Abstract
Human immunodeficiency virus type 1 (HIV-1)-infected individuals often exhibit multiple hematopoietic abnormalities reaching far beyond loss of CD4+ lymphocytes. We used the SCID-hu (Thy/Liv) mouse (severe combined immunodeficient mouse transplanted with human fetal thymus and liver tissues), which provides an in vivo system whereby human pluripotent hematopoietic progenitor cells can be maintained and undergo T-lymphoid differentiation and wherein HIV-1 infection causes severe depletion of CD4-bearing human thymocytes. Herein we show that HIV-1 infection rapidly and severely decreases the ex vivo recovery of human progenitor cells capable of differentiation into both erythroid and myeloid lineages. However, the total CD34+ cell population is not depleted. Combination antiretroviral therapy administered well after loss of multilineage progenitor activity reverses this inhibitory effect, establishing a causal role of viral replication. Taken together, our results suggest that pluripotent stem cells are not killed by HIV-1; rather, a later stage important in both myeloid and erythroid differentiation is affected. In addition, a primary virus isolated from a patient exhibiting multiple hematopoietic abnormalities preferentially depleted myeloid and erythroid colony-forming activity rather than CD4-bearing thymocytes in this system. Thus, HIV-1 infection perturbs multiple hematopoietic lineages in vivo, which may explain the many hematopoietic defects found in infected patients.
Human immunodeficiency virus type 1 (HIV-1)-infected individuals may exhibit multiple hematopoietic abnormalities including anemia, granulocytopenia, thrombocytopenia, and myelodysplastic/hyperplastic alterations of the bone marrow, suggesting virus-induced abnormalities in the bone marrow microenvironment (7, 9, 11, 35). Evidence of alteration of fetal hematopoiesis including leukopenia, anemia, and thrombocytopenia has also been found in aborted fetuses from HIV-1-seropositive women (6, 32). These observations suggest that HIV-1 infection may affect processes important during early stages of hematopoiesis. However, several different factors, including direct intracellular effects of virus infection, interaction with viral proteins at the cell surface, perturbation of the cytokine network, or immune-mediated effects, may play a role.
Following HIV-1 infection in vitro in long-term bone marrow cultures, inhibition of hematopoietic progenitor cell production occurs and alteration of production of cytokines relevant to hematopoiesis has been documented (14, 15, 27). These infected stromal cultures showed reduced production of the cytokines interleukin-6 (IL-6) and granulocyte colony-stimulating factor, which could affect regulatory signals important in hematopoiesis. Further in vitro studies suggested that HIV-1-induced suppression of hematopoiesis is mediated by the HIV-1-encoded envelope glycoprotein gp120 and the Nef regulatory protein, as well as by cellular proteins such as tumor necrosis factor alpha (8, 23). The p24 Gag protein of HIV-1 also was shown to inhibit myeloid colony formation of bone marrow cultures but had minor effects on erythroid colony formation (29). Purified CD34+ cells were reported to be susceptible to HIV-1 infection, as shown by the presence of proviral sequences in the ensuing colonies of erythroid and myeloid lineages generated from these cells (10). These effects could be influenced by the infection of microvascular endothelial cells of bone marrow stromal cultures from HIV-seropositive patients (27). Infection of these cells could affect the relevant neighboring microenvironment, by providing a continuing source of virus and by causing alteration of local cytokine levels. Therefore, HIV is likely to alter the stromal/progenitor cell microenvironment that supports hematopoiesis. However, these previous studies on in vitro consequences of virus infection could not determine how HIV-1 infection influences complex hematopoietic microenvironments in vivo.
To investigate how HIV-1 might affect hematopoiesis in vivo, we used the SCID-hu (Thy/Liv) mouse model, in which human fetal thymus and liver tissue are coimplanted into severe combined immunodeficient mice, resulting in a functional human hematopoietic organ (Thy/Liv) (24, 28). This model allows maintenance and differentiation through thymopoiesis of human hematopoietic progenitor cells (28) and also recapitulates the effects of HIV-1 infection in the human thymus. Direct infection of various strains of HIV-1 into Thy/Liv implants results in severe depletion of CD4-bearing human thymocytes which mirrors that seen in infected individuals (2, 5, 17, 20, 21, 30). Morphologic alterations of the thymic stroma, possibly due to infection of thymic epithelial cells, have been seen (30). These effects are precipitated by a large proviral burden, which may be as high as nearly one copy of the viral genome per CD4+ cell (18). Both direct virus-induced killing (18) and indirect apoptotic effects (31) have been implicated in the observed depletion of CD4+ thymocytes. Furthermore, this model has been used to determine the viral accessory genes involved in pathogenesis (3, 16) and the response of HIV-1 to antiviral therapies (19, 25, 26, 33). Previously, McCune et al. (24) reported that human pluripotent hematopoietic progenitor cells capable of giving rise to myeloid and erythroid colonies ex vivo in response to cytokines are maintained for extended periods of time in this model.
The SCID-hu system has thus proven relevant to the clinical situation, is amenable to manipulation and analyses, and offers an opportunity to investigate the effects of HIV-1 infection on multiple arms of hematopoiesis. Here we report that HIV-1 infection profoundly decreases the ability to recover hematopoietic colony-forming activity (CFA) from Thy/Liv implants. However, this effect is reversible following administration of combination antiretroviral therapy. Our studies thus establish a causal effect of viral replication on hematopoiesis of multiple lineages. In addition, the reversible nature of this inhibitory effect following therapy suggests that neither the very immature hematopoietic progenitor cell nor the differentiation-inducing microenvironment is destroyed by high levels of HIV-1. Furthermore, we identify a viral strain, isolated from a pediatric patient exhibiting severe hematologic abnormalities, which preferentially inhibits hematopoietic CFA rather than inducing CD4+ thymocyte depletion. Together our studies suggest that HIV-1 may be directly responsible for many of the hematopoietic perturbations seen in infected individuals.
MATERIALS AND METHODS
Construction and infection of SCID-hu mice.
SCID mice were transplanted with fragments of human fetal thymus and liver tissue as previously described and infected with 100 infectious units (IU) of HIV-1 by direct intraimplant injection (2, 28). At specific time intervals postinfection, sequential wedge biopsies (∼25% of each implant) were obtained, with mock-infected SCID-hu mice serving as negative controls.
Drug treatment of animals.
The combination drug treatment used was identical to that previously described (33) and included the following: protease inhibitor A77003 (Abbott Laboratories, Chicago, Ill.) at a dose of 50 mg/kg of body weight/day, delivered intraperitoneally; and ddI (Aldrich Chemical Co., Milwaukee, Wis.) at a dose of 50 mg/kg/day, also given intraperitoneally. Zidovudine (AZT; Aldrich) was delivered in drinking water (0.4 mg/ml) at a dose of 66 mg/kg/day, based on an estimate of consumption of 3 ml of water/day/animal and an average weight of 20 g/mouse.
Primary HIV-1 strains. (i) Virus isolates from hemophiliac patients.
Viruses were isolated from sequentially acquired cryopreserved peripheral blood mononuclear cells (PBMCs) obtained from two HIV-1-infected hemophiliacs who were monitored over approximately 4 years. Both subjects experienced a precipitous decline in CD4+ cells associated with a switch from non-syncytium-inducing (NSI) to syncytium-inducing (SI) strains. Viruses V26, V34, V19, and V22 were derived from one of these patients, and V1, V3, and V10460 were derived from the other.
(ii) Virus isolates from patient with hematopoietic abnormalities.
The isolates PT3MO and PT8MO were derived by coculture PBMCs from an infant patient at 3 and 8 months of age, respectively. The infant was a 1.6-kg 35-week-old premature female born of a 39-year-old gravida VII, para VII mother who was unaware of her HIV-1-seropositive status. The infant had an unremarkable stay in the nursery intensive care unit and did not require intubation or blood product transfusion. After discharge by 6 weeks of age, she developed upper lobe pneumonia and severe anemia and neutropenia with an absolute neutrophil count of 75/mm3. She had repeated episodes of respiratory distress, pneumonia, otitis media, and hepatosplenomegaly and severe pancytopenia. She had several documented leukocyte counts with no neutrophils on smear and 95% lymphocytes with a hemocrit of 18. HIV-1 infection was diagnosed by a positive virus culture and DNA PCR. She had a low CD4 cell count of 810 cells/mm3 at 3 months of age, which fell to 414/mm3 by 4 months and to below 100/mm3 by 8 months. At 4 months of age, she was started on a treatment protocol of AZT and nevirapine and given prophylactic sulfamethoxazole-trimethoprim (Bactrim). She progressed rapidly to AIDS with associated neurological developmental delay and with cytomegalovirus retinitis which was treated with ganciclovir. She continued to have problems with anemia and neutropenia. She developed cardiomyopathy and expired at 10 months of age due to complications of AIDS. Her mother and older sibling of 2 years of age were also diagnosed with HIV-1 infection.
Hematopoietic CFA in Thy/Liv implants.
Total Thy/Liv cells (107) were suspended in 2 ml of methylcellulose medium (Methocult H4320; Stem Cell Technologies Inc., Vancouver, British Columbia, Canada) containing 100 ng each of stem cell factor (SCF), granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-3, and IL-6 per ml and 2 U of erythropoietin per ml to allow the differentiation of progenitor cells. The cells were then seeded in duplicate 1-ml cultures, and hematopoietic colonies were enumerated by microscopy.
Quantitative PCR to detect HIV DNA.
To quantitate proviral burden in Thy/Liv implants, PCR was performed with end-radiolabeled primers specific for the R/U5 region of the viral long terminal repeat and for human β-globin as an internal standard. Quantitation (number of copies of HIV/100,000 cells) was achieved by comparison to standard curves consisting of cloned viral DNA and uninfected PBMC DNA, followed by radioanalytic image analysis as previously described (17, 33, 34).
To assess the presence of provirus in hematopoietic colonies, we collected single colonies of 500 cells or larger 2 weeks after plating, while viewing under a microscope. The cells were washed and lysed, and the DNA was subjected to phenol-chloroform extraction in the presence of tRNA (10 mg/ml) as a carrier, followed by ethanol precipitation. The resultant DNA was subjected to quantitative PCR analysis (25 cycles) for HIV-1 and human β-globin gene sequences, which confirmed the presence of human cellular DNA.
Flow cytometry.
Cells (0.5 × 106) from mock- or HIV-infected implants were stained with a three-color mixture of monoclonal antibodies consisting of mouse anti-human CD4-phycoerythrin (CD4-PE), CD8-fluorescein isothiocyanate (CD8-FITC), and CD3-peridinin chlorophyll protein (CD3-PerCP), obtained from Becton Dickinson, Mountain View, Calif. The cells were fixed in phosphate-buffered saline containing 1% formalin, and data were acquired with a Becton Dickinson FACScan flow cytometer. Isotype controls were used to set compensation for the analysis. The data were converted by a FACS Convert program for analysis, using CellQuest software to calculate the percentage of cells stained by each label. Other conjugated monoclonal antibodies purchased from Becton Dickinson and used similarly include anti-human CD34-FITC and CD45-PerCP and isotype control antibodies, γ1-PE, -FITC, and -PerCP. Other isotype control antibodies, goat anti-mouse (GAM) IgG2a (immunoglobulin G2a)-PE, IgG2b-PE, and IgG2b-FITC, were purchased from Caltag Laboratories (South San Francisco, Calif.). The anti-CXCR4 monoclonal antibody 12G5 (12) was from the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health (donated by J. Hoxie).
CD3+ cell depletion.
Total cells from mock- and HIVNL4-3-infected Thy/Liv implants were incubated with mouse anti-human monoclonal antibody OKT3 (UCLA Pharmacy) at 0.25 mg/106 cells on ice for 30 min. The cells were then washed and added to culture flasks previously coated with 5 ml of GAM antibody (100 mg/ml). The flasks were then centrifuged twice at 1,200 rpm for 5 min, to ensure attachment of cells to surface GAM antibody. The CD3-depleted nonadherent cells were collected, washed, and labeled with anti-CD3-PerCP, anti-CD4-PE, and anti-CD8-FITC monoclonal antibodies as described above. The panning procedure was repeated three times prior to labeling with the antibodies to attain maximum CD3+ cell depletion. For the cells derived from implants of 12 (6 mock and 6 infected) similarly treated mice, the recovery following CD3+ cell depletion ranged from 2.7 to 12.7%.
RESULTS AND DISCUSSION
Effects of HIV-1 infection on hematopoietic CFA.
To investigate the effects of HIV-1 infection on early events in myeloid and erythroid hematopoiesis, Thy/Liv cells were plated in methylcellulose in the presence of IL-3, IL-6, SCF, GM-CSF, and erythropoietin. Consequently, and also as previously reported (24), hematopoietic progenitor cells able to differentiate into myeloid and erythroid colonies in vitro were reproducibly obtained from cells from uninfected Thy/Liv implants. The fetal liver origin of these precursors was confirmed in that essentially no colonies were detectable from the same number of fetal thymocytes similarly cultured. Little variation in recovery of these precursor cells is seen following multiple simultaneous or sequential biopsies of the same implant or from multiple Thy/Liv implants generated from the same fetal tissue donor (Fig. 1A and B). Since recovery of cells from implants derived from different fetal tissue donors varied, each individual experiment herein was conducted with implants derived from the same fetal donor.
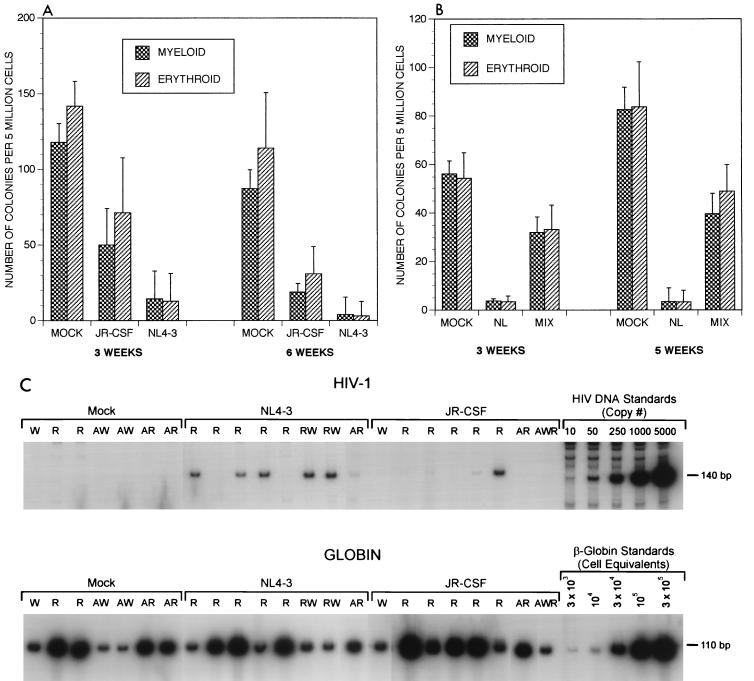
FIG. 1.
(A) HIV-1-induced inhibition of hematopoietic colony formation. Each bar represents data obtained from four uninfected or four HIV-1-infected animals. Sequential biopsies from the same implants were performed at both 3 and 6 weeks postinfection. Standard deviations were calculated by taking into account the duplicate sets of plates for each sample. The data from a second experiment (not shown) were similar to those shown. (B) Inhibition of hematopoiesis is an in vivo phenomenon. Cells from uninfected and HIV-1NL4-3-infected Thy/Liv implants (four animals each) obtained at 3 and 5 weeks postinfection were washed, mixed in equal numbers, and plated on methylcellulose. Hematopoietic colonies were evaluated as described in Materials and Methods. (C) PCR analyses of 23 representative myeloid and erythroid colonies, 7 derived from mock-infected and 16 derived from virus (8 of NL4-3 and 8 of JR-CSF strain)-infected Thy/Liv implants. These include six infected colonies. The lanes showing myeloid and erythroid colonies are indicated as W (white) and R (red), respectively. WR is a putative mixed progenitor colony. The letter A preceding W/R/WR indicates that the methylcellulose supporting the colony growth contained AZT (1 μg/ml). Quantitation for proviral DNA copies was done with a primer pair for β-globin as internal standard.
Following infection of Thy/Liv implants with each of two well-defined molecular clones of HIV-1, both myeloid and erythroid CFA were decreased (Fig. 1A). The colony numbers were reduced to minimal levels (<10%) by 3 weeks postinfection (Fig. 1A and Table 1) with the CXCR4-tropic SI HIV-1NL4-3 strain (1). The NSI CCR5-tropic strain, HIV-1JR-CSF (22), also inhibited CFA, but less aggressively than did the SI strain. Inclusion of AZT (1 μg/ml) in the methylcellulose did not increase the numbers of colonies formed, suggesting that virus infection in vitro was not causing this effect (not shown). To confirm that the observed hematopoietic inhibition occurred in vivo rather than in vitro due to the presence of infected thymocytes, equal numbers of cells from uninfected and infected implants were mixed ex vivo prior to culture in methylcellulose (Fig. 1B). The presence of infected thymocytes did not affect the CFA of cells from uninfected implants; thus, infection or exposure to the products of infected cells in vitro was not responsible for the observed inhibition.
TABLE 1.
HIV-1-induced inhibition of hematopoiesis at 3 and 6 weeks postinfectiona
| Virus infection (no. of animals) | HIV-1 phenotype | Mean ± SD
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 wk postinfection
|
6 wk postinfection
|
||||||||
| HIV-1 copies/ 100,000 cells | % Total CD4 | Myeloid colonies | Erythroid colonies | HIV-1 copies/ 100,000 cells | % Total CD4 | Myeloid colonies | Erythroid colonies | ||
| Mock (5) | 0 | 96 ± 1 | 134 ± 10 | 130 ± 12 | 0 | 90 ± 2 | 110 ± 27 | 96 ± 15 | |
| V3 (4) | NSI | 6.9 × 102 ± 8.0 × 102 | 96 ± 1 | 67 ± 32 | 50 ± 29 | 4.6 × 102 ± 1.7 × 102 | 90 ± 3 | 34 ± 19 | 23 ± 9 |
| V26 (4) | NSI | 7.6 × 102 ± 4.1 × 102 | 85 ± 21 | 17 ± 28 | 18 ± 23 | 4.5 × 102 ± 2.2 × 102 | 92 ± 1 | 27 ± 15 | 37 ± 31 |
| V19 (5) | NSI | 1.7 × 102 ± 2.4 × 102 | 96 ± 1 | 46 ± 28 | 42 ± 26 | 0.9 × 102 ± 0.5 × 102 | 91 ± 2 | 19 ± 4 | 24 ± 17 |
| V22 (5) | NSI | <0.5 × 102 | 96 ± 1 | 51 ± 56 | 39 ± 41 | 1.8 × 102 ± 1.1 × 102 | 92 ± 1 | 25 ± 11 | 28 ± 9 |
| V1 (4) | SI | 4.0 × 105 ± 1.3 × 105 | 56 ± 8 | 12 ± 7 | 6 ± 6 | 1.1 × 104 ± 0.5 × 104 | 10 ± 12 | 17 ± 15 | 16 ± 13 |
| V10460 (5) | SI | 2.2 × 105 ± 1.5 × 105 | 91 ± 5 | 9 ± 9 | 8 ± 13 | 6.7 × 104 ± 5.1 × 104 | 23 ± 6 | 4 ± 3 | 2 ± 1 |
| V34 (4) | SI | 1.6 × 105 ± 0.8 × 105 | 47 ± 27 | 2 ± 2 | 2 ± 2 | 1.0 × 104 ± 0.6 × 104 | 8 ± 4 | 1 ± 1 | 1 ± 1 |
| NL4-3 (6) | SI | 3.3 × 105 ± 2.0 × 105 | 85 ± 7 | 5 ± 3 | 2 ± 5 | 2.2 × 104 ± 0.1 × 104 | 9 ± 2 | 1 ± 1 | 1 ± 1 |
Pathogenicity of several primary HIV-1 isolates following infection of Thy/Liv tissue. The phenotype of each isolate as determined by MT2 cell tropism is indicated. HIV-1NL4-3 is used as a comparative control. Cells (the majority of which are thymocytes) from infected and control implants were obtained at 3 and 6 weeks postinfection. The primary virus isolates are compared for their effects on myeloid and erythroid CFA, CD4+ thymocyte depletion (percent total CD4), and virus replicative ability in thymocytes by quantitative PCR. Percent total CD4 includes both CD4+ CD8+ and CD4+ CD8− subsets.
To determine if direct infection of hematopoietic progenitor cells was occurring, resulting in loss of CFA, we performed quantitative PCR amplification using primers specific for viral sequences (34). These studies detected proviral DNA associated with only 10 of 100 myeloid and erythroid colonies derived from infected implants (Fig. 1C). Furthermore, proviral DNA was at a low copy number compared to quantitated human β-globin gene sequences (<0.05 copy/cell), indicating that provirus was not present in the original precursor cells forming these colonies. This low level of viral DNA is most likely contamination from infected thymocytes rather than genuine infection in most cases. Thus, surviving colonies are in general not HIV-1 infected. However, this apparent lack of infection does not rule out the possibility that any infected hematopoietic precursor cells may have been killed prior to colony formation. Alternatively, HIV-1 infection of the Thy/Liv implants may alter the hematopoietic microenvironment affecting progenitor cell differentiation.
Effects of different primary HIV-1 isolates on CFA.
To determine viral characteristics associated with this inhibitory phenomenon, seven additional primary HIV-1 isolates (three SI and four NSI [Table 1]) derived from hemophiliac patients infected during childhood were tested. The SI strains were more aggressive in both the inhibition of CFA as well as thymocyte depletion than were the NSI strains, correlating with a severalfold-greater replicative ability in thymocytes (Table 1). This is consistent with the pathogenicity profiles of the HIV-1JR-CSF and HIV-1NL4-3 molecular clones (Fig. 1A) and with previous studies of different virus strains on thymocytes (17, 20). Additionally, the inhibition of CFA kinetically preceded thymocyte depletion (Table 1). Since colony-forming progenitor cells are distinct from cells of the lymphoid lineage, this difference in kinetics suggests a fundamental difference in the pathogenic effects of HIV-1 on these two cell types.
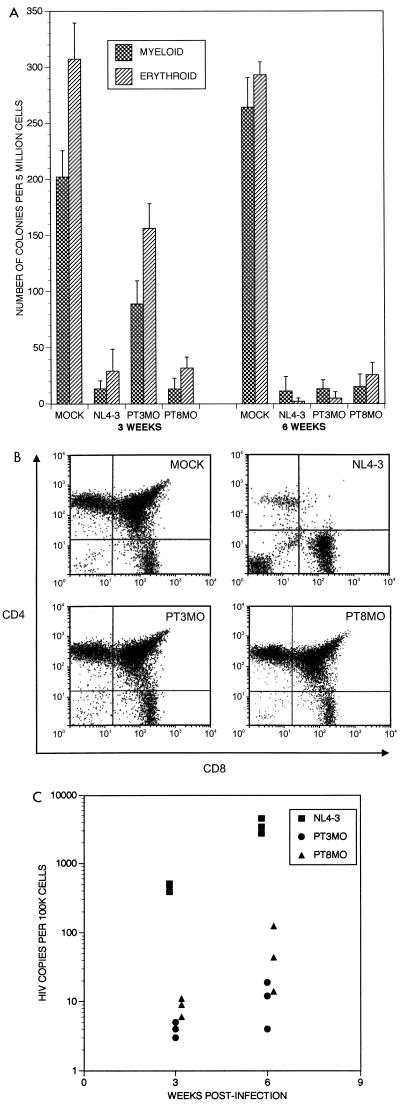
To investigate the relationship between effects on progenitor cells in SCID-hu mice and the clinical situation, two additional primary HIV-1 isolates (PT3MO and PT8MO) obtained from a pediatric AIDS patient exhibiting severe hematopoietic abnormalities (see Materials and Methods for clinical details) were assessed for the ability to deplete CFA (Fig. 2A). Interestingly, although the PT8MO isolate caused profound depletion of CFA, in contrast to other isolates aggressive against CFA, thymocyte depletion was not induced even by 6 weeks postinfection (Fig. 2B). This was likely due to a decreased replicative ability in thymocytes, as proviral loads were ∼350-fold lower at 3 weeks postinfection than those in implants infected with HIV-1NL4-3 (Fig. 2C). Thus, with the obvious exception of the PT8MO isolate, ability to deplete CFA correlated with thymocyte depletion caused by increased virus loads (Table 1), suggesting a unique phenotype or selective tropism of the PT8MO isolate toward progenitor cells of the non-T-cell lineages. Our data suggest that the severe hematological abnormalities seen in this patient were directly due to the presence of HIV-1. In addition, these results suggest, as did the kinetic study described above, that HIV-1 can have differential effects on thymocytes and hematopoietic progenitor cells. Future studies are planned to map the region of the viral genome responsible for these effects.
FIG. 2.
(A) Effects of pediatric viral isolates, PT3MO and PT8MO (see Materials and Methods for derivation and definition), on hematopoiesis in vivo. Titers of the isolates were determined by PCR on normal PBMCs in parallel, and 200 IU of each strain was injected into SCID-hu Thy/Liv implants. Each bar represents data obtained from four animals for each condition. Hematopoietic progenitor cell CFA was determined at 3 and 6 weeks postinfection. (B) The CD4/CD8 profiles of thymocytes from SCID-hu Thy/Liv implants at 6 weeks postinfection. Histograms from mock-infected implants and implants infected with HIV-1 strains NL4-3, PT3MO, and PT8MO are indicated. (C) Virus replication of pediatric isolates in Thy/Liv implants. The chart shows proviral loads in thymocytes (number of HIV copies/100,000 cells) from Thy/Liv implants infected with the pediatric isolates PT3MO and PT8MO and with HIV-1NL4-3. Determination of viral loads was performed by quantitative PCR at 3 and 6 weeks postinfection. Each symbol represents a single biopsy sample.
Effects of HIV-1 infection on levels of CD34+ cells.
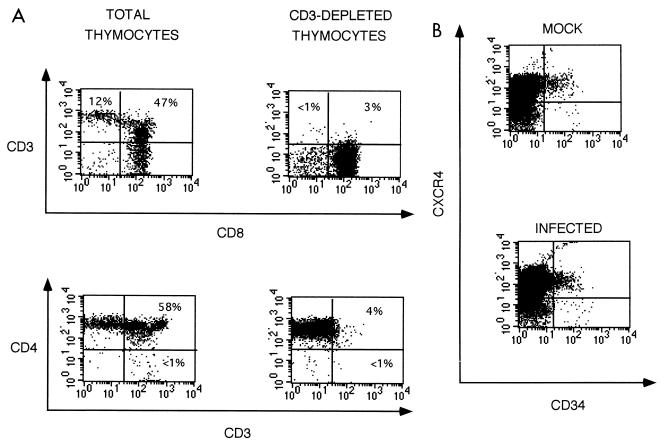
To help define the mechanism of HIV-1-induced inhibition of CFA, we focused our studies on the susceptibility of early progenitor cells to virus-mediated effects. Since we find that approximately 15% of fetal liver-derived CD34+ cells form colonies in vitro, we reasoned that if HIV killed these cells, their loss might be detected in vivo. We first determined that Thy/Liv cells depleted of CD3+ thymocytes by panning (Fig. 3A) and consequently enriched for CD34+ cells (Fig. 3B) exhibited a 50- to 100-fold enhancement of CFA (activities of 28,476 ± 3,086, 8 ± 2, and 342 ± 38 colonies/5 × 106 cells for CD3-depleted, CD3-enriched, and total Thy/Liv cells, respectively). Flow cytometry was then used to evaluate the effect of infection of Thy/Liv implants on CD34+ cells (Fig. 3B; Table 2). Total Thy/Liv cells derived from control and infected implants biopsied at 3 weeks postinfection prior to CD4+ cell loss but after inhibition of CFA were enriched for CD34+ cells by depletion of CD3+ thymocytes (Fig. 3A). Since it was difficult to detect CD4 on CD34+ cells, we colabeled the CD3-depleted cells with an anti-CXCR4 antibody (12) that binds to the HIV coreceptor (4, 13), along with common leukocyte antigen-specific anti-CD45 to ensure the human origin of all evaluated cells. HIV-1 infection did not deplete the levels of total CD34+ cells or those of CD34+ CXCR4+ cells, although the CFA was decreased to minimal levels (Fig. 3B; Table 2). This finding suggests either that the HIV-1-induced inhibitory effects occur late in the hematopoietic differentiation process or that CD34+ cell function (e.g., proliferation and maturation) is impaired by HIV-1 without altering CD34+ cell population size. Differentiation pathways via intermediate progenitor cells may be suppressed by the effect of infection on supportive elements, thereby preventing differentiation into their terminal entities. Alternatively, HIV-1-induced CFA inhibition may be the result of direct killing of precursor cells not detected in our assay. However, our data suggest that pluripotent stem cells are not killed, as this would likely result in a decrease of total CD34+ cells, which are progeny of pluripotent stem cells. Additional evidence for the survival of pluripotent stem cells during HIV-1 infection is presented below.
FIG. 3.
(A) Depletion of CD3+ thymocytes. SCID-hu Thy/Liv cells were subjected to CD3 cell depletion by panning. Staining profiles for CD3 versus CD8 and CD4 versus CD3 are illustrated. The flow cytometric profiles shown are representative of a mock-infected implant, but cells from all other implants in this experiment (six mock infected and six HIV-1 infected) produced similar profiles (Table 3). The percentage of cells in each quadrant is indicated. (B) Effect of HIV-1NL4-3 infection on the levels of CD34+ cells in Thy/Liv implants. Flow cytometric analysis was performed on CD3-depleted cells 3 weeks postinfection, using antibodies specific for CD45, CD34, and CXCR4. Gates were set on live cells positive for CD45, and the staining characteristics for CD34 and CXCR4 markers were analyzed (see Materials and Methods). Samples shown are representative of similar flow cytometric profiles generated for CD45+ cells from six mock-infected and six HIV-1-infected animals. Data obtained from all animals in this experiment are summarized in Table 3.
TABLE 2.
Effects of HIV-1 NL4-3 infection on CD34+ cell levels of Thy/Liv implants
| Group | No. (mean ± SD)a
|
|||
|---|---|---|---|---|
| % Total CD4+ cells | CD34+ CXCR4+ CD45+ cells/million | Myeloid colonies | Erythroid colonies | |
| Mock | 98 ± 1 | 23,000 ± 8,000 | 120 ± 12 | 134 ± 15 |
| Infected | 93 ± 3 | 34,000 ± 22,000 | 9 ± 12 | 6 ± 9 |
Total cells from the implants were depleted of CD3+ thymocytes by panning at 3 weeks postinfection. To determine CD34+ cell levels, the panned cells were costained with anti-CD34, anti-CD45, and anti-CXCR4 monoclonal antibodies. The CD34+ cell levels were analyzed by setting gates on CD45+ cells and determining the percentages of cells which were also positive for CD34 as well as for CXCR4. Cells derived from implants from six mock-infected and six virus-infected animals were analyzed. % total CD4+ cells includes both CD4+ CD8+ and CD4+ CD8− thymocytes as assessed by flow cytometry. To determine the number of CD34+ CXCR4+ human cells, the number of events detected in 50,000 panned cells was corrected for cell loss during the panning procedure and calculated for total recovery per 1 million input Thy/Liv cells.
Effect of antiretroviral therapy on hematopoietic inhibition.
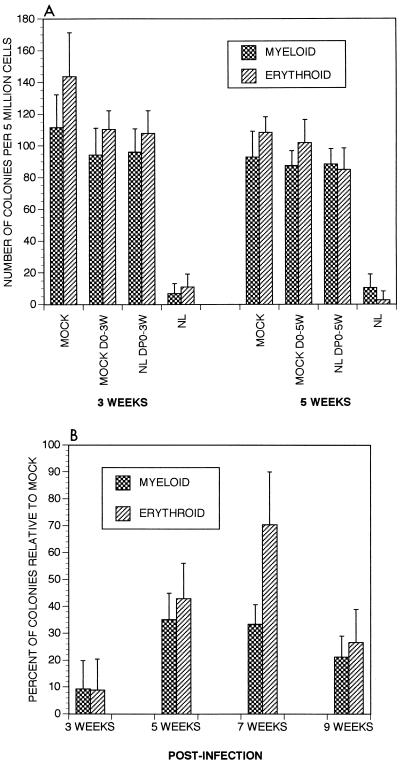
We previously established that we could inhibit HIV replication pharmacologically, using a three-drug combination of AZT, ddI, and a protease inhibitor (A77003) (33). To determine if halting ongoing virus replication would affect HIV-1-induced depletion of CFA, SCID-hu animals were treated for 2 successive days prior to HIV-1 infection, with therapy continued daily. This treatment resulted in an inability to detect proviral DNA sequences in three of four SCID-hu animals at 3 weeks postinfection, and prevented CD4+ thymocyte depletion in all four animals. Proviral DNA in the single animal that was positive 3 weeks postinfection (0.3% cells infected) was undetectable at 6 weeks postinfection (33), indicating that this drug combination can clear low-level infection. This treatment also prevented depletion of CFA in these implants (Fig. 4A). Thus, this drug combination was effective at preventing the pathogenic consequences of HIV infection in this in vivo system.
FIG. 4.
(A) Effect on CFA of antiretroviral therapy prior to infection. The three-drug combination consisting of AZT, ddI, and protease inhibitor was administered 2 days prior to infection. Recovery of myeloid and erythroid CFA at the indicated time points is shown. MOCK D0-3W, mock-infected mice treated with drugs daily for 3 weeks; NLDP0-3W, HIV-1NL4-3-infected mice which received drug treatment beginning 2 days prior to infection and continued daily for 3 weeks. Each group consisted of four animals, and assays were performed in duplicate. (B) Effect of combination drug treatment postinfection. Percent recovery of CFA of hematopoietic progenitor cells from HIV-1NL4-3-infected Thy/Liv implants relative to mock-infected implants following drug treatment is shown. The combination therapy was administered to infected animals beginning 3 weeks postinfection (when only approximately 10% of the original hematopoietic colonies could be recovered from infected implants), and therapy was continued daily. The median values obtained from each mock- and virus-infected implant at each time point were used to calculate the total percent recovery of hematopoietic colonies in drug-treated virus-infected implants relative to mock-infected implants. Each group consisted of four animals. Infected animals not receiving drug therapy showed values of less than 10% of mock at each time point (not shown). The 3-week time point indicates relative percent of CFA present immediately prior to initiation of therapy.
In a subsequent experiment, this combination therapy was administered to animals after colony inhibition (3 weeks postinfection) when virus loads were highest (Table 1; references 3 and 18). Animals not administered drugs exhibited continued decline of CFA (not shown). In contrast, we observed a resurgence of as much as 35% of myeloid and 70% of erythroid colonies in mice that received drugs following the initial loss of CFA (Fig. 4B). However, the rescue of hematopoietic colonies was transient, in that CFA diminished somewhat 6 weeks after therapy. While we were unable to detect a decrease in proviral load in implants of treated animals, we have seen elsewhere that drug treatment generally lowers plasma viremia within 1 week of administration (33). However, viremia is detectable in approximately 40% of infected animals 6 weeks following therapy, suggesting virus breakthrough leading to renewed depletion of CFA. We have noted in our other studies involving the response of T-lineage cells to combination therapies that inclusion of certain other protease inhibitors in the regimen may provide greater therapeutic benefit (33). Thus, it is highly likely that further optimization of the therapeutic regimen used might allow a more prolonged resurgence of CFA. Together, our results establish that the effect of HIV-1 on hematopoietic colony formation is reversible. This renewal of CFA further indicates that the pluripotent stem cell is not killed; rather, the effect is likely manifested following differentiation into lineage-specific colony-producing progenitor cells. In addition, our studies suggest that effective antiretroviral therapy might decrease the hematopoietic abnormalities associated with HIV-1 infection of humans.
In the SCID-hu system, there is no host immune response to HIV-1, which may in part explain virus breakthrough and why the drug-induced resurgence of CFA is only transient. In humans, the effects of drug therapy are likely aided by both cellular and humoral immune responses, which would help control virus replication. We are currently investigating mechanisms of HIV-induced hematopoietic alterations and optimizing therapeutic conditions to sustain multilineage progenitor cell activity in the SCID-hu model. These efforts may lead to the ability to maintain normal levels of hematopoiesis in HIV-infected individuals and may help in alleviating the virus-induced destruction of the immune system.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health to J.A.Z. (AI36554 and AI36059) and Y.B. (AI27550 and HD30629). J.A.Z. is an Elizabeth Glazer Scientist supported by the Pediatric AIDS Foundation. This work is also supported in part by a Pediatric AIDS Foundation Scholar Award (PF-77311) to P.S.K.
REFERENCES
- 1.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldrovandi G M, Feuer G, Gao L, Jamieson B, Kristeva M, Chen I S Y, Zack J A. The SCID-hu mouse as a model for HIV-1 infection. Nature. 1993;363:732–736. doi: 10.1038/363732a0. [DOI] [PubMed] [Google Scholar]
- 3.Aldrovandi G M, Zack J A. Replication and pathogenicity of human immunodeficiency virus type 1 accessory gene mutants in SCID-hu mice. J Virol. 1996;70:1505–1511. doi: 10.1128/jvi.70.3.1505-1511.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berson J F, Long D, Doranz B J, Rucker J, Jirik F R, Doms R W. A seven-transmembrane domain receptor involved in fusion and entry of T-cell-tropic human immunodeficiency virus type 1 strains. J Virol. 1996;70:6288–6295. doi: 10.1128/jvi.70.9.6288-6295.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonyhadi M L, Rabin L, Salimi S, Brown D A, Kosek J, McCune J M, Kaneshima H. HIV induces thymus depletion in vivo. Nature. 1993;363:728–732. doi: 10.1038/363728a0. [DOI] [PubMed] [Google Scholar]
- 6.Burstein Y, Rashbaum W K, Hatch W C, Calvelli T, Golodner M, Soeiro R, Lyman W D. Alterations in human fetal hematopoiesis are associated with HIV infection. Pediatr Res. 1992;32:155–159. doi: 10.1203/00006450-199208000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Calenda V, Chermann J C. The effects of HIV on haematopoiesis. Eur J Hematol. 1992;48:181–186. doi: 10.1111/j.1600-0609.1992.tb01582.x. [DOI] [PubMed] [Google Scholar]
- 8.Calenda V, Graber P, Delamarter J F, Chermann J C. Involvement of HIV nef protein in abnormal hematopoiesis in AIDS: in vitro study on bone marrow progenitor cells. Eur J Hematol. 1994;52:103–107. doi: 10.1111/j.1600-0609.1994.tb01294.x. [DOI] [PubMed] [Google Scholar]
- 9.Cen D, Zauli G, Szarnicki R, Davis B R. Effect of different human immunodeficiency virus type-1 (HIV-1) isolates on long-term bone marrow haemopoiesis. Br J Haematol. 1993;85:596–602. doi: 10.1111/j.1365-2141.1993.tb03353.x. [DOI] [PubMed] [Google Scholar]
- 10.Chelucci C, Hassan H J, Locardi C, Bulgarini D, Pelosi E, Mariani G, Testa U, Federico M, Valtieri M, Peschle C. In vitro human immunodeficiency virus-1 infection of purified hematopoietic progenitors in single-cell culture. Blood. 1995;85:1181–1187. [PubMed] [Google Scholar]
- 11.Davis B R, Zauli G. Effect of human immunodeficiency virus infection on haematopoiesis. Bailliere’s Clin Hematol. 1995;8:113–130. doi: 10.1016/s0950-3536(05)80234-3. [DOI] [PubMed] [Google Scholar]
- 12.Endres M J, Clapham P R, Marsh M, Ahuja M, Turner J D, McKnight A, Thomas J F, Stoebenau-Haggarty B, Choe S, Vance P J, Wells T N, Power C A, Sutterwala S S, Doms R W, Landau N R, Hoxie J A. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996;87:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 13.Feng Y, Broder C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 14.Geissler R G, Ganser A, Ottmann O G, Gute P, Morawetz A, Guba P, Helm E B, Hoelzer D. In vitro improvement of bone marrow-derived hematopoietic colony formation in HIV-positive patients by alpha-D-tocopherol and erythropoietin. Eur J Hematol. 1994;53:201–206. doi: 10.1111/j.1600-0609.1994.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 15.Geissler R G, Rossol R, Mentzel U, Ottmann O G, Klein A S, Gute P, Helm E B, Hoelzer D, Ganser A. Gamma delta-T-cell-receptor-positive lymphocytes inhibit human haematopoietic progenitor cell growth in HIV type 1-infected patients. AIDS Res Hum Retroviruses. 1996;12:577–584. doi: 10.1089/aid.1996.12.577. [DOI] [PubMed] [Google Scholar]
- 16.Jamieson B D, Aldrovandi G M, Planelles V, Jowett J B M, Gao L, Bloch L M, Chen I S Y, Zack J A. Requirement of human immunodeficiency virus type 1 nef for in vivo replication and pathogenicity. J Virol. 1994;68:3478–3485. doi: 10.1128/jvi.68.6.3478-3485.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jamieson B D, Pang S, Aldrovandi G M, Zha J, Zack J A. In vivo pathogenic properties of two clonal human immunodeficiency virus type 1 isolates. J Virol. 1995;69:6259–6264. doi: 10.1128/jvi.69.10.6259-6264.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jamieson B D, Uittenbogaart C H, Schmid I, Zack J A. High viral burden and rapid CD4+ cell depletion in human immunodeficiency virus type 1-infected SCID-hu mice suggest direct viral killing of thymocytes in vivo. J Virol. 1997;71:8245–8253. doi: 10.1128/jvi.71.11.8245-8253.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaneshima H, Shih C-C, Namikawa R, Rabin L, Outzen H, Machado S G, McCune J M. Human immunodeficiency virus infection of human lymph nodes in the SCID-hu mouse. Proc Natl Acad Sci USA. 1991;88:4523–4528. doi: 10.1073/pnas.88.10.4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaneshima H, Su L, Bonyhadi M L, Connor R I, Ho D D, McCune J M. Rapid-high, syncytium-inducing isolates of human immunodeficiency virus type 1 induce cytopathicity in the human thymus of the SCID-hu mouse. J Virol. 1994;68:8188–8192. doi: 10.1128/jvi.68.12.8188-8192.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kollmann T R, Kim A, Peettoello-Mantovani M, Hachamovitch M, Rubinstein A, Goldstein M M, Goldstein H. Divergent effects of chronic HIV-1 infection on human thymocyte maturation in SCID-hu mice. J Immunol. 1995;154:908–921. [PubMed] [Google Scholar]
- 22.Koyanagi Y, Miles S, Mitsuyasu R T, Merrill J E, Vinters H V, Chen I S Y. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science. 1987;236:819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- 23.Maciejewski J P, Weichold F F, Young N S. HIV-1 suppression of hematopoiesis in vitro mediated by envelope glycoprotein and TNF-alpha. J Immunol. 1994;153:4303–4310. [PubMed] [Google Scholar]
- 24.McCune J M, Namikawa R, Kaneshima H, Shultz L D, Lieberman M, Weissman I L. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 25.McCune J M, Namikawa R, Shih C-C, Rabin L, Kaneshima H. Suppression of HIV infection in AZT-treated SCID-hu mice. Science. 1990;247:564–566. doi: 10.1126/science.2300816. [DOI] [PubMed] [Google Scholar]
- 26.McCune J M, Peault B, Streeter P R, Rabin L. Preclinical evaluation of human hematolymphoid function in the SCID-hu mouse. Immunol Rev. 1991;124:45–62. doi: 10.1111/j.1600-065x.1991.tb00615.x. [DOI] [PubMed] [Google Scholar]
- 27.Moses A V, Williams S, Heneveld M L, Strussenberg J, Rarick M, Loveless M, Bagby G, Nelson J A. Human immunodeficiency virus infection of bone marrow endothelium reduces induction of stromal hematopoietic growth factors. Blood. 1996;87:919–925. [PubMed] [Google Scholar]
- 28.Namikawa R, Weilbaecher K N, Kaneshima H, Yee E J, McCune J M. Long-term human haematopoiesis in the SCID-hu mouse. J Exp Med. 1990;172:1055–1063. doi: 10.1084/jem.172.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rameshwar P, Denny T N, Gascon P. Enhanced HIV-1 activity in bone marrow can lead to myelopoietic suppression partially contributed by gag p24. J Immunol. 1996;157:4244–4250. [PubMed] [Google Scholar]
- 30.Stanley S K, McCune J M, Kaneshima H, Justement J S, Sullivan M, Boone E, Baseler M, Adelsberger J, Bonyhadi M, Orenstein J, Fox C H, Fauci A S. Human immunodeficiency virus infection of the human thymus and disruption of the thymic microenvironment in the SCID-hu mouse. J Exp Med. 1993;178:1151–1163. doi: 10.1084/jem.178.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su L, Kaneshima H, Bonyhadi M, Salimi S, Kraft D, Rabin L, McCune J M. HIV-1 induced thymocyte depletion is associated with indirect cytopathicity and infection of progenitor cells in vivo. Immunity. 1995;2:25–36. doi: 10.1016/1074-7613(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 32.Sugiura K, Oyaizu N, Pahwa R, Kalyanaraman V S, Pahwa S. Effect of human immunodeficiency virus-1 envelope glycoprotein on in vitro hematopoiesis of umbilical cord blood. Blood. 1992;80:1463–1469. [PubMed] [Google Scholar]
- 33.Withers-Ward E S, Amado R G, Koka P S, Jamieson B D, Kaplan A H, Chen I S Y, Zack J A. Transient renewal of thymopoiesis in HIV infected human thymic implants following antiviral therapy. Nat Med. 1997;3:1102–1109. doi: 10.1038/nm1097-1102. [DOI] [PubMed] [Google Scholar]
- 34.Zack J A, Arrigo S J, Weitsman S R, Go A S, Haislip A, Chen I S Y. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 35.Zauli G, Davis B R. Role of HIV infection in the hematologic manifestations of HIV. Crit Rev Oncol-Hematol. 1993;15:271–283. doi: 10.1016/1040-8428(93)90045-6. [DOI] [PubMed] [Google Scholar]