Abstract
Vaccination of experimental animals can provide efficient protection against ocular herpes simplex virus type 1 (HSV-1) challenge. Although it is suspected that local immune responses are important in protection against ocular HSV-1 infection, no definitive studies have been done to determine if local ocular vaccination would produce more efficacious protection against HSV-1 ocular challenge than systemic vaccination. To address this question, we vaccinated groups of rabbits either systemically or periocularly with recombinant HSV-2 glycoproteins B (gB2) and D (gD2) in MF59 emulsion or with live KOS (a nonneurovirulent strain of HSV-1). Three weeks after the final vaccination, all eyes were challenged with McKrae (a virulent, eye disease-producing strain of HSV-1). Systemic vaccination with either HSV-1 KOS or gB2/gD2 in MF59 did not provide significant protection against any of the four eye disease parameters measured (conjunctivitis, iritis, epithelial keratitis, and corneal clouding). In contrast, periocular vaccination with gB2/gD2 in MF59 provided significant protection against conjunctivitis and iritis, while ocular vaccination with live HSV-1 KOS provided significant protection against all four parameters. Thus, local ocular vaccination provided better protection than systemic vaccination against eye disease following ocular HSV-1 infection. Since local vaccination should produce a stronger local immune response than systemic vaccination, these results suggest that the local ocular immune response is very important in protecting against eye disease due to primary HSV-1 infection. Thus, for clinical protection against primary HSV-1-induced corneal disease, a local ocular vaccine may prove more effective than systemic vaccination.
Human herpes simplex virus (HSV) infections are common, occur at diverse sites, and have a wide range of symptoms, from inapparent to life-threatening encephalitis (2, 3). HSV infects mucosal surfaces, most commonly producing infections of the genitals, the mouth, or the eye. Greater than 90% of ocular HSV infections are due to HSV type 1 (HSV-1). HSV-1 infection of the eye can produce corneal inflammation and scarring as the result of an incompletely defined immunological response to the virus (1, 7, 14, 27, 32). This scarring is a major cause of corneal blindness (22, 35). In developed nations, HSV is the most frequent serious viral eye infection and is the most common cause of infectious blindness (22). In the United States, almost 500,000 people per year suffer primary or recurrent ocular HSV episodes that require doctor visits and medication (22). Over 1,000 corneal transplants per year are done in the United States as a direct result of HSV scarring (22, 35).
Experimental primary and recurrent ocular HSV-1 infection in the rabbit and the naturally occurring infection in humans share many characteristics. Primary infection is self-limited and is characterized by a benign transient infectious conjunctivitis. Infection of the cornea starts with epithelial keratitis, which destroys the corneal epithelium in a characteristic dendritic (neuron-like) and later geographic (amoeboid-like) pattern. As the keratitis, and its accompanying virus-induced immune-mediated inflammation, spread to the deeper part of the cornea (the stroma), the cornea becomes (temporarily) cloudy. Iritis (inflammation of the iris and anterior chamber) usually occurs only in eyes with severe keratitis. Corneal clouding and iritis follow, and are less common than, epithelial keratitis. In the rabbit, as in humans, HSV-1 epithelial keratitis with its dendritic and geographical spread is the hallmark of herpetic corneal infection. In fact, clinically, the dendritic ulcer is pathognomonic (i.e., characteristic or symptomatic of a particular disease) and requires no laboratory confirmation for the diagnosis of HSV infection. Epithelial keratitis is therefore the most relevant and important eye disease parameter in the studies described in this report.
The only Food and Drug Administration-approved treatments for primary HSV-1 ocular infection consist of the topical antivirals idoxuridine, vidarabine, and trifluorothymidine as well as oral acyclovir. Outside the United States, topical acyclovir is also used. Until recently, little attention has been given to the development of a vaccine against ocular HSV-1 infection. Virtually all work on HSV vaccine development has focused on the problem of genital HSV-2. Well-defined herpesvirus glycoprotein subunit vaccines have been developed by using recombinant DNA technology. These vaccines afford protective immunity when used prophylactically in mouse and guinea pig models of HSV-1 and HSV-2 disease (2, 8–11, 34, 36, 37).
Local immunity is likely to be especially important for mucosal surfaces such as the eye. In addition, previous HSV-1 infection at a nonocular site does not protect against nonprimary first episodes of ocular HSV-1 (4) or against recurrent ocular HSV-1 infection. Therefore, it was of interest to determine if ocular vaccine administration would be more effective than systemic vaccine administration as prophylaxis against primary infection and the resulting eye disease in rabbits following ocular challenge with a highly pathogenic HSV-1 strain (McKrae). Two different vaccines, both expected to be less than optimal, were used. Both vaccines afforded protection against lethal ocular challenge regardless of the route of administration. However, neither vaccine provided any protection against ocular disease when administered systemically. In contrast, both vaccines provided protection against ocular disease when administered periocularly, thus supporting the notion that ocular immunity is more important than systemic immunity in protecting against eye disease.
MATERIALS AND METHODS
Virus.
The challenge virus, HSV-1 McKrae, produces severe ocular disease in rabbits (25). The live-virus vaccine strain, HSV-1 KOS, is nonvirulent and produces no significant ocular disease in rabbits. Both viruses were triple plaque purified and prepared as previously described (25).
Rabbits.
Eight to ten-week-old New Zealand White male rabbits (Irish Farms) were used for all experiments. Rabbits were housed and handled in accordance with Association for Research in Vision and Ophthalmology, American Association for Laboratory Animal Care, and National Institutes of Health guidelines.
Glycoproteins and adjuvant for subunit vaccine.
HSV-2 glycoproteins B (gB2) and D (gD2) were prepared by expression of the modified genes in Chinese hamster ovary cells followed by purification to near homogeneity, using a series of traditional chromatographic steps as previously described and as previously used by Chiron Corp. in human clinical trials of a vaccine for genital herpes (20). The adjuvant MF59 was prepared as previously described (23). The vaccine was prepared by mixing 1 volume of gB2 plus gD2 in 2× phosphate-buffered saline with 1 volume of MF59.
Systemic vaccination.
Rabbits received three inoculations at 3-week intervals. Each inoculation with gB2/gD2/MF59 was delivered by a single intramuscular (i.m.) injection on one side of the lower back. Each dose contained 25 μg of each glycoprotein in a total volume of 0.1 ml. Three systemic vaccinations with live HSV-1 KOS were given intradermally 3 weeks apart. Each intradermal vaccine dose was divided into four or five 0.1-ml aliquots and was injected into separate sites on the back for a total dose of 2 × 107 PFU of live HSV-1 KOS.
Control vaccine.
The control vaccine was the adjuvant MF59 without glycoprotein (1 volume of MF59 plus 1 volume of 2× PBS) and was delivered identically and on the same schedule as the gB2/gD2/MF59 systemic vaccine.
Subconjunctival vaccinations with the gB2/gD2 vaccine.
Inoculations were given as previously described (23), with each eye receiving three inoculations at 3-week intervals. Each inoculation contained 7.5 μg of each glycoprotein in 0.1 ml. Vaccination resulted in approximately 25% of the eyes showing mild to moderate conjunctival inflammation for up to 7 days.
Topical ocular vaccination with HSV-1 KOS.
Eyes were vaccinated twice at a 3-week interval with 2 × 105 PFU of live KOS per eye as described below for HSV-1 challenge with McKrae. Since KOS produces no stromal keratitis and requires corneal scarification to produce epithelial keratitis (17, 26a, 38), the method used for inoculating with KOS resulted in no clinically recognizable disease.
Ocular challenge of rabbit eyes with HSV-1 McKrae.
Vaccinated and mock-vaccinated rabbits were bilaterally infected without scarification or anesthesia by placing 2 × 105 PFU (HSV-1 McKrae), in a total volume of 0.1 ml, into the conjunctival cul-de-sac, closing the eye, and rubbing the lid gently against the eye for 30 s (31). In naive rabbits, this dose of virus infects all eyes and produces moderate to severe ocular disease in about 90% of eyes. It results in the death of approximately 30 to 50% of the rabbits within 18 days (23, 25, 29, 30). Animals were challenged 3 weeks following the final dose of vaccine.
Measurement of titers.
HSV serum neutralizing antibody titers were measured as previously described (21), using an HSV-2 plaque reduction neutralization assay in the presence of added complement with twofold serum dilutions. The reported titer is the reciprocal of the serum dilution required to inhibit the cytolysis of a confluent monolayer of Vero cells by 50%.
HSV serum enzyme-linked immunosorbent assay (ELISA) titers were determined as described previously (21), using threefold serial dilutions of serum and either recombinant gB2 or recombinant gD2 as the capture antigen. The reported titers correspond to the reciprocal of the serum dilution producing an absorbance value of 1.0. ELISA titers are antigen specific, as shown by the absence of a measurable gD or gB ELISA titer in sera obtained before immunization of animals.
HSV tear ELISA titers. Tears were collected with a microcapillary pipette, and tear soluble immunoglobulin A (sIgA) ELISAs were performed as described above, using recombinant gD2 as the capture antigen.
Determination of clinical eye disease.
Clinical eye disease patterns were determined by examining the rabbit eyes in a masked fashion on days 3, 5, 7, 10, and 14 postinfection for scoring the incidence and severity of conjunctivitis, iritis, dendritic and geographic ulcers characteristic of HSV (epithelial keratitis), and acute transient stromal keratitis and edema (corneal clouding). Epithelial keratitis was evaluated by slit lamp biomicroscopy using 0.75% fluorescein stain (26). Conjunctivitis was determined by direct visual observation. The magnitude of epithelial disease was scored as 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 3.5, or 4, with 0, 1, 2, 3, and 4 representing no disease and disease involving 25, 50, 75, and 100% of the corneal surface, respectively. The levels of inflammatory severity of conjunctivitis, iritis, and stromal keratitis were assessed by using the same scale, with 0, 1, 2, 3, and 4 representing no inflammation, mild but recognizable inflammation, moderate easily recognizable inflammation, moderately severe inflammation, and very severe inflammation, respectively. To eliminate bias, these gradings were done in a masked fashion by readers highly experienced in this system.
Statistical analyses.
Statistical analyses were performed with Instat, a personal computer software program. Tests included the Student t test and the Fisher exact test.
RESULTS
Groups of naive rabbits were vaccinated either systemically or periocularly with either a gB2/gD2 subunit vaccine or live KOS, a nonvirulent HSV-1 strain that produces no eye disease in rabbits. The five experimental groups were as follows: KOS ocular vaccine, 11 rabbits; KOS systemic vaccine, 15 rabbits; gB2/gD2 ocular vaccine, 15 rabbits; gB2/gD2 systemic vaccine, 15 rabbits; and control (systemic adjuvant), 16 rabbits.
Protection against mortality.
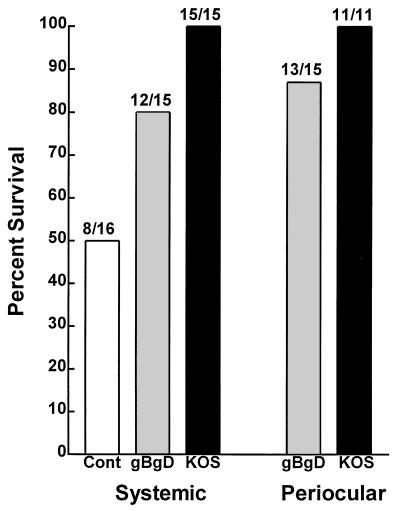
Three weeks after the final vaccination, rabbits were ocularly challenged with HSV-1 McKrae. Protection against mortality was similar for each vaccine, regardless of the vaccination route (Fig. 1). The KOS vaccines were significantly more efficacious than the control (P = 0.008 [ocular] and 0.002 [systemic]; Fisher exact test). Survival in the individual gB2/gD2 vaccine groups was not significantly more than for controls (P = 0.14 [systemic] and 0.054 [ocular]; Fisher exact test). However, since the two gB2/gD2 vaccine groups were similar, the data could be combined to increase the power of the analysis. This resulted in significant protection compared to mock vaccination (P = 0.036; Fisher exact test).
FIG. 1.
Survival of vaccinated rabbits following ocular challenge with HSV-1 McKrae. Rabbits were vaccinated either i.m. or ocularly with either gB2/gD2 or HSV-1 KOS as described in Materials and Methods. The mock-vaccinated control (Cont) group was vaccinated i.m. with adjuvant alone. Three weeks after the final vaccination, all rabbits were challenged with 2 × 105 PFU of HSV-1 McKrae in both eyes as described in Materials and Methods. Survival was determined 3 weeks after challenge. The ratio above each bar shows the number of surviving rabbits/the number of challenged rabbits. Comparisons were done as follows: periocular HSV-1 KOS group versus mock control group (P = 0.008 [Fisher exact test]; systemic HSV-1 KOS group versus mock control group (P = 0.002); periocular gB2/gD2 versus mock (P = 0.054); systemic gB2/gD2 versus mock (P = 0.14); combined gB2/gD2 groups versus mock (P = 0.036 [double-sided Fisher exact test]; P = 0.02 [single-sided Fisher exact test]).
Protection against conjunctivitis.
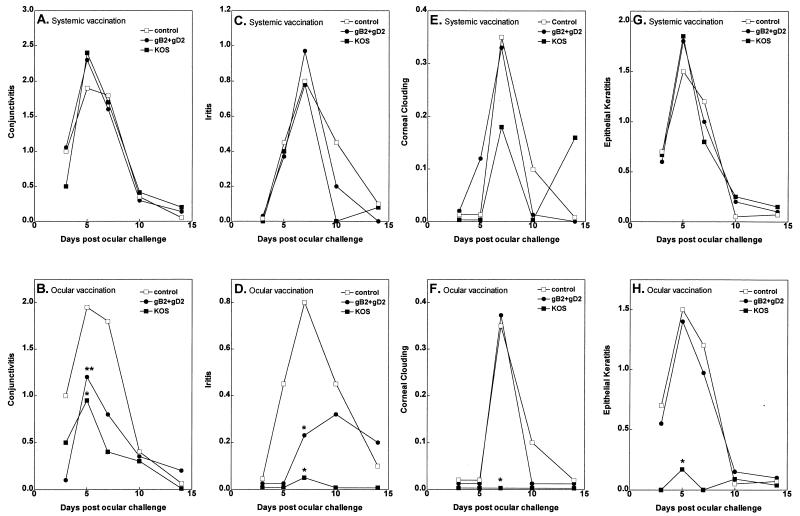
No differences in the average severity of conjunctivitis were observed between mock-vaccinated rabbits and rabbits vaccinated systemically with gB2/gD2 or KOS on any of the days examined (Fig. 2A) (P > 0.05; Student t test). In contrast to systemic vaccination, when the route of vaccination was periocular, both the subunit gD2/gB2 and the live KOS vaccines appeared to provide protection against conjunctivitis. In rabbits vaccinated ocularly with KOS, the average peak severity of conjunctivitis (day 5 following ocular challenge) was significantly less than in the control rabbits and in rabbits systemically vaccinated with KOS (Fig. 2B; P = 0.048 and 0.004, respectively; Student t test). Rabbits vaccinated ocularly with gB2/gD2 also appeared to have less conjunctivitis than control rabbits, but the difference did not quite reach statistical significance (P = 0.08; one sided). However, the gB2/gD2 ocularly vaccinated rabbits had significantly less conjunctivitis than the gB2/gD2 systemically vaccinated rabbits (Fig. 2B; P = 0.008). Thus, for both vaccines, compared to systemic vaccination, ocular vaccination provided significantly more protection against conjunctivitis.
FIG. 2.
Protection against eye disease. Rabbits were vaccinated three times either systemically or periocularly, and ocularly challenged, and eye disease was monitored on days 3, 5, 7, 10, and 14 as described in Materials and Methods. Each time point represents the average readings of both eyes from 11 to 16 rabbits from vaccinated or mock-vaccinated rabbits (ocular KOS vaccine, 11 rabbits; systemic KOS vaccine, 15 rabbits; ocular gB2/gD2 vaccine, 15 rabbits; systemic gB2/gD2 vaccine, 15 rabbits; adjuvant control, 16 rabbits). Single asterisks indicate points that are significantly less than for the mock-vaccinated control group at the 95% level, using the Student t test; double asterisks indicate values for the periocular vaccine group that are significantly less than values for the corresponding systemic vaccine group. Statistical analyses were done on a per-rabbit basis. Thus, the readings from both eyes were averaged for each rabbit, and the resulting value for each rabbit was used for the analyses (i.e., n for each group was the number of rabbits, not the number of eyes, in the group). The same groups showed statistical significance when analyses were done on a per-eye basis (not shown).
Protection against acute herpetic iritis.
Iritis is characterized by anterior chamber inflammation (cells and fibrin) and redness of the normally pink iris. Prophylactic systemic vaccination with gB2/gD2 or KOS did not reduce the peak severity of iritis (day 7 after ocular challenge) (Fig. 2C; P > 0.05). In contrast, prior periocular vaccination with gB2/gD2 resulted in significant reductions in average iritis severity compared to control and systemic gB2/gD2 vaccination on day 7 postchallenge (Fig. 2D; P = 0.02 and 0.03, respectively). Ocular vaccination with KOS also significantly reduced the peak average iritis severity compared to control and the corresponding systemic vaccination (Fig. 2D; P = 0.0008 and 0.03, respectively). Thus, as with conjunctivitis, periocular vaccination provided much more protection against manifestations of herpetic iritis than did systemic vaccination.
Protection against corneal clouding.
Corneal clouding (a transient corneal edema with corneal inflammation) is a measure of stromal keratitis. Systemic vaccination with gB2/gD2 or KOS did not affect the severity of corneal clouding (Fig. 2E). Periocular vaccination with gD2/gB2 also did not lessen stromal keratitis (Fig. 2F; P > 0.05). In contrast, periocular vaccination with KOS completely eliminated corneal clouding. Because of the low overall levels of corneal clouding in all groups, protection against the severity of corneal clouding by ocular KOS vaccination on the day of peak disease reached statistical significance only by a single-sided analysis (Fig. 2F; P = 0.04).
Protection against epithelial keratitis.
HSV-1 produces corneal epithelial cell loss in a characteristic dendritic pattern. As the dendrite expands, it develops smoother edges and takes on a geographic pattern. Epithelial keratitis is the combination of these dendritic and geographic lesions. Epithelial keratitis is the key pathologic finding indicative of ocular HSV-1 infection and is the clinical hallmark of acute herpetic corneal disease. Iritis and clouding are not present without epithelial keratitis.
Neither systemic gB2/gD2 nor systemic KOS vaccine provided protection against the average severity of epithelial keratitis (Fig. 2G). Local periocular vaccination with gB2/gD2 also did not provide protection against the average severity of epithelial keratitis (Fig. 2H). In contrast, periocular vaccination with KOS significantly reduced peak epithelial keratitis (Fig. 2H; P = 0.0006 compared to control; P < 0.0001 compared to systemic KOS vaccination).
Vaccine immunogenicity.
All rabbits were bled 3 weeks after the final vaccination, just prior to challenge with HSV-1. Neutralization and ELISAs (against HSV-2 and HSV-2 glycoproteins) were done individually on all sera (Tables 1 and 2). Both vaccines induced HSV-2 neutralizing antibody titers significantly greater than those for the adjuvant control, regardless of the route of vaccination (Tables 1 and 2). With both vaccines, the systemic vaccination showed a tendency to induce a higher neutralizing antibody titer than ocular vaccination, but the differences were not statistically significant.
TABLE 1.
Vaccine-induced humoral immune responses
| Vaccine (no. of rabbits) | Titer (geometric mean ± SD)a
|
||
|---|---|---|---|
| Neutralizationb | gD2 ELISA | gB2 ELISA | |
| KOS ocular (10 for neutralization, 8 for ELISA) | 39* ± 18 | 4,768 ± 4,630 | 12,617 ± 2,945 |
| KOS systemic (12) | 101* ± 81 | 8,571 ± 4,586 | 13,233 ± 10,363 |
| gB/gD ocular (13) | 303 ± 115 | 17,108 ± 9,657 | 20,698 ± 11,972 |
| gB/gD systemic (14) | 430 ± 357 | 27,097 ± 14,962 | 73,151 ± 67,048 |
| Adjuvant control (13) | 24* | 34* ± 16 | 45* ± 34 |
Neutralization titers followed by asterisks include one or more values of <24, the baseline of the assay. For the purpose of determining the mean and for statistical analyses (Table 2), 24 was substituted for <24. ELISA titers followed by asterisks include one or more values of <30, the baseline of that assay. For the purpose of determining the mean and for statistical analyses (Table 2), 30 was substituted for <30.
Neutralization assays were done against HSV-2.
TABLE 2.
Statistical analysis of data in Table 1
| Comparison | P valuea
|
||
|---|---|---|---|
| Neutralization | gD2 ELISA | gB2 ELISA | |
| KOS ocular vs control | 0.03 | <0.0001 | <0.0001 |
| KOS systemic vs control | 0.003 | <0.0001 | <0.0001 |
| KOS ocular vs KOS systemic | 0.07 | 0.016 | 0.85 |
| gB/gD ocular vs control | <0.0001 | <0.0001 | <0.0001 |
| gB/gD systemic vs control | <0.0001 | <0.0001 | <0.0001 |
| gB/gD ocular vs gBgD systemic | 0.46 | 0.069 | 0.0004 |
Determined by the Mann-Whitney rank sum test.
Both vaccines induced ELISA titers against gB2 and gD2 that were significantly above the control vaccine levels, regardless of the route of vaccination (Tables 1 and 2). Systemic vaccination with KOS induced an average gD2 ELISA titer significantly higher than that induced by ocular vaccination with the same vaccine. The KOS-induced gB2 ELISA titer also appeared slightly higher with systemic vaccination, but this difference was not significant. Systemic vaccination with gB2/gD2 induced an average gB2 ELISA titer that was significantly higher than that induced by ocular vaccination with gB2/gD2. Systemic vaccination with gB2/gD2 also appeared to induce a higher ELISA titer against gD2, but this difference was not statistically significant. Thus, systemic vaccination tended to induce stronger neutralizing antibody titers and stronger ELISA titers than ocular vaccination with the same vaccine.
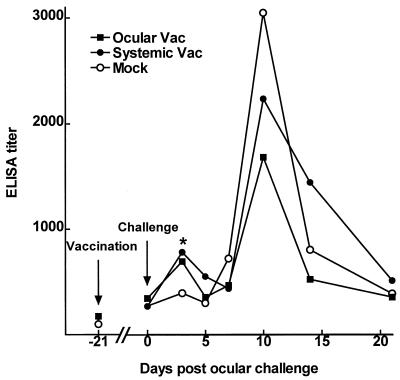
To examine the local ocular immune response to vaccination and challenge, groups of rabbits were vaccinated once, either periocularly or systemically, and then ocularly challenged as described above. Tear sIgA specific for gD2 was determined at various times by ELISAs (Fig. 3). Each point on days 3 to 21 represents the average titer of tears from 10 to 14 eyes per group. On day 3 postchallenge, the two vaccine groups were similar and had significantly more HSV-1-specific tear sIgA than the mock group (ocular versus mock, P = 0.045 [one-sided Student t test]; systemic versus mock, P = 0.01 [Mann-Whitney rank sum test]). This finding suggests early stimulation of a primed sIgA response in both vaccinated groups. There were no significant differences between the ocular and systemic vaccine groups or between either vaccine group and the mock group at any other time. However, at the time of peak HSV-1 specific tear sIgA response (day 10 postchallenge), there was a tendency for the mock group to have more sIgA than either vaccine group and for the systemic vaccine group to have more sIgA than the ocular vaccine group. This result was the reverse of the protective efficacy of the vaccines.
FIG. 3.
HSV-specific tear sIgA. Rabbits were vaccinated once, either systemically or periocularly, with gB2/gD2 and ocularly challenged 21 days later as described in Materials and Methods. Tears were collected at the times indicated, and the relative amount of HSV-1 gD2-specific sIgA in individual tear samples was determined by ELISA. The arrows indicate vaccination (day −21) and ocular challenge (day 0) as labeled. The asterisk indicates that both vaccine groups had significantly higher ELISA titers than the mock vaccine group (P < 0.05).
DISCUSSION
We previously showed that periocular vaccination of latently infected rabbits with gB2/gD2 in the adjuvant MF59/MTP-PE provided therapeutic protection against recurrent HSV-1, as judged by a statistically significant decrease in ocular spontaneous recurrent virus shedding (23) and spontaneous recurrent corneal disease (24). The present study, comparing the abilities of periocular and systemic vaccinations to protect against primary ocular HSV-1 challenge, was undertaken as part of our inquiries into the role and efficacy of periocular vaccination against primary and recurrent ocular herpes infection. The subconjunctival route used for periocular vaccination allows the use of adjuvant and ensures that the vaccine is delivered and retained at the local site. Subconjunctival injection is routinely used in clinical ophthalmology.
In this study, two vaccines that were expected to be less than completely effective were chosen. This was done because the goal was to determine if local periocular vaccination provided better ocular protection than systemic vaccination. Obviously, a vaccine that gave 100% protection against eye disease regardless of the vaccine route would not allow us to easily determine which route was more effective. A live and a subunit vaccine were both used to determine if any route specificity found would be consistent for different types of vaccines.
gB2/gD2 with MF59 was chosen as the suboptimal subunit vaccine because both glycoproteins are type 2 rather than type 1 (the McKrae challenge virus is type 1) and because the adjuvant lacks the added immunostimulant MTP-PE that was included in our recent successful therapeutic vaccine studies (23, 24). The avirulent HSV-1 KOS strain was chosen as the less than optimal live vaccine for this study because compared to other available wild-type HSV-1 strains (such as the virulent McKrae strain used as the challenge virus), its ability to replicate and spread in the rabbit is low. Thus, it was expected that HSV-1 KOS would be a suboptimal live HSV-1 vaccine. This hypothesis was supported by a small pilot study which showed that rabbits vaccinated systemically with HSV-1 McKrae, but not with HSV-1 KOS, were completely protected against eye disease (not shown).
The increased vaccine efficacy of periocular compared to systemic vaccines against HSV-1-induced ocular disease (summarized in Table 3) was even more impressive, since to strengthen the likelihood that any increased protection observed with ocular vaccination would be meaningful, the vaccinations were biased toward systemic vaccinations. Thus, a lower dose of gB/gD was used periocularly (7.5 μg of each glycoprotein per eye versus 25 μg of each glycoprotein given systemically) and a lower dose of virus and fewer periocular vaccinations were given with HSV-1 KOS (2 × 105 PFU/eye versus 2 × 107 PFU systemically; two periocular vaccinations versus three systemic vaccinations). Because suboptimal vaccines were specifically chosen for these studies, even ocular vaccination did not always provide significant protection against eye disease. However, in all situations in which differences in ocular protection were detected between the systemic and periocular routes of vaccine, the local periocular vaccine was superior.
TABLE 3.
Percent vaccine efficacy
| Vaccine | % Efficacya
|
||||
|---|---|---|---|---|---|
| Mortalityb | Conjunctivitisc | Iritisc | Cloudingc | Epithelial keratitisc | |
| KOS ocular | 100 | 53 | 94 | 100 | 89 |
| KOS systemic | 100 | 0d | 2 | 49 | 0 |
| gB/gD ocular | 73 | 40 | 60 | 0 | 7 |
| gB/gD systemic | 60 | 0 | 0 | 6 | 0 |
Calculated as 1 − (incidence rate in vaccine group/incidence rate in control group) × 100.
Incidence rate is defined as percent mortality.
Incidence rate is defined as average severity on the day of peak average severity.
Less than 0% efficacy.
As judged by neutralization and ELISA titers, both vaccines used in this study induced stronger humoral immune responses when the vaccine was delivered via a systemic route (i.m. or subcutaneous) than via an ocular route (topically on the cornea or subconjunctival injection). This finding suggests that in the rabbit ocular model of primary HSV-1 infection, the ability of a vaccine to induce serum neutralization titers and serum ELISA titers is not predictive of vaccine efficacy against eye disease and supports the hypothesis that vaccine efficacy against ocular disease is due to local/mucosal immunity and not systemic immunity.
The ability of these vaccines to induce tear sIgA specific for HSV-1 also did not appear to be predictive of vaccine efficacy against eye disease. In fact, there was an opposite tendency. Systemic vaccination tended to produce higher peak tear sIgA titers than did the more efficacious ocular vaccination. Furthermore, the peak tear sIgA titers produced following ocular challenge of mock-vaccinated rabbits tended to be higher than in either vaccine group. This type of result, in which following ocular challenge, the highest local ocular immune response occurs in the least well protected group, suggests that the higher immune response is the result of more virus replication in the eye due to the poorer efficacy of the vaccine. This, of course, can complicate the correlation of specific immune responses with vaccine efficacy and was similar to some of our previous findings in mice, in which following ocular HSV-1 challenge, fewer infiltrating immune cells were detected in the corneas of mice vaccinated with the more efficacious vaccines (14).
Interestingly, vaccine efficacy against mortality was similar regardless of vaccine route. Thus, vaccine efficacy against eye disease appeared to require local or mucosal immune responses at the eye, while vaccine efficacy against mortality could be obtained by systemic immunity. Systemic immunity could protect against mortality (due to viral encephalitis) without reducing eye disease by reducing viral replication in the trigeminal ganglia or the brain, or by reducing transit of virus between the eye and the trigeminal ganglia or between the trigeminal ganglia and the brain.
Various different immune responses have been implicated as being most important in protecting the mouse eye against HSV-1 infection. CD4+ T cells and CD8+ T cells have alternatively each been reported to protect against ocular HSV-1 and to be responsible for HSV-1 ocular disease (6, 13, 15, 16, 19, 27, 28, 33). In the mouse model, there is a very strong correlation between the ability of a vaccine to induce anti-HSV-1 serum antibody titers and vaccine efficacy against HSV-1 ocular infection (5, 12). Even intraperitoneal administration of neutralizing antibody can completely block HSV-1-induced ocular disease (18, 39). In contrast, in this report we did not find any correlation between serum antibody and protection against ocular disease in the rabbit. Serum antibody in humans also does not appear to protect against ocular HSV-1, since individuals with high rates of recurrent ocular HSV-1 often develop very high HSV-1 neutralizing antibody titers yet continue to have recurrent episodes. Thus, it appears that the immune responses (and vaccine efficacy) involved in protecting the mouse eye against HSV-1 may not be predictive of vaccine efficacy in humans. In particular, since serum antibody alone can protect the mouse eye against ocular HSV-1, using the mouse model as the sole basis of understanding vaccine efficacy against ocular HSV-1 may cause us to underestimate the importance of vaccine-induced local/mucosal immunity in humans. Thus, although the state of the art in rabbit immunology still lags significantly behind that of the mouse, the rabbit may be a more useful model for studying vaccine efficacy against primary and recurrent ocular HSV-1.
Why does humoral immunity protect the mouse eye and not the rabbit or human eye against ocular HSV-1? One possibility is the smaller size of the mouse eye. In rabbits and humans, capillaries are seen only in the outer 1 mm of the cornea, effectively isolating the central cornea from circulating immune factors. In the mouse, capillaries are also confined to the outer 1 mm of the cornea. However, because the mouse cornea is smaller than the corneas of rabbits and humans, circulating immune factors can rapidly diffuse from these peripheral capillaries into the central cornea, thus allowing serum antibody to protect the mouse cornea.
The ability to analyze cell-mediated immunity and ocular mucosal immune factors (other than tear sIgA) in the rabbit is still in its infancy compared to the situation for mice. Thus, the local/mucosal immune factors responsible for the vaccine efficacy against HSV-1-induced ocular disease in this study have not yet been determined. Although the sIgA analyses reported here suggest that tear sIgA is not a key protective immune response against primary HSV-1 ocular challenge, preliminary studies using a recurrent ocular HSV-1 vaccine model suggest that tear sIgA may play a role in protection against recurrent ocular HSV-1.
ACKNOWLEDGMENTS
We thank Anita Avery for excellent technical assistance, Jaleh Kilpatrick for measuring the serum ELISA antibody responses, and Philip Ng for measuring the neutralizing antibody responses.
This work was partially supported by Public Health Service grant EYO9392, the Discovery Fund for Eye Research, the Skirball Program in Molecular Ophthalmology, and the Factor Family Foundation.
REFERENCES
- 1.Bouley D M, Kanangat S, Rouse B T. The role of the innate immune system in the reconstituted SCID mouse model of herpetic stromal keratitis. Clin Immunol Immunopathol. 1996;80:23–30. doi: 10.1006/clin.1996.0090. [DOI] [PubMed] [Google Scholar]
- 2.Corey L, Spear P G. Infections with herpes simplex viruses (1) N Engl J Med. 1986;314:686–691. doi: 10.1056/NEJM198603133141105. [DOI] [PubMed] [Google Scholar]
- 3.Corey L, Spear P G. Infections with herpes simplex viruses (2) N Engl J Med. 1986;314:749–757. doi: 10.1056/NEJM198603203141205. [DOI] [PubMed] [Google Scholar]
- 4.Dawson C R, Togni B. Herpes simplex eye infections: clinical manifestations, pathogenesis and management. Surv Ophthalmol. 1976;21:121–135. doi: 10.1016/0039-6257(76)90090-4. [DOI] [PubMed] [Google Scholar]
- 5.Ghiasi H, Bahri S, Nesburn A B, Wechsler S L. Protection against herpes simplex virus-induced eye disease after vaccination with seven individually expressed herpes simplex virus 1 glycoproteins. Investig Ophthalmol Visual Sci. 1995;36:1352–1360. [PubMed] [Google Scholar]
- 6.Ghiasi H, Cai S, Nesburn A B, Wechsler S L. MHC-II but not MHC-I responses are required for vaccine-induced protection against ocular challenge with HSV-1. Curr Eye Res. 1997;16:1152–1158. doi: 10.1076/ceyr.16.11.1152.5104. [DOI] [PubMed] [Google Scholar]
- 7.Ghiasi H, Cai S, Nesburn A B, Wechsler S L. Vaccination with herpes simplex virus type 1 glycoprotein K impairs clearance of virus from the trigeminal ganglia resulting in chronic infection. Virology. 1996;224:330–333. doi: 10.1006/viro.1996.0537. [DOI] [PubMed] [Google Scholar]
- 8.Ghiasi H, Kaiwar R, Nesburn A B, Slanina S, Wechsler S L. Baculovirus-expressed glycoprotein E (gE) of herpes simplex virus type-1 (HSV-1) protects mice against lethal intraperitoneal and lethal ocular HSV-1 challenge. Virology. 1992;188:469–476. doi: 10.1016/0042-6822(92)90500-o. [DOI] [PubMed] [Google Scholar]
- 9.Ghiasi H, Kaiwar R, Nesburn A B, Wechsler S L. Baculovirus expressed herpes simplex virus type 1 glycoprotein C protects mice from lethal HSV-1 infection. Antiviral Res. 1992;18:291–302. doi: 10.1016/0166-3542(92)90062-a. [DOI] [PubMed] [Google Scholar]
- 10.Ghiasi H, Kaiwar R, Nesburn A B, Wechsler S L. Expression of herpes simplex virus type 1 glycoprotein B in insect cells. Initial analysis of its biochemical and immunological properties. Virus Res. 1992;22:25–39. doi: 10.1016/0168-1702(92)90087-p. [DOI] [PubMed] [Google Scholar]
- 11.Ghiasi H, Kaiwar R, Nesburn A B, Wechsler S L. Expression of herpes simplex virus type 1 glycoprotein I in baculovirus: preliminary biochemical characterization and protection studies. J Virol. 1992;66:2505–2509. doi: 10.1128/jvi.66.4.2505-2509.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghiasi H, Nesburn A B, Wechsler S L. Vaccination with a cocktail of seven recombinantly expressed HSV-1 glycoproteins protects against ocular HSV-1 challenge more efficiently than vaccination with any individual glycoprotein. Vaccine. 1996;14:107–112. doi: 10.1016/0264-410x(95)00169-2. [DOI] [PubMed] [Google Scholar]
- 13.Ghiasi H, Roopenian D C, Slanina S, Cai S, Nesburn A B, Wechsler S L. The importance of MHC-I and MHC-II responses in vaccine efficacy against lethal herpes simplex virus type 1 challenge. Immunology. 1997;91:430–435. doi: 10.1046/j.1365-2567.1997.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghiasi H, Wechsler S L, Kaiwar R, Nesburn A B, Hofman F M. Local expression of tumor necrosis factor alpha and interleukin-2 correlates with protection against corneal scarring after ocular challenge of vaccinated mice with herpes simplex virus type 1. J Virol. 1995;69:334–340. doi: 10.1128/jvi.69.1.334-340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hendricks R L, Janowicz M, Tumpey T M. Critical role of corneal Langerhans cells in the CD4- but not CD8-mediated immunopathology in herpes simplex virus-1-infected mouse corneas. J Immunol. 1992;148:2522–2529. [PubMed] [Google Scholar]
- 16.Hendricks R L, Tumpey T M. Concurrent regeneration of T lymphocytes and susceptibility to HSV-1 corneal stromal disease. Curr Eye Res. 1991;10:47–53. doi: 10.3109/02713689109020357. [DOI] [PubMed] [Google Scholar]
- 17.Hill J M, Rayfield M A, Haruta Y. Strain specificity of spontaneous and adrenergically induced HSV-1 ocular reactivation in latently infected rabbits. Curr Eye Res. 1987;6:91–97. doi: 10.3109/02713688709020074. [DOI] [PubMed] [Google Scholar]
- 18.Keadle T L, Laycock K A, Miller J K, Hook K K, Fenoglio E D, Francotte M, Slaoui M, Stuart P M, Pepose J S. Efficacy of a recombinant glycoprotein D subunit vaccine on the development of primary and recurrent ocular infection with herpes simplex virus type 1 in mice. J Infect Dis. 1997;176:331–338. doi: 10.1086/514049. [DOI] [PubMed] [Google Scholar]
- 19.Kolaitis G, Doymaz M, Rouse B T. Demonstration of MHC class II-restricted cytotoxic T lymphocytes in mice against herpes simplex virus. Immunology. 1990;71:101–106. [PMC free article] [PubMed] [Google Scholar]
- 20.Langenberg A G, Burke R L, Adair S F, Sekulovich R, Tigges M, Dekker C L, Corey L. A recombinant glycoprotein vaccine for herpes simplex virus type 2: safety and immunogenicity. Ann Intern Med. 1995;122:889–898. doi: 10.7326/0003-4819-122-12-199506150-00001. . (Erratum, 123:395.) [DOI] [PubMed] [Google Scholar]
- 21.Mertz G J, Ashley R, Burke R L, Benedetti J, Critchlow C, Jones C C, Corey L. Double-blind, placebo-controlled trial of a herpes simplex virus type 2 glycoprotein vaccine in persons at high risk for genital herpes infection. J Infect Dis. 1990;161:653–660. doi: 10.1093/infdis/161.4.653. [DOI] [PubMed] [Google Scholar]
- 22.Nesburn A B, editor. Report of the corneal disease panel: vision research: a national plan 1983–1987. II, part III. St. Louis, Mo: The C.V. Mosby Co.; 1983. [Google Scholar]
- 23.Nesburn A B, Burke R L, Ghiasi H, Slanina S, Bahri S, Wechsler S L. Vaccine therapy for ocular herpes simplex virus (HSV) infection: periocular vaccination reduces spontaneous ocular HSV type 1 shedding in latently infected rabbits. J Virol. 1994;68:5084–5092. doi: 10.1128/jvi.68.8.5084-5092.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nesburn A B, Burke R L, Ghiasi H, Slanina S M, Wechsler S L. A therapeutic vaccine that reduces recurrent herpes simplex virus corneal disease. Investig Ophthalmol Visual Sci, 1998;39:1163–1170. [PubMed] [Google Scholar]
- 25.Nesburn A B, Ghiasi H, Wechsler S L. Ocular safety and efficacy of an HSV-1 gD vaccine during primary and latent infection. Investig Ophthalmol Visual Sci. 1990;31:1497–1502. [PubMed] [Google Scholar]
- 26.Nesburn A B, Robinson C, Dickinson R. Adenine arabinoside effect on experimental idoxuridine-resistant herpes simplex infection. Investig Ophthalmol. 1974;13:302–304. [PubMed] [Google Scholar]
- 26a.Nesburn, A. B., and S. L. Wechsler. Unpublished observations.
- 27.Newell C K, Martin S, Sendele D, Mercadal C M, Rouse B T. Herpes simplex virus-induced stromal keratitis: role of T-lymphocyte subsets in immunopathology. J Virol. 1989;63:769–775. doi: 10.1128/jvi.63.2.769-775.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newell C K, Sendele D, Rouse B T. Effects of CD4+ and CD8+ T-lymphocyte depletion on the induction and expression of herpes simplex stromal keratitis. Regul Immunol. 1989;2:366–369. [PubMed] [Google Scholar]
- 29.Perng G C, Dunkel E C, Geary P A, Slanina S M, Ghiasi H, Kaiwar R, Nesburn A B, Wechsler S L. The latency-associated transcript gene of herpes simplex virus type 1 (HSV-1) is required for efficient in vivo spontaneous reactivation of HSV-1 from latency. J Virol. 1994;68:8045–8055. doi: 10.1128/jvi.68.12.8045-8055.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perng G C, Thompson R L, Sawtell N M, Taylor W E, Slanina S M, Ghiasi H, Kaiwar R, Nesburn A B, Wechsler S L. An avirulent ICP34.5 deletion mutant of herpes simplex virus type 1 is capable of in vivo spontaneous reactivation. J Virol. 1995;69:3033–3041. doi: 10.1128/jvi.69.5.3033-3041.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rock D L, Nesburn A B, Ghiasi H, Ong J, Lewis T L, Lokensgard J R, Wechsler S L. Detection of latency-related viral RNAs in trigeminal ganglia of rabbits latently infected with herpes simplex virus type 1. J Virol. 1987;61:3820–3826. doi: 10.1128/jvi.61.12.3820-3826.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rouse B T, Norley S, Martin S. Antiviral cytotoxic T lymphocyte induction and vaccination. Rev Infect Dis. 1988;10:16–33. doi: 10.1093/clinids/10.1.16. [DOI] [PubMed] [Google Scholar]
- 33.Russell R G, Nasisse M P, Larsen H S, Rouse B T. Role of T-lymphocytes in the pathogenesis of herpetic stromal keratitis. Investig Ophthalmol Visual Sci. 1984;25:938–944. [PubMed] [Google Scholar]
- 34.Sanchez-Pescador L, Burke R L, Ott G, Van Nest G. The effect of adjuvants on the efficacy of a recombinant herpes simplex virus glycoprotein vaccine. J Immunol. 1988;141:1720–1727. [PubMed] [Google Scholar]
- 35.Smith R E, McDonald H R, Nesburn A B, Minckler D S. Penetrating keratoplasty: changing indications, 1947 to 1978. Arch Ophthalmol. 1980;98:1226–1229. doi: 10.1001/archopht.1980.01020040078009. [DOI] [PubMed] [Google Scholar]
- 36.Stanberry L R, Bernstein D I, Burke R L, Pachl C, Myers M G. Vaccination with recombinant herpes simplex virus glycoproteins: protection against initial and recurrent genital herpes. J Infect Dis. 1987;155:914–920. doi: 10.1093/infdis/155.5.914. [DOI] [PubMed] [Google Scholar]
- 37.Stanberry L R, Myers M G, Stephanopoulos D E, Burke R L. Preinfection prophylaxis with herpes simplex virus glycoprotein immunogens: factors influencing efficacy. J Gen Virol. 1989;70:3177–3185. doi: 10.1099/0022-1317-70-12-3177. [DOI] [PubMed] [Google Scholar]
- 38.Stroop W G, Banks M C. Herpes simplex virus type 1 strain KOS-63 does not cause acute or recurrent ocular disease and does not reactivate ganglionic latency in vivo. Acta Neuropathol (Berlin) 1994;87:14–22. doi: 10.1007/BF00386250. [DOI] [PubMed] [Google Scholar]
- 39.Walker J, Laycock K, Pepose J, Leib D. Postexposure vaccination with a virion host shutoff defective mutant reduces UV-B radiation-induced ocular herpes virus shedding in mice. Vaccine. 1998;16:6–8. doi: 10.1016/s0264-410x(97)00177-1. [DOI] [PubMed] [Google Scholar]