Abstract
Infection and inflammation produce systemic responses that include hypozincemia and hypoferremia. The latter involves regulation of the iron transporter ferroportin 1 by hepcidin. The mechanism of reduced plasma zinc is not known. Transcripts of the two zinc transporter gene families (ZnT and Zip) were screened for regulation in mouse liver after turpentine-induced inflammation and LPS administration. Zip14 mRNA was the transporter transcript most up-regulated by inflammation and LPS. IL-6 knockout (IL-6–/–) mice did not exhibit either hypozincemia or the induction of Zip14 with turpentine inflammation. However, in IL-6–/– mice, LPS produced a milder hypozincemic response but no Zip14 induction. Northern analysis showed Zip14 up-regulation was specific for the liver, with one major transcript. Immunohistochemistry, using an antibody to an extracellular Zip14 epitope, showed both LPS and turpentine increased abundance of Zip14 at the plasma membrane of hepatocytes. IL-6 produced increased expression of Zip14 in primary hepatocytes cultures and localization of the protein to the plasma membrane. Transfection of mZip14 cDNA into human embryonic kidney cells increased zinc uptake as measured by both a fluorescent probe for free Zn2+ and 65Zn accumulation, as well as by metallothionein mRNA induction, all indicating that Zip14 functions as a zinc importer. Zip14 was localized in plasma membrane of the transfected cells. These in vivo and in vitro experiments demonstrate that Zip14 expression is up-regulated through IL-6, and that this zinc transporter most likely plays a major role in the mechanism responsible for hypozincemia that accompanies the acute-phase response to inflammation and infection.
Keywords: endotoxemia, inflammation, hepatic, Slc39a14, knockout mice
Classical features of the acute-phase response to infection and inflammation include fever, increased gluconeogenesis and protein catabolism, increased hepatic amino acid uptake, and markedly increased synthesis of acute-phase proteins by the liver (1, 2). Hypoferremia and hypozincemia are among the changes observed in the acute inflammatory response (1). The functions of these latter changes are believed to be host defensive. In the case of hypoferremia, the host is defended by the decreased availability of iron for pathogenic microorganisms (3). A beneficial role for corresponding hypozincemia has not been deduced. Zinc availability for hepatic synthesis of acute-phase proteins, regulation of gluconeogenesis, control of reactive species (e.g., nitric oxide or hydroxyl radicals), and control of microbial growth are among the postulated functions (4, 5).
Decreased serum zinc concentrations have been observed after acute administration of numerous agents, including endotoxin, dibutyryl cAMP, and glucocorticoid hormones (reviewed in ref. 4). Redistribution of zinc among various tissues, particularly to the liver, was observed after IL-1α administration to rats (6). Redistribution was accompanied by an increase in a hepatic metabolic zinc pool comprised of metallothionein (MT)-bound zinc. Hepatic MT expression is induced in rats by IL-6 through a mechanism that requires an adequate dietary zinc supply (7). In contrast to the integrative level, experiments with isolated liver parenchymal cells in culture showed IL-6, but not IL-1α, enhanced the increased cellular zinc accumulation and MT expression produced by glucocorticoids (8).
Inflammation produced by turpentine involves induction of IL-6 and leptin mediated by IL-1β. Cytokine induction by LPS is more complex, involving TNFα and IL-1α, in addition to IL-1β, leptin, and IL-6 (9). Nevertheless, IL-6 is viewed as the main proinflammatory cytokine regulating acute-phase genes (10). Recently, the hypoferremia of inflammation induced in mice by turpentine was demonstrated to be produced by IL-6 through induction of hepcidin synthesis in the liver (11). In mice, hepcidin transcription is also up-regulated by IL-1α and -1β (12). The mechanism accounting for the reduction in serum iron is through the influence of this regulatory hormone on expression of ferroportin 1 (13), the iron efflux transporter that controls iron release from macrophages (14). Similarly, increased hepatic zinc accumulation during inflammation or infection, which accompanies hypozincemia, may involve zinc transporter genes responsive to these cytokines. To explore possible mechanisms, experiments were carried out with mice of various genotypes to identify which genes of the ZnT (Slc30) and Zip (Slc39) zinc transporter families were differentially expressed in hepatocytes concurrently with the hypozincemia of turpentine-induced inflammation and after LPS, and to characterize transporter properties.
Materials and Methods
Mice and Treatments. Two strains of mice were used. Young adult male CD-1 strain mice (Charles River Breeding Laboratories) were used for some experiments. IL-6 knockout mice (IL-6–/–) and WT control mice of the C57BL/6 strain were also used. IL-6–/– and appropriate WT mice were from a colony maintained at the University of California, Los Angeles, or were obtained from The Jackson Laboratory. Mice were given free access to tap water and received standard rodent diets with adequate zinc [Harlan Teklad (Indianapolis)] with a 12-h light–dark cycle, as described (15, 16). Some IL-6–/– and WT mice were fed Prolab RMH 2000 diet (PMI Nutrition International, Richmond, IN) with an iron content of 440 mg/kg (11). Protocols were approved by Institutional Animal Care and Use Committees of both institutions.
Anesthesia was with isoflurane or halothane or, in the case of liver perfusions, sodium pentobarbital (60 mg/kg, i.p.). Blood was collected by cardiac puncture under anesthesia. Serum was obtained by two-stage centrifugation. Serum zinc concentrations were measured by atomic absorption. Turpentine abscess was initiated under anesthesia by injection (100 μl, s.c.) of pure gum spirits of turpentine. LPS (Escherichia coli serotype 055:B5; Sigma) was injected (5 μg/g body weight, i.p.). For these experiments, the mice were killed 16 h after injections.
Real-Time PCR and Northern Analysis. Tissues were excised rapidly after exsanguination by cardiac puncture and homogenized immediately in TRIzol reagent (Invitrogen), and total RNA was isolated. RNA from cultured hepatocytes was similarly obtained. All RNA was treated with DNase (Ambion, Austin, TX) before analysis. Sequences for transporter and other genes were obtained from GenBank, and primer/probe sets were designed by using primer express software (Version 2.0, Applied Biosystems) and were reported previously (15, 16). The primers and probe set for both Zip14 variants are presented in Table 2, which is published as supporting information on the PNAS web site. Fluorescence detection used TaqMan chemistry and one-step reverse transcriptase reactions (Applied Biosystems) for real-time quantitative PCR. Relative quantitation used five log10 standard curves with 18S rRNA as the normalizer (16, 17). Fluorescence was measured with a Bio-Rad iCycler detection system. Data on transcript abundance are expressed relative to 18S rRNA.
For Northern analysis, total RNA was denatured and fractionated by electrophoresis (1% agarose). The RNA was transferred to a nylon membrane and hybridized with a Zip14 cDNA probe labeled with α-32P by using methods described previously (18). 18S RNA was used as a control for loading. The 659-bp cDNA probe to detect mZip14 mRNA was generated by restriction digestion of the mZip14 clone with Msc I.
Antibody Production, Immunohistochemistry, and Western Analysis. The peptide CNSELDGKAPGTD was used to produce antibodies in rabbits to murine Zip14. It was commercially synthesized and analyzed by mass spectrometry to assess purity. The C-terminal cysteine was added to facilitate both crosslinking to keyhole limpet hemocyanin for antigen production in rabbits and for conjugation to SulfoLink (Pierce) for affinity purification (16). Liver was fixed with formalin and processed as described (16). Tissue sections (5 μM) were incubated with the Zip14 affinity-purified primary antibody, with IgG-Alexa 594 conjugate as the secondary antibody. The synthetic peptide used to prepare the antigen was incubated with the primary antibody as a negative control for specificity. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole. Fluorescence micrographs were obtained with an Axiovert 100 microscope (Zeiss) and charge-coupled device camera (Diagnostic Instruments, Sterling Heights, MI). For Western analysis, a membrane preparation was prepared from intact liver by centrifugation (100,000 × g), and the proteins were resolved by PAGE, as described (16). Chemiluminescence and phosphor imaging were used for detection.
DNA Organization, Amino Acid Sequence, and Protein Topology. Zip14 was identified as a novel gene induced during adipocyte development (GenBank accession no. AB177995). Data from GenBank show that mZip14 produces two transcripts that encode two slightly different proteins. TMpred was used to predict the protein topology and hydrophobicity plot. The short isoform transcript (BC021530) has 2,174 bp, whereas the longer form (AB177995) has 3,660 bp and a longer 3′ UTR. A cDNA clone containing the BC021530 sequence in the pCMV SPORT-6 mammalian expression vector was obtained [clone: 5353717 (I.M.A.G.E. Consortium) from American Type Tissue Collection]. Sequencing to confirm that the cDNA was BC021530 was conducted at the University of Florida Sequencing Core Facility.
Immunofluorescence Localization of Zip14 and IL-6-Stimulated Zn2+ Uptake in Murine Hepatocytes. Hepatocytes were isolated from anesthetized mice by using a two-step perfusion technique with perfusate allowed to flow retrograde from the portal vein without recirculation (8, 19). The cells were suspended in Williams' Medium E (Sigma, cat. no. W4125) buffered with 10 mM Hepes and 10 mM TES (Sigma, cat. no. T6541) and collected by centrifugation (50 × g; 3 min). After washes, the cells were suspended in medium supplemented with 10% FBS and 100 nM insulin, penicillin, and streptomycin. Only cells of ≥95% viability (trypan blue exclusion) were used. After cells were added to collagen-coated culture dishes, parenchymal cells attached in 2–3 h, and nonparenchymal cells were removed. After the parenchymal cells were in culture for 22 h, fresh medium with IL-6 (100 units/ml) or TNFα (30 and 100 units/ml), as recombinant murine peptides (PeproTech, Rocky Hill, NJ), were added for 4 h. Total RNA was obtained after the 4-h incubation. Hepatocytes were also attached to poly(l-lysine) and collagen-coated chambered glass coverslips (Lab-Tek). After 24 h in culture, they were treated with IL-6 (100 units/ml) or carrier for up to 8 h. Zip14 localization used the Zip14 antibody. Anti-rabbit IgG-Alexa 594 conjugate described above was used for fluorescence microscopy.
Cell Transfection and Immunofluorescence Measurement of Zn2+ Uptake. Human embryonic kidney (HEK) 293T cells were cultured in DMEM with 10% FBS. The short-form cDNA sequence in the mammalian expression vector described above was transfected into these cells by using Effectene reagent (Qiagen, Chatsworth, CA). Vector without the Zip14 sequence was the transfection control. After 24 h, the transfected cells were incubated with 5 μM FluoZin-3AM (Molecular Probes), a cell-permeable zinc fluorophore, in the medium without FBS for 30 min. The cells were then stimulated with Zn2+ (80 μM) to measure intracellular Zn2+ accumulation by fluorescence microscopy as above. Zinc uptake was also measured with 65Zn (Oak Ridge National Laboratory, Oak Ridge, TN). HEK cells were incubated in DMEM without FBS (pH 7.0), with Zn2+ (10 μM) containing 30 nCi (1 Ci = 37 GBq) 65Zn per 500 μl. The cells were washed with ice-cold 10 mM Hepes-buffered 0.9% saline (pH 7.4) containing 10 mM EDTA (20). Cells were solubilized in 0.2% SDS/0.2 M NaOH for 1 h (8). Protein content was measured colorimetrically with RC DC reagent (Bio-Rad). 65Zn content was measured with a Packard γ ray spectrometer. Expression of hMT mRNA in HEK cells was measured by real-time quantitative PCR using primer and probe sets described earlier (17).
Statistical Analysis. Data are expressed as means ± SEM and were analyzed by one-way ANOVA followed by the Student–Newman–Keuls multiple comparison test or Student's t test. The significance level was set at P < 0.05.
Results
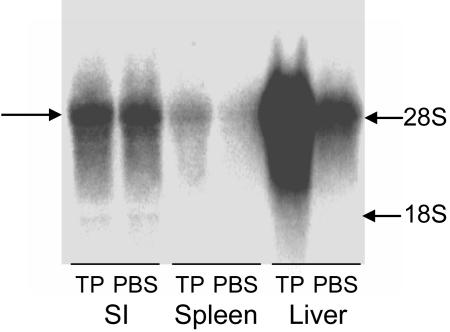
Response of Hepatic Zinc Transporter Genes of WT and IL-6–/– Mice to Inflammation. Inflammation induced by turpentine is accompanied by differential expression changes for specific ZnT and Zip genes expressed in liver. Of the 14 transporter transcripts measured, five were significantly up-regulated at this point (16 h) in the inflammatory process (Table 1). The 3.1-fold (P < 0.001) increase in Zip14 represented the transporter mRNA change of the greatest magnitude. Similarly, LPS administration also increased transcript abundance for ZnT5, Zip6, and Zip14 mRNAs. The 3.7-fold increase of mZip14 mRNA in response to LPS was the most robust response observed for any of the transporter genes surveyed. The increases in MT-1 mRNA of 33- to 37-fold, produced by inflammation and LPS, indicate the cytokine responsiveness of this MT gene. As shown by Northern analysis (Fig. 1), Zip14 is highly expressed in the liver compared with the small intestine and spleen. The increase of hepatic Zip14 mRNA during the acute-phase response to turpentine inflammation is clearly shown. At longer exposures, a smaller and much less abundant transcript, perhaps representing the short variant of Zip14, is observed. The marked hepatic Zip14 regulation during acute inflammation and LPS treatment led us to pursue more detailed studies of this transporter gene and its regulation.
Table 1. Effect of inflammation and LPS on relative zinc transporter transcript abundance.
| Gene | Turpentine | LPS |
|---|---|---|
| MT1 | 37.2 ± 4.7*** | 32.7 ± 1.1*** |
| ZnT1 | 1.0 ± 0.1 | 0.4 ± 0.02* |
| ZnT4 | 1.0 ± 0.1 | 1.5 ± 0.1 |
| ZnT5 | 2.9 ± 0.1*** | 1.5 ± 0.04** |
| ZnT6 | 1.0 ± 0.2 | 0.6 ± 0.01 |
| ZnT7 | 3.1 ± 1.1 | 1.0 ± 0.04 |
| Zip1 | 1.6 ± 0.2** | 1.1 ± 0.2 |
| Zip2 | 0.4 ± 0.04 | 0.4 ± 0.04** |
| Zip3 | 1.3 ± 0.2 | 1.3 ± 0.04 |
| Zip4 | 0.7 ± 0.1 | 1.2 ± 0.02 |
| Zip5 | 1.4 ± 0.6 | 0.8 ± 0.1 |
| Zip6 | 1.8 ± 0.2* | 2.6 ± 0.2*** |
| Zip7 | 1.8 ± 0.2* | 1.2 ± 0.03 |
| Zip8 | 0.7 ± 0.2 | 0.5 ± 0.02*** |
| Zip14 | 3.1 ± 0.4*** | 3.7 ± 0.3*** |
Mice were administered turpentine or LPS 16 h before being killed. Total RNA obtained from WT strain mice were used for transcript analysis by real-time quantitative PCR. MT-1 mRNA was measured on RNA from individual mice (n = 11) as a control for treatment responsiveness. Transporter mRNAs were measured in triplicate using RNA derived by pooling RNA from three to four mice from each treatment group. Values are transporter mRNA/18S mRNA expressed as mean ± SEM as relative to values from PBS-treated control mice with mean values set at 1. Significantly different from the two corresponding PBS groups at *, P < 0.05; **, P < 0.01; and ***, P < 0.001 as determined by ANOVA.
Fig. 1.
Northern analysis of total RNA from small intestine, spleen, and liver of WT mice administered turpentine (TP) or PBS 16 h before they were killed. The arrow points to the Zip14 transcript of ≈4 kb. Markers for 28S and 18S RNA are shown.
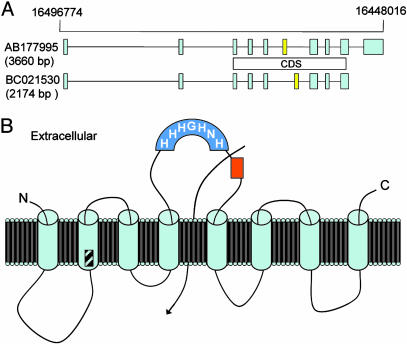
Identification and Organization of the mZip14 Gene. Homology of known genes of the Zip family to mammalian expressed sequence tags and reported cDNA sequences in GenBank suggest the Zip family may have 14 members. The reference sequence of the mouse gene designated as Zip14 (Slc39a14) has GenBank accession no. NM144808. The nucleotide sequence has been reported previously among 400 mouse cDNAs homologous to human KIAA genes derived from size-selected libraries (21). The amino acid sequence derived from GenBank is presented in Fig.7A, which is published as supporting information on the PNAS web site. The exon–intron organization of mZip14 on chromosome 14 is shown in Fig. 2A. The two variants are shown. The longer DNA sequence (identical to the reference sequence) has 3,660 bp with 10 exons and a shorter sequence of 9 exons within 2,174 bp. The coding sequence locations for the two transcripts produced are shown. Primer-pair sets for real-time quantitative PCR were derived from sequences in Exon 3 to measure all Zip14 transcripts and Exon 10 to measure only the reference sequence. Results for the mRNA increases observed were similar for the two primer pair sets (data not shown). Proposed topology with eight transmembrane-(TM) spanning domains is shown in Fig. 2B. Of note are the extracellular orientations of both the N and C termini and the histidine-rich domain. A potential ion channel and the extracellular location of the peptide sequence used to produce the polyclonal antibody are also shown. The region where protein sequences of the two Zip14 isoforms differ is shown in TM domain 2. A hydrophobicity plot of mZip14 is presented as Fig. 7B.
Fig. 2.
Features of the murine Zip14 gene. (A) The exon–intron organization of murine Zip14 on chromosome 14 is shown. Note the additional exon for the longer variant coding sequence (CDS) is shown. (B) Proposed topology for mZip14 showing eight transmembrane-spanning regions. The variable region (diagonal slashes) of the isoforms is shown. The N- and C-terminal domains, the histidine-rich region (darker blue), and the region used to generate the synthetic peptide (amino acids 288–299 from BAD16742) (orange) are extracellular. A potential ion channel location is also shown as an arrow.
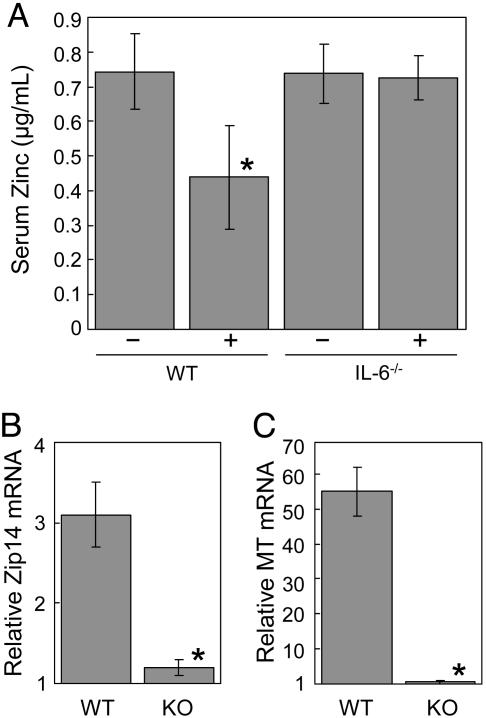
Response of Hepatic Zip14 to Turpentine and LPS. Turpentine and LPS share IL-6 as a common mediator. To further examine this relationship, the regulation of Zip14 in IL-6–/– mice was examined. As shown in Fig. 3A, turpentine-induced inflammation produces hypozincemia but not in the IL-6–/– mice. Similarly, Zip14 and MT expression do not increase in turpentine-treated IL-6–/– mice (Fig. 3 B and C). LPS administration also caused a significant reduction in serum zinc concentrations in both WT and IL-6–/– mice (Fig. 4A). The hypozincemic response was significantly less in the IL-6–/– mice, however. LPS markedly increased MT-1 mRNA in both genotypes, but the induction was less in the IL-6–/– mice (Fig. 4B). In contrast, Zip14 transcript levels were significantly elevated only in the WT mice (Fig. 4C). This suggests that the Zip14 induction by LPS requires IL-6 and is not influenced by MT or zinc accumulation in the liver. Western analysis showed an increase in a 50-kDa band, which is approximately the mass (54 kDa) expected for the Zip14 protein (Fig. 4D). Zip14 protein levels in liver from LPS-treated IL-6–/– mice were comparable to that found with PBS-treated WT mice (data not shown). Immunofluorescence shows Zip14 is distributed throughout the cytoplasm in liver cells of PBS-treated mice (Fig. 4E). After LPS, Zip14 is localized to the apical plasma membrane (Fig. 4F). Prior incubation of the antibody with Zip14 peptide blocked immunoreactivity (Fig. 4G). Zip14 localization to the apical plasma membrane was found after turpentine administration (Fig. 4H). The apical localization of Zip14 suggests that this transporter promotes zinc uptake from blood.
Fig. 3.
Influence of turpentine inflammation on serum zinc concentration and Zip14 and MT expression in IL-6 knockout (KO) and WT mice. Turpentine (TP) was administered 16 h before the mice were killed. (A) Serum Zn concentrations. Relative abundance of Zip14 (B) and MT-1 (C) mRNAs, respectively. Values are means ± SEM (n = 4–7 mice). *, P < 0.001 different from the nontreated WT mice.
Fig. 4.
Influence of LPS on serum zinc concentration and MT and Zip14 expression in IL-6 knockout (KO) and WT mice. LPS (5 μg/g of body weight) was administered 16 h before the mice were killed. (A) Serum Zn concentrations. Relative abundance of MT-1 (B) and Zip14 (C) mRNAs, respectively. (D) Western analysis of Zip14 expression in liver cytosol from PBS- and LPS-treated mice. Immunolocalization of Zip14 in liver sections from PBS-treated (E), LPS-treated (F), or turpentine-treated (H) mice. (G) Zip14 antibody was incubated with Zip14 peptide solution 1 h before use for immunohistochemistry. Values are means ± SEM (n = 4 mice). Values with different letters are significantly different (P < 0.05).
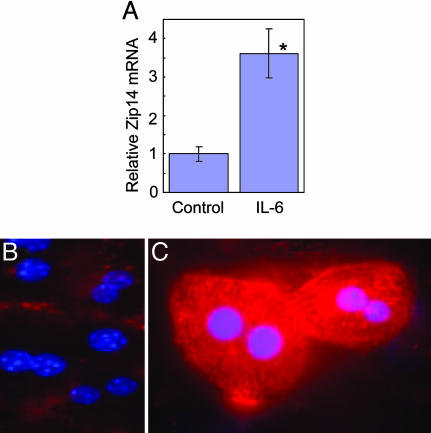
Zip14 Expression and Localization Regulated in Hepatocytes by IL-6 and TNFα. IL-6 produced a significant increase in Zip14 mRNA expression in cultured mouse hepatocytes (Fig. 5A). TNFα did not increase Zip14 mRNA levels at two different concentrations (30 ng/ml or 100 ng/ml) after 4 h (data not shown). Immunocytochemistry demonstrated that Zip14 protein was increased by IL-6 (Fig. 5 B vs. C). The protein clearly was localized to the plasma membrane of nonpermeabilized hepatocytes (Fig. 5C).
Fig. 5.
Influence of IL-6 on Zip14 expression in mouse liver parenchymal cells. (A) Relative abundance of Zip14 mRNA in hepatocytes treated or nontreated with IL-6 (100 units/ml). Immunolocalization of Zip14 in cultured hepatocytes from WT mice nontreated (B) and treated (C) with IL-6 (100 units/ml). Values are means ± SEM (n = 3). *, P < 0.01 different from nontreated hepatocytes.
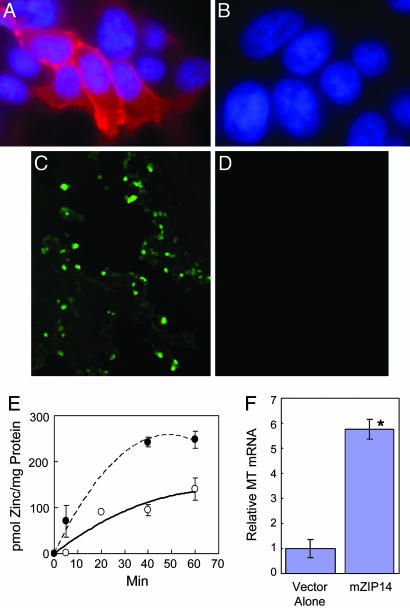
Functional Analysis of mZip14 Expression. To gain an appreciation of the role Zip14 may play in zinc transport, we expressed mZip14 in HEK 293T cells. The cells were transfected with vector or vector-containing mZip14 cDNA (short isoform). Zip14 protein, as detected by fluorescence, was clearly located in the plasma membrane of HEK cells overexpressing this protein (Fig. 6A). No fluorescence was detected in cells transfected with the vector alone (Fig. 6B). Zinc uptake was monitored through fluorescence generated when intracellular labile Zn2+ interacts with FluoZin-3AM. Five minutes after adding zinc at 80 μM, an intense fluorescence is detected in cells transfected with Zip14 (Fig. 6C), whereas virtually no fluorescence is produced in cells transfected with the vector alone (Fig. 6D). Zinc accumulation as a function of time was also measured using 65Zn in medium containing 10 μM of zinc (Fig. 6E). HEK cells overexpressing Zip14 accumulated more 65Zn than the controls. Further evidence that Zn2+ is being taken up by these human cells is through increased expression of hMT, a zinc-inducible gene. Substantially more hMT mRNA is found in Zip14 transfected cells 16 h after incubation in medium with 4 μM zinc than control cells (Fig. 6F).
Fig. 6.
Zip14 expression in HEK 293T cells transfected with mZIP14. Immunolocalization of ZIP14 in cells transfected with ZIP14 (A) or with the vector alone (B). Intracellular labile zinc measured with Fluozin-3AM in medium containing 80 μM of zinc in Zip14 transfected cells (C) or cells transfected with the vector alone (D). (E) Zinc accumulation, measured using 65Zn in medium containing 10 μM zinc. Transfection with Zip14 (##) or vector alone (○). Values are means ± SEM (n = 2). (F) Relative abundance of human MT-1 and –2 mRNAs in transfected cells 16 h after incubation in medium with a final concentration of 4 μM of zinc. Values are means ± SEM (n = 3). *, P < 0.001 different from cells transfected with the vector alone.
Discussion
The acute-phase response most frequently focuses on the orchestrated accelerated hepatic production of specific plasma proteins (1, 2). Although important, other systemic responses to inflammation and infection are equally important in defense against these challenges. These include increased gluconeogenesis and mobilization of amino acids to the liver. Hypoferremia and hypozincemia are also among features of the acute-phase response. As with all of these changes, the purpose is to ultimately restore homeostasis (1).
Hypoferremia, a defining component of the anemia of inflammation (also referred to as anemia of chronic disease), has recently been explained on a mechanistic level, although the functional significance of this response is not known. Specifically, IL-6, the proinflammatory cytokine responsible for inducing the majority of the acute-phase genes (1, 2, 10), has been shown to induce hepatic production of hepcidin, an iron regulatory hormone, in response to inflammation (11). Hepcidin then inhibits both iron release by macrophages and absorption of dietary iron from the intestine and thereby produces hypoferremia. Turpentine-induced inf lammation produces increases in IL-1β, which then signals IL-6 and leptin production (9). IL-6 signal transduction requires STAT3, which is activated by Jak-kinase 1, to up-regulate Type II acute-phase genes (1). IL-6 can also signal acute-phase genes of Types I and II via the mitogen-activated protein kinase-activated NF-IL-6 pathway (1, 22). Recently, hepcidin up-regulation by LPS has been demonstrated (12). LPS-stimulated cytokines include TNFα and IL-1α, as well as IL-1β, which, in turn, stimulate IL-6 production (9). During infections, multiple cytokines may contribute to hepcidin regulation.
Hypozincemia is frequently listed among the characteristic features of the acute-phase response (1, 2, 4). For more than two decades, studies have reported that numerous agents, including IL-1, endotoxins, turpentine, and some hormones, depress serum zinc concentrations in intact animals (reviewed in refs. 7 and 23). Evidence is available suggesting that the binding sites of MT, particularly those that comprise the N-terminal (β-cluster) metal-binding domain of the protein (24), may be the ultimate recipients of zinc ions lost from the plasma pool during stress and inflammation. Although IL-6 has been shown to enhance the induction of MT in hepatocytes (8, 9, 25), our data presented here suggest that both Zip14 and MT are components of the IL-6-mediated hypozincemia. Clearly, mice that cannot make IL-6 are not capable of lowering serum zinc concentrations during turpentine-induced inflammation. The involvement of IL-6, a master regulator of acute-phase responses, points to the liver as the organ responsible for the reduced serum zinc in inflammation. That Zip14 is up-regulated in mouse hepatocytes by IL-6 concomitantly with increased Zn2+ uptake supports that linkage.
LPS is also a strong inducer of hypozincemia in WT mice. However, ablation of the IL-6 gene only attenuates this response after LPS. These data suggest that, whereas IL-6 is a major cytokine regulating this transporter gene, other mediators contribute to the hypozincemic response from LPS.
The source of IL-6 in an aseptic inflammatory response is believed to be macrophages. The sequelae of events include ATP leakage from inflamed cells, changes in intracellular Ca2+, and membrane potential, which lead to enhanced IL-6 transcription in macrophages (26). Of note in this context is that the Zip14 promoter has response elements for numerous physiologic/immune mediators, suggesting other factors are involved in the regulation of Zip14 in specific cell types. The interplay of mediators may influence other zinc transporters. Although this report has focused on Zip14 because of its profound dependence on IL-6 for up-regulation, other hepatic transporters were also influenced, albeit to a lesser degree. Very recently, organic anion transporters localized to basolateral and canalicular membranes of hepatocytes have been shown to be down-regulated by IL-6 (27). This suggests IL-6 is an important modulator of hepatic solute transporters and thus controls metabolite and nutrient trafficking during the acute-phase response.
Murine Zip14 exists as two isoforms. The less abundant has 2,174 bp (BC021530), whereas the longer isoform has 3,660 (AB177995) and a longer 3′ UTR (21). Both isoforms produce proteins of 489 aa (albeit with 18-aa differences). Of potential importance is that the two human Zip14 isoforms have high (83–86%) homology to mouse Zip14 isoforms (Fig. 7 A) (28). Six single-nucleotide polymorphisms have been reported in the coding sequence of hZip14. Two of them are in the histidine-rich loop. Among the 14-member mZip protein family (Slc39a), Zip14 is most homologous to Zip8. The relationships among the various Zip genes have been reviewed in detail (29). Most Zip proteins have eight transmembrane domains (TMDs), with a loop region within the TMD III–V region. This histidine-rich loop has three to six histidines. The histidine-rich loop of Zip14 is between TMD IV and V (Fig. 2B). This is the aqueous cavity of the protein through which cations are predicted to pass (30). The evidence accumulated to date suggests Zip transporters function to elevate the zinc content of constituents found within the cytosolic compartment of cells (29, 31).
Very recently, array analysis of multiple human tissues revealed the liver and pancreas had the highest Zip14 transcript levels (32). Transfection of Chinese hamster ovary cells with hZIP14 cDNA increased intracellular zinc accumulation with increasing extracellular zinc concentrations and localization of ZIP14 at the plasma membrane. Of interest is that one of the transmembrane domains of hZIP14 may contain a metalloprotease motif. This motif is also present in the mouse Zip14 (Fig. 7 A).
The possible clinical significance of Zip14 regulation by IL-6 has yet to be evaluated. Because IL-6 is the key regulator of inflammation, the up-regulation of Zip14 may occur in many pathophysiologic situations. Hypozincemia is a frequent finding in acute and chronic inflammatory and infectious disease. The correlation of elevated plasma IL-6 with low plasma zinc to gastrointestinal disease occurrence in children with Down's syndrome is an example of such reports (10). Because hypozincemia is an IL-6-dependent physiologic effect, the response may vary in individuals with single-nucleotide polymorphisms in the promoter region of IL-6, which influences IL-6 production and function (33, 34). These could have implications with respect to geographic differences in manifestations of zinc deficiency. Similarly, a biological role for the depression of plasma zinc during infections has not yet been experimentally defined. Zinc is needed for microbial pathogenicity (5, 35), and a hypozincemia may limit zinc availability. Zn2+ mobilization induced by nitric oxide may also be an explanation for the up-regulation of Zip14 during inflammation and infection (24). Zinc influences cytokine production by leukocytic populations in vivo and in vitro (36, 37) and may be of therapeutic value during sepsis (38). Consequently, hypozincemia may be a way to limit these cytokines during the early acute phase.
Overall, these studies demonstrate that the zinc transporter Zip14 is regulated in the liver by IL-6; that Zip14 contributes to producing the hypozincemia of inflammation and infection; and that Zip14 is a major cytokine-responsive transporter localized at the plasma membrane of specific cells, including hepatocytes.
Supplementary Material
Acknowledgments
We thank Dr. Don A. Samuelson and Ms. Patricia A. Lewis for advice with histological techniques. Research described in this paper was supported by National Institutes of Health Grants DK 31127 (to R.J.C.) and DK 065029 (to T.G.), Boston Family Endowment Funds of the University of Florida Foundation, and the Florida Agricultural Experiment Station and was approved for publication as Journal Series No. R-10781.
Author contributions: J.P.L., L.A.L., and R.J.C. designed research; J.P.L., L.A.L., S.R., R.K.B., and T.B.A. performed research; S.R., R.K.B., M.D.K., T.G., and R.J.C. contributed new reagents/analytic tools; J.P.L., L.A.L., R.K.B., T.B.A., M.D.K., and R.J.C. analyzed data; and J.P.L., L.A.L., and R.J.C. wrote the paper.
Abbreviations: MT, metallothionein; HEK, human embryonic kidney.
References
- 1.Moshage, H. (1997) J. Pathol. 181, 257–266. [DOI] [PubMed] [Google Scholar]
- 2.Gabay, C. & Kushner, I. (1999) N. Engl. J. Med. 340, 448–454. [DOI] [PubMed] [Google Scholar]
- 3.Jurado, R. L. (1997) Clin. Infect. Dis. 25, 888–895. [DOI] [PubMed] [Google Scholar]
- 4.Cousins, R.J. (1996) in Present Knowledge in Nutrition, eds. Filer, L. J. & Ziegler, E. E. (Int. Life Sci. Inst. Nutr. Foundation, Washington, DC), 7th Ed., pp. 293–306.
- 5.Kim, S., Watanabe, K., Shirahata, T. & Watarai, M. (2004) J. Vet. Med. Sci. 66, 1059–1063. [DOI] [PubMed] [Google Scholar]
- 6.Cousins, R. J. & Leinart, A. S. (1988) FASEB J. 2, 2884–2890. [DOI] [PubMed] [Google Scholar]
- 7.Huber, K. L. & Cousins, R. J. (1993) J. Nutr. 123, 642–648. [DOI] [PubMed] [Google Scholar]
- 8.Schroeder, J. J. & Cousins R. J. (1990) Proc. Natl. Acad. Sci. USA 87, 3137–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faggioni, R., Fantuzzi, G., Fuller, J., Dinarello, C. A., Feingold, K. R. & Grunfeld, C. (1998) Am. J. Physiol. 274, R204–R208. [DOI] [PubMed] [Google Scholar]
- 10.Siewert, E., Dietrich, C. G., Lammert, F., Heinrich, P. C., Matern, S., Gartung, C. & Geier, A. (2004) Biochem. Biophys. Res. Commun. 322, 232–238. [DOI] [PubMed] [Google Scholar]
- 11.Nemeth, E., Rivera, S., Gabayan, V., Keller, C., Taudorf, S., Pedersen, B. K. & Ganz, T. (2004) J. Clin. Invest. 113, 1271–1276. [DOI] [PubMed] [Google Scholar]
- 12.Lee, P., Peng, H., Gelbart, T., Wang, L. & Beutler, E. (2005) Proc. Natl. Acad. Sci. USA 102, 1906–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nemeth, E., Tuttle, M. S., Powelson, J., Vaughn, M. B., Donovan, A., Ward, D. M., Ganz, T. & Kaplan, J. (2004) Science 306, 2090–2093. [DOI] [PubMed] [Google Scholar]
- 14.Knutson, M. D., Oukka, M., Koss, L. M., Aydemir, F. & Wessling-Resnick, M. (2005) Proc. Natl. Acad. Sci. USA 102, 1324–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore, J. B., Blanchard, R. K., McCormack, W. T. & Cousins, R. J. (2001) J. Nutr. 131, 3189–3196. [DOI] [PubMed] [Google Scholar]
- 16.Liuzzi, J. P., Bobo, J. A., Lichten, L. A., Samuelson, D. A. & Cousins, R. J. (2004) Proc. Natl. Acad. Sci. USA 101, 14355–14360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cousins, R. J., Blanchard, R. K., Popp, M. P., Liu, L., Cao, J., Moore, J. B. & Green, C. L. (2003) Proc. Natl. Acad. Sci. USA 100, 6952–6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liuzzi, J. P., Bobo, J. A., Cui, L., McMahon, R. J. & Cousins, R. J. (2003) J. Nutr. 133, 342–351. [DOI] [PubMed] [Google Scholar]
- 19.Yang, S. Q., Lin, H. Z., Lane, M. D., Clemens, M. & Diehl, A. M. (1997) Proc. Natl. Acad. Sci. USA 94, 2557–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Failla, M. L. & Cousins, R. J. (1978) Biochim. Biophys. Acta 538, 435–434. [DOI] [PubMed] [Google Scholar]
- 21.Okazaki, N., Kikuno, R., Ohara, R., Inamoto, S., Aizawa, H., Yuasa, S., Nakajima, D., Nagase, T., Ohara, O. & Koga, H. (2003) DNA Res. 10, 35–48. [DOI] [PubMed] [Google Scholar]
- 22.Stephanou, A., Isenberg, D. A., Akira, S., Kishimoto, T. & Latchman, D. S. (1998) Biochem. J. 330, 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cousins, R. J. (1985) Physiol. Rev. 65, 238–309. [DOI] [PubMed] [Google Scholar]
- 24.Zangger, K., Oz, G., Haslinger, E., Kunert, O. & Armitage, I. M. (2001) FASEB J. 15, 1303–1305. [DOI] [PubMed] [Google Scholar]
- 25.Hernández, J., Carrasco, J., Belloso, E., Giralt, M., Bluethmann, H., Kee Lee, D., Andrews, G. K. & Hidalgo, J. (2000) Cytokine 12, 791–796. [DOI] [PubMed] [Google Scholar]
- 26.Hanley, P. J., Musset, B., Renigunta, V., Limberg, S. H., Dalpke, A. H., Sus, R., Heeg, K. M., Preisig-Müller, R. & Daut, J. (2004) Proc. Natl. Acad. Sci. USA 101, 9479–9484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Licastro, F., Mariani, R. A., Faldella, G., Carpene, E., Guidicini, G., Rangoni, A., Grilli, T. & Bazzocchi, G. (2001) Brain Res. Bull. 55, 313–317. [DOI] [PubMed] [Google Scholar]
- 28.Taylor, K. M. & Nicholson, R. I. (2003) Biochim. Biophys. Acta 1611, 16–30. [DOI] [PubMed] [Google Scholar]
- 29.Eide, D. J. (2003) Pflügers Arch. 447, 796–800. [DOI] [PubMed] [Google Scholar]
- 30.Rogers, E. E., Eide, D. J. & Guerinot, M. L. (2000) Proc. Natl. Acad. Sci. USA 97, 12356–12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liuzzi, J. P. & Cousins, R. J. (2004) Annu. Rev. Nutr. 24, 151–172. [DOI] [PubMed] [Google Scholar]
- 32.Taylor, K. M., Morgan, H. E., Johnson, A. & Nicholson, R. I. (2005) FEBS Lett. 579, 427–432. [DOI] [PubMed] [Google Scholar]
- 33.Terry, C. F., Loukaci, V. & Green, F. R. (2000) J. Biol. Chem. 275, 18138–18144. [DOI] [PubMed] [Google Scholar]
- 34.Bennermo, M., Held, C., Stemme, S., Ericsson, C. G., Silveira, A., Green, F. & Tornvall, P. (2004) Clin. Chem. 50, 2136–2140. [DOI] [PubMed] [Google Scholar]
- 35.Schapiro, J. M., Libby, S. J. & Fang, F. C. (2003) Proc. Natl. Acad. Sci. USA 100, 8496–8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rink, L. & Kirchner, H. (2000) J. Nutr. 130, 1407S–1411S. [DOI] [PubMed] [Google Scholar]
- 37.Prasad, A. S. (2003) J. Trace Elem. Exp. Med. 16, 139–163. [Google Scholar]
- 38.Krones, C., Klosterhalfen, B., Fackeldey, V., Junge, K., Rosch, R., Schwab, R., Stumpf, M., Klinge, U. & Schumpelick, V. (2004) J. Invest. Surg. 17, 249–256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.