoptosis and the progression of PKD has not previously been described.
The rationale that apoptosis inhibition will attenuate PKD is based on studies that suggest that apoptosis may be causally linked to the development of renal cystic disease. Apoptosis is essential for Madin–Darby canine kidney (MDCK) cell cyst cavitation in collagen type 1 matrix. Cystogenesis in this system is inhibited by overexpression of the antiapoptotic gene Bcl-2 (25). Expression of the full-length cDNA for human polycystin 1 in MDCK cells induces resistance to apoptosis and spontaneous tubulogenesis rather than cyst formation (26). However, in cpk mice crossed with Pax2-deficient mice, reduced gene dosage increased interstitial cell apoptosis and slowed the progression of renal cystic disease (27).
In addition to apoptosis, tubular cell proliferation seems to be a prerequisite for cyst expansion (7). Recent reports have demonstrated that kidneys from patients with ADPKD have high levels of both apoptosis and cellular proliferation (18). Also, in SBM mice that overexpress c-myc and in Bcl-2 deficient mice, both models of renal cystic disease, there is a 10- to 100-fold increase in both apoptosis and proliferation (24, 28). In fact, increased apoptosis and proliferation occur early in the course of the disease and precede cystogenesis in the SBM mice (22). Thus, epithelial cell apoptosis and proliferation are dysregulated in ADPKD and may represent a general mechanism for cyst growth and tissue remodeling (18). The direct relationship between apoptosis and proliferation in PKD is also suggested by other studies. In Han:SPRD rats fed soy protein, the improved renal function and decreased cyst formation is accompanied by decreases in both tubular cell proliferation and apoptosis (29). Also, mice deficient in the antiapoptotic Bcl-2 gene have hyperproliferation as well as apoptosis that accompanies renal cysts (24, 30). For these reasons, in the present study, we determined the effect of inhibition of apoptosis on tubular proliferation and renal function in the Han:SPRD rat. Our results provide direct evidence that apoptosis inhibition ameliorates PKD.
In the present study, the processed form of caspase-3 was decreased as assessed by immunoblot analysis during caspase inhibition. This finding was associated with a significant decrease in apoptosis by TUNEL staining both in the noncystic tubules and in the tubular epithelial cells surrounding the cyst of the Han:SPRD rats. A relationship between apoptosis and tubular cell proliferation is known to occur, but the sequence of these events has not been elucidated. In this regard, in this model of renal cystic disease, caspase inhibition not only decreased apoptosis but also profoundly decreased tubular cell proliferation as assessed by PCNA staining. Similar to the effect on apoptosis, the decrease in cell proliferation was demonstrated both in the renal tubules and in the tubular epithelial cells surrounding the cysts.
The distribution of TUNEL staining demonstrates that most or all cells in a particular tubule section were apoptotic whereas other tubule sections showed no apoptosis. This finding may suggest that an entire tubule is being destroyed by apoptosis rather than individual tubular cells. In this regard, apoptosis was detected in noncystic tubules in preuremic human polycystic kidneys, suggesting that it may lead to the progressive loss of normal nephrons in PKD (19).
The combined effect of caspase inhibition to decrease renal apoptosis and cell proliferation was associated with an impressive decrease in kidney size and renal cyst volume. Most importantly, renal functional protection was observed in the caspase inhibitor-treated animals. The administration of the caspase inhibitor was not associated with detectable effects on PCNA, TUNEL staining, or BUN in the +/+ rats.
With caspase inhibition, it should be considered that apoptosis in ADPKD may be a double-edged sword (31). Whereas increased apoptosis may result in increased proliferation, cyst growth, and deterioration of renal function in PKD, apoptosis may also be a defense against oxidative or other forms of DNA damage and thus reduce the risk of neoplastic transformation (31). In this regard, the simultaneous induction of cell proliferation and apoptosis has been regarded as a safeguard against neoplastic transformation. In the present study, the rats were treated with the caspase inhibitor from a young age just after being weaned from their mothers. The IDN-8050 treatment had no detectable deleterious side effects as the rats appeared healthy and did not lose weight. No renal tumors were visualized either macroscopically or microscopically in the kidneys.
Caspases have been considered to be attractive potential targets for treatment of diseases because of their central role in apoptosis and the appealing prospect of small molecule inhibitor therapy (32). In animal studies, caspase inhibition has been shown to have remarkable protective effects on nonrenal organ injury due to apoptosis. Caspase inhibitors were demonstrated to be effective in decreasing ischemia-perfusion injury in heart (33), liver (34), and kidney (14) in experimental studies. The pan-caspase inhibitor IDN-1965 improved cardiac function and decreased mortality in mouse cardiomyopathy (35) and reduced apoptosis of sinusoidal endothelial cells during liver preservation injury (36). The pancaspase inhibitor IDN-6556 decreased the elevation of liver enzymes in patients with hepatic dysfunction (37).
There are currently no effective treatments for ADPKD. The ability of IDN-8050 in the present study to inhibit cyst formation and renal failure in Han:SPRD rats demonstrates its potential therapeutic usefulness. However, the usefulness of caspase inhibitors needs to be proven in other animal models of PKD before clinical trials in humans with ADPKD.
In summary, Han:SPRD rats were treated for 5 weeks with a pan-caspase inhibitor. The treatment significantly reduced apoptosis and proliferation in both cystic and noncystic tubules and slowed the morphological and functional disease progression. The treatment did not have major side effects. The relevance of these caspase inhibitor studies to clinical ADPKD is substantial, and the results may provide leads to altering the course of ADPKD. This result is particularly true because of the current availability of caspase inhibitors that are being tested in clinical trials in humans.
Acknowledgments
This work was supported by National Institutes of Health Grants DK56851 and DK34039 (Section 3) (to C.L.E.) and DK52599 and DK34039 (to R.W.S.).
Author contributions: Y.T., J.K., S.F., J.C.W., S.A.F., and C.L.E. performed research; Y.T. and J.C.W. contributed new reagents/analytic tools; S.F. and C.L.E. analyzed data; R.W.S. and C.L.E. designed research; and C.L.E. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: PKD, polycystic kidney disease; ADPKD, autosomal dominant PKD; Cy/+, heterozygous; +/+, control; BUN, blood urea nitrogen; PCNA, proliferating cell nuclear antigen; 2K/TBW, two-kidney/total body weight ratio.
References
- 1.Fick-Brosnahan, G, Ecder, T. & Schrier, R. W. (2001) in Diseases of the Kidney and Urinary Tract, ed. Schrier R. W (Lippincott, Williams & Wilkins, Philadelphia), pp. 547–588.
- 2.Cowley, B. D., Jr., Gudapaty, S., Kraybill, A. L., Barash, B. D., Harding, M. A., Calvet, J. P. & Gattone, V. H. 2. (1993) Kidney Int. 43, 522–534. [DOI] [PubMed] [Google Scholar]
- 3.Kranzlin, B., Schieren, G. & Gretz, N. (1997) Kidney Int. 51, 1160–1169. [DOI] [PubMed] [Google Scholar]
- 4.Schafer, K., Gretz, N., Bader, M., Oberbaumer, I., Eckardt, K. U., Kriz, W. & Bachmann, S. (1994) Kidney Int. 46, 134–152. [DOI] [PubMed] [Google Scholar]
- 5.Hocher, B., Zart, R., Schwarz, A., Vogt, V., Braun, C., Thone-reineke, C., Braun, N., Neumayer, H., Koppenhagen, K., Bauer, C., et al. (1999) J. Am. Soc. Nephrol. 9, 1169–1177. [DOI] [PubMed] [Google Scholar]
- 6.Ramasubbu, K., Gretz, N. & Bachmann, S. (1998) J. Am. Soc. Nephrol. 9, 937–945. [DOI] [PubMed] [Google Scholar]
- 7.Wilson, P. D. (2004) N. Engl. J. Med. 350, 151–164. [DOI] [PubMed] [Google Scholar]
- 8.Tao, Y., Kim, J., Schrier, R. W. & Edelstein, C. L. (2005) J. Am. Soc. Nephrol. 16, 46–51. [DOI] [PubMed] [Google Scholar]
- 9.Ecder, T., Melnikov, V. Y., Stanley, M., Korular, D., Lucia, M. S., Schrier, R. W. & Edelstein, C. L. (2002) Kidney Int. 61, 1220–1230. [DOI] [PubMed] [Google Scholar]
- 10.Tao, Y., Kim, J., Stanley M, He, Z., Faubel, S. G., Schrier, R. W. & Edelstein, C. L. (2004) Kidney Int. 67, 909–919. [DOI] [PubMed] [Google Scholar]
- 11.Cowley, B. D., Jr., Rupp, J. C., Muessel, M. J. & Gattone, V. H. 2. (1997) Am. J. Kidney Dis. 29, 265–272. [DOI] [PubMed] [Google Scholar]
- 12.Shi, Y., Melnikov, V. Y., Schrier, R. W. & Edelstein, C. L. (2000) Am. J. Physiol. 279, F509–F517. [DOI] [PubMed] [Google Scholar]
- 13.Chereau, D., Kodandapani, L., Tomaselli, K. J., Spada, A. P. & Wu, J. C. (2003) Biochemistry 42, 4151–4160. [DOI] [PubMed] [Google Scholar]
- 14.Melnikov, V. Y., Faubel, S. G., Siegmund B, Lucia, M. S., Ljubanovic, D. & Edelstein, C. L. (2002) J. Clin. Invest. 110, 1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edelstein, C. L., Wieder, E. D., Yaqoob, M. M., Gengaro, P. E., Burke, T. J. & Schrier, R. W. (1995) Proc. Natl. Acad. Sci. USA 92, 7662–7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu, J. C. & Fritz, L. C. (1999) Methods (Duluth) 17, 320–328. [DOI] [PubMed] [Google Scholar]
- 17.Zhou, X. J. & Kukes, G. (1998) Diagn. Mol. Pathol. 7, 65–68. [DOI] [PubMed] [Google Scholar]
- 18.Lanoix, J., D'Agati, V., Szabolcs, M. & Trudel, M. (1996) Oncogene 13, 1153–1160. [PubMed] [Google Scholar]
- 19.Woo, D. (1995) N. Engl. J. Med. 333, 18–25. [DOI] [PubMed] [Google Scholar]
- 20.Lager, D. J., Qian, Q., Bengal, R. J., Ishibashi, M. & Torres, V. E. (2001) Kidney Int. 59, 126–136. [DOI] [PubMed] [Google Scholar]
- 21.Winyard, P. J., Nauta, J., Lirenman, D. S., Hardman, P., Sams, V. R., Risdon, R. A. & Woolf, A. S. (1996) Kidney Int. 49, 135–146. [DOI] [PubMed] [Google Scholar]
- 22.Trudel, M., Barisoni, L., Lanoix, J. & D'Agati, V. (1998) Am. J. Pathol. 152, 219–229. [PMC free article] [PubMed] [Google Scholar]
- 23.Moser, M., Pscherer, A., Roth, C., Becker, J., Mucher, G., Zerres, K., Dixkens, C., Weis, J., Guay-Woodford, L., Buettner, R., et al. (1997) Genes Dev. 11, 1938–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veis, D. J., Sorenson, C. M., Shutter, J. R. & Korsmeyer, S. J. (1993) Cell 75, 229–240. [DOI] [PubMed] [Google Scholar]
- 25.Lin, H. H., Yang, T. P., Jiang, S. T., Yang, H. Y. & Tang, M. J. (1999) Kidney Int. 55, 168–178. [DOI] [PubMed] [Google Scholar]
- 26.Boletta, A., Qian, F., Onuchic, L. F., Bhunia, A. K., Phakdeekitcharoen, B., Hanaoka, K., Guggino, W., Monaco, L. & Germino, G. G. (2000) Mol. Cell 6, 1267–1273. [DOI] [PubMed] [Google Scholar]
- 27.Ostrom, L., Tang, M. J., Gruss, P. & Dressler, G. R. (2000) Dev. Biol. 219, 250–258. [DOI] [PubMed] [Google Scholar]
- 28.Trudel, M., Lanoix, J., Barisoni, L., Blouin, M. J., Desforges, M., L'Italien, C. & D'Agati, V. D. (1997) J. Exp. Med. 186, 1873–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogborn, M. R., Bankovic-Calic, N., Shoesmith, C., Buist, R. & Peeling, J. (1998) Am. J. Physiol. 274, F541–F549. [DOI] [PubMed] [Google Scholar]
- 30.Sorenson, C. M., Padanilam, B. J. & Hammerman, M. R. (1996) Am. J. Physiol. 271, F184–F193. [DOI] [PubMed] [Google Scholar]
- 31.Torres, V. E. (1999) Kidney Int. 55, 334–335. [DOI] [PubMed] [Google Scholar]
- 32.Thornberry, N. A. & Lazebnik, Y. (1998) Science 281, 1312–1316. [DOI] [PubMed] [Google Scholar]
- 33.Yaoita, H., Ogawa, K., Maehara, K. & Maruyama, Y. (1998) Circulation 97, 276–281. [DOI] [PubMed] [Google Scholar]
- 34.Rouquet, N., Pages, J., Molina, T., Briand, P. & Joulin, V. (1996) Curr. Biol. 6, 1192–1195. [DOI] [PubMed] [Google Scholar]
- 35.Hayakawa, Y., Chandra, M., Miao, W., Shirani, J., Brown, J. H., Dorn, G. W., Armstrong, R. C. & Kitsis, R. N. (2003) Circulation 108, 3036–3041. [DOI] [PubMed] [Google Scholar]
- 36.Natori, S., Selzner, M., Valentino, K. L., Fritz, L. C., Srinivasan, A., Clavien, P. A. & Gores, G. J. (1999) Transplantation 68, 89–96. [DOI] [PubMed] [Google Scholar]
- 37.Valentino, K. L., Gutierrez, M., Sanchez, R., Winship, M. J. & Shapiro, D. A. (2003) Int. J. Clin. Pharmacol. Ther. 41, 441–449. [DOI] [PubMed] [Google Scholar]