Abstract
Background
Inflammatory bowel disease (IBD) confers an increased lifetime risk of colorectal cancer (CRC). The pathogenesis of colitis-associated CRC is considered distinct from sporadic CRC, but existing data is mixed on long-term oncologic outcomes. This study aims to compare clinicopathological characteristics and survival between colitis-associated and sporadic CRC.
Methods
Data was retrospectively extracted and analyzed from a single institutional database of patients with surgically resected CRC between 2004 and 2015. Patients with IBD were identified as having colitis-associated CRC. The remainder were classified as sporadic CRC. Propensity score matching was performed. Univariate and survival analyses were carried out to estimate the differences between the two groups.
Results
Of 2275 patients included in this analysis, 65 carried a diagnosis of IBD (2.9%, 33 Crohn’s disease, 29 ulcerative colitis, 3 indeterminate colitis). Average age at CRC diagnosis was 62 years for colitis-associated CRC and 65 for sporadic CRC. The final propensity score matched cohort consisted of 65 colitis-associated and 130 sporadic CRC cases. Patients with colitis-associated CRC were more likely to undergo total proctocolectomy (p<0.01) and had higher incidence of locoregional recurrence (p=0.026) compared to sporadic CRC patients. There were no significant differences in time to recurrence, tumor grade, extramural vascular invasion, perineural invasion, or rate of R0 resections. Overall survival and disease-free survival did not differ between groups. On multiple Cox regression, IBD diagnosis was not a significant predictor of survival.
Conclusions
Patients with colitis-associated CRC who undergo surgical resection have comparable overall and disease-free survival to patients with sporadic CRC.
Keywords: inflammatory bowel disease, colitis-associated colorectal cancer, sporadic colorectal cancer, survival
Introduction
Inflammatory bowel disease (IBD) is a known lifetime risk factor for development of colorectal cancer (CRC). Colitis-associated colorectal cancer is considered a distinct entity from sporadic CRC. IBD patients require more frequent endoscopic surveillance and if found to develop a cancer they often undergo a more aggressive resection [1]. The molecular pathogenesis of cancer in IBD is thought to be distinct from the sporadic pathway due to the cumulative and multifocal neoplastic effects of chronic mucosal inflammation, although the exact mechanisms are yet to be understood. Whether these differences impact short- or long-term oncologic outcomes following appropriate treatment remains unanswered. Several studies report that, despite differences in tumor stage or location at diagnosis, IBD is not a prognostic factor for overall survival [2]–[4]. Others have found that colitis-associated CRC is associated with a higher incidence of synchronous and higher grade tumors, which translates to worse overall and disease-free survival [5]–[7]. However, with advancements in endoscopic surveillance and oncologic therapies, questions remain as to whether there is a true difference in prognosis regarding colitis-associated and sporadic CRC. This paper seeks to determine whether colitis-associated CRC carries a worse overall and disease-free survival compared to sporadic CRC.
Materials and methods
Study design
All data was extracted from a prospectively maintained and Institutional Review Board approved database, which included all patients who underwent surgical resection for colorectal cancer at a single tertiary care academic center between 2004 and 2015. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were followed and reported [8].
Patients were divided into two cohorts: 1) those with a history of colitis secondary to IBD and therefore characterized as having colitis-associated CRC, and 2) those with sporadic CRC. Demographic characteristics, pathologic features, and long-term disease outcomes were reviewed. Since this database is prospectively maintained, survival data were last updated in June 2022. Univariate analysis was done to compare demographics and tumor characteristics such as location, grade, and pathologic stage.
Propensity score matching analysis was performed using a 1:2 nearest neighbor matching method without replacement, such that two unique sporadic CRC patients were selected for each colitis-associated CRC patient in the database (Figure 1). The covariates included age, gender, ASA, tumor location (colon or rectum), neoadjuvant therapy, and AJCC pathologic stage.
Figure 1.
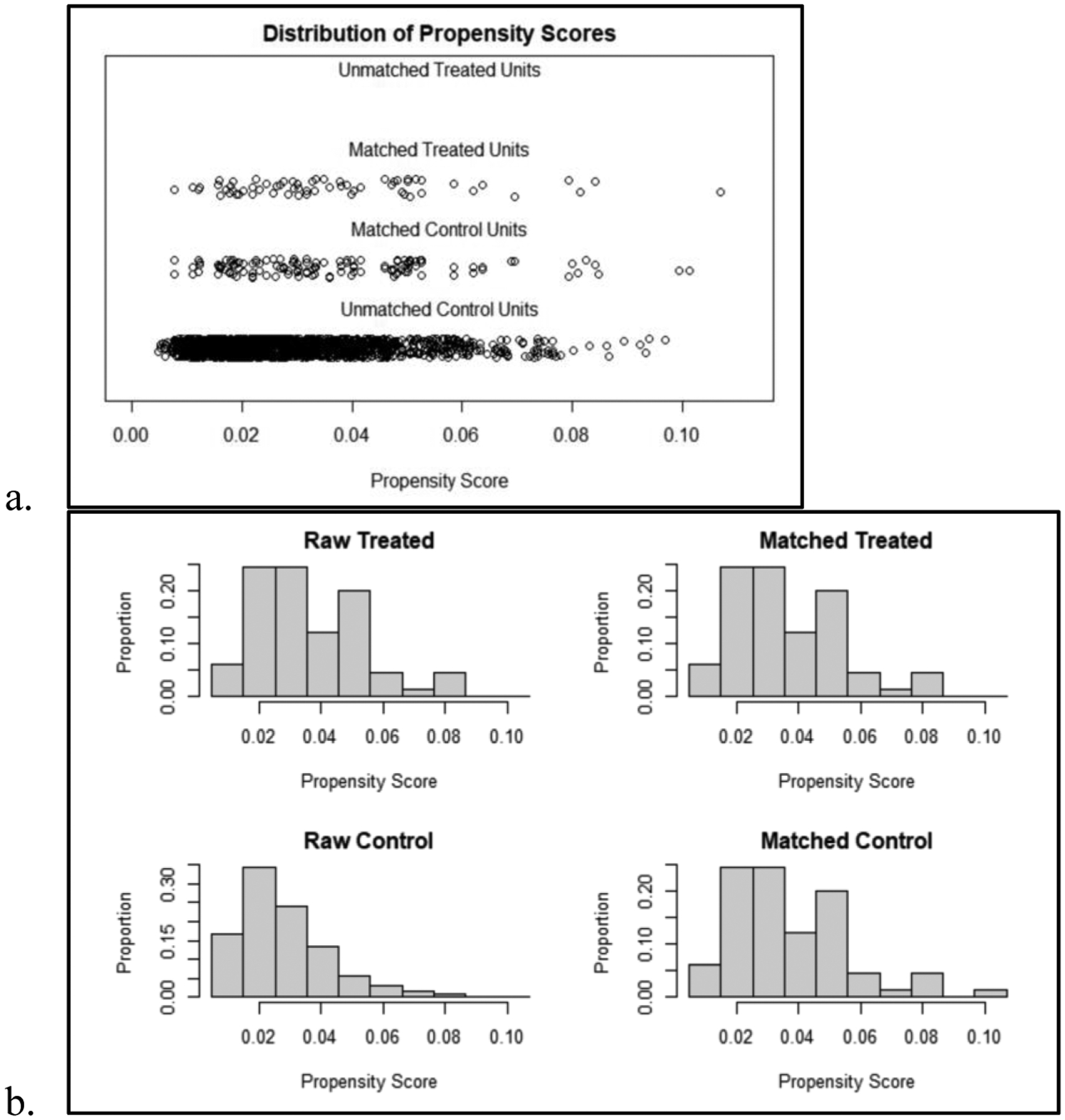
a) Distribution of all calculated propensity scores from 0 to 1.0 among matched and unmatched control and treated units. Control units refers to the sporadic CRC group, while treated units refers to the colitis-associated CRC group. b) Histogram representation of the distribution of propensity scores among all patients and among the final matched cohort.
The primary outcome measure was overall survival (OS). Secondary outcome measures included disease-free survival (DFS), operative characteristics such as resection type, and pathologic characteristics such as extramural vascular invasion (EMVI), perineural invasion (PNI), and tumor grade and stage.
Statistical analysis
Statistical analysis was performed using Stata/SE 17.0. Descriptive statistics were computed using univariate analysis. Overall survival and disease-free survival curves for both groups were constructed using the Kaplan-Meier method, and the curves were compared using the log-rank test. All time-to-event periods were expressed in months from the date of initial surgical resection. Multiple Cox regression analysis was performed to investigate the predictive value of IBD diagnosis in the context of other variables including patient demographics, neoadjuvant therapy, and pathologic stage.
Results
A total of 2275 patients were included in this analysis who underwent surgical resection for colorectal cancer at our institution between 2004 and 2015. Of these, 65 patients carried a diagnosis of IBD (2.9%). 33 patients had Crohn’s disease, 29 had ulcerative colitis, and 3 had indeterminate colitis. The average age at CRC diagnosis was 62 years for patients with colitis-associated CRC and 65 years for those with sporadic CRC. Prior to propensity score matching, there were no significant differences in age, gender breakdown, ASA, tumor site in colon vs. rectum, presence of synchronous tumors, pathologic stage, or receipt of neoadjuvant chemotherapy or radiation.
After propensity score matching, the final cohort consisted of 65 colitis-associated CRC patients and 130 sporadic CRC patients (Table 1). Mean age of each group was 62 and 61 years, respectively. The colitis-associated CRC group was 42% female, and the sporadic CRC group was 41% female. Both groups had 26.2% of tumors located in the rectum. Procedures were classified as total proctocolectomy, segmental colonic resection, proctectomy including abdominoperineal resection and low anterior resection, and transanal excision (Table 1). Patients with colitis-associated CRC were significantly more likely than their sporadic CRC counterparts to undergo a total proctocolectomy (27.7% vs. 0.8%, p<0.001), although both groups were matched for tumor location. The remaining procedure types did not differ significantly between groups.
Table 1.
Patient demographics and clinical characteristics by group. Data are presented as mean (SD) for continuous measures, and % (n) for categorical measures.
| Sporadic CRC | Colitis-associated CRC | P-value | |
|---|---|---|---|
| Age | 61 (14) | 62 (15) | 0.70 |
| Sex | 0.92 | ||
| Female | 41% (53) | 42% (27) | |
| Male | 59% (77) | 58% (38) | |
| Tumor Site | 1.00 | ||
| Colon | 72% (94) | 72% (47) | |
| Rectum | 26% (34) | 26% (17) | |
| Synchronous tumors | 2% (2) | 2% (1) | |
| Type of Resection | |||
| APR or LARa | 28% (37) | 15% (10) | 0.051 |
| Segmental colonic resection | 68% (88) | 55% (36) | 0.11 |
| Transanal excision | 3% (4) | 2% (1) | 0.67 |
| Total proctocolectomy | 1% (1) | 28% (18) | <0.001 |
APR = abdominoperineal resection. LAR = low anterior resection.
There were no differences between the groups in tumor grade, tumor stage, presence of extramural vascular invasion, small or large vessel invasion, perineural invasion, or rates of R0 resection achieved (Table 2). Patients with colitis-associated CRC were at greater risk of locoregional recurrence (RR 8.5, 95 CI (1.02, 71.51), p = 0.047). Locoregional recurrence was diagnosed in 7.7% of colitis-associated CRC patients, and in 0.7% of sporadic CRC patients (p=0.026). Only 1 patient with sporadic CRC in this cohort, who had a colonic tumor, developed locoregional recurrence. By contrast, 5 patients with colitis-associated CRC (3 colonic tumors, 2 rectal tumors) developed locoregional recurrence. However, there were no differences in rates of distant metastases (20% in colitis-associated CRC vs. 12.6% in sporadic CRC), or in the time to diagnosis of either type of recurrence. Median time to diagnosis of a locoregional recurrence was 12.2 months for colitis-associated CRC vs. 17.8 months for sporadic CRC. Median time to diagnosis of metastatic disease was 6.3 months for colitis-associated CRC vs. 10.2 months for sporadic CRC. Median follow up time was 5.0 years for the colitis-associated CRC group and 4.1 years for the sporadic CRC group. There were no differences in rates of adjuvant chemotherapy or radiation between the two groups. Among the patients who did not undergo a total proctocolectomy initially, 4 patients developed a metachronous colorectal adenocarcinoma detected during the follow up period (3 colitis-associated and 1 sporadic CRC), and 3 developed high-grade dysplasia (all sporadic CRC). There was no significant difference in the rate of either occurrence between groups. Definitive management of these cases included complete excision with polypectomy (in 2 sporadic CRC cases), additional segmental resection (in 2 colitis-associated and 1 sporadic CRC case), total abdominal colectomy (in 1 sporadic CRC case), and completion total proctocolectomy (in 1 colitis-associated CRC case).
Table 2.
Pathologic characteristics and oncologic outcomes by group. Data are presented as % (n).
| Sporadic CRC | Colitis-associated CRC | P-value | |
|---|---|---|---|
| Tumor Grade | 0.49 | ||
| Low-intermediate grade | 70% (91) | 62% (40) | |
| High grade | 20% (26) | 26% (17) | |
| Unspecified | 10% (13) | 12% (8) | |
| Tumor Stage | 0.38 | ||
| Tis | 8% (10) | 3% (2) | |
| T1 | 19% (25) | 25% (16) | |
| T2 | 18% (24) | 15% (10) | |
| T3 | 34% (44) | 28% (18) | |
| T4 | 21% (27) | 29% (19) | |
| Lymphovascular invasion | 48% (62) | 45% (29) | 0.68 |
| Extramural vascular invasion | 27% (35) | 31% (20) | 0.57 |
| Perineural invasion | 18% (24) | 20% (13) | 0.80 |
| R0 resection | 98% (127) | 94% (61) | 0.17 |
| AJCC pathologic stage | 0.99 | ||
| Stage 0 | 2% (3) | 2% (1) | |
| Stage 1 | 38% (50) | 38% (25) | |
| Stage 2 | 25% (32) | 28% (18) | |
| Stage 3 | 25% (32) | 23% (15) | |
| Stage 4 | 10% (13) | 9% (6) | |
| Adjuvant chemotherapy | 36% (47) | 43% (28) | 0.62 |
| Adjuvant radiation | 5% (6) | 3% (2) | 0.61 |
| Locoregional recurrence | 1% (1) | 8% (5) | 0.002 |
| Colon primary | 100% (1) | 60% (3) | |
| Rectum primary | - | 40% (2) | |
| Metastases | 13% (17) | 20% (13) | 0.29 |
| Median follow up (months) | 49 | 59.4 | 0.45 |
There were no significant differences in overall survival (OS) or disease-free survival (DFS) between the two groups (Figure 2). Overall survival rates at 1 year and at 5 years were 93.7% and 73.1% respectively in the colitis-associated CRC group, compared to 93.7% and 77.1% in the sporadic CRC group. Disease-free survival rates at 1 year and at 5 years were 92.2% and 67.5% respectively in the colitis-associated CRC group, compared to 93.7% and 74.1% in the sporadic CRC group. On multiple Cox regression analysis, a diagnosis of IBD was not found to be predictive of OS or DFS when adjusting for age, gender, receipt of neoadjuvant therapy, pathologic stage, and whether R0 resection was achieved.
Figure 2.
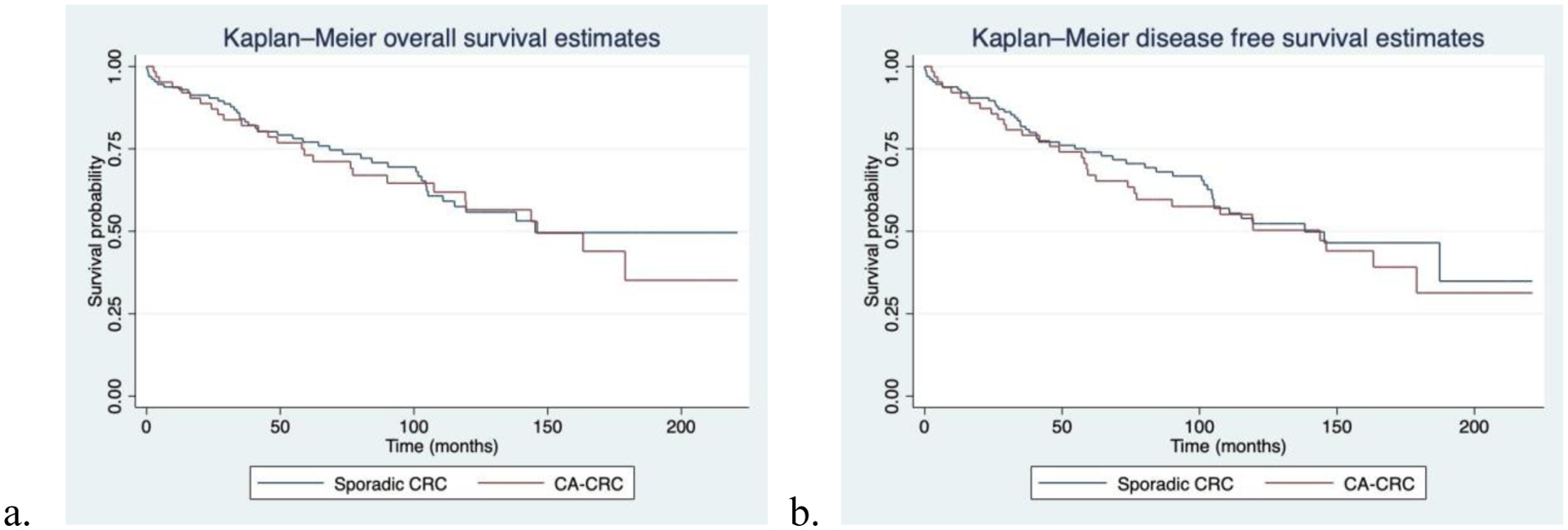
Kaplan-Meier survival estimates comparing sporadic CRC vs. colitis-associated CRC. a) Overall survival curve in months shows no difference between groups. b) Disease-free survival curve in months shows no difference between groups.
Discussion
Colitis-associated CRC and sporadic CRC are distinct in their presumed pathogenesis and approach to treatment, although there is no consensus as to whether this translates to a notable difference in outcomes. In this single-center retrospective study, IBD does not impact overall or disease-free CRC survival. Our findings are consistent with several previously published studies. A meta-analysis of 20 studies comparing outcomes of CRC patients with and without IBD found comparable 5-year overall survival, although IBD patients were found to have more synchronous tumors, more poorly differentiated tumors, and more rectal tumors [3]. Several prior studies also used propensity matching based on factors including age, sex, tumor stage, tumor location, and even time period of surgery. One such study which examined data from the Irish National Cancer Registry from 1994 to 2005 found no survival differences after propensity matching, and did not find IBD to be a significant prognostic factor for oncologic outcomes [2]. Thicoïpé et al. performed another 3:1 matched study over a similar time frame to the present study, which found no differences in 5-year disease-free survival or overall survival [4]. Data from matched studies spanning from the 1970s to 2015 have repeatedly demonstrated no overall or disease-free survival differences [9], [10], suggesting that despite potential morphological differences and advances over time in surveillance and treatment, long term outcomes remain comparable regardless of IBD.
On the other hand, multiple studies examining both matched and unmatched data over a similar time frame report worse 5-year overall survival for colitis-associated CRC [5]–[7]. One specifically found age-related 5-year survival differences, most pronounced in the 18–49-year age group (56.8% vs 71.4% in sporadic cancer, p<0.001) [11]. However, another large study analyzing the SEER database of CRC patients from 1991 to 2006 found only slightly worse, but not significantly worse, median cancer-specific survival among IBD patients (32.9 months vs. 42.4 months) [12], which could be multifactorial.
Amidst this background of mixed data, the prognosis of colitis-associated CRC remains unclear and the pathogenesis of CRC in IBD patients is incompletely understood. It is known that chronic intestinal inflammation leads to neoplastic changes over time, which is distinct from the pathways that precede sporadic CRC [13]–[15]. Recognized components of the sporadic CRC pathway, such as genetic mutations in APC, DNA hyper-methylation, and microsatellite instability, also appear to play a role in colitis-associated CRC. However, underlying chronic inflammation impacts the timing and frequency of these changes in some way, leading to varying patterns of carcinogenesis which can be multifocal. The question remains how these differences impact long-term oncologic outcomes. We suspect that although the risks of initial development of CRC, multifocal neoplasia, and local recurrence are greater in IBD patients, their long-term prognosis is evened out by rigorous surveillance and more extensive resections. Our findings support the growing body of literature that overall and disease-free survival are comparable between these two groups.
As expected, proctectomy was performed significantly more frequently in the IBD cohort then the sporadic CRC cohort. This difference was noted before and after propensity matching for tumor site, which aligns with both current guidelines on approach to CRC in patients with colitis [1] and with the increased risk of synchronous tumors among IBD patients [4], [12], [16], [17]. However, contrary to common practice and current guidelines, segmental resection was the most commonly performed operation not just for sporadic CRC cases but also for colitis-associated CRC patients. Use of segmental resection in colitis-associated CRC may contribute to our inability to identify higher incidence of synchronous tumors within the IBD patient cohort. Additionally, this operative strategy of limited resection may play an underlying role in the observed higher rate of locoregional recurrence in the colitis-associated CRC cohort, since we observed locoregional recurrence more frequently among patients with primary colonic tumors, rather than rectal tumors. In these cases, residual dysplasia may have been present but not removed at the time of segmental resection. The increased rate of locoregional recurrence in colitis-associated CRC in this analysis is similar to prior findings by Renz et al [6]. However, contrary to the study by Renz et al., the greater rate of locoregional recurrence did not translate to a difference in disease-free or overall survival. In addition, the rate of metachronous cancers diagnosed during the follow up period did not differ significantly between the sporadic and colitis-associated CRC cohorts. This supports the notion that even with less extensive resections, close endoscopic surveillance is a successful strategy in improving long-term outcomes in IBD patients.
We used propensity score matching to address the large sample size difference and potential presence of confounders between the colitis-associated CRC and sporadic CRC groups in this study. This approach can strengthen the analysis of observational studies when randomization is not possible, as in this case where the variable of interest is presence of inflammatory bowel disease. However, propensity score matching has some potential pitfalls due to the variability in matching methods that may be used, and due to the unavoidable reduction in sample size and therefore statistical power. To try to avoid these pitfalls, we ran multiple versions of logistic regression models with different covariates before choosing the final model with acceptable balance. As demonstrated by Figure 1, there is reasonable balance in propensity score distribution between the colitis-associated and sporadic CRC groups. All patients with colitis-associated CRC were included in the final matched cohort, and we used a 1:2 matching method without replacement to minimize how much the final sample size of sporadic CRC patients was reduced.
Other limitations besides sample size include the fact that this was a retrospective study, and therefore does not have certain data available that may have impacted our analysis such as use of biologics or steroids in IBD patients. This study was done at a single tertiary care center which inherently limits its external validity.
Conclusion
Although inflammatory bowel disease confers a higher lifetime risk of the development of colorectal cancer than the general population, it is not associated with worse disease-free or overall survival. This equivalence in survival remains true despite a higher risk of observed locoregional recurrence in patients with colitis-associated CRC, likely due to improvements in medical and surgical oncologic care.
Footnotes
Ethical Standards: Institutional Review Board approval was obtained for data collection and maintenance of the institutional research database which was used for this retrospective analysis.
Conflict of Interest Statement: All authors declare no competing interests.
References
- [1].Holubar SD et al. , “The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Surgical Management of Ulcerative Colitis,” Dis. Colon Rectum, vol. 64, no. 7, pp. 783–804, Jul. 2021, doi: 10.1097/DCR.0000000000002037. [DOI] [PubMed] [Google Scholar]
- [2].Ali RAR, Dooley C, Comber H, Newell J, and Egan LJ, “Clinical features, treatment, and survival of patients with colorectal cancer with or without inflammatory bowel disease,” Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc, vol. 9, no. 7, pp. 584–589.e1–2, Jul. 2011, doi: 10.1016/j.cgh.2011.04.016. [DOI] [PubMed] [Google Scholar]
- [3].Reynolds IS, O’Toole A, Deasy J, McNamara DA, and Burke JP, “A meta-analysis of the clinicopathological characteristics and survival outcomes of inflammatory bowel disease associated colorectal cancer,” Int. J. Colorectal Dis, vol. 32, no. 4, pp. 443–451, Apr. 2017, doi: 10.1007/s00384-017-2754-3. [DOI] [PubMed] [Google Scholar]
- [4].Thicoïpé A et al. , “Oncological outcomes of IBD-associated versus sporadic colorectal cancer in modern era: a matched case-control study,” Int. J. Colorectal Dis, vol. 33, no. 7, pp. 963–966, Jul. 2018, doi: 10.1007/s00384-018-3049-z. [DOI] [PubMed] [Google Scholar]
- [5].Hrabe JE, Byrn JC, Button AM, Zamba GK, Kapadia MR, and Mezhir JJ, “A matched case-control study of IBD-associated colorectal cancer: IBD portends worse outcome,” J. Surg. Oncol, vol. 109, no. 2, pp. 117–121, Feb. 2014, doi: 10.1002/jso.23465. [DOI] [PubMed] [Google Scholar]
- [6].Renz BW et al. , “Clinical outcome of IBD-associated versus sporadic colorectal cancer: a matched-pair analysis,” J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract, vol. 17, no. 5, pp. 981–990, May 2013, doi: 10.1007/s11605-013-2171-z. [DOI] [PubMed] [Google Scholar]
- [7].Taylor CC, Millien VO, Hou JK, and Massarweh NN, “Association Between Inflammatory Bowel Disease and Colorectal Cancer Stage of Disease and Survival,” J. Surg. Res, vol. 247, pp. 77–85, Mar. 2020, doi: 10.1016/j.jss.2019.10.040. [DOI] [PubMed] [Google Scholar]
- [8].Vandenbroucke JP et al. , “Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration,” PLoS Med, vol. 4, no. 10, p. e297, Oct. 2007, doi: 10.1371/journal.pmed.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vitali F et al. , “The outcome of patients with inflammatory bowel disease-associated colorectal cancer is not worse than that of patients with sporadic colorectal cancer-a matched-pair analysis of survival,” Int. J. Colorectal Dis, vol. 37, no. 2, pp. 381–391, Feb. 2022, doi: 10.1007/s00384-021-04072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Delaunoit T, Limburg PJ, Goldberg RM, Lymp JF, and Loftus EV, “Colorectal cancer prognosis among patients with inflammatory bowel disease,” Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc, vol. 4, no. 3, pp. 335–342, Mar. 2006, doi: 10.1016/j.cgh.2005.12.035. [DOI] [PubMed] [Google Scholar]
- [11].Bogach J, Pond G, Eskicioglu C, and Seow H, “Age-Related Survival Differences in Patients With Inflammatory Bowel Disease-Associated Colorectal Cancer: A Population-Based Cohort Study,” Inflamm. Bowel Dis, vol. 25, no. 12, pp. 1957–1965, Nov. 2019, doi: 10.1093/ibd/izz088. [DOI] [PubMed] [Google Scholar]
- [12].Gearhart SL, Nathan H, Pawlik TM, Wick E, Efron J, and Shore AD, “Outcomes from IBD-associated and non-IBD-associated colorectal cancer: a Surveillance Epidemiology and End Results Medicare study,” Dis. Colon Rectum, vol. 55, no. 3, pp. 270–277, Mar. 2012, doi: 10.1097/DCR.0b013e318242620f. [DOI] [PubMed] [Google Scholar]
- [13].Stidham RW and Higgins PDR, “Colorectal Cancer in Inflammatory Bowel Disease,” Clin. Colon Rectal Surg, vol. 31, no. 3, pp. 168–178, May 2018, doi: 10.1055/s-0037-1602237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Axelrad JE, Lichtiger S, and Yajnik V, “Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment,” World J. Gastroenterol, vol. 22, no. 20, pp. 4794–4801, May 2016, doi: 10.3748/wjg.v22.i20.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yashiro M, “Molecular Alterations of Colorectal Cancer with Inflammatory Bowel Disease,” Dig. Dis. Sci, vol. 60, no. 8, pp. 2251–2263, Aug. 2015, doi: 10.1007/s10620-015-3646-4. [DOI] [PubMed] [Google Scholar]
- [16].Kiran RP, Khoury W, Church JM, Lavery IC, Fazio VW, and Remzi FH, “Colorectal cancer complicating inflammatory bowel disease: similarities and differences between Crohn’s and ulcerative colitis based on three decades of experience,” Ann. Surg, vol. 252, no. 2, pp. 330–335, Aug. 2010, doi: 10.1097/SLA.0b013e3181e61e69. [DOI] [PubMed] [Google Scholar]
- [17].Leowardi C et al. , “Prognosis of Ulcerative Colitis-Associated Colorectal Carcinoma Compared to Sporadic Colorectal Carcinoma: A Matched Pair Analysis,” Ann. Surg. Oncol, vol. 23, no. 3, pp. 870–876, Mar. 2016, doi: 10.1245/s10434-015-4915-3. [DOI] [PubMed] [Google Scholar]


