Abstract

The global accumulation and adverse effects of nanoplastics (NPs) are a growing concern for the environment and human health. In recent years, more and more studies have begun to focus on the toxicity of plastic particles for early animal development. Different particle sizes of plastic particles have different toxicities to biological development. Nevertheless, the potential toxicological effects of 20 nm NPs, especially on neurodevelopment, have not been well investigated. In this paper, we used fluorescence microscopy to determine neurotoxicity in zebrafish at different concentrations of NPs. Moreover, the behavioral analysis demonstrated that NPs induced abnormal behavior of zebrafish. The results revealed developmental defects in zebrafish embryos after exposure to different concentrations (0, 0.3, 3, and 9 mg/L) of NPs. The morphological deformities, including abnormal body length and the rates of heart, survival, and hatching, were induced after NP exposure in zebrafish embryos. In addition, the development of primary motor neurons was observed the inhibitory effects of NPs on the length, occurrence, and development of primary motor neurons in Tg(hb9:GFP). Quantitative polymerase chain reaction analysis suggested that exposure to NPs significantly affects the expression of the genes involved in the occurrence and differentiation of primary motor neurons in zebrafish. Furthermore, the indicators associated with oxidative stress and apoptosis were found to be modified in zebrafish embryos at 24 and 48 h following exposure to NPs. Our findings demonstrated that NPs could cause toxicity in primary motor neurons by activating the oxidative stress response and inducing apoptosis, consequently impairing motor performance.
Introduction
Plastics were widely used in many aspects of our life which were also regarded as the world’s largest contribution to environmental contamination.1 In the last decades, plastics gradually threatened creatures in nature, including soil, aquatic, and land life or botany, and even humans. Since the 1950s, humans have made more than 8 billion tons of synthetic polymers, and forecasts indicate that this amount will rise to more than 30 billion tons by 2050.2,3 Nevertheless, the widespread production and insufficient management of plastics result in a paltry 10% being recycled, leading to the dissemination of these materials into a diverse environment.3,4
Microplastics (MPs) and nanoplastics (NPs) are tiny plastic particles that measure less than 5 mm in diameter.5 A study has revealed that microplastics can fragment even further into nanoparticles, which are particles smaller than 1 μm in diameter.6,7 NPs have attracted significant global attention due to their extensive use and detection in various environmental and biological samples. Moreover, NPs can accumulate in the cells and tissues of animals, resulting in notable impacts on biochemical processes such as cytotoxicity, inflammation, oxidative stress, fibrosis, and antioxidant activity.8,9 Recently, the investigation unveiled the existence of polystyrene nanoparticles (PS-NPs) in the brains of medaka fish.10 This finding suggests that PS-NPs have the ability to cross the blood-brain barrier and reach the brain. In another study, PS-NPs were found to migrate to the heart, causing reduced heart rate at early developmental stages.11 A conducted research on carp, which indicated that PS-NPs caused alterations in brain morphology as well as behavioral modifications.12 Nevertheless, the underlying regulatory mechanisms that drive NPs-induced neurodevelopmental damage are still unknown.
Zebrafish have been widely recognized as ideal model organisms for studying the impact of environmental pollutants. Their sensitivity during early development and locomotor activity at 4–5 days post fertilization make them suitable for ecological monitoring.13 Importantly, the utilization of fluorescent-labeled transgenic zebrafish presents a convenient means to observe the progression of nervous system development due to the inherent transparency of zebrafish embryos.14 This study aimed to use the distinctive characteristics of the zebrafish model to assess the neurotoxicity of NPs with a nominal diameter of 20 nm in vivo. To be clear, the hypothesis has been proposed that NPs may contribute to developmental disorders of the zebrafish embryonic nervous system by disturbing relevant signaling pathways in neural development. Consequently, we determined the influence of NPs on primary motor nerve growth in zebrafish embryos treated by NPs during the early stages of development in a dynamic manner. Furthermore, the investigation focused on the analysis of behavioral phenotypes and neural growth in zebrafish embryos treated by various concentrations of NPs. Afterward, we assessed oxidative stress levels, cell apoptosis levels, and molecular indicators related to neurodevelopment to gauge the neurotoxic impact of NPs with 20 nm particle size. Thus, our results indicated that 20 nm NPs induced neurodevelopmental toxicity via cell apoptosis and oxidative stress signaling pathways in vivo.
Materials and Methods
Ethics Statement
The research was conducted in compliance with the guidelines of the Guide for the Care and Use of Laboratory Animals at Sichuan University. All experiments were conducted in compliance with the guidelines and regulations established by the Ministry of Science and Technology of the People’s Republic of China (approval number 2006–398).
Nanoplastic (NP) Characterization
NP samples were procured by Huge Biotechnology Co., Ltd. (DS100, with a concentration of 1.04 g/cm3 and a coefficient of variation (CV %) of 3, located in Shanghai, China). The morphology of the NPs was examined by using scanning electron microscopy (SEM) (SU8100, manufactured by Hitachi in Japan). The zeta potential and DLS spectra were measured by a Zetasizer Nano ZS (Malvern Instruments, Worcestershire, United Kingdom). Then, the NPs were dissolved in deionized water. The average diameter of the polystyrene NPs particles was 20 nm. These particles were devoid of fluorescence and had a white appearance. The aggregation of NPs caused an increase in their hydration diameter, leading to larger sizes.
Zebrafish Maintenance and Embryo Collection
The adult zebrafish experimentalized in this study were obtained from the China Zebrafiah Resource Center in Wuhan, China. The zebrafish were of the transgenic Tg(hb9:GFP) strain and wild-type AB strain. The adult zebrafish were cultured in a controlled environment under specific conditions. The pH of the water in their tanks was maintained at 7.5 ± 0.5, the temperature was set at 27 ± 0.5 °C, and a 14 h light/10 h darkness photoperiod was followed. To ensure a nutritious diet, freshly hatched brine shrimp served as their primary feed to feed the adult zebrafish twice a day. For breeding purposes, equal numbers of male and female zebrafish were combined in a spawning tank in a 1:1 ratio. The next morning, spawning was induced and completed within a 30 min time frame at the beginning of the light cycle. Then, the fertilized embryos in the bottom of the spawning tank were absorbed and collected by a Pap tube. These embryos were then kept in an E3 embryonic medium, which consisted of 0.33 mM MgSO4, 0.33 mM CaCl2, 0.17 mM KCl, and 5 mM NaCl dissolved in 1000 mL distilled water. This medium provides the necessary nutrients and environment for the embryos to develop and grow. To ensure ethical treatment of the zebrafish used in the experiments, this study adhered to the local laws and the Chinese law for the Protection of Animals.
Embryonic Waterborne Exposure
To conduct the experiment, zebrafish embryos at 6 hpf were randomly distributed into 100 mm Petri dishes with each dish containing 100 embryos and 15 mL of the NP exposure solution. The exposure solutions had concentrations of 0.3, 3, and 9 mg/L. In order to maintain the freshness of exposure solutions throughout this experiment, 50% of the NP solutions were replaced every day. The embryos were exposed to the NPs until 72 hpf (3 days after fertilization). Throughout this time, the zebrafish embryos and larvae were examined twice a day in order to remove the deceased embryos or larvae from the Petri dishes. A group consisting of untreated zebrafish embryos was maintained alongside the exposed groups to serve as a control. All experiments were replicated three times independently.
Developmental Toxicity Test
Zebrafish embryos were exposed to 0.3, 3, and 9 mg/L of NPs solution at 6 hpf. The survival rates at various concentrations were recorded as the proportions of surviving embryos and larvae (n = 100 for each group) at 24, 48, and 72 hpf. The percentage of larvae hatched at different concentrations at 72 hpf (n = 100 per group) was calculated as the hatching rate. A stereo microscope (Leica M205FA, Leica Microsystems, Germany) was used to observe the heart rates of larvae at 48 and 72 hpf (n = 30 for each group). The same method and instrument were used to observe the body lengths of larvae at 72 hpf (n = 30 for each group), and ImageJ software was used to measure the body lengths of larvae at 72 hpf (n = 30 for each group). All experiments were replicated three times independently.
Quantifying Morphology and Locomotor Behavior Test of Zebrafish Larvae
Zebrafish embryos were exposed to 0.3, 3, and 9 mg/L of NPs solution at 6 hpf. Spontaneous movements was measured at 24 and 48 hpf by an optical microscope (OLYMPUS CX31)/stereo microscope (Leica M205FA, Leica microsystems, Germany) (n = 20 for each group). Using a medical needle as external stimuli to stick the larvae tail, zebrafish larvae at 72 hpf were observed by an optical microscope (OLYMPUS CX31) to detect whether they would respond to external stimuli (n = 20 for each group). The Zebralab video tracking system (ViewPoint Life Sciences, France) and high-throughput tracking analysis were used to measure the total swimming distances of zebrafish larvae at 96 hpf (n = 24 for each group). Zebrafish larvae from different concentrations were allocated to a 24-well plate (1 larva per well) and were measured by the above instruments. All experiments were replicated three times independently.
Image Observation of Transgenic Zebrafish
Zebrafish embryos of transgenic lines Tg(hb9:GFP) were exposed to 0.3 3 and 9 mg/L of NPs solution at 6 hpf (n = 30 for each group). Transgenic lines Tg(hb9:GFP) are the AB strain. We chose the early neurodevelopmental period: 36, 48, and 72 hpf to observe the development of central nerves and motor nerves. The central nerve and motor nerve images of transgenic lines Tg(hb9:GFP) larvae after anesthesia were obtained under a Leica DMi-8 fluorescence microscope. The motor nerve of transgenic line Tg(hb9:GFP) was measured using ImageJ software. All experiments were replicated three times independently.
Total RNA Extraction and qPCR Assay
Zebrafish embryos were exposed to 0.3, 3, and 9 mg/L of NPs solution at 6 hpf. According to the manufacturer’s instructions, TRIzol (Invitrogen, Thermo Fisher Scientific, Waltham, MA) was used to extract the total RNA of various groups of zebrafish embryos (n = 30 for each group). Hereafter, a spectrophotometer (Thermo Fisher Scientific) was used to detect the quality and purity of the RNA. The RNA was reverse-transcribed by using a reverse transcription PCR system on the CFX Maestro System (Bio-Rad Laboratories, Inc., CA) and qPCR assay. The primer sequences for qPCR detection are shown in Table 1.
Table 1. Primer Sequences Used to Target Neurodevelopmental-Related Genes, Oxidative Stress-Related Genes, and Apoptosis-related Genes in Zebrafish in This Study.
Statistical Analysis
Values were presented as means ± standard deviation (SD), and statistical significance was accepted at P < 0.05. The significant differences between mean values were determined by using one-way analysis of variance (ANOVA), and the Dunnett’s test was used to determine the significant difference between nanoplastic-treated and control groups (P < 0.05). The figures and the results of ANOVA were achieved by using Graphpad Prism 9 (GraphPad Software, San Diego, CA).
Results
Polystyrene NPs Affect the Survival Rates, Hatching Rates, Heart Rates, and Body Length of Zebrafish
In order to investigate how NPs affect zebrafish survival rates, zebrafish embryos at 6 hpf were exposed to various NP concentrations until 72 hpf. The results revealed that the survival rate of the zebrafish embryos in the high concentration group (9 mg/L) reduced significantly (Figure 1A). However, with the time under the condition of constant concentration increasing, the survival rates were steady, which suggests the effects of NPs on the survival was not a time-dependent manner in zebrafish embryos. Then, we detected the hatching rates in zebrafish embryos with NPs exposure. The results demonstrated that compared with the untreated group, NPs caused a significant dose-dependent reduction of hatching rates in zebrafish embryos which were exposed to NPs from 6 up to 72 hpf (Figure 1B).
Figure 1.
Effects of NPs exposure on the survival rate, hatching rate, heart rate, and body length of zebrafish embryos/larvae. (A) Survival rates of NPs-treated zebrafish embryos/larvae at different concentrations at 24, 48, and 72 hpf (n = 100 per group). (B) Hatching rates of NP-treated zebrafish larvae at various concentrations at 72 hpf (n = 100 per group). (C, D) Heart rates of NPs-treated zebrafish embryos/larvae at various concentrations at 48 and 72 hpf (n = 100 per group). (E) Body length diagram of various groups of NP-treated zebrafish larvae at 72 hpf. (F) Body length of various groups of NPs-treated zebrafish larvae at 72 hpf (n = 20 for each group). Scale bar, 0.5 mm. Data are presented as the mean ± SD *P < 0.05, *P < 0.01, ***P < 0.001, and ****P < 0.0001 compared with the control.
To further investigate the developmental toxicity of NPs exposure in zebrafish, we measured the heart rates and body lengths of zebrafish embryos exposed to NPs from 6 up to 48 and 72 hpf. The findings revealed that nanoplastic exposure dramatically reduced heart rates (P < 0.0001; Figures 1C,D). In addition, we observed a decrease in zebrafish body length after NP exposure in the 0.3 (P < 0.0001), 3 (P < 0.01), and 9 mg/L (P < 0.0001) at 72 hpf (Figure 1E,F), indicating that NP exposure may cause the developmental damage of zebrafish embryos.
NPs Significantly Inhibited Primary Motor Neuron Development
We employed the transgenic zebrafish line Tg(hb9:GFP), with intensive green fluorescent protein (eGFP) labeling of neuronal cells to evaluate the effects of NPs on the neural system. This line was utilized to observe neural development dynamically in zebrafish subjected to different concentrations of NPs at 36, 48, and 72 hpf (Figure 2A). The results showed that the neural pattern displayed a dose-dependent injury trend in the NPs-treated zebrafish embryos at 36, 48, and 72 hpf, compared with the control groups (red asterisks; Figure 2A). In addition, the number of synapses counted by quantitative analyses was significantly reduced in 9 mg/L (P < 0.0001; Figure 2B) at 36, 48, and 72 hpf of NP exposure. Furthermore, the lengths of synapses and spinal cords (Figure 2C,D) showed a dose-dependent reduction in NP-treated zebrafish embryos/larvae at 36, 48, and 72 hpf, indicating that NPs can injure zebrafish neuronal growth during the early developmental phase.
Figure 2.
Effects of NPs exposure on neural growth in zebrafish. (A) Neuronal morphology of zebrafish embryos/larvae at 36, 48, and 72 hpf of NP exposure. Scale bar, 200 um. (B) Quantification of the number of motor neurons in zebrafish embryos/larvae at 36, 48, and 72 hpf of NP exposure (n = 20 per group). (C) Quantification of the axon lengths of motor neurons in NP-treated zebrafish embryos/larvae at 36, 48, and 72 hpf (n = 20 per group). (D) Quantification of the spinal cord lengths of motor neurons in NPs-treated zebrafish embryos/larvae at 36, 48, and 72 hpf (n = 20 per group). Data are presented as the means ± SD *P < 0.05, ***P < 0.001, and ****P < 0.0001. Red asterisks represent axons.
NPs Induced Abnormal Locomotor Behavior
The neurological development is essential for locomotor behavior of zebrafish, and motility impairment typically follows neural system disruption. We conducted behavioral tests in order to assess the possible effects of NP exposure on zebrafish neural development. The motor abilities of the zebrafish larvae treated with NPs at different doses were assessed after exposure from 6 until 72 hpf. The results indicated that NPs significantly hindered the spontaneous motion of zebrafish larvae (Figure 3A). Furthermore, the findings demonstrate a concentration-dependent inhibitory effect on the locomotor behavior of zebrafish larvae (P < 0.05) (Figure 3B). Thus, these findings suggested that behavioral toxicity caused by NP exposure may have a positive correlation with the disturbance of neural growth in zebrafish larvae during the early developmental phase. In addition, we observed behavioral deficits in the majority of the larvae exposed to NPs at 48 hpf, including delayed or absent movement. We collected pools of zebrafish larvae with NPs exposure at various concentrations and measured the escape reaction of 50 randomly selected individual larvae at 48 hpf, an age at which a touch stimulus can induce stereotypical escape response behavior. The result showed that a touch stimulus at the dorsal tip of tail with a stereotypic escape response in zebrafish embryos was dose-dependent (Figure 3C and Supporting Information, Video S1), suggesting that NP-induced defect of motoneuron is the reason for the observed movement deficits at this age.
Figure 3.
NP exposure inhibits locomotor behavior in zebrafish larvae. (A) Moving track graphs of zebrafish larvae at 72 hpf of NPs exposure at different concentrations (n = 6 for each group). (B) Traveling distances of zebrafish larvae (n = 6 for each group). (C) Stereotypic escape response to different concentrations of NPs treatment (n = 6 per group). Data are presented as the mean ± SD *P < 0.05, *P < 0.01, ***P < 0.001 and ****P < 0.0001 compared with the control.
Identification of Downstream Signaling Pathways in NP-Induced Developmental Disruption in Zebrafish
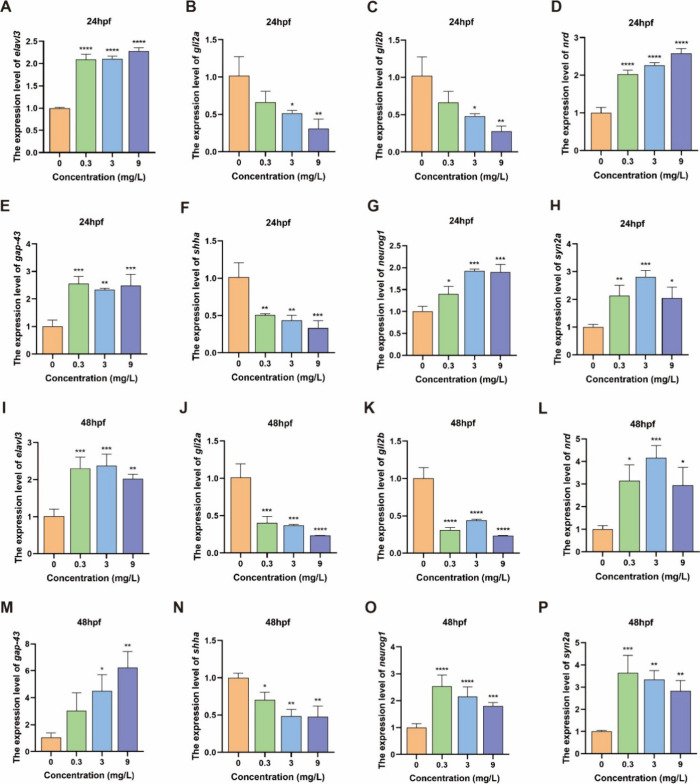
The impaired swimming ability and motor neuron phenotype observed in zebrafish larvae treated with NPs prompted us to investigate their potential neurotoxicity. The results showed that exposure to NPs disrupts the expression of neurodevelopmental genes in zebrafish larvae, potentially contributing to neurotoxic effects. We selected zebrafish larvae at two time points (24 and 48 hpf) to study the expression of genes involved in neurodevelopment (Figure 4). The result showed that most of the neurodevelopmental-related genes, including the expression levels of elavl3, nrd, gap-43, neurog1, and syn2a in larvae, were up-regulated and some of them were shown in a dose-dependent manner, compared to the control group. In contrast, the expression levels of gli2a, gli2b, and shha were significantly down-regulated in larvae exposed to NPs, compared to the control group. Together, all of the results revealed that NPs have toxic effects on the development of motor neurons in the early stage of zebrafish development.
Figure 4.
Expression of neural developmental genes. The expression of neurodevelopmental genes (elavl3, gli2a, gli2b, nrd, gap-43, shha, neurog1, and syn2a) in zebrafish larvae at 24 (A–H) and 48 hpf (I–P). Data are presented as the mean ± SD *P < 0.05, *P < 0.01, ***P < 0.001, and ****P < 0.0001 compared with the control.
NP Exposure Induces an Oxidative Stress and Apoptosis Response in Zebrafish Embryos
Since developmental toxicity is usually accompanied by an oxidative stress response and apoptosis,15−17 we then examined the impact of NPs exposure at various doses on ROS formation and the control of SOD activity in zebrafish embryos. Unsurprisingly, the NP-treated zebrafish embryos showed observably enhanced ROS accumulation (Figure 5A). The levels of ROS in NPs-treated zebrafish embryos were increased at 24 hpf, compared to the control group (Figure 5A). Moreover, after 24 h of exposure to zebrafish embryos, SOD activity decreased in groups exposed to NPs. Furthermore, the expression of the mRNA level of Cu/Zn-SOD was increased in the groups exposed to NPs (Figure 5B, section C).
Figure 5.
Effects of NP exposure on oxidative stress response and apoptosis in zebrafish embryos. (A) ROS accumulation in NP-treated zebrafish embryos at 24 hpf (n = 30 per group). (B) SOD activity in NP-treated zebrafish embryos at 24 hpf (n = 30 per group). (C) Expression level of Cu/Zn-SOD in NP-treated zebrafish embryos at 24 hpf (n = 30 per group). (D) Expression level of bcl2a in NP-treated zebrafish embryos at 24 hpf (n = 30 per group). (E) Expression level of caspase-3a in NP-treated zebrafish embryos at 24 hpf (n = 30 per group). (F) Expression level of bcl2a in NP-treated zebrafish embryos at 48 hpf (n = 30 per group). (G) Expression level of caspase-3a in NP-treated zebrafish embryos at 48 hpf (n = 30 per group). Data are presented as the mean ± SD *P < 0.05, *P < 0.01, ***P < 0.001, and ****P < 0.0001 compared with the control.
In order to provide additional evidence that NP-treated zebrafish embryos underwent apoptosis during the early developmental stage, we used the quantitative polymerase chain reaction (qPCR) to quantify the expression levels of apoptosis-related genes in the zebrafish embryos at 24 and 48 hpf exposure to NPs. The results showed that the expression of bcl2a gene, which regulates apoptotic in zebrafish, was significantly up-regulated (P < 0.05) in zebrafish embryos at 24 and 48 hpf exposed to different concentrations of NPs, compared to the control group (Figure 5D,F). Similarly, the expression of caspase-3a was significantly up-regulated in NPs-treated zebrafish embryos at 24 and 48 hpf (Figure 5E,G), compared to the control group. Thus, these results proved that the NP-induced developmental toxicity in zebrafish is linked to abnormal apoptosis.
Discussion
Plastics are extensively utilized for their versatility, lightweight, and affordability, and they fulfill an essential role in numerous sectors,1 but due to improper handling and mass production, a large amount of this plastic might eventually harm many environmental media.3,4 Consequently, plastic pollution stands as one of the most concerning issues.18,19 Due to the physical, chemical, and biological processes, plastics can degrade into microplastics (MPs) ranging in size from 1 μm to 5 mm in the environment.2,20 The concentrations of MPs in water, sediments, and organisms can achieve concentrations up to 25,800 items/m3,21 32,947 particles/kg,22 and 7527 items/fish, respectively.23 Among these, polystyrene (PS) is a particularly prevalent form of plastic fragments found in the environment.24−26 Over time, microplastics (MPs) can further break down into NPs with sizes smaller than 1 μm.27 NPs pose a considerable ecological challenge owing to their diminutive dimensions, expansive surface area, and capacity to infiltrate organisms. Duan discovered that the presence of PS-NPs on the zebrafish embryo’s chorionic membrane surface hindered the diffusion of oxygen between the embryo and surrounding water, causing internal hypoxia in the embryo.2 Consequently, this resulted in a deficient provision of oxygen necessary for the process of growth and delayed hatching. This discovery offers a potential rationale for the observed decrease in the survival rate and hatching rate across all experimental groups exposed to PS-NPs (Figure 1A,B). Multiple studies have provided evidence that NPs with small sizes were able to cross the chorionic membrane can induce deformities in zebrafish larvae. For instance, Liu discovered that 100 nm PS-NPs caused abnormality in zebrafish larvae, suggesting an adverse impact induced by the hypoxic microenvironment within the embryo on the cardiovascular system.28 Additionally, Park and Kim found that zebrafish embryos exposed to NPs with smaller granular sizes were more easily dispersed and accumulated throughout the embryo,3 which induced severe accumulation of NPs, cytokine stimulation, increased oxidative stress, and damage to organ cells, leading to larva developmental deformity. These are consistent with our results showing that 20 nm plastic particles exhibit developmental toxicity toward larvae, with a greater degree of toxicity associated with higher concentrations (Figure 1).
A number of studies have demonstrated that the vital phases for zebrafish neuronal cell differentiation, nervous system creation, and synaptogenesis are the early phases of the unhatched embryo.29 Because the blood-brain barrier has not formed during this period, contaminants into the body can impair the development, structural integrity, and functional capabilities of neural cells.30 The susceptibility of the neural system in the early developmental stage to environmental change has been well-documented. Alterations in locomotor behavior often function as a crucial marker of neurotoxicity given that locomotor activity exhibits greater susceptibility to the ecotoxicological impacts of pollutants in comparison to mortality rates. In our study, it was observed that the locomotor activity and neuronal development of zebrafish larvae were inhibited by nanoplastic particles at various concentrations (0.3, 3, and 9 mg/L). This indicates that nanoplastic particles can impair the function of the motor nerve (Figures 2 and 3). Similar findings were reported by Chen et al., who attributed the phenomenon to the increased oxidative stress and the reduced acetylcholinesterase (AChE) activity.31
Previous research has identified motor neuron abnormalities play a substantial role in modifying locomotor behavior elicited by different chemicals in zebrafish.32,33 The defects of neuronal development and motor neuron axons have also been associated with behavioral defects in larvae, as synaptic activity induced by neurons and motor neurons plays a crucial role in the development of swimming behavior.33,34 For example, BPA (bisphenol A) exposure in zebrafish was found to significantly impair motor function, as evidenced by reduced axon length and branching.35 These findings showed that the behavioral defects observed in zebrafish larvae are likely influenced by a complex interplay between the development of neurons and motor neurons as well as the response of the neurotransmitter system. The neuronal developmental processes in zebrafish embryos adheres to the consistent pattern of axonal growth and accurate targeting of axons toward certain muscle groups.36 In our study, we observed that a high concentration of NPs had a significant inhibitory effect on the development of motor neuron axons in transgenic Tg(hb9:GFP). This inhibition resulted in a shortened or irregular axon length, diminished synaptic density, and absence of axons (Figure 2). These findings showed that nanoplastic particles directly involved in the developmental damage of axons in motor neurons, presenting the dose-dependence. Consequently, abnormal axon growth and larval locomotor behavior deficit were correlated, which may help to explain why larvae exposed to varying concentrations of 20 nm NPs in this experiment showed less swimming activity (Figures 2 and 3).
Neurotoxicity induced by embryonic pollutants in zebrafish is largely dependent on the expression of apoptotic and developmental genes. For neurodevelopment-related genes, elavl3 is one of the earliest neuronal markers in the zebrafish larvae, encoding the neuron-specific RNA binding protein Huc.37 In this experiment, its expression level was up-regulated at 24 and 48 hpf, possibly due to compensatory effects on the neurotoxicity of NPs (Figure 4A,I). gli2a/2b mediates the development of the motor neurons38 ; significant down-regulation of gli2a/2b at 24 hpf and 48 hpf compared with control groups showed neurotoxicity with long NP exposure (Figure 4B,C,J,K). nrd and gap-43 play an important role in early neuronal formation, regeneration, and plasticity,39,40 which were significantly up-regulated in the 24 and 48 hpf NP exposure compared to the control group (Figure 4D,E,L,M), showing a compensatory effect of neurotoxicity upon exposure to NPs. shha forms a morphology in the nervous system, affecting the connection between spinal commissural axons and retinal ganglion cell axons.41 The expression of shha was significantly down-regulated at 24 and 48 hpf compared to the control group (Figure 4F,N), indicating that shha expression could be the primary reason for axonal damage caused by PS-NPs, leading to abnormal development of the motor nerves and motor behavior deficit in early larvae.42neurog1 is a key transcriptional regulator of neuronal differentiation.42 The expression of this gene was significantly up-regulated at both developmental stages at 24 and 48 hpf, indicating a compensatory effect of neurotoxicity upon exposure to NPs (Figure 4G,O). syn2a plays an important role in the neural development and synaptic transmission.43 The expression of syn2a was significantly up-regulated at both developmental stages at 24 and 48 hpf compared to controls (Figure 4H,P), again indicating a compensatory effect of juvenile zebrafish on neurotoxicity after prolonged exposure to NPs. Thus, all the results above indicated that 20 nm-NPs can inhibit the development of primary motor nerve in larval zebrafish.
The primary cause of oxidative stress in vivo is excessive oxygen radicals.44 Cells produce antioxidant enzymes in response to oxidative stress in vivo, and SOD is the most dominant component of antioxidant enzymes. In our study, various concentrations of 20 nm-NPs significantly reduced the activity of SOD (Figure 5B), which is similar to previous study that 100 nm-NPs significantly reduced the activity of SOD.45 Moreover, the expression level of the Cu/Zn-SOD gene showed a compensatory increase (Figure 5C), also illustrating that exposure to NPs causes abnormal SOD activity in oxidative stress. Several studies have shown that MPs and NPs can cause ROS metabolism and oxidative stress in zebrafish by regulating the antioxidants of SOD.46,47 In our study, the levels of increased ROS were measured in vitro after different concentrations of NPs exposure at 24 hpf (Figure 5A). Moreover, further studies showed that oxidative stress induced apoptosis by regulating the mRNA levels of bcl2a and caspase-3a in vitro (Figure 5D–G), indicating that exposure to NPs may lead to oxidative damage and apoptosis by inhibiting SOD activity or promoting ROS accumulation.
Conclusions
In conclusion, our study demonstrated that acute exposure to NPs can cause neural toxicity, which has detrimental impacts on neuron developmental processes (axon growth) in zebrafish embryos. Additionally, we discovered that zebrafish embryos exposed to NPs could experience developmental toxicity as a result of oxidative-stress-induced apoptosis and ROS accumulation. Further research unveiled aberrations in indicators associated with neurodevelopment, oxidative stress, and apoptosis, which could potentially represent the molecular mechanism underlying primary motor nerve injury. In summary, this paper explores the neurotoxic mechanism of NPs at different environmental concentrations and supplies data to support a comprehensive assessment of the ecotoxicity impacts of NPs.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (32072706 and U1901206).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.4c00231.
Stereotypic escape response of zebrafish under different concentrations of NPs (MP4)
Author Contributions
§ X.C. and W.X. contributed equally to this work
Author Contributions
J.L. and Y.W. established the research ideas and designed methods; X.C., W.X., J.Z., and J.C. conducted the experimental operations; X.C. and J.L. analyzed the data; X.C., J.L., and Y.W. wrote and revised the manuscript. J.L. and Y.W. supervised the research. All authors read and approved the manuscript for publication.
The authors declare no competing financial interest.
Supplementary Material
References
- Chisada S.; Yoshida M.; Karita K. Polyethylene microbeads are more critically toxic to the eyes and reproduction than the kidneys or growth in medaka, Oryzias latipes. Environ. Pollut. 2021, 268, 115957 10.1016/j.envpol.2020.115957. [DOI] [PubMed] [Google Scholar]
- Duan Z.; Duan X.; Zhao S.; et al. Barrier function of zebrafish embryonic chorions against microplastics and nanoplastics and its impact on embryo development. Journal of hazardous materials. 2020, 395, 122621 10.1016/j.jhazmat.2020.122621. [DOI] [PubMed] [Google Scholar]
- Park S. H.; Kim K. Microplastics induced developmental toxicity with microcirculation dysfunction in zebrafish embryos. Chemosphere. 2022, 286, 131868 10.1016/j.chemosphere.2021.131868. [DOI] [PubMed] [Google Scholar]
- Torres-Ruiz M.; De la Vieja A.; de Alba Gonzalez M.; Lopez M. E.; Calvo A. C.; Portilla A. I. C. Toxicity of nanoplastics for zebrafish embryos, what we know and where to go next. Sci. Total Environ. 2021, 797, 149125 10.1016/j.scitotenv.2021.149125. [DOI] [PubMed] [Google Scholar]
- Frias J. P.; Nash R. Microplastics: Finding a consensus on the definition. Marine pollution bulletin. 2019, 138, 145–147. 10.1016/j.marpolbul.2018.11.022. [DOI] [PubMed] [Google Scholar]
- Andrady A. Microplastics in the marine environment. Mar Pollute Bull. 2011, 62 (8), 1596–1605. 10.1016/j.marpolbul.2011.05.030. [DOI] [PubMed] [Google Scholar]
- Lambert S.; Wagner M. Characterisation of nanoplastics during the degradation of polystyrene. Chemosphere. 2016, 145, 265–268. 10.1016/j.chemosphere.2015.11.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H.; Xiong H.; Mi K.; Xue W.; Wei W.; Zhang Y. Toxicity comparison of nano-sized and micron-sized microplastics to Goldfish Carassius auratus Larvae. Journal of hazardous materials. 2020, 388, 122058 10.1016/j.jhazmat.2020.122058. [DOI] [PubMed] [Google Scholar]
- Yu J.; Chen L.; Gu W.; Liu S.; Wu B. Heterogeneity effects of nanoplastics and lead on zebrafish intestinal cells identified by single-cell sequencing. Chemosphere. 2022, 289, 133133 10.1016/j.chemosphere.2021.133133. [DOI] [PubMed] [Google Scholar]
- Kashiwada S. Distribution of nanoparticles in the see-through medaka (Oryzias latipes). Environmental health perspectives. 2006, 114 (11), 1697–1702. 10.1289/ehp.9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt J. A.; Kozal J. S.; Jayasundara N.; et al. Uptake, tissue distribution, and toxicity of polystyrene nanoparticles in developing zebrafish (Danio rerio). Aquatic Toxicology. 2018, 194, 185–194. 10.1016/j.aquatox.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson K.; Johnson E. V.; Malmendal A.; Linse S.; Hansson L.-A.; Cedervall T. Brain damage and behavioural disorders in fish induced by plastic nanoparticles delivered through the food chain. Sci. Rep. 2017, 7 (1), 11452. 10.1038/s41598-017-10813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y. J.; Jia Y. F.; Chen N.; et al. Zebrafish as a model system to study toxicology. Environmental toxicology and chemistry. 2014, 33 (1), 11–17. 10.1002/etc.2406. [DOI] [PubMed] [Google Scholar]
- Wu T.-S.; Cheng Y.-C.; Chen P.-J.; Huang Y.-T.; Yu F.-Y.; Liu B.-H. Exposure to aflatoxin B1 interferes with locomotion and neural development in zebrafish embryos and larvae. Chemosphere. 2019, 217, 905–913. 10.1016/j.chemosphere.2018.11.058. [DOI] [PubMed] [Google Scholar]
- Kupsco A.; Schlenk D. Oxidative stress, unfolded protein response, and apoptosis in developmental toxicity. International review of cell and molecular biology. 2015, 317, 1–66. 10.1016/bs.ircmb.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.; Wang H.; Duah P. A.; Retyunskiy V.; Liu Y.; Chen G. Zinc pyrithione (ZPT)-induced embryonic toxicogenomic responses reveal involvement of oxidative damage, apoptosis, endoplasmic reticulum (ER) stress and autophagy. Aquatic Toxicology. 2022, 248, 106195 10.1016/j.aquatox.2022.106195. [DOI] [PubMed] [Google Scholar]
- Lite C.; Guru A.; Juliet M.; Arockiaraj J. Embryonic exposure to butylparaben and propylparaben induced developmental toxicity and triggered anxiety-like neurobehavioral response associated with oxidative stress and apoptosis in the head of zebrafish larvae. Environmental Toxicology. 2022, 37 (8), 1988–2004. 10.1002/tox.23545. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Zhao H.; Xu Y.; et al. Early-life lead exposure induces long-term toxicity in the central nervous system: From zebrafish larvae to juveniles and adults. Sci. Total Environ. 2022, 804, 150185 10.1016/j.scitotenv.2021.150185. [DOI] [PubMed] [Google Scholar]
- Zhou R.; Lu G.; Yan Z.; Jiang R.; Sun Y.; Zhang P. Effects of polystyrene nanoplastics on the bioaccumulation, distribution and parental transfer of ethylhexyl salicylate. Environmental Science: Nano. 2022, 9 (3), 1025–1036. 10.1039/D1EN01004B. [DOI] [Google Scholar]
- Batel A.; Baumann L.; Carteny C. C.; Cormier B.; Keiter S. H.; Braunbeck T. Histological, enzymatic and chemical analyses of the potential effects of differently sized microplastic particles upon long-term ingestion in zebrafish (Danio rerio). Mar. Pollut. Bull. 2020, 153, 111022 10.1016/j.marpolbul.2020.111022. [DOI] [PubMed] [Google Scholar]
- Su L.; Xue Y.; Li L.; et al. Microplastics in taihu lake, China. Environ. Pollut. 2016, 216, 711–719. 10.1016/j.envpol.2016.06.036. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Su B.; Xu X.; et al. Preferential accumulation of small (< 300 μm) microplastics in the sediments of a coastal plain river network in eastern China. Water research. 2018, 144, 393–401. 10.1016/j.watres.2018.07.050. [DOI] [PubMed] [Google Scholar]
- Shabaka S. H.; Marey R. S.; Ghobashy M.; Abushady A. M.; Ismail G. A.; Khairy H. M. Thermal analysis and enhanced visual technique for assessment of microplastics in fish from an Urban Harbor, Mediterranean Coast of Egypt. Mar. Pollut. Bull. 2020, 159, 111465 10.1016/j.marpolbul.2020.111465. [DOI] [PubMed] [Google Scholar]
- Sendra M.; Pereiro P.; Yeste M. P.; Mercado L.; Figueras A.; Novoa B. Size matters: Zebrafish (Danio rerio) as a model to study toxicity of nanoplastics from cells to the whole organism. Environ. Pollut. 2021, 268, 115769 10.1016/j.envpol.2020.115769. [DOI] [PubMed] [Google Scholar]
- Atamanalp M.; Kokturk M.; Kırıcı M.; et al. Interaction of microplastic presence and oxidative stress in freshwater fish: a regional scale research, East Anatolia of Türkiye (Erzurum & Erzincan & Bingöl). Sustainability. 2022, 14 (19), 12009. 10.3390/su141912009. [DOI] [Google Scholar]
- Atamanalp M.; Köktürk M.; Parlak V.; Ucar A.; Arslan G.; Alak G. A new record for the presence of microplastics in dominant fish species of the Karasu River Erzurum, Turkey. Environ. Sci. Pollut. Res. 2022, 7866–7876. 10.1007/s11356-021-16243-w. [DOI] [PubMed] [Google Scholar]
- Gigault J.; Halle A. T.; Baudrimont M.; et al. Current opinion: what is a nanoplastic?. Environ. Pollut. 2018, 235, 1030–1034. 10.1016/j.envpol.2018.01.024. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Wang Y.; Li N.; Jiang S. Avobenzone and nanoplastics affect the development of zebrafish nervous system and retinal system and inhibit their locomotor behavior. Science of The Total Environment. 2022, 806, 150681 10.1016/j.scitotenv.2021.150681. [DOI] [PubMed] [Google Scholar]
- Grandjean P.; Landrigan P. J. Neurobehavioural effects of developmental toxicity. lancet neurology. 2014, 13 (3), 330–338. 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legradi J.; Di Paolo C.; Kraak M.; et al. An ecotoxicological view on neurotoxicity assessment. Environ. Sci. Eur. 2018, 30, 1–34. 10.1186/s12302-018-0173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q.; Gundlach M.; Yang S.; et al. Quantitative investigation of the mechanisms of microplastics and nanoplastics toward zebrafish larvae locomotor activity. Science of the total environment. 2017, 584, 1022–1031. 10.1016/j.scitotenv.2017.01.156. [DOI] [PubMed] [Google Scholar]
- Yuan L.; Qian L.; Qian Y.; et al. Bisphenol F-induced neurotoxicity toward zebrafish embryos. Environmental Science & Technology. 2019, 53 (24), 14638–14648. 10.1021/acs.est.9b04097. [DOI] [PubMed] [Google Scholar]
- Fan B.; Dai L.; Liu C.; Sun Q.; Yu L. Nano-TiO2 aggravates bioaccumulation and developmental neurotoxicity of triphenyl phosphate in zebrafish larvae. Chemosphere. 2022, 287, 132161 10.1016/j.chemosphere.2021.132161. [DOI] [PubMed] [Google Scholar]
- Jiang F.; Liu J.; Zeng X.; Yu L.; Liu C.; Wang J. Tris (2-butoxyethyl) phosphate affects motor behavior and axonal growth in zebrafish (Danio rerio) larvae. Aquatic Toxicology. 2018, 198, 215–223. 10.1016/j.aquatox.2018.03.012. [DOI] [PubMed] [Google Scholar]
- Morrice J. R.; Gregory-Evans C. Y.; Shaw C. A. Modeling environmentally-induced motor neuron degeneration in zebrafish. Sci. Rep. 2018, 8 (1), 4890. 10.1038/s41598-018-23018-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah G. K.; Al-Asmakh M.; Rasool K.; Mahmoud K. A. Ecotoxicological assessment of Ti 3 C 2 T x (MXene) using a zebrafish embryo model. Environmental Science: Nano. 2018, 5 (4), 1002–1011. 10.1039/C7EN01239J. [DOI] [Google Scholar]
- Kim C.-H.; Ueshima E.; Muraoka O.; et al. Zebrafish elav/HuC homologue as a very early neuronal marker. Neuroscience letters. 1996, 216 (2), 109–112. 10.1016/0304-3940(96)13021-4. [DOI] [PubMed] [Google Scholar]
- Ke Z.; Kondrichin I.; Gong Z.; Korzh V. Combined activity of the two Gli2 genes of zebrafish play a major role in Hedgehog signaling during zebrafish neurodevelopment. Molecular and Cellular Neuroscience. 2008, 37 (2), 388–401. 10.1016/j.mcn.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Korzh V.; Sleptsova I.; Liao J.; He J.; Gong Z. Expression of zebrafish bHLH genes ngn1and nrd defines distinct stages of neural differentiation. Developmental dynamics: an official publication of the American Association of Anatomists. 1998, 213 (1), 92–104. . [DOI] [PubMed] [Google Scholar]
- Udvadia A. J.; Köster R. W.; Skene J. P. GAP-43 promoter elements in transgenic zebrafish reveal a difference in signals for axon growth during CNS development and regeneration. Development. 2001, 128 (7), 1175–1182. 10.1242/dev.128.7.1175. [DOI] [PubMed] [Google Scholar]
- Sugimoto K.; Hui S. P.; Sheng D. Z.; Kikuchi K. Dissection of zebrafish shha function using site-specific targeting with a Cre-dependent genetic switch. Elife 2017, 6, e24635 10.7554/eLife.24635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw H. F.; Nechiporuk A.; Raible D. W. Zebrafish dorsal root ganglia neural precursor cells adopt a glial fate in the absence of neurogenin1. J. Neurosci. 2008, 28 (47), 12558–12569. 10.1523/JNEUROSCI.2079-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Hu M.; Li M.; et al. Effects of exposure to 3, 6-DBCZ on neurotoxicity and AhR pathway during early life stages of zebrafish (Danio rerio). Ecotoxicology and Environmental Safety 2024, 270, 115892 10.1016/j.ecoenv.2023.115892. [DOI] [PubMed] [Google Scholar]
- Zhao F.; Li H.; Cao F.; Chen X.; Liang Y.; Qiu L. Short-term developmental toxicity and potential mechanisms of the herbicide metamifop to zebrafish (Danio rerio) embryos. Chemosphere. 2019, 236, 124590 10.1016/j.chemosphere.2019.124590. [DOI] [PubMed] [Google Scholar]
- Feng M.; Luo J.; Wan Y.; et al. Polystyrene nanoplastic exposure induces developmental toxicity by activating the oxidative stress response and base excision repair pathway in zebrafish (Danio rerio). ACS omega. 2022, 7 (36), 32153–32163. 10.1021/acsomega.2c03378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao R.; Sheng C.; Lu Y.; Zhang Y.; Ren H.; Lemos B. Microplastics induce intestinal inflammation, oxidative stress, and disorders of metabolome and microbiome in zebrafish. Sci. Total Environ. 2019, 662, 246–253. 10.1016/j.scitotenv.2019.01.245. [DOI] [PubMed] [Google Scholar]
- Lu Y.; Zhang Y.; Deng Y.; et al. Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environmental science & technology. 2016, 50 (7), 4054–4060. 10.1021/acs.est.6b00183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.