Abstract
Background:
Pulmonary sarcoidosis is a systemic disease that can confound established follow-up tools. Pulmonary function tests (PFTs) are recommended in initial and follow-up patient evaluations yet are imperfect predictors of disease progression. The cardiopulmonary exercise test (CPET) is another potentially useful monitoring tool, although previous studies report conflicting findings regarding which variables are altered by the disease. Nuclear imaging tests are also employed to assess inflammatory activity and may be predictive of functional deterioration.
Aim:
We asked whether PFTs or CPET are more diagnostic of disease stage, which subsets of functional variables are impacted by the disease, and how these relate to nuclear imaging signs of active inflammation.
Study design and methods:
We collected retrospective data (spirometry, CPET, Gallium-67 scintigraphy, 18F-FDG PET/CT) from 48 patients and 10 controls. Disease severity was assessed following Scadding classification. First, we correlated individual PFTs and CPET parameters to Scadding stage and nuclear imaging data. Next, we performed Principal Component Analysis (PCA) on PFTs and CPET parameters, separated into respiratory, cardiovascular and metabolic subsets. Finally, we constructed multiple regression models to determine which variable subsets were the best predictors of Scadding stage and disease activity.
Results:
The majority of PFTs and CPET single parameters were significantly correlated with patient stage, while only few correlated with disease activity. Nevertheless, multiple regression models were able to significantly relate PFTs and CPET to both disease stage and activity. Additionally, these analyses highlighted CPET cardiovascular parameters as the best overall predictors of disease stage and activity.
Conclusions:
Our results display how CPET and spirometry data complement each other for sarcoidosis disease staging, and how these tests are able to detect disease activity. Our findings suggest that CPET, a repeatable and non-invasive functional test, should be more routinely performed and taken into account in sarcoidosis patient follow-up.
Keywords: sarcoidosis, cardiopulmonary exercise test, spirometry, scintigraphy, positron emission tomography imaging
Introduction
Sarcoidosis is a systemic disease with a wide spectrum of manifestations (1). While spontaneous remission occurs in two-thirds of patients, the remaining one-third experience progressive/relapsing disease (2). This is a significant burden for the patients as disease and treatments both cause adverse effects. Clinical guidelines recommend frequent follow-up, including physical examination, chest radiographs and spirometry (1). The complexity of sarcoidosis however makes it challenging for clinicians to assess disease stage and progression, and determine treatment strategies, purely based on resting-state pulmonary function tests (PFTs), laboratory data, and imaging (3–7).
Given these challenges, clinicians have often turned to nuclear imaging examinations to assess the presence of inflammatory activity, as the granuloma’s activity itself is correlated with worsening respiratory function (8–10). Gallium-67 scintigraphy (67Ga scanning) has been commonly used to detect sarcoidosis activity (8) and assess whether patients respond to treatment (1,11,12). However, although 67Ga scanning is still employed as a diagnostic tool (13), this technique suffers from limited sensitivity (14–16). When possible it is thus now recommended to perform imaging investigations through fluor-18 fluorodeoxyglucose positron emission tomography (18F-FDG PET), which yields a three-fold lower radiation dose and is more sensitive in detecting active sarcoidosis lesions, particularly when coupled with computer tomography (18F-FDG PET/CT) (12).
The cardiopulmonary exercise test (CPET) is another valuable tool, which is suggested on an individual basis if patients exhibit symptoms including breathlessness or impaired lung function (1). However, although CPET may yield prognostic insight about the progressive decline of pulmonary function (17,18), the procedure produces a slew of parameters (19), many of which are correlated with each other (20). Thus, although the value of exercise testing in sarcoidosis has been extensively studied (17,18,21–23), past results are multifaceted, with different variable subsets related to respiratory (22,24) or cardiovascular dysfunction (17, 20) exhibiting alterations that correlate with disease stage. This lack of a clear pattern of altered variables, coupled with the time consuming and complex nature of CPET, perhaps explains why this tool is not routinely adopted in sarcoidosis monitoring.
The objectives of the current study were thus two-fold: (i) to explore the diagnostic potential of CPET in addition to standard PFTs and (ii) to relate PFTs and CPET parameters to signs of disease activity assessed though nuclear imaging. Given the large number of parameters investigated, rather than attempting to identify which individual variables were most diagnostic we searched for the underlying dimensions that best (cor-)related to disease severity and activity. We thus performed Principal Component Analysis (PCA) on PFTs and CPET variables, separated into respiratory, cardiovascular and metabolic subsets, and regressed the recovered dimensions onto Scadding stage and nuclear imaging results.
Methods
Participants
We included data from forty-eight consecutive sarcoidosis patients (15 female; age: 45±6 mean±sd) and ten control participants (3 female; age: 46±9) referred to the University Hospital of Trieste between 2013 and 2018 for clinical exercise testing to assess dyspnea. Control participants had no known respiratory, cardiologic or neuromuscular disease. Tables 1 and 2 show participant demographics, the frequency of extrathoracic disease localizations and patient therapies at the time of the examinations. The study followed the tenets of the 6th revision of the Declaration of Helsinki (2008) and was approved by the Ethics Committee of CEUR (protocol 2019-58, approval date 09/09/2019). Written consent was waived due to the study’s retrospective nature. The study followed the “Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)” statement guidelines for observational case-control studies (25).
Table 1.
Participant demographics
| Sarcoidosis | Controls | Test | p | |
|---|---|---|---|---|
| Number (0/I/II/III/IV) | 48(2/14/21/9/2) | 10 | - | - |
| Sex (F/M) | 15/33 | 3/7 | χ2= .0060 | .94 |
| Age (mean ± sd) | 46 ± 9 | 43 ± 11 | t(56) = .91 | .37 |
| BMI (mean ± sd) | 27.3 ± 3.7 | 25.7 ± 3.8 | t(56) = 1.26 | .21 |
| Smokers (yes/no) | 13/35 | 3/7 | χ2= .0035 | .85 |
Table 2.
Instances of extrathoracic sarcoid localizations-manifestations and patient therapies for each disease stage. Number of patients at each stage
| Localization-manifestation\Stage | 0 | I | II | III | IV |
|---|---|---|---|---|---|
| Hypercalciuria | 0 | 7 | 10 | 3 | 0 |
| Cutaneous | 2 | 2 | 3 | 1 | 1 |
| Ocular | 0 | 1 | 3 | 0 | 0 |
| Articular | 2 | 2 | 2 | 0 | 0 |
| Hepatic | 0 | 0 | 2 | 2 | 0 |
| Splenic | 0 | 0 | 2 | 0 | 0 |
| Bone | 0 | 0 | 1 | 0 | 0 |
| Ovarian | 0 | 0 | 0 | 1 | 0 |
| Therapy\Stage | 0 | I | II | III | IV |
| Dapsone | 1 | 0 | 0 | 0 | 0 |
| Steroid | 0 | 6 | 18 | 4 | 2 |
| Methotrexate | 0 | 1 | 5 | 1 | 1 |
| Pentoxifylline | 0 | 0 | 1 | 2 | 0 |
| Hydroxychloroquine | 0 | 4 | 2 | 3 | 1 |
Forty-six patients had histologically proven sarcoidosis, two additional patients were diagnosed with sarcoidosis based on clinical, laboratory, and radiological findings following WASOG guidelines (2). Patients were classified into distinct disease stages from chest x-ray images following Scadding criteria (26). In our cohort, 2 patients were stage 0, 14 were stage I, 21 were stage II, 9 were stage III, and 2 were stage IV. Patients with ascertained or suspected cardiac localization of sarcoidosis or neurosarcoidosis were excluded. All patients at first visit in our Referral Centre underwent standard ECG.
Procedures
Spirometry
Forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), slow vital capacity (SVC) were recorded from flow-volume and volume-time curves. Total lung capacity (TLC) was assessed with multibreath nitrogen washout. The diffusing capacity of the lungs for carbon monoxide (DLCO) and the alveolar volume (VA) were measured with single-breath method (ULTIMA Series MedGraphics St. Paul Minnesota USA). PFTs followed ATS/ERS standard 2005 for both procedures and interpretation (27–29). If multiple spirometry examinations were available for a patient, we selected the one closest to the selected CPET examination (in all cases selected spirometry and CPET examinations were within four months of each other). In three control cases data from carbon monoxide diffusion tests and measurements of static pulmonary volumes were unavailable.
Cardiopulmonary exercise test (CPET)
Patients performed incremental exercise tests on an electromagnetically braked cycle ergometer (Corival LODE BV medical Technology, Groningen the Netherlands). The resistance ramp was chosen such that the predicted maximum work rate (W) for an individual patient would be reached within 8-12 minutes of exercise. Patient blood oxygen saturation (SaO2) was monitored with a finger pulse oximeter (7500 Nonin Sensor). Cardiorespiratory and metabolic state was measured continuously using a breath-by-breath automated exercise metabolic system (ULTIMA Series MedGraphics St. Paul Minnesota USA) interfaced with the cycle ergometer and oximeter. The system measured: oxygen uptake (VO2), carbon dioxide production (VCO2), minute ventilation (VE), tidal volume (VT), respiratory rate (RR) and derived the respiratory exchange ratio (RER), anaerobic threshold (AT), and end tidal partial pressure of CO2 (PetCO2). The breathing reserve (BR) was automatically computed by the system as BR=100×((MVV-VE))/MVV, with maximal voluntary ventilation estimated as MVV=FEV1×40. Systolic (PAS) and diastolic (PAD) blood pressure and electrocardiographic pattern were monitored continuously throughout the exercise test. Heart rate monitoring provided measurements of heart rate reserve (HRR), heart rate to VO2 gradient (HR/VO2), oxygen pulse (O2 pulse, ratio of O2 to heart rate), and Delta O2 pulse (the difference in O2 pulse between exercise peak and baseline). Oxyhemoglobin desaturation (DeSaO2) was calculated by subtracting basal SaO2 from SaO2 at exercise peak.
Table 3 shows all parameters derived from these measures and recorded for the study. Prior to performing analyses, the authors manually sorted CPET parameters into three groups primarily relating to either respiratory, cardiovascular, or metabolic function. If a patient had undergone multiple exercise tests, the oldest was selected and included in the analyses. Patient therapy at the time of the PFT was the same as at the time of the CPET.
Table 3.
Spirometry and CPET parameters
| Spirometry | CPET | ||
|---|---|---|---|
| Respiratory | Cardiovascular | Metabolic | |
| FVC | VEpeak | HRR% | RER |
| FVC% | TVinitial | O2 pulse% | VO2, peak |
| FEV1 | TVpeak | DELTA O2 pulse | VO2% |
| FEV1% | TVpeak/TVinitial | VO2/Wslope | AT |
| FEV1/SVC% | RR | HR/VO2 | AT% |
| TLC | BR% | PASinitial | W |
| TLC% | SAO2, initial | PASpeak | W% |
| DLCO | SAO2, peak | PADinitial | |
| DLCO% | DeSaO2 | PADpeak | |
| VA% | PetCO2, initial | ||
| DLCO/VA% | PetCO2, AT | ||
| PetCO2, peak | |||
| VE/VCO2, intercept | |||
| VE/VCO2, slope | |||
Abbreviations: FVC: forced vital capacity, FVC%: FVC in % of reference values, FEV1: forced expiratory volume in 1 second; FEV1% forced expiratory volume in 1 second in % of reference values; FEV1/SVC%: ratio of FEV1 to slow vital capacity in % of reference values; TLC: total lung capacity; TLC%: TLC in % of reference values; DLCO: diffusing capacity of the lungs for carbon monoxide; DLCO%: DLCO in % of reference values; VA: alveolar volume in % of reference values; DLCO/VA%: ratio of DLCO to VA; VEpeak peak minute ventilation; TVinitial: initial tidal volume; TVpeak: peak tidal volume; TVpeak/TVbaseline: ratio of peak to baseline tidal volume; RR: respiratory exchange ratio, the ratio between carbon dioxide production and oxygen intake; BR%: breathing reserve, the difference between the maximal voluntary ventilation and the minute ventilation (VE) as a % of the maximal voluntary ventilation; SAO2, initial: initial blood oxygen saturation; SaO2, peak: peak blood oxygen saturation; DeSaO2: blood oxygen desaturation, difference between SaO2 peak of the exercise SaO2 at baseline; PetCO2, initial: end tidal partial pressure of CO2 at baseline; PetCO2, AT: end tidal partial pressure of CO2 at anaerobic threshold; PetCO2, peak: end tidal partial pressure of CO2 at peak exercise; VE/VCO2, intercept: intercept of the linear relationship between minute ventilation and carbon dioxide production; VE/VCO2, slope: slope of the linear relationship between minute ventilation and carbon dioxide production; HRR%: heart rate reserve, the difference between maximum predicted heart rate and observed maximum heart rate, as a % of the maximum predicted heart rate; O2 pulse % predicted: O2 pulse % predicted: oxygen pulse in % of reference values; DELTA O2 pulse: the difference between oxygen pulse at the peak of the exercise and oxygen pulse at baseline; VO2/Wslope: the slope of the oxygen uptake to work rate linear relationship; HR/VO2: slope of the heart rate to oxygen uptake linear relationship; PASinitial: systolic blood pressure at baseline; PASpeak: systolic blood pressure at peak exercise; PADinitial: diastolic blood pressure at baseline; PADpeak: diastolic blood pressure at peak exercise; RER: respiratory exchange ratio, the ratio between carbon dioxide production and oxygen intake; VO2, peak: oxygen intake at peak exercise; VO2%: oxygen intake at peak in % of reference values; AT: anaerobic threshold; AT % predicted: anaerobic threshold in % of reference values; W: work rate at peak; W % predicted: work rate at peak in% of reference values.
Nuclear medicine examinations
A subset of sarcoidosis patients (n=40) underwent nuclear medicine tests: 20 underwent 18F-FDG PET/CT (CT/PET Discovery MI DR GE Healthcare), 20 underwent 67Ga scanning (gamma camera Symbia Intevo SPECT/TC Siemens). The choice of nuclear imaging technique was based on availability. Procedures followed acquisition and interpretation guidelines (30,31). Patients were classified as positive for active sarcoid sites if, at the pulmonary and/or mediastinal parenchymal level, 18F-FDG PET/CT exhibited standardized uptake values >2.5 (32) or 67Ga scanning showed at least moderate (+2) tracer uptake. Nuclear medicine examinations were performed within six months from CPET examinations. Patients did not undergo modifications in their therapeutic regimen between CPET and nuclear medicine tests. Steroid and/or methotrexate therapy was not significantly correlated with nuclear medicine findings (r=-.15, P=.37).
Statistical analyses
Analyses were performed using MATLAB (R2019b). Differences between group means and proportions for participant demographics were assessed via independent samples t-tests or χ2-test, as appropriate. Values of P<.05 were considered statistically significant throughout. No corrections for multiple comparisons were applied as analyses were exploratory/descriptive. Missing data were excluded from the analyses. Control participants were included, where possible, to provide a baseline reference against which to compare alterations in PFTs and CPET parameters in patients. In control analyses we verified that including or excluding control participants did not alter the pattern of results. Means and standard deviations for all parameters and all study groups are listed in Table 4.
Table 4.
PFTs and CPET parameter values (mean ± sd) for each participant group. Abbreviations in Table 3.
| Controls | 0 | I | II | III | IV | |
|---|---|---|---|---|---|---|
| FVC (L) | 4.73±1.05 | 3.75±0.76 | 4.54±0.96 | 4.22±1.06 | 3.92±0.78 | 3.86±0.61 |
| FVC% | 114.6±13.11 | 114.5±12.02 | 107.43±13.12 | 103.14±15.47 | 97±11.15 | 79±15.56 |
| FEV1 (L) | 3.84±1.01 | 2.83±0.37 | 3.66±0.74 | 3.34±0.86 | 3.01±0.55 | 2.48±0.56 |
| FEV1% | 110.8±13.15 | 105±14.14 | 105.5±9.3 | 98.95±16.65 | 89.89±10.24 | 61±9.9 |
| FEV1/SVC% | 105.33±7.39 | 104±4.24 | 103.36±5.4 | 102.29±7.09 | 98.67±12.46 | 82±24.04 |
| TLC (L) | 6.21±1.48 | 5.51±0.76 | 6.18±1.29 | 5.58±1.24 | 5.09±0.98 | 5.04±1.1 |
| TLC% | 98.71±9.93 | 105.5±16.26 | 95.86±14.7 | 88.19±14.87 | 80.78±8.89 | 70±14.14 |
| DLCO (ml/mmHg/min) | 25.87±11.82 | 22.03±7.35 | 27.75±5.78 | 27.34±6.29 | 22.79±6.65 | 18.86±5.23 |
| DLCO% | 94±20.61 | 87±15.56 | 93.5±17.26 | 94.1±16.2 | 78.11±19.59 | 57±18.38 |
| VA% | 100.43±12.49 | 92.5±0.71 | 94.14±15.01 | 91.33±14.97 | 84.22±8.24 | 74±14.14 |
| DLCO/VA% | 90.71±13.89 | 93±15.56 | 99.36±12.46 | 103.29±15.12 | 91.67±17.51 | 75.5±10.61 |
| VEpeak(L/min) | 86.92±26.18 | 83.35±27.51 | 80.16±22.17 | 75.9±25.26 | 63.1±15.88 | 68.65±36.98 |
| VTinitial (L) | 0.63±0.22 | 0.82±0.11 | 0.66±0.2 | 0.59±0.15 | 0.7±0.21 | 0.56±0.11 |
| VTpeak (L) | 2.54±0.74 | 1.67±0.79 | 2.18±0.57 | 2.04±0.7 | 1.87±0.47 | 1.54±0.25 |
| VTpeak/VTinitial | 4.09±0.52 | 2.13±1.25 | 3.43±0.79 | 3.45±0.84 | 2.73±0.49 | 2.84±1.02 |
| RR (1/min) | 35.1±8.66 | 51.5±7.78 | 37.86±9 | 37.43±7.54 | 34.11±6.31 | 43±16.97 |
| BR% | 38.43±11.21 | 19.7±11.88 | 36.1±16.45 | 39.49±13.84 | 45.09±10.02 | 30.5±20.51 |
| SAO2, initial (%) | 97±1.05 | 95.5±3.54 | 97.29±1.33 | 96.57±1.16 | 97±1.22 | 96±0 |
| SAO2, peak (%) | 96.3±1.16 | 96.5±3.54 | 96.71±1.9 | 96±2.53 | 95.67±1.87 | 86±4.24 |
| DeSaO2 (%) | -0.7±1.06 | 1±0 | -0.57±1.65 | -0.57±2.36 | -1.33±2.12 | -10±4.24 |
| PetCO2,initial(mmHg) | 38.1±2.33 | 34.5±0.71 | 35.21±4.66 | 35.81±2.73 | 33.78±4.06 | 33.5±3.54 |
| PetCO2, AT (mmHg) | 44.9±5.49 | 40±2.83 | 40.79±4.66 | 41±3.65 | 38.22±5.7 | 40.5±0.71 |
| PetCO2, peak (mmHg) | 38.3±5.93 | 30±7.07 | 36.71±4.07 | 36.38±3.76 | 36±5.05 | 38±11.31 |
| VE/VCO2, intercept | 0.04±2.15 | -3.79±5.85 | 0.98±1.54 | 0.54±2.07 | 1.03±2.09 | 2.58±10.42 |
| VE/VCO2, slope | 25.92±3.16 | 36.34±14.08 | 28.64±4.28 | 29.12±3.8 | 29.92±5.87 | 31.51±13.99 |
| HRR% | 5.99±9.56 | 19.3±4.53 | 8.46±7.58 | 7.91±11.14 | 11.09±12.24 | 10.6±10.47 |
| O2 pulse% | 113.5±19.28 | 117.5±20.51 | 104.93±18.18 | 92.86±18.01 | 92.56±18.77 | 68.5±3.54 |
| DELTA O2 pulse (ml/beat) | 10.7±3.37 | 11.5±6.36 | 10.57±2.77 | 9.29±2.95 | 7.56±1.33 | 7±0 |
| VO2/Wslope (ml/min/watt) | 10.04±1.18 | 9.95±0.86 | 9.47±1.11 | 9.04±1.37 | 8.81±1.21 | 7.91±0.8 |
| HR/VO2 (beat/ml) | 47.57±17.09 | 41.58±22.37 | 46.05±14.33 | 43.7±16.04 | 48.82±8.77 | 54.53±12.91 |
| PASinitial mmHg) | 120.4±14.18 | 122±1.41 | 125.86±20.75 | 121.9±13.35 | 122.89±21.54 | 117.5±3.54 |
| PASpeak (mmHg) | 177.9±22.04 | 164.5±0.71 | 177.43±30.42 | 178.33±22.24 | 169.33±28.96 | 167.5±50.2 |
| PADinitial (mmHg) | 75.4±8.76 | 80±8.49 | 81.43±6.61 | 86.48±9.76 | 83.33±10.51 | 88±9.9 |
| PADpeak (mmHg) | 80.7±10.73 | 76±5.66 | 94±10.9 | 96.1±12.39 | 92.11±18.34 | 92±14.14 |
| RER | 1.18±0.04 | 1.2±0.01 | 1.14±0.07 | 1.18±0.08 | 1.16±0.08 | 1.11±0.11 |
| VO2, peak (ml/kg/min) | 33.51±6.74 | 26.8±11.46 | 28.41±7.93 | 25.9±6.81 | 21.59±5.52 | 19.45±3.46 |
| VO2% | 109±15.37 | 95.5±21.92 | 95.5±15.79 | 85.38±16.54 | 78±14.47 | 60.5±3.54 |
| AT (ml/kg/min) | 19.68±3.12 | 13.9±5.94 | 16.87±4.27 | 15.8±3.75 | 13.38±3.65 | 13.5±0.42 |
| AT% | 64.5±9.44 | 49.5±12.02 | 57.14±8.93 | 52.38±10.51 | 48.33±11.35 | 42.5±6.36 |
| W (watt/min) | 198.7±55.19 | 138.5±88.39 | 177.29±54.96 | 161.24±55.87 | 134.33±34.08 | 162.5±24.75 |
| W% | 118.8±20.93 | 93.5±21.92 | 100.57±17.01 | 92.14±18.42 | 89.67±26.2 | 74±2.83 |
Predictors of patient stage
In all analyses, patient stage was coded 0-4 following Scadding classification for sarcoidosis patients, whereas control patients were coded as -1. To assess the relationship between patient stage and spirometry/CPET parameters, we first computed simple Pearson correlation coefficients (r) between each parameter and patient stage. The square of the correlation coefficient (r2, the coefficient of determination) represents the proportion of shared variance between correlated variables. Pearson correlation was also employed to assess the degree of interdependency between spirometry and CPET parameters.
To compare spirometry and CPET parameters as predictors of patient stage, we constructed and compared multiple regression models designed to predict patient stage from parameter subsets. To avoid issues of multicollinearity, prior to model fitting we performed principal component analysis (PCA) on each parameter subset to obtain a new set of uncorrelated predictors. To avoid fitting noise, we only employed PCA on principal components whose eigenvalues were greater than 1. To find the most parsimonious models that best accounted for patient stage, regression models were constructed using a stepwise inclusion procedure. We began from the most basic models that included only intercept terms. Predictors were added to the models one at a time and were retained only if the Bayesian Information Criterion decreased by more than 2 units.
One model, to be taken as comparison to all subsequent models, was constructed employing all spirometry and CPET parameters together. Next, we constructed models with only spirometry or only CPET parameters. Finally, we constructed models with only subsets of CPET parameters relating to respiratory, cardiovascular, or metabolic function (Table 3). To compare regression models that could have different numbers of predictors we employed the adjusted r2, a modified version of the coefficient of determination adjusted for the number of predictors in a model.
Predictors of disease activity
To investigate the relationship between spirometry and CPET parameters with nuclear medicine findings, the same analyses were performed as with patient stage, replacing patient stage with positive and negative nuclear medicine findings, coded as 1 or 0. The only difference was that we employed the point biserial correlation coefficient, which is however mathematically equivalent to the Pearson correlation coefficient with one dichotomous variable. Participants who did not undergo nuclear medicine examinations (8 sarcoidosis patients and all control participants) were excluded from these analyses.
Results
Predictors of patient stage
Figure 1a shows the strength and direction of the correlations (r, red for positive, blue for negative) between patient stage and all parameters, ordered by the proportion of variance explained by each parameter (r2, grey bars). The two parameters that best correlated with patient stage were VO2% (Figure 1b) and FEV1% (Figure 1c). However, most parameters (27/41) were significantly correlated with patient stage.
Figure 1.
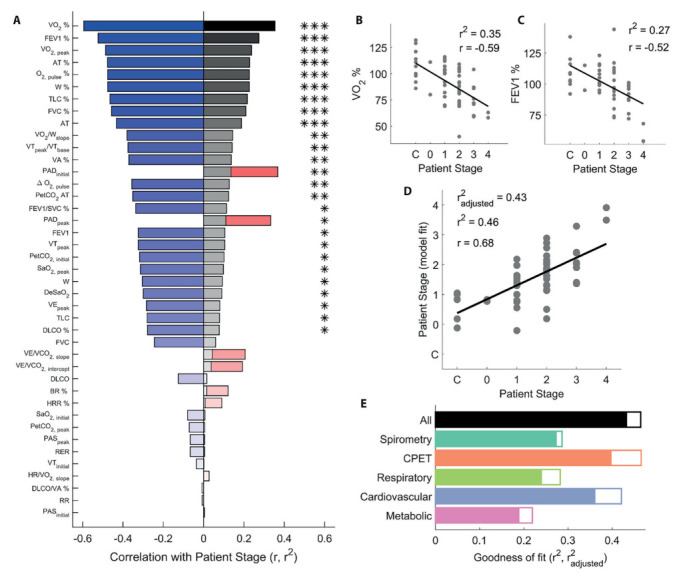
Relating spirometry and CPET to patient stage. (a) Correlation (r, red bars for positive, blue bars for negative) between all test parameters and patient stage, ordered by proportion of variance explained (r2, grey bars). (b) VO2% and (c) FEV1% plotted as a function of patient stage. (d) Patient stage recovered by fitting a multiple regression model to all parameters, as a function of ground truth patient stage. (e) Proportion of explained variance for all multiple regression models constructed. Empty bars are r2, filled bars are r2adjusted, i.e. corrected for the number of predictors in each model. In panels b-d, dots represent individual participants, lines are best fitting linear regression fits. *P<.05; **P<.01; ***P<.001.
As expected, measured parameters were highly correlated with each other (Figure 2).
Figure 2.
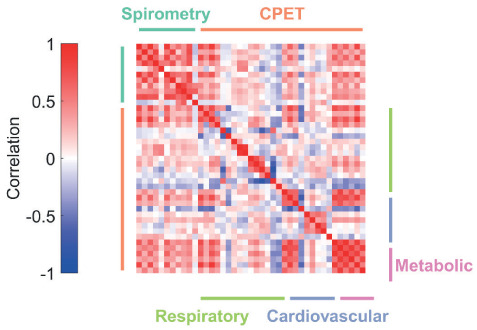
Correlations between spirometry and CPET parameters. The strength and direction of the correlation is color coded as shown in the legend. Parameters are ordered, left-to-right and top-to-bottom, as shown in Table 1. The color of each cell in the correlation matrix thus represents the strength of the correlation between two parameters.
To avoid issues of multicollinearity, we performed PCA on spirometry and CPET parameters. We then employed a stepwise procedure to fit multiple regression models to the recovered PCA dimensions, to assess which subset of test parameters best related to patient stage. Sensibly, the best model was obtained by employing all spirometry and CPET parameters (Figure 1d and 1e, black bar, F3,50=14.5, P<.001, r2=.46, r2adj=.43).
Figure 1e further shows that all multiple regression models fit to the spirometry and CPET data were able to significantly predict patient stage. A model employing CPET parameters alone was nearly as good as the full model (orange bar, F6,47=6.82, P<.001, r2=.47, r2adj=.40), and was noticeably better than a model containing only spirometry parameters (dark green bar, F1,52=20.9, P<.001, r2=.29, r2adj=.27). When restricting the models to CPET parameter subsets, the best model contained cardiovascular parameters (blue bar, F5,48=6.97, P<.001, r2=.42, r2adj=.36). Models employing only respiratory (light green bar, F3,50=6.65, P<.001, r2=.28, r2adj=.24) and only metabolic (purple bar, F2,51=7.16, P=.0018, r2=.22, r2adj=.19) parameters performed worst. In summary, cardiovascular parameters extracted from CPET data were the most informative for predicting pulmonary sarcoidosis patient stage.
Predictors of disease activity
Figure 3a shows the strength and direction of the correlations between nuclear medicine findings and all parameters, ordered by the proportion of variance explained by each parameter. Only forced expiratory volume parameters (FEV1, FEV1%, and FEV1/SVC%) were significantly correlated with nuclear medicine findings. Figure 3b-d shows these parameters plotted as a function of whether or not nuclear medicine examinations showed active sarcoid sites. Despite the relatively poor correlation between disease activity and individual parameters, a linear model was able to significantly regress all parameters onto disease activity (Figure 3e and 3g, black bar, F2,36=8.96, P<.001, r2=.42, r2adj=.38). This was not trivially related to disease stage, since positive and negative nuclear medicine findings were distributed across stages (Figure 3f).
Figure 3.
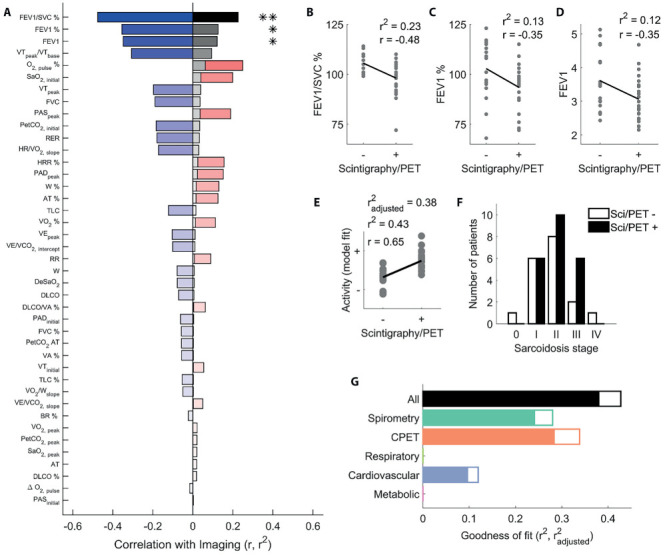
Relating spirometry and CPET to sarcoid disease activity. (a) Correlation (r, red bars for positive, blue bars for negative) between all test parameters and nuclear imaging findings, ordered by proportion of variance explained (r2, grey bars). (b) FEV1/SVC%, FEV1% (c), and FEV1 (d) parameters plotted as a function of whether nuclear medicine examinations were positive or negative. (e) Disease activity (+ active; - inactive) recovered by fitting a multiple regression model to all parameters, as a function of positive or negative nuclear imaging findings. (f) Number of patients with positive (black bars) and negative (white bars) nuclear medicine examinations at each disease stage in our sample. (g) Proportion of explained variance for all multiple regression models constructed. Empty bars are r2, filled bars are r2adjusted, i.e. corrected for the number of predictors in each model. In panels b-e, dots represent individual participants, lines are best fitting linear regression fits. *P<.05; **P<.01
Figure 3g further shows how all models trained on parameter subsets performed decidedly worse than the full model. The model employing CPET parameters alone (orange bar, F2,37=6.14, P<.01, r2=.34, r2adj=.28) performed only slightly better than one employing spirometry parameters alone (dark green bar, F1,37=7.2, P=.0018, r2=.28, r2adj=.24). When restricting the models to CPET parameter subsets, the model containing cardiovascular parameters was the only one that significantly related to nuclear medicine findings (blue bar, F1,36=5.15, P=.029, r2=.12, r2adj=.10), whereas no significant regression models were found for respiratory nor metabolic parameters alone.
Figure 3g further shows how all models trained on parameter subsets performed decidedly worse than the full model. The model employing CPET parameters alone (orange bar, F2,37=6.14, P<.01, r2=.34, r2adj=.28) performed only slightly better than one employing spirometry parameters alone (dark green bar, F1,37=7.2, P=.0018, r2=.28, r2adj=.24). When restricting the models to CPET parameter subsets, the model containing cardiovascular parameters was the only one that significantly related to nuclear medicine findings (blue bar, F1,36=5.15, P=.029, r2=.12, r2adj=.10), whereas no significant regression models were found for respiratory nor metabolic parameters alone.
Discussion
Our results demonstrate that taken together, spirometry and CPET variables are predictive of pulmonary sarcoidosis radiologic disease stage. We further observed that the parameters most strongly correlated to disease stage were CPET variables, specifically those related to cardiovascular function. Finally, and to our knowledge for first time in the literature, we were able to relate sarcoidosis inflammatory state—evaluated through nuclear imaging—to exercise capacity measured with CPET.
Assessing disease stage
Previous research has attempted to relate PFTs and CPET variables to disease stage with varying degrees of success. Barros et al (23) for example observed that increased radiographic stage was associated with worsening lung function in terms of FEV1/FVC and DLCO%. Zappala and colleagues (33) instead found that PFTs including FEV1, FVC, and DLCO were linked to changes in disease extent on chest radiography, but were not correlated with changes in disease stage. Winterbauer and Hutchinson (34) observed that DLCO was unable to differentiate the extent of the disease in early Scadding stages.
In our data, several spirometry parameters were significantly correlated with disease stage, including those previously identified in the literature (such as FEV1%, FVC%, and DLCO%). However, in our study the variables related to overall exercise capacity, namely VO2%, VO2, peak, and W%, were amongst the most strongly correlated with Scadding classification. VO2% was the best predictor of disease extent across all investigated parameters. This is in apparent contrast with previous studies where VO2 was mostly uncorrelated with disease stage (20,21,23,35). This apparent discrepancy however might be explained by the fact that previous studies investigated different cohorts from ours: Medinger and colleagues (21) recruited a larger proportion of stage I patients, whereas patients recruited by Wallaert et al (35) had uniformly normal VO2, peak compared to those included in our study. Our analyses also revealed a significant degree of interrelationship between CPET and spirometry data (which thus support each other in explaining disease stage). In previous research however, the relationship between CPET and PFTs has been unclear (21,36–38).
These considerations highlight a key aspect addressed by our study. Previous efforts—which focused on identifying specific individual variables related to radiologic extent and disease progression (17,18,21,35,38)—have yielded varied and even conflicting results. This is likely due to the heterogeneity of investigated cohorts and of the disease itself. To mitigate this issue, we reasoned that individual parameters were less important than the summary of a patient’s state that the parameters provide altogether. By performing PCA on the whole parameter set, we thus rid ourselves of individual parameters, and recovered the underlying dimensions that captured the state of respiratory, cardiovascular, and metabolic health of our cohort. By then performing multiple regression analyses on these recovered dimensions, we found that radiologic disease stage was best explained by a weighted combination of PFTs and CPET variables. Further, CPET variables captured disease stage better than spirometry variables. These results underline that CPET can assess sarcoidosis pulmonary involvement as well as, and likely better than, standard PFTs.
Assessing disease activity
Activity in the granulomas may lead to progression of the parenchymal destruction (39), and thus PET could be predictive of response to treatment and disease progression (39). However, it is well established that repeated radiological procedures increase the radiation risk for patients (40), and it is thus preferable to minimize nuclear imaging investigations to those strictly necessary. One option to minimize such exposures could be to perform preliminary assessments of disease activity through procedures such as PFTs and CPET.
However, to the best of our knowledge, previous research has only assessed the relationship between PTFs and PET positivity, and this is the first time that inflammatory state evaluated via nuclear imaging has been correlated to exercise capacity measured with incremental CPET. For example, Mostard et al (32) found that FVC% and DLCO% were statistically different between PET-positive and PET-negative patients, although these authors did not evaluate FEV1 and FEV1%. In our data instead, FEV1, FEV1%, and FEV1/SVC% were significantly correlated with nuclear imaging results, whereas DLCO was not. Differences across studies could again be related to differences in the cohorts investigated: most patients in stages 2/3 from our study had positive nuclear imaging, whereas stage 4 patients, who exhibited the worst DLCO, had negative nuclear imaging. More importantly however, a linear regression model incorporating both spirometry and CPET parameters correlated with nuclear imaging findings better than any individual functional parameter. These analyses thus further demonstrate that the sum of spirometry and CPET variables is more informative than any single parameter.
Limitations and future directions
We acknowledge limitations of our study relevant to future research efforts into understanding pulmonary sarcoidosis. First, most patients in our study were undergoing therapy. Although we verified that therapy was not significantly correlated with disease activity, therapy was nevertheless aimed at improving patient symptoms. Our findings should thus be considered as conservative estimates of the true relationships between investigated variables and disease stage/activity. Future research could assess the strength of these relationships before patients undergo treatment.
A second limitation is that we used Scadding classification, which is a longstanding and prognostically useful tool (26,41,42), yet has well-known shortcomings (4,33). Disease staging is not without merit of course; recent work has shown that staging at initial evaluation is predictive of whether the disease course will be self-limiting or persistent (43). Nevertheless, several authors suggest it may be useful for the field to move away from Scadding classification, towards phenotyping approaches (3,4). Here we used Scadding classification so that our results could be easily contextualized in previous literature. Future research could incorporate data-driven phenotyping (44) into the analyses developed here, potentially providing more fine-grained predictions of disease stage and progression.
A third limitation is that nuclear imaging was heterogeneous; half of our patients underwent 18F-FDG PET/CT exams, half underwent 67Ga scanning. Current best practices recommend performing imaging investigations through 18F-FDG PET/CT(14–16), although 67Ga scanning yields 88% sensitivity in detecting active sarcoidosis (12) and thus remains a valid tool when 18F-FDG PET/CT is unavailable. Since 18F-FDG PET/CT yields an even higher 97% sensitivity, future studies focusing exclusively on this latter technique may better characterize the observed relationship between PFTs/CPET and disease activity.
Finally, the current study was retrospective. Future prospective investigations are needed to confirm whether the statistical methods developed here can be employed to predict sarcoidosis disease progression and activity.
Conclusions
Our results provide a proof of concept demonstrating that spirometry and CPET variables are jointly predictive of disease stage and activity. Given a large enough sample, future work could robustly correlate spirometry and CPET variables with disease activity, providing clinicians with an additional diagnostic index to decide whether further nuclear imaging investigations are warranted. Such a tool could reduce costs, decrease unnecessary radiation doses, inform clinical decisions to treat, and assist in tailoring patient therapies.
In conclusion we showcase how CPET provides additional insights about disease extent, how CPET and spirometry data complement each other for disease staging, and how these tests are also able to detect sarcoidosis activity. For these reasons, we advocate that CPET, a repeatable and non-invasive functional test, should be more routinely performed and taken into account in sarcoidosis patient follow-up.
Author Contributions:
Conceptualization, C.T., M.R., M.C., F.D., C.C., E.B., F.S., P.C., B.R., and G.M.; methodology, C.T., M.R., and G.M.; software, G.M.; formal analysis, G.M.; investigation, C.T. and M.R.; resources, M.C. and B.R.; data curation, C.T., M.R., and G.M.; writing—original draft preparation, C.T., M.R., and G.M.; writing—review and editing, C.T., M.R., M.C., B.R., G.M.; visualization, C.T., M.R., and G.M.; project administration, C.T. and M.R.; funding acquisition, B.R. All authors have read and agreed to the published version of the manuscript.
Guarantor Statement:
C.T. is the corresponding author and is responsible for all content of the manuscript.
Data Availability Statement:
Data and analysis code is made available from the Zenodo repository (doi: 10.5281/zenodo.10472163).
Conflict of Interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- Statement on Sarcoidosis. Am J Respir Crit Care Med. 1999 Aug 1;160(2):736–55. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- Costabel U, Hunninghake GW. On behalf of the Sarcoidosis Statement Committee. ATS/ERS/WASOG statement on sarcoidosis. Eur Respir J. 1999 Oct;14(4):735. doi: 10.1034/j.1399-3003.1999.14d02.x. [DOI] [PubMed] [Google Scholar]
- Pereira CAC, Dornfeld MC, Baughman R, Judson MA. Clinical phenotypes in sarcoidosis. Current Opinion in Pulmonary Medicine. 2014 Sep;20(5):496–502. doi: 10.1097/MCP.0000000000000077. [DOI] [PubMed] [Google Scholar]
- Culver DA, Baughman RP. It’s time to evolve from Scadding: phenotyping sarcoidosis. Eur Respir J. 2018 Jan;51(1):1800050. doi: 10.1183/13993003.00050-2018. [DOI] [PubMed] [Google Scholar]
- Judson MA. The treatment of pulmonary sarcoidosis. Respiratory Medicine. 2012 Oct;106(10):1351–61. doi: 10.1016/j.rmed.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Ruaro B, Confalonieri P, Santagiuliana M, et al. Correlation between Potential Risk Factors and Pulmonary Embolism in Sarcoidosis Patients Timely Treated. JCM. 2021 Jun 2;10(11):2462. doi: 10.3390/jcm10112462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifaldi R, Salton F, Confalonieri P, et al. Pulmonary Sarcoidosis and Immune Dysregulation: A Pilot Study on Possible Correlation. Diagnostics. 2023 Sep 11;13(18):2899. doi: 10.3390/diagnostics13182899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klech H, Köhn H, Huppmann M, Pohl W. Thoracic imaging with gallium-67. Eur J Nucl Med. 1987 Jun;13(S1):S24–36. doi: 10.1007/BF00253288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keijsers RG, Verzijlbergen EJ, van den Bosch JM, et al. 18F-FDG PET as a predictor of pulmonary function in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2011 Oct;28(2):123–9. [PubMed] [Google Scholar]
- Drent M, Jacobs JA, de Vries J, Lamers RJS, Liem IH, Wouters EFM. Does the cellular bronchoalveolar lavage fluid profile reflect the severity of sarcoidosis? Eur Respir J. 1999 Jun 1;13(6):1338–44. doi: 10.1183/09031936.99.13613459. [DOI] [PubMed] [Google Scholar]
- Prabhakar HB, Rabinowitz CB, Gibbons FK, O’Donnell WJ, Shepard JAO, Aquino SL. Imaging Features of Sarcoidosis on MDCT, FDG PET, and PET/CT. American Journal of Roentgenology. 2008 Mar;190(3_supplement):S1–6. doi: 10.2214/AJR.07.7001. [DOI] [PubMed] [Google Scholar]
- Keijsers RGM, van den Heuvel DAF, Grutters JC. Imaging the inflammatory activity of sarcoidosis. Eur Respir J. 2013 Mar;41(3):743–51. doi: 10.1183/09031936.00088612. [DOI] [PubMed] [Google Scholar]
- Crouser ED, Maier LA, Wilson KC, et al. Diagnosis and Detection of Sarcoidosis. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020 Apr 15;201(8):e26–51. doi: 10.1164/rccm.202002-0251ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel HL, Albertine KH, Park CH, Patrick H. Whole-body Gallium 67 Scans: Role in Diagnosis of Sarcoidosis. Am Rev Respir Dis. 1991 Nov;144(5):1182–6. doi: 10.1164/ajrccm/144.5.1182. [DOI] [PubMed] [Google Scholar]
- Sulavik SB, Spencer RP, Weed DA, Shapiro HR, Shiue ST, Castriotta RJ. Recognition of distinctive patterns of gallium-67 distribution in sarcoidosis. J Nucl Med. 1990 Dec;31(12):1909–14. [PubMed] [Google Scholar]
- Sulavik SB, Spencer RP, Palestro CJ, Swyer AJ, Teirstein AS, Goldsmith SJ. Specificity and Sensitivity of Distinctive Chest Radiographic and/or 67Ga Images in the Noninvasive Diagnosis of Sarcoidosis. Chest. 1993 Feb;103(2):403–9. doi: 10.1378/chest.103.2.403. [DOI] [PubMed] [Google Scholar]
- Lopes AJ, Menezes SLS, Dias CM, Oliveira JF, Mainenti MRM, Guimarães FS. Cardiopulmonary exercise testing variables as predictors of long-term outcome in thoracic sarcoidosis. Braz J Med Biol Res. 2012 Mar;45(3):256–63. doi: 10.1590/S0100-879X2012007500018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcellis RGJ, Lenssen AF, de Vries GJ, et al. Is There an Added Value of Cardiopulmonary Exercise Testing in Sarcoidosis Patients? Lung. 2013 Feb;191(1):43–52. doi: 10.1007/s00408-012-9432-6. [DOI] [PubMed] [Google Scholar]
- Mezzani A. Cardiopulmonary Exercise Testing: Basics of Methodology and Measurements. Annals ATS. 2017 Jul;14(Supplement_1):S3–11. doi: 10.1513/AnnalsATS.201612-997FR. [DOI] [PubMed] [Google Scholar]
- Kallianos A, Zarogoulidis P, Ampatzoglou F, et al. Reduction of exercise capacity in sarcoidosis in relation to disease severity. Patient Prefer Adherence. 2015;9:1179–88. doi: 10.2147/PPA.S86465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medinger AE, Khouri S, Rohatgi PK. Sarcoidosis: The Value of Exercise Testing. Chest. 2001 Jul;120(1):93–101. doi: 10.1378/chest.120.1.93. [DOI] [PubMed] [Google Scholar]
- Miller A, Brown LK, Sloane MF, Bhuptani A, Teirstein AS. Cardiorespiratory Responses to Incremental Exercise in Sarcoidosis Patients With Normal Spirometry. Chest. 1995 Feb;107(2):323–9. doi: 10.1378/chest.107.2.323. [DOI] [PubMed] [Google Scholar]
- Barros WGP, Neder JA, Pereira CAC, Nery LE. Clinical. Radiographic and Functional Predictors of Pulmonary Gas Exchange Impairment at Moderate Exercise in Patients with Sarcoidosis. Respiration. 2004;71(4):367–73. doi: 10.1159/000079641. [DOI] [PubMed] [Google Scholar]
- Kiani A, Eslaminejad A, Shafeipour M, et al. Spirometry, cardiopulmonary exercise testing and the six-minute walk test results in sarcoidosis patients. Sarcoidosis vasculitis and diffuse lung disease. 2019 Sep 16;36(3):185–94. doi: 10.36141/svdld.v36i3.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Elm E, Altman DG, Egger M, Pocock SJ, G⊘tzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Journal of Clinical Epidemiology. 2008 Apr;61(4):344–9. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Scadding JG. Prognosis of Intrathoracic Sarcoidosis in England. BMJ. 1961 Nov 4;2(5261):1165–72. doi: 10.1136/bmj.2.5261.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR. Standardisation of spirometry. European Respiratory Journal. 2005 Aug 1;26(2):319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005 Sep;26(3):511–22. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- Tailor V, Bossi M, Bunce C, Greenwood JA, Dahlmann-Noor A. Binocular versus standard occlusion or blurring treatment for unilateral amblyopia in children aged three to eight years. In: Cochrane Database of Systematic Reviews [Internet] John Wiley & Sons, Ltd; 2015 [cited 2017 Dec 31]. Available from: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD011347.pub2/abstract . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casali M, Lauri C, Altini C, et al. State of the art of 18F-FDG PET/CT application in inflammation and infection: a guide for image acquisition and interpretation. Clin Transl Imaging. 2021 Aug;9(4):299–339. doi: 10.1007/s40336-021-00445-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabold JE, Palestro CJ, Brown ML, et al. Procedure guideline for gallium scintigraphy in inflammation. Society of Nuclear Medicine. J Nucl Med. 1997 Jun;38(6):994–7. [PubMed] [Google Scholar]
- Mostard RLM, Verschakelen JA, van Kroonenburgh MJPG, et al. Severity of pulmonary involvement and 18F-FDG PET activity in sarcoidosis. Respiratory Medicine. 2013 Mar;107(3):439–47. doi: 10.1016/j.rmed.2012.11.011. [DOI] [PubMed] [Google Scholar]
- Zappala CJ, Desai SR, Copley SJ, et al. Optimal scoring of serial change on chest radiography in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2011 Oct;28(2):130–8. [PubMed] [Google Scholar]
- Winterbauer RH, Hutchinson JF. Use of Pulmonary Function Tests in the Management of Sarcoidosis. Chest. 1980 Oct;78(4):640–7. doi: 10.1378/chest.78.4.640. [DOI] [PubMed] [Google Scholar]
- Wallaert B, Talleu C, Wemeau-Stervinou L, Duhamel A, Robin S, Aguilaniu B. Reduction of Maximal Oxygen Uptake in Sarcoidosis: Relationship with Disease Severity. Respiration. 2011;82(6):501–8. doi: 10.1159/000330050. [DOI] [PubMed] [Google Scholar]
- Delobbe A, Perrault H, Maitre J, et al. Impaired exercise response in sarcoid patients with normal pulmonary functio. Sarcoidosis Vasc Diffuse Lung Dis. 2002 Jun;19(2):148–53. [PubMed] [Google Scholar]
- Matthews JI, Hooper RG. Exercise Testing in Pulmonary Sarcoidosis. Chest. 1983 Jan;83(1):75–81. doi: 10.1378/chest.83.1.75. [DOI] [PubMed] [Google Scholar]
- Kollert F, Geck B, Suchy R, et al. The impact of gas exchange measurement during exercise in pulmonary sarcoidosis. Respiratory Medicine. 2011 Jan;105(1):122–9. doi: 10.1016/j.rmed.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Keijsers RGM, Grutters JC. In Which Patients with Sarcoidosis Is FDG PET/CT Indicated? JCM. 2020 Mar 24;9(3):890. doi: 10.3390/jcm9030890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Law MWM, Khong PL. Whole-Body PET/CT Scanning: Estimation of Radiation Dose and Cancer Risk. Radiology. 2009 Apr;251(1):166–74. doi: 10.1148/radiol.2511081300. [DOI] [PubMed] [Google Scholar]
- Demirkok SS, Basaranoglu M, Akinci ED, Karayel T. Analysis of 275 patients with sarcoidosis over a 38 year period; a single-institution experience. Respiratory Medicine. 2007 Jun;101(6):1147–54. doi: 10.1016/j.rmed.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lynch J, Ma Y, Koss M, White E. Pulmonary Sarcoidosis. Semin Respir Crit Care Med. 2007 Feb;28(1):053–74. doi: 10.1055/s-2007-970333. [DOI] [PubMed] [Google Scholar]
- Castro MDC, Pereira CA, de C, Soares MR. Prognostic features of sarcoidosis course in a Brazilian cohort. J Bras Pneumol. 2022;48(1):e20210366. doi: 10.36416/1806-3756/e20210366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagts C, Ascoli C, Fraidenburg DR, et al. Unsupervised Clustering Reveals Sarcoidosis Phenotypes Marked by a Reduction in Lymphocytes Relate to Increased Inflammatory Activity on 18FDG-PET/CT. Front Med. 2021 Feb 24;8:595077. doi: 10.3389/fmed.2021.595077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and analysis code is made available from the Zenodo repository (doi: 10.5281/zenodo.10472163).


