Abstract
Although gait disorders are common in Alzheimer’s disease (AD), determining which brain structures and related lesions are specifically involved is a goal yet to be reached. Our objective was to systematically review all published data that examined associations between gait disorders and brain imaging in AD. Of 486 selected studies, 4 observational studies met the selection criteria. The number of participants ranged from 2 to 61 community dwellers (29%-100% female) with prodromal or dementia-stage AD. Quantitative gait disorders (ie, slower gait velocity explained by shorter stride length) were associated with white matter lesions, mainly in the medial frontal lobes and basal ganglia. The nigrostriatal dopamine system was unaffected. Qualitative gait disorders (ie, higher stride length variability) correlated with lower hippocampal volume and function. Gait disorders in AD could be explained by a high burden of age-related subcortical hyperintensities on the frontal–subcortical circuits (nonspecific) together with hippocampal atrophy and hypometabolism (specific).
Keywords: gait, Alzheimer’s disease, brain imaging, leukoencephalopathies, hippocampus
Introduction
Gait disorders are common throughout the course of Alzheimer’s disease (AD), with a prevalence increasing with the stages of AD. 1,2 Interestingly, it has been suggested that AD-related gait disorders (ADRGDs) could be not only a companion event of AD but also rather a specific sign of AD-related cognitive decline. 1 The study of ADRGDs is attractive in the sense that they predict adverse outcomes including falls, loss of independency, institutionalization, hospitalization, and death, 3,4 and also because they are thought to be useful for the early diagnosis of AD. For instance, it was recently reported that gait disorders illustrated by increased gait variability could be used as a clinical marker of prodromal AD, also called amnestic mild cognitive impairment (aMCI). 5 –8 The ADRGDs may even appear first in the course of AD, before memory impairments. Two longitudinal studies 9,10 conducted on healthy elderly participants found that reduced gait velocity could be observed prior to the development of cognitive impairment in the oldest old. Also, Stark et al 11 recently reported more falls, in most cases resulting from gait disorders, during an 8-month follow-up in 125 cognitively healthy seniors (mean age 75 years) when positron emission tomography scans showed large deposits of amyloid-β plaques indicating preclinical AD. Unfortunately, the authors did not specify which brain structures were most affected by the amyloid-β deposits among fallers.
Highlighting that gait disorders may help early detection of AD is informative; however, understanding the mechanisms responsible for gait disorders in AD seems even more important to develop new strategies for the early management of the disease and to avoid further mobility decline and falls. 12 In functional terms, ADRGDs have been related to the impairment of higher levels of gait control at subcortical and cortical levels. 13 –15 Yet, it remains unclear which brain structures and related lesions are specifically involved in AD and could explain ADRGDs. This question has not received yet a structured critical evaluation, unlike gait disorders in vascular dementias or in subcortical degenerative dementias and Parkinsonism, whose links with, respectively, vascular ischemic lesions 16 and disorders of the basal ganglia 17 are well described. Our purpose was to systematically review all published literature that examined the relationships between gait disorders and morphological, metabolic, or functional brain imaging in patients with AD.
Methods
Literature Search
A systematic Medline literature search was conducted in July 2011, without limit of date and language restriction, using the Medical Subject Heading terms “Gait” OR “Gait Disorders, Neurologic” OR “Walking” combined with “Brain Mapping” OR “Magnetic Resonance Imaging” OR “Tomography, X-Ray Computed” OR “Tomography, Emission-Computed, Single-Photon” OR “Positron-Emission Tomography” OR “Nuclear Medicine” OR “Brain” combined with “Alzheimer disease” OR “Dementia” OR “Mild cognitive impairment.” The search also included the Cochrane Library (Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effectiveness, and Cochrane Controlled Trials). An iterative process was used to ensure all relevant articles had been obtained. A further hand search of bibliographic references of extracted articles and existing reviews was also conducted to identify potential studies not captured in the electronic database searches.
Study Selection and Analysis
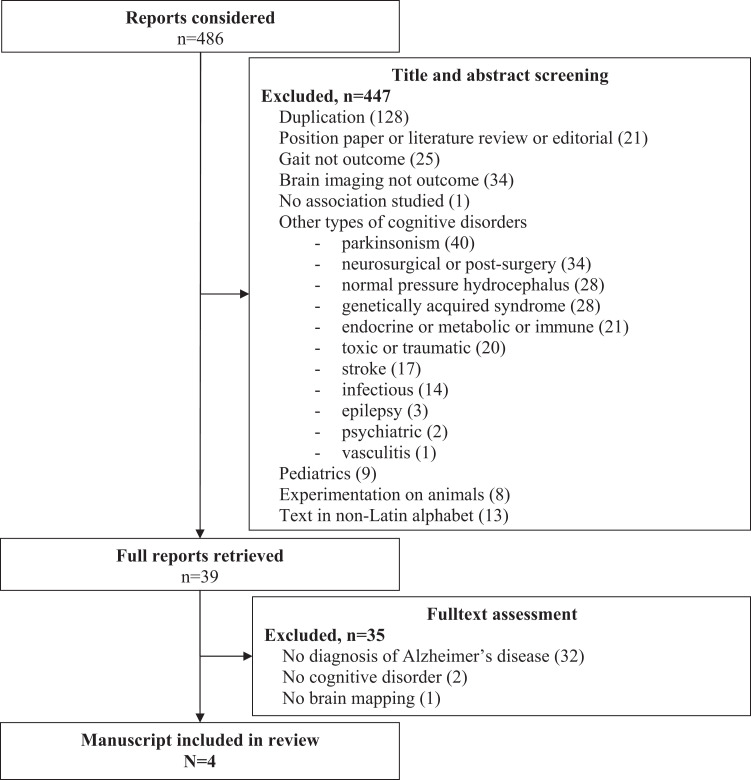
One member of the team (C.A.) screened abstracts from the initial search and obtained articles deemed potentially relevant. Initial screening criteria for the abstracts were (1) observation studies (ie, case report, case series, cross-sectional, case–control, and cohort studies); (2) intervention studies; (3) data collection of gait as outcome; (4) data collection of brain mapping (whether morphological, metabolic, or functional) as outcome; (5) diagnosis of degenerative cortical dementia and/or white matter hyperintensities (WMHs); (6) involvement of adult human participants aged 18 years and older; and (7) article written in Latin alphabet. If a study met the initial selection criteria or its eligibility could not be determined from the title and abstract (or abstract not provided), the full text was retrieved. Two reviewers (C.A. and O.B.) then independently assessed the full text for inclusion status. Disagreements were resolved by a third reviewer (M.M.O.). The full articles were screened using the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) checklist which describes items that should be included in reports of cohort studies 18 and the Consolidated Standards of Reporting Trials (CONSORT) statement for clinical trials. 19 Final selection criteria were, therefore, applied when gait assessment and brain mapping were provided and when the participants presented with AD (ie, preclinical AD, prodromal AD, or dementia-stage AD). The study selection is shown on a flow diagram (Figure 1).
Figure 1.
Flow diagram of selection of studies focusing on gait disorders and brain mapping in Alzheimer’s disease.
Of the 486 originally identified abstracts, 39 met the initial inclusion criteria. 20 –58 Following thorough examination, we excluded 35 of those 39 studies because no diagnosis of AD was made (vascular lesions as single outcome, n = 22; other nondegenerative dementias, n = 6; other degenerative dementias, n = 4; no cognitive disorder, n = 2) or because no data were available for brain imaging (n = 1). The remaining 4 studies were included in this review. 21 –24 Articles selected for the full review had the following information extracted: authors, date of publication, study design, settings and study population, diagnosis criteria for dementia-stage AD or prodromal AD, brain imaging techniques, gait assessment methods, and description of brain imaging and gait parameters.
Results
Table 1 summarizes the 4 observational studies included in this review. 21 –24 The number of participants with AD ranged from 2 to 61. 21,23 Women represented 28.6% to 100% of participants with AD. 21,23 Participants were more likely to be older adults, with a mean age calculated at 74.95 years. Data collection was based on 1 case series 21 and 3 cross-sectional studies. 22 –24 No clinical trials were found. Two studies involved patients with dementia-stage AD21,22 and the other 2 focused on prodromal AD. 23,24 The cross-sectional studies also included either cognitively healthy controls 22,24 or people with nonamnestic MCI. 23 Regarding brain mapping, all studies used magnetic resonance imaging (MRI). Rossor et al 21 also utilized other brain imaging techniques (ie, computed tomography scan [CT scan], electroencephalography, and positron emission tomography [PET]). Zimmerman et al 24 also used proton magnetic resonance spectroscopy (H 1 -MRS). Studies focused either on brain morphology 21 –23 or vascular burden, 21 –23 or brain metabolism. 21,24 With the exception of the case series that evaluated gait only clinically, 21 cross-sectional studies assessed gait disturbances with the use of functional tests (gait velocity at normal or fast pace, Timed Up and Go test (TUG), Walking while talking test, and Tinetti test) 22 –24 or computerized treadmill with embedded pressure sensors (GAITRite, CIR Systems, Pennsylvania). 22,24 All parameters were measured at steady state walking and were averaged over 2 or 3 walking tests 22,24 . The parameters studied reflect gait either quantitatively (ie, gait velocity in cm/s, 22 –24 stride length in cm, 22,24 cadence in steps/min, 22 stride width in cm 22 and swing time in seconds 24 ) or qualitatively (ie, dynamic stability assessed with stride length variability in standard deviation 24 ). Due to its design, the case series only described the brain abnormalities observed in AD cases with gait disorders, 21 while cross-sectional studies used simple comparisons and models of correlation and regression to examine the relationships between gait and brain. 22 –24 Due to the heterogeneity of the methods, the results could not be meta-analyzed.
Table 1.
Main Characteristics of the 4 Studies Exploring the Association Between Gait Disorders and Brain Imaging in Patients With Alzheimer’s Disease
| Study |
Outcomes |
Results | |||||
|---|---|---|---|---|---|---|---|
| References | Design | Settings/population | Brain imaging technique | Brain outcome | Gait assessment | Gait outcome | Relationships between gait parameters and brain imaging |
| Rossor et al 21 | Case series |
|
|
Clinical examination: ability to get up and walk | Inability to walk without assistance and confinement to wheelchair, with a quick installation in 2 (case 1) or 3 years (case 2) |
|
|
| Nadkarni et al 22 Sunnybrook Dementia Study | Cross- sectional |
|
MRI
|
Total and regional subcortical WMH scored on the 4-point ARWMC visual rating scale
|
|
|
|
| Onen et al 23 MRI CODE study | Cross-sectional |
|
|
Presence of periventricular WMH (defined as WMH directly contiguous to the ventricles, with thickness > 5 mm) |
|
|
|
| Zimmerman et al 24 Einstein Aging Study | Cross-sectional |
|
|
|
Walk test at most comfortable pace on a computerized 180 × 35.5 inches long walkway mat with embedded pressure sensors (GAITRite, CIR Systems) | Parameters (steady-state walking; mean values of 2 walk tests):
|
|
|
|||||||
Abbreviations: AD, Alzheimer’s disease; ARWMC, age-related white matter change; BIMC, Blessed Information-Memory-Concentration test; CDR, Clinical Dementia Rating scale; CMRO2, cerebral metabolic rate for oxygen; CT scan, computed tomography scan; EEG, electroencephalography; FCSRT-IR, Free and Cued Selective Reminding Test with Immediate Recall; IADL, Instrumental Activities of Daily Living; IST, Isaac Set Test; Ki, influx constants; MMSE, Mini-Mental State Examination; MCI, Mild Cognitive Impairment; MRI, magnetic resonance imaging; H 1 -MRS, proton magnetic resonance spectroscopy; MRI CODE study, Magnetic Resonance Imaging of COgnitive DEcline study; NAA/Cr, N-acetylaspartate to creatine ratio; NINCDS/ADRDA, National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer Disease and Related Disorders Association; PET, positron emission tomography; SD, standard deviation; SPGR, spoiled gradient recalled; TUG, Timed Up and Go; WAIS, Wechsler Adult Intelligence Scale; WMH, white matter hyperintensities; IQ, intelligent quotient; DSM III, Diagnostic and Statistical Manual of Mental Disorder (Third Edition); 3D, three-dimensional.
From the morphological point of view, 3 studies focused on the subcortical WMH 21 –23 and the third one tested the midsagittal area and hippocampal volume on morphological MRI. 24 Regarding the WMH, Rossor et al 21 found, among patients with poor gait function, increased signal intensity around the anterior parts of the lateral ventricles only. In line with this, Onen et al 23 found that participants with periventricular WMH underperformed the TUG and the Walking while talking test and walked slower than those without periventricular WMH. These associations were yet weakened by adjustment for age and brain atrophy (Table 1). In the study by Nadkarni et al, 22 the WMHs were more precisely rated with the use of the 4-point Age-Related White Matter Change (ARWMC) visual scale. The authors found that the gait parameter most related to the ARWMC score on the whole brain was stride length (r = −.4, P = .01; Table 1). More precisely, they showed that the stride length correlated with the ARWMC score in the frontal area (r = −.4, P < .05) and the basal ganglia (r = −.4, P = .01), but not in other brain areas. Nevertheless, when comparing participants with AD with a low total ARWMC score to cognitively healthy controls with the same low total ARWMC score, the authors highlighted that participants with AD still had slower gait velocity (P < .001), shorter stride length (P = .04), and lower cadence (P < .01) than controls. 22 Regarding the level of brain atrophy, Zimmerman et al 24 found that neither the stride length nor the stride length variability correlated significantly with MRI-derived midsagittal area measurement, a substitute for whole brain volume (P = .39 and P = .97, respectively; Table 1). Then, focusing on the hippocampus—the brain area more specifically affected in AD—the same authors showed that the hippocampal volume correlated with gait velocity (r = .5, P < .01), was associated with stride length (β = .36, P = .03), and showed a trend toward significance for stride length variability (β = −.33, P = .08). 24
From the functional point of view, 15 O2 PET metabolism measurements made by Rossor et al 21 from the brain were divided into 5 areas and the nigrostriatal system specifically. Both cases exhibited symmetric areas of low cerebral metabolic rate for oxygen in the medial frontal lobes bilaterally, more particularly in the anterior frontal gyrus and the cingulate gyrus (Table 1). There was also a trend for hypometabolism in some temporal and parietal areas compared with a control group. Conversely, the metabolism of the nigrostriatal dopamine system was unaffected in AD cases with gait disorders as the influx constants for 18 F-Dopa were normal in caudate and putamen. 19 In another study using H 1 -MRS, Zimmerman et al 24 found that the stride length and the stride length variability correlated with hippocampal metabolism measured by a decreased N-acetylaspartate to creatine ratio in the case of short stride length (r = .56, P = .04) or high stride length variability (r = −.56, P = .04; Table 1).
Discussion
Although this systematic review was able to select a limited number of studies, the highlighted findings remained consistent within them and suggested subcortical white matter as well as hippocampus, but not nigrostriatal system, involvement in ADRGDs.
First, Rossor et al, 21 Nadkarni et al, 22 and Onen et al 23 reported the presence of WMH among the patients with AD with quantitative ADRGDs relative to motor power and propulsion abilities, such as slower gait velocity or shorter stride length. WMH are usually considered as evidence of ischemic disease while aging with changes predominantly affecting small vessels and parenchyma in the deep white matter, thereby resulting in the disruption in tracts connecting different parts of the brain. 32,59 The WMH typically begins proximal to the lateral ventricles and then grows radially outward. 32 Based on studies cited in this review, it appears that quantitative ADRGDs are associated with WMH specifically in the frontal area and the basal ganglia, which are both part of the frontal–subcortical circuits. Disruption in these neuronal circuits may explain the finding of symmetric areas of hypometabolism in the medial frontal lobes of patients with AD unable to walk in Rossor et al’s series, 21 and is likely to explain ADRGD of slowdown type as these circuits are involved in upright posture, 60 spatial information, 61 movement initiation, and planning. 62 Increasing epidemiological, neuroimaging, and clinical studies suggest that the vascular burden plays a key role in the onset and progression of AD, which is primarily a neurodegenerative disease. 63 It is, therefore, not surprising that the vascular component of AD appears to be involved in gait disorders among patients with AD. Yet, it is interesting to note that, while adjusting for age and brain atrophy, the association of WMH with gait disorders was weakened (Table 1). Moreover, while matching participants for the level of vascular burden, participants with AD still had worse performance on gait velocity (P < .001), stride length (P = .04), and cadence (P < .01) compared with controls (Table 1). These observations are in line with previous studies reporting that the WMH correlated with locomotor performance even among high-functioning elderly individuals. 42,45,59 Therefore, we suggest that the finding of quantitative gait disorders explained by WMH in AD may be fortuitous and not specific of AD but rather explained by the older age of participants with AD.
Besides this propulsion aspect, our review also provided compelling evidence that qualitative ADRGDs relative to gait dynamic stability and regularity, illustrated by increased stride length variability, may be explained by AD-related cortical misprocessing. Gait variability is generally thought to be a surrogate marker of the efficiency of higher-level motor control supported by gray matter. 1 In this perspective, Zimmerman et al 24 demonstrated that ADRGDs could not be explained by the atrophy of the brain as a whole, justifying further investigations of specific gray matter areas. In particular, the study by Rossor et al 21 showed that ADRGDs may not be attributed to a dysfunction of the deep dopaminergic system since the 18 F-Dopa uptake into the caudate and putamen was normal in both reported cases. Conversely, Zimmerman et al 24 reported that, in AD, higher stride length variability was associated with lower metabolism in hippocampal cortex, which is precisely the first cortical region damaged in AD. 63 Of note, a prior PET study in healthy younger adults consistently showed an involvement of hippocampus in gait performance and reported an association between higher activity in hippocampal region and increasing complexity of gait (eg, walking and avoiding obstacles). 64 Animal models were also consistent with this finding as it has been shown that hippocampus lesions generated memory disorders as well as limb coordination impairments illustrated by higher gait variability. 65 Two main explanations may be proposed for this finding. First, the hippocampus has a functional relationship with the prefrontal cortex through the entorhinal cortex and the nigrostriatal system. 66 Degeneration of the hippocampus thus causes a disintegration of visual, vestibular, and proprioceptive sensory and contextual information into spatial maps, which is a necessary cognitive resource to maintain normal walking pattern since walking in the real world is a complex task requiring paying attention to environmental features to avoid stumbles and falls. 13 Second, the hippocampus is involved in memory of past strategies and retrieval of complex foot movement sequences. 67,68 As a consequence, learning and memory disorders arising from hippocampus lesion in the course of AD may also explain uncoordinated movements and qualitative ADRGDs.
Gait disorders are common across AD spectrum. Their gravity is related to their high prevalence and their adverse consequences including disability and mortality. 3,4 Thus, understanding the determinants of ADRGDs, and in particular which part of the brain is involved and by which mechanism, may help elucidate how to maintain function late in life and prevent adverse health events as long as possible in the course of AD. The present systematic review emphasizes that gait disorders in AD are explained by age-related vascular ischemic burden independent of AD, as well as by AD-related cortical misprocessing. As a consequence, strategies for ADRGDs management should fix each of these domains—for example, by combining cerebrovascular prevention 69 with pharmaceutical or nonpharmaceutical cognitive enhancement 70,71 —or both at once. In this perspective, cross-domains therapies such as physical training, which limits vascular risk and improves performance in physical and cognitive domains at once, 72,73 appear of particular interest.
The findings of our systematic review need to be tempered by a number of limitations. First, this is a relatively new and emerging area of research, and few studies have been conducted to date, which narrowed the amount of studies to be included in this systematic review. Therefore, our conclusions need to be confirmed in larger studies. The heterogeneity of the studied populations must also be considered. For instance, the stage of AD differed depending on the study; Onen et al 23 and Zimmerman et al 24 included prodromal participants with AD while Nadkarni et al 22 examined participants with mild AD, and Rossor et al 21 reported 2 severe atypical AD—corticobasal degeneration cases. Moreover, the 4 studies included in this literature review did not assess the same morphological measurements and did not use the same imaging techniques, with the exception of MRI. Harmonization of outcome measures seems desirable.
Conclusions
In conclusion, the present systematic review shows that ADRGD relative to propulsion appear to be explained by age-related vascular burden, while ADRGD relative to dynamic stability are more likely explained by AD-related cortical misprocessing in hippocampus. These associations should be considered with caution due to the limited number of studies on this topic. The role of the dopaminergic system and determining whether hippocampus and/or vascular lesions are specifically responsible for ADRGDs should be assessed in bigger samples. Future studies should include a clear description of the population stratified by AD severity, standardized protocols for quantitative gait analysis, and robust structural, functional and metabolic imaging techniques. Understanding neuroanatomical correlates of ADRGDs may offer a powerful mechanism to act on the health care needs of older adults with AD and to maintain function late in life.
Footnotes
Cédric Annweiler has full access to the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses. He was also involved in the study concept and design. Acquisition of data was obtained by Cédric Annweiler and Olivier Beauchet. Analysis and interpretation of data were done by Cédric Annweiler, Manuel Montero-Odasso, and Olivier Beauchet. Drafting of the manuscript was done by Cédric Annweiler, Olivier Beauchet, Manuel Montero-Odasso, and Gilles Allali. Critical revision of the manuscript for important intellectual content was done by Robert Bartha, Sébastien Celle, Frédéric Roche and Thierry Annweiler. Cédric Annweiler involved in administrative, technical, or material support.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Cédric Annweiler is supported by a grant from the Canadian Institutes for Health and Research–Institute of Aging (CIHR-IA) and holds a research grant from Servier Institute in France. Manuel Montero-Odasso is supported, in part, by grants from the Canadian Institutes for Health and Research–Institute of Aging (CIHR-IA), the Drummond Foundation, and the Physician Services Incorporated Foundation of Canada (PSI). He is the first recipient of the Schulich Clinician-Scientist Award and recipient of the CIHR New Investigator Award (2011-2016). Gilles Allali was supported by a grant from the Swiss National Science Foundation (No 33CM30-124115). The sponsors had no role in the design and conduct of the study, in the collection, management, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
References
- 1. Beauchet O, Allali G, Berrut G, Hommet C, Dubost V, Assal F. Gait analysis in demented subjects: interests and perspectives. Neuropsychiatr Dis Treat. 2008;4(1):155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mazoteras Munoz V, Abellan van Kan G, Cantet C, et al. Gait and balance impairments in Alzheimer disease patients. Alzheimer Dis Assoc Disord. 2010;24(1):79–84. [DOI] [PubMed] [Google Scholar]
- 3. Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13(10):881–889. [DOI] [PubMed] [Google Scholar]
- 4. Montero-Odasso M, Schapira M, Soriano ER, et al. Gait velocity as a single predictor of adverse events in healthy seniors aged 75 years and older. J Gerontol A Biol Sci Med Sci. 2005;60(10):1304–1309. [DOI] [PubMed] [Google Scholar]
- 5. Montero-Odasso M, Casas A, Hansen KT, et al. Quantitative gait analysis under dual-task in older people with mild cognitive impairment: a reliability study. J Neuroeng Rehabil. 2009;6:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maquet D, Lekeu F, Warzee E, et al. Gait analysis in elderly adult patients with mild cognitive impairment and patients with mild Alzheimer’s disease: simple versus dual task: a preliminary report. Clin Physiol Funct Imaging. 2010;30(1):51–56. [DOI] [PubMed] [Google Scholar]
- 7. Beauchet O, Allali G, Thiery S, Gautier J, Fantino B, Annweiler C. Association between high variability of gait speed and mild cognitive impairment: a cross-sectional pilot study. J Am Geriatr Soc. 2011;59(10):1973–1974. [DOI] [PubMed] [Google Scholar]
- 8. Montero-Odasso M, Muir SW, Speechley M. Dual-task complexity affects gait in people with mild cognitive impairment: the interplay between gait variability, dual tasking, and risk of falls. Arch Phys Med Rehabil. 2012;93(2):293–299. [DOI] [PubMed] [Google Scholar]
- 9. Camicioli R, Howieson D, Oken B, Sexton G, Kaye J. Motor slowing precedes cognitive impairment in the oldest old. Neurology. 1998;50(5):1496–1498. [DOI] [PubMed] [Google Scholar]
- 10. Barberger-Gateau P, Fabrigoule C, Helmer C, Rouch I, Dartigues JF. Functional impairment in instrumental activities of daily living: an early clinical sign of dementia? J Am Geriatr Soc. 1999;47(4):456–462. [DOI] [PubMed] [Google Scholar]
- 11. Stark S, Roe C, Grant E, Morris J. Risk of falls among older adults with preclinical Alzheimer’s disease. Alzheimers Dement. 2011;7:S176. [Google Scholar]
- 12. Muir SW, Speechley M, Wells J, Borrie M, Gopaul K, Montero-Odasso M. Gait assessment in mild cognitive impairment and Alzheimer’s disease: the effect of dual-task challenges across the cognitive spectrum. Gait posture. 2012;35(1):96–100. [DOI] [PubMed] [Google Scholar]
- 13. Sheridan PL, Solomont J, Kowall N, Hausdorff JM. Influence of executive function on locomotor function: divided attention increases gait variability in Alzheimer’s disease. J Am Geriatr Soc. 2003;51(11):1633–1637. [DOI] [PubMed] [Google Scholar]
- 14. Ble A, Volpato S, Zuliani G, et al. Executive function correlates with walking speed in older persons: the InCHIANTI study. J Am Geriatr Soc. 2005;53(3):410–415. [DOI] [PubMed] [Google Scholar]
- 15. Allali G, Dubois B, Assal F, et al. Frontotemporal dementia: pathology of gait? Mov Disord. 2010;25(6):731–737. [DOI] [PubMed] [Google Scholar]
- 16. Roman GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC. Subcortical ischaemic vascular dementia. Lancet Neurol. 2002;1(7):426–436. [DOI] [PubMed] [Google Scholar]
- 17. Bohnen NI, Cham R. Postural control, gait, and dopamine functions in parkinsonian movements disorders. Clin Geriatr Med. 2006;22(4):797–812. [DOI] [PubMed] [Google Scholar]
- 18. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. [DOI] [PubMed] [Google Scholar]
- 19. Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet. 2001;357(9263):1191–1194. [PubMed] [Google Scholar]
- 20. Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer’s disease. Lancet. 2011;377(9770):1019–1031. [DOI] [PubMed] [Google Scholar]
- 21. Rossor MN, Tyrrell PJ, Warrington EK, Thompson PD, Marsden CD, Lantos P. Progressive frontal gait disturbance with atypical Alzheimer’s disease and corticobasal degeneration. J Neurol Neurosurg Psychiatry 1999;67(3):345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nadkarni NK, McIlroy WE, Mawji E, Black SE. Gait and subcortical hyperintensities in mild Alzheimer’s disease and aging. Dement Geriatr Cogn Disord. 2009;28(4):295–301. [DOI] [PubMed] [Google Scholar]
- 23. Onen F, Henry-Feugeas MC, Roy C, Baron G, Ravaud P. Mobility decline of unknown origin in mild cognitive impairment: an MRI-based clinical study of the pathogenesis. Brain Res. 2008;1222:79–86. [DOI] [PubMed] [Google Scholar]
- 24. Zimmerman ME, Lipton RB, Pan JW, Hetherington HP, Verghese J. MRI- and MRS-derived hippocampal correlates of quantitative locomotor function in older adults. Brain Res. 2009;1291:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huff FJ, Boller F, Lucchelli F, Querriera R, Beyer J, Belle S. The neurologic examination in patients with probable Alzheimer’s disease. Arch Neurol. 1987;44(9):929–932. [DOI] [PubMed] [Google Scholar]
- 26. Hennerici MG, Oster M, Cohen S, Schwartz A, Motsch L, Daffertshofer M. Are gait disturbances and white matter degeneration early indicators of vascular dementia? Dementia. 1994;5(3-4):197–202. [DOI] [PubMed] [Google Scholar]
- 27. Baloh RW, Yue Q, Socotch TM, Jacobson KM. White matter lesions and disequilibrium in older people. I. Case-control comparison. Arch Neurol. 1995;52(10):970–974. [DOI] [PubMed] [Google Scholar]
- 28. Longstreth WT, Jr, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27(8):1274–1282. [DOI] [PubMed] [Google Scholar]
- 29. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 26-1997. A 64-year-old man with progressive dementia, seizures, and unstable gait. N Engl J Med. 1997;337(8):549–556. [DOI] [PubMed] [Google Scholar]
- 30. Gass A, Oster M, Cohen S, Daffertshofer M, Schwartz A, Hennerici MG. Assessment of T2- and T1-weighted MRI brain lesion load in patients with subcortical vascular encephalopathy. Neuroradiology. 1998;40(8):503–506. [DOI] [PubMed] [Google Scholar]
- 31. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 28-1999. A 68-year-old woman with rapidly progressive dementia and a gait disorder. N Engl J Med. 1999;341(12):901–908. [DOI] [PubMed] [Google Scholar]
- 32. Whitman GT, Tang Y, Lin A, Baloh RW. A prospective study of cerebral white matter abnormalities in older people with gait dysfunction. Neurology. 2001;57(6):990–994. [DOI] [PubMed] [Google Scholar]
- 33. Frisoni GB, Galluzzi S, Bresciani L, Geroldi Cristina Zanetti O. Mild cognitive impairment with subcortical vascular features: clinical characteristics and outcome. J Neurol. 2002;249(10):1423–1432. [DOI] [PubMed] [Google Scholar]
- 34. Szatmari S, Csiba L, Fekete I, Bereczki D. Subcortical features and cognitive performance in Hungarian cerebrovascular outpatients. J Neurol Sci. 2002;203-204:99–101. [DOI] [PubMed] [Google Scholar]
- 35. Starr JM, Leaper SA, Murray AD, et al. Brain white matter lesions detected by magnetic resonance imaging are associated with balance and gait speed. J Neurol Neurosurg Psychiatry. 2003;74(1):94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schlossmacher MG, Hamann C, Cole AG, Gonzalez RG, Frosch MP. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 27-2004. A 79-year-old woman with disturbances in gait, cognition, and autonomic function. N Engl J Med. 2004;351(9):912–922. [DOI] [PubMed] [Google Scholar]
- 37. Buscaglia J, Faris J. Unsteady, unfocused, and unable to hear. Am J Med. 2005;118(11):1215–1217. [DOI] [PubMed] [Google Scholar]
- 38. Dickerson BC, Holtzman D, Grant PE, Tian D. Case records of the Massachusetts General Hospital. Case 36-2005. A 61-year-old woman with seizure, disturbed gait, and altered mental status. N Engl J Med. 2005;353(21):2271–2280. [DOI] [PubMed] [Google Scholar]
- 39. Johnson RT, Gonzalez RG, Frosch MP. Case records of the Massachusetts General Hospital. Case 27-2005. An 80-year-old man with fatigue, unsteady gait, and confusion. N Engl J Med. 2005;353(10):1042–1050. [DOI] [PubMed] [Google Scholar]
- 40. Onen F, Feugeas MC, De Marco G, et al. Cerebrospinal fluid MR dynamics and risk of falls in the elderly. J Neuroradiol. 2005;32(1):3–9. [DOI] [PubMed] [Google Scholar]
- 41. Wolfson L, Wei X, Hall CB, et al. Accrual of MRI white matter abnormalities in elderly with normal and impaired mobility. J Neurol Sci. 2005;232(1-2):23–27. [DOI] [PubMed] [Google Scholar]
- 42. Rosano C, Brach J, Longstreth WT, Jr, Newman AB. Quantitative measures of gait characteristics indicate prevalence of underlying subclinical structural brain abnormalities in high-functioning older adults. Neuroepidemiology. 2006;26(1):52–60. [DOI] [PubMed] [Google Scholar]
- 43. Guerini F, Frisoni GB, Bellelli G, Trabucchi M. Subcortical vascular lesions and functional recovery in older patients with gait disorders. Arch Gerontol Geriatr. 2007;45(1):87–96. [DOI] [PubMed] [Google Scholar]
- 44. Lippa CF, Boeve BF, Parisi JE, Keegan BM. A 75-year-old man with cognitive impairment and gait changes. Neurology. 2007;69(11):1183–1189. [DOI] [PubMed] [Google Scholar]
- 45. Rosano C, Brach J, Studenski S, Longstreth WT, Jr, Newman AB. Gait variability is associated with subclinical brain vascular abnormalities in high-functioning older adults. Neuroepidemiology. 2007;29(3-4):193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Diehl-Schmid J, Schulte-Overberg J, Hartmann J, Forstl H, Kurz A, Haussermann P. Extrapyramidal signs, primitive reflexes and incontinence in fronto-temporal dementia. Eur J Neurol. 2007;14(8):860–864. [DOI] [PubMed] [Google Scholar]
- 47. Silbert LC, Nelson C, Howieson DB, Moore MM, Kaye JA. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology. 2008;71(2):108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Staekenborg SS, van der Flier WM, van Straaten EC, Lane R, Barkhof F, Scheltens P. Neurological signs in relation to type of cerebrovascular disease in vascular dementia. Stroke. 2008;39(2):317–322. [DOI] [PubMed] [Google Scholar]
- 49. Bhadelia RA, Price LL, Tedesco KL, et al. Diffusion tensor imaging, white matter lesions, the corpus callosum, and gait in the elderly. Stroke. 2009;40(12):3816–3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Blahak C, Baezner H, Pantoni L, et al. Deep frontal and periventricular age related white matter changes but not basal ganglia and infratentorial hyperintensities are associated with falls: cross sectional results from the LADIS study. J Neurol Neurosurg Psychiatry. 2009;80(6):608–613. [DOI] [PubMed] [Google Scholar]
- 51. Carboncini MC, Volterrani D, Bonfiglio L, et al. Higher level gait disorders in subcortical chronic vascular encephalopathy: a single photon emission computed tomography study. Age Ageing. 2009;38(3):302–307. [DOI] [PubMed] [Google Scholar]
- 52. Dimauro S, Milone M, Keegan BM. A 41-year-old woman with progressive leg weakness and numbness, dizziness, and myalgia. Neurology. 2009;72(14):1262–1268. [DOI] [PubMed] [Google Scholar]
- 53. Lossos A, Klein CJ, McEvoy KM, Keegan BM. A 63-year-old woman with urinary incontinence and progressive gait disorder. Neurology. 2009;72(18):1607–1613. [DOI] [PubMed] [Google Scholar]
- 54. Srikanth V, Beare R, Blizzard L, et al. Cerebral white matter lesions, gait, and the risk of incident falls: a prospective population-based study. Stroke. 2009;40(1):175–180. [DOI] [PubMed] [Google Scholar]
- 55. Jonsson M, Edman A, Lind K, Rolstad S, Sjogren M, Wallin A. Apathy is a prominent neuropsychiatric feature of radiological white-matter changes in patients with dementia. Int J Geriatr Psychiatry. 2010;25(6):588–595. [DOI] [PubMed] [Google Scholar]
- 56. Wakefield DB, Moscufo N, Guttmann CR, et al. White matter hyperintensities predict functional decline in voiding, mobility, and cognition in older adults. J Am Geriatr Soc. 2010;58(2):275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rosano C, Sigurdsson S, Siggeirsdottir K, et al. Magnetization transfer imaging, white matter hyperintensities, brain atrophy and slower gait in older men and women. Neurobiol Aging. 2010;31(7):1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. de Laat KF, Tuladhar AM, van Norden AG, Norris DG, Zwiers MP, de Leeuw FE. Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain. 2011;134(pt 1):73–83. [DOI] [PubMed] [Google Scholar]
- 59. Annweiler C, Montero-Odasso M. Vascular burden as a substrate for higher-level gait disorders in older adults. A review of brain mapping literature. Panminerva Med. 2012. In press. [PubMed] [Google Scholar]
- 60. Ouchi Y, Yoshikawa E, Futatsubashi M, Okada H, Torizuka T, Kaneko M. Activation in the premotor cortex during mental calculation in patients with Alzheimer’s disease: relevance of reduction in posterior cingulate metabolism. Neuroimage. 2004;22(1):155–163. [DOI] [PubMed] [Google Scholar]
- 61. Bartels AL, Leenders KL. Brain imaging in patients with freezing of gait. Mov Disord. 2008;23(suppl 2):S461–S467. [DOI] [PubMed] [Google Scholar]
- 62. Herrero MT, Barcia C, Navarro JM. Functional anatomy of thalamus and basal ganglia. Childs Nerv Syst. 2002;18(8):386–404. [DOI] [PubMed] [Google Scholar]
- 63. van Norden AG, van Dijk EJ, de Laat KF, Scheltens P, Olderikkert MG, de Leeuw FE. Dementia: Alzheimer pathology and vascular factors: from mutually exclusive to interaction. Biochim Biophys Acta. 2012;1822(3):340–349. [DOI] [PubMed] [Google Scholar]
- 64. Malouin F, Richards CL, Jackson PL, Dumas F, Doyon J. Brain activations during motor imagery of locomotor-related tasks: a PET study. Hum Brain Mapp. 2003;19(1):47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bland BH, Oddie SD. Theta bands oscillation and synchrony in the hippocampal formation and associated structures: the case for its role in sensorimotor integration. Behav Brain Res. 2001;127(1-2):119–136. [DOI] [PubMed] [Google Scholar]
- 66. Scherder E, Eggermont L, Swaab D, et al. Gait in ageing and associated dementias; its relationship with cognition. Neurosci Biobehav Rev. 2007;31(4):485–497. [DOI] [PubMed] [Google Scholar]
- 67. Lafleur MF, Jackson PL, Malouin F, Richards CL, Evans AC, Doyon J. Motor learning produces parallel dynamic functional changes during the execution and imagination of sequential foot movements. Neuroimage. 2002;16(1):142–157. [DOI] [PubMed] [Google Scholar]
- 68. Heuninckx S, Wenderoth N, Debaere F, Peeters R, Swinnen SP. Neural basis of aging: the penetration of cognition into action control. J Neurosci. 2005;25(29):6787–6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rolita L, Verghese J. Neurovascular coupling: key to gait slowing in aging? Ann Neurol. 2011;70(2):189–191. [DOI] [PubMed] [Google Scholar]
- 70. Montero-Odasso M, Wells JL, Borrie MJ, Speechley M. Can cognitive enhancers reduce the risk of falls in older people with mild cognitive impairment? A protocol for a randomized controlled double blind trial. BMC Neurol. 2009;9:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Beauchet O, Launay C, Fantino B, Annweiler C, Allali G. Does memantine improve the gait of individuals with Alzheimer’s disease? J Am Geriatr Soc. 2011;59(11):2181–2182. [DOI] [PubMed] [Google Scholar]
- 72. Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348(25):2508–2516. [DOI] [PubMed] [Google Scholar]
- 73. Matsumura BA, Ambrose AF. Balance in the elderly. Clin Geriatr Med. 2006;22(2):395–412. [DOI] [PubMed] [Google Scholar]