Abstract
Background
Prior studies on vitamin D and dementia outcomes yielded mixed results and had several important limitations.
Objectives
We aimed to assess the associations of both serum vitamin D status and supplementation with all-cause dementia, Alzheimer’s disease (AD), and vascular dementia (VD) incidence.
Methods
With a prospective cohort study design, we comprehensively assessed the associations of vitamin D and multivitamin supplementation, as well as vitamin D deficiency {25-hydroxyvitamin D [25(OH)D] <30 nmol/L}, and insufficiency [25(OH)D 30 to <50 nmol/L], with the 14-year incidence of all-cause dementia, AD, and VD in 269,229 participants, aged 55 to 69, from the UK Biobank.
Results
Although 5.0% reported regular vitamin D use and 19.8% reported multivitamin use, the majority of participants exhibited either vitamin D deficiency (18.3%) or insufficiency (34.0%). However, vitamin D deficiency was less prevalent among users of vitamin D (6.9%) or multivitamin preparations (9.5%) than among nonusers (21.5%). Adjusted Cox regression models demonstrated 19% to 25% increased risk of all 3 dementia outcomes for those with vitamin D deficiency [hazard ratio (HR) 95% confidence interval (CI)]: 1.25 (1.16, 1.34) for all-cause dementia; 1.19 (1.07–1.31) for AD; 1.24 (1.08–1.43) for VD] and 10% to 15% increased risk of those with vitamin D insufficiency [HR (95% CI): 1.11 (1.05, 1.18) for all-cause dementia; 1.10 (1.02–1.19) for AD; 1.15 (1.03–1.29) for VD]. Regular users of vitamin D and multivitamins had 17% and 14% lower risk of AD [HR (95% CI): 0.83 (0.71, 0.98)] and VD [HR (95% CI): 0.86 (0.75, 0.98)] incidence, respectively.
Conclusions
Although our findings indicate the potential benefits of vitamin D supplementation for dementia prevention, randomized controlled trials are essential for definitive evidence.
Keywords: vitamin D, dementia, Alzheimer’s disease, vascular dementia, cohort study
Introduction
Dementia affects over 55 million people worldwide, and this number is predicted to triple by 2050 [1,2]. As a result, the search for effective treatments has led to an explosion of research. Yet, despite these efforts, effective therapeutics for all-cause dementia that can halt or reverse its progression remain elusive [3,4]. Therefore, the importance of preventing dementia through the control of modifiable risk factors is imperative [5].
Research into the role of vitamin D in Alzheimer’s disease (AD) suggests a potential involvement in the modulation of amyloid beta (Aβ) plaques, a key factor in AD pathology [6]. Moreover, evidence indicates that vitamin D might provide neuroprotection against Aβ-induced tau hyperphosphorylation [7].
Vitamin D deficiency and insufficiency, typically defined by serum 25-hydroxyvitamin D (25(OH)D) levels of <30 nmol/L and <50 nmol/L respectively [8], have high prevalence rates—40% in Europe, 24% in the United States, and 37% in Canada [9]. A recent meta-analysis of observational studies suggests that 25(OH)D levels <50 nmol/L may be a risk factor for dementia, specifically for AD [10]. Addressing this may positively influence dementia prevention, especially since dietary supplements can safely and affordably manage it [11]. However, it is essential to mention that many of the included observational studies omitted to consider pivotal confounders, such as outdoor activity duration, seasonal variations in 25(OH)D levels, body mass index (BMI), dietary habits, and smoking status [12,13].
The results from past clinical trials on vitamin D supplementation vary considerably. Some have shown cognitive improvements following vitamin D supplementation, whereas others have found no significant benefits [[14], [15], [16], [17], [18], [19], [20]]. The disparities in findings could be attributed to factors like dosing differences, small sample sizes, and, notably, insufficient follow-up durations—vital for preventive dementia research. Although extended follow-up periods are more feasible in observational studies on vitamin D supplementation, their results have been similarly inconsistent. For instance, one study reported a reduced incidence of dementia linked to vitamin D [11], contrasting another that found an increased risk [21].
With data from the large UK Biobank cohort study with a median follow-up of 13.6 years, we aimed to assess the associations of serum vitamin D status and supplementation with all-cause dementia, AD, and vascular dementia (VD) incidence. Our analysis is the first to adjust for a wide range of potential confounders, encompassing demographic, socioeconomic, lifestyle, biomarker, healthcare-related, and genetic factors. Furthermore, the size of our data set permitted stratified analyses by age, sex, skin pigmentation, and Apolipoprotein E (APOE) ε4 allele status, helping to identify potential subgroups that might benefit most from vitamin D supplementation.
Methods
Data source and study population
In this study, data were sourced from a prospective cohort, the UK Biobank from the United Kingdom. The UK Biobank stands as an expansive, large-scale longitudinal study that took shape by enlisting over half a million study participants, aged between 40 and 69 y, with a few outliers of the predetermined age range, aged 37 to 40 y or 70 to 73 y. Although we did not exclude the outliers, we used the aspired minimum and maximum age in texts and tables. Recruitment spanned the years between 2006 and 2010, encompassing 22 study assessment centers strategically positioned across the United Kingdom [22]. At the baseline assessment visit, participants completed an electronically signed consent, a touch-screen questionnaire, a brief computer-assisted interview, physical and functional measures, and a collection of biological samples, including blood, urine, and saliva [23]. Follow-up for health-related outcomes was carried out mainly through linkages to routinely available data from the UK National Health Service (e.g., death registrations, cancer registrations, hospital inpatient/outpatient records, and primary care data) [23].
Out of the initial cohort of 502,366 participants enlisted in the UK Biobank, n = 194,109 individuals below the age of 55 were excluded. This decision was based on the significantly lower dementia prevalence at baseline age <55 vs. ≥55 y (0.2% vs. 2.6%) and the distinct etiology of early-onset dementia with predominantly AD cases caused by mutations in the presenilin 1, presenilin 2, or the amyloid precursor protein gene [24]. Furthermore, those who lacked essential baseline data concerning vitamin D usage and serum 25(OH)D concentration or with pre-existing dementia at baseline were excluded (refer to Supplemental Figure 1 for a flow-chart of the selection of the study population). Consequently, a refined cohort of 269,229 participants remained as the focal point for the analysis of dementia outcomes in this study.
Exposure variables
The predictors enlisted in this study encompassed measured vitamin D status, coupled with the self-reported utilization of vitamin D and/or multivitamin supplements during the baseline visit. Vitamin D deficiency was defined as serum 25(OH)D levels <30 nmol/L, whereas insufficiency was characterized by levels <50 nmol/L, but ≥30 nmol/L [8]. The quantification of serum 25(OH)D concentrations employed the Chemiluminescent Immunoassay technique, a direct competitive method executed using the DiaSorin Liaison XL system (manufactured by Diasorin S.p.A). Furthermore, to ensure rigorous accuracy, the method underwent external validation via participation in the RIQAS Immunoassay Speciality I scheme, attaining an impeccable quality assurance rating of 100% [25,26].
Essential insights concerning the habitual consumption of vitamin D and multivitamin supplements were drawn from the comprehensive UK Biobank baseline questionnaire. The question inquired, “Do you regularly take any of the following (you can select more than one answer)?” offering a spectrum of answer categories: “Vitamin A/Vitamin B/Vitamin C/Vitamin D/Vitamin E/Folic acid or Folate/Multivitamins ± minerals/None of the above/Prefer not to answer.”
For classification, participants indicating “Vitamin D” were categorized as vitamin D users. These participants included those who obtained vitamin D through over-the-counter (OTC) sources (83.7%) and those with prescribed vitamin D medications (16.3%). Those selecting “Multivitamins ± minerals” were categorized as multivitamin users and considered separate because most multivitamin preparations contain low-dose vitamin D (usually the daily recommended intake of 400 IU per tablet or capsule). Few participants reported using vitamin D and multivitamin products, and these individuals were included in the vitamin D user category. Those selecting “None of the above” or “Other specific vitamin products than vitamin D or multivitamins” were classified as nonusers [12].
Assessment of covariates
The selection of covariates for this study was primarily informed by a prior research project conducted within the UK Biobank by our group, which identified 49 determinants of vitamin D deficiency and 49 determinants of vitamin D supplementation [12]. These determinants were used as covariates and extended by the APOE ε4 genotype, given its significance as a specific risk factor for dementia. The covariates spanned diverse domains, including demographic, socioeconomic, lifestyle, biomarker, healthcare-related, and genetic dimensions, and their assessments and categorizations are described in more detail in Supplemental Text 1 and Supplemental Table 1.
Ascertainment of incident dementia outcomes
Within the scope of the UK Biobank, occurrences of incident all-cause dementia, AD, and VD were acquired through algorithmic amalgamations of interlinked data derived from hospital admission records and death registries [27]. The minimum and maximum follow-up time were 12.7 and 16.2 years, respectively, counted either from March 3, 2006 (first recruited participant) or October 1, 2010 (last recruited participant) to November 30, 2022 (end of data collection by the UK Biobank at the time of analysis).
Statistical analyses
General remarks.
We utilized SAS statistical software to execute all statistical analyses (version 9.4; SAS Institute, Inc, Cary, NC, USA). Descriptive statistics stood as our foundation for comprehending the demographics and baseline characteristics of the cohort. Person-time calculations commenced from the enrollment date and continued until the onset of dementia, death, date of loss of follow-up, or the end of the last follow-up on November 30, 2022. Considering missing values, a rigorous approach of multiple imputation (MI) was embraced, generating 5 imputed data sets. The highest missingness was observed for physical activity (24.2%), followed by income (16.9%), APOE ε4 status (16.6%), and the forced expiratory volume in one second (10.0%). All other variables had below 10% of missingness. Although there is no established guideline for an acceptable percentage of missing data for MI, 50% of missingness is frequently suggested, which would be far above the missingness rates of all variables used in this study [28]. Of note, the MI excluded exposure and outcome variables, which needed completion. This preparation paved the way for subsequent regression analyses [29]. The SAS program PROC MIANALYZE took the role of orchestrating the analysis and harmonizing outcomes stemming from the corresponding imputed data sets. A significance threshold of 0.05 was employed for all analyses.
Associations of vitamin D deficiency and insufficiency with dementia outcomes.
Cox proportional hazards regression models were conducted to explore potential associations of vitamin D deficiency and insufficiency with dementia outcomes. To effectively mitigate confounding, a comprehensive set of determinants for vitamin D deficiency and dementia was integrated. To facilitate this endeavor, the selected variables were partitioned across 5 distinct models, each progressively enhancing the degree of adjustment. Model 1 encapsulated quintessential factors, including age, sex, skin color, the geographical latitude of the assessment center, and the specific calendar month of attendance at the assessment center. Model 2 expanded its adjustment by incorporating socioeconomic dimensions. Model 3 additionally integrated lifestyle attributes and factors specific to vitamin D. Model 4 included parameters associated with weight measurements, and model 5 encompassed a comprehensive spectrum of factors, spanning diseases, manifestations of diseases, biomarkers, variables providing insight into overall health status, and the APOE genotype. A detailed listing of the covariates adjusted for in the various models is provided in the footnotes of pertinent tables.
Associations of vitamin D supplementation with dementia outcomes.
Drawing a parallel from the analyses conducted for vitamin D deficiency and insufficiency, a similar approach was adopted. In this context, we again employed 5 Cox proportional hazards regression models to investigate the plausible associations between the utilization of vitamin D and multivitamin supplements and the occurrence of all-cause dementia, AD, and VD. Employing the same variable categories utilized for vitamin D deficiency and insufficiency, the individual variables partly differed (for details, see the footnotes of pertinent tables).
Subgroup and sensitivity analyses.
Subgroup analyses were carried out by sex (female/male), age (55 to <65 or ≥65 to 69 y), skin color (non-black and non-brown/black or brown), APOE ε4 allele status (APOE ε4 noncarrier or APOE ε4 carrier), and BMI of <30 or ≥30 kg/m2. Furthermore, to address potential concerns of reverse causation in the analysis of vitamin D status and protopathic bias in the analysis of vitamin D supplementation, we performed sensitivity analyses. In these analyses, subjects who developed dementia in the first 5 years of follow-up were excluded [30,31].
Results
Characteristics of the study population
The cohort for the dementia outcomes analysis comprised a total of 269,229 participants (as depicted in Supplemental Figure 1). The mean age of the participants included in the study was 62.1 years, with an IQR of 59 to 65 y at the baseline assessment. Among these participants, 140,857 (52.3%) were female. Notably, a significant proportion of the cohort fell within the categories of overweight (BMI: 25 to <30 kg/m2; 44.1%) or obese (BMI: ≥30 kg/m2; 25.2%). Within the cohort, current smokers constituted 8.8%, whereas 12.0% reported high alcohol consumption. Furthermore, almost one-fifth of the participants reported low levels of physical activity. The prevalence for hypertension, diabetes mellitus, and coronary artery disease was 35.5%, 6.3%, and 8.3%, respectively. The median count of chronic diseases was 2, with an IQR spanning from 1 to 3. In terms of vitamin D status, the majority of study participants demonstrated either vitamin D deficiency (18.3%) or insufficiency (34.0%). Only a small subset, specifically 5.0%, reported regular consumption of vitamin D, whereas an additional 19.8% revealed regular usage of a multivitamin (± minerals) preparations. Further baseline characteristics of the study population are presented in Table 1.
TABLE 1.
Baseline characteristics of the study population (N = 269,229)
| Baseline characteristics | Ntotal (%)1 unless otherwise specified |
|---|---|
| 25(OH)D levels (nmol/L), mean (SD) | 50.1 (20.8) |
| Vitamin D status | |
| Vitamin D deficiency (25(OH)D <30 nmol/L) | 49,210 (18.3) |
| Vitamin D insufficiency (25(OH)D 30–<50 nmol/L) | 91,463 (34.0) |
| Vitamin D sufficiency (25(OH)D ≥50 nmol/L) | 128,556 (47.8) |
| Vitamin D / multivitamin supplements use | |
| No | 202,532 (75.2) |
| Yes, vitamin D preparations regularly | 13,372 (5.0) |
| Yes, multivitamin (± minerals) preparations regularly | 53,325 (19.8) |
| Sex | |
| Female | 140,857 (52.3) |
| Male | 128,372 (47.7) |
| Age (y), mean (SD) | 62.1 (4.1) |
| Skin color | |
| Very fair | 19,492 (7.4) |
| Fair | 187,908 (70.8) |
| Light olive | 46,621 (17.6) |
| Dark olive | 4633 (1.8) |
| Brown | 5446 (2.0) |
| Black | 1236 (0.5) |
| Y of education | |
| ≤9 | 78,795 (29.7) |
| 10–11 | 69,570 (26.2) |
| ≥12 | 117,037 (44.1) |
| Indices of multiple deprivation, mean (SD) | −0.06 (0.96) |
| Average household income (£) | |
| <18,000 | 64,525 (28.8) |
| 18,000–30,999 | 66,834 (29.9) |
| 31,000–51,999 | 52,203 (23.3) |
| 52,000–100,000 | 32,243 (14.4) |
| >100,000 | 7934 (3.6) |
| Disability2 | 18,885 (7.1) |
| BMI (kg/m2) | |
| <18.5 | 1233 (0.5) |
| 18.5–<25 | 81,210 (30.3) |
| 25–<30 | 118,276 (44.1) |
| 30–<35 | 49,168 (18.3) |
| 35–<40 | 13,551 (5.1) |
| ≥40 | 4751 (1.8) |
| Waist circumference (cm), mean (SD) | 91.4 (13.3) |
| Smoking status | |
| Never smoker | 138,192 (51.3) |
| Former smoker, occasionally | 32,823 (12.2) |
| Former smoker, regularly | 74,514 (27.7) |
| Current smoker, occasionally | 5926 (2.2) |
| Current smoker, regularly | 17,698 (6.6) |
| Alcohol consumption3 | |
| Abstainer | 82,012 (30.5) |
| Low | 109,576 (40.7) |
| Moderate | 45,334 (16.8) |
| High | 32,307 (12.0) |
| Physical activity4 | |
| Low | 36,420 (17.9) |
| Moderate | 84,558 (41.5) |
| High | 83,038 (40.7) |
| Visiting friends/family | |
| Almost daily | 35,931 (13.4) |
| 2–4 times/wk | 89,563 (33.5) |
| Once/wk | 89,846 (33.6) |
| Once every few months/rare | 52,333 (19.6) |
| Oily fish consumption | |
| Never/less than once a week | 104,915 (39.2) |
| At least once a week | 162,962 (60.8) |
| Cereal consumption (bowls/wk) | |
| Never | 42,428 (15.8) |
| <7 | 109,613 (40.8) |
| ≥7 | 116,485 (43.4) |
| Processed meat intake | |
| Never/less than once a week | 108,204 (40.3) |
| At least once a week | 160,538 (59.7) |
| Milk consumption | |
| Never/rarely | 8536 (3.17) |
| Occasionally/regularly | 260,530 (96.8) |
| Spread consumption | |
| Never/rarely | 28,264 (10.5) |
| Butter | 93,719 (34.9) |
| Margarine/others | 146,900 (54.6) |
| Preferred bread type | |
| White | 64,733 (24.9) |
| Wholemeal/wholegrain/brown | 184,663 (70.9) |
| Other | 11,040 (4.2) |
| eGFR (mL/min/1.73 m2) | |
| ≥90 | 131,857 (49.0) |
| <90 | 137,040 (51.0) |
| HbA1c (%) | |
| <6 | 228,987 (89.7) |
| 6–<6.5 | 13,957 (5.5) |
| 6.5–<7 | 4879 (1.9) |
| 7–<8 | 4613 (1.8) |
| ≥8 | 2957 (1.2) |
| HDL cholesterol (mg/dL) | |
| <40 | 30,538 (12.4) |
| ≥40 | 215,789 (87.6) |
| SBP (mmHg) | |
| <140 | 116,744 (43.4) |
| 140–<160 | 97,036 (36.1) |
| 160–<180 | 43,122 (16.0) |
| ≥180 | 12,049 (4.5) |
| DBP (mmHg) | |
| <60 | 2792 (1.0) |
| 60–<100 | 250,602 (93.2) |
| ≥100 | 15,564 (5.8) |
| C-reactive protein (mg/L) | |
| <3 | 203,202 (75.7) |
| 3–<10 | 53,217 (19.8) |
| ≥10 | 12,052 (4.5) |
| FEV1 (L), mean (SD) | 2.65 (0.75) |
| Hand grip strength (kg), mean (SD) | 31.5 (11.0) |
| APOE e4 status | - |
| ε2/ε2, ε2/ε3, ε3/ε3 | 161,912 (72.1) |
| ε2/ε4 | 5745 (2.6) |
| ε3/ε4 | 51,681 (23.0) |
| ε4/ε4 | 5142 (2.3) |
| No. of comorbidities, median (IQR) | 2 (1–3) |
| History of any cancer excl. nonmelanoma skin cancer | 35,965 (13.4) |
| Hypertension | 95,642 (35.5) |
| Type 2 diabetes mellitus | 16,952 (6.3) |
| History of stroke | 9720 (3.6) |
| Coronary artery disease | 22,435 (8.3) |
| COPD | 14,115 (5.2) |
| Asthma | 29,240 (10.9) |
| Osteoporosis | 8500 (3.2) |
| Fracture in last 5 years | 25,843 (9.6) |
| Arthritis | 54,587 (20.3) |
| Gout | 5614 (2.1) |
| Parkinson | 819 (0.3) |
| Depressed mood in last 2 wks | 10,038 (3.9) |
| Tiredness/lethargy in last 2 wks | 27,789 (10.7) |
| Chronic fatigue syndrome | 1036 (0.4) |
| Hypothyroidism | 17,179 (6.4) |
| No. of drugs, median (IQR) | 2 (1–4) |
Abbreviations: 25(OH)D: 25-hydroxyvitamin D; APOE, Apolipoprotein E; BMI, body mass index; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FEV1, Forced expiratory volume in 1-second; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; IPAQ, International Physical Activity Questionnaire; SBP, systolic blood pressure; SD, standard deviation.
Data are based on unimputed data set.
Disability is defined as having attendance allowance, disability living allowance, or blue badge.
Alcohol consumption levels: low (females: 0–19.99 g/d, males: 0–39.99 g/d); medium (females: 20–39.99 g/d, males: 40–59.99 g/d); high (females: ≥40 g/d, males: ≥60 g/d).
IPAQ activity levels: low (not meeting the criteria for medium and high levels of IPAQ activity); medium (≥0.5 h/d of moderate-intensity activity on most days); high (≥1 h/d of moderate-intensity activity).
Baseline characteristics varied across serum vitamin D status (Supplemental Table 1) and vitamin D supplementation groups (Supplemental Table 2). Just to name a few, the vitamin D deficiency group, compared to the group with sufficient levels, had more frequently brown or black skin color and were more frequently obese (BMI: ≥30 kg/m2), regularly smoking, and engaged in low amounts of physical activity. Vitamin D users, in contrast to nonusers, were more frequently female, more often had a higher education level (≥12 years of school), and were less frequently regular smokers. Of note, the 25(OH)D levels were higher among vitamin D users (59.3 nmol/L) and multivitamin users (56.0 nmol/L) than nonusers (47.9 nmol/L). Consequently, the prevalence of vitamin D deficiency was much lower among subjects using vitamin D supplements (6.9%) or multivitamin supplements (9.5%) than among nonusers (21.3%).
Associations of vitamin D deficiency and insufficiency with dementia outcomes
Within the entire cohort, a total of 7087 participants (2.6%) experienced all-cause dementia over a median follow-up period of 13.6 y (IQR: 12.7–14.3 y). Among these, 3616 participants were diagnosed with AD, whereas 1815 had a diagnosis of VD. As portrayed in Table 2, both vitamin D deficiency and insufficiency exhibited statistically significant associations with all-cause dementia, AD, and VD across all levels of covariate adjustments. Of note, the hazard ratios (HRs) demonstrated attenuation as adjustments progressed from model 1 to model 5. Within model 5, compared to individuals with sufficient 25(OH)D levels, a 25% and 11% increased risk of all-cause dementia were observed for individuals with vitamin D deficiency {HR [95% confidence interval (CI)]: 1.25 [1.16, 1.34]} and individuals with vitamin D insufficiency [1.11 (1.05–1.18)], respectively. The augmented risk associated with vitamin D deficiency and insufficiency exhibited a consistent pattern for AD [HRs (95% CI): 1.19 (1.07, 1.31) and 1.10 (1.02, 1.19), respectively] and for VD [HRs (95% CI): 1.24 (1.08, 1.43) and 1.15 (1.03, 1.29), respectively].
TABLE 2.
Associations of vitamin D deficiency and insufficiency with all-cause dementia, Alzheimer’s disease, and vascular dementia in 269,229 UK Biobank participants
| Dementia outcomes | Vitamin D deficiency (n = 49,210) |
Vitamin D insufficiency (n = 91,463) |
Vitamin D sufficiency (n = 128,556) |
|||
|---|---|---|---|---|---|---|
| Ncases (%) | HR (95% CI)6 | Ncases (%) | HR (95% CI)6 | Ncases (%) | HR (95% CI) | |
| All-cause dementia | 1538 (3.1) | 2422 (2.7) | 3127 (2.4) | |||
| Model 11 | 1.58 (1.48, 1.68) | 1.18 (1.12, 1.25) | Ref | |||
| Model 22 | 1.49 (1.39, 1.59) | 1.16 (1.10, 1.23) | Ref | |||
| Model 33 | 1.37 (1.28, 1.47) | 1.12 (1.06, 1.19) | Ref | |||
| Model 44 | 1.36 (1.26, 1.46) | 1.12 (1.06, 1.18) | Ref | |||
| Model 55 | 1.25 (1.16, 1.34) | 1.11 (1.05, 1.18) | Ref | |||
| Alzheimer’s disease | 726 (1.5) | 1237 (1.4) | 1653 (1.3) | |||
| Model 11 | 1.24 (1.13, 1.36) | 1.09 (1.01, 1.17) | Ref | |||
| Model 22 | 1.21 (1.10, 1.33) | 1.08 (1.00, 1.17) | Ref | |||
| Model 33 | 1.23 (1.11, 1.35) | 1.09 (1.01, 1.18) | Ref | |||
| Model 44 | 1.25 (1.13, 1.39) | 1.11 (1.03, 1.20) | Ref | |||
| Model 55 | 1.19 (1.07, 1.31) | 1.10 (1.02, 1.19) | Ref | |||
| Vascular dementia | 413 (0.8) | 633 (0.7) | 769 (0.6) | |||
| Model 11 | 1.68 (1.47, 1.91) | 1.26 (1.13, 1.40) | Ref | |||
| Model 22 | 1.56 (1.36, 1.77) | 1.24 (1.11, 1.38) | Ref | |||
| Model 33 | 1.44 (1.26, 1.66) | 1.20 (1.07, 1.34) | Ref | |||
| Model 44 | 1.37 (1.19, 1.57) | 1.16 (1.04, 1.30) | Ref | |||
| Model 55 | 1.24 (1.08, 1.43) | 1.15 (1.03, 1.29) | Ref | |||
The covariates were modeled as continuous or categorical variables as shown in Supplemental Table 1, which displays their distributions according to the vitamin D status groups. 6 HRs with 95% CIs were derived from Cox proportional hazards models.
Abbreviations: CI: confidence interval, HR: hazard ratio, Ref: reference
Cox proportional hazards model 1: Age, sex, skin color, latitude of study center and calendar month of attending the assessment center.
Cox proportional hazards model 2: Model 1 variables plus socioeconomic factors (education, indices of multiple deprivation, no of individuals in household, and household income).
Cox proportional hazards model 3: Model 2 variables plus lifestyle factors (smoking, alcohol consumption, physical activity, frequency of visiting friends/family and consumption of oily fish, cereal, processed meat, milk, bread, and spread), and vitamin D specific factors (time spent outdoors in summer and winter, ease of skin tanning, use of sunscreen/UV protection, and solarium/sunlamp use).
Cox proportional hazards model 4: Model 3 variables plus weight variables (body mass index and waist circumference).
Cox proportional hazards model 5: Model 4 variables plus diseases and disease symptoms (diabetes, stroke, coronary artery disease, chronic obstructive pulmonary disease, osteoporosis, arthritis, gout, Parkinson, depressed mood, and tiredness/lethargy), biomarkers (estimated glomerular filtration rate, HbA1c, HDL cholesterol, systolic blood pressure, diastolic blood pressure, C-reactive protein, forced expiratory volume in 1 s, and hand grip strength), general health status (no. of drugs, no. of chronic diseases, disability, and general self-rated health), and Apolipoprotein E ε4 status.
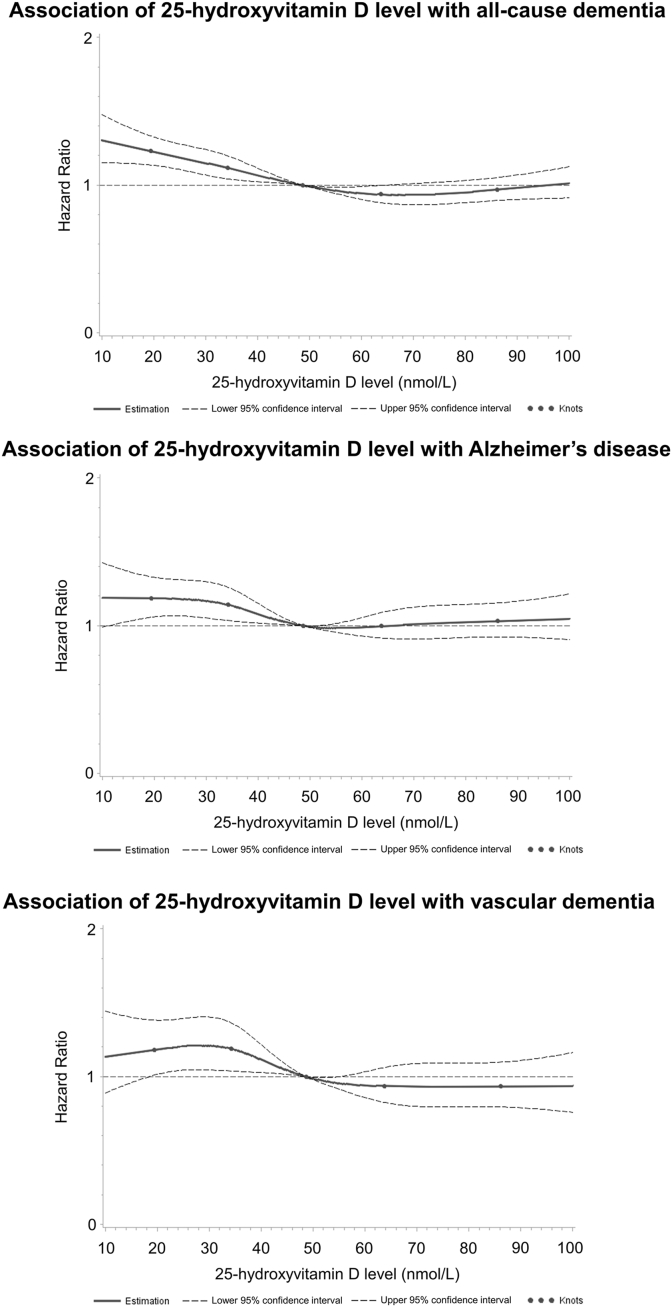
In dose-response analyses, we extended our investigations. Among individuals with 25(OH)D levels <50 nmol/L, there appeared to be an inverse dose-response relationship with all-cause dementia, whereas there was no association in subjects with higher 25(OH)D levels (Figure 1). The latter was the same for AD and VD, but the curve plateaued at 25(OH)D levels <30 nmol/L.
FIGURE 1.
Dose-response curves illustrating the relationship between 25-hydroxyvitamin D levels and dementia outcomes in 269,229 UK Biobank participants. Dose-response curves were generated from the imputation data set 1.
In a sensitivity analysis, excluding dementia events in the first 5 years, the HRs for both vitamin D deficiency and insufficiency demonstrated a slight decrease or remained comparable to the HRs in the main analysis. Importantly, the statistically significant associations with dementia outcomes persisted (Supplemental Table 3).
Subgroup analyses on the associations of vitamin D deficiency and insufficiency with dementia outcomes
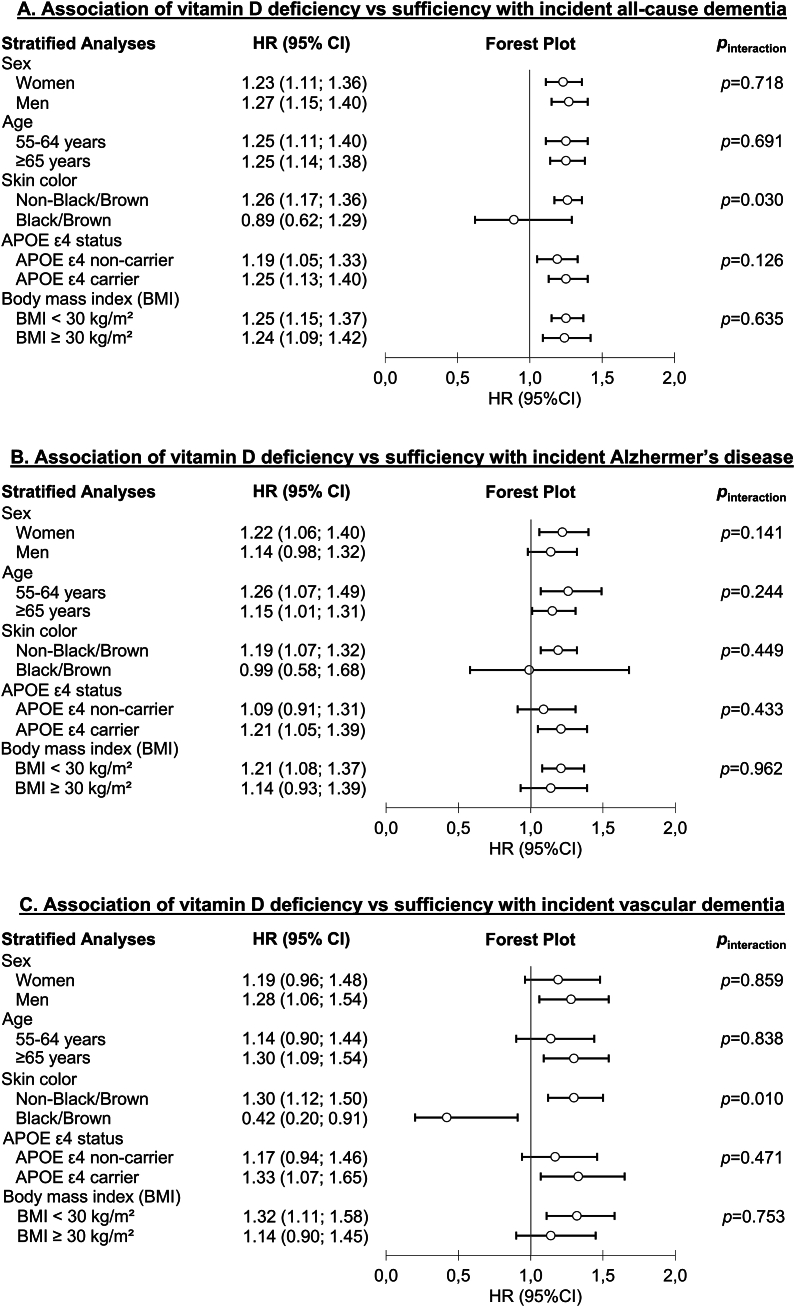
The results of subgroup analyses, stratified by sex, age, APOE ε4 allele status, and BMI, did not unveil substantial differences in the associations between vitamin D status and dementia outcomes (Figure 2, Supplemental Tables 4, 5, 7, and 8). However, a particularly intriguing pattern emerged from our subgroup analysis based on skin color. It appeared that neither vitamin D deficiency nor vitamin D insufficiency were associated with the dementia outcomes in study participants with darker skin tones (Supplemental Table 6). For VD, the association of vitamin D deficiency was even reversed compared to the main analysis, but this analysis must be considered with caution because it is only based on 17 VD events among 2711 subjects with vitamin D deficiency. The effect modification by skin color was substantiated by statistically significant interaction effects for the associations of vitamin D deficiency with all-cause dementia (pinteraction = 0.030) and VD (pinteraction = 0.010) (Figure 2, Supplemental Table 6).
FIGURE 2.
Forest plots presenting subgroup analyses investigating the associations of vitamin D deficiency with incident all-cause dementia (A), Alzheimer’s disease (B), and vascular dementia (C) in 269,229 UK Biobank participants. HRs with 95% CIs were derived from Cox proportional hazards models. APOE, Apolipoprotein E; BMI, body mass index; CI, confidence interval; HR, hazard ratio.
Associations of vitamin D supplementation with dementia outcomes
Table 3 presents the association between the utilization of vitamin D and multivitamin supplements and all-cause dementia, AD, and VD. For vitamin D use, the associations gained strength from models 1 to 5, and in the most comprehensively adjusted model 5, a statistically significant inverse association between vitamin D use and AD became apparent [HR (95% CI): 0.83 (0.71, 0.98)]. The HR point estimates (95% CI) for all-cause dementia [0.90 (0.81, 1.01)] and VD [0.90 (0.72, 1.12)] were quite close to the one of AD but were not statistically significant.
TABLE 3.
Associations of vitamin D supplement use and multivitamin use with all-cause dementia, Alzheimer’s disease, and vascular dementia in 269,229 UK Biobank participants
| Dementia outcomes | Vitamin D users (n = 13,372) |
Multivitamin users (n = 53,325) |
Nonusers (n = 202,532) |
|||
|---|---|---|---|---|---|---|
| Ncases (%) | HR (95% CI)6 | Ncases (%) | HR (95% CI)6 | Ncases (%) | HR (95% CI) | |
| All-cause dementia | 353 (2.6) | 1314 (2.5) | 5420 (2.7) | |||
| Model 11 | 0.97 (0.87, 1.08) | 0.96 (0.90, 1.02) | Ref | |||
| Model 22 | 0.97 (0.87, 1.08) | 0.96 (0.90, 1.02) | Ref | |||
| Model 33 | 1.00 (0.90, 1.11) | 0.98 (0.93, 1.05) | Ref | |||
| Model 44 | 0.99 (0.89, 1.11) | 0.99 (0.93, 1.05) | Ref | |||
| Model 55 | 0.90 (0.81, 1.01) | 0.98 (0.92, 1.04) | Ref | |||
| Alzheimer’s disease | 170 (1.3) | 729 (1.4) | 2717 (1.3) | |||
| Model 11 | 0.90 (0.77, 1.05) | 1.05 (0.97, 1.14) | Ref | |||
| Model 22 | 0.90 (0.77, 1.05) | 1.06 (0.98, 1.15) | Ref | |||
| Model 33 | 0.91 (0.78, 1.06) | 1.07 (0.98, 1.16) | Ref | |||
| Model 44 | 0.89 (0.76, 1.04) | 1.06 (0.98, 1.15) | Ref | |||
| Model 55 | 0.83 (0.71, 0.98) | 1.05 (0.97, 1.14) | Ref | |||
| Vascular dementia | 88 (0.7) | 290 (0.5) | 1437 (0.7) | |||
| Model 11 | 0.96 (0.77, 1.19) | 0.82 (0.73, 0.94) | Ref | |||
| Model 22 | 0.97 (0.78, 1.20) | 0.83 (0.73, 0.95) | Ref | |||
| Model 33 | 0.99 (0.80, 1.24) | 0.86 (0.76, 0.98) | Ref | |||
| Model 44 | 1.02 (0.82, 1.27) | 0.87 (0.76, 0.99) | Ref | |||
| Model 55 | 0.90 (0.72, 1.12) | 0.86 (0.75, 0.98) | Ref | |||
The covariates were modeled as continuous or categorical variables as shown in Supplemental Table 2, which displays their distributions according to the vitamin D status groups. 6 HRs with 95% CIs were derived from Cox proportional hazards models.
Abbreviations: CI: confidence interval, HR: hazard ratio, Ref: reference
Cox proportional hazards model 1: Age, sex, skin color, latitude of study center and calendar month of attending the assessment center.
Cox proportional hazards model 2: Model 1 variables plus socioeconomic factors (indices of multiple deprivation, no of individuals in household, and household income).
Cox proportional hazards model 3: Model 2 variables plus lifestyle factors (smoking, alcohol consumption, physical activity, venturesome personality, frequency of visiting friends/family) and vitamin D specific factors (consumption of oily fish, processed meat, milk, bread, spread, time spent outdoors in summer, ease of skin tanning, use of sunscreen/UV protection, and solarium/sunlamp use).
Cox proportional hazards model 4: Model 3 variables plus weight variables (body mass index and waist circumference).
Cox proportional hazards model 5: Model 4 variables plus diseases & disease symptoms (cancer, hypertension, stroke, coronary artery disease, chronic obstructive pulmonary disease, asthma, osteoporosis, fractured in last 5 years, arthritis, gout, diabetes, hypothyroidism, chronic fatigue syndrome, tiredness/lethargy in last 2 wks, Parkinson, and depressed mood), biomarkers (estimated glomerular filtration rate, C-reactive protein), general health status (disability, general self-rated health and no. of drugs), and medication intake (low-dose aspirin, lipid-lowering drugs, and anti-depression drugs), and Apolipoprotein E ε4 status.
The risk estimates remained relatively stable for multivitamin use from models 1 to 5. In the most comprehensively adjusted model (model 5), a statistically significant inverse association was observed between multivitamin use and VD, reflecting a 14% reduction in risk [HR (95% CI): 0.86 (0.75, 0.98)]. The HR point estimates (95% CI) for the association of multivitamin use with all-cause dementia [0.98 (0.92, 1.04)] and AD [1.05 (0.97, 1.14)] were not statistically significant and closer to the null effect value of HR=1.0 than to the HR point estimate observed for VD.
The sensitivity analysis excluding events from the first 5 y did not result in substantially different results (Supplemental Table 9). Of note, the association between vitamin D use and all-cause dementia became statistically significant [HR (95% CI): 0.89 (0.79, 0.99); P = 0.041].
Subgroup analyses on the associations of vitamin D supplementation with dementia outcomes
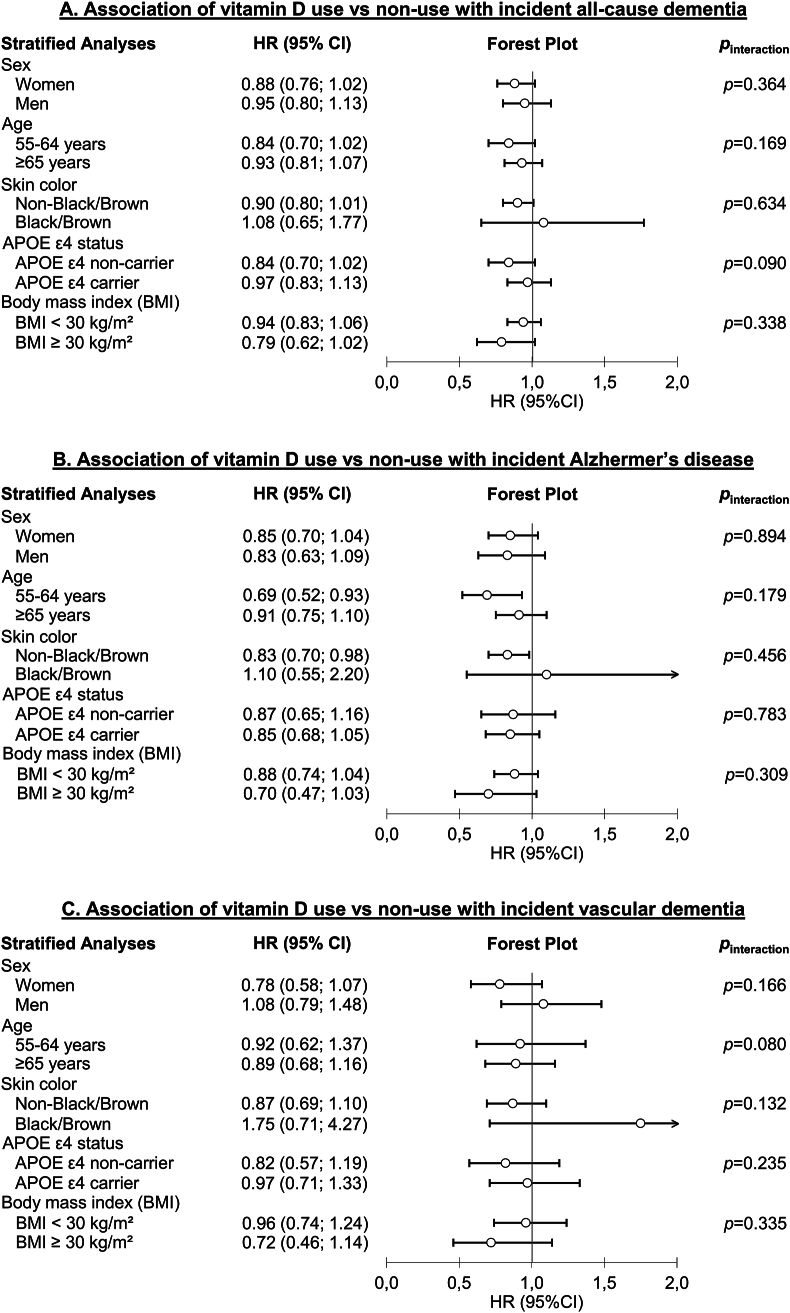
In subgroup analyses, no differences were observed regarding sex and APOE ε4 allele status (Figure 3, Supplemental Tables 10 and 13). However, the inverse association of vitamin D use and AD was stronger among individuals aged 55–64 y [HR (95% CI): 0.69 (0.52, 0.93)] than among individuals aged 65–69 y (0.91 [0.75, 1.10]), but the interaction test results were not statistically significant (P = 0.179) (Figure 3, Supplemental Table 11). Furthermore, this association was absent among people with black or brown skin color [HR (95% CI): 1.10 (0.55, 2.20)] and only observed among subjects with lighter skin colors [HR (95% CI): 0.83 (0.70, 0.98)]. However, the interaction test was not statistically significant (P = 0.456) (Figure 3, Supplemental Table 12). The latter pattern for skin color was also observed for the association of multivitamin use and VD; however, there was no detectable age difference for this association (Figure 3, Supplemental Table 12). Furthermore, the inverse associations of vitamin D supplement use with all-cause dementia, AD, and VD were more pronounced in individuals with a BMI ≥30 kg/m2 [HRs (95% CI): 0.79 (0.62, 1.02), 0.70 (0.47, 1.03), and 0.72 (0.46, 1.14), respectively] compared to those with a BMI <30 kg/m2 [0.94 (0.83, 1.06), 0.88 (0.74, 1.04), and 0.96 (0.74, 1.24), respectively], but interaction tests were not statistically significant (Figure 3, Supplemental Table 14). Interestingly, similar patterns were observed for multivitamin supplement use and its associations with all-cause dementia, AD, and VD (Supplemental Table 14), with all interaction tests showing statistical significance (P = 0.024, P = 0.013, and P = 0.032, respectively).
FIGURE 3.
Forest plots illustrating subgroup analyses exploring the associations of vitamin D supplementation with incident all-cause dementia (A), Alzheimer’s disease (B), and vascular dementia (C) in 269,229 UK Biobank participants. HRs with 95% CIs were derived from Cox proportional hazards models. APOE, Apolipoprotein E; BMI, body mass index; CI, confidence interval; HR: hazard ratio.
Discussion
Summary of the findings
Utilizing data from 55- to 69-year-old study participants of the population-based UK Biobank with 14 years of follow-up, our study revealed consistent associations between objectively measured vitamin D deficiency and insufficiency (via serum samples), as well as self-reported regular use of vitamin D supplements, with all-cause, AD, and VD dementia, although some results for vitamin D supplements use did not reach statistical significance. A 25(OH)D level cut-off of 50 nmol/L appeared appropriate for predicting dementia outcomes, but the dementia risk was particularly pronounced in individuals with 25(OH)D level cut-off below 30 nmol/L (e.g., 25% increased all-cause dementia risk). No or even reversed associations of vitamin D deficiency, vitamin D insufficiency, and vitamin D supplementation with dementia outcomes were observed for individuals with darker skin tones. Furthermore, vitamin D users of younger age (55–64 y) had more robust risk reduction for AD than older individuals (65–69 y), which was consistent with a higher AD risk of subjects with vitamin D deficiency in the younger than in the older age group. In addition, vitamin D supplement use was strongly associated with all dementia outcomes among participants with and without obesity. Lastly, it is worth noting that we observed a statistically significant association between multivitamin supplement use and reduced VD risk—again, especially among subjects with obesity.
Associations of vitamin D deficiency and insufficiency with dementia outcomes
The associations between vitamin D status and dementia outcomes have been subject to extensive investigation over the past decade [10,13,[32], [33], [34]]. However, these studies have yielded inconsistent results. In the most recent systematic review and meta-analysis conducted by Chai et al. [10], a total of 12 prospective cohort studies and 4 cross-sectional studies were included. The pooled HRs (95% CI) for all-cause dementia and AD were reported as 1.48 (1.19–1.85) and 1.51 (1.04–2.18), respectively, for severe vitamin D deficiency, which they defined as a level <25 nmol/L. Additionally, they observed the pooled HRs (95% CI) for moderate vitamin D deficiency, which they defined as 25 to 50 nmol/L, to be 1.20 (0.99–1.44) for all-cause dementia and 1.36 (1.01–1.84) for AD. These estimates are much higher than those observed in our study. Although the disparity in the applied cut-off for severe vitamin D deficiency does not likely explain this divergence, the inclusion of cross-sectional studies, studies with a short follow-up time, and/or a much older population in the meta-analysis may have led to more substantial effect estimates. In the cross-sectional studies, prevalent dementia may have caused vitamin D deficiency, and in studies with short follow-up time or an old population, undiagnosed dementia or its precursors already prevalent at baseline could be the reason for the lower 25(OH)D levels.
Additionally, an important distinction arises from the comprehensive adjustment for potential confounders in several models. This facet was more rigorously undertaken in our analysis, leading to progressively lower effect estimates. Among the studies included in the systematic review and meta-analysis, 2 studies—Licher et al. [35] and Afzal et al. [36]—adjusted for various confounders, including socioeconomic status, biomarkers, lifestyle factors, and disease-related factors. Notably, these studies reported similar increases in the risk of all-cause dementia [HR (95% CI): 1.22 (0.97, 1.52) for 25(OH)D <25 nmol/L] [35], AD [1.25 (0.95, 1.64) for 25(OH)D <25 nmol/L] [36], and VD [1.22 (0.77, 1.91) for 25(OH)D <50 nmol/L] [36] as compared to our study’s findings. Hence, we are confident that associations of vitamin D deficiency with dementia outcomes in the 19%- to 25%-risk increase are closer to the actual effect than the more significant estimates reported by other studies.
Subgroup analyses on the associations of vitamin D deficiency and insufficiency with dementia outcomes
Prior research has indicated a higher prevalence of vitamin D deficiency and insufficiency among people with black when compared to those with white skin color [37,38], and has shown associations of vitamin D deficiency and insufficiency with poor cognitive performance only in subjects with black skin color [38,39]. However, in longitudinal analysis, vitamin D insufficiency was not associated with poor cognitive performance in older black adults [38].
Our study is the first prospective study on dementia outcomes with a subgroup analysis among people with darker skin tones with a sufficient sample size (n = 6682) [40]. In line with earlier investigations, our study revealed that participants with darker skin tones had a lower mean 25(OH)D level (38.1 nmol/L) when compared to other participants (50.4 nmol/L). In contrast to the cross-sectional study’s results on cognitive performance and our findings for subjects with lighter skin tones [38], vitamin D deficiency and insufficiency were not associated with all-cause dementia and AD in individuals with black or brown skin color and even a risk factor for VD in the latter. However, this finding should be interpreted with caution due to the small case numbers in this analysis. Nevertheless, the statistically significant interaction tests of skin color and vitamin D deficiency in the analyses regarding all-cause dementia and VD support the observation that skin color might be an effect modifier.
The same phenomenon has been identified previously in the context of the association between vitamin D deficiency and bone mineral density (BMD) as well as the risk of fragility fractures [[41], [42], [43]]. Despite having lower 25(OH)D levels, individuals with black skin tones tend to exhibit higher BMD and a lower risk of fractures. A polymorphism in the vitamin D–binding protein gene, which is more prevalent among blacks and is associated with lower levels of the vitamin D–binding protein, might be an explanation [44]. Consequently, when vitamin D–binding protein levels are low, a lower total 25(OH)D level is required for a sufficient level of the bio-available pharmacologically active metabolite 1,25-dihydroxyvitamin D. Thus, low total 25(OH)D levels may not necessarily indicate true vitamin D deficiency when vitamin D–binding protein levels are concurrently low.
Associations of vitamin D supplementation with dementia outcomes
Vitamin D supplementation can effectively counteract vitamin D deficiency and insufficiency. In this study, the prevalence of vitamin D deficiency was much lower among subjects using vitamin D supplements (6.9%) or multivitamin supplements (9.5%) than among nonusers (21.3%). Moreover, it is highly likely that a potential effect of vitamin D supplementation and dementia outcomes is mediated by an increase of various vitamin D metabolites, of which 25(OH)D is by far the most abundant, in the blood circulation as well as in storage depots in fat tissue. Given the previous research establishing an association between vitamin D deficiency and an elevated risk of dementia outcomes, there has been a recent surge in investigating whether vitamin D supplementation could serve as a potential preventive measure against this significant challenge [11,14,21,45]. However, these studies have presented conflicting results. Lai et al. [21] not only observed increased Aβ deposition and exacerbated AD in mice due to vitamin D supplementation but also found that dementia-free older adults taking vitamin D supplements were 1.8 times more likely to develop dementia than those not taking the supplements. Conversely, Ghahremani et al. [11] observed that vitamin D supplementation was associated with a 40% lower dementia incidence, and females and APOE ε4 noncarriers significantly benefited more from vitamin D supplementation. However, both studies had certain limitations. Important confounders, such as sun exposure and socioeconomic status, were not adjusted for in both studies, and adjustment for diseases was limited. Our study comprehensively considered a wide range of confounders in 5 different models. A comprehensive adjustment for the diseases and the general health status was primarily shown to be essential to detect an inverse association between vitamin D supplement use and dementia outcomes because people with poor health have a higher tendency to be vitamin D users.
Interestingly, effect modification by age was suggested in the UK Biobank regarding the association between vitamin D use and AD. The effect estimate was more pronounced in individuals aged 55–64 than those aged 65–69 y. One potential explanation for this discrepancy might be the competing risk of death—older participants had a higher likelihood of death, which could attenuate the effect estimates. Additionally, we speculate that the younger age group took vitamin D for a longer time before they reached the age of potential dementia onset. It might be important to start vitamin D supplementation in middle age to prevent Aß plaques from forming years before dementia symptoms are evident.
Our study observed that participants with obesity (BMI ≥30 kg/m2) showed a more pronounced risk reduction for all-cause dementia, AD, and VD, which is biologically plausible. Obesity is a strong risk factor for a low vitamin D status [46]. In the analyzed UK Biobank sample, the prevalence of obesity was 34.7%, 28.4%, and 19.2% among subjects with vitamin D deficiency, insufficiency, and a sufficient vitamin D status, respectively. This difference can be attributed to the sequestration of vitamin D metabolites into adipose tissue [46]. Therefore, subjects with obesity have a higher need for vitamin D supplementation to maintain adequate 25(OH)D levels and might profit more from this intervention [47].
In the realm of multivitamin supplementation, an intriguing discovery surfaced: the utilization of multivitamin supplements was linked to a reduced risk of VD, and obesity was an effect modifier, with substantially stronger results among individuals with obesity. This phenomenon could potentially be ascribed to the beneficial effects of vitamin D supplementation, as previously discussed, or it might stem from the influence of other components within the multivitamins. Notably, the supplementation of folic acid, vitamin B12, vitamin C, and vitamin E has been associated with cognitive enhancement in existing literature [45,48,49]. Future investigations should delve into the relationship between multivitamin supplementation and the risk of VD to gain a more comprehensive understanding, with a particular focus on exploring potential interactions with cardiovascular disease [50,51].
Potential role of vitamin D in dementia pathogenesis
Multiple potential mechanisms linking vitamin D deficiency to dementia development have been suggested in previous studies. Vitamin D might promote the clearance and breakdown of amyloid plaques [6], mitigate amyloid-induced cytotoxicity and apoptosis in primary cortical neurons [52], and potentially participate in the Aβ-triggered induction of nitric oxide synthase, a component of AD’s inflammatory process [53]. Moreover, vitamin D deficiency has been associated with cerebrovascular pathology, including an increased risk of stroke, particularly ischemic stroke [54], and a higher occurrence of white matter hyperintensities or large vessel infarcts [55,56]. Additionally, links between vitamin D deficiency and vascular risk factors, such as hypertension and diabetes mellitus [57,58], both of which are associated with dementia, point to a complex, multifaceted mechanism of action.
Future analyses of the UK Biobank’s wealth of data could explore these potential mechanisms. In addition to a comprehensive assessment of diseases and lifestyle factors, magnetic resonance imaging images of the brain have been made, and blood samples are available for measuring AD-specific biomarkers (eg, Aβ, glial fibrillary acidic protein, and phosphorylated protein tau).
Strengths and limitations
Our study benefits from a substantial sample size and comprehensive coverage of serum 25(OH)D levels, vitamin D supplement usage, and potential confounders. Nonetheless, several limitations deserve attention. Although our analysis accounted for a broad range of confounding factors, it is essential to acknowledge the persistent limitations of residual confounding inherent in observational studies. The UK Biobank sample might not fully represent the broader UK population due to a potential “healthy volunteer” bias. Additionally, participants were recruited primarily from areas proximate to study centers and marked by a low response rate [59]. However, the UK Biobank’s relative associations between exposures and disease outcomes are reliable, providing a basis for some extrapolation [60].
Dementia diagnoses were derived from hospital and death records within the UK Biobank, leading to potential issues of underdiagnosis and misdiagnosis. Yet, the extensive scope of the UK Biobank may mitigate some of this bias. Furthermore, vitamin D supplement use was ascertained only at the baseline visit for all UK Biobank study participants, leaving a gap of uncertainty about vitamin D supplement use during follow-up. Another notable limitation is the absence of data on medication adherence and the reliance on self-reported information regarding regular intake of vitamin D and multivitamin supplements, gathered only at baseline. Nonadherence, changes of exposure during follow-up and an underdiagnosis of dementia in the UK Biobank could have been causes of an underestimation of the true effect estimates by our study. Moreover, specific information on the vitamin D dosages was not available.
The generalizability of our findings is limited to the age range 55 to 69 y (median: 62 y). The average study participant reached an age of 74 y because the median follow-up time was 13.6 y. This is below the age at which the most dementia diagnoses are made because most affected individuals get diagnosed in their 80s or 90s. A longer follow-up time would have been desirable also because of the long latency period for dementia development [61]. Thus, reverse causation cannot be excluded despite the long follow-up time in this study and the robust results after excluding early events in the first 5 y.
Need for further research
The ideal cohort study would need to assess the average daily vitamin D intake by supplements, which would sum up vitamin D intake from vitamin D specific products (purchased as OTC or as a prescription drug for daily, weekly, monthly, or other frequency of use) and multivitamin products. Furthermore, it should include study participants in mid-life (40–59 y) because lifestyle factors are best addressed in this age group for dementia prevention [61]. However, such a study would need to have ≥30 years of follow-up because most people get diagnosed with dementia at the age of ≥80 y and would need to have repeated 25(OH)D measurements and assessments of vitamin D intake by supplements because it is unlikely that the exposures at baseline are constant over such a long time. A randomized controlled trial (RCT) would be even better than a cohort study, which would avoid problematic confounding and establish a causal relationship. However, it is uncertain whether such an elaborate study (either RCT or cohort study) with decades of follow-up will ever be conducted.
Conclusion
Our study illuminates consistent associations between various facets of vitamin D and multivitamin intake, objectively measured vitamin D deficiency and insufficiency from blood samples, and 14-y dementia incidence in a study population aged 55 to 69 y at baseline. Subgroup analyses revealed effect modification by skin color with associations only observed in the non-brown/non-black skin color group and stronger effect estimates for vitamin D supplementation in younger compared to older study participants.
Although our results are encouraging and suggest a potential role for vitamin D supplementation in dementia prevention, particularly for those with vitamin D deficiency, we advocate caution due to the observational nature of this study. RCTs with long follow-up periods are indispensable to establishing the efficacy of dementia prevention strategies.
Acknowledgments
We are thankful to the study participants featured in this manuscript.
Author contributions
L-JC and BS: contributed to the concept and design of this research; L-JC: performed the statistical analyses; L-JC: drafted the manuscript and BS revised and edited it; SS, HS, and HB: commented critically on an advanced manuscript version regarding the interpretation of the results and the discussion; L-JC and BS: take responsibility for the integrity and accuracy of the data and the statistical analysis; and all authors: read and approved the final version of the manuscript.
Conflict of interest
The authors report no conflicts of interest.
Funding
This research was conducted using the UK Biobank Resource under application 89329. UK Biobank was established by the Wellcome Trust, Medical Research Council, Department of Health, Scottish government, and Northwest Regional Development Agency. It has also had funding from the Welsh assembly government and the British Heart Foundation. The sponsors had no role in data acquisition or the decision to publish the data.
Consent for publication
Not applicable.
Ethical approval
UK Biobank received ethical approval from the North West Multicentre Research Ethics Committee (REC reference: 11/NW/03820). UK Biobank is conducted in accordance with the 1964 Helsinki declaration and its later amendments.
Data availability
Data from the UK Biobank (https://www.ukbiobank.ac.uk/) are available to researchers on application. This research was conducted using the UK Biobank Resource under application 89329.
Dissemination to participants and related patient and public communities
Results from UK Biobank are routinely disseminated to study participants via the study website and Twitter feed.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajcnut.2024.01.020.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.World Health Organization . World Health Organization; Geneva, Switzerland: 2023. fact sheets of dementia.https://www.who.int/news-room/fact-sheets/detail/dementia [Internet] [cited 2023 April 9]. Available from: [Google Scholar]
- 2.GBD 2019 Dementia Forecasting Collaborators Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet. Public. Health. 2022;7(2):e105–e125. doi: 10.1016/s2468-2667(21)00249-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cummings J., Zhou Y., Lee G., Zhong K., Fonseca J., Cheng F. Alzheimer’s disease drug development pipeline: 2023. Alzheimers. Dement. 2023;9(2) doi: 10.1002/trc2.12385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gauthier S., Albert M., Fox N., Goedert M., Kivipelto M., Mestre-Ferrandiz J., et al. Why has therapy development for dementia failed in the last two decades? Alzheimers. Dement. 2016;12(1):60–64. doi: 10.1016/j.jalz.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Livingston G., Huntley J., Sommerlad A., Ames D., Ballard C., Banerjee S., et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. doi: 10.1016/s0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grimm M.O.W., Thiel A., Lauer A.A., Winkler J., Lehmann J., Regner L., et al. Vitamin D and its analogues decrease amyloid-β (Aβ) formation and increase Aβ-degradation. Int. J. Mol. Sci. 2017;18(12) doi: 10.3390/ijms18122764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin C.I., Chang Y.C., Kao N.J., Lee W.J., Cross T.W., Lin S.H. 1,25(OH)(2)D(3) Alleviates Aβ(25-35)-induced tau hyperphosphorylation, excessive reactive oxygen species, and apoptosis through interplay with glial cell line-derived neurotrophic factor signaling in SH-SY5Y Cells. Int. J. Mol. Sci. 2020;21(12) doi: 10.3390/ijms21124215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Institute of Medicine (US) In: Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary reference intakes for calcium and vitamin D. Ross AC. Taylor C.L., Yaktine A.L., Del Valle H.B., editors. National Academies Press (US); Washington (DC): 2011. [PubMed] [Google Scholar]
- 9.Amrein K., Scherkl M., Hoffmann M., Neuwersch-Sommeregger S., Köstenberger M., Tmava Berisha A., et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur. J. Clin. Nutr. 2020;74(11):1498–1513. doi: 10.1038/s41430-020-0558-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chai B., Gao F., Wu R., Dong T., Gu C., Lin Q., et al. Vitamin D deficiency as a risk factor for dementia and Alzheimer’s disease: an updated meta-analysis. BMC. Neurol. 2019;19(1):284. doi: 10.1186/s12883-019-1500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghahremani M., Smith E.E., Chen H.Y., Creese B., Goodarzi Z., Ismail Z. Vitamin D supplementation and incident dementia: effects of sex, APOE, and baseline cognitive status. Alzheimers. Dement (Amst). 2023;15(1) doi: 10.1002/dad2.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sha S., Nguyen T.M.N., Kuznia S., Niedermaier T., Zhu A., Brenner H., et al. Real-world evidence for the effectiveness of vitamin D supplementation in reduction of total and cause-specific mortality. J Intern Med. 2023;293(3):384–397. doi: 10.1111/joim.13578. [DOI] [PubMed] [Google Scholar]
- 13.Littlejohns T.J., Henley W.E., Lang I.A., Annweiler C., Beauchet O., Chaves P.H., et al. Vitamin D and the risk of dementia and Alzheimer disease. Neurology. 2014;83(10):920–928. doi: 10.1212/wnl.0000000000000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang J.H., Vyas C.M., Okereke O.I., Ogata S., Albert M., Lee I.M., et al. Effect of vitamin D on cognitive decline: results from two ancillary studies of the VITAL randomized trial. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-02485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossom R.C., Espeland M.A., Manson J.E., Dysken M.W., Johnson K.C., Lane D.S., et al. Calcium and vitamin D supplementation and cognitive impairment in the women’s health initiative. J. Am. Geriatr. Soc. 2012;60(12) doi: 10.1111/jgs.12032. 2197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bischoff-Ferrari H.A., Vellas B., Rizzoli R., Kressig R.W., da Silva J.A.P., Blauth M., et al. Effect of vitamin D supplementation, omega-3 fatty acid supplementation, or a strength-training exercise program on clinical outcomes in older adults: the DO-HEALTH randomized clinical trial. JAMA. 2020;324(18):1855–1868. doi: 10.1001/jama.2020.16909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schietzel S., Fischer K., Brugger P., Orav E.J., Renerts K., Gagesch M M., et al. Effect of 2000 IU compared with 800 IU vitamin D on cognitive performance among adults age 60 years and older: a randomized controlled trial. Am. J. Clin. Nutr. 2019;110(1):246–253. doi: 10.1093/ajcn/nqz081. [DOI] [PubMed] [Google Scholar]
- 18.Jorde R., Kubiak J., Svartberg J., Fuskevåg O.M., Figenschau Y., Martinaityte I., et al. Vitamin D supplementation has no effect on cognitive performance after four months in mid-aged and older subjects. J. Neurol. Sci. 2019;396:165–171. doi: 10.1016/j.jns.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Owusu J.E., Islam S., Katumuluwa S.S., Stolberg A.R., Usera G.L., Anwarullah A.A., et al. Cognition and vitamin D in Older African-American Women- Physical performance and Osteoporosis prevention with vitamin D in older African Americans Trial and Dementia. J. Am. Geriatr. Soc. 2019;67(1):81–86. doi: 10.1111/jgs.15607. [DOI] [PubMed] [Google Scholar]
- 20.Pettersen J.A. Does high dose vitamin D supplementation enhance cognition?: a randomized trial in healthy adults. Exp. Gerontol. 2017;90:90–97. doi: 10.1016/j.exger.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 21.Lai R.H., Hsu C.C., Yu B.H., Lo Y.R., Hsu Y.Y., Chen M.H., et al. Vitamin D supplementation worsens Alzheimer’s progression: animal model and human cohort studies. Aging. Cell. 2022;21(8) doi: 10.1111/acel.13670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen N., Sudlow C., Downey P., Peakman T., Danesh J., Elliott P., et al. UK Biobank: current status and what it means for epidemiology. Health. Policy. Technol. 2012;1(3):123–126. doi: 10.1016/j.hlpt.2012.07.003. [DOI] [Google Scholar]
- 23.Sudlow C., Gallacher J., Allen N., Beral V., Burton P., Danesh J., et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS. Med. 2015;12(3) doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrade-Guerrero J., Santiago-Balmaseda A., Jeronimo-Aguilar P., Vargas-Rodríguez I., Cadena-Suárez A.R., Sánchez-Garibay C., et al. Alzheimer’s disease: an updated overview of its genetics. Int. J. Mol. Sci. 2023;24(4) doi: 10.3390/ijms24043754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Immunoassay Speciality 1 EQA. RIQAS: Randox Laboratories. 2021. https://www.randox.com/immunoassay-speciality-i-eqa/ [Internet] [cited 2023 April 10th]. Available from: [Google Scholar]
- 26.Fry D., Almond R., Moffat S., Gordon M., Singh P. 2019. UK Biobank biomarker project companion document to accompany serum biomarker data: UK Biobank.https://biobank.ndph.ox.ac.uk/showcase/showcase/docs/serum_biochemistry.pdf [Internet] [cited 2023 April 10]. Available from: [Google Scholar]
- 27.Bush K., Wilkinson T., Schnier C., Nolan J., Sudlow C. 2018. Definitions of dementia and the major diagnostic pathologies, UK Biobank phase 1 outcomes adjudication.https://biobank.ndph.ox.ac.uk/showcase/showcase/docs/alg_outcome_dementia.pdf [Internet] [cited 2023 April 11]. Available from: [Google Scholar]
- 28.Madley-Dowd P., Hughes R., Tilling K., Heron J. The proportion of missing data should not be used to guide decisions on multiple imputation. J. Clin. Epidemiol. 2019;110:63–73. doi: 10.1016/j.jclinepi.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sterne J.A., White I.R., Carlin J.B., Spratt M., Royston P., Kenward M.G., et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Besser L.M., Brenowitz W.D., Meyer O.L., Hoermann S., Renne J. Methods to address self-selection and reverse causation in studies of neighborhood environments and brain health. Int. J. Environ. Res. Public. Health. 2021;18(12) doi: 10.3390/ijerph18126484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamim H., Monfared A.A., LeLorier J. Application of lag-time into exposure definitions to control for protopathic bias. Pharmacoepidemiol. Drug. Saf. 2007;16(3):250–258. doi: 10.1002/pds.1360. [DOI] [PubMed] [Google Scholar]
- 32.Jayedi A., Rashidy-Pour A., Shab-Bidar S. Vitamin D status and risk of dementia and Alzheimer's disease: a meta-analysis of dose-response (†) Nutr. Neurosci. 2019;22(11):750–759. doi: 10.1080/1028415x.2018.1436639. [DOI] [PubMed] [Google Scholar]
- 33.Sommer I., Griebler U., Kien C., Auer S., Klerings I., Hammer R., et al. Vitamin D deficiency as a risk factor for dementia: a systematic review and meta-analysis. BMC. Geriatr. 2017;17(1):16. doi: 10.1186/s12877-016-0405-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Etgen T., Sander D., Bickel H., Sander K., Förstl H. Vitamin D deficiency, cognitive impairment and dementia: a systematic review and meta-analysis. Dement. Geriatr. Cogn. Disord. 2012;33(5):297–305. doi: 10.1159/000339702. [DOI] [PubMed] [Google Scholar]
- 35.Licher S., de Bruijn R.F.A.G., Wolters F.J., Zillikens M.C., Ikram M.A., Ikram M.K. Vitamin D and the risk of dementia: the Rotterdam study. J. Alzheimers. Dis. 2017;60(3):989–997. doi: 10.3233/jad-170407. [DOI] [PubMed] [Google Scholar]
- 36.Afzal S., Bojesen S.E., Nordestgaard B.G. reduced 25-hydroxyvitamin D and risk of Alzheimer's disease and vascular dementia. Alzheimers. Dement. 2014;10(3):296–302. doi: 10.1016/j.jalz.2013.05.1765. [DOI] [PubMed] [Google Scholar]
- 37.Liu X., Baylin A., Levy P.D. Vitamin D deficiency and insufficiency among US adults: prevalence, predictors and clinical implications. Br. J. Nutr. 2018;119(8):928–936. doi: 10.1017/s0007114518000491. [DOI] [PubMed] [Google Scholar]
- 38.Kilpatrick L., Houston D.K., Wilson V.K., Lovato J., Ayonayon H.N., Cauley J.A., et al. Low 25-Hydroxyvitamin D concentrations and risk of incident cognitive impairment in Black and White older adults: the Health ABC study. J. Nutr. Gerontol. Geriatr. 2018;37(1):1–13. doi: 10.1080/21551197.2017.1419899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilkins C.H., Birge S.J., Sheline Y.I., Morris J.C. Vitamin D deficiency is associated with worse cognitive performance and lower bone density in older African Americans. J. Natl. Med. Assoc. 2009;101(4):349–354. doi: 10.1016/s0027-9684(15)30883-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ames B.N., Grant W.B., Willett W.C. Does the high prevalence of vitamin D deficiency in African Americans contribute to health disparities? Nutrients. 2021;13(2) doi: 10.3390/nu13020499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bischoff-Ferrari H.A., Dietrich T., Orav E.J., Dawson-Hughes B. Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am. J. Med. 2004;116(9):634–639. doi: 10.1016/j.amjmed.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 42.Cauley J.A., Lui L.Y., Ensrud K.E., Zmuda J.M., Stone K.L., Hochberg M.C., et al. Bone mineral density and the risk of incident nonspinal fractures in black and white women. JAMA. 2005;293(17):2102–2108. doi: 10.1001/jama.293.17.2102. [DOI] [PubMed] [Google Scholar]
- 43.Hannan M.T., Litman H.J., Araujo A.B., McLennan C.E., McLean R.R., McKinlay J.B., et al. Serum 25-hydroxyvitamin D and bone mineral density in a racially and ethnically diverse group of men. J. Clin. Endocrinol. Metab. 2008;93(1):40–46. doi: 10.1210/jc.2007-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Powe C.E., Evans M.K., Wenger J., Zonderman A.B., Berg A.H., Nalls M., et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N. Engl. J. Med. 2013;369(21):1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gil Martínez V., Avedillo Salas A., Santander Ballestín S. Vitamin supplementation and dementia: a systematic review. Nutrients. 2022;14(5) doi: 10.3390/nu14051033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karampela I., Sakelliou A., Vallianou N., Christodoulatos G.S., Magkos F., Dalamaga M. Vitamin D and obesity: current evidence and controversies. Curr. Obes. Rep. 2021;10(2):162–180. doi: 10.1007/s13679-021-00433-1. [DOI] [PubMed] [Google Scholar]
- 47.Opdenoordt S., van Sorge A., Telting D., Giesen A., de Boer H., de Boer H. Cholecalciferol loading dose guideline for vitamin D-deficient adults. Eur. J. Endocrinol. 2010;162(4):805–811. doi: 10.1530/eje-09-0932. [DOI] [PubMed] [Google Scholar]
- 48.Baker L.D., Manson J.E., Rapp S.R., Sesso H.D., Gaussoin S.A., Shumaker S.A., et al. Effects of cocoa extract and a multivitamin on cognitive function: a randomized clinical trial. Alzheimers. Dement. 2023;19(4):1308–1319. doi: 10.1002/alz.12767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yeung L.K., Alschuler D.M., Wall M., Luttmann-Gibson H., Copeland T., Hale C., et al. Multivitamin supplementation improves memory in older adults: a randomized clinical trial. Am. J. Clin. Nutr. 2023;118(1):273–282. doi: 10.1016/j.ajcnut.2023.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boban M., Bulj N., Kolačević Zeljković M., Radeljić V., Krcmar T., Trbusic M M., et al. Nutritional considerations of cardiovascular diseases and treatments. Nutr. Metab. Insights. 2019;12 doi: 10.1177/1178638819833705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cvetinovic N., Loncar G., Isakovic A.M., von Haehling S., Doehner W., Lainscak M., et al. Micronutrient depletion in heart failure: common, clinically relevant and treatable. Int. J. Mol. Sci. 2019;20(22) doi: 10.3390/ijms20225627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dursun E., Gezen-Ak D., Yilmazer S. A novel perspective for Alzheimer's disease: vitamin D receptor suppression by amyloid-β and preventing the amyloid-β induced alterations by vitamin D in cortical neurons. J. Alzheimers. Dis. 2011;23(2):207–219. doi: 10.3233/jad-2010-101377. [DOI] [PubMed] [Google Scholar]
- 53.Dursun E., Gezen-Ak D., Yilmazer S. A new mechanism for amyloid-β induction of iNOS: vitamin D-VDR pathway disruption. J. Alzheimers. Dis. 2013;36(3):459–474. doi: 10.3233/jad-130416. [DOI] [PubMed] [Google Scholar]
- 54.Judd S.E., Morgan C.J., Panwar B., Howard V.J., Wadley V.G., Jenny N.S., et al. Vitamin D deficiency and incident stroke risk in community-living black and white adults. Int. J. Stroke. 2016;11(1):93–102. doi: 10.1177/1747493015607515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schramm S., Schliephake L., Himpfen H., Caspers S., Erbel R., Jöckel K.H., et al. Vitamin D and white matter hyperintensities: results of the population-based Heinz Nixdorf Recall Study and 1000BRAINS. Eur. J. Neurol. 2021;28(6):1849–1858. doi: 10.1111/ene.14810. [DOI] [PubMed] [Google Scholar]
- 56.Buell J.S., Dawson-Hughes B., Scott T.M., Weiner D.E., Dallal G.E., Qui W.Q., et al. 25-Hydroxyvitamin D, dementia, and cerebrovascular pathology in elders receiving home services. Neurology. 2010;74(1):18–26. doi: 10.1212/WNL.0b013e3181beecb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karadeniz Y., Özpamuk-Karadeniz F., Ahbab S., Ataoğlu E., Can G. Vitamin D deficiency is a potential risk for blood pressure elevation and the development of hypertension. Medicina (Kaunas). 2021;57(12) doi: 10.3390/medicina57121297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berridge M.J. Vitamin D deficiency and diabetes. Biochem. J. 2017;474(8):1321–1332. doi: 10.1042/bcj20170042. [DOI] [PubMed] [Google Scholar]
- 59.Batty G.D., Gale C.R., Kivimäki M., Deary I.J., Bell S. Comparison of risk factor associations in UK Biobank against representative, general population based studies with conventional response rates: prospective cohort study and individual participant meta-analysis. BMJ. 2020;368:131. doi: 10.1136/bmj.m131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fry A., Littlejohns T.J., Sudlow C., Doherty N., Adamska L., Sprosen T T., et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am. J. Epidemiol. 2017;186(9):1026–1034. doi: 10.1093/aje/kwx246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bartali B., Devore E., Grodstein F., Kang J.H. Plasma vitamin D levels and cognitive function in aging women: the nurses' health study. J. Nutr. Health. Aging. 2014;18(4):400–406. doi: 10.1007/s12603-013-0409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the UK Biobank (https://www.ukbiobank.ac.uk/) are available to researchers on application. This research was conducted using the UK Biobank Resource under application 89329.