Abstract
Nocturnal blood pressure and nighttime dipping patterns are associated with the occurrence of cardiovascular events. However, there is few research on whether riser pattern is associated with the poor prognosis of patients with coronary heart disease (CHD) and hypertension independent of nighttime systolic blood pressure (SBP). This prospective and observational clinical study included 568 hospitalized patients with CHD and hypertension. All patients underwent 24‐h ambulatory blood pressure (BP) monitoring during their hospitalization. Multivariate adjusted Cox proportional hazard models were utilized to examine the associations of nocturnal blood pressure and dipping status with primary endpoint events. Additionally, Harrell's C‐statistics were employed to compare the discriminative ability of each model. During the 1‐year follow‐up period, 64 (11.3%) primary endpoint events were recorded, including 55 (9.7%) atherosclerotic cardiovascular disease (ASCVD) events. After adjusting for demographic and clinical risk variables, nighttime SBP was significantly related to the risk of incident primary endpoint events [per 20 mm Hg increase: hazard ratio (HR) = 1.775, 95% confidence interval (CI) 1.256–2.507]. The riser pattern group exhibited a significantly higher risk for primary endpoint events compared to the dipper pattern group, even after adjusting for office SBP (HR: 2.687, 95% CI: 1.015–7.110, p = .047). Furthermore, the addition of nighttime SBP or dipping status to the base model yielded statistically significant increments in C‐statistic values (p = .036 and p = .007). However, adding both nighttime SBP and dipping status did not significantly enhance the model's performance in predicting the risk of primary endpoint events and ASCVD events according to the C‐index (p = .053 and p = .054), which meant that the riser pattern group did not exhibit a significantly higher risk for primary endpoint events compared to the dipper pattern group after adjusting for nighttime SBP. In conclusion, nocturnal SBP and riser pattern demonstrated an association with adverse prognosis in patients with CHD and hypertension. Notably, nocturnal SBP proved to be a more reliable predictor than dipping status.
Keywords: ambulatory blood pressure monitoring, coronary heart disease, dipping status, hypertension, nocturnal blood pressure
1. INTRODUCTION
The global prevalence of coronary heart disease (CHD) is increasing due to factors such as obesity, unhealthy lifestyles, and an aging population. 1 Hypertension is recognized as one of the leading risk factors for CHD and has a high prevalence globally. 2 Therefore, it is crucial to accurately diagnose and manage hypertension in patients with CHD.
Ambulatory blood pressure monitoring (ABPM) is a valuable method that provides various parameters, including 24‐h, daytime, and nighttime blood pressure, as well as diurnal blood pressure fluctuations. In Asian populations, ABPM plays a particularly important role due to specific features of hypertension, such as a higher prevalence of masked hypertension and nocturnal hypertension. 3 Previous studies have shown that nighttime systolic blood pressure (SBP) is a more sensitive prognostic indicator for cardiovascular disease risk compared to daytime SBP or 24‐h SBP measured by ABPM. 4 , 5 Normally, blood pressure is higher during the day and lower at night, with a normal dip of BP at night defined as a reduction of less than 20% and at least 10%. This is known as a dipping pattern. Other patterns include extreme dipping (reduction of over 20%), nondipping (reduction of less than 10%), and riser pattern (any riser) based on the decrease in SBP during nighttime compared to daytime. 3 The riser pattern has been associated with a significantly worse prognosis for stroke and cardiovascular events. 6 However, limited research has investigated whether riser pattern is associated with the poor prognosis of patients with CHD and hypertension independent of nighttime SBP.
The objective of this study was to examine the association between nocturnal blood pressure, nighttime dipping patterns, and the occurrence of major adverse events in patients with both CHD and hypertension.
2. METHODS
2.1. Study design and participants
The single‐center, prospective, observational study was approved by the ethics committee of Zhengzhou University Central China Fuwai Hospital (Approval No. 2021‐20). Recruitment occurred from January 2021 to December 2021, and follow‐up was completed by December 31, 2022. The study adhered to the principles of the Declaration of Helsinki, and all participants provided written informed consent. Patients with both CHD and hypertension, whether previously diagnosed or diagnosed for the first time in the inpatient department, were enrolled based on inclusion and exclusion criteria (see full inclusion and exclusion criteria in Data Supplement). Only patients who underwent ABPM examination were included in the final analysis.
2.2. Clinical outcomes
The primary endpoint of the study was a compound endpoint that included the first occurrence of all‐cause mortality or total nonfatal cardiovascular disease (CVD) events. Total CVDs were defined as follows: (1) nonfatal stroke/TIA, which was characterized by the sudden onset of a neurological deficit lasting for ≥24 h without any other underlying condition explaining the symptoms, confirmed through brain computed tomography or magnetic resonance imaging; (2) nonfatal CHD, which encompassed acute MI and unstable angina requiring coronary revascularization, either percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG); (3) HF, referring to hospitalization and treatment for clinical manifestations of HF. The secondary endpoint of the study focused on ASCVD events, including stroke/TIA and CHD events. All endpoint events were reviewed and determined by three specialists who were blind to the patient's ambulatory BP profile and clinical characteristics. If events occurred multiple times, only the first occurrence was considered in the analysis. CAD events in patients with HF were classified as CAD.
2.3. Follow‐up and assessments
Demographic information, medical history, biochemical examination results, drug usage, and blood pressure measurements were obtained from the medical records. Patients were followed up for 1 year through clinic visits or telephone conversations to determine their vital status and the occurrence of cardiovascular events at 1, 6, and 12 months. Standard computerized case report forms were completed at each interval. The nighttime dipping was calculated as (1 minus average nighttime SBP/average daytime SBP) × 100 (%). Patients were divided into three groups based on the decrease in SBP during sleep compared to when awake: extreme dippers (≥20%), dippers (10%–<20%), nondippers (0%–<10%), and risers (any increase).
2.4. Blood pressure measurements
The measurement of blood pressure during admission was mainly based on the 2017 ACC/AHA guidelines. The measurement of blood pressure in the inpatient department of our hospital uses the Omron HEM‐7064 device (Omron HealthCare, Kyoto, Japan). 3 The main measurement points include the following: Before the measurement, inform the patient: (1) Avoid caffeine, exercise, and smoking for at least 30 min; (2) Emptying the bladder; and (3) Relax and sit in a chair for at least 5 min (feet on the floor, back supported). Measurement: (1) Place the middle of the cuff at the right atrial level of the patient's upper arm (the midpoint of the sternum); (2) During the rest period or measurement period, patients and observers should not gossip; (3) During the first measurement, the blood pressure of both arms was recorded, and the arm with higher blood pressure reading was used in the subsequent reading; three blood pressure measurements should be interval of 1−2 min. The first blood pressure reading was discarded, and the average of the second and third blood pressure readings was taken as the final analysis value.
For ABPM, a calibrated and validated device (BI5000; BI Co, Shenzhen, China) was used to measure SBP, diastolic blood pressure (DBP), and heart rate. 3 Measurements were taken every 15 min between 07:00 and 23:00 h and every 30 min during the night, covering a total of 24 consecutive hours. Participants were instructed to maintain their usual activities with minimal restrictions, following a similar activity‐rest schedule and avoiding daytime napping throughout the evaluation day. A diary was maintained by each participant to record bedtime and wake‐up time, providing accurate data for calculating awake and asleep blood pressure averages.
2.5. Definitions
Smoking was categorized as never/ever/current smoking, while drinking was categorized as never/ever/current drinking. Current smoking patients were defined as those who have smoked cigarettes in the 30 days prior to the history collection, and current drinking patients were defined as who have consumed alcohol in the 30 days prior to history collection. The criteria for diagnosing diabetes were as follows: Fasting plasma glucose ≥7.0 mmol/L, or HbA1c ≥6.5%, or 2‐h post‐prandial glucose ≥11.1 mmol/L, or taking anti‐diabetic medication. 7 CKD is defined as having at least two of the following conditions and presents for >3 months: albuminuria (AER ≥30 mg/g), urine sediment abnormalities, electrolyte and other abnormalities due to tubular disorders, abnormalities detected by histology, structural abnormalities detected by imaging, history of kidney transplantation, and GFR decreased to 60 mL/min per 1.73 m2. 8
2.6. Statistical analysis
Continuous data are presented as mean values with standard deviation (SD) and categorical variables are presented as percentages. Demographic variables and clinical characteristics among the different groups based on the nocturnal blood pressure (BP) dipping pattern were compared using one‐way analysis of variance for continuous variables or χ2 test for categorical variables. Logistic regression analyses with adjustments were conducted to identify variables that were highly correlated or predictive of the riser pattern. The cumulative incidence of endpoint events in the subgroups with different dipping patterns was analyzed using Kaplan–Meier curves.
Cox proportional hazards models were utilized to assess the risk of endpoint events associated with a 20‐mm Hg increase in SBP, and a 10% increase in nocturnal SBP dipping, adjusted for statistically significant variables and clinical factors that may influence patients' prognosis. The dipper group served as the reference in the dipping status models. Additional adjustments included office SBP (model 1), office SBP and 24‐hour SBP (model 2), office SBP and daytime SBP (model 3), and office SBP and nighttime SBP (model 4). Harrell's C‐statistics were used to compare the discriminative ability of each model.
Subgroup analyses were conducted to assess whether the association between dipping status and primary endpoint events differed among different subgroups based on sex, age (<65 years or ≥65 years), current smoking (yes or no), current drinking (yes or no), diabetes status (yes or no), blood pressure control status (controlled or uncontrolled), presence of myocardial infarction (yes or no), peripheral artery disease (PAD) (yes or no), and chronic kidney disease (yes or no). Based on 24‐h blood pressure control, patients were classified into two groups: controlled hypertension (blood pressure <130/80 mmHg with three or fewer antihypertensive drugs) and uncontrolled hypertension (blood pressure ≥130/80 mmHg with unlimited types of antihypertensive drugs or <130/80 mmHg with four or more antihypertensive drugs, including diuretics). All statistical analyses were performed using IBM SPSS Statistics 26.0 software (SPSS Inc, Chicago, IL) and R version 4.3.1. Statistical significance was defined as two‐sided p values <.05.
3. RESULTS
3.1. Study population
Total 1467 patients with CHD and hypertension were enrolled in our study. Among them, 608 patients underwent ABPM, while 9 patients were excluded due to missing nighttime ABPM results and 31 patients were lost to follow‐up. A final cohort of 568 patients was divided into three groups based on their nocturnal SBP dipping pattern, as shown in Figure 1. Baseline clinical characteristics of the three groups were displayed in Table 1. The proportions of patients with stroke/transient ischemic attack (TIA), PAD, diabetes was significantly higher in the riser pattern group. Additionally, the riser pattern group exhibited older age, wider ascending aorta (AAO), and higher 24‐h SBP, office SBP, nighttime SBP, and nighttime DBP compared to the other groups (all p < .05). No significant differences were found in terms of sex, body mass index (BMI), usage of antihypertensive drugs, and other laboratory results (all p > .05).
FIGURE 1.
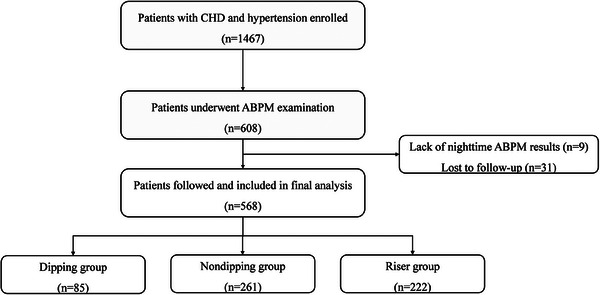
Study flow chart. ABPM, ambulatory blood pressure monitoring; CHD, coronary heart disease.
TABLE 1.
Baseline characteristics of overall and groups by dipping status.
| Variables | Overall (n = 568) | Dipper (n = 85) | Nondipper (n = 261) | Riser (n = 222) | p value |
|---|---|---|---|---|---|
| Age (years) | 59.76 ± 9.99 | 58.63 ± 9.52 | 58.63 ± 10.38 | 61.58 ± 9.46 | .004 |
| Male (n, %) | 387 (68.1) | 61 (71.8) | 183 (70.1) | 143 (64.4) | .301 |
| BMI (kg/m2) | 27.52 ± 6.18 | 27.12 ± 3.29 | 27.20 ± 3.61 | 28.07 ± 8.93 | .328 |
| Current smoking (n, %) | 145 (25.5) | 22 (25.9) | 73 (28.0) | 50 (22.5) | .391 |
| Current drinking (n, %) | 116 (20.4) | 21 (24.7) | 60 (23.0) | 35 (15.8) | .083 |
| Revascularization (n, %) | 164 (28.9) | 17 (20.0) | 80 (30.7) | 67 (30.2) | .146 |
| Case history (n, %) | |||||
| MI | 94 (16.5) | 14 (16.5) | 40 (15.3) | 40 (16.5) | .730 |
| HF | 38 (6.7) | 5 (5.9) | 16 (6.1) | 17 (7.7) | .759 |
| Stroke/TIA | 76 (13.4) | 4 (4.7) | 34 (13.4) | 38 (17.1) | .016 |
| PAD | 107 (18.8) | 15 (17.6) | 39 (14.9) | 53 (23.9) | .042 |
| CKD a | 27 (4.8) | 2 (2.4) | 13 (5.0) | 12 (5.4) | .517 |
| Diabetes | 225 (39.6) | 34 (40.0) | 89 (34.1) | 102 (45.9) | .030 |
| AF | 39 (6.9) | 4 (4.7) | 15 (5.7) | 20 (9.0) | .256 |
| LVH by ECG b | 38 (6.7) | 6 (7.1) | 16 (6.1) | 16 (7.2) | .885 |
| Cardiovascular medications (n, %) | |||||
| ACEI | 62 (10.9) | 5 (5.9) | 31 (11.9) | 26 (11.7) | .272 |
| ARB | 345 (60.7) | 57 (67.1) | 154 (59.0) | 134 (60.4) | .413 |
| β‐Blocker | 426 (75.0) | 61 (71.8) | 190 (72.8) | 175 (78.8) | .236 |
| Calcium channel blockers | 297 (52.3) | 40 (47.1) | 137 (52.5) | 120 (54.1) | .545 |
| Diuretic | 124 (21.8) | 21 (24.7) | 57 (21.8) | 46 (20.7) | .751 |
| Antiplatelet medication | 566 (99.6) | 85 (100.0) | 260 (99.6) | 221 (99.5) | .832 |
| Antidiabetic agents | 206 (36.3) | 30 (35.3) | 87 (33.3) | 89 (40.1) | .300 |
| Stains | 550 (96.8) | 81 (95.3) | 254 (97.3) | 215 (96.8) | .652 |
| Laboratory variables | |||||
| LDL cholesterol (mmol/L) | 2.12 ± 0.84 | 2.16 ± 0.78 | 2.17 ± 0.88 | 2.07 ± 0.81 | .411 |
| HbA1c (%) | 6.33 ± 1.43 | 6.28 ± 1.89 | 6.22 ± 1.26 | 6.47 ± 1.42 | .194 |
| eGFR (mL/min/1.73 m2) | 88.65 ± 16.92 | 90.64 ± 14.76 | 89.91 ± 17.53 | 86.34 ± 16.73 | .051 |
| Echocardiography variables | |||||
| LVEF (%) | 60.72 ± 7.21 | 61.01 ± 5.66 | 60.73 ± 7.82 | 60.58 ± 7.04 | .907 |
| LV (mm) | 47.28 ± 5.25 | 46.30 ± 4.45 | 47.40 ± 5.81 | 47.51 ± 4.82 | .216 |
| AAO (mm) | 34.07 ± 3.60 | 33.76 ± 3.07 | 33.75 ± 3.43 | 34.57 ± 3.93 | .049 |
| Blood pressure variables (mmHg) | |||||
| Office SBP | 134.59 ± 15.73 | 132.44 ± 17.22 | 133.62 ± 14.45 | 136.54 ± 16.39 | .049 |
| Office DBP | 81.87 ± 11.18 | 81.45 ± 11.49 | 83.04 ± 10.95 | 82.87 ± 11.18 | .438 |
| 24‐h SBP | 127.73 ± 14.73 | 125.31 ± 13.66 | 126.09 ± 13.88 | 130.57 ± 15.66 | .001 |
| 24‐h DBP | 74.74 ± 10.01 | 74.33 ± 9.93 | 74.48 ± 9.86 | 75.22 ± 10.25 | .662 |
| Daytime SBP | 128.25 ± 14.65 | 129.45 ± 14.17 | 127.28 ± 14.23 | 128.93 ± 15.31 | .336 |
| Daytime DBP | 75.26 ± 10.61 | 76.74 ± 10.26 | 75.12 ± 11.07 | 74.85 ± 10.18 | .362 |
| Nighttime SBP | 125.68 ± 17.67 | 111.79 ± 12.92 | 121.72 ± 13.91 | 135.70 ± 17.80 | <.001 |
| Nighttime DBP | 72.27 ± 11.11 | 66.31 ± 9.09 | 71.23 ± 9.65 | 75.79 ± 12.19 | <.001 |
Abbreviations: AAO, ascending aorta; ACEI, angiotensin‐converting enzyme inhibitors; AF, atrial fibrillation; ARB, angiotensin II receptor blockers; BMI, body mass index; CKD, chronic kidney disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin A1c; HF, heart failure; LDL, low density lipoprotein; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; MI, myocardial infarction; PAD, peripheral artery disease; SBP, systolic blood pressure; TIA, transient ischemic attack.
Defined as eGFR <60 mL/min/1.73 m2 and markers of kidney damage (≥1 for >3 months) on the basis of The KDIGO CKD Clinical Guideline.
Defined as the sum of voltage amplitudes of SV1 and RV5 was equal to or greater than 3.5 mV according to the Sokolow–Lyon criterion.
3.2. Baseline characteristics of the groups stratified by the occurrence of primary endpoint events
The baseline characteristics of the groups categorized based on the occurrence of primary endpoint events were presented in Table 2. In the group with events, there were significantly higher proportions of patients with HF, stroke/TIA, chronic kidney disease (CKD), and left ventricular hypertrophy (LVH). Meanwhile, with‐events group exhibited lower estimated glomerular filtration rate (eGFR), lower left ventricular ejection fraction (LVEF), and higher 24‐h SBP, nighttime SBP, and nighttime DBP (all p < .05).
TABLE 2.
Baseline characteristics of patients with and without primary endpoint events.
| Variables | Without events (n = 504) | With events (n = 64) | p value |
|---|---|---|---|
| Age (years) | 59.59 ± 9.95 | 61.08 ± 10.33 | .277 |
| Male (n, %) | 343 (68.1) | 44 (68.8%) | .911 |
| BMI (kg/m2) | 27.60 ± 6.47 | 27.00 ± 3.45 | .497 |
| Current smoking (n, %) | 131 (26.0) | 14 (21.9) | .477 |
| Current drinking (n, %) | 104 (20.6) | 12 (18.8) | .725 |
| Revascularization (n, %) | 146 (29.0) | 18 (28.1) | .888 |
| Case history (n, %) | |||
| MI | 80 (15.9) | 14 (21.9) | .224 |
| HF | 29 (5.8) | 9 (14.1) | .012 |
| Stroke/TIA | 62 (12.3) | 14 (21.9) | .034 |
| PAD | 93 (18.5) | 14 (21.9) | .509 |
| CKD a | 20 (4.0) | 7 (10.9) | .014 |
| Diabetes | 198 (39.3) | 27 (42.2) | .655 |
| AF | 33 (6.5) | 6 (9.4) | .399 |
| LVH by ECG b | 28 (5.6) | 10 (15.6) | .006 |
| Medications (n, %) | |||
| ACEI | 56 (11.1) | 6 (9.4) | .675 |
| ARB | 304 (60.3) | 41 (64.1) | .563 |
| β‐Blocker | 374 (74.2) | 52 (81.3) | .220 |
| Calcium channel blockers | 263 (52.2) | 34 (53.1) | .887 |
| Diuretic | 106 (21.0) | 18 (28.1) | .196 |
| Antiplatelet medication | 502 (99.6) | 64 (100.0) | .614 |
| Antidiabetic agents | 182 (36.1) | 24 (37.5) | .828 |
| Stains | 486 (96.4) | 64 (100.0) | .124 |
| Laboratory variables | |||
| LDL cholesterol (mmol/L) | 2.13 ± 0.80 | 2.11 ± 1.11 | .882 |
| HbA1c (%) | 6.35 ± 1.47 | 6.13 ± 1.07 | .297 |
| eGFR (mL/min/1.73 m2) | 89.53 ± 16.64 | 81.76 ± 17.70 | .001 |
| Echocardiography variables | |||
| LVEF (%) | 61.08 ± 6.94 | 57.89 ± 8.67 | .002 |
| LV (mm) | 47.16 ± 5.09 | 48.16 ± 6.36 | .182 |
| AAO width (mm) | 34.06 ± 3.57 | 34.16 ± 3.87 | .841 |
| Blood pressure variables (mmHg) | |||
| Office SBP | 134.45 ± 15.62 | 135.70 ± 16.59 | .549 |
| Office DBP | 82.76 ± 11.12 | 83.69 ± 11.70 | .535 |
| 24‐h SBP | 127.19 ± 14.23 | 131.95 ± 17.73 | .015 |
| 24‐h DBP | 74.58 ± 9.89 | 76.00 ± 10.94 | .287 |
| Daytime SBP | 127.83 ± 14.29 | 131.58 ± 17.03 | .054 |
| Daytime DBP | 75.12 ± 10.60 | 76.38 ± 10.68 | .371 |
| Nighttime SBP | 124.70 ± 16.76 | 133.49 ± 22.36 | <.001 |
| Nighttime DBP | 71.93 ± 10.87 | 75.00 ± 12.63 | .038 |
Abbreviations: AAO, ascending aorta; ACEI, angiotensin‐converting enzyme inhibitors; AF, atrial fibrillation; ARB, angiotensin II receptor blockers; BMI, body mass index; CKD, chronic kidney disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin A1c; HF, heart failure; LDL, low density lipoprotein; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; MI, myocardial infarction; SBP, systolic blood pressure; TIA, transient ischemic attack; PAD, peripheral artery disease.
Defined as eGFR <60 mL/min/1.73 m2 and markers of kidney damage (≥1 for >3 months) on the basis of The KDIGO CKD Clinical Guideline.
Defined as the sum of voltage amplitudes of SV1 and RV5 was equal to or greater than 3.5 mV according to the Sokolow–Lyon criterion.
3.3. Baseline variables associated with riser pattern
Univariate and multivariate logistic regression analysis were preformed to identify variables that were highly correlated to the riser pattern. The results demonstrated that 8 variables were significantly related to the riser pattern with p value <.1 in univariate logistic regression analysis. Among them, PAD (OR: 1.950, 95% CI: 1.126–3.376, p = .017) and AAO width (OR: 1.066, 95% CI: 1.005–1.130, p = .033) exhibited an increased risk of the riser pattern, as shown in Table 3.
TABLE 3.
Logistic regression analysis with variables associated with riser pattern.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Diabetes | 1.569 | 1.113–2.212 | .010 | |||
| PAD | 1.845 | 1.207–2.818 | .005 | 1.950 | 1.126–3.376 | .017 |
| Stroke/TIA | 1.561 | 0.961–2.536 | .072 | |||
| AF | 1.889 | 0.982–3.631 | .057 | |||
| Smoking | 0.829 | 0.679–1.013 | .066 | |||
| Drinking | 0.802 | 0.650–0.990 | .040 | |||
| AAO width | 1.079 | 1.025–1.137 | .004 | 1.066 | 1.005–1.130 | .033 |
| eGFR | 0.990 | 0.980–1.001 | .075 | |||
Abbreviations: AAO, ascending aorta; AF, atrial fibrillation; eGFR, estimated glomerular filtration rate; OR, odds ratio; PAD, peripheral artery disease.
3.4. Clinical outcomes and cardiovascular events
During the 1‐year follow‐up period, 64 (11.3%) primary endpoint events were recorded, including 55 (9.7%) ASCVD events. The numbers of death and heart failure are 6 (1.1%) and 3 (0.5%). The cumulative incidence rates of the primary endpoint and ASCVD are shown in Figure 2. The riser versus dipper pattern was significantly associated with an increased risk of the primary endpoint (p = .009) and ASCVD (p = .005), similar to the riser versus nondipper pattern.
FIGURE 2.
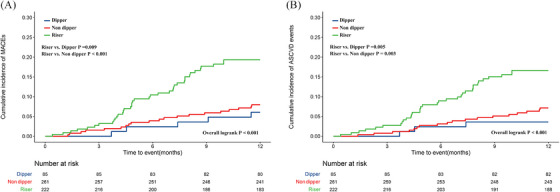
Cumulative incidence of total and ASCVD events in three groups. Kaplan–Meier curves showing the cumulative incidence of total events (A) and ASCVD events (B) in patients with dipper, nondipper, and riser groups.
The base Cox proportional hazards model was primarily based on statistically significant variables and clinical factors that may influence patients' prognosis. These variables included age, sex, body mass index, smoking, alcohol use, diabetes, dyslipidemia, HF, history of cardiovascular disease (including myocardial infarction and revascularization therapy), types of antihypertensive drugs and eGFR. After adjusting for these variables, nighttime SBP was found to be significantly associated with the risk of incident primary endpoint events (per 20 mmHg increase: HR = 1.775, 95% CI: 1.256–2.507) (Table 4). However, the ambulatory blood pressure measures of DBP were not associated with the risk of incident primary endpoint events after adjusting for these variables (Table S1). Furthermore, we constructed four models to assess the impact of dipping status by adding office SBP and different ambulatory blood pressure measures to the base model. The risk of primary endpoint events in the riser pattern group remained significantly higher compared to the dipper pattern group after adjusting for office SBP in model 1 (HR: 2.687, 95% CI: 1.015–7.110, p = .047). Additionally, the risk remained significant after further adjustments for office SBP and daytime SBP (HR: 2.970, 95% CI: 1.108–7.961, p = .031). The risk remained significant after further adjustments for office DBP and daytime DBP (HR: 2.757, 95% CI: 1.037–7.730, p = .042) (Table S1). The base model exhibited a C‐index value of .715 (95% CI, 0.637–0.793) for primary endpoint events and 0.707 (95% CI, 0.613–0.801) for ASCVD events (Table 5). There were statistically improvements in the C‐index values when nighttime SBP or dipping status was added to the base model (p = .036 and p = .007). However, adding both nighttime SBP and dipping status did not significantly enhance the model's performance in predicting the risk of primary endpoint events and ASCVD events according to the C‐index (p = .053 and p = .054) (Table 5), which meant that the riser pattern group did not exhibit a significantly higher risk for primary endpoint events compared to the dipper pattern group after adjusting for nighttime SBP. However, the similar model improvement results of DBP were not found in our study (Table S2).
TABLE 4.
Association between different ambulatory blood pressure measures (per 20‐mm Hg increase in SBP) or dipping status of nighttime blood pressure and risk of cardiovascular disease.
| Primary endpoint events | ASCVD events | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Office blood pressure measures a | ||||||
| Office SBP | 1.242 | 0.918–1.679 | .160 | 1.328 | 0.935–1.886 | .113 |
| Ambulatory blood pressure measures b | ||||||
| 24‐h SBP | 1.576 | 1.023–2.429 | .039 | 1.748 | 1.080–2.831 | .023 |
| Daytime SBP | 1.397 | 0.908–2.148 | .128 | 1.509 | 0.932–2.443 | .094 |
| Nighttime SBP | 1.775 | 1.256–2.507 | .001 | 1.986 | 1.356–2.910 | <.001 |
| Dipping, % | 0.959 | 0.923–0.995 | .027 | 0.950 | 0.911–0.990 | .015 |
| Dipping status c | ||||||
| Model 1 | ||||||
| Office SBP | 1.241 | 0.898–1.714 | .121 | 1.336 | 0.910–1.959 | .139 |
| Dipper | 1 | Reference | – | 1 | Reference | – |
| Nondipper | 1.237 | 0.449–3.404 | .681 | 1.862 | 0.534–6.498 | .329 |
| Riser | 2.687 | 1.015–7.110 | .047 | 3.757 | 1.116–12.651 | .033 |
| Model 2 | ||||||
| Office SBP | 1.108 | 0.822–1.494 | .500 | 1.123 | 0.776–1.625 | .382 |
| 24‐h SBP | 1.565 | 1.010–2.424 | .045 | 1.684 | 1.032–2.747 | .037 |
| Dipper | 1 | Reference | – | 1 | Reference | – |
| Nondipper | 1.198 | 0.431–3.329 | .729 | 1.753 | 0.500–6.148 | .381 |
| Riser | 2.603 | 0.976–6.940 | .056 | 3.480 | 1.027–11.786 | .045 |
| Model 3 | ||||||
| Office SBP | 1.111 | 0.822–1.502 | .494 | 1.133 | 0.779–1.646 | .514 |
| Daytime SBP | 1.538 | 0.989–2.392 | .056 | 1.630 | 0.997–2.665 | .051 |
| Dipper | 1 | Reference | – | 1 | Reference | – |
| Nondipper | 1.287 | 0.463–3.577 | .629 | 1.900 | 0.542–6.655 | .316 |
| Riser | 2.970 | 1.108–7.961 | .031 | 4.042 | 1.193–13.695 | .025 |
| Model 4 | ||||||
| Office SBP | 1.113 | 0.831–1.491 | .474 | 1.117 | 0.780–1.600 | .547 |
| Nighttime SBP | 1.571 | 1.071–2.303 | .021 | 1.734 | 1.128–2.667 | .012 |
| Dipper | 1 | Reference | – | 1 | Reference | – |
| Nondipper | 0.956 | 0.337–2.717 | .933 | 1.341 | 0.373–4.822 | .653 |
| Riser | 1.668 | 0.576–4.830 | .346 | 2.033 | 0.547–7.552 | .289 |
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; CI, confidence interval; HF, heart failure; HR, hazard ratio; MI, myocardial infarction; SBP, systolic blood pressure.
HR values for office SBP were adjusted for age, sex, body mass index, smoking, alcohol use, diabetes, dyslipidemia, HF, history of cardiovascular disease (including MI, and revascularization therapy), types of antihypertensive drugs, and eGFR.
HR values for different ambulatory blood pressure measures were adjusted for office SBP, and other covariates (age, sex, body mass index, smoking, alcohol use, diabetes, dyslipidemia, HF, history of cardiovascular disease (including MI and revascularization therapy), types of antihypertensive drugs, and eGFR).
HR values for dipping status were adjusted for age, sex, body mass index, smoking, alcohol use, diabetes, dyslipidemia, HF, history of cardiovascular disease (including MI and revascularization therapy), types of antihypertensive drugs, and eGFR, plus office SBP (model 1), plus office SBP and 24‐hour SBP (model 2), plus office SBP and daytime SBP (model 3), and plus office SBP and nighttime SBP (model 4).
TABLE 5.
Improvements in model performance (C‐index).
| Primary endpoint events | ASCVD events | |||
|---|---|---|---|---|
| C‐index (95% CI) | p value | C‐index (95% CI) | p value | |
| Base model | 0.715 (0.637–0.793) | 0.707 (0.613–0.801) | ||
| Base model + 24‐h SBP a | 0.721 (0.645–0.797) | .521 | 0.711 (0.617–0.805) | .503 |
| Base model + daytime SBP a | 0.716 (0.638–0.794) | .935 | 0.707 (0.614–0.801) | .969 |
| Base model + nighttime SBP a | 0.734 (0.660–0.808) | .036 | 0.731 (0.641–0.821) | .024 |
| Base model + dipping status a | 0.726 (0.646–0.806) | .007 | 0.726 (0.634–0.817) | .004 |
| Base model + 24‐h SBP + dipping status b | 0.728 (0.648–0.808) | .008 | 0.727 (0.637–0.817) | .005 |
| Base model + daytime SBP + dipping status b | 0.728 (0.648–0.807) | .007 | 0.726 (0.635–0.818) | .004 |
| Base model + nighttime SBP + dipping status b | 0.731 (0.653–0.809) | .053 | 0.733 (0.643–0.823) | .054 |
Note: Base model includes age, sex, body mass index, smoking, alcohol use, diabetes, dyslipidemia, HF, history of cardiovascular disease (including MI, and revascularization therapy), types of antihypertensive drugs, eGFR and office SBP.
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; CI, confidence interval; eGFR, estimated glomerular filtration rate; HF, heart failure; MI, myocardial infarction; SBP, systolic blood pressure.
p values are differences of base model versus base model + 24‐h SBP or + daytime SBP or + nighttime SBP or + dipping status.
p values are differences of base model + ambulatory blood pressure monitoring indices versus base model + ambulatory blood pressure monitoring measures + dipping status.
3.5. Subgroup analysis
The relationship between dipping status and primary endpoint events was analyzed in various subgroups based on sex (male or female), age (>65 or ≤65 years), current smoking (yes or no), current drinking (yes or no), diabetes (yes or no), blood pressure control status (controlled or uncontrolled), MI (yes or no), PAD (yes or no), and CKD (yes or no). There were no statistically significant interactions observed among these subgroups (All p for interaction >.05). The statistical significance was found only in male patients, patients below the age of 65, patients without current smoking, patients without diabetes, MI, PAD, and CKD (all p < .05) (Figure 3).
FIGURE 3.

Cox proportional hazards analysis evaluating prognostic implication of dipping status in various subgroups. HR was evaluated by per 10% increase in nocturnal SBP dipping. HR was adjusted for office SBP, and other covariates (age, sex, body mass index, smoking, alcohol use, diabetes, dyslipidemia, HF, history of cardiovascular disease (including MI, and revascularization therapy), and types of antihypertensive drugs). CKD, chronic kidney disease; HF, heart failure; MI, myocardial infarction; PAD, peripheral artery disease.
4. DISCUSSION
Although guidelines and clinical practitioners have a propensity to concentrate on ABPM daytime values, the importance of nighttime blood pressure phenotype is gradually recognized by researchers in recent years. 3 , 9 , 10 , 11 Our prospective, observational, and ABPM‐based clinical study evaluated the association between nighttime blood pressure phenotype and prognosis in patients with CHD and hypertension. The results revealed a significant association between the riser pattern and nocturnal high blood pressure with major adverse events, including ASCVD events. It's worth noting that the association between the riser pattern and poor prognosis was not independent of nighttime SBP. Nighttime SBP was found to be a more sensitive prognostic parameter for ASCVD events compared to the riser pattern alone. Furthermore, the risk associated with the riser pattern and nocturnal high blood pressure remained significant across different subgroups.
Our findings revealed that a 20‐mm Hg increase in nighttime SBP was associated with a higher risk of total events (HR = 1.775, p = .001) and ASCVD events (HR = 1.986, p < .001), whereas an increase in daytime SBP did not show the same association. This phenomenon also appeared in the improvement of the model. The addition of nighttime SBP and dipping status significantly enhanced the model's predictive ability for major adverse events and ASCVD events. On the other hand, incorporating daytime BP did not improve the model's performance in predicting either total events or ASCVD risk (p = .935 and p = .969). These results suggest that elevated nocturnal SBP and dipping status have a greater impact than daytime SBP in patients with CHD and hypertension, which was align with the findings of the following two studies. 11 , 12
In a Japanese study, the riser pattern and nighttime blood pressure (BP) were found to be significantly associated with the risk of total cardiovascular disease (CVD) events in individuals with at least one cardiovascular risk factor. 12 Additionally, a large multicenter controlled prospective clinical trial demonstrated that reducing asleep SBP and increasing SBP dipping can reduce the occurrence of major cardiovascular events. 11 However, it remains unclear whether the riser pattern is independently linked to a poor prognosis, irrespective of nighttime SBP in patients with CHD and hypertension. In our study, we observed that adding dipping status did not improve the performance of the base model plus nighttime SBP. This suggests that nocturnal SBP is the key factor influencing the prognosis in patients with CHD and hypertension, rather than dipping status. These findings align with previous studies. 13 , 14
Reducing nocturnal hypertension is crucial for maintaining optimal 24‐h BP management and reducing cardiovascular events. In addition, nocturnal BP is considered the most reliable parameter for risk stratification in ABPM due to the consistent reproducibility of nighttime BP compared to measures of dipping status. 15 A study conducted among hypertensive patients without antihypertensive treatment showed that the results of repeat 24‐h ABPM within 1 month changed in the diagnosis of nocturnal hypertension in 18% of patients, as well as alterations in the classification of dipping and nondipping in 24% of patients. 16 Therefore, it is best to repeat the 24‐h ABPM within a relatively short timeframe of 3–6 months, especially in patients who experienced sleep disturbances during monitoring. 17
Non‐dipping is particularly frequent in individuals with diabetes mellitus and has been related to an increased risk of target organ damage, stroke, cardiovascular events, and mortality in several studies. 18 , 19 , 20 Although several studies have investigated the predictive significance of non‐dipping status in both the general population and patients with hypertension, the findings have not always been consistent. In the initial study that defined non‐dipping, patients with a non‐dipping BP profile showed a significantly higher risk of stroke. 21 Some subsequent studies have supported a connection between non‐dipping status (elevated nocturnal BP) and a higher incidence of cardiovascular events, 22 , 23 , 24 while others have failed to establish such an association. 25 , 26 , 27 Similarly, we were unable to identify an association between nondipping and poor prognosis in patients with CHD and hypertension.
The previous studies revealed that nighttime SBP and dipping status were significantly associated with adverse prognosis in patients with CHD and hypertension. 28 , 29 Pierdomenico et al also reported that different therapeutic approaches based on circadian BP profile may decrease the incidence of nocturnal ischemia. 30 However, these studies did not investigate which factor, nocturnal blood pressure or dipping status, had a greater impact on the prognosis of CHD patients with hypertension. A number of studies with larger sample sizes have concluded that nighttime blood pressure may be considered optimal measurements for estimating cardiovascular risk through modeling improvements in the general of low‐risk population. 12 , 14 Our study also used similar statistical methodology to further explore whether the findings apply to patients with CHD and hypertension.
Two Spanish studies have demonstrated that taking antihypertensive medications at bedtime not only improves blood pressure control but also reduces the occurrence of cardiovascular events and cardiovascular death. 11 , 31 However, recent RCT results indicated that nighttime use of antihypertensive medications did not further improve blood pressure control. 32 , 33 , 34 The divergent outcomes can be attributed to variations in the pharmacokinetic properties of the drugs used in the different studies. In studies employing long half‐life and controlled‐release agents, no significant difference in antihypertensive effects between dosing times was observed. 34 , 35 Consequently, the use of long‐acting antihypertensive drugs serves as the foundation for achieving 24‐h blood pressure control.
There are several limitations of this study that should be acknowledged. Firstly, it was a single‐center, prospective study with a small sample size and limited follow‐up time, which may result in the different findings for DBP in comparison to SBP and selection bias. Secondly, our study did not include an analysis of patients with an extreme dipping pattern as there were only four such patients, and none of them experienced the primary endpoint events or ASCVD events. Thirdly, most patients only underwent ABPM once during their hospitalization, and the lack of subsequent repeat measurements may introduce analysis discrepancies. However, it is important to note that the effectiveness of antihypertensive medication would likely strengthen, rather than weaken, the relationship between ABPM results and major adverse cardiovascular events. Finally, there is currently no peer‐reviewed clinical validation information for the ABPM device used in this study. In conclusion, our results still warrant further multicenter and large‐sample investigation of nocturnal hypertension and dipping status for the treatment of patients with CHD and hypertension.
5. CONCLUSIONS
Nocturnal SBP and riser pattern were associated with adverse prognosis in patients with CHD and hypertension, and nocturnal SBP was a more reliable predictor than dipping status. Thus, the management and monitoring of nocturnal blood pressure is particularly important for patients with CHD and hypertension.
AUTHOR CONTRIBUTIONS
Yao Du: Study design; collection of data; statistical analyses; drafting of the manuscript. Binbin Zhu and Yahui Liu; Weicen Zhou; Zhou Du: Collection of data; statistical analyses. Wei Yang: Study design; critical revision for important intellectual content. Chuanyu Gao: Study conception and design; critical revision for important intellectual content; and final approval of the submitted manuscript.
CONFLICT OF INTEREST STATEMENT
All authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supporting information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Du Y, Zhu B, Liu Y, et al. Association between nocturnal blood pressure phenotype and adverse cardiovascular prognosis in patients with coronary heart disease and hypertension. J Clin Hypertens. 2024;26:405–415. 10.1111/jch.14790
DATA AVAILABILITY STATEMENT
The date that supports the findings of this study are available from the corresponding author upon reasonable request. The date is not publicly due to them containing information that could compromise research participant privacy.
REFERENCES
- 1. Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics‐2021 update: a report from the American Heart Association. Circulation. 2021;143:e254‐e743. [DOI] [PubMed] [Google Scholar]
- 2. Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertens Dallas Tex 1979. 2020;75:285‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kario K, Shin J, Chen C‐H, et al. Expert panel consensus recommendations for ambulatory blood pressure monitoring in Asia: the HOPE Asia Network. J Clin Hypertens Greenwich Conn. 2019;21:1250‐1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hermida RC, Ayala DE, Smolensky MH, et al. Sleep‐time blood pressure: unique sensitive prognostic marker of vascular risk and therapeutic target for prevention. Sleep Med Rev. 2017;33:17‐27. [DOI] [PubMed] [Google Scholar]
- 5. ABC‐H Investigators , Roush GC, Fagard RH, et al. Prognostic impact from clinic, daytime, and night‐time systolic blood pressure in nine cohorts of 13,844 patients with hypertension. J Hypertens. 2014;32:2332‐2340. discussion 2340. [DOI] [PubMed] [Google Scholar]
- 6. Ohkubo T, Hozawa A, Yamaguchi J, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24‐h blood pressure: the Ohasama study. J Hypertens. 2002;20:2183‐2189. [DOI] [PubMed] [Google Scholar]
- 7. ElSayed NA, Aleppo G, Aroda VR, et al. 2. Classification and diagnosis of diabetes: standards of care in diabetes‐2023. Diabetes Care. 2023;46:S19‐S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members . Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825‐830. [DOI] [PubMed] [Google Scholar]
- 9. Hermida RC, Crespo JJ, Otero A, et al. Asleep blood pressure: significant prognostic marker of vascular risk and therapeutic target for prevention. Eur Heart J. 2018;39:4159‐4171. [DOI] [PubMed] [Google Scholar]
- 10. Narita K, Hoshide S, Kario K. Nighttime home blood pressure is associated with the cardiovascular disease events risk in treatment‐resistant hypertension. Hypertens Dallas Tex 1979. 2022;79:e18‐e20. [DOI] [PubMed] [Google Scholar]
- 11. Hermida RC, Crespo JJ, Domínguez‐Sardiña M, et al. Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial. Eur Heart J. 2020;41:4565‐4576. [DOI] [PubMed] [Google Scholar]
- 12. Kario K, Hoshide S, Mizuno H, et al. Nighttime blood pressure phenotype and cardiovascular prognosis: practitioner‐based nationwide JAMP study. Circulation. 2020;142:1810‐1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Asayama K, Fujiwara T, Hoshide S, et al. Nocturnal blood pressure measured by home devices: evidence and perspective for clinical application. J Hypertens. 2019;37:905‐916. [DOI] [PubMed] [Google Scholar]
- 14. Yang W‐Y, Melgarejo JD, Thijs L, et al. Association of office and ambulatory blood pressure with mortality and cardiovascular outcomes. JAMA. 2019;322:409‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hoshide S, Cheng H‐M, Huang Q, et al. Role of ambulatory blood pressure monitoring for the management of hypertension in Asian populations. J Clin Hypertens Greenwich Conn. 2017;19:1240‐1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cuspidi C, Sala C, Valerio C, Negri F, Mancia G. Nocturnal blood pressure in untreated essential hypertensives. Blood Press. 2011;20:335‐341. [DOI] [PubMed] [Google Scholar]
- 17. Parati G, Stergiou G, O'Brien E, et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens. 2014;32:1359‐1366. [DOI] [PubMed] [Google Scholar]
- 18. Mousa T, el‐Sayed MA, Motawea AK, Salama MA, Elhendy A. Association of blunted nighttime blood pressure dipping with coronary artery stenosis in men. Am J Hypertens. 2004;17:977‐980. [DOI] [PubMed] [Google Scholar]
- 19. Hoshide S, Kario K, Hoshide Y, et al. Associations between nondipping of nocturnal blood pressure decrease and cardiovascular target organ damage in strictly selected community‐dwelling normotensives. Am J Hypertens. 2003;16:434‐438. [DOI] [PubMed] [Google Scholar]
- 20. Fan H‐Q, Li Y, Thijs L, et al. Prognostic value of isolated nocturnal hypertension on ambulatory measurement in 8711 individuals from 10 populations. J Hypertens. 2010;28:2036‐2045. [DOI] [PubMed] [Google Scholar]
- 21. O'Brien E, Sheridan J, O'Malley K. Dippers and non‐dippers. Lancet Lond Engl. 1988;2:397. doi: 10.1016/s0140-6736(88)92867-x [DOI] [PubMed] [Google Scholar]
- 22. Boggia J, Li Y, Thijs L, et al. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet Lond Engl. 2007;370:1219‐1229. [DOI] [PubMed] [Google Scholar]
- 23. Androulakis E, Papageorgiou N, Lioudaki E, et al. Subclinical organ damage in white‐coat hypertension: the possible role of cystatin C. J Clin Hypertens Greenwich Conn. 2017;19:190‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Palmas W, Pickering TG, Teresi J, et al. Ambulatory blood pressure monitoring and all‐cause mortality in elderly people with diabetes mellitus. Hypertens Dallas Tex 1979. 2009;53:120‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eguchi K, Pickering TG, Hoshide S, et al. Ambulatory blood pressure is a better marker than clinic blood pressure in predicting cardiovascular events in patients with/without type 2 diabetes. Am J Hypertens. 2008;21:443‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Björklund K, Lind L, Zethelius B, Berglund L, Lithell H. Prognostic significance of 24‐h ambulatory blood pressure characteristics for cardiovascular morbidity in a population of elderly men. J Hypertens. 2004;22:1691‐1697. [DOI] [PubMed] [Google Scholar]
- 27. Hansen TW, Jeppesen J, Rasmussen S, Ibsen H, Torp‐Pedersen C. Ambulatory blood pressure monitoring and risk of cardiovascular disease: a population based study. Am J Hypertens. 2006;19:243‐250. [DOI] [PubMed] [Google Scholar]
- 28. Dzielińska Z, Prejbisz A, Makowiecka‐Cieśla M, et al. The 24‐h blood pressure measurement may predict mortality and cardiovascular events in hypertensive patients with coronary artery disease. Blood Press Monit. 2009;14:99‐102. [DOI] [PubMed] [Google Scholar]
- 29. Kurpesa M, Trzos E, Drozdz J, Bednarkiewicz Z, Krzemińska‐Pakuła M. Myocardial ischemia and autonomic activity in dippers and non‐dippers with coronary artery disease: assessment of normotensive and hypertensive patients. Int J Cardiol. 2002;83:133‐142. [DOI] [PubMed] [Google Scholar]
- 30. Pierdomenico SD, Bucci A, Costantini F, et al. Circadian blood pressure changes and myocardial ischemia in hypertensive patients with coronary artery disease. J Am Coll Cardiol. 1998;31:1627‐1634. [DOI] [PubMed] [Google Scholar]
- 31. Hermida RC, Calvo C, Ayala DE, et al. Administration time‐dependent effects of valsartan on ambulatory blood pressure in elderly hypertensive subjects. Chronobiol Int. 2005;22:755‐776. [DOI] [PubMed] [Google Scholar]
- 32. Mackenzie IS, Rogers A, Poulter NR, et al. Cardiovascular outcomes in adults with hypertension with evening versus morning dosing of usual antihypertensives in the UK (TIME study): a prospective, randomised, open‐label, blinded‐endpoint clinical trial. Lancet Lond Engl. 2022;400:1417‐1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Poulter NR, Savopoulos C, Anjum A, et al. Randomized crossover trial of the impact of morning or evening dosing of antihypertensive agents on 24‐hour ambulatory blood pressure. Hypertens Dallas Tex 1979. 2018;72:870‐873. [DOI] [PubMed] [Google Scholar]
- 34. Liu J, Su X, Nie Y, Zeng Z, Chen H. Dosing time matters? Nighttime vs. daytime administration of nifedipine gastrointestinal therapeutic system (GITS) or amlodipine on non‐dipper hypertension: a randomized controlled trial of NARRAS. Front Cardiovasc Med. 2021;8:755403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Serinel Y, Yee BJ, Grunstein RR, et al. Chronotherapy for hypertension in obstructive sleep apnoea (CHOSA): a randomised, double‐blind, placebo‐controlled crossover trial. Thorax. 2017;72:550‐558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Data Availability Statement
The date that supports the findings of this study are available from the corresponding author upon reasonable request. The date is not publicly due to them containing information that could compromise research participant privacy.


