Abstract
Cytomegalovirus (CMV) infection during the transient immunodeficiency after bone marrow transplantation (BMT) develops into disease unless antiviral CD8 T cells are restored in due course. Histoincompatibility between donor and recipient is associated with increased risk. Complications may include a rejection response against the foreign major histocompatibility complex (MHC) antigens and a lack of antiviral control resulting from a misfit between donor-derived T cells and the antigenic viral peptides presented in recipient tissues. Here we have established a murine model of CMV disease after experimental BMT performed across a single MHC class I disparity. Specifically, BALB/c bone marrow cells expressing the prevailing antigen-presenting molecule Ld were transplanted into the Ld gene deletion mutant BALB/c-H-2dm2, an experimental setting that entails a selective risk of host-versus-graft but not graft-versus-host response. The reconstituted T-cell population proved to be chimeric in that it consisted of Ld-positive donor-derived and Ld-negative recipient-derived cells. Pulmonary infiltrates did not include cytolytic T cells directed against Ld. This finding implies that the infection did not trigger a host-versus-graft response. Notably, upon adoptive transfer, donor-derived CD8 T cells preferentially protected tissues of donor genotype, whereas recipient-derived CD8 T cells protected tissues of either genotype. We infer from these data that the focus on immunodominant antigens presented by Ld within the donor cell population distracted the donor T cells from protecting recipient tissues and that protection in the chimeras was therefore primarily based on recipient T cells. As a consequence, T-cell chimerism after BMT should give a positive prognosis with respect to control of CMV.
Cytomegaloviruses (CMV) are kept under tight immune control (for reviews, see references 22 and 23). As a consequence, acute CMV infection is resolved rapidly and does not result in disease unless the host is immunologically immature or immunocompromised. Bone marrow (BM) transplantation (BMT) as a therapy of hematological malignancies is associated with a transient immunodeficiency. Accordingly, during the period of immunocompromise, transmission of donor-type CMV with the transplant as well as recurrence of CMV from latency established within the organs of the transplantation recipient both entail a risk for destructive virus replication in tissues resulting in multiple-organ CMV disease (16). In BMT recipients, CMV-induced interstitial pneumonia is a frequent and endangering manifestation of CMV disease (11, 27). However, CMV infection does not inevitably result in fatal disease. It appears that CD8 T-cell reconstitution is the decisive parameter in the control of CMV after BMT. Clinical data have shown that both efficient reconstitution of CD8 T cells (41) and supplementation of antiviral CD8 T cells by preemptive cytoimmunotherapy with T-cell lines (42, 50) correlate with a reduced risk of human CMV disease, whereas combined in vivo-ex vivo T-cell depletion, intended as a prophylaxis against graft-versus-host (GvH) disease, accidentally resulted in an increased incidence of CMV infections in BMT patients (14). Aspects of these clinical problems can be approached experimentally in a murine model of BMT and concurrent infection with murine CMV (for an overview, see reference 35). Specifically, depletion of CD8 T cells, but not of CD4 T cells, performed in vivo during the phase of reconstitution after BMT abolished the development of protective antiviral immunity, with an inevitably lethal outcome (34, 47) resulting from multiple-organ pathology (34), including BM aplasia (29, 30). Likewise, an insufficient endogenous reconstitution was successfully supplemented by experimental adoptive cytoimmunotherapy with antiviral CD8 T cells. Again, CD4 T cells were not effective (36, 37, 39, 47). Altogether, clinical data on human CMV infection and experimental data from the murine model have so far been concordant and have identified CD8 T cells as the principal effectors controlling CMV infections after BMT.
These findings imply that all conditions which lower the efficacy of CD8 T-cell reconstitution will increase the risk for progression of asymptomatic CMV infection to fatal CMV disease. Histoincompatibility between graft and recipient is a factor likely to negatively influence the restoration of antiviral immunity. Accordingly, even though cases of severe human CMV disease have been reported also after autologous BMT (27, 40), the incidence of CMV-related complications is generally higher after histoincompatible BMT (51). In clinical BMT, donor and recipient are usually matched in major histocompatibility complex (MHC) class II molecules, whereas differences in minor histocompatibility loci and in MHC class I loci are tolerated if unavoidable. Complications caused in the CMV-infected recipient by histoincompatibility may include (i) an impaired engraftment of transplanted cells in the recipient BM stroma, (ii) an immunological GvH response as well as a host-versus-graft (HvG) response directed against the foreign minor or major histocompatibility molecules, and (iii) a lack of antiviral T-cell control resulting from an inappropriate repertoire of viral antigenic peptides presented by infected tissue cells of the transplantation recipient.
In a first attempt to dissect these possibilities, we have established a murine model of experimental BMT performed across a single MHC class I disparity, namely, the presence and absence of the Ld molecule in BALB/c mice (MHC class I molecules Kd, Dd, and Ld) and the Ld gene deletion mutant BALB/c-H-2dm2 (44), respectively. Depending on the choice of donor and recipient for the BMT, immunogenetical GvH and HvG conditions can be studied separately (35). Work presented herein focuses on the HvG setting with BALB/c as the donor strain and the mutant as the recipient. Hence, after incomplete depletion of hematopoietic cells of the recipients, this model entails a risk for graft rejection caused by a recipient response directed against the donor MHC class I molecule Ld. In addition, presentation of viral peptides by Ld, including the immunodominant IE1 nonapeptide of murine CMV (18, 38), is confined to donor-derived hematopoietic cells and their progeny, whereas the parenchymal and stromal sites of cytocidal infection (34) lack Ld as the prevailing peptide presenter. The aim of the study was to investigate the influence of this particular MHC class I disparity on the control of murine CMV after BMT.
MATERIALS AND METHODS
HvG-BMT, adoptive cell transfer, and murine CMV infection.
Animal experiments were approved by the Ethics Commission, permission no. 177-07/931-17, according to German federal law. For an HvG setting of MHC-mismatched BMT across a single MHC class I disparity, female mice of the inbred strains BALB/c (MHC class I molecules Kd, Dd, and Ld) and BALB/c-H-2dm2 (MHC class I molecules Kd and Dd only) were used at the age of 8 weeks as donors and recipients, respectively, of BM cells (BMC). For hematoablative conditioning, recipients were total-body γ irradiated with a dose of 6 Gy delivered by a 137Cs γ-ray source. Donor femoral and tibial BMC were isolated by flushing medium through the bone shafts (30) and were depleted of contaminating intravascular and sinusoidal CD8 T cells (<2% in normal BALB/c BM) by three cycles of treatment with rat anti-murine CD8 monoclonal antibody (MAb) clone YTS 169.4 (7) and magnetic beads coated with sheep anti-rat immunoglobulin (Ig) antibody (Ab) (Dynabeads M-450; Dynal, Oslo, Norway) at a bead-to-cell ratio of 2:1. The indicated numbers of BMC were infused intravenously into the tail veins of recipients about 6 h after irradiation. Survival rates were determined for groups of 20 recipients by daily monitoring. For adoptive cell transfer, indicator recipients were irradiated with 6 Gy and received the indicated numbers of unseparated, T-cell subset-depleted, or sorted pulmonary infiltrate lymphocytes in place of BMC. The in vitro depletion of CD8 and CD4 T cells was achieved by two cycles of treatment with the relevant MAbs and complement (47). Recipients were infected with 105 PFU of purified murine CMV (25) strain Smith (ATCC VR-194), injected subcutaneously in the left hind footpad about 2 h after BMT or T-cell transfer.
Determination of virus titers in organs.
The titer of infectious virus in organs was determined by a plaque assay performed with centrifugal enhancement of infectivity as described previously (25). In brief, organs were frozen and thawed to disrupt the cells and were homogenized by passage through a steel mesh. Appropriate dilutions of the homogenate were used in duplicate to infect permissive murine embryonic fibroblasts grown to near-confluent monolayers in flat-bottomed 48-well Falcon plates. Infection was performed at a centrifugal force of 1,000 × g for 30 min at 20°C. A methylcellulose overlay medium served to prevent the formation of secondary plaques. Primary plaques were counted after an incubation of the cultures for 4 to 5 days. The virus titers represent PFU per organ and are indexed as PFU* to indicate centrifugal enhancement.
Two-color immunohistochemistry for the analysis of tissue infection and T-cell infiltration.
Livers were fixed with phosphate-buffered saline (PBS; pH 7.4) containing 4% (vol/vol) formalin. The tissue was then processed by standard procedures for paraffin embedding. Sections of 2 μm were dewaxed in xylene and subjected to two-color immunohistochemistry essentially as described recently (18). In brief, T cells expressing CD3ɛ were labeled by an incubation of the sections for 1 h with a rat IgG1 MAb, clone CD3-12. Rat IgG1 (catalog no. 344 71A; Pharmingen, San Diego, Calif.) was used for the isotype control. The staining was performed by using a biotinylated goat anti-rat Ig Ab and the avidin-biotin-peroxidase complex with diaminobenzidine tetrahydrochloride as the substrate. The staining was enhanced by ammonium nickel sulfate hexahydrate, resulting in a black precipitate. The viral IE1 protein pp89 (21) was labeled by incubation for 1 h with MAb CROMA 101 (murine IgG1), followed by staining with rabbit anti-mouse Ig Ab and the alkaline phosphatase–anti-alkaline phosphatase (APAAP) complex with new fuchsin as the substrate, yielding a red precipitate. The isotype control was performed with mouse IgG1 (catalog no. X-0931; Dako, Hamburg, Germany). Counterstaining was done with hematoxylin. Infected cells as well as liver-infiltrating cells were counted for representative, randomly selected 10-mm2 areas of tissue.
Detection of viral DNA by ISH.
Deparaffinized sections of liver and spleen were subjected to in situ hybridization (ISH) as described previously (29). In essence, viral DNA accumulated in an intranuclear inclusion body in infected cells was stained with a digoxigenin-11-dUTP-labeled probe of 1,534 bp specific for the murine CMV gB gene. Labeling was performed with alkaline phosphatase-conjugated antidigoxigenin Ab with new fuchsin as the substrate. Counterstaining was done with hematoxylin.
Isolation of lymphocytes from pulmonary infiltrates.
Lymphocytes were isolated from the lung parenchyma by using a modification (18) of the method described by Holt et al. (17). Mice were lethally anesthetized by inhalation of carbon dioxide. For removing intravascular leukocytes, the vascular bed of the lungs was perfused with 5 to 10 ml of PBS devoid of Ca2+ and Mg2+ and containing heparin (10 U/ml). Alveolar leukocytes were removed by bronchoalveolar lavage. The lungs were then excised, and trachea, bronchi, bronchioles, and peritracheal as well as hilar lymph nodes were discarded. The lung parenchyma was minced, and a single-cell suspension was prepared by collagenase-DNase digestion. Specifically, tissue pooled from several lungs was incubated under permanent stirring at a volume of 5 ml per lung for 1 h at 37°C in high-glucose Dulbecco’s modified Eagle’s medium (catalog no. 41965-039; Gibco BRL, Eggenstein, Germany) supplemented with 10% fetal calf serum, penicillin, and streptomycin and containing type I collagenase (200 U/ml; Biochrom, Berlin, Germany) and type I DNase (50 μg/ml; DN-25; Sigma). Cells were washed and resuspended in RPMI (catalog no. 31870-025; Gibco BRL) supplemented as described previously (18). Lymphocytes were enriched by density gradient centrifugation for 30 min at 760 × g on Ficoll (1.077 g/ml; Sigma).
Cytofluorometric analysis.
Cell surface antigen expression was analyzed by fluorescence-activated cell sorting (FACS) with a FACSort (Becton Dickinson, San Jose, Calif.), using CellQuest software (Becton Dickinson) for data processing. Compensation of the overlap in the emission spectra of fluorescent dyes was done throughout. For all measurements, a threshold was set in the forward scatter (FSC)-versus-side scatter (SSC) plot to exclude events of the size of erythrocytes and to exclude dead cells. If not indicated otherwise, a lymphocyte gate was set in the FSC-versus-SSC plot to exclude macrophages and residual granulocytes from analysis.
Spleen cells were prepared according to established procedures, including lysis of erythrocytes in Gey’s buffer. Pulmonary infiltrate cells and BMC were isolated as described above. Cells were thoroughly washed in FACS buffer (Dulbecco’s PBS devoid of Ca2+ and Mg2+ [pH 7.4], supplemented with 0.4% [wt/vol] bovine serum albumin, 10 mM EDTA, 20 mM HEPES, and 0.05% NaN3). Nonspecific binding sites were saturated by incubation of the cells in blocking solution, which is FACS buffer supplemented with 1.6% (wt/vol) bovine serum albumin, 2 mg of rabbit serum Ig per liter, and 2 mg of rat anti-mouse FcγII/III receptor MAb (CD32/CD16; IgG2b; clone 2.4G2, catalog no. 01241; Dianova, Hamburg, Germany) per liter.
(i) Analysis of chimerism in BM.
Ld was labeled indirectly with mouse anti-mouse H-2Ld MAb (IgG2a; clone 30-5-75, catalog no. CL9011-A; Cedarlane, Hornby, Ontario, Canada) followed by polyclonal fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG2a Ab (catalog no. M32301; Caltag, Burlingame, Calif.).
(ii) Analysis of T-cell chimerism.
Three-color labeling with fluorochromated antibodies was performed in the following order. (i) Ld or Dd cell surface molecules were labeled. Ld was labeled as described above; labeling of Dd was performed directly with FITC-conjugated mouse MAb anti-mouse H-2Dd MAb (IgG2a; clone 34-5-85, catalog no. 9009F; Cedarlane). (ii) T-cell receptor (TCR) α/β was labeled with phycoerythrin (PE)-conjugated hamster anti-mouse TCR α/β MAb (clone H57-597, catalog no. 01305A; Pharmingen). (iii) Labeling of surface CD8 and CD4 was performed with duochrome (RED613)-conjugated rat anti-mouse CD8a MAb (IgG2a; clone 53-6.7, catalog no. 19870-021; Gibco BRL) and CD4 (IgG2a; clone H129.19, catalog no. 19862-028; Gibco BRL), respectively.
(iii) Determination of CD8/CD4 ratios.
Three-color labeling was performed with FITC-conjugated rat anti-mouse CD8a MAb (IgG2a; clone 53-6.7, catalog no. 1353; Becton Dickinson), PE-conjugated hamster anti-mouse TCR α/β MAb, and duochrome-conjugated rat anti-mouse CD4 MAb. The analysis of CD8 and CD4 expression was restricted to α/β T cells by setting an electronic gate on signals with positive PE fluorescence.
Sorting of donor-derived and recipient-derived CD8 T cells.
CD8 T cells of donor or recipient origin were preparatively purified by a combination of positive immunomagnetic selection and cytofluorometric cell sorting. Cell washing steps and incubations were performed with FACS buffer devoid of NaN3.
(i) Immunomagnetic enrichment of CD8 T cells.
Ficoll gradient-enriched lymphocytes derived from pulmonary infiltrates of BMT chimeras were first incubated with blocking solution devoid of NaN3 (ca. 3 × 107 cells in 90 μl) and 10 μl of superparamagnetic 50-nm-diameter beads (MicroBeads, catalog no. 481-01; Miltenyi Biotec Systems, Bergisch-Gladbach, Germany) and then passed through a magnetic separation column (MiniMacs separation unit, column type MS, catalog no. 422-01; Miltenyi Biotec Systems) to remove debris and cells binding nonspecifically to the magnetic beads. Effluent cells were collected, incubated with beads (10 μl per ca. 2 × 107 cells in 90 μl of FACS buffer) that were conjugated with rat MAb (IgG2a; clone 53-6.7) directed against mouse CD8a (MicroBeads, catalog no. 494-01; Miltenyi Biotec Systems), and separated in the magnetic column. It should be noted that the load of beads per cell is below the threshold critical for functional blocking of CD8 molecules. The CD8-negative effluent cells were discarded. The positively selected CD8-positive cells were recovered by elution with FACS buffer after disconnection of the magnetic field. The purity of the cells was determined by two-color cytofluorometric analysis. For this, an aliquot of the cell yield was labeled with mouse anti-mouse H-2Ld MAb (IgG2a; clone 30-5-75), FITC-conjugated goat anti-mouse IgG2a Ab, and PE-conjugated rat anti-mouse CD8a MAb (clone 53-6.7, catalog no. 1271 237; Boehringer, Mannheim, Germany).
(ii) Cytofluorometric cell sorting.
The CD8 T cells derived from the immunomagnetic sorting were labeled indirectly with mouse anti-mouse H-2Ld MAb and FITC-conjugated goat anti-mouse IgG2a Ab. Sorting was performed at a sort rate of ca. 10,000 cells/min with a FACSort equipped with a cell concentrator module (Becton Dickinson). The purity of the sorted cell populations was determined by two-color analytic cytofluorometry. For this, aliquots of the sorted cells were additionally labeled with PE-conjugated hamster anti-mouse TCR α/β MAb. Further aliquots were used to determine the cytolytic activity. Finally, the remaining cells were used for adoptive transfer.
Cytolytic assays.
For measuring the lytic activity of cytolytic T lymphocytes (CTL), standard 4-h 51Cr release assays were performed at the indicated effector/target cell ratios with a constant number (103) of 51Cr-labeled target cells and graded numbers of effector cells in 0.2-ml round-bottomed 96-microwell plates. Throughout, reported cytolytic activity represents the mean percentage of specific 51Cr release from three replicate microcultures.
(i) Antigen-independent assessment of cytolytic activity by TCR α/β-redirected lysis.
The strategy of redirected lysis (24) was used to measure the total cytolytic activity of CTL populations. For this, Fc receptor-expressing P815 cells (ATCC TIB-64; mastocytoma cell line derived from the mouse strain DBA/2; H-2d) were armed with Abs by incubation for 15 min at 20°C with an optimized dose of hamster MAb (IgG) specific for murine TCR α/β (clone H57-597, catalog no. 01301D; Dianova) before use as target cells.
(ii) Assessment of HvG-reactive cytolytic activity.
CTL activity directed against Ld was monitored with the Ld-expressing target cells P815 and the P815-derived transfectants P815-B7 and P815-Fas, expressing B7-1 (CD80) (1, 2) and Fas (CD95) (43), respectively. The transfectant cell lines were propagated in selection media containing 1 mg of Geneticin (G418) per ml and 1 mM l-histidinol, respectively. The expression of CD80 and CD95 was verified by cytofluorometric analysis using FITC-conjugated mouse anti-human B7-1 MAb (clone BB1, catalog no. 33514; Dianova) and FITC-conjugated hamster anti-mouse Fas MAb (clone Jo2, catalog no. 15404D; Dianova), respectively.
RESULTS
Establishment of T-cell chimerism after MHC-disparate BMT following incomplete lymphohematoablation.
By definition, recipients of a nonautologous BMT become chimeric in that their own hematopoietic cells are replaced by donor-derived hematopoietic cells, whereas stromal and parenchymal cells of all tissues remain of recipient genotype. It is known that incomplete hematoablation of BMT recipients can lead to a donor-recipient chimerism also within the population of hematopoietic cells and their progeny. This situation is referred to as mixed chimerism, as opposed to the complete chimerism established after complete hematoablation of the recipients followed by lymphohematopoietic repopulation exclusively with donor-derived cells. Notably, a stable mixed chimerism is associated with donor-specific central transplantation tolerance (for reviews, see references 5, 31, and 49).
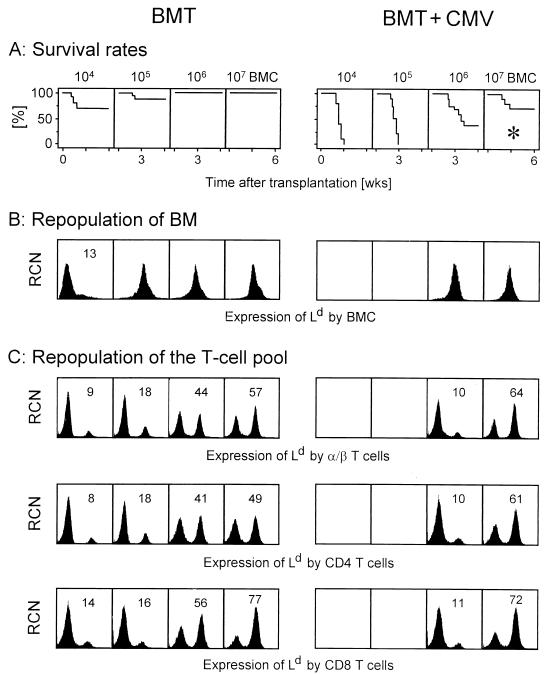
We therefore wished to investigate the contributions of donor-derived and recipient-derived hematopoietic cells to the lymphohematopoietic reconstitution in our experimental model of MHC-disparate BMT using BALB/c mice (MHC class I molecules Kd, Dd, and Ld) as donors and sublethally (6 Gy) irradiated BALB/c-H-2dm2 mice (MHC class I molecules Kd and Dd only) as recipients. In the absence of infection, a low amount of transplanted donor BMC suffices for achieving survival of all recipients (Fig. 1A, left). After transplantation of 104 donor BMC, the recipient BM is repopulated primarily by endogenous reconstitution with recipient-derived, Ld-negative hematopoietic cells, but higher numbers of donor BMC compete with and replace endogenous reconstitution, resulting in complete repopulation of recipient BM with donor-derived, Ld-positive hematopoietic cells (Fig. 1B, left). Thus, by the definition stated above, the recipients became complete chimeras with regard to the BM. It is instructive to note that B220 (CD45)-positive B cells and all cells of the myelomonocytic lineage, which includes Gr-1-positive granulocytes and CD11b-positive monocytes, were also found to be of donor origin (data not shown). By contrast, the reconstituted T-cell population, measured for the spleen, was found to be chimeric over the whole range of transplanted donor BMC. This chimerism represented a very stable balance, since 10-fold increases in the numbers of donor BMC resulted in only moderate shifts of the chimerism towards donor-derived T cells (Fig. 1C, top left). This chimerism was not T-cell subset specific but applied likewise to CD4 T cells (Fig. 1C, center left) and to CD8 T cells, with a slight preponderance of donor-derived CD8 T cells (Fig. 1C, bottom left). Specifically, there was no evidence for a selective amplification of recipient-derived CD8 T cells, as one would expect in case of an MHC class I-directed HvG response. The extrapolation predicts that unreasonable numbers of donor BMC would be needed to completely replace recipient-derived T cells. Thus, by the definition presented above, the recipients became mixed chimeras with regard to the T-cell pool. Such a difference in the chimeric state of different cell populations is referred to as a split chimerism. The origin of the recipient-derived T cells is an issue beyond the scope of this report. Regarding this question, it may be helpful to outline the current state of knowledge. Apparently, these cells cannot be derived from BM that is occupied by donor-derived hematopoietic cells. We have evidence that they are derived from radiation-resistant thymic progenitors, since their reconstitution can be prevented by thymectomy but not by in vivo depletion of peripheral T cells of either subset (not shown).
FIG. 1.
Establishment of chimerism in an HvG setting of MHC class I-mismatched BMT after incomplete lymphohematoablative conditioning. BALB/c-H-2dm2 recipients (uninfected [BMT] and infected with murine CMV on the day of BMT [BMT + CMV]) were γ irradiated with a sublethal dose of 6 Gy and then reconstituted with the indicated graded numbers of BMC derived from BALB/c donors. (A) Kaplan-Meier survival plots for groups of 20 mice. The asterisk marks the conditions of BMT under which all subsequent experiments were performed. (B) Origin of BMC in the repopulated recipient BM measured for 6-week survivors. Femoral BMC of three mice were pooled for analysis. Positive expression of Ld identifies donor (BALB/c)-derived BMC. The histograms are based on the analysis of 10,000 BMC. RCN, relative cell number. Expression of Ld (abscissa) is given in log FITC fluorescence (FL-1) intensity. (C) Analysis of chimerism among T cells and T-cell subsets derived from the spleens of 6-week survivors. Lymphocytes derived from three spleens were pooled for analysis. Individual testing of aliquots taken before forming the pools gave essentially the same results (not depicted). Three-color fluorescence (FL) analyses were performed for the marker combinations FITC (FL-1)-Ld plus PE (FL-2)-TCR α/β plus RED613 (FL-3)-CD4 or RED613 (FL-3)-CD8. A first gate was set on lymphocytes; a second gate was set on α/β T cells positive in FL-2 (top), on CD4 T cells simultaneously positive in FL-2 and FL-3 (center), and on CD8 T cells simultaneously positive in FL-2 and FL-3 (bottom). FL-1 histograms are shown for 10,000 gated cells. For chimeric cell populations, the percentage of donor-derived, Ld-positive cells is indicated. Controls of Dd expression in the chimeras and of Ld expression in BALB/c-BALB/c isochimeras gave single peaks with positive FL-1 (not shown); these controls were made to make sure that we were not misled by MHC class I-negative cells and by Ld-low/high-expressing cells, respectively.
How does murine CMV infection interfere with this highly balanced system? In accordance with previous findings in models of MHC-compatible sex-matched (18) as well as sex-mismatched BMT (48), murine CMV infection dramatically increased the death rates, but sufficiently high numbers of BMC protected against fatal CMV disease (Fig. 1A, right). Compared with syngeneic BMT performed with BALB/c mice as donors and recipients under otherwise identical conditions (18), the death rates were found to be slightly increased, suggesting a reduced efficacy of antiviral control as a result of the MHC class I mismatch. Fatal CMV disease is characterized by viral histopathology in almost all vital organs (34) and is associated with BM aplasia (29, 30). As we have shown recently, murine CMV infection inhibits the engraftment of low numbers of donor BMC, whereas high numbers of donor BMC support hematopoietic reconstitution by preventing hemopoietin deficiency of recipient stroma (48). Accordingly, the BM of survivors was found to be repopulated with donor-derived hematopoietic cells (Fig. 1B, right). Regarding T cells, the reconstitution of donor-derived T cells was inhibited by viral pathogenesis in the BM, but this effect was overcome by transplantation of 107 BMC. Again, there was no marked difference between the two T-cell subsets (Fig. 1C, right).
Control of murine CMV infection in the lungs correlates with infiltration of CD8 T cells.
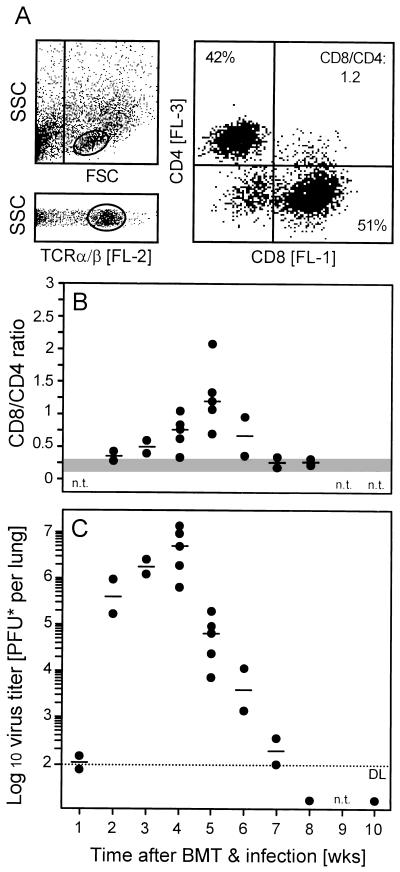
Among multiple organs infected by murine CMV in immunocompromised recipients (34), the lungs represent a major organ site of viral pathogenesis, latency, and recurrence (3, 25, 39). Previous work has identified CD8 T cells as the principal antiviral effector cells controlling murine CMV in the lungs (18, 39). Specifically, the model of syngeneic BMT with BALB/c donors and recipients had revealed a massive infiltration of the infected lungs preferentially by CD8 T cells, and the peak of this infiltration was found to correlate with the resolution of productive infection (18). Here we have performed the corresponding experiments for the MHC-disparate setting (6 Gy, 107 BMC; corresponding to Fig. 1A, asterisk) with MHC class I Ld-negative recipients (Fig. 2). At the peak of the infiltration, CD8/CD4 ratios ranged between 0.7 and 2.1 in infected lungs, as opposed to 0.15 to 0.3 in uninfected lungs (Fig. 2A and B). In absolute terms, yields of T lymphocytes per lung were 1.2 × 106 to 2.2 × 106 and 0.1 × 106 to 0.3 × 106 in infected and uninfected lungs, respectively. This massive expansion of the pulmonary T-cell pool coincided with the resolution of lung infection (compare Fig. 2B and C). In essence, the previous experience using syngeneic BMT was thus fully reproduced, with the notable difference that CD8 T-cell infiltration and resolution of lung infection were both delayed by about 1 week. In accordance with the slight increase in the death rates (Fig. 1A), this finding indicated a lower efficacy of antiviral control in the MHC-disparate setting. However, the important finding is that the infiltrating cells did not fail to control the infection in the Ld-negative lung parenchyma.
FIG. 2.
Preferential recruitment of CD8 T cells to the infected lungs and control of pulmonary infection. (A) Three-color cytofluorometric analysis of lung infiltrate lymphocytes in the combination FITC (FL-1)-CD8 plus PE (FL-2)-TCR α/β plus RED613 (FL-3)-CD4. A lymphocyte gate was set in the FSC-versus-SSC plot, representing cell size and cell granularity, respectively (upper left); a second gate was set on positive FL-2 to select cells expressing TCR α/β (lower left). (Right) Expression of CD4 and CD8 among the FL-2-positive α/β T cells, shown in a two-dimensional dot plot of FL-3 and FL-1 fluorescence, respectively. The percentages of CD4 and CD8 T cells as well as the CD8/CD4 ratio are indicated. The analysis of the subpopulations was based on 20,000 α/β T cells. (B) Kinetics of lung infiltration. For independent but analogous transplantations, CD8/CD4 ratios within pulmonary α/β T cells were determined in weekly intervals. Each closed circle represents the analysis at the indicated time point of a particular transplantation. Data refer to a pool of pulmonary infiltrate cells derived from five recipients. The median values for the independent transplantations are indicated by dashes; the shaded area indicates the range of CD8/CD4 ratios observed in uninfected recipients under otherwise corresponding conditions. n.t., not tested. (C) Kinetics of murine CMV replication in the lungs. Virus titers in the lungs of infected recipients were determined for the transplantations shown in panel B. Closed circles represent the median values of the virus titers of three recipients per transplantation and time point. The median values for the independent transplantations are marked by dashes. The dotted line indicates the detection limit (DL) of the plaque assay. n.t., not tested.
CD8 T cells in pulmonary infiltrates have the propensity to resolve murine CMV infection in various target tissues.
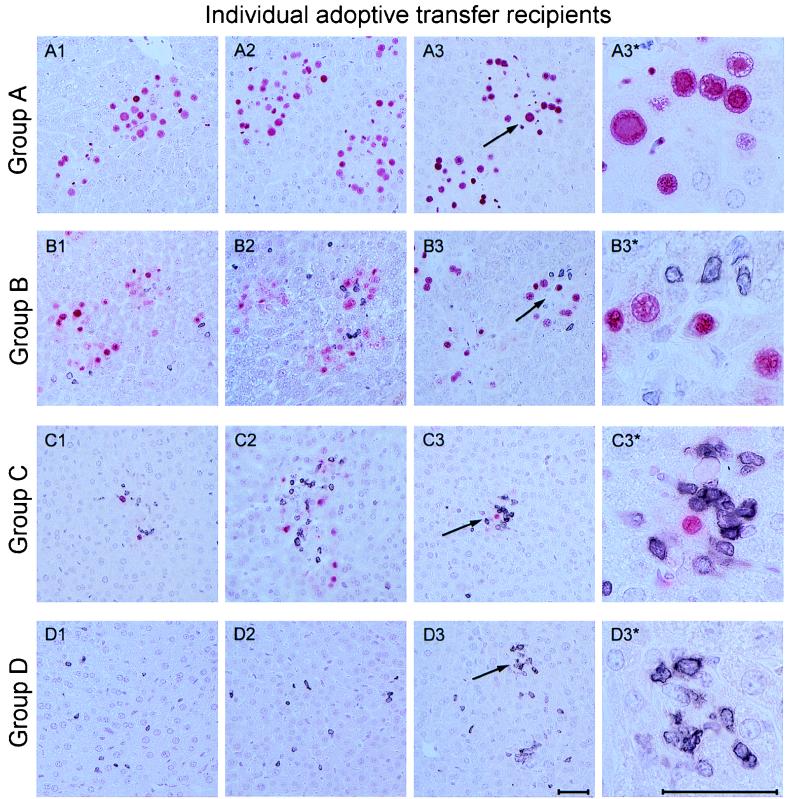
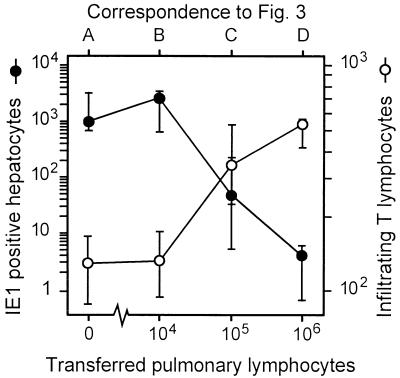
The coincidence between resolution of productive lung infection and the massive expansion of CD8 T cells in the lungs strongly suggested an antiviral function of these CD8 T cells, but correlative evidence is not a formal proof. We therefore directly tested the in vivo antiviral function of lung infiltrate cells by an approach of preemptive cytoimmunotherapy of murine CMV organ disease. Lymphocytes were isolated from pulmonary infiltrates at 5 weeks after BMT and infection and were adoptively transferred into immunocompromised and infected BALB/c indicator recipients. Protection against viral histopathology in the indicator recipients was monitored on day 14 after the cell transfer by two-color immunohistology of liver tissue, simultaneously detecting infected, IE1 protein-expressing liver cells and liver-infiltrating CD3ɛ-positive T lymphocytes (Fig. 3). In the experimental group with no cells transferred (group A), foci of infection were frequent and caused a viral hepatitis, characterized by plaque-like tissue lesions with no prominent inflammatory infiltrates. Note the absence of nucleated cells in the center of the plaques, which indicates a cytocidal infection of the liver. The main target cell of murine CMV infection in the liver is the hepatocyte (Fig. A3* [high resolution] shows details), while bile duct epithelial cells were usually not infected (not shown). As we anticipated from the design of the experiment, only few CD3ɛ-positive T lymphocytes were found in the liver parenchyma. Transfer of pulmonary infiltrate cells was associated with a cell dose-dependent infiltration of the recipient liver by CD3ɛ-positive T lymphocytes. This infiltration correlated with a decline in the number of infected cells and, as a consequence, with prevention of tissue destruction (groups B to D). Notably, infiltrating T cells were not randomly distributed in the liver parenchyma but were arranged in clusters, forming inflammatory foci that sequester the infected hepatocytes (Fig. 3C1 to C3 [overview] and C3* [details]). These inflammatory foci are reminiscent of foci formed in infected liver tissue by natural killer (NK) cells in another model of murine CMV hepatitis (45), suggesting that trafficking of T cells and NK cells to the infected liver may be regulated similarly. After transfer of 106 cells, the livers were found to be free of virus, and there were no signs of histopathology except a remainder of T cells (Fig. 3, group D). Quantitative immunohistology, compiling cell counts for three individual recipients per group and for representative areas of liver tissue sections, clearly documents the inverse relation between infection of liver tissue and infiltration by CD3ɛ-positive T cells (Fig. 4).
FIG. 3.
Two-color immunohistological analysis of liver tissue infection and T-cell infiltration. Pulmonary lymphocytes derived from lung infiltrates of infected chimeras at 5 weeks after BMT were tested for their antiviral and protective ability by intravenous adoptive transfer into infected BALB/c indicator recipients that were immunocompromised by 6 Gy of γ irradiation. The analysis was performed on day 14 after cell transfer. Group A, controls given no pulmonary T cells; groups B, C, and D, recipients of adoptive transfer of 104, 105, and 106 pulmonary lymphocytes, respectively. Panels A1 through D3 represent liver sections from individual recipients, three per experimental group. Infected cells, mostly hepatocytes, are identified by red nuclear staining of the IE1 protein pp89 of murine CMV. Infiltrating T cells are identified by black staining of CD3ɛ. Arrows mark areas of interest shown in overview (A3 through D3) and resolved to greater detail (A3* through D3*). Note the cytomegalic cells with prominent intranuclear inclusion bodies in panel A3* and the inflammatory foci visible under the conditions of group C. Photographs cover 0.08 mm2 (overview panels) and 0.005 mm2 (detail panels) of liver sections. The bar markers represent 50 μm.
FIG. 4.
Inverse relation between T-cell infiltration and infection of liver tissue. The two-color immunohistology shown in Fig. 3, groups A to D, was analyzed quantitatively by counting infected, IE1 protein-expressing hepatocytes and infiltrating CD3ɛ-expressing T lymphocytes for representative 10-mm2 areas of liver sections. Symbols represent median values, and bars indicate ranges of the counted cell numbers for three recipients per experimental group.
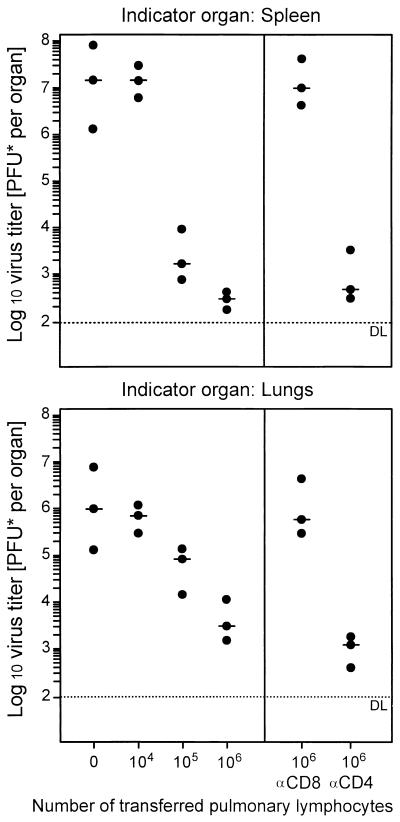
The antiviral function of the pulmonary infiltrate cells was not restricted to the liver. Upon adoptive transfer, they limited virus replication, as measured by virus titers, in the spleen as well as in the lungs (Fig. 5). The apparently higher efficacy of antiviral control in the spleen than in the lungs is not peculiar to pulmonary infiltrate-derived T cells but is a consistent finding made previously also with T cells derived from lymphoid tissues (reviewed in reference 22). In accordance with these earlier studies, the antiviral function was abolished by depletion of CD8 T cells but not of CD4 T cells (Fig. 5, right). This result identified the antiviral effector cells in lung infiltrates as CD8 T cells. In conclusion, this set of experiments demonstrated the antiviral function of pulmonary CD8 T cells.
FIG. 5.
The antiviral function of pulmonary infiltrate-derived lymphocytes is accomplished by CD8-positive effector cells. The dose-dependent antiviral effect of pulmonary lymphocytes upon adoptive transfer of graded cell numbers is documented for the spleens (top) and lungs (bottom) of infected BALB/c indicator recipients immunocompromised by 6 Gy of γ irradiation. The antiviral T-cell subset was identified by adoptive transfer of 106 pulmonary lymphocytes depleted of either CD8 or CD4 T cells by treatment with anti-CD8 (αCD8) or anti-CD4 (αCD4) MAb and complement, respectively. Closed circles represent virus titers per organ for three recipients per experimental group. The median values are marked by dashes; each dotted line indicates the detection limit (DL) of the plaque assay.
Recipient-derived CD8 T cells are preferentially recruited to the lungs.
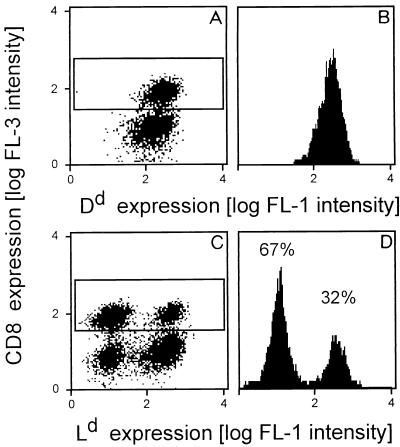
The chimerism of the T-cell pool detected in the spleen (Fig. 1C) raised the question of which T cells were recruited to the lungs. As the extremes, either donor-derived or recipient-derived T cells could be recruited selectively. We addressed this question by a cytofluorometric analysis of donor-recipient chimerism among CD8 T cells isolated from the infected lungs at the peak of infiltration. The pulmonary CD8 T-cell pool was found to be chimeric (Fig. 6), but notably, recipient-derived CD8 T cells were in this case preponderant. This finding indicated a preferential involvement of recipient-derived CD8 T cells in the pulmonary immune response.
FIG. 6.
Chimerism among CD8 T cells in pulmonary infiltrates. Pulmonary lymphocytes were isolated at the peak of infiltration after BMT (6 Gy, 107 BMC) and infection. Three-color cytofluorometric analysis was performed for the combination FITC (FL-1)-Dd or FITC (FL-1)-Ld plus PE (FL-2)-TCR α/β plus RED613 (FL-3)-CD8. A gate was set on lymphocytes, and the analysis was restricted to α/β T cells by a second gate set on positive FL-2. (A and C) Two-dimensional dot plots of FL-3 (CD8) versus FL-1 (Dd [A] and Ld [C]) for 20,000 α/β T cells. CD8-positive cells are marked by a frame. (B and D) Histograms of FL-1 for the framed FL-2- and FL-3-positive CD8 T cells. The percentages of Ld-negative, recipient-derived and Ld-positive, donor-derived cells are indicated.
Absence of HvG-reactive CTL in lung infiltrates.
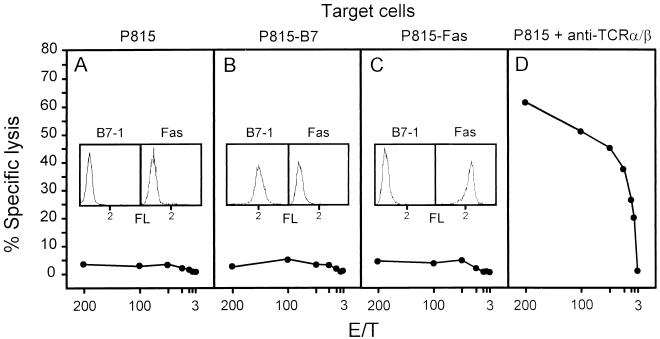
One possible reason for a selective amplification of recipient-derived T cells in the infected lungs could be an HvG response directed against the Ld molecule expressed by the infiltrating donor-derived cells. Such a response could be supported by the cytokine milieu generated during the CMV-induced inflammatory process. We have investigated this possibility by ex vivo testing of Ld-specific CTL activity in pulmonary infiltrates at the peak of the infiltration. Pulmonary T lymphocytes did not lyse Ld-expressing P815 target cells (Fig. 7A). Thus, lung infiltrates did not include HvG-reactive, ex vivo cytolytic effector cells. While engagement of the TCR-CD3 complex suffices for eliciting the cytolytic effector function of mature CTL, less mature CTL precursors can be triggered to exert cytolytic activity only if target cells express B7-1 (CD80) for costimulatory signalling by B7-CD28 interaction (1, 2). Since P815 mastocytoma cells do not express B7, CTL precursors were not detected by the assay used. Donor-derived activated B and T cells as well as monocytes in the infiltrates are potential targets for an HvG reactivity of B7-dependent effector cells because they express Ld as well as B7. Notably, however, provision of B7 by using the transfectant P815-B7 as the target cell in the cytolytic assay did not enable detection of B7-dependent Ld-specific effector cells in the lung infiltrates (Fig. 7B). Finally, an HvG reactivity operating via Fas-Fas ligand interaction inducing apoptosis (43) was excluded by the use of transfectant P815-Fas as the target cell (Fig. 7C). These negative results raised the question of whether CTL activity had developed at all within lung infiltrates. Redirected lysis of Fc receptor-expressing P815 target cells armed with antibody specific for TCR or CD3 (24) is a method for detecting all CTL regardless of their antigen specificity. The TCR α/β redirected lysis assay indeed revealed a very strong CTL activity within lung infiltrates (Fig. 7D). In conclusion, lung infiltrates contained CTL, but HvG-reactive CTL were not present in detectable numbers. We therefore concluded that the amplification of recipient-derived T cells resulted from their participation in the antiviral response.
FIG. 7.
Absence of MHC class I Ld-specific, HvG-directed cytolytic activity in the lungs of mixed chimeras. Pulmonary infiltrate lymphocytes isolated at the peak of lung infiltration were tested for ex vivo cytolytic activity at the indicated effector-to-target (E/T) cell ratios on target cells expressing Ld (A; parental P815 mastocytoma), Ld plus B7-1 (B; transfectant P815-B7), and Ld plus Fas (C; transfectant P815-Fas). Insets show the cytofluorometric analyses verifying the expression of B7-1 and Fas by the respective transfectants, as well as their absence on parental P815. FL, log FITC fluorescence intensity. The total CTL activity contained in the tested lymphocyte population was determined by the TCR redirected lysis assay using a MAb directed against TCR α/β bound to Fc receptors that are expressed constitutively by P815 (D). All target cells expressed surface Ld as detected by cytofluorometry, and all were lysed by a CTL line specific for Ld (not shown).
Sorted donor-derived and recipient-derived pulmonary CD8 T cells are both cytolytically active.
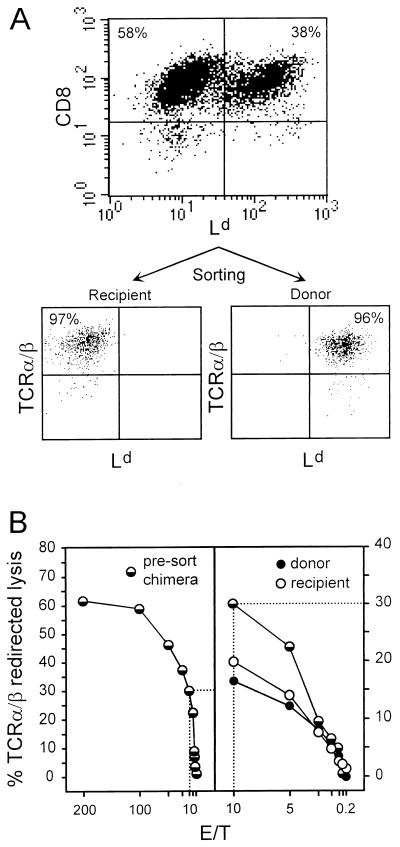
For testing the functional properties of the two sets of CD8 T cells, they had to be separated and purified preparatively. To this end, CD8 T cells among pulmonary infiltrate lymphocytes were positively enriched to >95% purity by immunomagnetic selection. The recovered cells were then labeled with anti-Ld antibody and were separated into Ld-negative recipient-derived and Ld-positive donor-derived CD8 T cells by cytofluorometric sorting. Cytofluorometric reanalysis documented the efficacy of the separation and verified that all CD8-positive cells coexpressed TCR α/β (Fig. 8A). The cytolytic activity of the sorted sets of CD8 T cells was tested by the TCR α/β redirected lysis assay (Fig. 8B). Compared with the presorting cytolytic activity of the chimeric CD8 T-cell population (Fig. 8B, left panel), there was some loss of activity by the sorting procedure, as one would expect, but the sets of CD8 T cells showed comparable activities (Fig. 8B, right panel). It should be noted that control experiments performed with Ld-positive CD8 T cells derived from BALB/c recipients of syngeneic BMT did not reveal any influence of anti-Ld antibody binding on the function of the cells (not shown).
FIG. 8.
Cytolytic activity of sorted recipient-derived and donor-derived CD8 T cells. (A) Pulmonary lymphocytes were isolated at the peak of infiltration, and CD8-positive cells were enriched by immunomagnetic selection. (Top) The efficacy of the positive selection was monitored for an aliquot of the cells by a two-color cytofluorometric analysis of CD8 (PE, FL-2) versus Ld (FITC, FL-1) expression performed for 20,000 cells with no preselection of gates. (Bottom) The majority of the CD8 T cells were labeled for Ld only and were then sorted into Ld-negative and Ld-positive sets. The efficacy of the sorting was monitored for 2,000 cells of each set by two-color cytofluorometric analysis of TCR α/β (PE, FL-2) and Ld (FITC, FL-1) expression performed with no preselection of gates. (B) The cytolytic activity of pulmonary CD8 T cells was determined by TCR α/β redirected lysis at the indicated effector-to-target (E/T) cell ratios. (Left) Cytolytic activity of the chimeric CD8 T-cell population after immunomagnetic purification but before the cytofluorometric sorting; (right) cytolytic activity of the sorted cells. For comparison, the presort activity is included for the part of the titration marked by dotted lines.
MHC preferences in the antiviral function of pulmonary CD8 T cells.
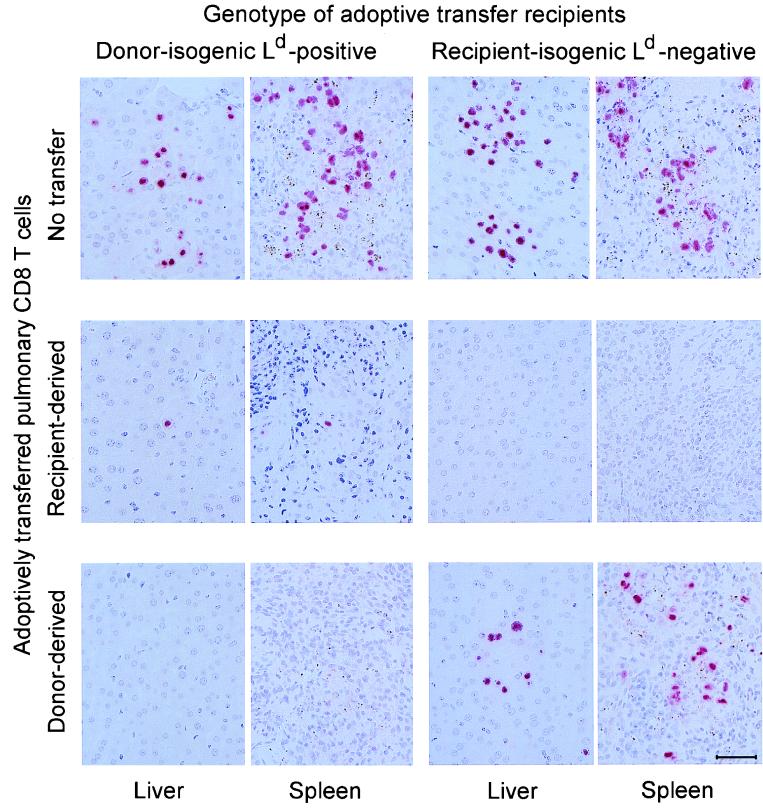
The in vivo function of the sorted Ld-positive donor-derived and Ld-negative recipient-derived CD8 T cells was tested by adoptive transfer into infected indicator mice of either MHC genotype, that is, into donor-matched, Ld-positive BALB/c mice and into recipient-matched, Ld-negative BALB/c-H-2dm2 mice. Since the recipient-derived CD8 T cells in the mixed chimeras did not include Ld-specific HvG-reactive CTL (Fig. 7), a GvH complication did not occur in Ld-positive indicator mice. Antiviral activity in liver and spleen was monitored by ISH detecting productively infected cells characterized by viral DNA accumulated in an intranuclear inclusion body (Fig. 9). In the absence of protecting cells, the degrees of infection and histopathology were comparable in both genotypes of indicator mice (Fig. 9, top row). Notably, recipient-derived pulmonary CD8 T cells protected indicator tissues of either genotype with similar efficacies (Fig. 9, center row). By contrast, donor-derived pulmonary CD8 T cells virtually failed to protect the recipient-matched indicator tissues (Fig. 9, bottom row), even though they were effectual in donor-matched indicator tissues. It should be noted that this failure was not absolute, but that higher numbers of donor-derived CD8 T cells were needed to control murine CMV also in the Ld-negative recipient (not shown).
FIG. 9.
Control of viral replication in tissues of adoptive transfer recipients by sorted donor-derived and recipient-derived pulmonary CD8 T cells. The in vivo antiviral activity of the sorted CD8 T cells was tested by adoptive transfer of 50,000 cells into immunocompromised, γ-irradiated (6 Gy) indicator recipients, which were chosen to be isogenic either with the original BMT donor (BALB/c) or with the original BMT recipient (BALB/c-H-2dm2). Virus replication in liver and spleen of the indicator recipients was monitored on day 14 after the cell transfer by ISH specific for a gB gene DNA sequence of murine CMV. The red staining visualizes infected cells identified by the accumulation of viral DNA in an intranuclear inclusion body. Each photograph covers a 0.05-mm2 area of tissue section. The bar marker represents 50 μm.
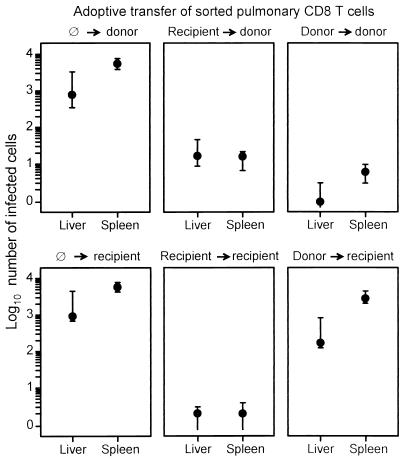
These findings were substantiated by counting the infected cells for a representative area of liver and spleen tissue sections and by documenting the variance for three individually tested indicator mice per experimental group (Fig. 10). The quantitative data gave us a more qualified view. In principle, CD8 T cells of either MHC genotype showed a preference for the MHC-matched indicator tissues, but this preference was far more pronounced for the Ld-positive donor-derived CD8 T cells.
FIG. 10.
MHC genotype-specific, differential antiviral function of sorted donor-derived and recipient-derived pulmonary CD8 T cells. The results of the histological ISH analyses shown in Fig. 9 for selected 0.05-mm2 areas of tissue were substantiated by counting the numbers of infected cells in representative 10-mm2 areas of liver and spleen sections. Closed circles represent median values, and bars indicate ranges of the counted cell numbers for three adoptive transfer recipients per experimental group.
Since the infected tissue cells in the mixed BMT chimeras are of recipient genotype, we conclude that control of murine CMV infection in their lungs and other organs was predominantly but not exclusively a function of recipient-derived, i.e., of endogenously reconstituted, antiviral CD8 T cells. As a consequence, we propose that the establishment of a mixed chimerism after BMT gives an advantage to the BMT recipient with respect to the control of CMV infection.
DISCUSSION
Despite routine preemptive antiviral chemotherapy, human CMV infection continues to be a major complication in patients receiving BMT. Even though the case-fatality rate of CMV disease, pneumonia in particular, is high also in patients receiving syngeneic BMT or a stem cell autograft, it is established clinical experience that minor and especially major histocompatibility antigen disparities are associated with an increased incidence of severe CMV disease (reviewed in reference 10). A pathogenic link between GvH disease and CMV infection is indicated by clinical data (10) and by murine models involving transfer of mature, immunoreactive allogeneic T cells into infected recipients (8, 9, 13). While CMV infection may promote GvH disease by inducing inflammatory cytokines or by altering the expression of MHC molecules and of cell adhesion molecules, recent work by Soderberg-Naucler et al. has provided evidence also for a cooperative effect in the opposite direction, namely for the reactivation of latent CMV by allogeneic stimulation (46).
If donor and recipient differ at an MHC locus, allogeneic stimulation can theoretically occur in either direction, resulting in simultaneous GvH and HvG responses. It is therefore difficult to dissect the contributions of GvH and HvG reactions to CMV pathogenesis in transplantation chimeras. This problem has prompted us to develop the murine model described herein, using a pair of mouse strains, BALB/c and BALB/c-H-2dm2, that differ by the presence and absence of the MHC class I molecule Ld, respectively. Depending on the choice of donor and recipient for the experimental BMT, GvH- and HvG-related complications can thus be analyzed separately. The present study was focused on the HvG version of this model, with the aim to characterize the influence of a singular MHC class I disparity on the course of murine CMV disease in the specific context of the reconstitution of antiviral immunity after BMT.
One notable finding from the data is the absence of an immunological HvG response in recipients after incomplete lymphohematoablation followed by MHC class-I disparate BMT. Specifically, the donor BMC mediated protection against death and repopulated the recipient with all hematopoietic lineages, including α/β T lymphocytes. However, over a wide range of experimental conditions, residual recipient T lymphopoiesis led to a chimeric T-cell population in the survivors. Since mixed chimerism can develop only after incomplete lymphohematoablation of the BMT recipient, one might ask whether the chosen setting of experimental BMT is appropriate as a model for clinical BMT. We think it is, because mixed chimerism is reportedly a frequent feature after clinical BMT, in particular after transplantation of T-cell-depleted BMC (15, 19, 28, 33). Interestingly, recent clinical data suggest a survival advantage of mixed chimeric patients (19).
In the model system described here, evidence against an immunological HvG response was provided by the long-term coexistence of donor-derived and recipient-derived T cells and by the apparent absence of Ld-specific ex vivo CTL in the pulmonary infiltrates. While the association between mixed chimerism and donor-MHC specific transplantation tolerance had been known since the pioneering work of Billingham et al. (4), the molecular mechanisms leading to transplantation tolerance had remained enigmatic. Only recently, the expression of Fas ligand (CD95L) on transplanted BMC was found to be essential for tolerance induction, relating tolerance to apoptotic deletion of graft-reactive cells (12).
An interplay between transplantation tolerance and viruses is an interesting possibility. Since CMV is a virus of known pathogenic relevance in BMT recipients, it is a primary candidate for such an interplay. The data presented herein show that murine CMV infection can alter the chimeric balance to the disfavor of the donor-derived repopulation, and this could reduce the tolerogenic donor cell dose. However, the murine model presented here did not provide evidence for CMV being a trigger of an acute HvG response. In conclusion, residual recipient T lymphopoiesis does not result in rejection of the BM graft.
What, then, are the consequences of T-cell chimerism for the control of CMV? Specifically, is mixed chimerism beneficial or deleterious for the infected recipient? We infer from the data that residual recipient T lymphopoiesis can indeed be beneficial by controlling CMV infection. A vital role for donor BMC in the hematopoietic repopulation became obvious from the dose-dependent improvement of the survival rates in both groups of recipients, uninfected as well as infected. Clearly, hematopoietic donor cells are of immediate need to overcome the BM aplasia caused by the hematoablative treatment in that they repopulate the recipient BM with all hematopoietic cell lineages. In addition, hematopoietic donor cells protect the recipient BM stroma against CMV-induced hemopoietin downregulation by an undefined mechanism that is unrelated to control of infection (48). Without these basic protective functions of the transplanted donor BMC, neither donor-derived nor recipient-derived immunocompetent T cells would be reconstituted. A significant participation of recipient-derived CD8 T cells in the antiviral response was first indicated by their preferential expansion in the infected lungs. Firm evidence was finally provided by cell sorting followed by the in vivo functional analysis of the purified donor-derived and recipient-derived CD8 T cells. Upon adoptive transfer into infected indicator mice, both sorted populations were effectual in the respective MHC-matched indicator tissues. However, whereas the Ld-negative recipient-derived CD8 T cells controlled the infection also in Ld-positive donor-isogenic tissues, the Ld-positive donor-derived CD8 T cells were much less efficient within Ld-negative recipient-isogenic tissues. Since the infected tissue cells of the mixed chimeras are of recipient genotype, we conclude that the control of murine CMV infection in the mixed chimeras was based preferentially on the endogenously reconstituted recipient-derived T cells.
This data imply that Ld-negative recipient-derived CD8 T cells recognized antigenic viral peptides presented by Kd and/or Dd molecules shared by donor and recipient, whereas the response by Ld-positive donor-derived CD8 T cells was focused on antigenic viral peptides presented by the Ld molecule that, in this model, was missing in recipient tissue. The phenomenon is likely to be related to the known role of Ld as the prevailing peptide presenter molecule in the H-2d haplotype (6, 32), a feature that has been explained by low loading of Ld with self peptides providing accessible binding sites for foreign peptides (26). Accordingly, the immunodominant IE1 nonapeptide of murine CMV is presented by Ld (38). Recent work has shown that IE1-specific CTL dominate the pulmonary CTL response to murine CMV after syngeneic BMT performed with BALB/c mice as donors and recipients, even though this dominance was relative rather than absolute (18). The data shown here do indicate that peptides presented by Ld, but not necessarily only the immunodominant IE1 peptide, account for a biologically relevant part of the CD8 T-cell response to murine CMV in the H-2d haplotype.
Since the tissue cells in the mixed chimeras did not express Ld, the focus of the donor-derived CD8 T cells to Ld implies that they have acquired immunodominance from an intrinsic viral peptide presentation by Ld within the population of donor cells. This is surprising, because productive murine CMV infection occurs primarily in stromal and parenchymal tissue cells (34). We must now propose that donor-derived lung infiltrate cells included a sufficient number of infected cells presenting viral peptides processed from endogenously synthesized proteins, or that they presented antigenic viral peptides generated by exogenous loading of the MHC class I pathway of antigen presentation (for a review, see reference 20) with viral proteins derived from the infected Ld-negative lung tissue cells. Work is in progress to identify and quantitate the antigenic viral peptides processed in the different compartments of the mixed chimeric lungs.
In conclusion, the mixed chimera model has shown that an MHC preference leading to an immunodominant antiviral response within the donor-derived cell population can prevent the donor CD8 T cells from protecting recipient tissues. In such a scenario, BMT recipients depend on the otherwise subdominant immune response that is provided by residual own lymphopoietic reconstitution. Since MHC-governed immunodominance of antigens is not the exception, our results may apply to other mixed chimeric systems as well. We therefore propose that establishment of a T-cell chimerism after MHC-disparate BMT is beneficial with respect to the control of infections.
ACKNOWLEDGMENTS
We thank L. L. Lanier, DNAX, Palo Alto, Calif., and E. R. Podack, University of Miami, Miami, Fla., for permission to use transfectants P815-B7 and P815-Fas, respectively. S. Jonjic, University of Rijeka, Rijeka, Croatia, provided CROMA antibody specific for the murine CMV IE1 protein.
This work was supported by grants to M. J. Reddehase by the Deutsche Forschungsgemeinschaft (projects RE 712/3-2 and RE 712/4-1) and Sonderforschungsbereich 311.
REFERENCES
- 1.Azuma M, Cayabyab M, Buck D, Philipps J H, Lanier L L. CD28 interaction with B7 costimulates primary allogeneic proliferative responses and cytotoxicity mediated by small, resting T lymphocytes. J Exp Med. 1992;175:353–360. doi: 10.1084/jem.175.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azuma M, Cayabyab M, Phillips J H, Lanier L L. Requirements for CD28-dependent T cell-mediated cytotoxicity. J Immunol. 1993;150:2091–2101. [PubMed] [Google Scholar]
- 3.Balthesen M, Messerle M, Reddehase M J. Lungs are a major organ site of cytomegalovirus latency and recurrence. J Virol. 1993;67:5360–5366. doi: 10.1128/jvi.67.9.5360-5366.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Billingham R E, Brent L, Medawar P B. Actively acquired tolerance of foreign cells. Nature (London) 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 5.Charlton B, Auchincloss H, Jr, Fathman C G. Mechanisms of transplantation tolerance. Annu Rev Immunol. 1994;12:707–734. doi: 10.1146/annurev.iy.12.040194.003423. [DOI] [PubMed] [Google Scholar]
- 6.Ciavarra R, Forman J. H-2L-restricted recognition of viral antigens: in the H-2d haplotype, anti-vesicular stomatitis virus cytotoxic T cells are restricted solely by H-2L. J Exp Med. 1982;156:778–790. doi: 10.1084/jem.156.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cobbold S P, Jayasuriya A, Nash A, Prospero T D, Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature (London) 1984;312:548–550. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- 8.Cray C, Levy R B. The ability of murine cytomegalovirus and class I major histocompatibility complex-disparate parental cells to induce alterations characteristic of severe graft-versus-host reactions. Transplantation. 1989;48:1057–1063. doi: 10.1097/00007890-198912000-00033. [DOI] [PubMed] [Google Scholar]
- 9.Cray C, Levy R B. CD8+ and CD4+ T cells contribute to the exacerbation of class I MHC disparate graft-vs-host reaction by concurrent murine cytomegalovirus infection. Clin Immunol Immunopathol. 1993;67:84–90. doi: 10.1006/clin.1993.1048. [DOI] [PubMed] [Google Scholar]
- 10.Einsele H, Hebart H, Bokemeyer C, Kanz L, Jahn G, Müller C A. Cytomegalovirus infection following hematopoietic stem cell transplantation. Monogr Virol. 1998;21:106–118. [Google Scholar]
- 11.Forman S J, Zaia J A. Treatment and prevention of cytomegalovirus pneumonia after bone marrow transplantation. Where do we stand? Blood. 1994;83:2392–2398. [PubMed] [Google Scholar]
- 12.George J F, Sweeney S D, Kirklin J K, Simpson E M, Goldstein D R, Thomas J M. An essential role for Fas ligand in transplantation tolerance induced by donor bone marrow. Nat Med. 1998;4:333–335. doi: 10.1038/nm0398-333. [DOI] [PubMed] [Google Scholar]
- 13.Grundy J E, Shanley J D, Shearer G M. Augmentation of graft-versus-host reaction by cytomegalovirus infection resulting in interstitial pneumonitis. Transplantation. 1985;39:548–553. doi: 10.1097/00007890-198505000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Hertenstein B, Hampl W, Bunjes D, Wiesneth M, Duncker C, Koszinowski U H, Heimpel H, Arnold R, Mertens T. In vivo/ex vivo T cell depletion for GvHD prophylaxis influences onset and course of active cytomegalovirus infection and disease after BMT. Bone Marrow Transplant. 1995;15:387–393. [PubMed] [Google Scholar]
- 15.Hill R S, Petersen F B, Storb R, Appelbaum F R, Doney K, Dahlberg S, Ramberg R, Thomas E D. Mixed hematologic chimerism after allogeneic marrow transplantation for severe aplastic anemia is associated with a higher risk of graft rejection and a lessened incidence of acute graft-versus-host disease. Blood. 1986;67:811–816. [PubMed] [Google Scholar]
- 16.Ho M. Cytomegaloviruses. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. New York, N.Y: Churchill Livingstone; 1995. pp. 1351–1364. [Google Scholar]
- 17.Holt P G, Degebrodt A, Venaille T, O’Leary C, Krska K, Flexman J, Farrell H, Shellam G, Young P, Penhale J, Robertson T, Papadimitriou J M. Preparation of interstitial lung cells by enzymatic digestion of tissue slices: preliminary characterization by morphology and performance in functional assays. Immunology. 1985;54:139–147. [PMC free article] [PubMed] [Google Scholar]
- 18.Holtappels R, Podlech J, Geginat G, Steffens H-P, Thomas D, Reddehase M J. Control of murine cytomegalovirus in the lungs: relative but not absolute immunodominance of the immediate-early 1 nonapeptide during the antiviral cytolytic T-lymphocyte response in pulmonary infiltrates. J Virol. 1998;72:7201–7212. doi: 10.1128/jvi.72.9.7201-7212.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huss R, Deeg H J, Gooley T, Bryant E, Leisenring W, Clift R, Buckner C D, Martin P, Storb R, Appelbaum F R. Effect of mixed chimerism on graft-versus-host disease, disease recurrence, and survival after HLA-identical marrow transplantation for aplastic anemia or chronic myelogenous leukemia. Bone Marrow Transplant. 1996;18:767–776. [PubMed] [Google Scholar]
- 20.Jondal M, Schirmbeck R, Reimann J. MHC class-I-restricted CTL responses to exogenous antigens. Immunity. 1996;5:295–302. doi: 10.1016/s1074-7613(00)80255-1. [DOI] [PubMed] [Google Scholar]
- 21.Keil G M, Fibi M R, Koszinowski U H. Characterization of the major immediate-early polypeptides encoded by murine cytomegalovirus. J Virol. 1985;54:422–428. doi: 10.1128/jvi.54.2.422-428.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koszinowski U H, Del Val M, Reddehase M J. Cellular and molecular basis of the protective immune response to cytomegalovirus infection. Curr Top Microbiol Immunol. 1990;154:189–220. doi: 10.1007/978-3-642-74980-3_8. [DOI] [PubMed] [Google Scholar]
- 23.Koszinowski U H, Reddehase M J, Jonjic S. The role of T-lymphocyte subsets in the control of cytomegalovirus infection. In: Thomas D B, editor. Viruses and the cellular immune response. New York, N.Y: Marcel Dekker; 1993. pp. 429–445. [Google Scholar]
- 24.Kranz D M, Tonegawa S, Eisen H N. Attachment of an anti-receptor antibody to non-target cells renders them susceptible to lysis by a clone of cytotoxic T lymphocytes. Proc Natl Acad Sci USA. 1984;81:7922–7926. doi: 10.1073/pnas.81.24.7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurz S, Steffens H-P, Mayer A, Harris J R, Reddehase M J. Latency versus persistence or intermittent recurrences: evidence for a latent state of murine cytomegalovirus in the lungs. J Virol. 1997;71:2980–2987. doi: 10.1128/jvi.71.4.2980-2987.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lie W-R, Myers N B, Connolly J M, Gorka J, Lee D R, Hansen T H. The specific binding of peptide ligand to Ld class I major histocompatibility complex molecules determines their antigenic structure. J Exp Med. 1991;173:449–459. doi: 10.1084/jem.173.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ljungman P. Cytomegalovirus interstitial pneumonia in autologous bone marrow transplant recipients. Bone Marrow Transplant. 1994;13:209–212. [PubMed] [Google Scholar]
- 28.Mangioni S, Balduzzi A, Rivolta A, Rovelli A, Nesi F, Rossi V, Busca A, Uderzo C, Miniero R, Biondi A. Long-term persistence of hemopoietic chimerism following sex-mismatched bone marrow transplantation. Bone Marrow Transplant. 1997;20:969–973. doi: 10.1038/sj.bmt.1701011. [DOI] [PubMed] [Google Scholar]
- 29.Mayer A, Podlech J, Kurz S, Steffens H-P, Maiberger S, Thalmeier K, Angele P, Dreher L, Reddehase M J. Bone marrow failure by cytomegalovirus is associated with an in vivo deficiency in the expression of essential stromal hemopoietin genes. J Virol. 1997;71:4589–4598. doi: 10.1128/jvi.71.6.4589-4598.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mutter W, Reddehase M J, Busch F W, Bühring H-J, Koszinowski U H. Failure in generating hemopoietic stem cells is the primary cause of death from cytomegalovirus disease in the immunocompromised host. J Exp Med. 1988;167:1645–1658. doi: 10.1084/jem.167.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikolic B, Sykes M. Bone marrow chimerism and transplantation tolerance. Curr Opin Immunol. 1997;9:634–640. doi: 10.1016/s0952-7915(97)80042-8. [DOI] [PubMed] [Google Scholar]
- 32.Orn A, Goodnow R S, Hood L, Brayton P R, Woodward J G, Harmon R C, Frelinger J A. Product of a transferred H-2Ld gene acts as restriction element for LCMV-specific killer T cells. Nature (London) 1982;297:415–417. doi: 10.1038/297415a0. [DOI] [PubMed] [Google Scholar]
- 33.Petz L D, Yam P, Wallace R B, Stock A D, de Lange G, Knowlton R G, Brown V A, Donis-Keller H, Hill L R, Forman S J, Blume K G. Mixed hematopoietic chimerism following bone marrow transplantation for hematologic malignancies. Blood. 1987;70:1331–1337. [PubMed] [Google Scholar]
- 34.Podlech J, Holtappels R, Wirtz N, Steffens H-P, Reddehase M J. Reconstitution of CD8 T cells is essential for the prevention of multiple-organ cytomegalovirus histopathology after bone marrow transplantation. J Gen Virol, 1998;79:2099–2104. doi: 10.1099/0022-1317-79-9-2099. [DOI] [PubMed] [Google Scholar]
- 35.Podlech J, Steffens H-P, Holtappels R, Mayer A, Alterio de Goss M, Oettel O, Wirtz N, Maiberger S, Reddehase M J. Cytomegalovirus pathogenesis after experimental bone marrow transplantation. Monogr Virol. 1998;21:119–128. [Google Scholar]
- 36.Reddehase M J, Jonjic S, Weiland F, Mutter W, Koszinowski U H. Adoptive immunotherapy of murine cytomegalovirus adrenalitis in the immunocompromised host: CD4-helper-independent antiviral function of CD8-positive memory T lymphocytes derived from latently infected donors. J Virol. 1988;62:1061–1065. doi: 10.1128/jvi.62.3.1061-1065.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reddehase M J, Mutter W, Koszinowski U H. In vivo application of recombinant interleukin 2 in the immunotherapy of established cytomegalovirus infection. J Exp Med. 1987;165:650–656. doi: 10.1084/jem.165.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reddehase M J, Rothbard J B, Koszinowski U H. A pentapeptide as minimal antigenic determinant for MHC class-I-restricted T lymphocytes. Nature (London) 1989;337:651–653. doi: 10.1038/337651a0. [DOI] [PubMed] [Google Scholar]
- 39.Reddehase M J, Weiland F, Münch K, Jonjic S, Lüske A, Koszinowski U H. Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J Virol. 1985;55:264–273. doi: 10.1128/jvi.55.2.264-273.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reusser P, Fisher L D, Buckner C D, Thomas E D, Meyers J D. Cytomegalovirus infection after autologous bone marrow transplantation: occurrence of cytomegalovirus disease and effect on engraftment. Blood. 1990;75:1888–1894. [PubMed] [Google Scholar]
- 41.Reusser P, Riddell S R, Meyers J D, Greenberg P D. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood. 1991;78:1373–1380. [PubMed] [Google Scholar]
- 42.Riddell S R, Watanabe K S, Goodrich J M, Li C R, Agha M E, Greenberg P D. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257:238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 43.Rouvier E, Luciani M F, Goldstein P. Fas involvement in Ca2+-independent T cell-mediated cytotoxicity. J Exp Med. 1993;177:195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rubocki R J, Hansen T H, Lee D R. Molecular studies of murine mutant BALB/c-H-2-dm2 define a deletion of several class I genes including the entire H-2Ld gene. Proc Natl Acad Sci USA. 1986;83:9606–9610. doi: 10.1073/pnas.83.24.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salazar-Mather T P, Orange J S, Biron C A. Early murine cytomegalovirus (MCMV) infection induces liver natural killer (NK) cell inflammation and protection through macrophage inflammatory protein 1α (MIP-1α)-dependent pathways. J Exp Med. 1998;187:1–14. doi: 10.1084/jem.187.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soderberg-Naucler C, Fish K N, Nelson J A. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell. 1997;91:119–126. doi: 10.1016/s0092-8674(01)80014-3. [DOI] [PubMed] [Google Scholar]
- 47.Steffens H-P, Kurz S, Holtappels R, Reddehase M J. Preemptive CD8 T-cell immunotherapy of acute cytomegalovirus infection prevents lethal disease, limits the burden of latent viral genomes, and reduces the risk of virus recurrence. J Virol. 1998;72:1797–1804. doi: 10.1128/jvi.72.3.1797-1804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steffens H-P, Podlech J, Kurz S, Angele P, Dreis D, Reddehase M J. Cytomegalovirus inhibits the engraftment of donor bone marrow cells by downregulation of hemopoietin gene expression in recipient stroma. J Virol. 1998;72:5006–5115. doi: 10.1128/jvi.72.6.5006-5015.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sykes M. Chimerism and central tolerance. Curr Opin Immunol. 1996;8:694–703. doi: 10.1016/s0952-7915(96)80088-4. [DOI] [PubMed] [Google Scholar]
- 50.Walter E A, Greenberg P D, Gilbert M J, Finch R J, Watanabe K S, Thomas E D, Riddell S R. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 51.Wingard J R, Chen D Y, Burns W H, Fuller D J, Braine H G, Yeager A M, Kaiser H, Burke P J, Graham M L, Santos G W. Cytomegalovirus infection after autologous bone marrow transplantation with comparison to infection after allogeneic bone marrow transplantation. Blood. 1988;71:1432–1437. [PubMed] [Google Scholar]