Abstract
The tumor-associated antigen EpCAM (GA733-2) is a highly expressed target on adenocarcinoma cells, as defined by murine mAb CO17-1A. We recently developed a transgenic plant system for the safe and inexpensive production of large quantities of mAb CO17-1A as a future source of clinical-grade protein. Although the glycosylation pattern of plant-derived mAb (mAbP) CO17-1A differs considerably from that of the mammalian-derived mAb (mAbM), we show here that the biological activity of both mAbs is quite similar. mAbP heavy and light chains assembled to bind the recombinant antigen GA733-2E and specifically bound to human SW948 colorectal carcinoma cells expressing the antigen GA733-2 to the same extent as mAbM. mAbP was as effective as mAbM CO17-1A in inhibiting tumor growth of xenotransplanted SW948 cells in nude mice. These results suggest the promise of transgenic plants as a useful alternative way to produce full-size mAb for cancer immunotherapy.
Keywords: endoplasmic reticulum, glycosylation, Lys-Asp-Glu-Leu, mammalian-derived mAb
The mAb CO17-1A (IgG2a) recognizes the tumor-associated antigen GA733, which is highly expressed on human colorectal carcinomas (1). This mAb has proven to be efficacious in treating micrometastases and in preventing the recurrence of colorectal cancer in high-risk patients (Duke's C) (2, 3). mAb CO17-1A was the first murine anticarcinoma antibody tested in humans and has a well documented safety record. However, mAb production using hybridoma technology is limited by the high cost associated with obtaining sufficient quantities and by the potential presence of animal pathogens (4, 5). mAb can be produced in several other recombinant protein production systems, such as bacteria, yeast, insect cells, transgenic mammals, and plants (5). The plant system for mAb production offers several advantages, such as the lack of animal pathogenic contaminants, relatively inexpensive plant cultivation, low cost of scale-up for agricultural production, and glycosylation (5–7). Thus, transgenic plants are considered to be efficient systems for the production of functional therapeutic agents (6). The recombinant mAb CO17-1A has been expressed and assembled in tobacco plants by using the tobacco mosaic virus vector system (8). Although such plant virus expression systems are potentially more rapid and efficient than transgenic plants, they have several drawbacks, including the requirement for virus transcript inoculation due to the transient gene expression and a frequently high mutation and deletion rate of a foreign gene during plant RNA virus replication (9). In contrast, transgenic plants provide stable gene insertion and ease of propagation through tissue culture or seedlings (10).
Topically applied plant-derived mAb (mAbP) has been shown to protect against infectious diseases such as Streptococcus mutans (11) and herpes simplex virus (12). Recently, we described mAbP against rabies virus (13) for systemic postexposure prophylaxis. Despite the efficient expression of numerous mAbPs for use against infectious diseases, there has been no study of these reagents for use in cancer immunotherapy.
mAbs are glycosylated molecules, and the pattern of glycosylation influences mAb stability, variable region-dependent binding activity, and the interaction between Fc regions and Fc receptors that play a pivotal role in IgG effector functions (14, 15). In both plant and mammalian cells, N-glycosylation begins in the endoplasmic reticulum and is followed by the production of glycan backbone structure (GlcNAc2Man3GlcNAc2) in cis-Golgi (7, 17). However, the plant glycosylation processing in the medial- and trans-Golgi yields β (1,2)-xylose (Xyl) and core α (1,3)-fucose (Fuc) instead of the α (1,6)-Fuc and sialic acid produced during mammalian glycosylation, leading to a difference in glycan structure between the two kingdoms (7). Because variations in glycan composition influence the effector functions through which mAbs act on cancer cells (14, 15), we compared the antitumor biological activity of mAb CO17-1A produced in plants vs. that of the mammalian-derived mAb (mAbM).
We show here that mAb CO17-1A is expressed and assembled in transgenic plants, and that mAbP CO17-1A with altered glycosylation shows levels of specific binding activity to colon cancer cells and tumor inhibition activity in nude mice similar to that of its mammalian counterpart.
Materials and Methods
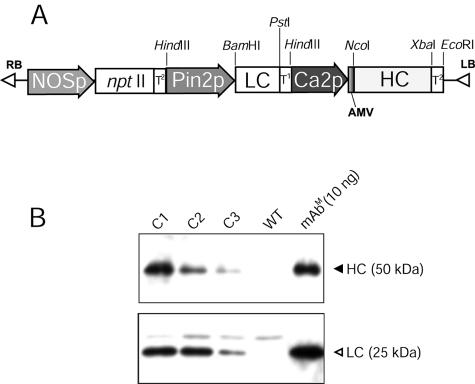
Construction of Plant Transformation Vector. cDNA for the mAb CO17-1A heavy chain (HC, 1,459 bp) and light chain (LC, 764 bp) (8) was cloned into the pGEM-T vector (Promega). PCR was used to clone the HC gene under the control of the cauliflower mosaic virus 35S promoter with duplicated upstream B domains (Ca2 promoter) and the untranslated leader sequence of alfalfa mosaic virus RNA4 (Fig. 1A). To create restriction sites for cloning, forward and reverse primers were designed to contain NcoI and XbaI restriction sites in the 5′- and -3′ ends of the HC gene, respectively (NcoI-HCF, 5′-cggccatggaatggagcagagtcttt-3′; XbaI-HCR, 5′-cgtctagattagtgatggtgatggtgatgatc-3′). The LC gene was PCR-cloned under the control of the potato proteinase inhibitor II (Pin2) promoter (19) and inserted into the HindIII restriction site of pGEM-T vector (Fig. 1 A). PCR was carried out to create BamHI and PstI restriction by using primers BamHI-LCF (5′-cggggcccatgggcatcaagatggaatca-3′) and PstI-LCR (5′-cgtctagactaacactcattcctgttgaa-3′). The HC and LC expression cassettes were cloned into the plant expression binary vector pBI121 to yield pBICO17 (Fig. 1 A).
Fig. 1.
Expression of HC and LC genes in transgenic plant generated by Agrobacterium-mediated transformation. (A) The T-DNA region in a binary vector (pBICO17) was transferred to tobacco by using A. tumefaciens. Pin2p, promoter of Pin2 gene from potato; T1, terminator of Pin2 gene from potato; LBA4404 and cDNA of LC and HC of mAb CO17-1A, respectively; Ca2p, cauliflower mosaic virus 35S promoter with duplicated upstream B domain; AMV, untranslated leader sequence of alfalfa mosaic virus RNA4; T2, terminator of nopaline synthase (NOS) gene. This binary vector contains the nptII gene under the control of the NOS promoter (NOSp) for a selectable marker to confer resistance to antibiotic kanamycin. (B) Western blots of HC, anti-murine Fcγ mAb (Upper) and LC, anti-murine F(ab′)2 (Lower) of mAb CO17-1A in transgenic tobacco lines. C1, transgenic tobacco line C1; C2, transgenic tobacco line C2; C3, transgenic tobacco line C3; WT, wild-type tobacco; mAbm(10 ng), 10 ng of mAbm CO17-1A. Ten microliters of leaf extract (0.2 mg of leaf fresh weight per μl) was loaded.
Plant Transformation. Tobacco (Nicotiana tabacum cv. Xanthi) leaf pieces were used for Agrobacterium-mediated transformation (Agrobacterium tumefaciens LBA4404) (13). After transformation, leaf pieces were transferred to Murashige and Skoog-based medium containing kinetin (1 μg/ml), indoleacetic acid (0.1 μg/ml), carbenicillin (500 μg/ml), and kanamycin (100 μg/ml). Established transgenic tobacco lines were later transferred to soil for subsequent generations (T1 and T2) by self-fertilization in a greenhouse.
SDS/PAGE and Western Blot Analysis. SDS/PAGE and Western blot analysis were conducted as described in refs. 13 and 20. Ten milligrams of leaf tissue was homogenized in 50 μl of extraction buffer (50 mM Tris, pH 7.5) containing protease inhibitor mixture (Roche). Proteins in homogenates were separated on 12% SDS/PAGE and transferred to an Immobilon-P transfer membrane (Millipore). Membranes were incubated in blocking solution [0.5% (wt/vol) I-Block (Tropix, Bedford, MA) in 1× PBS plus 0.1% (vol/vol) Tween 20], followed by rabbit anti-mouse mAb [Fcγ- and F(ab′)2-specific] conjugated to horseradish peroxidase (Jackson ImmunoResearch) to detect HC and LC, respectively. mAbM CO17-1A (Centocor) was used as a positive control.
ELISA. Binding of transgenic tobacco-expressed mAb CO17-1A to the recombinant colorectal carcinoma-associated antigen GA733-2E (21) was assessed by ELISA. Briefly, 96-well NuncImmuno MaxiSorp surface plates (Nunc) were coated with 1 μg/ml GA733-2E in 50 mM sodium carbonate (pH 9.6). Leaf tissue (20 mg) was homogenized in 100 μl of extraction buffer containing 10 mM sodium sulfite, 2% (wt/vol) polyvinylpyrrolidone (molecular weight 40,000), 3 mM sodium azide, and 2% (vol/vol) Tween 20. Plates were loaded with 50 μl of serial 3-fold dilutions of leaf homogenate of transgenic/WT tobacco and mAbM CO17-1A (2 μg/ml) as a positive control. After the addition of horseradish peroxidase-conjugated goat anti-mouse mAb (Jackson ImmunoResearch), plates were treated with 50 μl of O-phenylenediamine dihydrochloride as peroxidase substrate to detect mAb CO17-1A binding to GA733-2E.
Purification of mAb Expressed in Tobacco Plants. Tobacco plant leaves (1 kg) were homogenized with extraction buffer (2.5 liters) containing 9 ml of Rohament CL and 9 ml of Rohapect CM (both from AB Enzymes, Fort Mill, SC) according to previous protocols (20). Protein in the homogenates was precipitated by using ammonium sulfate. Soluble protein was applied to HiTrap protein G column (Amersham Pharmacia). mAbs were eluted with 0.1 M glycine-HCl (pH 2.7) and dialyzed overnight with 1× PBS.
Cell ELISA. Flat-bottom Nunclon Δ MicroWell plates (Nalge Nunc International, Rochester, NY) were coated with SW948 and SW620 colorectal carcinoma cells, negative control WM115 melanoma cells, and A431 human epidermal carcinoma cells (100 μl; 1 × 106 cells per ml) and incubated overnight at 37°C. After fixation in 50 μl of 0.05% glutaraldehyde in 1× PBS for 20 min at room temperature, cells were washed once with 1× PBS, blocked with 25 μl of 0.7% glycine, and incubated with 50 ng of purified mAbP CO17-1A, mAbM CO17-1A positive control, and anti-hepatitis B virus IgG negative control. Reactivity was visualized as described above.
Assay for Tumor Growth Inhibition in Vivo. Thymus-deficient BALB/c nu/nu mice (6–8 weeks old; Charles River Laboratories) were inoculated s.c. with 2 × 106 SW948 human colorectal carcinoma cells. Immediately after tumor cell inoculation, three groups of five mice each were injected i.p. with 100 μg of mAbP, mAbM (positive control), and antirabies mAb (negative control), respectively, followed by the same injections given every 2 days for a total of 6 days. Tumor volumes were calculated based on the three major diameters measured with graduated calipers and were recorded 12, 19, 26, 33, and 40 days after injection. Mice were killed by CO2 inhalation on day 40 after tumor observation. Statistical analysis with Student's t test was performed to test for the different tumor volume of each group by using minitab software (Minitab, State College, PA).
Results
Generation of Transgenic Plants Expressing mAb CO17-1A. Transgenic tobacco plants were obtained by Agrobacterium-mediated transformation with the plant binary vector pBICO17 (Fig. 1). Two different promoters, cauliflower mosaic virus 35S promoter and Pin2 promoter, were used for the expression of the HC and LC genes, respectively, to yield the plant binary vector pBICO17 (Fig. 1 A). Nine putative transformants were selected on medium containing kanamycin. The presence of the transgene and the physical size of the expressed HC and LC were confirmed in the nine transformants by PCR and Western blot, respectively (data not shown). Both HC and LC protein bands (Fig. 1B) were identified in leaf extracts of transgenic lines on Western blot. Transgenic line C1 with the highest density of HC and LC (lane 1) was used to obtain subsequent generations (T1 and T2) by self-fertilization. In the T1 generation, one copy of the gene insert was confirmed in line C1 by Mendelian segregation (data not shown). The line C1 T2 generation was established for the production of mAb CO17-1A.
Assembly of mAbP CO17-1A and Binding to Antigen GA733. Leaf extracts of three representative transgenic lines (C1, C2, and C3) and WT tobacco were applied to ELISA plates coated with recombinant antigen GA733-2E. Absorbance values of leaf extracts (at dilutions 1:1) of all transgenic lines were significantly greater than that of the WT tobacco (Fig. 2), and transgenic line C1 with the highest intensity of HC and LC protein bands (Fig. 1B) showed significantly greater absorbance (P < 0.05) up to the 1:27 dilution. These results confirm the assembly of HC and LC of mAb CO17-1A and its binding activity to colorectal carcinoma-associated antigen GA733.
Fig. 2.
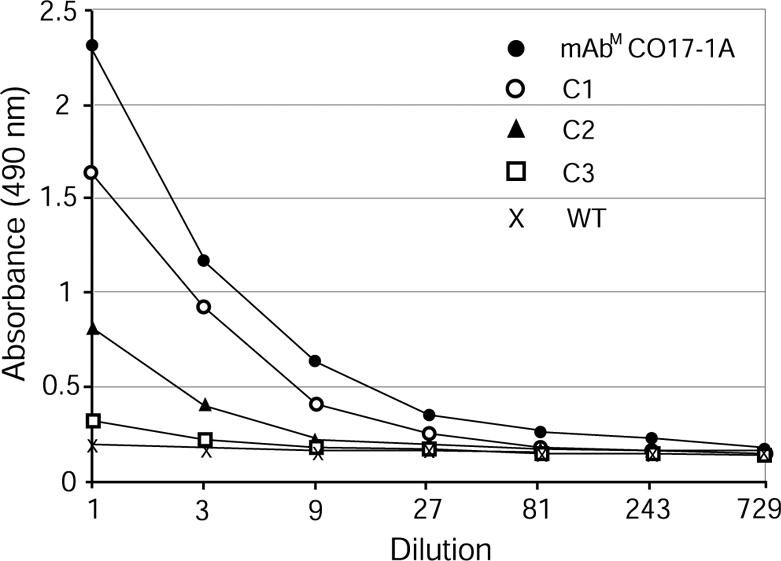
ELISA analysis of expression of assembled mAb CO17-1A produced in transgenic tobacco lines. ELISA was conducted by using 96-well plates coated with the recombinant Ag GA733-2E. mAbm CO17-1A, 50 μl (2.0 μg/ml) of purified mAb CO17-1A from the hybridoma supernatant; C1, C2, and C3, 50 μl of leaf extracts of tobacco transgenic lines expressing the HC and LC; WT, 50 μl of leaf extract of a WT tobacco.
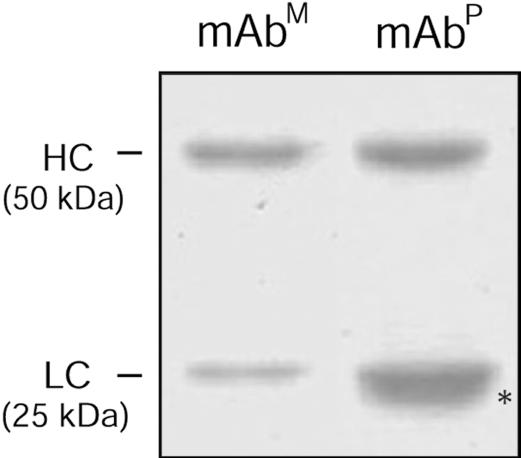
Specific Binding Activity of Purified mAbP CO17-1A. mAbP CO17-1A was purified from leaves from line C1 tobacco plants (T2 generation) and resolved by SDS/PAGE (Fig. 3). One extra protein band below the LC band was observed (asterisk, lane 2), which was recognized by anti-murine F(ab′)2 mAb, but not anti-murine Fcγ mAb, indicating that this extra band is an LC-related protein as described in ref. 22. Purification with protein G yielded 310 μg of purified mAbP from 1 kg of fresh leaf weight. In vitro cell ELISA in which mAbM and mAbP were applied to ELISA plates coated with SW948 colorectal carcinoma cells expressing Ag GA733, colorectal carcinoma metastatic cells SW620, WM115 negative control cells, or human A431 squamous carcinoma cells (Fig. 4) revealed significantly higher absorbance (0.19) for mAbP CO17-1A when reacted with SW948 cells, compared with other cell lines, and levels were similar to those of the parental mAbM. For SW620 cells, both mAbP and mAbM showed similar low absorbance (≈0.05), whereas low or no reactivity of either mAb with A431 and WM115 cells was detected. Absorbance of anti-hepatitis virus IgG used as a negative control was low with all cell lines.
Fig. 3.
SDS/PAGE analysis of mAbP purified from transgenic tobacco leaf. Lanes: mAbm, purified mAbm CO17-1A (1.5 μg); purified mAbP (2.5 μg). The asterisk indicates an additional band below light chain band.
Fig. 4.
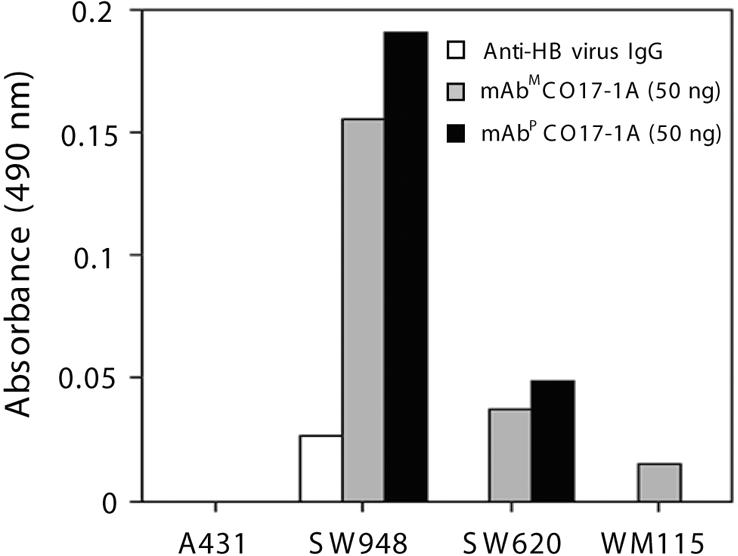
Specific binding activity of mAbP CO17-1A to colorectal cancer cells. Purified mAbm and mAbP and anti-hepatitis B (HB) virus IgG were applied to ELISA plates coated with different cell lines. A431, negative control cell line; SW948, colorectal carcinoma cell line highly expressing Ag GA733 and Ag CO17-1A; SW620, colorectal cancer cell line; WM115, melanoma cell line as a negative control.
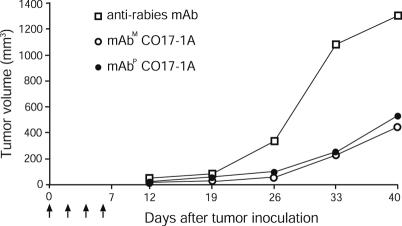
In Vivo Tumor Growth Inhibition by mAbP CO17-1A. The effect of mAbP CO17-1A on tumor growth was analyzed in nude mice injected with colorectal carcinoma cells (Fig. 5). At 12 and 19 days after tumor injection, there was no significant difference in tumor volumes among all mouse groups. However, after 19 days, tumor volume in mAbP-ormAbM-treated mice was significantly lower than that of the negative control group treated with antirabies mAb. In the negative control group, the kinetics of tumor growth was much faster than in the mAbM or mAbP CO17-1A-treated mice. At 26 days, the mean tumor volumes of mAbP- and mAbM-treated mice were significantly lower than that of the control group (99.5 and 99.0 mm3 vs. 334.7 mm3, P < 0.005). At 40 days, mean tumor volumes of mAbP- and mAbM-treated mice were 2.5 times less than that of the control group (527.8 and 439.5 mm3 vs. 1,290.0 mm3) (P < 0.05 and P < 0.1, respectively). At no time during the observation period did the mean tumor volume of mAbP-treated mice differ significantly from that of mAbM-treated mice.
Fig. 5.
In vivo efficacy of mAbP CO17-1A for inhibition of tumor growth in nude mice. SW948 human colon carcinoma cells (2 × 106) were s.c. injected into each mouse (thymus-deficient BALB/c nu/nu mice). After tumor-cell inoculation, each experimental group of five mice received i.p. injections of a total of 400 μgofmAbP, mAbm as a positive control, and antirabies mAb as a negative control, respectively (100 μg of the mAbs every 2 days as indicated by arrows). Tumor volumes were recorded 12, 19, 26, 33, and 40 days after injection.
Discussion
In this study, we demonstrate that anti-colorectal cancer mAbP CO17-1A and mAbM CO17-1A have similar specific binding activity on colorectal cancer cells and similar efficacy in inhibiting human colorectal tumor growth in vivo. As in a previous study (13), the HC and LC of mAbP CO17-1A were expressed under the two different promoters, cauliflower mosaic virus promoter and Pin2 promoter. The expression level of mAb CO17-1A in transgenic line C1 was 0.9 mg/kg of fresh leaf weight, representing 0.02% of total soluble leaf protein, compared with 0.07% reported for the antirabies mAb expressed in tobacco (13). The higher yield of antirabies mAbP might reflect the use of the endoplasmic reticulum retrieval motif KDEL (Lys-Asp-Glu-Leu), which was fused to the HC to retain mAb in the endoplasmic reticulum (13) but was not used in the present study. We have observed lower expression of the antirabies mAb when the KDEL sequences is absent in HC (K.K. and H.K., unpublished data), suggesting that the endoplasmic reticulum is a good subcellular compartment for the accumulation of antibody (23).
Western blot analysis and ELISA demonstrated the expression of mAb CO17-1A in plants and its assembly/binding to recombinant carcinoma-associated antigen GA733-2E. Because GA733-2E is a truncated recombinant antigen produced in a baculovirus-insect cell expression system (21), we addressed the possibility that the conformation of GA733-2E differs from that of the native antigen GA733-2 expressed on colorectal carcinoma cells, leading to altered immunoreactivity with antibody. However, in ELISA with SW948 colorectal carcinoma cells expressing native GA733-2, the purified mAbP and mAbM CO17-1A showed similar specific binding activity on the cells. mAbP CO17-1A has a different glycosylation profile than that of the parental mAbM, despite the same biantennary structure (GlcNAc2Man3GlcNAc2) in both plants and animals (Fig. 6) (22). Specifically, mAbP has β (1,2)-Xyl, whereas mAbM has α (1,6)-Fuc and an additional α-galactose (Gal) residue (Fig. 6). Although it is well known that variations in antibody glycan structures influence the biological functions of the Fab region, such as binding activity to its antigen (15, 16), we found that mAbP CO17-1A retains its specific binding activity to colorectal cancer cells. This result was not unexpected, because mAb CO17-1A does not carry the glycosylation site on the LC and HC variable region crucial for its specific binding activity. In accord, our previous study (13) showed that altered glycosylation in the Fc region of human antirabies mAb 57 does not affect antigen-specific binding activity.
Fig. 6.
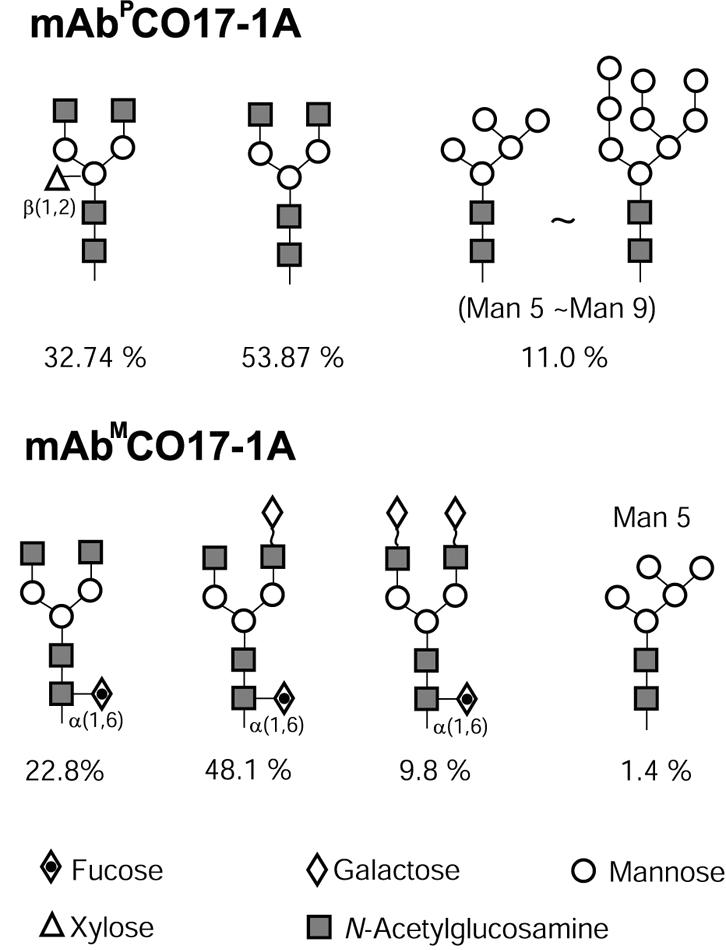
Profile of plant glycosylation pattern on mAbP and mAbm. The percent of normal-phase HPLC areas of each glycan structure was analyzed with previously reported data (22).
Although altered glycosylation of the Fc region of mAb CO17-1A does not affect specific binding activity, it may lead to loss of mAb affinity for the Fc receptors, with consequent compromise of effector functions (15, 24, 25). However, in the nude mouse model, the mAbP CO17-1A was as effective as the parental mAb CO17-1A in suppressing human colorectal tumor growth. Tumor inhibition by anticancer mAbs is due mainly to the effector functions of antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-mediated lysis (26). For mAb CO17-1A, tumor growth inhibition is due mainly to the ADCC effector function (14). Thus, the altered glycosylation pattern on the Fc region of mAbP CO17-1A did not affect adversely ADCC effector functions. In contrast with mAbM CO17-1A, which contains α(1,6)-Fuc and an additional α-Gal residue (Fig. 6) (22), the N-glycosylation profile of mAbP shows GlcNAc2Man3GlcNAc2 (53.87%), GlcNAc2Man3[β (1,2)-Xyl] GlcNAc2 (32.74%), and Man5–8GlcNAc2 (11%) (22). This glycosylation profile of mAbP CO17-1A differs from that in previous reports of other mAbPs (17, 27, 28). The profile discrepancy reflects the intrinsic properties of the Fc region of mAbs, which affect the accessibility of the individual glycans and inherent efficiency of the mAb secretion in plants (7). Fuc does not appear on the mAb CO17-1A expressed in transgenic tobacco (22), suggesting that secretion of this mAb CO17-1A is not fully processed in tobacco plants, unlike alfalfa, which shows highly efficient secretion and/or folding machinery (7, 28). This absence of Fuc on mAb CO17-1A might benefit its ADCC effector function (29). Modification of mAb glycosylation structures has been reported to enhance effector functions (28, 29). Other studies (14, 31) have reported that removal of Gal reduced complement-mediated lysis of antigen-bearing target cells but does not affect ADCC for a certain IgG subclass. Thus, when major effector functions of a given mAb are actually mediated by ADCC, the absence of Fuc and Gal from mAbP in N-glycosylation might increase the mAb antitumor activity by enhancing ADCC effector function (14, 29). Indeed, our mAbP CO17-1A lacking Fuc and Gal but containing the plant-specific sugar β(1,2)-Xyl exhibited tumor inhibition activity comparable with that of the parental mAbM. Further studies to directly compare mAbP and mAbM for binding activity to Fc receptors and ADCC will help to determine whether plant-altered glycosylation of Fc influences effector functions.
Our findings indicate that anti-colorectal cancer mAbP CO17-1A with the plant-altered glycosylation on the mAb Fc has biological activities similar to those of the mAbM in vitro and in vivo. Modification of mAb N-glycosylation in plants has been well characterized so that the plant production system is amenable to study of the effect of glycosylation on mAb biological activities.
Acknowledgments
We thank Dr. D. Deka and Ms. P. Matyszczuk for technical assistance. This work was supported by the U.S. Department of Agriculture.
Abbreviations: mAbP, plant-derived mAb; mAbm, mammalian-derived mAb; Pin2, potato proteinase inhibitor II; HC, heavy chain; LC, light chain; ADCC, antibody-dependent cell-mediated cytotoxicity; Fuc, fucose.
References
- 1.Koprowski, H., Steplewski, Z., Mitchell, K., Herlyn, M., Herlyn, D. & Fuhrer, P. (1979) Somatic Cell Genet. 5, 957–971. [DOI] [PubMed] [Google Scholar]
- 2.Riethmüller, G., Schneider-Gadicke, E., Schlimok, G., Schmiegel, W., Raab, R., Hoffken, K., Gruber, R., Pichlmaier, H., Hirche, H. & Pichlmayr, R. (1994) Lancet 343, 1177–1183. [DOI] [PubMed] [Google Scholar]
- 3.Riethmüller, G., Holz, E., Schlimok, G., Schmiegel, W., Raab, R., Hoffken, K., Gruber, R., Funke, I., Pichlmaier, H., Hirche, H., et al. (1998) J. Clin. Oncol. 16, 1788–1794. [DOI] [PubMed] [Google Scholar]
- 4.Stoger, E., Sack, M., Fischer, R. & Christou, P. (2002) Curr. Opin. Biotechnol. 13, 161–166. [DOI] [PubMed] [Google Scholar]
- 5.Ma, J. K., Drake, P. M. & Christou, P. (2003) Nat. Rev. Genet. 4, 794–805. [DOI] [PubMed] [Google Scholar]
- 6.Daniell, H., Streatfield, S. J. & Wycoff, K. (2001) Trends Plant Sci. 6, 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomord, V., Sourrouille, C., Fitchette, A.-C., Bardor, M., Pagny, S., Lerouge, P. & Faye, L. (2004) Plant Biotechnol. J. 2, 83–100. [DOI] [PubMed] [Google Scholar]
- 8.Verch, T., Yusibov, V. & Koprowski, H. (1998) J. Immunol. Methods 220, 69–75. [DOI] [PubMed] [Google Scholar]
- 9.Smith, G., Walmsley, A. & Polkinghorne, I. (1997) Reprod. Fertil. Dev. 9, 85–89. [DOI] [PubMed] [Google Scholar]
- 10.Koprowski, H. & Yusibov, V. (2001) Vaccine 19, 2745–2741. [DOI] [PubMed] [Google Scholar]
- 11.Ma, J. K., Hikmat, B. Y., Wycoff, K., Vine, N. D., Chargelegue, D., Yu, L., Hein, M. B. & Lehner, T. (1998) Nat. Med. 4, 601–606. [DOI] [PubMed] [Google Scholar]
- 12.Zeitlin, L., Olmsted, S. S., Moench, T. R., Co, M. S., Martinell, B. J., Paradkar, V. M., Russell, D. R., Queen, C., Cone, R. A. & Whaley, K. J. (1998) Nat. Biotechnol. 16, 1361–1364. [DOI] [PubMed] [Google Scholar]
- 13.Ko, K., Tekoah, Y., Rudd, P. M., Harvey, D. J., Dwek, R. A., Spitsin, S., Hanlon, C. A., Rupprecht, C., Dietzschold, B., Golovkin, M. & Koprowski, H. (2003) Proc. Natl. Acad. Sci. USA 100, 8013–8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herlyn, M., Steplewski, Z., Herlyn, D. & Koprowski, H. (1986) Hybridoma 5, S3–S10. [PubMed] [Google Scholar]
- 15.Wright, A. & Morrison, S. L. (1997) Trends Biotechnol. 15, 26–32. [DOI] [PubMed] [Google Scholar]
- 16.Rudd, P. M., Elliott, T., Cresswell, P., Wilson, I. A. & Dwek, R. A. (2001) Science 291, 2370–2376. [DOI] [PubMed] [Google Scholar]
- 17.Cabanes-Macheteau, M., Fitchette-Laine, A. C., Loutelier-Bourhis, C., Lange, C., Vine, N. D., Ma, J. K., Lerouge, P. & Faye, L. (1999) Glycobiology 9, 365–372. [DOI] [PubMed] [Google Scholar]
- 18.Datla, R. S. S., Bekkaoui, F., Hammerlindl, J. K., Pilate, G., Dunstan, D. I. & Crosby, W. L. (1993) Plant Sci. (Limerick, Irel.) 94, 139–149. [Google Scholar]
- 19.Ko, K., Norelli, J. L., Reynoird, J.-P., Boresjza-Wysocka, E., Brown, S. K. & Aldwinckle, H. S. (2000) Biotechnol. Lett. 22, 373–381. [Google Scholar]
- 20.Ko, K., Wei, X., Crooks, P. A. & Koprowski, H. (2004) J. Immunol. Methods 286, 79–85. [DOI] [PubMed] [Google Scholar]
- 21.Strassburg, C. P., Kasai, Y., Seng, B. A., Miniou, P., Zaloudik, J., Herlyn, D., Koprowski, H. & Linnenbach, A. J. (1992) Cancer Res. 52, 815–821. [PubMed] [Google Scholar]
- 22.Tekoah, Y., Ko, K., Koprowski, H., Harvey, D. J., Wormald, M. R., Dwek, R. A. & Rudd, P. M. (2004) Arch. Biochem. Biophys. 426, 266–278. [DOI] [PubMed] [Google Scholar]
- 23.Conrad, U. & Fiedler, U. (1998) Plant Mol. Biol. 38, 101–109. [PubMed] [Google Scholar]
- 24.Jefferis, R. & Lund, J. (2002) Immunol. Lett. 82, 57–65. [DOI] [PubMed] [Google Scholar]
- 25.Krapp, S., Mimura, Y., Jefferis, R., Huber, P. & Sondermann, J. (2003) J. Mol. Biol. 325, 979–989. [DOI] [PubMed] [Google Scholar]
- 26.Gelderman, K. A., Tomlinson, S., Ross, G. D. & Gorter, A. (2004) Trends Immunol. 25, 158–164. [DOI] [PubMed] [Google Scholar]
- 27.Bardor, M., Loutelier-Bourhis, C., Paccalet, T., Cosette, P., Fitchette, A.-C., Vezina, L. P., Trepanier, S., Dargis, M., Lemieux, G., Lange, C., et al. (2003) Plant Biotechnol. J. 1, 451–462. [DOI] [PubMed] [Google Scholar]
- 28.Wright, A. & Morrison, S. L. (1998) J. Immunol. 160, 3393–3402. [PubMed] [Google Scholar]
- 29.Shields, R. L., Lai, J., Keck, R., O'Connell, L. Y., Hong, K., Meng, Y. G., Weikert, S. H. A. & Presta, L. (2002) J. Biol. Chem. 277, 26733–26740. [DOI] [PubMed] [Google Scholar]
- 30.Boyd, P. N., Lines, A. C. & Patel, A. K. (1995) Mol. Immunol. 32, 1311–1318. [DOI] [PubMed] [Google Scholar]
- 31.Bakker, H., Bardor, M., Molthoff, J. W., Gomord, V., Elbers, I., Stevens, L. H., Jordi, W., Lommen, A., Faye, L., Lerouge, P. & Bosch, D. (2001) Proc. Natl. Acad. Sci. USA 98, 2899–2904. [DOI] [PMC free article] [PubMed] [Google Scholar]