Abstract
The BZLF1 gene of Epstein-Barr virus (EBV), which encodes a transcription factor, Zta, is transcribed into monocistronic and bicistronic mRNAs from two different promoters during the immediate-early stage of the EBV lytic cycle. It is generally accepted that the Zta protein translated from the monocistronic mRNA profoundly influences the activation of the EBV lytic cycle. In this study, we constructed a plasmid, pCMV-RZLUC, which can transcribe a bicistronic mRNA consisting of BRLF1 and a BZLF1-luc fusion gene under latent conditions. P3HR1 cells transfected with this plasmid produce a luciferase activity which is approximately 17-fold higher than the activity exhibited by pRZLUC, a plasmid incapable of transcribing the bicistronic mRNA. Genetic analyses indicated that mutations in BRLF1 not only can decrease the translation of the fusion gene from the bicistronic mRNA but can also be complemented by a functional BRLF1 gene in cis. This observation implies that the product of BRLF1, Rta, is involved in the translation of the downstream gene. Results presented herein also demonstrate that these mutations cannot be complemented in trans with a plasmid overexpressing Rta, suggesting that the amount of Rta in the vicinity of the intercistronic region may be crucial for the translation. Furthermore, our results correspond to those of previous investigations indicating that the Zta protein can be translated from the bicistronic mRNA and that, similar to the translation of bicistronic ZLUC, mutations in BRLF1 also hinder the translation of Zta from the BRLF1-BZLF1 bicistronic mRNA. Translation of Zta from the bicistronic mRNA may play an essential role in the activation of the EBV lytic cycle.
Epstein-Barr virus (EBV) is a herpesvirus which infects human B lymphocytes, T lymphocytes, and epithelial cells. This virus is the etiological agent of infectious mononucleosis and is closely associated with several neoplastic diseases, including nasopharyngeal carcinoma, Burkitt’s lymphoma, Hodgkin’s disease, and T-cell lymphoma (35). After infection of B lymphocytes, EBV immortalizes the cells and is maintained as an episome under latent conditions. During this stage, only a few genes, including six EBV nuclear antigens, three membrane proteins, and two small RNA species, are expressed (21). EBV can be switched from latency to a lytic cycle when the latently infected cells are exposed to extracellular stimuli, including phorbol esters, butyrate, calcium ionophores, and anti-immunoglobulin (3, 9, 25, 42, 47). When EBV enters a lytic cycle, the first genes expressed from the viral genome are BZLF1 and BRLF1 (4, 14, 42). BZLF1 encodes a transcriptional factor called Zta or ZEBRA. This protein is a transcription factor of the b-Zip family which exhibits homology to c-Fos and C/EBP (10, 22). The Zta protein can upregulate the expression of EBV lytic genes that are necessary for lytic DNA replication (6, 12, 19, 20, 39). The Zta protein also binds to the lytic origin of replication of EBV; this binding is a prerequisite for EBV replication (11, 38). Therefore, expression of BZLF1 is necessary for activation of the EBV lytic cycle.
BZLF1 is transcribed from a promoter, PZ, located immediately upstream from BZLF1. The mRNA transcribed from this promoter is monocistronic, consisting of only BZLF1 (27). BZLF1 is also transcribed from the BRLF1 promoter (PR). The mRNA transcribed from this promoter is bicistronic, consisting of BRLF1 and BZLF1 (27). Previous studies have shown that the Zta protein is translated from the monocistronic BZLF1 mRNA during the immediate-early stage and that the activation of the PZ promoter is crucial for the disruption of EBV latency, allowing the virus to enter the lytic cycle (41). The PZ promoter comprises an AP-1-responsive element, Zta-binding sites or Zta-responsive elements, and negative regulatory elements (13, 28, 29). Zta-responsive elements allow Zta to upregulate the expression of BZLF1 (12). Like PZ, PR is activated at the immediate-early stage of the EBV lytic cycle. The activation of PR requires Sp1 (46) and is mediated by the transcription factors Zif268 and Zta (41, 45). The mRNA transcribed from PR, 4.0 kb in length, is spliced into a 3.3-kb mRNA and a 0.8-kb mRNA (27). The 0.8-kb mRNA encodes an Rta-Zta fusion protein called RAZ, which is an inhibitor of Zta (15). The 3.3-kb transcript is bicistronic, consisting of both BRLF1 and BZLF1. In addition, although the 4.0- and 3.3-kb bicistronic mRNAs are not spliced into a monocistronic BZLF1 mRNA, they can translate Rta and Zta in COS cells (27). However, according to a more recent study in which transfection of EBV-positive cells with a Zta-overexpressing plasmid activated PR but not PZ, the bicistronic mRNA transcribed under these conditions is not translated efficiently (24). In this study, we substituted a luciferase (luc) gene for the BZLF1 component of the BRLF1-BZLF1 translational unit transcribed from a cytomegalovirus (CMV) immediate-early promoter (PC). The results showed that the reporter gene can be efficiently translated from the bicistronic mRNA. Genetic analysis also revealed that a functional Rta protein is also necessary for this translation.
MATERIALS AND METHODS
Cell lines.
P3HR1, Rael, and Akata are EBV-positive Burkitt’s lymphoma cell lines. An EBV-negative Akata cell line was derived from Akata cells (40). Liang is a lymphoblastoid cell line obtained by transformation of human normal primary B lymphocytes with EBV strain B95-8. Cells, except Rael, were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum. Rael cells were cultured in the RPMI 1640 medium containing 20% fetal calf serum.
Transfection and luciferase assay procedures.
Plasmids were prepared from Escherichia coli by CsCl gradient centrifugation in accordance with a method described elsewhere (36). For plasmid transfection, 10 μg of plasmid DNA was mixed with 5 × 106 cells in 300 ml of culture medium. Electroporation was performed at 960 μF and 0.2 V with a Bio-Rad (Richmond, Calif.) Gene-Pulser electroporator. To closely examine gene expression under latent conditions, cells were transferred to 10 ml of fresh culture medium. Gene expression under lytic conditions was studied by transferring cells to 10 ml of medium containing 30 ng of 12-O-tetradecanoylphorbol-13-acetate (TPA) and 3 mM sodium butyrate. Cell lysate was prepared 24 h after transfection in accordance with the method of de Wet et al. (8) but with slight modifications. Briefly, cells were harvested by centrifugation and were washed with phosphate-buffered saline. Cells were then lysed in 200 μl of lysis buffer containing 25 mM Tris-phosphate (pH 7.8), 2 mM dithiothreitol, 2 mM 1,2-diaminocyclohexane-N,N,N′,N′-tetraacetic acid, 10% glycerol, and 1% Triton X-100. The lysate was centrifuged with a microcentrifuge at 11,750 × g for 5 min at room temperature, and the supernatant was used for the determination of luciferase activity. Luciferase assay solution (100 μl), containing 20 mM Tricine, 1.07 mM (MgCO3)4Mg(OH)2 · 5H2O, 2.67 mM MgSO4, 0.1 mM EDTA, 33.3 mM dithiothreitol, 270 μM coenzyme A, 470 μM luciferin, and 530 μM ATP, was automatically injected into a test tube containing 20 μl of the cell lysate. Luciferase activity was then monitored for 10 s with a luminometer (model LB953; Berthod, Bad Wildbad, Germany). Each transfection experiment was repeated at least three times, and each sample in the experiment was prepared in duplicate.
Plasmid construction.
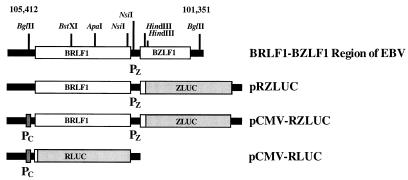
A DNA fragment containing BRLF1 and BZLF1 (from nucleotide [nt] 105412 to nt 101351 of the EBV genome) (Fig. 1) was isolated from cosmid CM302-23 (34) by BglII digestion. This fragment was inserted into the SmaI site of pGEM-7Zf(−) (Promega Corp., Madison, Wis.) to generate plasmid pRZ. Plasmid pCMV-RZ was constructed by inserting the CMV immediate-early promoter (PC), isolated from pCMV-CAT by HindIII digestion, into the HindIII site of pRZ. Plasmid pCMV-LUC was constructed by inserting PC into the HindIII site of pGL2-Basic (Promega Corp.). Plasmid pRZLUC contained BRLF1 and a BZLF1-luc fusion gene (ZLUC). This plasmid was constructed by inserting the 2.4-kb HindIII fragment of pRZ, consisting of the entire BRLF1 gene, the intercistronic region between BRLF1 and BZLF1, and the 5′ 75-bp region of BZLF1, into the HindIII site of pGL2-Basic. Plasmid pCMV-RZLUC was constructed by inserting a repaired PC fragment into the repaired BglII site of pRZLUC. APA-FS and BST-FS were two −1-frameshift mutations (4-bp deletion) generated at the ApaI and BstXI sites of BRLF1, respectively, by treating the ApaI- or BstXI-digested DNA fragment with T4 DNA polymerase and then self-ligating the DNA fragments with T4 DNA ligase. The APA-linker A and APA-linker B mutations were generated by inserting a double-stranded 18-bp linker into the ApaI site of BRLF1. The 18-bp double-stranded linker consisted of two oligonucleotides: 5′-TGAGCTCGAATTCTGGCC and 5′-AGAATTCGAGCTCAGGCC. This linker, when inserted in the linker A orientation, contained a translational termination codon, TGA. The linker did not contain a termination codon if inserted in the opposite orientation (linker B). Next, deletion mutations in BRLF1 were generated by restriction digestion. The deletion mutation ΔStuI was generated by StuI digestion. This deletion changed the reading frame of BRLF1. The ΔStuI-L mutation was then constructed by replacing the StuI fragment of BRLF1 with the linker 5′-CATCCTTTTTCGG. This insertion restored the original reading frame of BRLF1. The deletion mutation ΔBA was generated by double digestion with BstXI and ApaI and replacement of the deleted fragment with the linker 5′-AGAATTCGAGCTCAGGCC. The ΔAB mutation was generated by deleting the ApaI-BamHI fragment of BRLF1, treating the digested plasmid with T4 DNA polymerase, and inserting an 8-bp linker, 5′-GGCCCCAA, at the deletion site. Neither ΔBA nor ΔAB changed the reading frame of BRLF1. The ΔNsiI mutation consisted of an 85-bp deletion which removed the termination codon of BRLF1. Tricistronic constructs pCMV-RRZLUC(APA-FS), pCMV-RRZLUC(ΔAB), and pCMV-RRZLUC(2APA-FS) were prepared by inserting the DNA fragment containing PC and BRLF1, isolated from pCMV-RZLUC(KspI) by MluI-KspI double digestion and repaired by T4 DNA polymerase, into the repaired MluI sites of the bicistronic pRZLUC mutant constructs. The KspI site in pCMV-RZLUC(KspI) was previously created by site-directed mutagenesis at a location 5 bp downstream from the termination codon of BRLF1. Plasmid pCMV was constructed by inserting PC into the HindIII site of pGEM-7Zf(−). Plasmid pCMV-R was generated by inserting the repaired BglII-HindIII fragment of pRZLUC (nt 105412 to 103080 of the EBV genome) into the SmaI site of pCMV. Plasmid pBMLF1-LUC contained a luc gene driven by the BMLF1 promoter (nt 84805 to 84289 of the EBV genome). Plasmid pZLUC contained a luc gene driven by the promoter of BZLF1 (nt 104040 to 103141 of the EBV genome). Finally, plasmid pCMV-RLUC was constructed by inserting the XmnI fragment of pCMV-RZLUC, which contained PC and the 5′-most 29 bp of BRLF1, into the XmnI-HindIII sites of pGL2-Basic. Finally, plasmids pCMV-RRZ(APA-FS) and pCMV-RRZ(2APA-FS) were constructed by inserting the SalI fragments of pCMV-RRZLUC(APA-FS) and pCMV-RRZLUC(2APA-FS), which contained wild-type BRLF1 and mutated BRLF1 (APA-FS), respectively, into the SalI site of pCMV-RZ(APA-FS).
FIG. 1.
Restriction map of the BRLF1-BZLF1 region of the EBV genome and the plasmids used in this study. The numbers correspond to nucleotide positions of the EBV genome. ZLUC, a fusion gene consisting of BZLF1 and luc; RLUC, a fusion gene consisting of BRLF1 and luc; PC, immediate-early promoter of CMV; PZ, promoter of BZLF1.
Northern blot analysis.
P3HR1 cells were cultured for 24 h after transfection. Total RNA was extracted from P3HR1 cells with guanidinium isothiocyanate and was purified by CsCl centrifugation in accordance with a method described elsewhere (36). Alternatively, total RNA was purified from the cells with an RNeasy kit purchased from Qiagen (Hilden, Germany). RNA was separated by electrophoresis on a 1% agarose–formaldehyde gel and was then transferred to a nylon membrane (Amersham). Hybridization was performed in a solution containing 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 0.5% sodium dodecyl sulfate (SDS), and 100 μg of denatured salmon sperm DNA per ml. A β-actin probe (codons 220 to 303) and a luc probe (codons 16 to 221) were prepared by in vitro transcription with T7 RNA polymerase. Hybridization was performed at 68°C for 16 h. After hybridization, the membrane was initially washed at 25°C for 15 min with 2× SSC containing 0.1% SDS and then washed at 68°C for 1 h with 0.1× SSC containing 0.5% SDS. Finally, autoradiography was performed in accordance with a method described elsewhere (36).
Western blot analysis.
Zta protein was detected by Western blot analysis with 4F10 monoclonal antibody (provided by C.-H. Tsai) and an ECL detection kit (Amersham) in accordance with a method described elsewhere (42a).
Site-directed mutagenesis.
Site-directed mutagenesis was performed by the method of Ho et al. (18). Mutant TAA contained a nonsense mutation at nt 103403 of the EBV genome, which converted a TCA codon to TAA. This mutation resulted in premature termination of BRLF1 translation, and the protein encoded by the mutant gene was 12 amino acids shorter at the C terminus than wild-type Rta. Mutant CAG contained a mutation at the termination codon of BRLF1, at nt 103368 of the EBV genome. This mutation extended the translation of BRLF1 into the intercistronic region and resulted in an Rta protein which was 15 amino acids longer at the C terminus than wild-type Rta. The TATA sequence of PZ in mutant pCMV-RZ(XbaI) was mutated from TTTAAA to TCTAGA.
DNA sequencing.
DNA sequencing was performed with Sequenase (U.S. Biochemical Corp. Inc.) in accordance with the chain termination method of Sanger et al. (37). The constructs reported herein were confirmed by DNA sequencing.
RESULTS
Transcription of ZLUC mRNA.
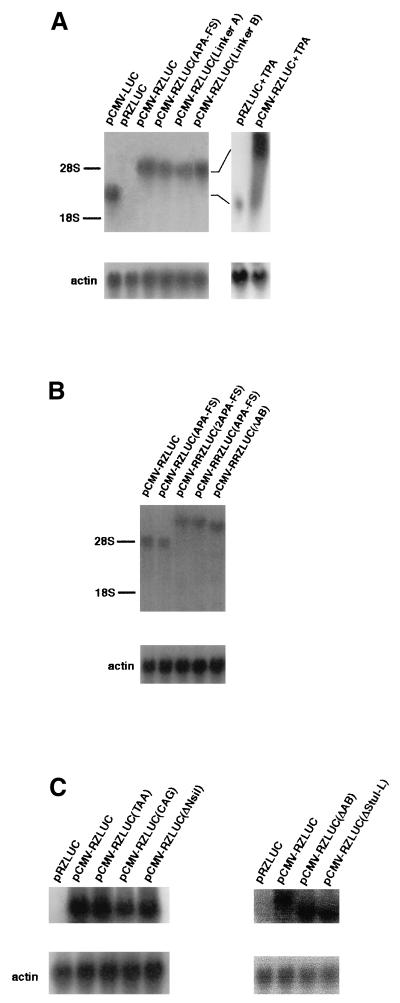
Plasmid pCMV-RZLUC contains PC, BRLF1, PZ, and a BZLF1-luc fusion gene (ZLUC) (Fig. 1). In this study, we transfected P3HR1 cells with this plasmid to determine whether a bicistronic BRLF1-ZLUC mRNA was transcribed from this plasmid. Northern analysis, using the luc sequence as a probe, revealed that under latent conditions the ZLUC mRNA transcribed from pCMV-RZLUC had a size of approximately 5 kb, i.e., approximately 2 kb larger than the monocistronic luc mRNA transcribed from pCMV-LUC (Fig. 2A). However, ZLUC mRNA was not detected in the cells transfected with pRZLUC (Fig. 2A), a plasmid identical to pCMV-RZLUC except that it lacks PC. We have also transfected P3HR1 cells with plasmids containing two copies of BRLF1 and one copy of ZLUC (see below). These plasmids transcribed a 7-kb ZLUC transcript (Fig. 2B).
FIG. 2.
Analysis of luc transcripts in P3HR1 cells by Northern blot hybridization. RNA was prepared from cells transfected with the following: bicistronic constructs, including pRZLUC, pCMV-RZLUC, pCMV-RZLUC(APA-FS), pCMV-RZLUC(Linker A), and pCMV-RZLUC(Linker B) (A); tricistronic constructs, including pCMV-RRZLUC(APA-FS), pCMV-RRZLUC(2APA-FS), and pCMV-RRZLUC(ΔAB) (B); and bicistronic constructs with mutations in BRLF1, including pCMV-RZLUC(CAG), pCMV-RZLUC(TAA), pCMV-RZLUC(ΔNsiI), pCMV-RZLUC(ΔAB), and pCMV-RZLUC(ΔStuI-L) (C). pRZLUC+TPA and pCMV-RZLUC+TPA are cells transfected with pRZLUC and pCMV-RZLUC respectively, and treated with TPA and sodium butyrate for 24 h. In panel A, pCMV-LUC, which transcribed a monocistronic luc transcript, was used as a control. In panel B, pCMV-RZLUC and pCMV-RZLUC(APA-FS), which transcribed bicistronic mRNAs, were used as controls. In panel C, pRZLUC and pCMV-RZLUC were used as controls. The sizes of mRNAs transcribed from pCMV-LUC, bicistronic constructs, and tricistronic constructs are approximately 3 kb, 5 kb (28S), and 7 kb, respectively.
Translation of bicistronic ZLUC.
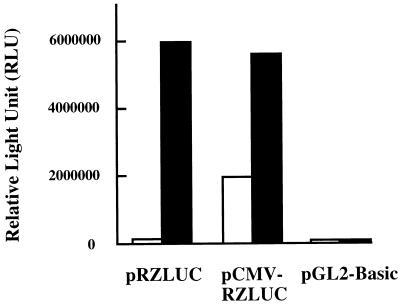
We have used luciferase activity as an indicator of ZLUC translation. Cells transfected with pRZLUC or pCMV-RZLUC were induced for lytic replication by TPA-sodium butyrate treatment. Those results showed that both plasmids exhibited a high level of luciferase activity (Fig. 3), indicating that PZ was activated by the treatment. Northern blot analysis revealed that a monocistronic ZLUC transcript was transcribed from the plasmids after TPA-sodium butyrate treatment (Fig. 2A), indicating that the luciferase activity observed was attributed to translation of monocistronic ZLUC mRNA transcribed from the promoter. On the other hand, these two plasmids expressed luciferase activity at different levels under latent conditions. The activity of pRZLUC was close to the background level; the pCMV-RZLUC luciferase activity was 17-fold higher than the pRZLUC activity (Fig. 3). Since PZ is inactive and monocistronic ZLUC mRNA was not transcribed under latent conditions (Fig. 2), the luciferase activity expressed by pCMV-RZLUC could be attributed to the translation of ZLUC from the bicistronic BRLF1-ZLUC mRNA.
FIG. 3.
Translation of monocistronic and bicistronic ZLUC in P3HR1 cells. Translation was examined under latent (white bar) or lytic (black bar) conditions. Plasmid pGL2-Basic was used as a negative control.
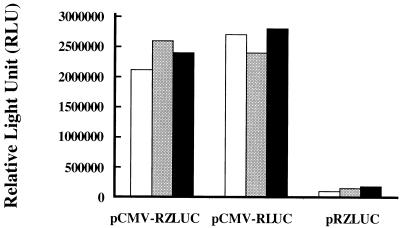
As mentioned earlier, ZLUC can be translated from the bicistronic mRNA under latent conditions. Therefore, in this study, we compared the translation of BRLF1 and ZLUC of the bicistronic mRNA. In doing so, the translation of BRLF1-luc (RLUC) and that of bicistronic ZLUC fusion genes were compared at different time intervals after transfection. The experimental results indicated that the luciferase activity exhibited by pCMV-RLUC (Fig. 1) was 1.3-fold higher than that of pCMV-RZLUC at 24 h and that these activities were approximately equal at 36 and 48 h (Fig. 4). These results demonstrated that despite the presence of a 1.8-kb BRLF1 in the bicistronic mRNA, ZLUC was still translated at approximately the same level as BRLF1. Plasmid pRZLUC did not transcribe a ZLUC transcript, and therefore the construct exhibited a background level of luciferase activity during this period (Fig. 4).
FIG. 4.
Expression of ZLUC under latent conditions by pCMV-RLUC, pCMV-RZLUC, and pRZLUC. Activities were examined 24 h (white bars), 36 h (gray bars), and 48 h (black bars) after transfection.
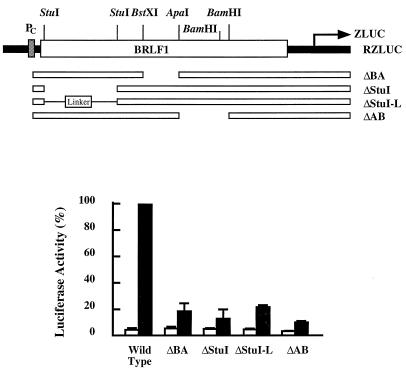
Mutations in BRLF1 and the translation of bicistronic ZLUC.
In this study we generated two −1-frameshift mutations, APA-FS and BST-FS, at the ApaI and BstXI sites in BRLF1, respectively, to examine whether mutations in BRLF1 affected the translation of ZLUC. Similar to the results shown in Fig. 3, a 16-fold difference in the luciferase activities of pCMV-RZLUC and pRZLUC was observed under latent conditions (Fig. 5). The APA-FS and BST-FS mutations in pCMV-RZLUC, on the other hand, decreased ZLUC expression by approximately 85% (Fig. 5A). Next, the effects of mutations in BRLF1 were more closely examined by inserting an 18-bp linker (linker A), consisting of a termination codon, into the ApaI site of BRLF1 (Fig. 1). This insertion decreased ZLUC translation by approximately 85% (Fig. 5A). This linker was also inserted in the opposite orientation (linker B). In this orientation, the linker did not stop the translation of BRLF1 and did not lower the expression of ZLUC (Fig. 5A), indicating that translation of the entire BRLF1 is necessary for the translation of bicistronic ZLUC. We also generated three mutations in the 3′ region of BRLF1. Mutation TAA was a nonsense mutation generated at a location 36 nt upstream from the termination codon of BRLF1. Mutant CAG had a mutation at the termination codon of BRLF1 which changed the TAG sequence to CAG and extended the translation of BRLF1 into the intercistronic region. Mutant ΔNsiI had an NsiI deletion which removed the termination codon of BRLF1 (Fig. 1). These three mutations lowered the level of ZLUC expression by approximately 70 to 90% (Fig. 5B). Based on the above observations, we could conclude that precise termination of translation at the termination codon of BRLF1 is essential for the translation of ZLUC from the bicistronic mRNA. To further demonstrate exactly how BRLF1 mutations influence bicistronic ZLUC translation, we also generated deletions in BRLF1. The ΔStuI mutation consisted of a deletion of the StuI fragment of BRLF1 (Fig. 6). This deletion resulted in a reading frame shift in BRLF1. In addition, this mutation in pCMV-RZLUC decreased the translation of ZLUC by approximately 85% (Fig. 6). The StuI fragment was replaced with a 13-bp linker (ΔStuI-L) to restore the reading frame of BRLF1. Interestingly, this mutation in pCMV-RZLUC still resulted in the mutant phenotype and did not restore the luciferase activity (Fig. 6). We also generated two in-frame deletion mutations, ΔBA and ΔAB (Fig. 6). Similar to ΔStuI-L, these two deletions decreased the luciferase activity by approximately 80%. Unlike pCMV-RZLUC, pRZLUC and its mutant constructs expressed ZLUC activity at the background level (Fig. 5 and 6).
FIG. 5.
Effect of BRLF1 frameshift mutations and nonsense mutations on translation of bicistronic ZLUC. In panel A, APA-FS and BST-FS were −1-frameshift mutations generated at the ApaI and BstXI sites of BRLF1, respectively; linker A consisted of a termination codon, and its insertion terminated the translation of BRLF1 at the insertion site; linker B had a sequence identical to that of linker A but was inserted in the opposite orientation, such that there was not a termination codon and the reading frame of BRLF1 was not changed. In panel B, the CAG mutation changed the termination codon of BRLF1 to a CAG codon, the TAA mutation prematurely terminated translation of BRLF1 at a location 36 nt upstream from the termination codon of BRLF1, and ΔNsiI was a deletion which removed the termination codon of BRLF1. ZLUC expression from pCMV-RZLUC (black bars) and pRZLUC (white bars) containing these mutations was examined in P3HR1 cells under latent conditions.
FIG. 6.
Deletion in BRLF1 and the expression of bicistronic ZLUC. Deletion ΔStuI changed the reading frame of BRLF1, but deletions ΔBA, ΔAB, and ΔStuI-L did not. ZLUC expression from pCMV-RZLUC (black bars) and pRZLUC (white bars) containing these mutations was examined under latent conditions.
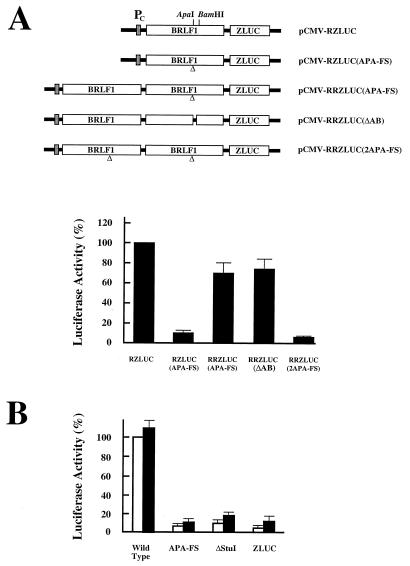
Involvement of Rta in translation of bicistronic ZLUC.
In the study described in the preceding section, mutations in BRLF1 were found to decrease the translation of ZLUC from the bicistronic mRNA. Therefore, complementation experiments were performed to investigate the possible involvement of Rta in the translation. For the cis-complementation study, a DNA fragment containing PC and BRLF1 was inserted in pRZLUC(APA-FS) and pRZLUC(ΔAB) (Fig. 7A). This insertion restored the ZLUC translation of the APA-FS mutant construct, albeit at a reduced level (Fig. 7A). Similarly, the ΔAB mutation in pCMV-RZLUC was also complemented by a BRLF1 gene in cis (Fig. 7A). ZLUC was not translated, however, if two copies of a mutant BRLF1 were placed upstream from ZLUC (Fig. 7A). Also used in this study was a plasmid, pCMV-R, which consisted of an intact BRLF1 gene driven by PC. However, this plasmid could not rescue the function of BRLF1 mutations in bicistronic translation in trans (Fig. 7B). According to our results, the pCMV-R construct could also activate the BMLF1 promoter in pBMLF1-LUC by roughly 30- to 60-fold in P3HR1 cell (data not shown), indicating that a lack of complementation in trans was not attributable to a lack of Rta expression in the cells.
FIG. 7.
Complementation studies. (A) A cis-complementation study. A functional BRLF1 was located between PC and the BRLF1 mutant gene. (B) A trans study. pCMV-R was cotransfected with wild-type pCMV-RZLUC, mutant pCMV-RZLUC plasmids, and pZLUC. White bars, plasmid (5 μg) cotransfected with pCMV (5 μg); black bars, plasmid (5 μg) cotransfected with pCMV-R (5 μg).
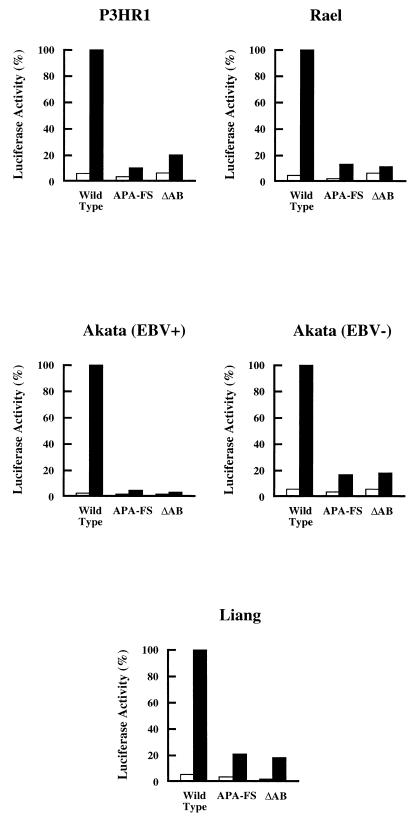
Translation of bicistronic ZLUC in different cell lines.
In addition to its expression in P3HR1 cells, ZLUC was also translated from the bicistronic mRNA in two EBV-positive cell lines, Rael and Akata, in EBV-negative Akata cells, and in Liang cells (Fig. 8). With approximately the same numbers of cells and the same amount of pCMV-RZLUC, ZLUC was consistently expressed at the highest level in P3HR1 cells. The 100% values in Fig. 8 represent approximately 107, 5 × 105, 106, 5 × 105, and 106 relative light units (RLU) for the activity detected in P3HR1, Rael, Akata, EBV-negative Akata, and Liang cells, respectively; the background values exhibited by pGL2-Basic were consistently approximately 1% of the value exhibited by pCMV-RZLUC. Similar to the expression in P3HR1 cells, the APA-FS and ΔAB mutations in pCMV-RZLUC also decreased ZLUC translation in Rael, Akata, EBV-negative Akata, and Liang cells (Fig. 8). In addition to these four cell lines, the expression of ZLUC from pCMV-RZLUC was also examined in two other EBV-negative cell lines, CA46 and BJAB. The RLU values observed in CA46 cells were approximately 500- to 1,000-fold lower than that expressed in P3HR1 cells, at a level of approximately 103 RLU. In addition, in contrast to the expression in the two EBV-positive cell lines and in the EBV-negative Akata cell line, in which the differences in the luciferase activities of the wild-type and mutant plasmids were obvious, no difference in the luciferase activities of CA46 cells transfected with these plasmids could be detected (data not shown). In BJAB cells, the luciferase activity translated from pCMV-RZLUC was extremely low. The RLU readings from pCMV-RZLUC and its mutant derivatives were usually less than 100 (data not shown).
FIG. 8.
Translation of ZLUC from BRLF1-ZLUC bicistronic mRNA in Akata, EBV-negative Akata, Liang, P3HR1, and Rael cells. APA-FS and ΔAB were mutants that could not translate ZLUC from bicistronic mRNA. The results shown represent the averages of values obtained from two independent experiments. White bars, pRZLUC and its mutant derivatives; black bars, pCMV-RZLUC and its mutant derivatives.
Translation of Zta from bicistronic and tricistronic mRNAs.
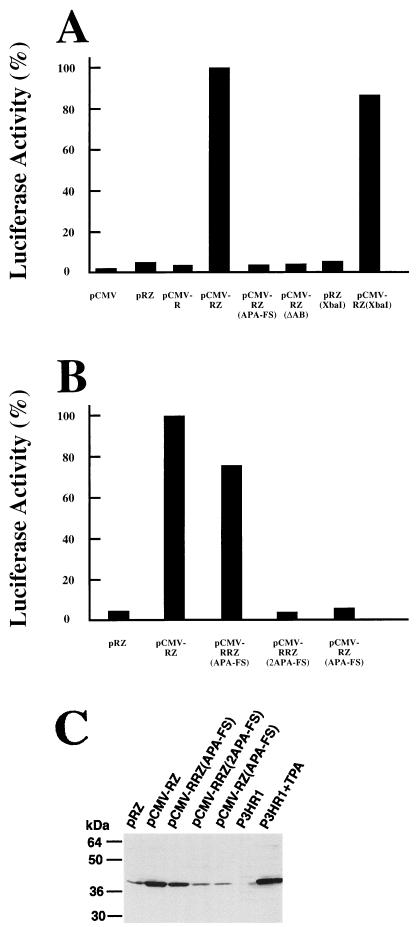
In this study, we also examined whether the BRLF1 mutations actually affect the translation of Zta from the BRLF1-BZLF1 bicistronic mRNA. This determination was made by cotransfecting EBV-negative Akata cells with an effector plasmid which could transcribe the BRLF1-BZLF1 bicistronic mRNA and with a reporter plasmid containing a luc gene driven by PZ (pZLUC). Activation of the PZ promoter of pZLUC by the effector plasmid was used as an indicator of the expression of Zta from the bicistronic mRNA. Our results showed that plasmid pRZ, which lacked PC and PR, did not activate the PZ promoter of pZLUC (Fig. 9A). The luc gene in pZLUC was also not activated by a plasmid containing a BRLF1 gene transcribed from PC (pCMV-R) or by a plasmid containing only PC (pCMV). On the other hand, plasmid pCMV-RZ, which could transcribe the BRLF1-BZLF1 bicistronic mRNA, activated the PZ promoter at a level approximately 22-fold higher than that exhibited by pRZ (Fig. 9A).
FIG. 9.
(A and B) Translation of BZLF1 from bicistronic and tricistronic mRNA. The reporter plasmid pZLUC contained a luc reporter gene transcribed from PZ. The effector plasmids used in this study are indicated on the x axes. This study was carried out in EBV-negative Akata cells transfected with 5 μg each of effector and reporter plasmids under latent conditions. The results shown represent the averages of values obtained from two independent experiments. (C) Zta protein translated in the cells was analyzed by Western blotting. P3HR1, cells were maintained under latent conditions; P3HR1+TPA, cells were treated with TPA and sodium butyrate for 24 h.
Also examined in this study was the effect of the APA-FS and ΔAB mutations on the translation of bicistronic BZLF1. According to our results, pCMV-RZ containing the mutations could not activate the PZ promoter of the reporter plasmid (Fig. 9A). We also changed the TATA sequence of PZ from TTTAAA (a DraI cutting sequence) to TCTAGA (an XbaI cutting sequence). In a separate experiment, we demonstrated that this mutation in pRZLUC destroyed the function of PZ and prevented the plasmid from transcribing the monocistronic BZLF1 mRNA under lytic conditions (data not shown). This mutation in pCMV-RZ, however, only slightly decreased the plasmid’s ability to activate the PZ promoter of pZLUC under latent conditions (Fig. 9A). Western blot analysis also revealed that Zta protein was translated from pCMV-RZ in EBV-negative Akata cells under latent conditions at a level only slightly lower than that expressed by TPA-treated P3HR1 cells (Fig. 9C). On the other hand, the amount of Zta expressed by pCMV-RZ(APA-FS) was significantly less, approximately equal to the amount of Zta expressed by P3HR1 cells under latent conditions (Fig. 9C). These results confirm that Zta is indeed translated from the BRLF1-BZLF1 bicistronic mRNA and that BRLF1 is involved in the translation.
We also constructed a tricistronic plasmid, pCMV-RRZ(APA-FS), which contained a functional BRLF1, in cis, upstream from the mutated BRLF1 (APA-FS). We found that the Zta protein was efficiently translated from this plasmid under latent conditions in EBV-negative Akata cells (Fig. 9C). This plasmid has a structure similar to that of pCMV-RRZLUC(APA-FS) (Fig. 7A), except that the ZLUC gene has been replaced with BZLF1. Notably, this plasmid could activate the PZ promoter of pZLUC at a level approximately 76% of that exhibited by the control plasmid, pCMV-RZ (Fig. 9B). On the other hand, cells cotransfected with pZLUC and pCMV-RRZLUC(2APA-FS), which contains two copies of mutant BRFL1, exhibited luciferase activity at the background level (Fig. 9B). This observation implies that Zta protein was not translated from the plasmid. Western blot analysis also confirmed this, further indicating that Zta protein was efficiently translated by pCMV-RRZ(APA-FS) but not by pCMV-RRZ(2APA-FS) (Fig. 9C). The above results demonstrate that BRLF1 mutations can be complemented by a wild-type BRLF1 gene in cis.
DISCUSSION
BZLF1 is an important gene expressed during the EBV lytic cycle. The protein encoded by this gene, Zta, binds to the lytic origin of replication, which not only is required for EBV DNA replication during the lytic cycle but also functions as a transcription factor to activate early viral genes (6, 12, 19, 20, 39). Therefore, expression of Zta is a prerequisite for activation of the EBV lytic cycle. BZLF1 can be transcribed from PZ as a monocistronic mRNA or from PR as a part of the BRLF1-BZLF1 bicistronic mRNA (27). In this study, we have constructed a plasmid, pCMV-RZLUC, to examine the translation of the bicistronic mRNA. Northern hybridization was initially used to examine the ZLUC mRNA transcribed under latent conditions in P3HR1 cells. According to those results, the cells transfected with pCMV-RZLUC transcribed a 5-kb ZLUC mRNA (Fig. 2A). On the other hand, ZLUC mRNA was not detected in the cells transfected with pRZLUC (Fig. 2A). These results indicated that although ZLUC was transcribed from PC by pCMV-RZLUC, the mRNA either was not transcribed or was transcribed from pRZLUC at a level undetectable by Northern blot analysis. In addition, the size of the ZLUC mRNA detected in the cells transfected with pCMV-RZLUC correlated with the distance between the PC promoter and a polyadenylation signal located downstream from the RZLUC fragment, implying that the mRNA is probably bicistronic, with BRLF1 located upstream and ZLUC located downstream. We also found that pCMV-RRZLUC(APA-FS) and pCMV-RRZLUC(ΔAB) transcribed mRNAs of approximately 7 kb (Fig. 2B), indicating that these transcripts are tricistronic. These results confirm that transcription does not terminate within the BRLF1-BZLF1 region.
At the immediate-early stage of its lytic cycle, EBV is known to transcribe the BRLF1-BZLF1 bicistronic mRNA from PR. We speculated that at the time when EBV switches from latency to the lytic cycle, most viral lytic genes would not have been expressed and the cells might still maintain an environment resembling the conditions of the latent stage. Therefore, examination of ZLUC translation from the bicistronic mRNA under latent conditions might reveal whether BZLF1 is translated from the bicistronic mRNA at the immediate-early stage of the lytic cycle. More importantly, experimentally defining the immediate-early stage after lytic induction and examining the expression under such conditions could be difficult. Therefore, based on these reasons, we examined the translation of the bicistronic mRNA under latent conditions. In this study, we first compared the luciferase activities exhibited by pRZLUC and by pCMV-RZLUC under lytic conditions in P3HR1 cells. Since both plasmids contain PZ, which can be activated under lytic conditions, these two plasmids can transcribe the monocistronic ZLUC mRNA and express luciferase activity at a high level after TPA-sodium butyrate treatment (Fig. 3). On the other hand, under latent conditions, the luciferase activity exhibited by pCMV-RZLUC is 17-fold higher than the activity exhibited by pRZLUC (Fig. 3). Northern blotting results indicated that pRZLUC does not transcribe a detectable amount of bicistronic or monocistronic ZLUC mRNA. This finding suggests that the difference in luciferase activity might be attributed to the transcription and the translation of ZLUC from the BRLF1-ZLUC bicistronic mRNA. On the other hand, it is possible that the PC promoter in pCMV-RZLUC somehow elevates the transcription of monocistronic ZLUC, albeit at a level undetectable by Northern hybridization (Fig. 2); this elevated transcription could have contributed to the difference observed herein. However, our mutagenesis experiments revealed that frameshift and deletion mutations in BRLF1 can significantly reduce the luciferase activity expressed from pCMV-RZLUC (Fig. 5 and 6). These mutations are unlikely to affect the hypothetical activation of PZ by PC. The possibility of RNA splicing can also be ruled out, since Northern blot hybridization did not detect such a spliced mRNA species (Fig. 2).
Having demonstrated that the ZLUC fusion gene can be translated from BRLF1-ZLUC bicistronic mRNA, we also examined the efficiency of this translation. In doing so, the luciferase activity exhibited by pCMV-RLUC was compared with that exhibited by pCMV-RZLUC. According to those results, cells transfected with these two plasmids expressed luciferase at approximately the same level during a 2-day period (Fig. 4). Comparing the translation of two different genes simply on the basis of the amount of luciferase detected in the cells can occasionally be difficult, since the stability of the mRNA and the fusion protein can also affect expression. However, in RLUC and ZLUC, the genes consist of only 29 and 75 nucleotides of the BRLF1 and BZLF1 sequences, respectively. Inserting such short sequences into the luc sequence may not significantly affect the stability of the mRNA and the proteins. Therefore, if the RLUC mRNA and the Rta-Luc fusion protein are not less stable than ZLUC and the Zta-Luc fusion protein, Fig. 4 indicates that ZLUC is translated from the bicistronic mRNA as efficiently as RLUC. In fact, this finding is somewhat unexpected, since in nearly all cases known so far the translation of the downstream gene of a bicistronic mRNA in eukaryotic cells is always affected by the presence of an upstream open reading frame (uORF) and is less efficient than the translation of the uORF (23). Our finding also appears to contradict data from a previous investigation which demonstrated that although the BRLF1-BZLF1 bicistronic mRNA is transcribed at the lytic stage, only a small amount of Zta protein can be detected in the cells (24). The discrepancy may originate from the fact that in our system we used the pCMV-RZLUC construct, which does not produce Zta and does not activate the EBV lytic cycle. Moreover, there exists the possibility that translation of Zta from the bicistronic mRNA may be inhibited under lytic conditions when Zta is expressed at an excessive level.
Translation of bicistronic ZLUC could theoretically involve one of three translation mechanisms. One of those mechanisms is internal initiation (31). An internal ribosome entry site, such as the one present in the RNA of picornaviruses (17, 33, 43), may be located upstream from the initiation codon of BZLF1. The 40S ribosome may directly bind to the site and start to scan the downstream region for the initiation codon to initiate translation. However, ZLUC is unlikely to be translated by this mechanism, since if an internal ribosome entry site does exist, the 40S ribosome should recognize this entry site to initiate the translation of ZLUC; moreover, the mutations in BRLF1 should not have affected the translation of ZLUC from the bicistronic mRNA (Fig. 5 and 6).
The second mechanism known to be involved in the translation of bicistronic mRNA is leaky scanning (5, 23). When a bicistronic mRNA is translated by this mechanism, the initiation codon of a uORF is frequently surrounded by a sequence which is unfavorable for translation initiation (23). Consequently, only some ribosomes start translation from the initiation codon of the uORF; the others may skip the initiation codon and use an AUG codon further downstream to start protein synthesis. In the case of BRLF1-ZLUC bicistronic mRNA, 41 AUG codons are present upstream from the initiation codon of BZLF1, 5 of which are located in the intercistronic region. If ZLUC is translated by the leaky scanning mechanism, the 40S ribosome must neglect these AUG codons and start translation from the initiation codon of BZLF1, an event unlikely to occur under normal circumstances. In fact, we have changed the five AUG codons in the intercistronic region by site-directed mutagenesis, and none of the mutations affected ZLUC translation (unpublished data). These results suggest that translation of ZLUC probably does not involve leaky scanning.
The third mechanism known to be involved in bicistronic mRNA translation is reinitiation (1, 23, 30, 32). In fact, this is the translation mechanism used most frequently by eukaryotic bicistronic mRNAs. A bicistronic mRNA that uses this mechanism for translation usually has a uORF of less than 120 nt (26). As generally accepted, a ribosome may retain some of the initiation factors after translating a short uORF, which may allow the ribosome to scan the intercistronic region to initiate the translation of the downstream gene. Under such conditions, the translation of the downstream gene can be significantly reduced by the translation of a uORF (23, 26). A uORF consisting of more than 120 nt may cause the ribosome to lose all of the translation initiation factors during translation, resulting in the dissociation of the ribosome from the mRNA after translation of the uORF. Under these circumstances, the downstream gene of the bicistronic mRNA is not translated (26). In the BRLF1-ZLUC bicistronic mRNA, the length of the uORF (BRLF1) is 1.8 kb; i.e., it markedly exceeds the limit of 120 nt. However, this length does not seem to affect the translation of ZLUC (Fig. 4). Furthermore, in-frame deletions in pCMV-RZLUC can abolish the translation of ZLUC from the bicistronic mRNA (Fig. 6). This finding suggests that although the translation of the uORF is terminated at the termination codon of BRLF1, the ribosome cannot reinitiate the translation of the ZLUC gene, indicating that ZLUC translation probably does not involve the reinitiation mechanism. In fact, our cis-complementation experiment (Fig. 7A) indicated that ZLUC is probably translated by a ribosome shunting mechanism (16, 44). This is owing to the fact that in the tricistronic mRNA, the BRLF1 frameshift and deletion mutations, which are situated in the middle of the construct, do not seem to block the action of the ribosome and prevent the translation of downstream ZLUC. Our results (not shown) also suggest that the 40S ribosome actually jumps to an acceptor site in the intercistronic region after the translation of BRLF1 and then initiates the translation of ZLUC.
Results from this study indicated that frameshift mutations and deletions in BRLF1 can affect the translation of ZLUC from the bicistronic mRNA (Fig. 5 and 6). In fact, the most interesting finding of this study is that even in-frame deletions (ΔBA, ΔAB, and ΔStuI-L) in BRLF1 can abolish the translation of ZLUC (Fig. 6). These mutations allow translation to terminate precisely at the end of BRLF1, and yet the downstream gene is not translated. The only logical explanation for this is that the function of BRLF1 is also necessary for the translation of ZLUC. Actually, the strongest evidence suggesting the involvement of Rta in the translation of bicistronic ZLUC comes from the fact that a functional BRLF1 in cis can complement the BRLF1 mutations (Fig. 7A). Therefore, the Rta protein may actually assist the ribosome in initiating the translation of ZLUC from bicistronic mRNA. As Fig. 2C depicts, the lack of expression of ZLUC by a CAG, TAA, ΔNsi, ΔAB, or ΔStu-L mutant could not be attributed to mRNA instability caused by the mutation since the amount of mRNA in the cells was approximately equal to the ZLUC mRNA in the cells transfected with wild-type plasmid pCMV-RZLUC. On the other hand, the mRNAs transcribed from the frameshift mutants seem to be present to a lesser extent in the cells, suggesting that the mutant mRNAs are probably less stable than the mRNA transcribed from the wild-type plasmid. This may only partially account for the decreased luciferase activity of the mutant plasmids. In fact, the complementation study suggests that this instability may not be the major cause of the low-level expression, since the instability is unlikely to be corrected by placing a functional BRLF1 in cis (Fig. 7A). Notably, a wild-type BRLF1 in cis can partially complement the mutations, to a level approximately 80% of that exhibited by the wild-type construct. It is possible that in the tricistronic constructs the wild-type BRLF1 is 1.8 kb away from ZLUC and that this distance affected the translation. Furthermore, the tricistronic constructs used in this study are approximately 20% larger than the bicistronic plasmids. Since we have used the same amount of plasmid in all of the transfection experiments, the number of molecules of the tricistronic plasmids used in the study is smaller than that of the bicistronic plasmids. This may also explain why the activity exhibited by pCMV-RRZLUC was lower than that of pCMV-RZLUC. Our results also confirm that BRLF1 mutations cannot be complemented by a functional BRLF1 in trans (Fig. 7B). Therefore, it is likely that the concentration of Rta in the vicinity of the intercistronic region must be sufficiently high to allow the bicistronic translation to occur. If this is indeed the case, the newly synthesized Rta protein may immediately assist the ribosome in initiating the translation of the downstream gene.
The notion of Rta being a translational initiation factor that assists in the translation of ZLUC is intriguing, and it is not without a basis. According to our analyses, the N-terminal 482 amino acids of Rta actually exhibits 21% identity and 53% similarity to the sequence of elF-4B (STM1 protein) of Saccharomyces cerevisiae (2, 7). However, the significance of this similarity to the bicistronic translation remains unclear. Since we have determined that bicistronic ZLUC translation relies on Rta, the similarity may imply that Rta has a role in translation initiation. However, further studies are necessary to elucidate the function of Rta in translation. Furthermore, experimental results indicate that bicistronic ZLUC can be translated in Akata, P3HR1, Rael, an EBV-negative cell line derived from Akata, and a lymphoblastoid cell line, Liang (Fig. 8), but cannot be translated in two EBV-negative cell lines, CA46 and BJAB. These data suggest that in addition to Rta, the bicistronic translation may also require EBV-induced cellular factors.
In addition to showing that the ZLUC gene is translated from bicistronic mRNA, the data presented herein also indicate that BZLF1 is translated from the BRLF1-BZLF1 bicistronic mRNA (Fig. 9). This conclusion comes from the fact that in EBV-negative Akata cells, a PZ-luc reporter plasmid (pZLUC) is activated by an effector plasmid (pCMV-RZ) which can transcribe BRLF1-BZLF1 bicistronic mRNA. Moreover, this activation is only slightly affected by a TATA mutation in PZ [pCMV-RZ(XbaI)] (Fig. 9A). Since the monocistronic BZLF1 is not transcribed from pCMV-RZ(XbaI), the activation of the reporter plasmid by this mutant indicates that Zta protein is translated from the bicistronic mRNA. The question that now arises is why EBV translates Zta protein from two different types of BZLF1 transcripts during the lytic cycle. It is generally believed that the EBV lytic cycle is triggered by the Zta protein translated from the monocistronic mRNA transcribed from PZ (41). However, the mechanism which activates the transcription of PZ in vivo still remains unclear. Since PR is activated in B lymphocytes by Zif268 or by Zta, activation of PR may involve a mechanism which differs from that by which PZ is activated (45). It is possible that under natural conditions, when PR is activated at the immediate-early stage of the EBV lytic cycle, Zta protein is translated from the bicistronic mRNA and then upregulates the transcription from PZ. If this hypothesis is correct, the translation of BZLF1 from the bicistronic mRNA may profoundly influence the lytic development of EBV.
ACKNOWLEDGMENTS
We thank C.-H. Tsai for providing the Zta monoclonal antibody.
This work was supported by a medical research grant from the Chang-Gung Memorial Hospital (CMRP-348) and by a grant from the National Science Council of the Republic of China (NSC83-0412-B-182-030) to S.-T.L. P.-J.C. is a recipient of a graduate research scholarship from the National Science Council of the Republic of China.
REFERENCES
- 1.Abastado J-P, Miller P F, Jackson B M, Hinnebusch A G. Suppression of ribosomal reinitiation at upstream open reading frames in amino acid-starved cells forms the basis for GCN4 translational control. Mol Cell Biol. 1991;11:486–496. doi: 10.1128/mcb.11.1.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altmann M, Muller P P, Wittmer B, Ruchti F, Lanker S, Trachsel H. A Saccharomyces cerevisiae homologue of mammalian translation initiation factor 4B contributes to RNA helicase activity. EMBO J. 1993;12:3997–4003. doi: 10.1002/j.1460-2075.1993.tb06077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer G, Hofler P, zur Hausen H. Epstein-Barr virus induction by a serum factor. I. Induction and cooperation with additional inducer. Virology. 1982;121:184–194. doi: 10.1016/0042-6822(82)90128-3. [DOI] [PubMed] [Google Scholar]
- 4.Biggin M, Bodescot M, Perricaudet M, Farrell P. Epstein-Barr virus gene expression in P3HR1-superinfected Raji cells. J Virol. 1987;61:3120–3132. doi: 10.1128/jvi.61.10.3120-3132.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chenik M, Chebli K, Blondel D. Translation initiation at alternate in-frame AUG codons in the rabies virus phosphoprotein mRNA is mediated by a ribosomal leaky scanning mechanism. J Virol. 1995;69:707–712. doi: 10.1128/jvi.69.2.707-712.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chevallier-Greco A, Gruffat H, Manet E, Calender A, Sergeant A. The Epstein-Barr virus (EBV) DR enhancer contains two functionally different domains: domain A is constitutive and cell specific, domain B is transactivated by the EBV early protein R. J Virol. 1989;63:615–623. doi: 10.1128/jvi.63.2.615-623.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coppolecchia R, Buser P, Stotz A, Linder P. A new yeast translation initiation factor suppresses a mutation in the elF-4A RNA helicase. EMBO J. 1993;12:4005–4011. doi: 10.1002/j.1460-2075.1993.tb06078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Wet J R, Wood K V, DeLuca M, Helinski D R, Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987;7:725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faggioni A, Zompetta C, Grimaldi S, Barile G, Frati L, Lazdins J. Calcium modulation activates Epstein-Barr virus genome in latently infected cells. Science. 1986;232:1554–1556. doi: 10.1126/science.3012779. [DOI] [PubMed] [Google Scholar]
- 10.Farrell P J, Rowe D T, Rooney C M, Kouzarides T. Epstein-Barr virus BZLF1 transactivator specifically binds to a consensus AP-1 site and is related to c-fos. EMBO J. 1989;8:127–132. doi: 10.1002/j.1460-2075.1989.tb03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fixman E D, Hayward G S, Hayward S D. Replication of Epstein-Barr virus oriLyt: lack of a dedicated virally encoded origin-binding protein and dependence on Zta in cotransfection assays. J Virol. 1995;69:2998–3006. doi: 10.1128/jvi.69.5.2998-3006.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flemington E, Speck S H. Autoregulation of Epstein-Barr virus putative lytic switch gene BZLF1. J Virol. 1990;64:1227–1232. doi: 10.1128/jvi.64.3.1227-1232.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flemington E, Speck S H. Identification of phorbol ester response elements in the promoter of the Epstein-Barr virus putative lytic switch gene BZLF1. J Virol. 1990;64:1217–1226. doi: 10.1128/jvi.64.3.1217-1226.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flemington E K, Goldfeld A E, Speck S H. Efficient transcription of the Epstein-Barr virus immediate-early BZLF1 and BRLF1 genes requires protein synthesis. J Virol. 1991;65:7073–7077. doi: 10.1128/jvi.65.12.7073-7077.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furnari F B, Zacny V, Quinlivan E B, Kenney S, Pagano J S. RAZ, an Epstein-Barr virus transdominant repressor that modulates the viral reactivation mechanism. J Virol. 1994;68:1827–1836. doi: 10.1128/jvi.68.3.1827-1836.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Futterer J, Kiss-Laszio Z, Hohn T. Nonlinear ribosome migration on cauliflower mosaic virus 35S RNA. Cell. 1993;73:789–802. doi: 10.1016/0092-8674(93)90257-q. [DOI] [PubMed] [Google Scholar]
- 17.Hellen C U T, Wimmer E. Translation of encephalomyocarditis virus RNA by internal ribosomal entry. Curr Top Microbiol Immunol. 1995;203:31–64. doi: 10.1007/978-3-642-79663-0_2. [DOI] [PubMed] [Google Scholar]
- 18.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 19.Holley-Guthrie E A, Quinlivan E B, Mar E-C, Kenney S. The Epstein-Barr virus (EBV) BMRF1 promoter for early antigen (EA-D) is regulated by the EBV transactivators, BRLF1 and BZLF1, in a cell-specific manner. J Virol. 1990;64:3753–3759. doi: 10.1128/jvi.64.8.3753-3759.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kenney S, Holley-Guthrie E, Mar E-C, Smith M. The Epstein-Barr virus BMLF1 promoter contains an enhancer element that is responsive to the BZLF1 and BRLF1 transactivator. J Virol. 1989;63:3878–3883. doi: 10.1128/jvi.63.9.3878-3883.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kieff E. Epstein-Barr virus and its replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2343–2396. [Google Scholar]
- 22.Kouzarides T, Packham G, Cook A, Farrell P J. The BZLF1 protein of EBV has a coiled coil dimerization domain without a heptad leucine repeat but with homology to the C/EBP leucine zipper. Oncogene. 1991;6:195–204. [PubMed] [Google Scholar]
- 23.Kozak M. The scanning model for translation: an update. J Cell Biol. 1989;108:229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Roux F, Sergeant A, Corbo L. Epstein-Barr virus (EBV) EB1/Zta protein provided in trans and competent for activation of productive cycle genes does not activate the BZLF1 gene in the EBV genome. J Gen Virol. 1996;77:501–509. doi: 10.1099/0022-1317-77-3-501. [DOI] [PubMed] [Google Scholar]
- 25.Luka J, Kallin B, Klein G. Induction of the Epstein-Barr virus (EBV) cycle in latently infected cells by n-butyrate. Virology. 1979;94:228–231. doi: 10.1016/0042-6822(79)90455-0. [DOI] [PubMed] [Google Scholar]
- 26.Luukkonen B G M, Tan W, Schwartz S. Efficiency of reinitiation of translation on human immunodeficiency virus type 1 mRNAs is determined by the length of the upstream open reading frame and by intercistronic distance. J Virol. 1995;69:4086–4094. doi: 10.1128/jvi.69.7.4086-4094.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manet E, Gruffat H, Trescol-Biemont M C, Moreno N, Chambard P, Giot J F, Sergeant A. Epstein-Barr virus bicistronic mRNA generated by facultative splicing code for two transcriptional transactivators. EMBO J. 1989;8:1819–1826. doi: 10.1002/j.1460-2075.1989.tb03576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montalvo E A, Cottam M, Hill S, Wang Y-C J. YY1 binds to and regulates cis-acting negative elements in the Epstein-Barr virus BZLF1 promoter. J Virol. 1995;69:4158–4165. doi: 10.1128/jvi.69.7.4158-4165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montalvo E A, Shi Y, Shenk T E, Levine A J. Negative regulation of the BZLF1 promoter of Epstein-Barr virus. J Virol. 1991;65:3647–3655. doi: 10.1128/jvi.65.7.3647-3655.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mueller P P, Hinnebusch A G. Multiple upstream AUG codons mediate translational control of GCN4. Cell. 1986;45:201–207. doi: 10.1016/0092-8674(86)90384-3. [DOI] [PubMed] [Google Scholar]
- 31.Oh S K, Sarnow P. Gene regulation: translational initiation by internal ribosome binding. Curr Opin Genet Dev. 1993;3:295–300. doi: 10.1016/0959-437X(93)90037-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peabody D S, Berg P. Termination-reinitiation occurs in the translation of mammalian cell mRNAs. Mol Cell Biol. 1986;6:2695–2703. doi: 10.1128/mcb.6.7.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pelletier J, Sonenberg N. Internal initiation of translation of eukatyotic mRNA directed by a sequence from poliovirus. Nature (London) 1988;334:320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- 34.Pokack A, Hartl G, Zimber U, Freese U-K, Laux G, Takaki K, Hohn B, Gissmann L, Bornkamm G W. A complete set of overlapping cosmid clones of M-ABA virus derived from nasopharyngeal carcinoma and its similarity to other Epstein-Barr virus isolates. Gene. 1984;27:279–288. doi: 10.1016/0378-1119(84)90072-6. [DOI] [PubMed] [Google Scholar]
- 35.Rickinson A B, Kieff E. Epstein-Barr virus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2397–2446. [Google Scholar]
- 36.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 37.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schepers A, Pich D, Hammerschmidt W. A transcription factor with homology to the AP-1 family links RNA transcription and DNA replication in the lytic cycle of Epstein-Barr virus. EMBO J. 1993;12:3921–3929. doi: 10.1002/j.1460-2075.1993.tb06070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimizu N, Sakura S, Ono Y, Takada K. Identification of an enhancer-type sequence that is responsive to Z and R trans-activators of Epstein-Barr virus. Virology. 1989;172:655–658. doi: 10.1016/0042-6822(89)90210-9. [DOI] [PubMed] [Google Scholar]
- 40.Shimizu N, Tanabe-Tochikura A, Kuroiwa Y, Takada K. Isolation of Epstein-Barr virus (EBV)-negative cell clones from the EBV-positive Burkitt’s lymphoma (BL) line Akata: malignant phenotypes of BL cells are dependent on EBV. J Virol. 1994;68:6069–6073. doi: 10.1128/jvi.68.9.6069-6073.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sinclair A J, Brimmell M, Shanahan F, Farrell P J. Pathways of activation of the Epstein-Barr virus productive cycle. J Virol. 1991;65:2237–2244. doi: 10.1128/jvi.65.5.2237-2244.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takada K, Ono Y. Synchronous and sequential activation of latently infected Epstein-Barr virus genomes. J Virol. 1989;63:445–449. doi: 10.1128/jvi.63.1.445-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42a.Tsai C-H, Liu M-T, Chen M-R, Lu J, Yang H-L, Chen J-Y, Yang C-S. Characterization of monoclonal antibodies to the Zta and DNase proteins of Epstein-Barr virus. J Biomed Sci. 1997;4:69–77. doi: 10.1007/BF02255596. [DOI] [PubMed] [Google Scholar]
- 43.Tsukiyama-Kohara K, Iizuka N, Kohara M, Nomoto A. Internal ribosomal entry site within hepatitis C virus RNA. J Virol. 1992;66:1476–1483. doi: 10.1128/jvi.66.3.1476-1483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yueh A, Schneider R J. Selective translation initiation by ribosome jumping in adenovirus-infected and heat-shocked cells. Genes Dev. 1996;10:1557–1567. doi: 10.1101/gad.10.12.1557. [DOI] [PubMed] [Google Scholar]
- 45.Zalani S, Holley-Guthrie E, Kenney S. The Zif268 cellular transcription factor activates expression of the Epstein-Barr virus immediate-early BRLF1 promoter. J Virol. 1995;69:3816–3823. doi: 10.1128/jvi.69.6.3816-3823.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zalani S, Holley-Guthrie E A, Gutsch D E, Kenney S C. The Epstein-Barr virus immediate-early promoter BRLF1 can be activated by the cellular Sp1 transcription factor. J Virol. 1992;66:7282–7292. doi: 10.1128/jvi.66.12.7282-7292.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.zur Hausen H, O’Neill F J, Freese U K, Hecker E. Persisting oncogenic herpes-virus induced by tumor promoter TPA. Nature (London) 1978;272:373–375. doi: 10.1038/272373a0. [DOI] [PubMed] [Google Scholar]