Abstract
The cytomegalovirus (CMV) assembly protein precursor (pAP) interacts with the major capsid protein (MCP), and this interaction is required for nuclear translocation of the MCP, which otherwise remains in the cytoplasm of transfected cells (L. J. Wood et al., J. Virol. 71:179–190, 1997). We have interpreted this finding to indicate that the CMV MCP lacks its own nuclear localization signal (NLS) and utilizes the pAP as an NLS-bearing escort into the nucleus. The CMV pAP amino acid sequence has two clusters of basic residues (e.g., KRRRER [NLS1] and KARKRLK [NLS2], for simian CMV) that resemble the simian virus 40 large-T-antigen NLS (D. Kalderon et al., Cell 39:499–509, 1984) and one of these (NLS1) has a counterpart in the pAP homologs of other herpesviruses. The work described here establishes that NLS1 and NLS2 are mutually independent NLS that can act (i) in cis to translocate pAP and the related proteinase precursor (pNP1) into the nucleus and (ii) in trans to transport MCP into the nucleus. By using combinations of NLS mutants and carboxy-terminal deletion constructs, we demonstrated a self-interaction of pAP and cytoplasmic interactions of pAP with pNP1 and of pNP1 with itself. The relevance of these findings to early steps in capsid assembly, the mechanism of MCP nuclear transport, and the possible cytoplasmic formation of protocapsomeric substructures is discussed.
Herpesvirus capsids assemble in the nucleus during the late phase of infection. The outer shell of the capsid is composed of four protein species; in cytomegalovirus (CMV) these are called the major capsid protein (MCP; e.g., human CMV [HCMV] UL86, 154 kDa), the minor capsid protein (mCP; e.g., HCMV UL85, 34 kDa), the minor capsid-binding protein (mCBP; e.g., HCMV UL46, 33 kDa), and the smallest capsid protein (e.g., HCMV UL48/49, 8.5 kDa). Organization of these four proteins into a capsid appears to be coordinated by two genetically related, internally situated proteins, in CMV called the proteinase precursor (pNP1; e.g., HCMV UL80a, 74 kDa) and the assembly protein precursor (pAP; e.g., HCMV UL80.5, 38 kDa). During CMV capsid maturation, the internal proteins are cleaved by the autocatalytic proteinase [(pNP1→NP1 + tail; NP1→NP1c + NP1n; NP1n→An + Ac) and (pAP→AP + tail)], and most, if not all, of the cleavage products are eliminated from the particle in conjunction with DNA packaging (reviewed in reference 22).
In addition to the transient but essential roles played by the CMV proteinase and assembly protein precursors, both are distinguished among the capsid proteins in several ways: (i) pNP1 and pAP are genetically related by the overlapping, 3′ coterminal arrangement of the genes encoding them (60) (thus, pNP1 is an amino-terminal, in-frame extension of pAP) (Fig. 1); (ii) both are proteolytically processed (23, 62) and are modified by phosphorylation (21, 23, 32, 44b, 49); and (iii) both interact with the CMV MCP through a 21-amino-acid carboxyl conserved domain and self-interact through a 19-amino-acid amino conserved domain (63). The herpes simplex virus (HSV) pAP homolog, pVP22a (ICP35c,d), has similar characteristics (14, 24, 31, 38, 44, 45).
FIG. 1.
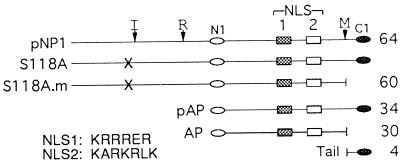
Landmarks of the SCMV pNP1 and pAP. Landmarks and abbreviations are as follows: NLS1 (dark gray rectangles) and NLS2 (light gray rectangles); N1 (empty oval) and C1 (filled oval), which represent, respectively, the 13-amino-acid amino-terminal sequence and 21-amino-acid carboxy-terminal sequence used to prepare the antipeptide antisera, anti-N1 and anti-C1 (53); the maturational (M; VNA↓S), release (R; YVKA↓S), and internal (I; INA↓A) sites that are cleaved autoproteolytically by pNP1 (or assemblin) to eliminate the tail (61, 62), free the amino terminal 28-kDa proteolytic domain (assemblin) (61), and convert assemblin from a one-chain to a two-chain enzyme (29, 30); and X, which represents mutation S118A that replaces the catalytic nucleophile and inactivates the proteinase (61). Designations for the proteinase precursor (pNP1), a proteolytically inactive form of pNP1 (S118A) and its mature form cleaved at the M site (S118A.m), the assembly protein precursor (pAP) and its mature form (AP), and the carboxyl tail removed by M-site cleavage are indicated at the left of each line, and the corresponding molecular weight (103) is indicated at the right. The amino acid sequences of NLS1 and NLS2 are given at the lower left.
Insight into the functional significance of the intermolecular interaction between pAP and MCP was obtained by using a transient transfection/intracellular localization assay based on indirect immunofluorescence (IF). In this assay system, the CMV MCP does not enter the nucleus when expressed alone but is efficiently transported into the nucleus by pAP and not by its cleavage product, AP (63). Similar results were found with the counterpart proteins of HSV, although exclusion of HSV MCP from the nucleus appears to be less complete (36, 42, 47) (see Fig. 5B). We interpreted these observations as indicating that the CMV MCP lacks its own nuclear localization signal (NLS), forms an MCP-pAP complex in the cytoplasm, and through this complex takes advantage of NLS present in pAP for transport into the nucleus.
FIG. 5.
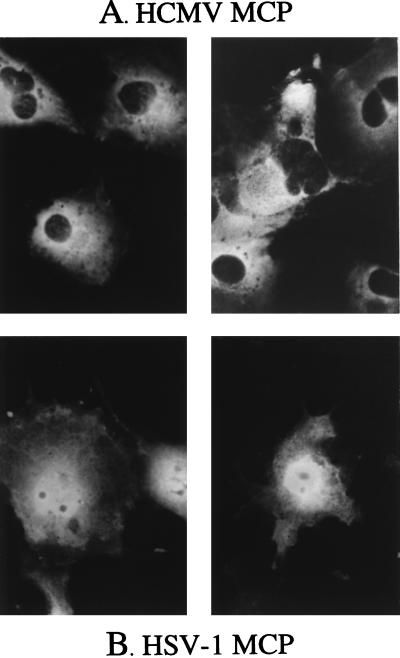
When expressed alone, the HCMV MCP is excluded from the nucleus, but the HSV-1 MCP is not. COS-7 cells were transfected with plasmids encoding either the HCMV MCP or its HSV-1 counterpart, VP5, and analyzed 3 days later by indirect IF assay using antisera to the HCMV MCP (A) or to the HSV MCP, VP5 (B), all as described in the text. Shown are photomicrographs of representative immunopositive cells.
Inspection of the amino acid sequences of the HCMV and simian CMV (SCMV) proteinase and assembly protein precursors revealed two well-conserved sequences, designated NLS1 and NLS2 (Fig. 1), resembling the simian virus 40 (SV40) large-T-antigen NLS. The work described here was done to determine whether these sequences have a role in mediating the nuclear translocation of pNP1 and pAP and identify the complexes they form with themselves and with MCP.
MATERIALS AND METHODS
Cells and transfection.
African green monkey kidney cells transformed with the SV40 large-T-antigen gene (COS-7 cells; ATCC CRL 1651; American Type Culture Collection, Rockville, Md.) were grown in Dulbecco’s modified eagle medium (catalog no. 12100-061; GIBCO, Grand Island, N.Y.) supplemented with fetal calf serum (10%), penicillin (100 U/ml), streptomycin (100 U/ml), and nystatin (10 U/ml). Transfections were done in COS-7 cells by using either a calcium phosphate (61) or DEAE-dextran (63) procedure, as described before.
Plasmid construction.
The construction, cloning, and propagation of plasmids were carried out by standard techniques (52), and mutations were confirmed by automated DNA sequence analysis at the JHMI Core DNA Analysis Facility or Protein/Peptide/DNA Laboratory.
The parental plasmids were (i) AW2, the SCMV pAP gene in pGEM-4Z (62); (ii) AW48, the SCMV gene for inactive proteinase precursor (S118A.L) in pRSV.5neo (61); and (iii) SP1, the AW2 plasmid modified to contain a unique XhoI site and encoding a serine codon-to-glycine codon mutation at residue 144. Other plasmids used included MB30, a pcDNA/AMP I construct encoding the HCMV MCP (63), and AW1, which contains the SCMV pAP gene in pRSV.5neo (62).
NLS mutations were made as follows. pAP/NLS1− was made in SP1 by changing NLS1 from KRRRER153 to AAAAEA through oligonucleotide-directed mutagenesis. The sequence between XhoI and MluI was replaced with the oligonucleotide TCGAGATCTGGGGCCCTTGCTGCTGCTGCTGAAGCTGA annealed to a complementary strand such that a 5′ XhoI and a 3′ MluI overhang resulted. This oligonucleotide returns Ala144 to wild-type Ser144. NLS2− was made in AW2 by changing NLS2 from KARKRLK179 to AAAAALA by replacing the wild-type fragment between MluI and StyI with the oligonucleotide CGCGTCGAGTGATGAGGAAGAGGACATGAGTTTTCCCGGGGAAGCTGATCATGGCGCGGCTGCTGCAGCGCTGGCTGCTCATCACGGTCGCGATAATAACAACTCAGGTTCAGATGC annealed to a complementary strand such that a 5′ MluI and a 3′ StyI overhang resulted. pAP/NLS1,2− contains both the NLS1− and NLS2− mutations and was made by replacing the wild-type fragment between MluI and StyI of the NLS1− construct with the NLS2− mutational oligonucleotide. All three mutants were subcloned into pRSV.5neo by cleaving the pGEM-4Z plasmids with SalI and BamHI and ligating the respective genes into the SalI/BamHI polylinker sites of pRSV.5neo.
Each of the three NLS mutations was subcloned into the proteolytically inactive SCMV proteinase precursor, S118A.L, to generate S118A/NLS1−, S118A/NLS2−, and S118A/NLS1,2−. This was done by replacing the DraIII/BamHI fragment of AW48 with the corresponding fragment from each of the three mutants.
The mature form of the inactive, full-length proteinase (S118A.m; stops at VNA_ of the M site [Fig. 1]), was constructed by replacing the small DraIII/BamHI fragment of AW48 with the corresponding DraIII/BamHI fragment from AP.RSV.5neo, a plasmid encoding the SCMV AP (63). S118A.m containing the NLS1,2 mutation was made by replacing the small DraIII/BamHI fragment of S118A.m. with the corresponding DraIII/BamHI fragment from AP/NLS1,2−.
AP/NLS1−, AP/NLS2−, and AP/NLS1,2− were made by replacing the 1,117-bp Bsu36I fragment of AP.RSV.5neo with the corresponding 1,117-bp Bsu36I fragment from the respective pAP mutants.
The β-galactosidase (β-Gal) fusion constructs were made by first ligating a PCR-generated lacZ gene into the BamHI site of the pRSV.5neo polylinker to give LacZ.RSV.5neo. The NLS1, NLS2, or NLS1,2 coding sequences, preceded by a translational start codon, were then inserted, in frame, at the NotI site 5′ of the lacZ sequence. This cloning strategy introduced an Ala triplet between the NLS and lacZ sequence.
IF.
Seventy-two hours after addition of the DNA, transfected cells were processed for indirect IF as described previously (63) and typically photographed at a magnification of ×40 and exposure time of 40 s, using an Olympus BH-2 microscope and Kodak Ecktachrome Elite, ASA 400 film. The primary antibodies were (i) anti-N1, a polyclonal antipeptide rabbit antiserum raised against a peptide representing residues 2 to 14 of the SCMV AP (53), diluted at 1:80 in 5% bovine serum albumin–10 mM Tris–0.15 M NaCl–0.02% sodium azide, pH 7.4 (TN/BSA); (ii) anti-C1, a polyclonal antipeptide rabbit antiserum raised against a peptide representing the carboxy-terminal 21 residues of the SCMV pAP (53), diluted at 1:80 in TN/BSA; (iii) anti-MCP, a polyclonal antipeptide rabbit antiserum raised against a peptide representing the carboxy-terminal 15 residues of the AD169 HCMV MCP (63), diluted at 1:500 in TN/BSA; and (iv) anti-β-Gal, a mouse monoclonal antibody to Escherichia coli β-Gal (catalog no. 1083104; Boehringer Mannheim, Indianapolis, Ind.), diluted at 1:250 in TN/BSA. The secondary antibodies were (i) fluorescein isothiocyanate-labeled goat anti-rabbit antibodies (catalog no. 111-095-144; Jackson Immunolaboratories, Inc., West Grove, Pa.) diluted 1:150 in TN/BSA and (ii) fluorescein isothiocyanate-labeled goat anti-mouse antibodies (catalog no. 115-095-062; Jackson Immunolaboratories) diluted 1:150 in TN/BSA.
RESULTS
NLS1 and NLS2 functionality was evaluated by testing their ability to translocate target proteins from the cytoplasm into the nucleus, as determined by IF assays of transfected cells. The SCMV assembly protein and proteinase precursors used in this study are illustrated in Fig. 1. To ensure that interpretation of results would not be complicated by the ability of the 34-kDa pAP to enter the nucleus by non-NLS-mediated diffusion (i.e., excluded size of ≥40 to 60 kDa) (5, 43), experiments to study these NLS in their native context were done first by using the genetically related 64-kDa proteinase precursor (pNP1). An inactive mutant of the proteinase (S118A [61]) was used to prevent its autoproteolytic cleavage at the M, R, and I sites. Corresponding experiments done with pAP, despite its small size, are also described.
NLS1 and NLS2 promote nuclear translocation of β-Gal.
Initial experiments tested the ability of NLS1 and NLS2 to direct a normally cytoplasmic protein, β-Gal, into the nucleus. NLS1, NLS2, or the entire NLS1,2 region (i.e., amino acids 148 to 179) was fused to the amino terminus of β-Gal to create β-Gal/NLS1, β-Gal-NLS2, and β-Gal/NLS1,2, respectively. The fusion proteins were separately expressed by transfecting COS-7 cells, and 72 h later their intracellular distribution was determined by IF assay.
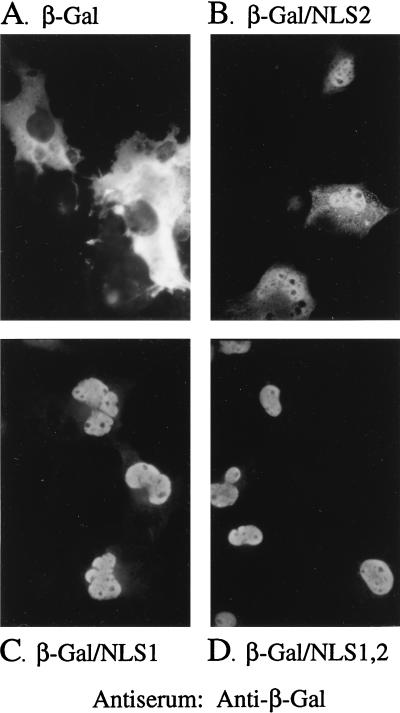
Wild-type β-Gal was essentially excluded from the nucleus (Fig. 2A), due to its large size (116 kDa) and lack of an NLS (25). β-Gal/NLS1, in contrast, was detected primarily in the nucleus (Fig. 2C) but with some cytoplasmic fluorescence evident; β-Gal/NLS2 was also mainly nuclear (Fig. 2B) but showed more cytoplasmic fluorescence than β-Gal/NLS1, indicating that both of these CMV NLS are functional and that NLS1 is inherently stronger than NLS2. β-Gal/NLS1,2 showed the strongest nuclear localization (Fig. 2D), indicating that the two signals work additively, as observed for other NLS (reviewed in reference 20).
FIG. 2.
β-Gal fusions with NLS1 or NLS2 localize to the nucleus. COS-7 cells were transfected with plasmids encoding β-Gal/NLS fusion proteins and analyzed 3 days later by indirect IF using a monoclonal antibody to β-Gal, all as described in the text. Shown are photomicrographs of the resulting cells expressing β-Gal (A), β-Gal/NLS2 (B), β-Gal/NLS1 (C), or β-Gal/NLS1,2 (D).
Mutation of NLS alters intracellular distribution of pNP1 and pAP.
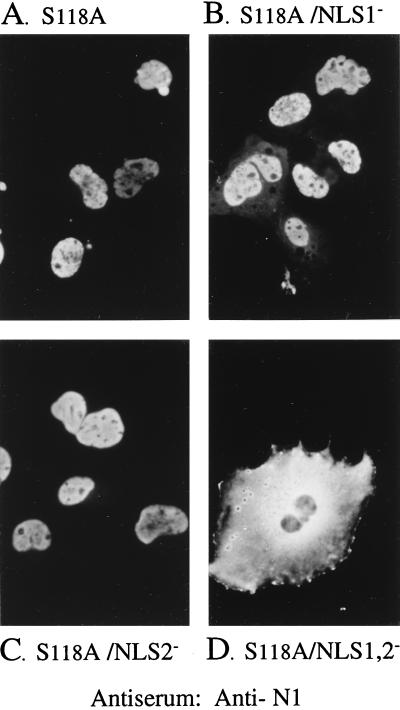
NLS1 and NLS2 were mutated individually or together to determine their influence on the intracellular localization of the mutant proteinase precursor, S118A. The basic residues of each NLS were replaced with alanines, and the intracellular distribution of the mutants was determined by IF assay. S118A and S118A/NLS2− both localized to the nucleus (Fig. 3A and C, respectively), and S118A/NLS1− also distributed primarily to the nucleus but showed some fluorescence in the cytoplasm (Fig. 3B). However, when neither NLS was present (S118A/NLS1,2−), the protein was essentially excluded from the nucleus and distributed throughout the cytoplasm (Fig. 3D), demonstrating that either NLS1 or NLS2 is required for nuclear localization of the proteinase precursor. These results also indicate that pNP1 does not contain NLS other than NLS1 and NLS2.
FIG. 3.
S118A requires NLS1 or NLS2 for its nuclear transport. COS-7 cells were transfected with plasmids encoding either S118A or its NLS mutants and analyzed 3 days later by indirect IF assay using anti-N1, as described in the text. Shown are photomicrographs of representative cells expressing S118A (A), S118A/NLS1− (B), S118A/NLS2− (C), and S118A/NLS1,2− (D).
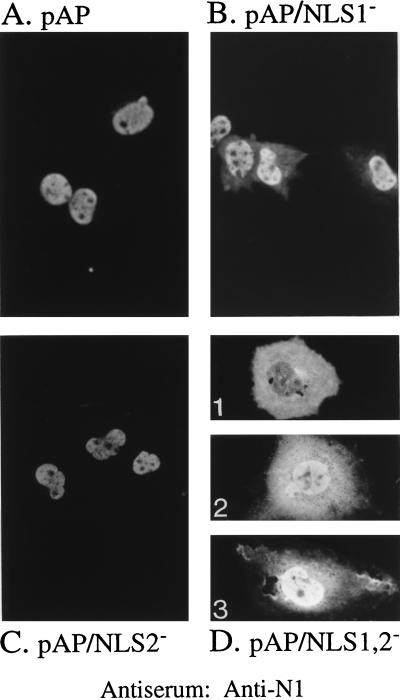
The experiment was repeated with the NLS mutations in the context of pAP. Cells were transfected with plasmids encoding either wild-type pAP or its NLS mutants, and the intracellular distribution of the proteins was determined. When both NLS were present (pAP [Fig. 4A]) or just NLS1 was present (pAP/NLS2− [Fig. 4C]), the protein localized strongly to the nucleus. When only NLS2 was present (pAP/NLS1− [Fig. 4B]), the protein also localized to the nucleus but some cytoplasmic fluorescence was detected, as noted with the corresponding NLS1− constructs, β-Gal/NLS2 (Fig. 2B) and S118A/NLS1− (Fig. 3B). When both NLS were mutated to give pAP/NLS1,2−, all cells showed increased levels of cytoplasmic fluorescence, due to the absence of active NLS-mediated nuclear translocation. However, because pAP/NLS1,2−, unlike S118A/NLS1,2−, can enter the nucleus by passive diffusion, its phenotype was varied: in some cells cytoplasmic fluorescence was predominant (Fig. 4D1); in others cytoplasmic and nuclear fluorescence were comparable (Fig. 4D2); and in some nuclear fluorescence was predominant (Fig. 4D3), but no cells showed the essentially exclusive nuclear fluorescence observed with wild-type pAP (Fig. 4A).
FIG. 4.
NLS1 and NLS2 enhance nuclear localization of pAP. COS-7 cells were transfected with plasmids encoding either pAP or its NLS mutants and analyzed 3 days later by indirect IF assay using anti-N1, all as described in the text. Shown are photomicrographs of representative cells expressing wild-type pAP (A), pAP/NLS1− (B), pAP/NLS2− (C), and pAP/NLS1,2−, which had a comparatively heterogeneous distribution of fluorescence (D).
NLS mutations in the proteinase and assembly protein precursors affect nuclear translocation of MCP.
Nuclear translocation of the CMV MCP requires its interaction with pAP (63) or pNP1 (shown below), suggesting that NLS1 or NLS2, or both, functions in trans to enable the resulting MCP complexes to enter the nucleus. This was tested by comparing the intracellular distribution of MCP expressed alone or coexpressed with S118A or its NLS mutants. The HCMV MCP was used in place of the SCMV MCP in these studies because it was more easily detected and photographed in the IF assays. This substitution is justified on the basis that (i) the amino acid sequence of the SCMV and HCMV MCPs are 78% identical (44a); (ii) the SCMV pAP interacts specifically (i.e., via its carboxyl end) with the HCMV MCP (63); (iii) NLS1 and NLS2 are highly conserved between the SCMV and HCMV pAP homologs (see Fig. 11); and (iv) comparable, but essentially undocumentable (i.e., fluorescence too weak), results were obtained in assays using the homologous SCMV MCP-pAP pair (data not shown). The HSV MCP was also expressed alone for comparison.
FIG. 11.
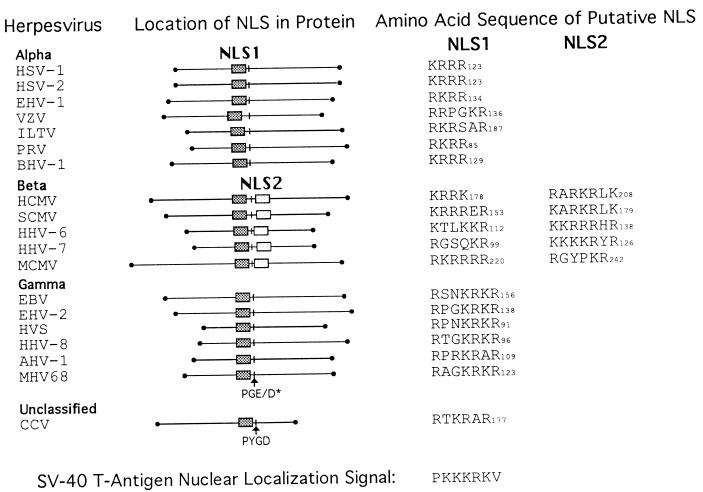
Positional homologs of NLS1 and NLS2 in the pAP homologs of herpes-group viruses. Amino acid sequences of available pAP homologs were aligned by using the Pima program (Baylor College of Medicine), and positional homologs of CMV pAP NLS1 and NLS2 were identified as indicated. A conserved Pro-Gly-Glu/Asp (PGE/D) sequence is indicated as an internal reference, and the carboxy-terminal amino acid number for each sequence is given. Sequences were obtained from GenBank; the viruses are as follows: HSV-1 (40), HSV-2 (54), equine herpesvirus 1 (EHV-1) (57), varicella-zoster virus (VZV) (12), infectious laryngotracheitis virus (ILTV) (27), pseudorabies virus (PRV) (7), bovine herpesvirus 1 (BHV-1) (28), HCMV strain AD169 (9), SCMV strain Colburn (60), human herpesvirus 6 (HHV-6) (26), human herpesvirus 7 (HHV-7) (41), murine CMV (MCMV) (39), Epstein-Barr virus (EBV) (2), equine herpesvirus 2 (EHV-2) (56), herpesvirus saimiri (HVS) (1), human herpesvirus 8 (HHV-8) (51), wildebeest herpesvirus (AHV-1) (17), murine herpesvirus 68 (MHV68) (59), and channel catfish virus (CCV) (11). Classification as alpha-, beta-, or gammaherpesvirus is based on the nomenclature of Roizman et al. (50). The amino acid sequence of the SV40 large-T-antigen NLS (33, 34) is shown at the bottom.
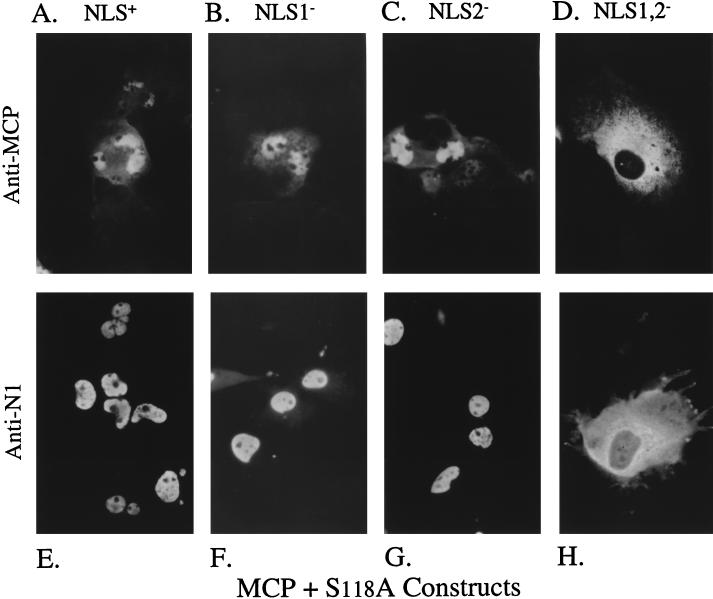
When the HCMV MCP was expressed alone, it distributed throughout the cytoplasm but was excluded from the nucleus (Fig. 5A). In contrast, the distribution of the HSV MCP was both cytoplasmic and nuclear (Fig. 5B), as observed by others (36, 42, 47). When the HCMV MCP was coexpressed with S118A, S118A/NLS1−, or S118A/NLS2−, both MCP (Fig. 6A, B, or C, respectively) and the corresponding NLS-bearing escort (Fig. 6E, F, or G, respectively) localized to the nucleus. However, when MCP was coexpressed with the NLS-deficient escort, S118A/NLS1,2−, neither protein entered the nucleus and predominantly cytoplasmic immunofluorescence was observed (Fig. 6D and H). These findings are consistent with both NLS1 and NLS2 being able to act in trans, via S118A-MCP complex formation, as signals for HCMV MCP nuclear translocation. Because both NLS function in this capacity, these results also indicate that neither is selectively masked by the S118A-MCP interaction.
FIG. 6.
Nuclear transport of MCP by S118A requires NLS1 or NLS2. COS-7 cells were cotransfected with plasmids encoding the HCMV MCP, together with either S118A or its NLS mutants, and analyzed 3 days later by indirect IF assay using either anti-MCP (63) (A to D) or anti-N1 (E to H), all as described in the text. Shown are photomicrographs of representative cells coexpressing MCP with S118A (A and E), S118A/NLS1− (B and F), S118A/NLS2− (C and G), or S118A/NLS1,2− (D and H).
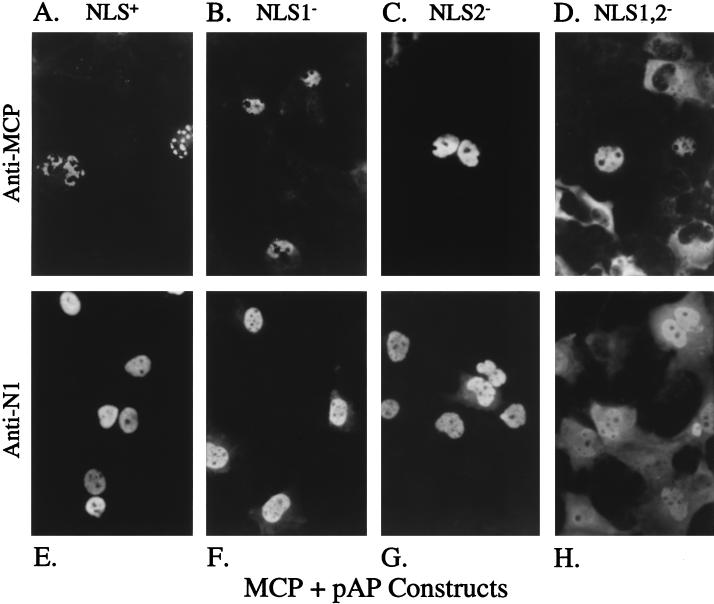
When this experiment was repeated using the same NLS mutations in the context of pAP, both MCP (Fig. 7A to C) and its NLS-bearing escorts (pAP, pAP/NLS1−, and pAP/NLS2−) (Fig. 7E to G) localized to the nucleus, as observed above for the corresponding S118A constructs. Unexpectedly, MCP-specific fluorescence was also detected in the nuclei of some cells coexpressing MCP and the NLS− mutant, pAP/NLS1,2− (Fig. 7D). Such nuclear fluorescence was not observed for the corresponding proteinase construct (S118A/NLS1,2− [Fig. 6D]) and is attributed to diffusion of the comparatively smaller pAP/NLS1,2− into the nucleus (Fig. 7H) and its trapping there of immunoreactive breakdown fragments of MCP, as discussed below.
FIG. 7.
NLS present in pAP mediate nuclear transport of MCP. COS-7 cells were cotransfected with plasmids encoding the HCMV MCP and either wild-type or NLS mutant pAP proteins and analyzed 3 days later by indirect IF assay using anti-MCP (A to D) or anti-N1 (E to H), all as described in the text. Shown are photomicrographs of representative cells coexpressing MCP with pAP (A and E), pAP/NLS1− (B and F), pAP/NLS2− (C and G), and pAP/NLS1,2− (D and H). Note focal pattern of intranuclear MCP fluorescence when expressed with pAP (A).
Proteinase precursor interacts with itself and with pAP in the cytoplasm.
The proteinase precursor interacted with itself and with the pAP when tested in the GAL4 two-hybrid system (63). The experiments described below were done to determine whether these interactions can take place in the cytoplasm and, if so, whether they mask the function of either NLS. The NLS+ M-site-cleaved forms of S118A and pAP (i.e., S118A.m and AP, terminating at the M-site P1 alanine [Fig. 1]) were used as test escorts. Because these escorts lack the 33-amino-acid carboxyl tail, which includes the 21-amino-acid sequence used to prepare the anti-C1 antiserum (53), they are immunologically invisible to anti-C1 and enable the intracellular distribution of the S118A/NLS1,2− target to be selectively determined by IF assay.
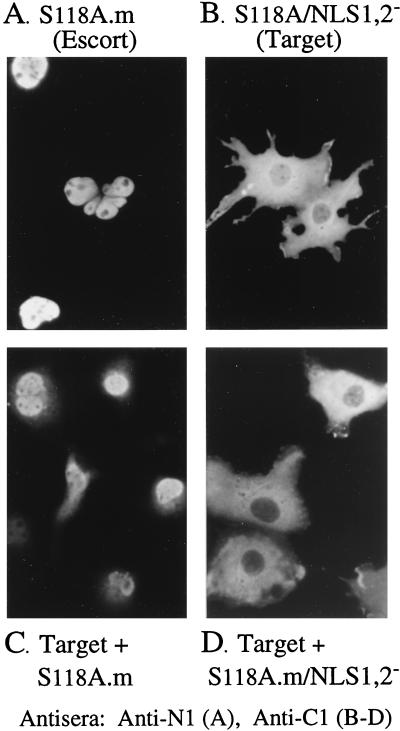
The proteinase self-interaction was tested by using S118A/NLS1,2− as the NLS− target and S118A.m as the NLS+ escort. When expressed alone, the S118A/NLS1,2− target was excluded from the nucleus (Fig. 8B), as observed above (Fig. 3D), and the S118A.m escort localized entirely within the nucleus (Fig. 8A). But when the two were coexpressed, the S118A/NLS1,2− target was translocated into the nucleus (Fig. 8C). Nuclear fluorescence was not detected when S118A/NLS1,2− was coexpressed with the same escort lacking NLS (i.e., S118A.m/NLS1,2− [Fig. 8D]), indicating that at least one NLS per complex is needed for its nuclear translocation. These data indicate that the S118A/NLS1,2− mutant is sufficiently soluble to undergo nuclear translocation, corroborate the S118A self-interaction detected in GAL4 two-hybrid assays (63), and demonstrate that the interaction can take place in the cytoplasm.
FIG. 8.
Precursor proteinase can self-interact in the cytoplasm of transfected cells. COS-7 cells were transfected with plasmids encoding different constructs of the proteinase precursor and analyzed 3 days later by indirect IF using anti-N1 (A) or anti-C1 (B to D), all as described in the text. Shown are photomicrographs of representative cells expressing the NLS+ S118A.m escort alone (A), the S118A/NLS1,2− target alone (B), and the S118A/NLS1,2− target together with the S118A.m escort (C) or together with the NLS− S118A.m/NLS1,2− escort (D).
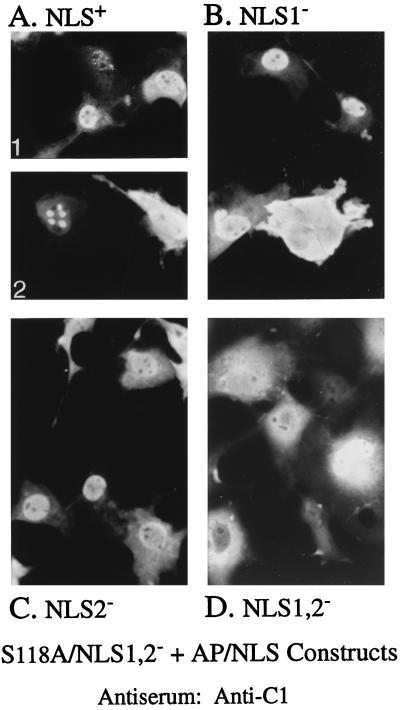
Interaction of the proteinase with pAP was tested next by expressing S118A/NLS1,2− alone (e.g., Fig. 3D and 8B) or together with an AP escort containing both, one, or neither NLS. When S118A/NLS1,2− was coexpressed with AP (Fig. 9A1 and A2), AP/NLS1− (Fig. 9B), or AP/NLS2− (Fig. 9C), most of the fluorescence was nuclear, indicating the formation and nuclear translocation of cytoplasmic complexes composed of the two proteins. Some nuclei in cells coexpressing S118A/NLS1,2− and AP showed a pattern of focally concentrated fluorescence (e.g., Fig. 9A2) that was not observed with any of the three AP/NLS mutants. Some nuclear fluorescence of S118A/NLS1,2− was also observed when it was coexpressed with an NLS-deficient AP escort (i.e., AP/NLS1,2− [Fig. 9D]). This result may be due to a trapping effect similar to that suggested above to explain the unanticipated nuclear fluorescence observed when the MCP target and pAP/NLS1,2− escort were coexpressed and is discussed below.
FIG. 9.
Demonstration of AP-proteinase precursor interaction in cytoplasm. COS-7 cells were cotransfected with plasmids encoding S118A/NLS1,2− and AP or its NLS mutants and analyzed 3 days later by indirect IF assay using anti-C1 to determine the intracellular distribution of S118A/NLS1,2−, all as described in the text. Shown are photomicrographs of representative cells coexpressing S118A/NLS1,2− with AP (A), AP/NLS1− (B), AP/NLS2− (C), or AP/NLS1,2− (D). Note greater coalescence of S118A/NLS1,2− fluorescence when the AP escort contained both NLS (e.g., top cell in panel A1 and left cell in panel A2).
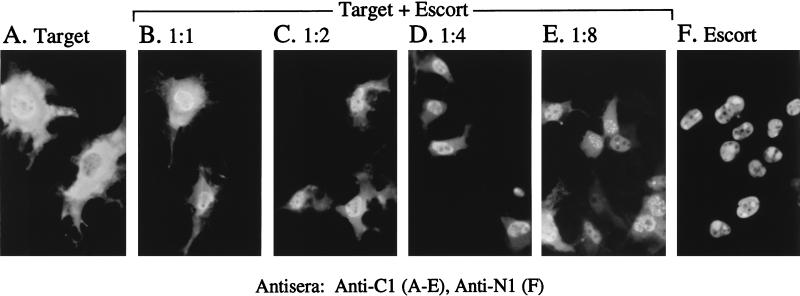
The pAP self-interaction was also tested, even though both target and escort can enter the nucleus by diffusion (e.g., Fig. 4D). The target protein was NLS− pAP (i.e., mutant pAP/NLS1,2−), the escort was NLS+ mature AP, and the target-specific antiserum was anti-C1. Expression of the NLS− target alone resulted in both cytoplasmic and nuclear fluorescence (Fig. 10A), as observed in Fig. 4D. Expression of the NLS+ escort alone gave a distribution of nucleus-only fluorescence (Fig. 10F), with what appeared to be a more punctate or coalesced pattern than was observed with the precursor, pAP (e.g., Fig. 4A). Coexpression of the target and escort in an equimolar ratio (i.e., based on input plasmid concentrations) resulted in a noticeable shift toward a more nuclear distribution of fluorescence (Fig. 10B). When the amount of escort-encoding plasmid was increased to two-, four-, and eightfold above that of the target (Fig. 10C, D, and E, respectively), the distribution of fluorescence became progressively more nuclear (compare Fig. 10B and C) and then more punctate (compare Fig. 10C and D with Fig. 10E). Thus, despite the small size of these proteins, a pAP-AP interaction was evident from the more complete nuclear localization of the NLS− pAP target when it was coexpressed with NLS+ AP escorts. We observed that the number of fluorescent cells was consistently higher in the coexpressions than when pAP/NLS1,2− was expressed alone, and we suspect that this phenomenon is caused by masking of the C1 epitope when pAP is expressed alone. Consistent with this explanation, Western immunoassays done as part of a similar experiment revealed that although pAP target protein fluorescence increased in the coexpressions, the amount of target protein itself did not increase; in fact, it decreased as the amount of escort increased (data not shown).
FIG. 10.
pAP self-interacts in the absence of other viral proteins. COS-7 cells were transfected with plasmids encoding pAP/NLS1,2− (target) alone, AP (escort) alone (F), or pAP/NLS1,2− together with increasing amounts of AP (B to E). The intracellular localization of pAP/NLS1,2− was determined by indirect IF assay using anti-C1 (A to E), and the distribution of AP alone was determined by using anti-N1 (F), all as described in the text. In cotransfections, the molar ratios of the pAP/NLS1,2− to AP plasmid were 1:1 (B), 1:2 (C), 1:4 (D), and 1:8 (E). Shown are representative cells from each transfection. Note the greater coalescence of fluorescence (i.e., speckles and spots) in panels E and F.
DISCUSSION
We have identified two SV40 large-T-antigen-like NLS in the CMV pAP. The functionality and relative strengths of these sequences, called NLS1 and NLS2, were established by showing that each could change the localization of β-Gal (116 kDa) from cytoplasmic to nuclear when fused to its amino terminus.
Mutagenesis experiments showed that both NLS were also functional in their native context. An enzymatically inactive mutant of the proteinase precursor pNP1 (i.e., S118A [61]), which has the entire pAP sequence as its carboxyl half (Fig. 1), was used because its larger size (64 kDa, versus 34 kDa for the pAP) prevents it from entering the nucleus by non-NLS-mediated diffusion. Nuclear localization of S118A occurred when either NLS1 or NLS2 was intact; when both were mutated, the protein remained in the cytoplasm (Fig. 3D). Consistent results were obtained when these experiments were done with pAP (Fig. 4), but, as expected, their interpretation was complicated by its smaller size and ability to diffuse into the nucleus.
NLS1 was functionally somewhat stronger than NLS2, as evidenced by its better ability to promote nuclear translocation of β-Gal, S118A, and pAP when acting in cis. Only NLS1 has an apparent counterpart among the pAP homologs of all herpesviruses (Fig. 11), indicating that it may be the functionally more important of the two. NLS2, however, is conserved among all betaherpesvirus pAP homologs, suggesting that it satisfies a group-specific requirement. Noteworthy in this regard is that, in addition to their NLS being closely symmetric with respect to the putative helix-breaking PGE/D motif, the termini of the betaherpesvirus pAP homologs are also more symmetric about this motif than are those of the pAP homologs of most other herpesviruses (Fig. 11).
Having established that NLS1 and NLS2 can each function independently as a transport signal, experiments were done to test whether these sequences provide a mechanism, through pAP-MCP complex formation, to enable MCP nuclear translocation. This possibility was suggested to explain the findings that for both CMV and HSV type 1 (HSV-1), nuclear transport of MCP requires (63) or is enhanced by (42) coexpression with their respective pAP, both of which contain an NLS1 motif (Fig. 11 and reference 19). This mechanism is supported for CMV by the finding that either NLS1 or NLS2 must be present in pAP (or pNP1) to mediate MCP nuclear translocation, and as observed in the β-Gal and S118A self-translocation experiments, both must be present for maximal effect. Although this finding ascribes a functional role to at least one of these NLS (i.e., promote nuclear translocation of MCP), it does not explain the presence of two in the CMV pAP.
Other proteins with multiple NLS have been described. In some instances, one NLS overlaps a DNA- or protein-binding domain (4, 58), whose interaction may block that NLS and create the need for a compensating second NLS. In another instance, a sequence that functioned alone as an NLS in a heterologous fusion protein did not function alone as an NLS in its native context (10). We considered the possibility that multiple NLS could be required to maintain presentation of at least one signal as the protein changes conformation or interacts with other molecules, but our experiments provided no support for this. Both NLS1 and NLS2 were functional in their native context, and their strengths were NLS1 + NLS2 > NLS1 ≥ NLS2, whether their effect was exerted in cis (Fig. 2 to 4) or in trans (Fig. 6, 7, and 9).
The possibility that the two NLS act additively to enhance the efficiency of transporting the large cytoplasmic complexes that result from pAP, pNP1, and MCP interactions could not be readily tested in these IF assays but is fully compatible with our results. The ability of multiple NLS to enlarge the channel of the nuclear pore complex (16), or increase the rate and extent of nuclear accumulation (15, 16, 37, 48), has been demonstrated in other systems (reviewed in reference 20). This explanation for the two NLS in CMV pAP could account for the difference in nuclear localization between the CMV and HSV MCPs, if (i) optimal localization of pAP-MCP complexes requires two NLS equivalents, as indicated here for CMV, and (ii) these signals can be contributed by either protein. Thus, the CMV MCP, which has no intrinsic ability to enter the nucleus when expressed alone (Fig. 5A and reference 63), has no NLS and depends on its pAP or pNP1 escort to provide both. In contrast, the HSV MCP, which does have some ability to enter the nucleus when expressed alone (Fig. 5B and references 36, 42, and 47), may contribute one NLS equivalent itself and depend on its pAP or pNP1 escort homolog for only one additional NLS. This interpretation could include as yet undetected MCP sequences (e.g., an endogenous nuclear export signal that overrides a possible endogenous NLS) that may be involved in the overall transport process. Alternatively, the apparent nuclear localization difference between the CMV and HSV MCPs expressed alone could result from (i) a comparatively greater breakdown of the HSV MCP, resulting in the diffusion of immunoreactive fragments into the nucleus, or (ii) an interaction of the HSV MCP with a host escort (55) that partially substitutes for the HSV pAP homolog, pVP22a (ICP35c,d).
The ability of pAP and pNP1 to participate in self-interactions, and to interact with one another and with MCP, was demonstrated previously by using the GAL4 two-hybrid system (63). In the same study, these interactions were related to the capsid assembly pathway by two other observations, namely, that nuclear transport of MCP depends on pAP-MCP complex formation and that pAP multimerization promotes the pAP-MCP interaction. These findings, and similar studies with the HSV-1 counterparts of pAP and MCP (i.e., pVP22a and VP5, respectively) (42, 44), suggest that an ordered association of capsid proteins takes place in the cytoplasm, proceeding from initial interactions of pAP and pNP1 to form homo- and/or hetero-oligomers, to their interaction with MCP to form precapsomeric complexes, and possibly to the formation of protocapsomers that could be translocated into the nucleus for capsid assembly. Features of this model provide mechanisms for (i) potentiating or stabilizing pAP (or pNP1) interaction with MCP, (ii) promoting delivery of MCP to the nucleus, and (iii) ensuring incorporation of the maturational proteinase precursor, pNP1, into nascently forming capsids.
As a means of determining whether the putative nucleating interactions in this cascade (i.e., formation of pAP and pNP1 homo- and hetero-oligomers) can take place in the cytoplasm, as would be predicted, we assayed for a shift in the intracellular distribution of NLS mutants of pAP and pNP1 when coexpressed with NLS+ escorts. Our results demonstrated that the pNP1 self-interaction (Fig. 8) and the pAP-pNP1 interaction (Fig. 9) can take place in the cytoplasm, but they did not give unambiguous evidence for cytoplasmic interaction of pAP because both the target and escort proteins can diffuse into the nucleus. It would be surprising if pAP did not self-interact in the cytoplasm, however, given that the closely related pNP1 (S118A) does (Fig. 8C and 9). Moreover, the concentration-dependent shift of pAP/NLS1,2− from the cytoplasm to the nucleus when coexpressed with increasing amounts of NLS+ escort (Fig. 10) is fully consistent with a cytoplasmic interaction.
We were surprised that pAP lacking NLS showed some ability to influence the intracellular distribution of MCP and S118A/NLS1,2− (Fig. 7D and 9D, respectively) and suspect that this is due to a fraction of the indicator protein (i.e., MCP or S118A/NLS1,2−) being broken down by proteolysis to immunologically reactive fragments able to diffuse into the nucleus. These fragments could then interact with NLS− pAP that had also diffused into the nucleus (Fig. 4D) and form a complex too large to diffuse back out. Consistent with this interpretation, (i) there is some breakdown of MCP and S118A/NLS1,2− to smaller fragments when expressed in transfected cells (Western immunoassay [data not shown]) and (ii) the effect was not observed when either MCP or S118A/NLS1,2− was expressed alone (i.e., no nuclear pAP or AP to entrap immunoreactive target fragments). An alternative explanation, namely, that a cryptic NLS is expressed in the pAP-MCP or AP-S118A complexes, is weakened by the fact that this effect was not observed when the escort was S118A/NLS1,2− or S118A.m/NLS1,2− (Fig. 6D and 8D), which contain the entire pAP/NLS1,2− or AP/NLS1,2− sequence, respectively (Fig. 1).
In summary, we have shown that the two SV40 T-antigen-like NLS, present in the CMV pAP and its genetically related pNP1, can effect the nuclear translocation of pAP and pNP1 by acting in cis and that of MCP by acting in trans. In addition, our data demonstrate that multimeric complexes of capsid proteins can form in the cytoplasm and that their nuclear transport can be mediated by NLS1 and NLS2. These complexes can consist of pAP-pAP, pNP1-pNP1, pAP-pNP1, pNP1-MCP, pAP-MCP, and theoretically capsomeric or subcapsomeric assemblages (e.g., [pAP-MCP]6 protohexamer).
Evidence that such complexes form and are required for nuclear translocation of specific CMV (63) and HSV (36, 42, 47) proteins has been reported before. The experiments described here indicate that the underlying reason for this requirement is that not all of the capsid proteins contain the NLS required for efficient translocation into the nucleus. In the case of CMV, NLS present in pAP and pNP1 act in trans, through pAP-MCP (and pNP1-MCP) complexes, to enable nuclear translocation of the MCP (Fig. 6 and 7; reference 63). Similarly, NLS present in another outer shell protein, the mCBP component of the triplex, also appears to act in trans, through mCBP-mCP complexes, to enable nuclear translocation of mCP—its triplex partner (3, 3a). Similar pairwise interactions between NLS+ and NLS− or weak NLS+ capsid proteins have been reported for the DNA-containing papovaviruses, polyomavirus (13, 18) and SV40 (35), and adenovirus (8), suggesting this may be a general mechanism for nuclear replicating viruses but not explaining why.
The presence of NLS in proteins destined for incorporation into capsids forming in the nucleus would be expected to maximize their concentration in the assembly compartment and to minimize their concentration in the cytoplasm, where premature capsid formation could decrease the efficiency of the assembly pathway. Further, by endowing only one partner (e.g., pAP) of a protein pair (e.g., pAP-MCP) with an NLS, an additional level of control could be imposed on the flow of events leading to capsid assembly. For example, if the efficiency or fidelity of capsid formation were enhanced by the availability of correctly preassembled subunits (e.g., pAP-MCP complexes), compartmentalizing the early (e.g., cytoplasmic protein-protein interactions) and late (e.g., nuclear subunit-subunit interactions) steps in the pathway could be advantageous. Thus, in CMV the essential capsid proteins would be supplied to the nucleus as preformed pAP-(and/or pNP1-)-MCP or mCP-mCBP subunits, thereby reducing the chances of incorporating a misfolded substituent into the nascent capsid or having a stoichiometric imbalance of capsid proteins in the assembly compartment. These speculations predict that a CMV mutant virus lacking NLS in both pAP and mCBP would give rise to cytoplasmic capsids and have a higher frequency of aberrant particles. Similar mechanisms could help regulate the flow and incorporation of cellular protein subunits into nuclear structures, such as transcription complexes (6) and spliceosomes (46).
ACKNOWLEDGMENTS
We thank Lisa Cimakasky for help in constructing the β-Gal constructs during a research rotation in the laboratory, Kendra Clopper Plafker for excellent technical assistance, and Pamela Harvey and Michael McElwaine for patience and diligence in printing the photomicrographs.
S.M.P. was a student in the Pharmacology and Molecular Sciences training program and was supported by USPHS grant GM07626. This work was aided by USPHS research grants AI13718 and AI32957.
REFERENCES
- 1.Albrecht J-C, Nicholas J, Biller D, Cameron K R, Biesinger B, Newman C, Wettmann S, Craxton M A, Coleman H, Fleckenstein B, Honess R W. Primary structure of the herpesvirus saimiri genome. J Virol. 1992;66:5047–5058. doi: 10.1128/jvi.66.8.5047-5058.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baer R, Bankier A T, Biggin M D, Deininger P L, Farrell P J, Gibson T J, Hatfull G, Hudson G S, Satchwell S C, Sequin C, Tufnell P S, Barrell B G. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature (London) 1984;310:207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- 3.Baxter M K, Gibson W. Presented at the 22nd International Herpesvirus Workshop, La Jolla, Calif. 1997. The putative human cytomegalovirus triplex proteins, minor capsid protein (mCP) and mCP-binding protein (mC-BP), form a heterotrimeric complex that localizes to the cell nucleus in the absence of other viral proteins. [Google Scholar]
- 3a.Baxter, M. K., and W. Gibson. Unpublished data.
- 4.Boehm U, Heinlein M, Behrens U, Kunze R. One of three nuclear localization signals of maize Activator (Ac) transposase overlaps the DNA-binding domain. Plant J. 1995;7:441–451. doi: 10.1046/j.1365-313x.1995.7030441.x. [DOI] [PubMed] [Google Scholar]
- 5.Bonner W M. Protein migration into nuclei. J Cell Biol. 1975;64:421–430. doi: 10.1083/jcb.64.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulikas T. Nuclear import of DNA repair proteins. Anticancer Res. 1997;17:843–864. [PubMed] [Google Scholar]
- 7.Camacho A, Tabares E. Characterization of the genes, including that encoding the viral proteinase, contained in BamHI restriction fragment 9 of the pseudorabies virus genome. J Gen Virol. 1996;77(Pt.8):1865–1874. doi: 10.1099/0022-1317-77-8-1865. [DOI] [PubMed] [Google Scholar]
- 8.Cepko C L, Sharp P A. Analysis of Ad5 hexon and 100K temperature sensitive mutants using conformation-specific monoclonal antibodies. Virology. 1983;129:137–154. doi: 10.1016/0042-6822(83)90402-6. [DOI] [PubMed] [Google Scholar]
- 9.Chee M S, Bankier A T, Beck S, Bohni R, Brown C M, Cerny R, Horsnell T, Hutchison C A, Kouzarides T, Martignetti J A, Preddie E, Satchwell S C, Tomlinson P, Weston K M, Barrell B G. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr Top Microbiol Immun. 1990;154:125–169. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- 10.Dang C V, Lee W M F. Identification of the human c-myc protein nuclear translocation signal. Mol Cell Biol. 1988;8:4048–4054. doi: 10.1128/mcb.8.10.4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davison A J. Channel catfish virus: a new type of herpesvirus. Virology. 1992;186:9–14. doi: 10.1016/0042-6822(92)90056-u. [DOI] [PubMed] [Google Scholar]
- 12.Davison A J, Scott J E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986;67:1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- 13.Delos S E, Montroll L, Moreland R B, Garcea R L. Expression of the polyomavirus VP2 and VP3 proteins in insect cells: coexpression with the major capsid protein VP1 alters VP2/VP3 subcellular localization. Virology. 1993;194:393–398. doi: 10.1006/viro.1993.1274. [DOI] [PubMed] [Google Scholar]
- 14.Desai P, Person S. Molecular interactions between the HSV-1 capsid proteins as measured by the yeast two-hybrid system. Virology. 1996;220:516–521. doi: 10.1006/viro.1996.0341. [DOI] [PubMed] [Google Scholar]
- 15.Dingwall C, Sharnick S V, Laskey R A. A polypeptide domain that specifies migration of nucleoplasmin into the nucleus. Cell. 1982;30:449–458. doi: 10.1016/0092-8674(82)90242-2. [DOI] [PubMed] [Google Scholar]
- 16.Dworetzky S I, Lanford R E, Feldherr C M. The effects of variations in the number and sequence of targeting signals on nuclear uptake. J Cell Biol. 1988;107:1279–1287. doi: 10.1083/jcb.107.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ensser A, Pflanz R, Fleckenstein B. Primary structure of the alcelaphine herpesvirus 1 genome. J Virol. 1997;71:6517–6525. doi: 10.1128/jvi.71.9.6517-6525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forstova F, Krauzewicz N, Wallace S, Street A J, Dilworth S M, Beard S, Griffin B E. Cooperation of structural proteins during late events in the life cycle of polyomavirus. J Virol. 1993;67:1405–1413. doi: 10.1128/jvi.67.3.1405-1413.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao M, Matusick-Kuman L, Hurlburt W, DiTusa S F, Newcomb W W, Brown J C, McCann P J, Deckman I, Colonno R J. The protease of herpes simplex virus type 1 is essential for functional capsid formation and viral growth. J Virol. 1994;68:3702–3712. doi: 10.1128/jvi.68.6.3702-3712.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia-Bustos J, Heitman J, Hall M N. Nuclear protein localization. Biochim Biophys Acta. 1991;1071:83–101. doi: 10.1016/0304-4157(91)90013-m. [DOI] [PubMed] [Google Scholar]
- 21.Gibson W. Structural and nonstructural proteins of strain Colburn cytomegalovirus. Virology. 1981;111:516–537. doi: 10.1016/0042-6822(81)90354-8. [DOI] [PubMed] [Google Scholar]
- 22.Gibson W. Structure and assembly of the virion. Intervirology. 1996;39:389–400. doi: 10.1159/000150509. [DOI] [PubMed] [Google Scholar]
- 23.Gibson W, Marcy A, Comolli J C, Lee J. Identification of precursor to cytomegalovirus capsid assembly protein and evidence that processing results in loss of its carboxy-terminal end. J Virol. 1990;64:1241–1249. doi: 10.1128/jvi.64.3.1241-1249.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibson W, Roizman B. Proteins specified by herpes simplex virus. X. Staining and radiolabeling properties of B capsid and virion proteins in polyacrylamide gels. J Virol. 1974;13:155–165. doi: 10.1128/jvi.13.1.155-165.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilmore T D, Temin H M. v-rel oncoproteins in the nucleus and in the cytoplasm transform chicken spleen cells. J Virol. 1988;62:703–714. doi: 10.1128/jvi.62.3.703-714.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gompels U A, Nicholas J, Lawrence G, Jones M, Thomson B J, Martin M E D, Efstathiou S, Craxton M, Macaulay H A. The DNA sequence of human herpesvirus-6: structure, coding content, and genome evolution. Virology. 1995;209:29–51. doi: 10.1006/viro.1995.1228. [DOI] [PubMed] [Google Scholar]
- 27.Griffin A M. The complete sequence of the capsid p40 gene from infectious laryngotracheitis virus. Nucleic Acids Res. 1990;18:3664. doi: 10.1093/nar/18.12.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haanes E J, Thomsen D R, Martin S, Homa F L, Lowery D E. Bovine herpesvirus 1 maturational proteinase and scaffold proteins can substitute for the homologous herpes simplex virus type 1 proteins in the formation of hybrid type B capsids. J Virol. 1995;69:7375–7379. doi: 10.1128/jvi.69.11.7375-7379.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall M R T, Gibson W. Cytomegalovirus assemblin: the amino and carboxyl domains of the proteinase form active enzyme when separately cloned and coexpressed in eukaryotic cells. J Virol. 1996;70:5395–5404. doi: 10.1128/jvi.70.8.5395-5404.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holwerda B C, Wittwer A J, Duffin K L, Smith C, Toth M V, Carr L S, Wiegand R C, Bryant M L. Activity of two-chain recombinant human cytomegalovirus protease. J Biol Chem. 1994;269:25911–25915. [PubMed] [Google Scholar]
- 31.Hong Z, Beaudet-Miller M, Burkin J, Zhang R, Kwong A D. Identification of a minimal hydrophobic domain in the herpes simplex virus type 1 scaffolding protein which is required for interaction with the major capsid protein. J Virol. 1996;70:533–540. doi: 10.1128/jvi.70.1.533-540.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irmiere A, Gibson W. Isolation and characterization of a noninfectious virion-like particle released from cells infected with human strains of cytomegalovirus. Virology. 1983;130:118–133. doi: 10.1016/0042-6822(83)90122-8. [DOI] [PubMed] [Google Scholar]
- 33.Kalderon D, Richardson W D, Markham A F. Sequence requirements for nuclear location of simian virus 40 large-Ta antigen. Nature. 1984;311:33–38. doi: 10.1038/311033a0. [DOI] [PubMed] [Google Scholar]
- 34.Kalderon D, Roberts B L, Richardson W D, Smith A E. A short amino acid sequence able to specify nuclear location. Cell. 1984;39:499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- 35.Kasamatsu H, Nehorayan A. Vp1 affects intracellular localization of Vp3 polypeptide during simian virus 40 infection. Proc Natl Acad Sci USA. 1979;76:2808–2812. doi: 10.1073/pnas.76.6.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kennard J, Rixon F J, McDougall I S, Tatman J D, Preston V G. The 25 amino acid residues at the carboxy terminus of the herpes simplex virus type 1 UL26.5 protein are required for the formation of the capsid shell around the scaffold. J Gen Virol. 1995;76:1611–1621. doi: 10.1099/0022-1317-76-7-1611. [DOI] [PubMed] [Google Scholar]
- 37.Knauf J A, Pendergrass S H, Marrone B L, Strmiste G F, MacInne M A, Park M S. Multiple nuclear localization signals in XPG nuclease. Mutat Res. 1996;363:67–75. doi: 10.1016/0921-8777(95)00062-3. [DOI] [PubMed] [Google Scholar]
- 38.Liu F, Roizman B. The herpes simplex virus 1 gene encoding a protease also contains within its coding domain the gene encoding the more abundant substrate. J Virol. 1991;65:5149–5156. doi: 10.1128/jvi.65.10.5149-5156.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loutsch J M, Galvin N J, Bryant M L, Holwerda B C. Cloning and sequence analysis of murine cytomegalovirus protease and capsid assembly protein genes. Biochem Biophys Res Commun. 1994;203:472–478. doi: 10.1006/bbrc.1994.2206. [DOI] [PubMed] [Google Scholar]
- 40.McGeoch D J, Dalrymple M A, Davison A J, Dolan A, Frame M C, McNab D, Perry L J, Scott J E, Taylor P. The complete sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988;69:1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- 41.Nicholas J. Determination and analysis of the complete nucleotide sequence of human herpesvirus 7. J Virol. 1996;70:5975–5989. doi: 10.1128/jvi.70.9.5975-5989.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nicholson P, Addison C, Cross A M, Kennard J, Preston V G, Rixon F J. Localization of the herpes simplex virus type 1 major capsid protein VP5 to the cell nucleus requires the abundant scaffolding protein VP22a. J Gen Virol. 1994;75:1091–1099. doi: 10.1099/0022-1317-75-5-1091. [DOI] [PubMed] [Google Scholar]
- 43.Paine P L, Feldherr C M. Nucleocytoplasmic exchange of macromolecules. Exp Cell Res. 1972;74:81–98. doi: 10.1016/0014-4827(72)90483-1. [DOI] [PubMed] [Google Scholar]
- 44.Pelletier A, Do F, Brisebois J J, Legace L, Cordingley M G. Self-association of herpes simplex virus type 1 ICP35 is via coiled-coil interactions and promotes stable interaction with the major capsid protein. J Virol. 1997;71:5197–5208. doi: 10.1128/jvi.71.7.5197-5208.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44a.Plafker, K. C., M.-E. Harmon, and W. Gibson. Unpublished data.
- 44b.Plafker, S. M., and W. Gibson. Unpublished data.
- 45.Preston V G, Coates J A, Rixon F J. Identification and characterization of a herpes simplex virus gene product required for encapsidation of virus DNA. J Virol. 1983;45:1056–1064. doi: 10.1128/jvi.45.3.1056-1064.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raker V A, Plessel G, Luhrmann R. The snRNP core assembly pathway: identification of stable core protein heteromeric complexes and an snRNP subcore particle in vitro. EMBO J. 1996;15:2256–2269. [PMC free article] [PubMed] [Google Scholar]
- 47.Rixon F J, Addison C, McGregor A, Macnab S L, Nicholson P, Preston V G, Tatman J D. Multiple interactions control the intracellular localization of the herpes simplex virus type 1 capsid proteins. J Gen Virol. 1996;77:2251–2260. doi: 10.1099/0022-1317-77-9-2251. [DOI] [PubMed] [Google Scholar]
- 48.Roberts B L, Richardson W D, Smith A E. The effect of protein context on nuclear location signal function. Cell. 1987;50:465–475. doi: 10.1016/0092-8674(87)90500-9. [DOI] [PubMed] [Google Scholar]
- 49.Roby C, Gibson W. Characterization of phosphoproteins and protein kinase activity of virions, noninfectious enveloped particles, and dense bodies of human cytomegalovirus. J Virol. 1986;59:714–727. doi: 10.1128/jvi.59.3.714-727.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roizman B, Carmichael L E, Deinhardt F, de-The G, Nahmias A J, Plowright W, Rapp F, Sheldrick P, Takahashi M, Wolf K. Herpesviridae. Definition, provisional nomenclature and taxonomy. Intervirology. 1981;16:201–217. doi: 10.1159/000149269. [DOI] [PubMed] [Google Scholar]
- 51.Russo J J, Bohenzky R A, Chien M-C, Chen J, Yan M, Maddalena D, Parry J P, Peruzzi D, Edelman I S, Chang Y, Moore P S. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8) Proc Natl Acad Sci USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 53.Schenk P, Woods A S, Gibson W. The 45-kDa protein of cytomegalovirus (Colburn) B-capsids is an amino-terminal extension form of the assembly protein. J Virol. 1991;65:1525–1529. doi: 10.1128/jvi.65.3.1525-1529.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steffy K R, Schoen S, Chen C M. Nucleotide sequence of the herpes simplex virus type 2 gene encoding the protease and capsid protein ICP35. J Gen Virol. 1995;76:1069–1072. doi: 10.1099/0022-1317-76-4-1069. [DOI] [PubMed] [Google Scholar]
- 55.Szebeni A, Mehrotra B, Baumann A, Adam S A, Wingfield P T, Olson M O J. Nucleolar protein B23 stimulates nuclear import of the HIV-1 Rev protein and NLS-conjugated albumin. Biochemistry. 1997;36:3941–3949. doi: 10.1021/bi9627931. [DOI] [PubMed] [Google Scholar]
- 56.Telford E A, Watson M S, Aird H C, Perry J, Davison A J. The DNA sequence of equine herpesvirus type 2. J Mol Biol. 1995;249:520–528. doi: 10.1006/jmbi.1995.0314. [DOI] [PubMed] [Google Scholar]
- 57.Telford E A R, Watson M S, McBride K, Davison A J. The DNA sequence of equine herpes 1. Virology. 1992;189:304–316. doi: 10.1016/0042-6822(92)90706-u. [DOI] [PubMed] [Google Scholar]
- 58.Vandromme M, Cavadore J-C, Bonnieu A, Froeschle A, Lamb N, Fernandez A. Two nuclear localization signals present in the basic-helix 1 domains of MyoD promote its active nuclear translocation and can function independently. Proc Natl Acad Sci USA. 1995;92:4646–4650. doi: 10.1073/pnas.92.10.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Virgin H W I, Latreille P, Wamsley P, Hallsworth K, Weck K E, Dal Canto A J, Speck S H. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J Virol. 1997;71:5894–5904. doi: 10.1128/jvi.71.8.5894-5904.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Welch A R, McNally L M, Gibson W. Cytomegalovirus assembly protein nested gene family: four 3′-coterminal transcripts encode four in-frame, overlapping proteins. J Virol. 1991;65:4091–4100. doi: 10.1128/jvi.65.8.4091-4100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Welch A R, McNally L M, Hall M R T, Gibson W. Herpesvirus proteinase: site-directed mutagenesis used to study maturational, release, and inactivation cleavage sites of precursor and to identify a possible catalytic site serine and histidine. J Virol. 1993;67:7360–7372. doi: 10.1128/jvi.67.12.7360-7372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Welch A R, Woods A S, McNally L M, Cotter R J, Gibson W. A herpesvirus maturational proteinase, assemblin: identification of its gene, putative active site domain, and cleavage site. Proc Natl Acad Sci USA. 1991;88:10792–10796. doi: 10.1073/pnas.88.23.10792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wood L J, Baxter M K, Plafker S M, Gibson W. Human cytomegalovirus capsid assembly protein precursor (pUL80.5) interacts with itself and with the major capsid protein (pUL86) through two different domains. J Virol. 1997;71:179–190. doi: 10.1128/jvi.71.1.179-190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]