Abstract
We have investigated the ability of 5-methyltetrahydrofolate (5-MTHF) and tetrahydrobiopterin (BH4) to modulate nitric oxide (NO)-independent vascular relaxations that are mediated by the sequential spread of endothelial hyperpolarization through the wall of the rabbit iliac artery by means of myoendothelial and homocellular smooth muscle gap junctions. Relaxations and subintimal smooth muscle hyperpolarizations evoked by cyclopiazonic acid were depressed by the gap junction inhibitor 2-aminoethoxydiphenyl borate, whose effects were prevented by 5-MTHF and BH4, but not by their oxidized forms folic acid and 7,8-dihydrobiopterin. Analogously, 5-MTHF and BH4, but not folic acid or 7,8-dihydrobiopterin, attenuated the depression of subintimal hyperpolarization by a connexin-mimetic peptide targeted against Cx37 and Cx40 (37,40Gap 26) and the depression of subadventitial hyperpolarization by a peptide targeted against Cx43 (43Gap 26), thus reflecting the known differential expression of Cx37 and Cx40 in the endothelium and Cx43 in the media of the rabbit iliac artery. The inhibitory effects of 2-aminoethoxydiphenyl borate and 37,40Gap 26 against subintimal hyperpolarization were prevented by catalase, which destroys H2O2. 5-MTHF and BH4 thus appear capable of modulating electrotonic signaling by means of myoendothelial and smooth muscle gap junctions by reducing oxidant stress, potentially conferring an ability to reverse the endothelial dysfunction found in disease states through mechanisms that are independent of NO.
Keywords: connexin, folic acid, methotrexate, gap junction, endothelium-derived hyperpolarizing factor
The endothelium can mediate vascular relaxation not only through the release of nitric oxide (NO) and vasoactive prostanoids but also by promoting smooth muscle hyperpolarization, and there is growing evidence that electrotonic spread of endothelial hyperpolarization through gap junctions, rather than a transferable endothelium-derived hyperpolarizing factor (EDHF), may underpin this electrical response (reviewed in ref. 1). NO-independent, “EDHF-type” responses may thus be inhibited by pharmacological probes that interrupt gap junctional communication and by blockade of the Ca2+-activated K+ channels that mediate endothelial hyperpolarization (1–4). EDHF-type relaxations are depressed in many conditions that predispose to the development of vascular disease, including diabetes, hypertension, hypercholesterolemia, and hyperhomocysteinemia (5–7). In such pathophysiological states, NO-mediated endothelium-dependent relaxations may also be impaired but can be normalized by 5-methyltetrahydrofolate [5-MTHF; a reduced, biologically active form of folic acid (FA)] and by (6R)-5,6,7,8-tetrahydrobiopterin [(6R)-BH4]. Both agents prevent uncoupling of the NADPH oxidase activity of endothelial NO synthase, thereby enhancing production of NO relative to the superoxide anion, and may also increase NO bioavailability by scavenging this radical directly (8–12). Recent evidence suggests that the depressed NO-independent, EDHF-type relaxations found in diabetes and hyperhomocysteinemia can also be normalized by 5-MTHF, although underlying mechanisms remain unknown (7, 13).
In many cell types, redox mechanisms modulate the functionality of gap junctions by regulating the phosphorylation status of the connexin proteins that form these communication channels, such that the impaired cell–cell coupling and increased connexin phosphorylation associated with oxidative stress can be prevented by a wide range of antioxidants, including the enzyme catalase that destroys H2O2 (14–22). Because electrotonic signaling may underpin the EDHF phenomenon, in the present study, we have investigated whether the intrinsic antioxidant activity of 5-MTHF and (6R)-BH4 can preserve NO-independent relaxations and hyperpolarizations of the rabbit iliac artery by influencing cell–cell coupling. This result was achieved by comparing the effects of 5-MTHF and (6R)-BH4 and their oxidized forms FA and 7,8-dihydrobiopterin (BH2) on EDHF-type responses in the presence of gap junction inhibitors. Two structurally unrelated classes of probe were used, namely, 2-aminoethoxydiphenyl borate (APB) (23) and synthetic peptides homologous to the Gap 26 domain of the first extracellular loops of the major vascular connexin proteins, Cxs 37, 40, and 43 (1). Blockade of gap junctions by such agents is effected from the outside of the cell membrane and is reversible, with the peptides selectively targeting specific connexin subtypes according to sequence homology (1, 4, 23). Because it is conceivable that 5-MTHF and (6R)-BH4 interact with signaling pathways that are activated when agonists occupy specific endothelial membrane receptors, EDHF-type responses were evoked by the sarcoplasmic/endoplasmic reticulum Ca2+ ATPase inhibitor cyclopiazonic acid (CPA) (4). This agent depletes Ca2+ stores by preventing Ca2+ uptake and thereby stimulates store-operated Ca2+ entry and secondary Ca2+-activated K+ channel activation (1, 24). Immunostaining has shown that Cx37 and Cx40 are the dominant connexin subtypes present in the endothelium of the rabbit iliac artery, whereas Cx43 dominates in its media (4). Connexin-mimetic peptides homologous to the Gap 26 domain of the first extracellular loop of Cx37/Cx40 and Cx43 (denoted as 37,40Gap 26 and 43Gap 26) were therefore used to differentiate between myoendothelial and smooth muscle communication pathways and to localize the sites of action of 5-MTHF and (6R)-BH4. The findings suggest that these compounds can modulate electrotonic signaling by opposing an apparent ability of oxidant stress to depress cell–cell coupling in the vascular wall.
Materials and Methods
Mechanical Responses. Iliac arteries were obtained from male NZW rabbits (2–2.5 kg) killed with sodium pentobarbitone (120 mg/kg i.v.). Rings 2–3 mm wide were mounted in a myograph containing oxygenated (95% O2 and 5% CO2) Holman's buffer (120 mM NaCl/5 mM KCl/2.5 mM CaCl2/1.3 mM NaH2PO4/25 mM NaHCO3/11 mM glucose/10 mM sucrose) at 37°C and maintained at a resting tension of 2 mN during a 1-h equilibration period, followed by incubation for 30 min with the NO synthase inhibitor NG-nitro-l-arginine methyl ester (l-NAME; 300 μM) and the cyclooxygenase inhibitor indomethacin (10 μM). The rings were incubated for another 40 min in the additional presence of APB (10 μM), 5-MTHF, FA or its structural analogue methotrexate (25), (6R)-BH4 or its stereoisomer (6S)-BH4,BH2 (each at 100 μM), and catalase (2,000 units/ml) as required. Tone was induced by phenylephrine (1 μM) to construct cumulative concentration-relaxation curves for CPA. It should be noted that the two BH4 stereoisomers possess identical antioxidant activity, whereas only (6R)-BH4 is an effective cofactor for NO synthase (10, 11).
Calcium Imaging. APB has been reported to impair gap junctional communication and electrical coupling with an IC50 of 5–10 μM, whereas higher concentrations may additionally attenuate store-operated Ca2+ entry and/or inositol trisphosphate-induced Ca2+ release (23, 26, 27). The isolated rabbit aortic valve was therefore used to confirm that 10 μM APB did not affect Ca2+ mobilization by CPA. This preparation consists of endothelial cells supported by a collagenous matrix (24), and thereby avoids alterations in endothelial Ca2+ homeostasis by electrical and/or chemical signals transmitted from smooth muscle cells via myoendothelial gap junctions in arterial segments (28, 29). Valve leaflets were incubated with Fura-2AM (5 μM) for 2 h in oxygenated Holman's buffer at room temperature (25°C), followed by a washout, and then maintained in buffer containing l-NAME (300 μM) and indomethacin (10 μM) for 30 min before additional incubation with APB, 5-MTHF, and BH4 as required for 40 min. To study Ca2+ mobilization by CPA, the preparations were alternately excited at 340/380 nm, and images were acquired at 2-s intervals with an exposure time of 100 ms at each wavelength. Background-corrected Fura-2 fluorescent ratio (F340/380) was calculated to give an index of intracellular [Ca2+] (24). Data are presented as the percentage change in this ratio after exposure to 30 μM CPA.
Microelectrode Studies. Endothelial and smooth muscle membrane potential were recorded with glass capillary microelectrodes filled with 3M KCl by using conventional whole-cell patch-clamp or intracellular sharp electrode techniques in an organ chamber superfused (2 ml/min at 37°C) with oxygenated Holman's buffer containing l-NAME (300 μM) and indomethacin (10 μM). Arterial strips were held adventitia down for endothelial patching and subintimal smooth muscle impalement, and intima down for subadventitial smooth muscle impalement as described (3, 4). Other drugs were administered directly into the organ chamber and incubated for 40 min as above under conditions of no flow before stimulation by CPA at a concentration (30 μM) that does not affect smooth muscle membrane potential in endothelium-denuded rabbit iliac arteries (4). APB (10 μM), 37,40Gap 26 (VCYDQAFPISHIR, 600 μM), and 43Gap 26 (VCYDKSFPISHVR, 100 μM) were used to block gap junctional communication, with pilot studies showing that 100 μM 43Gap 26 inhibited electrotonic signaling between smooth muscle cells as effectively as the concentration of 600 μM used in previous studies (4). In some experiments, CPA-evoked hyperpolarizations were studied in the presence of the NO donor spermine-NONOate (10 μM) or the NO scavenger hemoglobin (20 μM), prepared by lysis of rabbit erythrocytes in HPLC grade water and purification on a Sephadex G25 column.
Statistics. EC50 values are expressed as mean with 95% confidence intervals; results are otherwise given as mean ± SEM, where n denotes the number of animals studied for each data point. Data were compared by the Student t test for paired or unpaired data as appropriate and concentration–response curves assessed by ANOVA followed by the Tukey–Cramer posttest. Maximal hyperpolarizations evoked by CPA under different experimental conditions were compared by ANOVA followed by Dunnett's multiple comparison test. P < 0.05 was considered significant.
Results
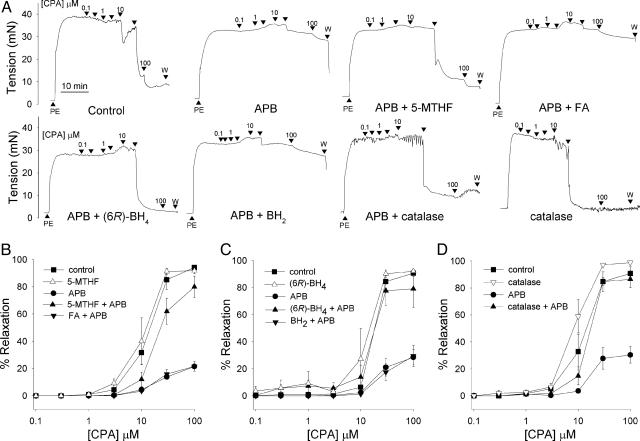
Mechanical Responses. In each experimental group, EDHF-type relaxations evoked by CPA attained a maximum of 90–95% of phenylephrine-induced tone with an EC50 of ≈15 μM, and concentration–relaxation curves were not significantly affected by 100 μM 5-MTHF, (6R)-BH4, or 2,000 units/ml catalase (Fig. 1 and Table 1). After incubation with 10 μM APB, maximal relaxations to CPA were reduced to 25–30% of phenylephrine-induced tone without systematic change in EC50 (Fig. 1 and Table 1). Coincubation with 100 μM 5-MTHF, 100 μM(6R)-BH4, or 2,000 units/ml catalase prevented the loss of CPA-evoked relaxation observed in the presence of APB, whereas 100 μM FA, methotrexate, and BH2 were ineffective (Fig. 1 and Table 1).
Fig. 1.
Mechanical studies with APB. (A) Original recordings showing that inhibition of CPA-evoked EDHF-type relaxations by 10 μM APB in iliac artery rings was prevented by 100 μM 5-MTHF, (6R)-BH4, and 2,000 units/ml catalase but not by 100 μM FA or BH2. PE, 1 μM phenylephrine. (B–D) Concentration–response curves for CPA-evoked relaxation in the presence and absence of 10 μM APB under corresponding experimental conditions. Control relaxations were unaffected by 5-MTHF, (6R)-BH4, and catalase.
Table 1. Maximal relaxations to CPA as a percent of PE-induced tone (Rmax) and concentrations of CPA causing half-maximal relaxation (EC50) under different experimental conditions.
| Group (n) | Rmax, % | EC50, μM |
|---|---|---|
| Control (8) | 95.1 ± 4.7 | 13.2 (10.7-16.3) |
| 5-MTHF (4) | 94.2 ± 6.6 | 11.2 (8.5-14.6) |
| APB (8) | 23.5 ± 1.9 | 24.1 (17.7-32.7) |
| 5-MTHF plus APB (12) | 81.0 ± 5.8 | 19.3 (14.5-25.6) |
| FA plus APB (6) | 22.1 ± 2.3 | 20.2 (13.4-30.4) |
| Methotrexate plus APB (6) | 31.9 ± 6.3 | 23.2 (11.5-46.7) |
| Control (9) | 90.7 ± 2.0 | 17.6 (15.3-20.1) |
| (6R)-BH4 (3) | 91.9 ± 8.9 | 12.7 (5.5-29.3) |
| APB (9) | 28.8 ± 3.4 | 21.7 (13.7-34.3) |
| (6R)-BH4 plus APB (5) | 79.1 ± 7.4 | 14.4 (5.2-40.0) |
| BH2 plus APB (4) | 30.0 ± 4.2 | 26.9 (17.9-40.3) |
| Control (7) | 91.5 ± 6.3 | 12.5 (9.3-16.6) |
| Catalase (4) | 99.6 ± 3.9 | 8.8 (7.4-10.5) |
| APB (7) | 30.4 ± 3.8 | 16.8 (8.7-32.4) |
| Catalase plus APB (4) | 86.5 ± 3.4 | 13.9 (9.7-19.8) |
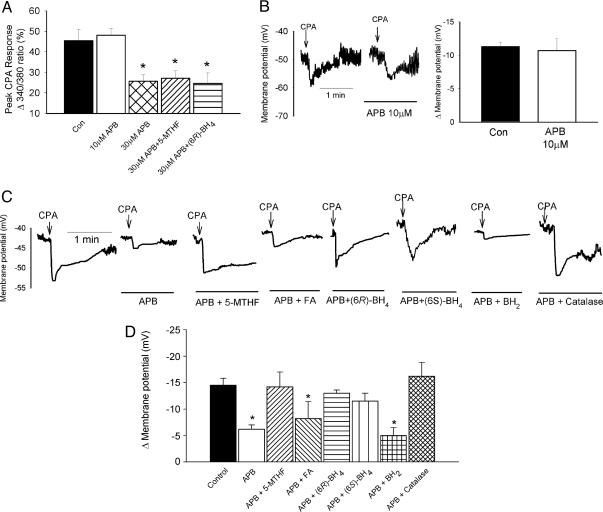
Aortic Valve [Ca2+]i. The peak increase in F340/380 ratio evoked by CPA was 45.4 ± 5.6% (n = 17) and was not affected by 10 μMAPB (n = 6), whereas 30 μM APB reduced this response to 25.6 ± 3.3% (n = 18, P < 0.05; Fig. 2A). The inhibitory effects of 30 μM APB on CPA-evoked increases in [Ca2+]i were not prevented by 100 μM 5-MTHF or (6R)-BH4 (n = 6 and 14, respectively; Fig. 2A).
Fig. 2.
Imaging and electrophysiological studies with APB. (A) Histogram showing that 10 μM APB did not affect Ca2+ mobilization by 30 μM CPA in the endothelium of the aortic valve, whereas 30 μM APB caused a significant reduction in the 380:340 fluorescent ratio that was insensitive to 100 μM 5-MTHF or (6R)-BH4.(B) Whole-cell patch-clamp recordings and histogram showing that 10 μM APB did not depress endothelial hyperpolarizations evoked by 30 μM CPA. (C and D) Original recordings and histogram showing that attenuation of CPA-evoked subintimal smooth muscle hyperpolarization by 10 μM APB was prevented by 100 μM 5-MTHF, (6R)-BH4,(6S)-BH4, or 2,000 units/ml catalase but not by 100 μM FA or BH2. *, P < 0.05, compared with control.
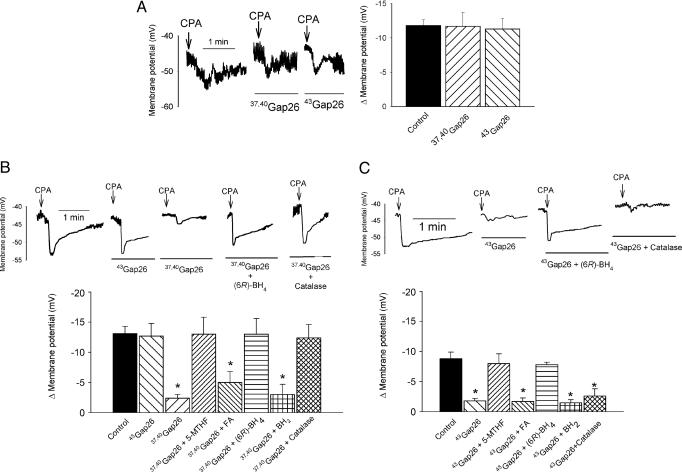
Endothelial Membrane Potential. Resting endothelial membrane potential was –44.8 ± 2.8 mV and 30 μM CPA evoked a peak hyperpolarization of 11.6 ± 0.7mV(n = 9). Neither parameter was significantly affected by 10 μM APB, 600 μM 37,40Gap 26, or 100 μM 43Gap 26 (n = 3 for each; Figs. 2B and 3A).
Fig. 3.
Electrophysiological studies with connexin-mimetic peptides. (A) Whole-cell patch-clamp recordings and histogram confirming that 600 μM 37,40Gap 26 and 100 μM 43Gap 26 did not depress endothelial hyperpolarizations evoked by 30 μM CPA. (B and C) Original recordings and histograms comparing the effects of the peptides and 2,000 units/ml catalase on subintimal (B) and subadventitial (C) hyperpolarizations evoked by CPA. 37,40Gap 26 attenuated the transmission of endothelial hyperpolarization to subintimal smooth muscle, whereas 43Gap 26 selectively impaired transmission of subintimal hyperpolarization across the vessel wall. The effects of both peptides were prevented by 100 μM 5-MTHF and (6R)-BH4 but not by 100 μM FA or BH2. Catalase prevented the effects of 37,40Gap 26 on subintimal hyperpolarization, but not those of 43Gap 26 on subadventitial hyperpolarization. *, P < 0.05, compared with control.
Effects of APB on Smooth Muscle Hyperpolarization. Resting subintimal membrane potential was –46.0 ± 1.2 mV (n = 22) and was unaffected by 10 μMAPB(n = 22) or the combination of APB with 100 μM 5-MTHF, FA, methotrexate, (6R)-BH4, (6S)-BH4, or BH2 (n = 4 in each case, Fig. 2C; numerical data not shown). Incubation with 10 μM APB reduced the peak subintimal hyperpolarization evoked by 30 μM CPA from 14.5 ± 1.3 mV to 6.2 ± 0.8 mV (n = 22, P < 0.05; Fig. 2 C and D), and this effect of APB was prevented by 100 μM 5-MTHF, (6R)-BH4, or (6S)-BH4, and with use of 2,000 units/ml catalase (n = 4 for each; Fig. 2 C and D). By contrast, in preparations incubated with 100 μM FA, methotrexate, or BH2, subintimal hyperpolarizations were 8.2 ± 2.2, 8.4 ± 2.8, and 5.0 ± 0.5 mV, respectively (n = 4, P < 0.05 for each; Fig. 2 C and D) and statistically similar to those observed in the presence of 10 μMAPB alone. Denatured catalase, obtained by heating a stock solution of catalase in Holman's buffer to 75°C for 5 min, did not prevent the inhibition of subintimal hyperpolarization by 10 μM APB (n = 3, data not shown).
Effects of Connexin-Mimetic Peptides on Smooth Muscle Hyperpolarization. Resting subintimal and subadventitial membrane potentials were –41.4 ± 1.0 mV and –42.1 ± 1.1 mV (n = 28 and 18, respectively) and unaffected by 600 μM 37,40Gap 26 or 100 μM 43Gap 26 or by the combination of 100 μM 5-MTHF, (6R)-BH4, FA, or BH2 with these peptides (Fig. 3 B and C; numerical data not shown). Peak subintimal hyperpolarizations evoked by 30 μM CPA were reduced from 13.1 ± 1.2 mV to 2.4 ± 0.6 mV by 600 μM 37,40Gap 26 (n = 20, P < 0.05; Fig. 3B). This inhibition was prevented by 100 μM 5-MTHF or (6R)-BH4 (n = 4 for both), but not with 100 μM FA or BH2, when responses were reduced to 5.0 ± 1.8 mV and 3.7 ± 1.7 mV, respectively, and did not differ from those observed in the presence of 37,40Gap 26 alone (n = 4 for both; Fig. 3B). Incubation with 100 μM 43Gap 26 did not significantly affect CPA-evoked subintimal hyperpolarization (n = 4), whereas recordings from subadventitial smooth muscle cells were reduced from 8.8 ± 1.1 mV to 1.8 ± 0.4 mV (n = 18, P < 0.05; Fig. 3 B and C). The ability of 43Gap 26 to depress subadventitial hyperpolarization was prevented by 100 μM 5-MTHF or (6R)-BH4 (n = 4 for both; Fig. 3C), but was unaffected with 100 μM FA or BH2, when reductions to 1.7 ± 0.6 mV and 1.5 ± 0.2 mV were recorded, and did not differ from those observed in the presence of 37,40Gap 26 alone (n = 4 for both; Fig. 3C). Catalase (2,000 units/ml) prevented the inhibitory effects of 600 μM 37,40Gap 26 on subintimal hyperpolarization, but did not significantly affect the reduction in subadventitial responses caused by 100 μM 43Gap 26 (n = 3 for both; Fig. 3 B and C).
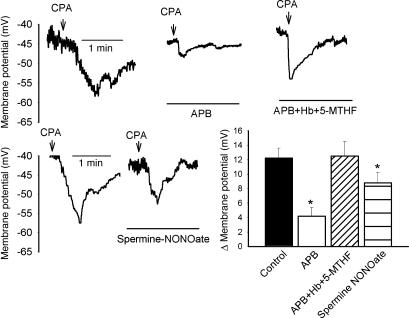
Effects of Hemoglobin and Exogenous NO. To exclude the possibility that NO released from endothelial stores contributes to l-NAME-insensitive EDHF-type responses (30), experiments were performed with the NO scavenger hemoglobin and the NO donor spermine-NONOate. In this series of experiments, resting subintimal membrane potential was –44.4 ± 3.8 mV (n = 8) and was unaffected by incubation with 20 μM hemoglobin or 10 μM spermine-NONOate (n = 4 for both; Fig. 4, data not shown). Incubation with 10 μM APB reduced CPA-evoked smooth muscle hyperpolarizations from 12.2 ± 1.2 mV to 4.2 ± 1.2 mV(n = 8 and 4, respectively; P < 0.05) and 20 μM hemoglobin did not affect the ability of 100 μM 5-MTHF to prevent this decrease in subintimal response (n = 4; Fig. 4). Administration of 10 μM spermine-NONOate significantly reduced CPA-evoked subintimal smooth muscle hyperpolarizations to 8.8 ± 1.4mV(n = 4, P < 0.05; Fig. 4).
Fig. 4.
Original recordings and histogram showing that the ability of 100 μM 5-MTHF to prevent inhibition of CPA-evoked subintimal hyperpolarization by 10 μM APB was not diminished to 10 μM hemoglobin, and that 10 μM spermine-NONOate depressed the hyperpolarizing response to CPA without altering resting membrane potential. *, P < 0.05, compared with control.
Discussion
The present study has provided evidence that the redox status of endothelial and smooth muscle cells can play a key role in regulating the electrotonic transmission of endothelial hyperpolarization through the wall of the rabbit iliac artery under specific experimental conditions. Although control relaxations were unaffected by 5-MTHF, (6R)-BH4, and catalase, each of these antioxidants was able to prevent the suppression of EDHF-type responses by two structurally unrelated classes of gap junction blocker that act from the outside of the cell membrane. The findings are likely to reflect interactions between distinct regulatory mechanisms because redox status is thought to modulate cell–cell coupling via intracellular signaling pathways (14–22).
NO-independent relaxations and subintimal smooth muscle hyperpolarizations evoked by a nearly maximally effective concentration of CPA (30 μM) were reduced by ≈75% in the presence of 10 μM APB. This inhibition was prevented by 5-MTHF and (6R)-BH4, but not by their oxidized forms FA and BH2, or by methotrexate, an analogue of FA that inhibits dihydrofolate reductase (25). The ability of 10 μM APB to inhibit gap junctional communication appeared to be specific, because at this concentration, the compound did not impair endothelial hyperpolarization in the iliac artery or Ca2+ mobilization in the endothelium of the rabbit aortic valve. By contrast, 30 μM APB depressed CPA-evoked increases in Ca2+ in the valvular endothelium through mechanisms that were insensitive to 5-MTHF and (6R)-BH4, consistent with additional effects of APB on pathways that do not contribute directly to intercellular communication (23, 26, 27). In parallel experiments, the sites of action of 5-MTHF and (6R)-BH4 were localized by exploiting the ability of 37,40Gap 26 to attenuate the transmission of endothelial hyperpolarization to subintimal smooth muscle and 43Gap 26 to attenuate electrotonic signaling across the media of the rabbit iliac artery without affecting CPA-evoked changes in endothelial membrane potential. Subintimal and subadventitial hyperpolarizations evoked by CPA were both reduced by ≈75%, with the effects of the peptides being prevented with 5-MTHF and (6R)-BH4, but not with FA or BH2. Taken together, the findings suggest that redox mechanisms can modulate the EDHF phenomenon by affecting the functionality of both myoendothelial and homocellular smooth muscle gap junction channels.
It is well established that authentic H2O2, organic hydroperoxides, and H2O2-generating enzyme systems impair dye transfer via gap junctions (e.g.) in hepatocytes, liver epithelial cells, and myometrial smooth muscle cells (14, 16, 19, 21), and in cochlear Henson cells, oxidative stress depresses electrical coupling through mechanisms that are sensitive to catalase, suggesting a specific role for endogenous H2O2 (18). In the present study, the inhibitory effects of APB against CPA-evoked relaxation and subintimal hyperpolarization and 37,40Gap 26 against subintimal hyperpolarization were prevented by catalase, consistent with the hypothesis that endogenous H2O2 acts synergistically with these probes to depress myoendothelial communication. Although catalase did not prevent the inhibition of electrotonic smooth muscle signaling by 43Gap 26, 5-MTHF, and (6R)-BH4 were both effective against this peptide. A possible explanation for these observations is that catalase, which reduces intracellular oxidant stress by destroying H2O2 in the extracellular space, is unable to penetrate into the media of the rabbit iliac artery. This thick-walled vessel possesses ≈10 layers of smooth muscle cells, and its internal elastic lamina and substantial adventitia (4) would be expected to restrict the entry of large proteins such as catalase.
The concentration of l-NAME (300 μM) used the present study has previously been shown to result in nearly complete loss of endothelial NO synthase activity because it abolishes endothelium-dependent elevations in cGMP in the rabbit iliac artery (31). Because l-NAME also inhibits superoxide formation by NO synthase (32), the ability of 5-MTHF and (6R)-BH4 to modulate the EDHF phenomenon is unlikely to reflect decreased superoxide production as a result of enhanced cofactor activity (8, 10). Indeed, the (6R)- and (6S)-BH4 stereoisomers possess equivalent antioxidant activity, and both compounds preserved CPA-evoked subintimal hyperpolarization in the presence of APB, whereas only (6R)-BH4 is an effective cofactor for NO synthase (10, 11). In specific artery types, release of NO from endothelial “stores” has been hypothesized to mediate l-NAME-insensitive EDHF-type responses (30), thus raising the theoretical possibility that direct scavenging of superoxide by 5-MTHF, (6R)-BH4, or (6S)-BH4 might stabilize NO derived from this source. However, this mechanism was excluded by observations that: (i) the NO scavenger hemoglobin did not attenuate subintimal hyperpolarizations evoked by CPA in the combined presence of APB and 5-MTHF, and (ii) the NO donor spermine-NONOate depressed subintimal hyperpolarizations to CPA without affecting resting smooth muscle membrane potential, thus suggesting that NO may attenuate endothelial hyperpolarization and/or electrotonic coupling via myoendothelial gap junctions in the rabbit iliac artery.
Potential vascular sources of superoxide anions and H2O2 include mitochondria and NADPH/xanthine oxidases (reviewed in refs. 33 and 34), but which contribute to the redox status of endothelial and smooth muscle cells under the present experimental conditions remains to be elucidated. The mechanisms through which oxidant stress impairs communication via gap junctions also remain incompletely characterized, although there is evidence that oxidants such as H2O2 and depletion of the endogenous antioxidant glutathione attenuate cell–cell coupling through similar signaling pathways (16, 20, 22). In the case of gap junctions constructed from Cx43, oxidant stress can depress channel functionality by promoting phosphorylation of residues present in the cytoplasmic carboxyl tail of this connexin protein by the tyrosine kinase Src, serine/threonine kinases such as mitogen-activated protein kinases and protein kinase C, and by inactivating tyrosine-protein phosphatases (22, 35, 36). In liver epithelial cells, authentic H2O2 reversibly attenuates intercellular communication through an action that has been attributed to hyperphosphorylation of Cx43 secondary to activation of extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinases (16, 21), with impairment of gap junctional communication being prevented by antioxidants such as N-acetylcysteine and α-tocopherol (17). By contrast, the ability of the tumor promoter 12-O-tetradecanoyl phorbol acetate to attenuate coupling in the same cell line involves hyperphosphorylation of Cx43 by protein kinase C, with the protective role of the antioxidants boldine and probucol then reflecting reduced translocation of this enzyme to the cell membrane (15). Because 5-MTHF, (6R)-BH4, and catalase each prevented the inhibition of myoendothelial signaling by APB and 37,40Gap 26 in the rabbit iliac artery, oxidant stress also appears to modulate the functionality of gap junctions containing Cx37 or Cx40, although no previous reports appear to have investigated the role of redox mechanisms in regulating communication by means of channels constructed from these connexin subtypes. Whereas the selective effects of 37,40Gap26 and 43Gap26 against signaling via myoendothelial and homocellular smooth muscle gap junctions in the rabbit iliac artery imply that these peptides do not themselves alter redox status by means of nonspecific membrane effects, it should be noted that pharmacological inhibition of cell–cell coupling can markedly exacerbate the cellular effects of superimposed oxidant stress (e.g., in hippocampal cell cultures) without increasing oxidant stress under basal conditions (37). Observations that EDHF-type responses evoked by CPA were unaffected by 5-MTHF, (6R)-BH4, and catalase in control experiments nevertheless suggest that redox mechanisms play a prominent role in regulating electrotonic signaling through the vascular wall only under conditions where gap junctional communication is already impaired (e.g., in the presence of APB or connexinmimetic peptides). Indeed, previous studies (38) have shown that the Ca2+ ionophore A23187 is capable of promoting extracellular release of H2O2 from the endothelium of the rabbit iliac artery at levels that are sufficient to evoke catalase-sensitive smooth muscle relaxations, but are insufficient to reduce the magnitude of a coexistent subintimal hyperpolarization that is transmitted from the endothelium via myoendothelial gap junctions.
In conclusion, we have demonstrated that 5-MTHF and BH4, but not FA or BH2, can modulate arterial function through effects on gap junctional communication and electrotonic signaling. The findings thus raise the possibility that pharmacological manipulation of oxidant stress may offer therapeutic potential in the normalization of endothelium-dependent responses in the many disease states in which the EDHF phenomenon is impaired.
Acknowledgments
This work was supported in part by the British Heart Foundation (A.T.C.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: EDHF, endothelium-derived hyperpolarizing factor; 5-MTHF, 5-methyltetrahydrofolate; BH4, tetrahydrobiopterin; BH2, 7,8-dihydrobiopterin; (6R), (6R)-5,6,7,8; APB, 2-aminoethoxydiphenyl borate; l-NAME; NG-nitro-l-arginine methyl ester; CPA, cyclopiazonic acid; FA, folic acid.
References
- 1.Griffith, T. M. (2004) Br. J. Pharmacol. 141, 881–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaytor, A. T., Evans, W. H. & Griffith, T. M. (1998) J. Physiol. 508, 561–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffith, T. M., Chaytor, A. T., Taylor, H. J., Giddings, B. D. & Edwards D. H. (2002) Proc. Natl. Acad. Sci. USA 99, 6392–6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaytor, A. T., Bakker, L. M., Edwards, D. H. & Griffith, T. M. (2005) Br. J. Pharmacol. 144, 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feletou, M. & Vanhoutte, P. M. (2004) Pharmacol. Res. 49, 565–580. [DOI] [PubMed] [Google Scholar]
- 6.Li, H., Brodsky, S., Kumari, S., Valiunas, V., Brink, P., Kaide, J., Nasjletti, A. & Goligorsky, M. S. (2002) Am. J. Physiol. 282, H2124–H2133. [DOI] [PubMed] [Google Scholar]
- 7.De Vriese, A. S., Blom, H. J., Heil, S. G., Mortier, S., Kluijtmans, L. A., Van de Voorde, J. & Lameire, N. H. (2004) Circulation 109, 2331–2336. [DOI] [PubMed] [Google Scholar]
- 8.Verhaar, M. C., Stroes, E. & Rabelink, T. J. (2002) Arterioscler. Thromb. Vasc. Biol. 22, 6–13. [DOI] [PubMed] [Google Scholar]
- 9.Werner, E. R., Gorren, A. C., Heller, R., Werner-Felmayer, G. & Mayer, B. (2003) Exp. Biol. Med. 228, 1291–1302. [DOI] [PubMed] [Google Scholar]
- 10.Ihlemann, N., Rask-Madsen, C., Perner, A., Dominguez, H., Hermann, T., Kober, L. & Torp-Pedersen, C. (2003) Am. J. Physiol. 285, H875–H882. [DOI] [PubMed] [Google Scholar]
- 11.Klatt, P., Schmid, M., Leopold, E., Schmidt, K., Werner, E. R. & Mayer, B. (1994) J. Biol. Chem. 269, 13861–13866. [PubMed] [Google Scholar]
- 12.Hyndman, M. E., Verma, S., Rosenfeld, R. J., Anderson, T. J. & Parsons, H. G. (2002) Am. J. Physiol. 282, H2167–H2172. [DOI] [PubMed] [Google Scholar]
- 13.De Vriese, A. S., Van de Voorde, J., Blom, H. J., Vanhoutte, P. M., Verbeke, M. & Lameire, N. H. (2000) Diabetologia 43, 1116–1125. [DOI] [PubMed] [Google Scholar]
- 14.Ruch, R. J. & Klaunig, J. E. (1988) Toxicol. Appl. Pharmacol. 94, 427–436. [DOI] [PubMed] [Google Scholar]
- 15.Hu, J., Speisky, H. & Cotgreave, I. A. (1995) Biochem. Pharmacol. 50, 1635–1643. [DOI] [PubMed] [Google Scholar]
- 16.Upham, B. L., Kang, K. S., Cho, H. Y. & Trosko, J. E. (1997) Carcinogenesis 18, 37–42. [DOI] [PubMed] [Google Scholar]
- 17.Huang, R. P., Peng, A., Hossain, M. Z., Fan, Y., Jagdale, A. & Boynton, A. L. (1999) Carcinogenesis 20, 485–492. [DOI] [PubMed] [Google Scholar]
- 18.Todt, I., Ngezahayo, A., Ernst, A. & Kolb, H. A. (1999) Pflügers Arch. 438, 865–867. [DOI] [PubMed] [Google Scholar]
- 19.Krieger, T. R. & Loch-Caruso, R. (2001) Biol. Reprod. 64, 537–547. [DOI] [PubMed] [Google Scholar]
- 20.Zhou, Z. Y., Sugawara, K., Hashi, R., Muramoto, K., Mawatari, K., Matsukawa, T., Liu, Z. W., Devadas, M. & Kato, S. (2001) Neuroscience 102, 959–967. [DOI] [PubMed] [Google Scholar]
- 21.Cho, J. H., Cho, S. D., Hu, H., Kim, S. H., Lee, S. K., Lee, Y. S. & Kang, K. S. (2002) Carcinogenesis 23, 1163–1169. [DOI] [PubMed] [Google Scholar]
- 22.Abdelmohsen, K., Gerber, P. A., von Montfort, C., Sies, H. & Klotz, L. O. (2003) J. Biol. Chem. 278, 38360–38367. [DOI] [PubMed] [Google Scholar]
- 23.Harks, E. G., Camina, J. P., Peters, P. H., Ypey, D. L., Scheenen, W. J., van Zoelen, E. J. & Theuvenet, A. P. (2003) FASEB J. 17, 941–943. [DOI] [PubMed] [Google Scholar]
- 24.Li, L. & van Breemen, C. (1996) Am. J. Physiol. 270, H837–H848. [DOI] [PubMed] [Google Scholar]
- 25.Goodsell, D. S. (1999) Stem Cells (Dayton) 17, 314–315. [DOI] [PubMed] [Google Scholar]
- 26.Bootman, M. D., Collins, T. J., Mackenzie, L., Roderick, H. L., Berridge, M. J. & Peppiatt, C. M. (2002) FASEB J. 16, 1145–1150. [DOI] [PubMed] [Google Scholar]
- 27.Soulsby, M. D. & Wojcikiewicz, R. J. (2002) Cell Calcium 32, 175–181. [DOI] [PubMed] [Google Scholar]
- 28.Dora, K., Doyle, M. P. & Duling, B. R. (1997) Proc. Natl. Acad. Sci. USA 94, 6529–6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuster, A., Oishi, H., Beny, J.-L, Stergiopulos, N. & Meister, J. J. (2001) Am. J. Physiol. 280, H1088–H1096. [DOI] [PubMed] [Google Scholar]
- 30.Chauhan, S., Rahman, A., Nilsson, H., Clapp, L., MacAllister, R. & Ahluwalia, A. (2003) Cardiovasc. Res. 57, 207–216. [DOI] [PubMed] [Google Scholar]
- 31.Taylor, H. J., Chaytor, A. T., Edwards, D. H. & Griffith, T. M. (2001) Biochem. Biophys. Res. Commun. 283, 583–589. [DOI] [PubMed] [Google Scholar]
- 32.Xia, Y., Tsai, A. L., Berka, V. & Zweier, J. L. (1998) J. Biol. Chem. 273, 25804–25808. [DOI] [PubMed] [Google Scholar]
- 33.Li, J. M. & Shah, A. M. (2004) Am. J. Physiol. 287, R1014–R1030. [DOI] [PubMed] [Google Scholar]
- 34.Griendling, K. K., Sorescum D., Lasseguem B. & Ushio-Fukai, M. (2000) Arterioscler. Thromb. Vasc. Biol. 20, 2175–2183. [DOI] [PubMed] [Google Scholar]
- 35.Cottrell, G. T., Lin, R., Warn-Cramer, B. J., Lau, A. F. & Burt, J. M. (2003) Am. J. Physiol. 284, C511–C520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lampe, P. D. & Lau, A. F. (2004) Int. J. Biochem. Cell Biol. 36, 1171–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blanc, E. M., Bruce-Keller, A. J. & Mattson, M. P. (2004) J. Neurochem. 70, 958–970. [DOI] [PubMed] [Google Scholar]
- 38.Chaytor, A. T., Edwards, D. H., Bakker, L. M. & Griffith, T. M. (2003) Proc. Natl. Acad. Sci. USA 100, 15212–15217. [DOI] [PMC free article] [PubMed] [Google Scholar]